Abstract
Scientific evidence related to the aromatase reaction in various biological processes spanning from mid-1960 to today is abundant; however, as our analytical sensitivity increases, a new look at the old chemical reaction is necessary. Here, we review an irreversible aromatase reaction from the substrate androstenedione. It proceeds in 3 consecutive steps. In the first 2 steps, 19-hydroxy steroids are produced. In the third step, estrone is produced. They can dissociate from the enzyme complex and either accumulate in tissues or enter the blood.
In this review, we want to highlight the potential importance of these 19-hydroxy steroids in various physiological and pathological conditions. We focus primarily on 19-hydroxy steroids, and in particular on the 19-hydroxyandrostenedione produced by the incomplete aromatase reaction. Using a PubMed database and the search term “aromatase reaction,” 19-hydroxylation of androgens and steroid measurements, we detail the chemistry of the aromatase reaction and list previous and current methods used to measure 19-hydroxy steroids.
We present evidence of the existence of 19-hydroxy steroids in brain tissue, ovaries, testes, adrenal glands, prostate cancer, as well as during pregnancy and parturition and in Cushing’s disease. Based on the available literature, a potential involvement of 19-hydroxy steroids in the brain differentiation process, sperm motility, ovarian function, and hypertension is suggested and warrants future research.
We hope that with the advancement of highly specific and sensitive analytical methods, future research into 19-hydroxy steroids will be encouraged, as much remains to be learned and discovered.
Keywords: 19-hydroxyandrostenedione, 19-oxoandrostenedione, ACTH, POR, HPA and HPG axes
The functional aromatase enzyme is a product of the CYP19A1 gene and consists of 503 amino acid residues and a heme group (protoporphyrin IX). Aromatase is a monooxygenase that transfers 1 oxygen atom from molecular dioxygen to the substrate and 1 to the water. There are several endogenous substrates for aromatase: androstenedione, testosterone (T), 16-α hydroxytestosterone, and dihydrotestosterone (DHT), although DHT is only oxidized and not aromatized [1–3].
Aromatase complex consists of 2 highly conserved components: P450 aromatase and NADPH P450 reductase, a product of the POR gene. The expression of POR starts at the 2-cell stage of embryonic development, and a germ-line deletion of this gene in mice results in embryonic lethality, indicating its importance for embryogenesis [4]. POR contains flavin adenine dinucleotide (FAD) and Flavin mononucleotide (FMN), which bind FAD and FMN, and act as a port of entry and exit, respectively, for the electrons transferred from the NADPH to POR gene [5]. Binding of NADPH induces a conformational change in POR to a “closed form” ready for interflavin electron transfer. When the connecting domain loop extends, the whole structure changes to an “open form,” which is also a preferred form when interacting with aromatase [6, 7].
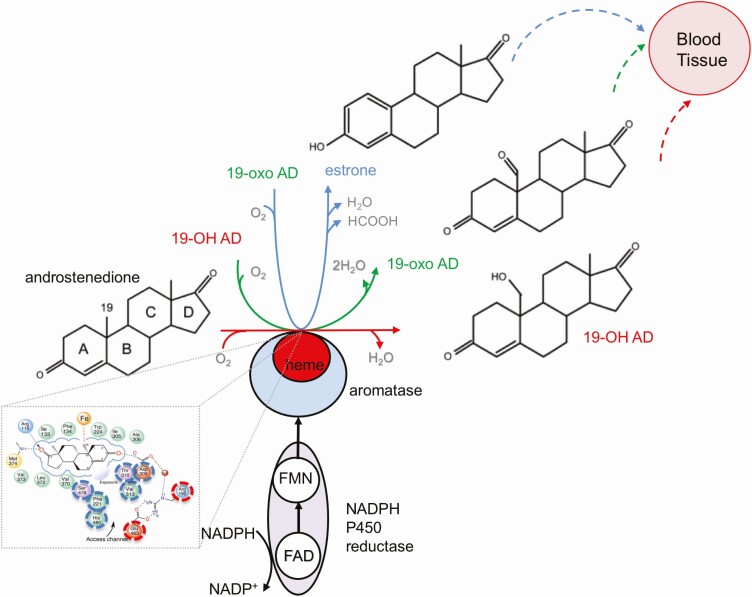
Aromatase catalyzes an irreversible and complex transformation of androgens to estrogens, and it is the only enzyme in vertebrates known to catalyze the aromatization of a six-membered ring [8–11] (Fig. 1). This reaction, first reported in 1959, involves 3 consecutive hydroxylations. When androstenedione is the substrate, the first 2 steps produce two 19-hydroxy steroids, 19-hydroxyandrostendione (19-OH AD), and 19-oxoandrostenedione (19-oxo AD). The third step of aromatase reaction is aromatization of the steroid A ring, which results in the formation of estrone (E1) and formic acid [12–17]. The interaction between aromatase and POR is critical for this reaction and the extent of hydroxylations are highly dependent on this reductase [18]. Limitation of POR results in reduced electron supply and increases the production of 19-OH AD and 19-Oxo AD relative to E1 [19]. Aromatase exists as a homodimer and oligomer, and forms heterodimers only with POR [20, 21]. A proposed ratio of 2:2 (1:1 aromatase homodimer × 2 POR) was suggested, as this ratio leads to the greater activity and reduced release of 19-hydroxy steroids [20].
Figure 1.
Aromatase reaction. The oxidation of androstenedione to estrone by aromatase complex involves NADPH, NADPH P450 reductase, and aromatase. It does not follow a clear linear trajectory of sequential reactions but has a distributive character, where 19-OH AD and 19-oxo AD freely dissociate from the aromatase binding site and enter blood and/or tissue, or re-enter the aromatase reaction again for further oxidation and estrone production (adapted from [154] and [25]). Inset: A closeup look at the aromatase binding pocket with a substrate androstenedione. The residues lining binding pocket are labelled as: hydrophobic-green, acidic-red, basic blue, polar-purple and S-containing yellow. Residues associated with a doorway/access channel are dash-circled (adapted from [22]) and in dash-circled red Arg 192 and Glu 483 gatekeepers are indicated.
Abbreviations: 19-OH AD, 19-hydroxyandrostenedione, 19-oxo AD, 19-oxo-androstenedione.
Aromatase binding pocket is about 400 Å 3 big and consists of heme porphyrin rings and hydrophobic side chains, which form van der Waals contacts and tightly bind androstenedione, with C19 of the methyl group of the substrate androstenedione only 4 Å away from the Fe atom [2, 22]. Binding pocket hosts the proton relay network, and a doorway/access channel through which oxygen, water, and steroids can pass (Fig. 1 inset, dash-circled blue, the “gatekeepers” residues Arg192 and Glu483 are dash-circled red [22]). The aromatase/membrane interface is critical for these access/egress channels and studies have shown that this access channel “breathes,” thus allowing steroids, oxygen, and water to enter and exit the binding pocket [23, 24].
Detailed analysis of aromatase reaction steps demonstrated that aromatase allows a free dissociation of 19-hydroxy steroids, which has been attributed to its distributive-dissociative nature [10, 25]. Similar results were obtained from kinetic studies [14], and also in reconstitution assays [26]. Thus, aromatase is a distributive enzyme, and 19-OH AD and 19-Oxo AD as an aromatase reaction product can dissociate from the complex and may accumulate in the blood and tissues.
Documented Presence and Measurements of 19-hydroxy Steroids in Different Cell Lines, Tissue, and Blood
The first report of the existence of 19-hydroxy steroids dates back to 1955, when Meyer incubated dihydroepiandrostenedione (DHEA) and androstenedione with bovine adrenal homogenate preparations and discovered a 19-OH AD among a “wide galaxy of conversion products” [27]. Later, it was demonstrated that 19-OH AD is also produced by placental and brain aromatase [28–30]. These early measurements employed a radiometric method, in which a tritiated substrate androstenedione was used, and both (1) a transfer of tritium to the water as an indicator of hydroxylation, as well as (2) expulsion of the tritiated C19-fragment with the generation of 3H-formic acid, as an indicator of aromatization were measured [30–34]. These experiments demonstrated much higher concentrations of tritiated 19-OH AD and 19-oxo AD than tritiated estrogens, indicating that these substantial hydroxylations were not followed by the aromatization step. However, it seems that results in these early studies were not corrected for the kinetic isotope effect known to be present in radiometric studies [14]; thus, they were later largely abandoned. In parallel to the radiometric methods, a radioimmunoassay (RIA) was developed and used for measurements of 19-hydroxy steroids in human plasma [35–38]. The RIA method was also used to observe an age-related decrease in endogenous plasma 19-OH AD and androstenedione levels [39].
Because different steroids with similar structures—and differing only in their hydroxyl or carbonyl groups—can cross-react with specific antibodies, their reliability have been recently questioned [40, 41]. Furthermore, radioimmunoassay can measure only 1 analyte at a time.
Currently, gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) are considered accurate, efficient, and reliable methods for the measurement of steroids and their various metabolites [42]. These methods have sufficient analytical selectivity and specificity and can overcome immunoassay deficiencies. Here, we review literature related to measurements of 19-hydroxy steroids in the plasma, tissues, and cell lines.
The GC-MS method was used to measure 19-OH AD in serum from pregnant women and in placenta samples [43]. The high performance liquid chromatography (HPLC) method was used to measure 19-OH AD production from kidney fibroblast-like cells with stable expression of porcine ovarian aromatase [44]. The HPLC method was also used to evaluate the production of 19-hydroxy steroids from HEK293 cells expressing either porcine placental or gonadal aromatase [45, 46]. We have also recently applied the LC-MS method to measure the release of 19-OH AD from the prostate cancer cells [47]. LC-MS method was recently applied to quantify the release of many steroids (using both targeted and untargeted approaches), and among them also 19-OH AD and 16-hydroxytestosterone, from the human adrenal H295R cells [48], which also express aromatase transcripts [49] and are positive for aromatase activity [50].
Production of 19-hydroxy steroids and estrone was also measured using a bioelectrochemical method [51]. In addition, ultra performance liquid chromatography (UPLC)/MS-MS method was used to assess changes in steroid profiles from boar testis tissue, including 19-OH AD, in the presence and absence of aromatase inhibitors [52].
The detectable presence of 19-hydroxy steroids in these studies demonstrate they are not just short-lived intermediates of the aromatase reaction but are likely active metabolites, of which their functions have yet to be revealed.
The aromatase enzyme is expressed in brain, ovary, testis, placenta, adrenal gland, adipose tissue, bone, olfactory system, and also in some malignancies (such as breast cancer [53], prostate cancer [54], malignant human liver cell line HepG2 and HuH7 [55], malignant human lung carcinoma cell line A549 and LK87 [56], and human endometrial carcinoma [57]), and its expression is regulated by tissue-specific promoters [1, 9, 58–60]. Several excellent reviews and manuscripts on aromatase and steroidogenesis have been already published [1, 13, 58, 61–65], and here we will only review literature related to the measurements of 19-hydroxy steroids and their potential effects in various physiological and pathophysiological processes.
Neurosteroidogenesis and Potential Roles for 19-hydroxy Steroids
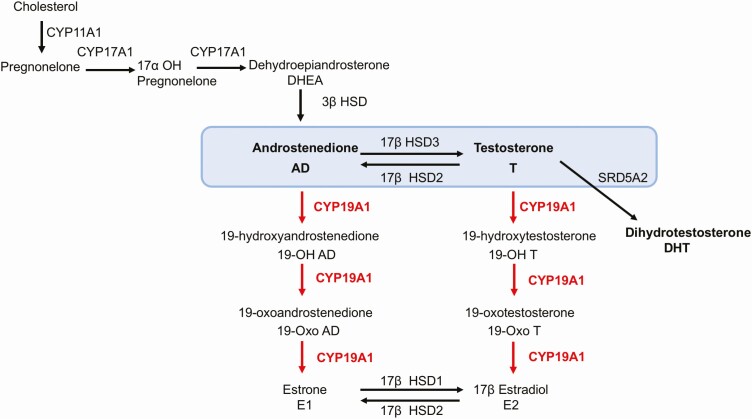
All steroidogenesis enzymes are detected in fetal and adult brain [66–68], and aromatase in particular is present in the hypothalamus, preoptic area (POA), limbic lobe, the olfactory bulb, hippocampus, lateral septum, amygdala, bed nucleus of the stria terminalis, and nucleus accumbens [59, 69–74]. Aromatase substrate androstenedione was also detected in the human adult brain [75]. Furthermore, 3β hydroxysteroid dehydrogenase (3β HSD), an enzyme that converts DHEA to androstenedione (Fig. 2), has been also detected, indicating that oxidation of DHEA to androstenedione is indeed possible in the adult brain [76–81]. Recent mass spectrometry analysis of human proteome was also detected 3β HSD in fetal brain, indicating that androstenedione can be also synthesized in the fetal brain [82]. These results also suggest the potential presence of 19-hydroxy steroids in both fetal and adult brain, and that was indeed demonstrated.
Figure 2.
Aromatase acts on substrates androstenedione and testosterone, producing a variety of 19-hydroxy steroids. 17β HSD enzymes catalyzing the conversion of T to AD and AD to T, as well as E1 to E2 and E2 to E1 are indicated. 3β HSD mediates oxidation of DHEA to AD. The third substrate for aromatase reaction is 16-α hydroxytestosterone. It has been omitted here, as very little is known about it, and it has not been discussed. T is converted to DHT by the enzyme SRD5A2 (steroid 5-alpha reductase A2). Abbreviations: AD, androstenedione; DHEA, dehydroepiandrostenedione; DHT, dihydrotestosterone; E1, estrone; E2, estradiol; HSD, hydroxysteroid dehydrogenase; SRD5A2, steroid 5-alpha reductase A2; T, testosterone.
Aromatization of androstenedione by human fetal hypothalamus and amygdala was first reported in 1971 [83]. Studies revealed a 2-fold greater aromatization in the male vs female hypothalamus [84, 85]. These results provided a basis for the aromatization hypothesis [86], according to which T synthesized by fetal testis diffuses into the male brain, where it is locally aromatized and thus initiates the process of masculinization. In male mice, this process starts at E17 and extends until postnatal day P10 [61, 87–89]. It is believed that the differences that emerge during this initial phase result in sexually dimorphic circuits [90], and aromatase plays an essential role in brain development and sexual differentiation [91]. Aromatase expression in sexually dimorphic regions of the brain is at its highest level during this perinatal period, demonstrating its critical role in the development of sexually dimorphic patterning [74, 92, 93]. In female embryos, steroid-secreting ovaries develop after the first postnatal week, and the process of feminization has been classically viewed as “default” [94, 95]. Estradiol presence was, however, detected in the female newborn brain, suggesting that female rat fetal brain can also synthesize estrogens de novo [68, 90]. This intriguing finding is hard to explain by the aromatization hypothesis, which implies that only circulating T is a precursor for estrogen, because in the female fetal brain T is nondetectable [75]. These results suggest potential roles for aromatase reaction products derived from androstenedione, warrant future research, and call for a re-evaluation of the initial aromatization hypothesis. A recent study by Nugent et al demonstrated active repression of male-typical genes mediated by DNA methylation during the brain feminization process [96]. Thus, the brain differentiation process is far from being clearly defined, and novel mechanisms, such as epigenetic control, is currently being actively investigated.
Do 19-hydroxy steroids accumulate during a critical perinatal period to contribute to the brain differentiation?
Brain aromatase activity is developmentally regulated and expressed in different regions of the male rat brain [93]. The number of aromatase-positive neurons in rodent studies of both sexes increases during gestation and peaks around birth, with higher expression in males, but estrogen content (although increased at birth in the male hypothalamus) decreases significantly already at 2 hours after birth, at the time when aromatase activity is still high [97–101]. Thus, there is a lack of a clear correlation between aromatase activity and estrogen content during this critical period, which may be indicative of potentially incomplete aromatase reaction. The evidence that the first 19-hydroxylation step can exceed the final third aromatization step in male rat neonatal hypothalamus and amygdala was first published in 1985 [34]. These results demonstrated increased accumulation of 19-OH AD and 19-Oxo AD when compared with E1 [34]. In addition, it was demonstrated that the ratio of 19-hydroxylation/aromatization was similar in the neonate and adult rat hypothalamus in both sexes [33]. However, since these results were obtained using a radiometric method, a more accurate and specific analysis using a more specific LC-MS method is now necessary to confirm these early results.
Estrogen injection in male rats 4 days after birth produced adults unable to achieve intromission, although they mounted as frequently as control animals [102]. On the contrary, androstenedione injection during this period resulted in normal patterns of sexual behavior in adulthood [103]. Blocking aromatase blocked the brain differentiation of the male rodent brain [104]. These results indicate that aromatase reaction products derived from androstenedione may be implicated in brain differentiation during the critical perinatal period.
The sexually dimorphic nucleus of the POA (sexually dimorphic nucleus [SDN]-POA) in the hypothalamus is important for male copulatory behavior, and it is several-fold larger in male rats than in female rats [105]. Testosterone treatment of females during a perinatal period produced larger SDN-POA, similar to the one seen in males, and the application of aromatase inhibitor during this critical period reduced the size of SDN-POA and changed male copulatory behavior [105, 106]. As no sex differences in estrogen receptor expression (ERs) in SDN-POA has been detected, the current view is that ER expression has not been proven informative as the basis of sex differences during brain development [90, 107, 108]. We suggest that conversion of T to androstenedione by 17β-hydroxysteroid dehydrogenase (HSD17B2) may occur [64, 109]. HSD17B2 is present in the fetal brain, where it can convert T to androstenedione, thus making it available to act as a substrate for aromatase during this critical period (Fig. 2) [82, 93]. Recent elegant experiments using a brain-specific ArKO model demonstrated the importance of brain aromatase for T-dependent male sexual activity and feedback regulation of T of testicular origin in the adult mice [65].
Ovaries
Human ovarian follicles synthesize estrogen in a compartmentalized fashion; androgens are produced in the outer theca interna cells layer, while estrogens are produced in the inner granulosa layer [110]. This “2-cell” organization of follicular estrogen synthesis may have its basis in avoiding the competition between CYP17A1 and CYP19A1 for reducing equivalents provided by POR if both enzymes are expressed in the same cell [111]. Follicle stimulating hormone (FSH) increases both aromatase and POR activity, and it induces differentiation of rat granulosa cells into steroidogenic cells [112].
Do ovaries produce 19-hydroxy steroids?
Ovarian synthesis of estrogens in ovarian granulosa cells is associated with the parallel synthesis of 19-OH AD and 19-Oxo AD, and tritiated water assay indicated that these metabolites accumulate in higher quantities than estrogens [113, 114]. Production of 19-OH AD was also reported in the human ovarian HOSE 17 cells using an reverse-phase-HPLC method [115]. How exactly 19-hydroxy steroids affect ovarian function has not been investigated, but an intriguing hypothesis of their potential role in sperm chemotaxis described below is proposed.
Testes
Adult testicular germ cells express aromatase, and estrogens play an important role in sperm maturation [1, 116]. Association between aromatase and sperm count and motility has been clearly established, and both 19-OH AD and 19-OH T were also detected in the testicular vein blood, suggesting their role in in sperm motility [117–122]. Recently, it has been demonstrated that prolonged treatment with letrozole decreased 19-OH AD levels in testis when analyzed by the highly specific UPLC-MS/MS method; however, the effects on sperm motility were not analyzed [52].
Is 19-OH AD involved in sperm motility and chemotaxis?
It is interesting to mention that about 90 transcripts of olfactory receptors (ORs) have been found in human spermatozoa [123]. Previously, another olfactory receptor, hOR17-4, has been implicated in sperm chemotaxis [124], and more recently several highly expressed ORs have been detected in seminal plasma, sperm, testes, and epididymis using a high-resolution mass-spectrometer approach [125]. Olfactory receptor OR51E2 is highly expressed on the acrosome cap, the midpiece, and the entire flagella in spermatozoa [123]. Recently, 19-OH AD has been identified as a potent agonist for olfactory receptor OR51E2 [47]. Thus, activation of this receptor by 19-OH AD may contribute to sperm motility. In addition, secretion of 19-OH AD from the ovarian cells has also been reported [115]. Taken together, these studies suggest that: (1) 19-OH AD and 19-OH T originating from testes may contribute to sperm motility, and (2) 19-OH AD secreted from the ovarian cells may activate OR51E2 in the sperm, and thus contribute to sperm chemotaxis. Future studies are necessary to test these assumptions.
Pregnancy and Parturition
An increase of 19-OH AD measured by the GC-MS method during pregnancy was reported and 6-fold higher concentrations were detected at the end of the third trimester [43]. This increase of 19-OH AD in the maternal blood is also combined with its dramatic decrease in the umbilical artery at delivery, indicating that all 19-OH AD is completely transferred and taken up by the baby and/or placenta at delivery, while no such effect was observed with either T or E1 [43]. High amounts of 19-OH AD were also detected in the end-term placenta tissue [43], indicating that the placenta may be the major source of 19-hydroxy steroids production. Unfortunately, there was no follow-up on this study.
Taken together, these results suggest that 19-OH AD is likely to originate from the placenta. What is the function of this newly produced steroid and is it important for parturition? These remain open questions, as we still lack a full understanding of the role of different steroids in the parturition process.
A recent transcriptomic study of the fetal–maternal interface from Vento-Tormo et al. demonstrate an overlapping aromatase and OR51E2 receptor presence in the syncytiotrophoblasts [126]. As 19-OH AD is a ligand for OR51E2 receptor, it would be interesting to study their potential molecular interactions with respect to aromatase during early placentation period (https://maternal-fetal-interface.cellgeni.sanger.ac.uk/).
No correlation between androstenedione levels and gestation in normotensive pregnant women was found; however, in hypertensive women, a highly significant correlation was demonstrated [127]. Furthermore, increased levels of circulating T were found in women with preeclampsia and although the early studies reported an increase in circulating 19-OH AD in the hypertensive pregnant women, a subsequent study on the small number of participants did not support this claim [43, 127–130]. Thus, future studies on a larger sample-set are warranted to clarify if 19-hydroxy steroids play any role in pregnancy and parturition.
Adrenal Glands
Adrenal steroidogenesis is regulated by sympathetic innervation, ACTH, and by complex paracrine interactions of interdispersed medulla and cortex cells. As aromatase is expressed in the adrenal gland, it is highly likely that this gland also produces 19-OH AD [131, 132]. This has been indeed demonstrated in several early studies using the RIA method.
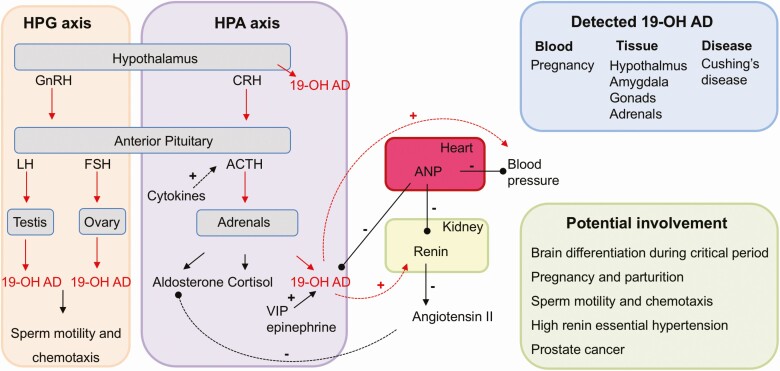
The secretion of 19-OH AD increased during ACTH and angiotensin II stimulation [35, 36, 133]. These results support the view that 19-OH has an adrenal origin. In addition, these studies further strengthen the notion that the first C-19 hydroxylation step (in which 19-OH AD is produced under ACTH control) is to a certain extent unaccompanied by subsequent aromatization and thus may represent a potentially significant physiologically relevant transformation. The regulation of 19-OH AD secretion from cultured human adrenal cells by ACTH was also demonstrated [134]. Of note, when ACTH is suppressed, angiotensin II acts to stimulate secretion of 19-OH AD [37, 133]. Since the highest expression of aromatase in the adrenal gland was detected in zona reticularis, the synthesis of 19-OH AD is most likely to be in that area, however direct evidence for this is still lacking [135]. Positive correlations between basal plasma 19-OH AD and androstenedione, as well as cortisol, were reported, and the suppression of 19-OH AD secretion by dexamethasone indicates that 19-OH AD secretion is regulated by the hypothalamic pituitary adrenal axis (HPA) axis (Fig. 3) [39, 136].
Figure 3.
Regulation, detection, and the potential involvement of 19-OH AD in human physiology and pathophysiology. Secretion of 19-OH AD is under control of the hypothalamic pituitary adrenal axis (HPA) and is directly stimulated by ACTH. LH and FSH acting on testis and ovary also increase 19-OH AD secretion via the hypothalamic pituitary gonadal (HPG) axis. Cytokines likely indirectly increase 19-OH AD via ACTH. Positive and negative regulation of 19-OH AD secretion by VIP, epinephrine, and ANP are indicated. 19-OH AD increases blood pressure and renin secretion and is increased during pregnancy and Cushing’s disease. Tissues, where 19-OH AD has been measured, are indicated, and a list of potential involvement in several physiological and pathophysiological processes is presented. Abbreviations: 19-OH AD, 19-hydroxyandrostenedione; ACTH, adrenocorticotropic hormone; ANP, atrial natriuretic peptide; FSH, follicle stimulating hormone; LH, luteinizing hormone; VIP, vasoactive intestinal peptide.
Various neuroendocrine modulators, such as epinephrine and vasoactive intestinal peptide (VIP) are released from the adrenal nerves, while androstenedione and other C19-androgens are released from the adrenocortical cells [137, 138], suggesting that these cells may also potentially release 19-OH AD. In contrast, it was demonstrated that atrial natriuretic peptide (ANP) decreases the secretion of 19-OH AD [134]. Cytokines produced by either immune cells within the gland or by adrenal cells can also affect adrenal steroidogenesis. For example, IL-6 activates the HPA axis and stimulates a release of ACTH, and also stimulates a release of aldosterone, cortisol, and DHEA [139, 140]. Thus, cytokines are likely to also increase 19-OH AD secretion. Further research is needed to prove these assumptions.
What are the functional consequences of increased 19-OH AD secretion in Cushing’s disease?
High levels of 19-OH AD were detected in Cushing’s disease, a benign pituitary adenoma, characterized by the increased secretion of ACTH, which stimulates adrenal glands to secrete cortisol and 19-OH AD, while decreased levels of 19-OH AD are seen in Cushing’s syndrome [141]. Whether this secretion exerts a negative feedback loop in the hypothalamus is currently unknown (Fig. 3).
Obesity is one of the symptoms of Cushing’s disease, and since aromatase is abundantly expressed in adipose tissue, increased production of 19-OH AD from the adipose tissue is also likely. Future research is needed to unravel the functional consequences of high 19-OH AD found in the blood of patients with Cushing’s disease, who also develop hypertension.
Hypertension and 19-OH AD
19-OH AD–treated rats developed hypertension, and elevated 19-OH AD was reported in patients with high renin essential hypertension [36, 38]. 19-OH AD and 19-Oxo AD also amplify the sodium-retaining action of aldosterone [142, 143]. These results suggested a potential role of the renin-angiotensin system (RAS) in 19-OH AD secretion [37, 144]. Aldosterone-producing adenoma patients have lower levels of circulating 19-OH AD, likely due to a suppressed RAS; however, it seems that 19-OH AD does not play a causative role in hypertension seen in these patients [145]. Future experiments are warranted to dissect the role of this steroid in hypertension. It is interesting to note that Olfr78, a mouse ortholog of human olfactory receptor OR51E2, is activated by 19-OH AD and is also expressed in renal afferent arterioles where it can affect renin release when stimulated with short chain fatty acids (SCFA) [47, 146]. Olfr78 knock-out mice also have lower circulating plasma renin [146, 147]. Olfactory receptors, like most G-protein coupled receptors (GPCRs), are quite promiscuous, and it is possible that their activation by various agonists including 19-OH AD contributes to blood pressure regulation.
19-OH AD, POR, and Cancer
Aromatase overexpression in tumor tissue results from a shift in promoter use, which allows for the activation of cAMP-dependent signaling pathway and results in increased estrogen synthesis [58]. Both aromatase and CYP17A1 require POR for their electron transport and catalysis, and if expressed in the same cell, which is the case in the cancer cell, these 2 cytochrome enzymes compete with one another for POR, reducing equivalents NADPH and O2. A 30-fold increase in CYP19A1 and a 17-fold increase in CYP17A1 was measured in the metastatic prostate cancer tissue, while the enzyme SRD5A2 (5α reductase), which converts T to DHT, is decreased 9 fold (Fig. 3) [148]. A disbalance in the T:E ratio has been associated with prostate cancer progression, and increased secretion of 19-OH AD was detected in the prostate cancer cells following activation of olfactory receptor OR51E2, indicating a potential role for aromatase reaction products in prostate carcinogenesis [47, 149]. Administration of abiraterone acetate (a CYP17A1 inhibitor) may actually save NADPH and POR for the aromatase reaction, thus driving androgen metabolism via aromatase with a consequent release of 19-OH AD, 19-Oxo AD, and estrone. This may potentially contribute to chemotherapy resistance. Steroid hormones stimulate prostate cancer progression and ArKO mice have reduced prostatic hyperplasia and incidence of prostate cancer following exposure to T and estrogens, indicating that 19-hydroxy steroids are likely involved in prostate carcinogenesis [150, 151].
Aromatase inhibitors have major roles in the treatment of hormone-sensitive breast cancer and, recently, POR was identified as an independent prognostic biomarker of short recurrence-free survival of triple-negative breast cancer patients [152]. It has been demonstrated that patients with triple-negative breast cancer and with high POR expression in the primary tumors have a 2-fold higher risk of tumor recurrence [153].
Conclusion
A list of potential roles of 19-hydroxy steroids, and of 19-OH AD in particular, in various physiological and pathophysiological processes is presented in Fig. 3.
Research in the brain differentiation process started over 5 decades ago, and although much has been learned, it is still not completely understood. We believe that future studies of steroid metabolites, and in particular 19-hydroxy steroids, using state-of-the-art analytical tools will help to better understand this extremely complex process.
Striking data from pregnancy studies indicate an underappreciated role of 19-OH AD and should be followed by future studies.
Both ovarian and testicular synthesis of 19-OH AD has been documented, but its role has not been examined so far.
Increased secretion of 19-OH AD in Cushing’s disease may warrant future research to determine its role in disease pathology. Could 19-OH AD serve as a diagnostic biomarker in Cushing’s disease? As 19-OH AD secretion from the adrenal gland is under the HPA axis, we assume it will be also involved in stress-related behaviors. Does it contribute to hypertension?
Many questions still remaining unanswered. Is 19-OH AD an androgen? Does 19-OH AD produced by adrenals send negative feedback to the hypothalamus? Do 19-hydroxy steroids act as ligands for other GPCRs (except OR51E2), transporters, or channels? Which signaling pathways are regulated by 19-hydroxy steroids? This review raises interesting questions that merit further investigation. We hope that it will stimulate future studies related to the roles of 19-OH AD and 19-Oxo AD in the brain, pregnancy, blood pressure regulation, Cushing’s disease, and cancer.
Numerous studies related to aromatase reaction have been listed here, and many more are certainly missing, but we hope that the information provided will stir discussion and stimulate future research endeavors. As our analytical sensitivity and methodology are nowadays significantly improved, it is time to re-examine 19-hydroxy steroids, the products of aromatase reaction.
Acknowledgments
We are grateful to Dr Maira Harume Nagai and Professors Jennifer L. Pluznick and F. Peter Guengerich for suggestions and their critical reading of the manuscript.
Financial Support: National Institute of Health/National Institute on Deafness and Other Communication Disorders R01DC016224.
Glossary
Abbreviations
- 19-OH AD
, 19-hydroxyandrostenedione
- 19-Oxo AD
19-oxoandrostenedione
- 3β HSD
3β hydroxysteroid dehydrogenase
- ACTH
adenocorticotropic hormone
- ANP
atrial natriuretic peptide
- ArKO
aromatase-deficient
- BNST
bed nucleus of the stria terminalis
- CRH
corticotropin releasing factor
- DHEA
dehydroepiandrosterone
- DHT
dihydrotestosterone
- E1
estrone
- E2
17β-estradiol
- E3
17β,16α-estriol
- FSH
follicle stimulating hormone
- GnRH
gonadotropin releasing hormone
- HPA
hypothalamic pituitary adrenal axis
- HPG
hypothalamic pituitary gonadal axis
- HSD17B
17β-hydroxysteroid dehydrogenase
- LH
luteinizing hormone
- PE
pre-eclampsia
- POA
preoptic area
- POR
cytochrome P450 oxidoreductase NADPH cytochrome reductase
- RAAS
renin-angiotensin-aldosterone system
- SDN
sexually dimorphic nucleus of the POA
- T
testosterone
- VIP
vasoactive intestinal peptide.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
References
- 1. Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77(1-2):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457(7226):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng Q, Sohl CD, Yoshimoto FK, Guengerich FP. Oxidation of dihydrotestosterone by human cytochromes P450 19A1 and 3A4. J Biol Chem. 2012;287(35):29554–29567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen AL, O’Leary KA, Kasper CB. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem. 2002;277(8):6536–6541. [DOI] [PubMed] [Google Scholar]
- 5. Aigrain L, Fatemi F, Frances O, Lescop E, Truan G. Dynamic control of electron transfers in diflavin reductases. Int J Mol Sci. 2012;13(11):15012–15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ. Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc Natl Acad Sci U S A. 1997;94(16):8411–8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamdane D, Xia C, Im SC, Zhang H, Kim JJ, Waskell L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J Biol Chem. 2009;284(17):11374–11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghosh D, Griswold J, Erman M, Pangborn W. X-ray structure of human aromatase reveals an androgen-specific active site. J Steroid Biochem Mol Biol. 2010;118(4-5):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342–355. [DOI] [PubMed] [Google Scholar]
- 10. Guengerich FP. Mechanisms of cytochrome P450-catalyzed oxidations. ACS Catal. 2018;8(12):10964–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rendic SP, Peter Guengerich F. Human cytochrome P450 enzymes 5-51 as targets of drugs and natural and environmental compounds: mechanisms, induction, and inhibition - toxic effects and benefits. Drug Metab Rev. 2018;50(3):256–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryan KJ. Biological aromatization of steroids. J Biol Chem. 1959;234(2):268–272. [PubMed] [Google Scholar]
- 13. Blakemore J, Naftolin F. Aromatase: contributions to physiology and disease in women and men. Physiology (Bethesda). 2016;31(4):258–269. [DOI] [PubMed] [Google Scholar]
- 14. Khatri Y, Luthra A, Duggal R, Sligar SG. Kinetic solvent isotope effect in steady-state turnover by CYP19A1 suggests involvement of Compound 1 for both hydroxylation and aromatization steps. FEBS Lett. 2014;588(17):3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gantt SL, Denisov IG, Grinkova YV, Sligar SG. The critical iron-oxygen intermediate in human aromatase. Biochem Biophys Res Commun. 2009;387(1):169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mak PJ, Luthra A, Sligar SG, Kincaid JR. Resonance raman spectroscopy of the oxygenated intermediates of human CYP19A1 implicates a compound i intermediate in the final lyase step. J Am Chem Soc. 2014;136(13):4825–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshimoto FK, Guengerich FP. Mechanism of the third oxidative step in the conversion of androgens to estrogens by cytochrome P450 19A1 steroid aromatase. J Am Chem Soc. 2014;136(42):15016–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murataliev MB, Feyereisen R, Walker FA. Electron transfer by diflavin reductases. Biochim Biophys Acta. 2004;1698(1):1–26. [DOI] [PubMed] [Google Scholar]
- 19. Grogan J, Shou M, Zhou D, Chen S, Korzekwa KR. Use of aromatase (CYP19) metabolite ratios to characterize electron transfer from NADPH-cytochrome P450 reductase. Biochemistry. 1993;32(45):12007–12012. [DOI] [PubMed] [Google Scholar]
- 20. Martin LL, Holien JK, Mizrachi D, et al. Evolutionary comparisons predict that dimerization of human cytochrome P450 aromatase increases its enzymatic activity and efficiency. J Steroid Biochem Mol Biol. 2015;154:294–301. [DOI] [PubMed] [Google Scholar]
- 21. Praporski S, Ng SM, Nguyen AD, et al. Organization of cytochrome P450 enzymes involved in sex steroid synthesis: protein–protein ineractions in lipid membranes. J Biol Chem. 2009;284(48):33224–33232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghosh D, Lo J, Egbuta C. Recent progress in the discovery of next generation inhibitors of aromatase from the structure-function perspective. J Med Chem. 2016;59(11):5131–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sgrignani J, Magistrato A. Influence of the membrane lipophilic environment on the structure and on the substrate access/egress routes of the human aromatase enzyme. a computational study. J Chem Inf Model. 2012;52(6):1595–1606. [DOI] [PubMed] [Google Scholar]
- 24. Jiang W, Ghosh D. Motion and flexibility in human cytochrome p450 aromatase. Plos One. 2012;7(2):e32565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sohl CD, Guengerich FP. Kinetic analysis of the three-step steroid aromatase reaction of human cytochrome P450 19A1. J Biol Chem. 2010;285(23):17734–17743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sethumadhavan K, Bellino FL. Human placental estrogen synthetase (aromatase). Effect of environment on the kinetics of protein-protein and substrate-protein interactions and the production of 19-oxygenated androgen intermediates in the purified reconstituted cytochrome P450 enzyme system. J Steroid Biochem Mol Biol. 1991;39(3):381–394. [DOI] [PubMed] [Google Scholar]
- 27. Meyer AS. 19-Hydroxylation of delta 4-Androstene-3,17-dione and dehydroepiandrosterone by bovine adrenals. Experientia. 1955;11(3):99–102. [DOI] [PubMed] [Google Scholar]
- 28. Fishman J, Goto J. Mechanism of estrogen biosynthesis. Participation of multiple enzyme sites in placental aromatase hydroxylations. J Biol Chem. 1981;256(9):4466–4471. [PubMed] [Google Scholar]
- 29. Fishman J, Raju MS. Mechanism of estrogen biosynthesis. Stereochemistry of C-1 hydrogen elimination in the aromatization of 2 beta-hydroxy-19-oxoandrostenedione. J Biol Chem. 1981;256(9):4472–4477. [PubMed] [Google Scholar]
- 30. Hahn EF, Fishman J. Stereochemistry of 1,2-hydrogen loss during aromatization in the brain. J Steroid Biochem. 1985;22(5):597–600. [DOI] [PubMed] [Google Scholar]
- 31. Thompson EA Jr, Siiteri PK. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J Biol Chem. 1974;249(17):5364–5372. [PubMed] [Google Scholar]
- 32. Miyairi S, Sugita O, Sassa S, Fishman J. Aromatization and 19-hydroxylation of androgens by rat brain cytochrome P-450. Biochem Biophys Res Commun. 1988;150(1):311–315. [DOI] [PubMed] [Google Scholar]
- 33. Michnovicz JJ, Hahn EF, Fishman J. 19-Hydroxylation and aromatization of androgens in the developing rat brain. Endocrinology. 1987;121(4):1209–1214. [DOI] [PubMed] [Google Scholar]
- 34. Hahn EF, Miyairi S, Fishman J. 19-Hydroxylation of androgens in the rat brain. Proc Natl Acad Sci U S A. 1985;82(9):2728–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sekihara H. 19-hydroxyandrostenedione as a new hypertensinogenic agent. J Steroid Biochem. 1982;16(2):329–331. [DOI] [PubMed] [Google Scholar]
- 36. Sekihara H, Ohsawa N. 19-hydroxyandrostenedione, a new amplifier of the action of aldosterone, in low renin essential hypertension. Jpn J Med. 1982;21(2):154–155. [DOI] [PubMed] [Google Scholar]
- 37. Sekihara H, Torii R, Osawa Y, Takaku F. Angiotensin II induces the release of 19-hydroxyandrostenedione in man. J Clin Endocrinol Metab. 1985;61(2):291–296. [DOI] [PubMed] [Google Scholar]
- 38. Sekihara H, Yonemitsu K, Yazaki Y. Plasma 19-hydroxyandrostenedione is elevated in patients with high renin essential hypertension. Clin Endocrinol (Oxf). 1993;39(5):557–560. [DOI] [PubMed] [Google Scholar]
- 39. Higuchi K, Ogo A, Maki T, et al. Evidence for age-related change in plasma 19-hydroxyandrostenedione. Endocrinol Jpn. 1989;36(6):881–885. [DOI] [PubMed] [Google Scholar]
- 40. Penning TM, Lee SH, Jin Y, Gutierrez A, Blair IA. Liquid chromatography-mass spectrometry (LC-MS) of steroid hormone metabolites and its applications. J Steroid Biochem Mol Biol. 2010;121(3-5):546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahmed KEM, Frøysa HG, Karlsen OA, et al. LC-MS/MS based profiling and dynamic modelling of the steroidogenesis pathway in adrenocarcinoma H295R cells. Toxicol in Vitro. 2018;52:332–341. [DOI] [PubMed] [Google Scholar]
- 42. Auchus RJ. Steroid assays and endocrinology: best practices for basic scientists. Endocrinology. 2014;155(6):2049–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Osawa Y, Ohnishi S, Yarborough C, et al. Serum level of 19-hydroxyandrostenedione during pregnancy and at delivery determined by gas chromatography/mass spectrometry. Steroids. 1990;55(4):165–169. [DOI] [PubMed] [Google Scholar]
- 44. Corbin CJ, Khalil MW, Conley AJ. Functional ovarian and placental isoforms of porcine aromatase. Mol Cell Endocrinol. 1995;113(1):29–37. [DOI] [PubMed] [Google Scholar]
- 45. Corbin CJ, Trant JM, Walters KW, Conley AJ. Changes in testosterone metabolism associated with the evolution of placental and gonadal isozymes of porcine aromatase cytochrome P450. Endocrinology. 1999;140(11):5202–5210. [DOI] [PubMed] [Google Scholar]
- 46. Corbin CJ, Trant JM, Conley AJ. Porcine gonadal and placental isozymes of aromatase cytochrome P450: sub-cellular distribution and support by NADPH-cytochrome P450 reductase. Mol Cell Endocrinol. 2001;172(1-2):115–124. [DOI] [PubMed] [Google Scholar]
- 47. Abaffy T, Bain JR, Muehlbauer MJ, et al. A testosterone metabolite 19-hydroxyandrostenedione induces neuroendocrine trans-differentiation of prostate cancer cells via an ectopic olfactory receptor. Front Oncol. 2018;8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strajhar P, Tonoli D, Jeanneret F, et al. Steroid profiling in H295R cells to identify chemicals potentially disrupting the production of adrenal steroids. Toxicology. 2017;381:51–63. [DOI] [PubMed] [Google Scholar]
- 49. Staels B, Hum DW, Miller WL. Regulation of steroidogenesis in NCI-H295 cells: a cellular model of the human fetal adrenal. Mol Endocrinol. 1993;7(3):423–433. [DOI] [PubMed] [Google Scholar]
- 50. Sanderson JT, Hordijk J, Denison MS, Springsteel MF, Nantz MH, van den Berg M. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2004;82(1):70–79. [DOI] [PubMed] [Google Scholar]
- 51. Di Nardo G, Castrignanò S, Sadeghi SJ, Baravalle R, Gilardi G. Bioelectrochemistry as a tool for the study of aromatization of steroids by human aromatase. Electrochem Commun. 2015;52:25–28. [Google Scholar]
- 52. Kucera H, Puschner B, Conley A, Berger T. Tissue steroid levels in response to reduced testicular estrogen synthesis in the male pig, Sus scrofa. Plos One. 2019;14(4):e0215390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kijima I, Ye J, Glackin C, Chen S. CCAAT/enhancer binding protein delta up-regulates aromatase promoters I.3/II in breast cancer epithelial cells. Cancer Res. 2008;68(11):4455–4464. [DOI] [PubMed] [Google Scholar]
- 54. Ellem SJ, Risbridger GP. Aromatase and prostate cancer. Minerva Endocrinol. 2006;31(1):1–12. [PubMed] [Google Scholar]
- 55. Carruba G. Aromatase in nontumoral and malignant human liver tissues and cells. Ann N Y Acad Sci. 2009;1155:187–193. [DOI] [PubMed] [Google Scholar]
- 56. Miki Y, Suzuki T, Abe K, et al. Intratumoral localization of aromatase and interaction between stromal and parenchymal cells in the non-small cell lung carcinoma microenvironment. Cancer Res. 2010;70(16):6659–6669. [DOI] [PubMed] [Google Scholar]
- 57. Watanabe K, Sasano H, Harada N, et al. Aromatase in human endometrial carcinoma and hyperplasia. Immunohistochemical, in situ hybridization, and biochemical studies. Am J Pathol. 1995;146(2):491–500. [PMC free article] [PubMed] [Google Scholar]
- 58. Simpson ER, Clyne C, Rubin G, et al. Aromatase–a brief overview. Annu Rev Physiol. 2002;64:93–127. [DOI] [PubMed] [Google Scholar]
- 59. Shay DA, Vieira-Potter VJ, Rosenfeld CS. Sexually dimorphic effects of aromatase on neurobehavioral responses. Front Mol Neurosci. 2018;11:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kamat A, Hinshelwood MM, Murry BA, Mendelson CR. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab. 2002;13(3):122–128. [DOI] [PubMed] [Google Scholar]
- 61. Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Brain Res Rev. 1996;22(1):1–26. [PubMed] [Google Scholar]
- 62. Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30(4):343–375. [DOI] [PubMed] [Google Scholar]
- 63. Bakker J. The sexual differentiation of the human brain: role of sex hormones versus sex chromosomes. In: Coolen LM, Grattan DR, eds. Neuroendocrine Regulation of Behavior. Cham: Springer International Publishing; 2019:45–67. [DOI] [PubMed] [Google Scholar]
- 64. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brooks DC, Coon VJ, Ercan CM, et al. Brain aromatase and the regulation of sexual activity in male mice. Endocrinology. 2020;161(10):bqaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shibuya K, Takata N, Hojo Y, et al. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim Biophys Acta. 2003;1619(3):301–316. [DOI] [PubMed] [Google Scholar]
- 67. Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56. [DOI] [PubMed] [Google Scholar]
- 68. Kretz O, Fester L, Wehrenberg U, et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24(26):5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Callard GV, Petro Z, Ryan KJ. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology. 1978;103(6):2283–2290. [DOI] [PubMed] [Google Scholar]
- 70. Balthazart J, Foidart A, Surlemont C, Harada N. Neuroanatomical specificity in the co-localization of aromatase and estrogen receptors. J Neurobiol. 1991;22(2):143–157. [DOI] [PubMed] [Google Scholar]
- 71. Foidart A, Harada N, Balthazart J. Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res. 1995;280(3):561–574. [DOI] [PubMed] [Google Scholar]
- 72. Cisternas CD, Garcia-Segura LM, Cambiasso MJ. Hormonal and genetic factors interact to control aromatase expression in the developing brain. J Neuroendocrinol. 2018;30(2):1–8. [DOI] [PubMed] [Google Scholar]
- 73. Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27(3):207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu MV, Manoli DS, Fraser EJ, et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hammond GL, Hirvonen J, Vihko R. Progesterone, androstenedione, testosterone, 5 alpha-dihydrotestosterone and androsterone concentrations in specific regions of the human brain. J Steroid Biochem. 1983;18(2):185–189. [DOI] [PubMed] [Google Scholar]
- 76. Weidenfeld J, Siegel RA, Chowers I. In vitro conversion of pregnenolone to progesterone by discrete brain areas of the male rat. J Steroid Biochem. 1980;13(8):961–963. [DOI] [PubMed] [Google Scholar]
- 77. Dupont E, Simard J, Luu-The V, Labrie F, Pelletier G. Localization of 3 beta-hydroxysteroid dehydrogenase in rat brain as studied by in situ hybridization. Mol Cell Neurosci. 1994;5(2):119–123. [DOI] [PubMed] [Google Scholar]
- 78. Guennoun R, Fiddes RJ, Gouézou M, Lombès M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30(2):287–300. [DOI] [PubMed] [Google Scholar]
- 79. Meffre D, Delespierre B, Gouézou M, Schumacher M, Stein DG, Guennoun R. 3beta-Hydroxysteroid dehydrogenase/5-ene-4-ene isomerase mRNA expression in rat brain: effect of pseudopregnancy and traumatic brain injury. J Steroid Biochem Mol Biol. 2007;104(3-5):293–300. [DOI] [PubMed] [Google Scholar]
- 80. Pelletier G. Steroidogenic enzymes in the brain: morphological aspects. Prog Brain Res. 2010;181:193–207. [DOI] [PubMed] [Google Scholar]
- 81. Brown RC, Cascio C, Papadopoulos V. Pathways of neurosteroid biosynthesis in cell lines from human brain: regulation of dehydroepiandrosterone formation by oxidative stress and beta-amyloid peptide. J Neurochem. 2000;74(2):847–859. [DOI] [PubMed] [Google Scholar]
- 82. Kim MS, Pinto SM, Getnet D, et al. A draft map of the human proteome. Nature. 2014;509(7502):575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol. 1971;51(4):795–796. [DOI] [PubMed] [Google Scholar]
- 84. Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology. 1972;90(1):295–298. [DOI] [PubMed] [Google Scholar]
- 85. Callard GV, Hoffman RA, Petro Z, Ryan KJ. In vitro aromatization and other androgen transformations in the brain of the hamster (Mesocricetus auratus). Biol Reprod. 1979;21(1):33–38. [DOI] [PubMed] [Google Scholar]
- 86. Naftolin F, Ryan KJ, Davies IJ, et al. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. [DOI] [PubMed] [Google Scholar]
- 87. Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. [DOI] [PubMed] [Google Scholar]
- 88. Colciago A, Celotti F, Pravettoni A, Mornati O, Martini L, Negri-Cesi P. Dimorphic expression of testosterone metabolizing enzymes in the hypothalamic area of developing rats. Brain Res Dev Brain Res. 2005;155(2):107–116. [DOI] [PubMed] [Google Scholar]
- 89. Harada N, Yamada K. Ontogeny of aromatase messenger ribonucleic acid in mouse brain: fluorometrical quantitation by polymerase chain reaction. Endocrinology. 1992;131(5):2306–2312. [DOI] [PubMed] [Google Scholar]
- 90. McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211(4488):1294–1302. [DOI] [PubMed] [Google Scholar]
- 92. George FW, Ojeda SR. Changes in aromatase activity in the rat brain during embryonic, neonatal, and infantile development. Endocrinology. 1982;111(2):522–529. [DOI] [PubMed] [Google Scholar]
- 93. Lauber ME, Lichtensteiger W. Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135(4):1661–1668. [DOI] [PubMed] [Google Scholar]
- 94. Mannan MA, O’Shaughnessy PJ. Steroidogenesis during postnatal development in the mouse ovary. J Endocrinol. 1991;130(1):101–106. [DOI] [PubMed] [Google Scholar]
- 95. Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: dogma and beyond. Front Neurosci. 2012;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nugent BM, Wright CL, Shetty AC, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18(5):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tsuruo Y, Ishimura K, Fujita H, Osawa Y. Immunocytochemical localization of aromatase-containing neurons in the rat brain during pre- and postnatal development. Cell Tissue Res. 1994;278(1):29–39. [DOI] [PubMed] [Google Scholar]
- 98. MacLusky NJ, Philip A, Hurlburt C, Naftolin F. Estrogen formation in the developing rat brain: sex differences in aromatase activity during early post-natal life. Psychoneuroendocrinology. 1985;10(3):355–361. [DOI] [PubMed] [Google Scholar]
- 99. Beyer C, Wozniak A, Hutchison JB. Sex-specific aromatization of testosterone in mouse hypothalamic neurons. Neuroendocrinology. 1993;58(6):673–681. [DOI] [PubMed] [Google Scholar]
- 100. Beyer C, Green SJ, Hutchison JB. Androgens influence sexual differentiation of embryonic mouse hypothalamic aromatase neurons in vitro. Endocrinology. 1994;135(3):1220–1226. [DOI] [PubMed] [Google Scholar]
- 101. Rhoda J, Corbier P, Roffi J. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17 beta-estradiol. Endocrinology. 1984;114(5):1754–1760. [DOI] [PubMed] [Google Scholar]
- 102. Levine S, Mullins R Jr. Estrogen administered neonatally affects adult sexual behavior in male and female rats. Science. 1964;144(3615):185–187. [DOI] [PubMed] [Google Scholar]
- 103. Gilroy AF, Ward IL. Effects of perinatal androstenedione on sexual behavior differentiation in male rats. Behav Biol. 1978;23(2):243–248. [DOI] [PubMed] [Google Scholar]
- 104. Bakker J, Brand T, van Ophemert J, Slob AK. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav Neurosci. 1993;107(3):480–487. [DOI] [PubMed] [Google Scholar]
- 105. Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, van de Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56(3):535–541. [DOI] [PubMed] [Google Scholar]
- 106. Döhler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Pre- and postnatal influence of testosterone propionate and diethylstilbestrol on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res. 1984;302(2):291–295. [DOI] [PubMed] [Google Scholar]
- 107. Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–1039. [DOI] [PubMed] [Google Scholar]
- 108. Cintra A, Fuxe K, Härfstrand A, et al. Rapid important paper on the cellular localization and distribution of estrogen receptors in the rat tel- and diencephalon using monoclonal antibodies to human estrogen receptor. Neurochem Int. 1986;8(4):587–595. [DOI] [PubMed] [Google Scholar]
- 109. Porcu P, Barron AM, Frye CA, et al. Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J Neuroendocrinol. 2016;28(2):12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Conley AJ, Corbin CJ, Thomas JL, et al. Costs and consequences of cellular compartmentalization and substrate competition among human enzymes involved in androgen and estrogen synthesis. Biol Reprod. 2012;86(1):1–8. [DOI] [PubMed] [Google Scholar]
- 111. Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the “two-cell, two-gonadotrophin” model revisited. Mol Cell Endocrinol. 1994;100(1-2):51–54. [DOI] [PubMed] [Google Scholar]
- 112. Inaoka Y, Yazawa T, Mizutani T, et al. Regulation of P450 oxidoreductase by gonadotropins in rat ovary and its effect on estrogen production. Reprod Biol Endocrinol. 2008;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Norton BI, Miyairi S, Fishman J. 19-hydroxylation of androgens by rat granulosa cells. Endocrinology. 1988;122(3):1047–1052. [DOI] [PubMed] [Google Scholar]
- 114. Kautsky MP, Thurman GW, Hagerman DD. A convenient biological synthesis of 19-hydroxy(4-14C)4-androstene-3,17-dione from (4-14C)4-androstene-3,17-dione with sow ovarian 19-hydroxylase. J Chromatogr. 1975;114(2):472–475. [DOI] [PubMed] [Google Scholar]
- 115. Okubo T, Mok SC, Chen S. Regulation of aromatase expression in human ovarian surface epithelial cells. J Clin Endocrinol Metab. 2000;85(12):4889–4899. [DOI] [PubMed] [Google Scholar]
- 116. Nitta H, Bunick D, Hess RA, et al. Germ cells of the mouse testis express P450 aromatase. Endocrinology. 1993;132(3):1396–1401. [DOI] [PubMed] [Google Scholar]
- 117. Lazaros L, Xita N, Kaponis A, et al. The association of aromatase (CYP19) gene variants with sperm concentration and motility. Asian J Androl. 2011;13(2):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S. Aromatase expression and role of estrogens in male gonad: a review. Reprod Biol Endocrinol. 2003;1:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Galeraud-Denis I, Travert C, de Vienne C, Said L, Saad A, Carreau S. New insights about the evaluation of human sperm quality: the aromatase example. Folia Histochem Cytobiol. 2009;47(5):S13–S17. [DOI] [PubMed] [Google Scholar]
- 120. Aquila S, Sisci D, Gentile M, Middea E, Siciliano L, Andò S. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab. 2002;87(7):3385–3390. [DOI] [PubMed] [Google Scholar]
- 121. Xu X, Sun M, Ye J, et al. The effect of aromatase on the reproductive function of obese males. Horm Metab Res. 2017;49(8):572–579. [DOI] [PubMed] [Google Scholar]
- 122. Raeside JI, Renaud RL, Friendship RM, Khalil MW. Secretion of 19-hydroxyandrostenedione and 19-hydroxytestosterone by porcine Leydig cells in vitro and in vivo. J Endocrinol. 1993;137(2):281–289. [DOI] [PubMed] [Google Scholar]
- 123. Flegel C, Vogel F, Hofreuter A, et al. Characterization of the olfactory receptors expressed in human spermatozoa. Front Mol Biosci. 2015;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Spehr M, Gisselmann G, Poplawski A, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299(5615):2054–2058. [DOI] [PubMed] [Google Scholar]
- 125. Milardi D, Colussi C, Grande G, et al. Olfactory receptors in semen and in the male tract: from proteome to proteins. Front Endocrinol (Lausanne). 2018;8:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vento-Tormo R, Efremova M, Botting RA, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563(7731):347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Martin JD, Hähnel ME, Hähnel R. Plasma androstenedione in normotensive and hypertensive pregnancy. Steroids. 1986;48(5-6):315–329. [DOI] [PubMed] [Google Scholar]
- 128. Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet Gynecol. 1999;180(1 Pt 1):60–63. [DOI] [PubMed] [Google Scholar]
- 129. Kumar S, Gordon GH, Abbott DH, Mishra JS. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction. 2018;156(5):R155–R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Martin JD, Hähnel ME, Hähnel R. 19-hydroxyandrostenedione—a factor in pregnancy hypertension. Clin Exp Hypertens Part B. 1985;4(2-3):127–139. [Google Scholar]
- 131. Belgorosky A, Baquedano MS, Guercio G, Rivarola MA. Adrenarche: postnatal adrenal zonation and hormonal and metabolic regulation. Horm Res. 2008;70(5):257–267. [DOI] [PubMed] [Google Scholar]
- 132. Baquedano MS, Saraco N, Berensztein E, et al. Identification and developmental changes of aromatase and estrogen receptor expression in prepubertal and pubertal human adrenal tissues. J Clin Endocrinol Metab. 2007;92(6):2215–2222. [DOI] [PubMed] [Google Scholar]
- 133. Sekihara H. Evidence that 19-hydroxyandrostenedione is secreted by the adrenal cortex and is under the control of ACTH and the renin-angiotensin system in man. Biochem Biophys Res Commun. 1982;105(2):610–614. [DOI] [PubMed] [Google Scholar]
- 134. Higuchi K, Nawata H, Kato K, Ibayashi H. Alpha-human atrial natriuretic polypeptide inhibits 19-hydroxy-androstenedione secretion by human adrenal cells. Horm Metab Res. 1989;21(2):92–95. [DOI] [PubMed] [Google Scholar]
- 135. Moreau F, Mittre H, Benhaim A, et al. Aromatase expression in the normal human adult adrenal and in adrenocortical tumors: biochemical, immunohistochemical, and molecular studies. Eur J Endocrinol. 2009;160(1):93–99. [DOI] [PubMed] [Google Scholar]
- 136. Morita H, Mune T, Yasuda K, et al. Secretory regulation of 19-hydroxyandrostenedione in normal man. Endocrinol Jpn. 1992;39(5):431–438. [DOI] [PubMed] [Google Scholar]
- 137. Ehrhart-Bornstein M, Bornstein SR, Güse-Behling H, et al. Sympathoadrenal regulation of adrenal androstenedione release. Neuroendocrinology. 1994;59(4):406–412. [DOI] [PubMed] [Google Scholar]
- 138. Bornstein SR, Haidan A, Ehrhart-Bornstein M. Cellular communication in the neuro-adrenocortical axis: role of vasoactive intestinal polypeptide (VIP). Endocr Res. 1996;22(4):819–829. [DOI] [PubMed] [Google Scholar]
- 139. Päth G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J Clin Endocrinol Metab. 1997;82(7):2343–2349. [DOI] [PubMed] [Google Scholar]
- 140. Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19(2):101–143. [DOI] [PubMed] [Google Scholar]
- 141. Mune T, Morita H, Yasuda K, Murayama M, Yamakita N, Miura K. Elevated plasma 19-hydroxyandrostenedione levels in Cushing’s disease: stimulation with ACTH and inhibition with metyrapone. Clin Endocrinol (Oxf). 1993;38(3):265–272. [DOI] [PubMed] [Google Scholar]
- 142. Sekihara H, Yazaki Y, Kojima T. 19-Hydroxyandrostenedione amplifies the hypertensive action of mineralocorticoids in rats. J Endocrinol. 1993;138(1):31–40. [DOI] [PubMed] [Google Scholar]
- 143. Sekihara H, Yazaki Y. 19-Oxoandrost-4-ene-3,17-dione amplifies the action of aldosterone. J Steroid Biochem Mol Biol. 1993;46(1):69–72. [DOI] [PubMed] [Google Scholar]
- 144. Sekihara H. 19-hydroxyandrostenedione and 6 beta-hydroxyandrostenedione: new steroids regulated by the renin-angiotensin system in man. J Steroid Biochem. 1984;20(1):383–385. [DOI] [PubMed] [Google Scholar]
- 145. Morita H, Mune T, Yasuda K, Yamakita N, Miyazaki S, Miura K. Low plasma 19-hydroxyandrostenedione levels in patients with aldosterone-producing adenoma. Endocr J. 1993;40(1):89–97. [DOI] [PubMed] [Google Scholar]
- 146. Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110(11):4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Poll BG, Cheema MU, Pluznick JL. Gut microbial metabolites and blood pressure regulation: focus on SCFAs and TMAO. Physiology (Bethesda). 2020;35(4):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J Steroid Biochem Mol Biol. 2010;118(4-5):246–251. [DOI] [PubMed] [Google Scholar]
- 150. Ricke WA, Ishii K, Ricke EA, et al. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118(9):2123–2131. [DOI] [PubMed] [Google Scholar]
- 151. Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. Faseb J. 2008;22(5):1512–1520. [DOI] [PubMed] [Google Scholar]
- 152. Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. [DOI] [PubMed] [Google Scholar]
- 153. Pedersen MH, Hood BL, Ehmsen S, et al. CYPOR is a novel and independent prognostic biomarker of recurrence-free survival in triple-negative breast cancer patients. Int J Cancer. 2019;144(3):631–640. [DOI] [PubMed] [Google Scholar]
- 154. Conley A, Hinshelwood M. Mammalian aromatases. Reproduction. 2001;121(5):685–695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.