Abstract
Aims
Atrioventricular block (AVB) of unknown aetiology is rare in the young, and outcome in these patients is unknown. We aimed to assess long-term morbidity and mortality in young patients with AVB of unknown aetiology.
Methods and results
We identified all Danish patients younger than 50 years receiving a first pacemaker due to AVB between January 1996 and December 2015. By reviewing medical records, we included patients with AVB of unknown aetiology. A matched control cohort was established. Follow-up was performed using national registries. The primary outcome was a composite endpoint consisting of death, heart failure hospitalization, ventricular tachyarrhythmia, and cardiac arrest with successful resuscitation. We included 517 patients, and 5170 controls. Median age at first pacemaker implantation was 41.3 years [interquartile range (IQR) 32.7–46.2 years]. After a median follow-up of 9.8 years (IQR 5.7–14.5 years), the primary endpoint had occurred in 14.9% of patients and 3.2% of controls [hazard ratio (HR) 3.8; 95% confidence interval (CI) 2.9–5.1; P < 0.001]. Patients with persistent AVB at time of diagnosis had a higher risk of the primary endpoint (HR 10.6; 95% CI 5.7–20.0; P < 0.001), and risk was highest early in the follow-up period (HR 6.8; 95% CI 4.6–10.0; P < 0.001, during 0–5 years of follow-up).
Conclusion
Atrioventricular block of unknown aetiology presenting before the age of 50 years and treated with pacemaker implantation was associated with a three- to four-fold higher rate of the composite endpoint of death or hospitalization for heart failure, ventricular tachyarrhythmia, or cardiac arrest with successful resuscitation. Patients with persistent AVB were at higher risk. These findings warrant improved follow-up strategies for young patients with AVB of unknown aetiology.
Keywords: Atrioventricular block, Long-term outcomes, Young, Pacemaker implantation, Unknown aetiology, Follow-up
Graphical Abstract
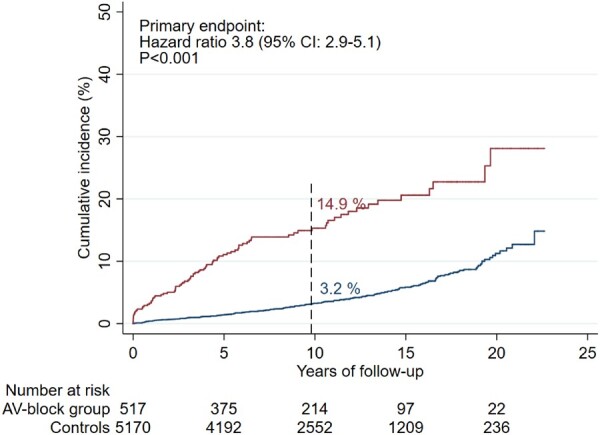
Atrioventricular block of unknown aetiology presenting before the age of 50 years and treated with pacemaker implantation was associated with a three- to four-fold higher rate of the composite endpoint of death or hospitalization for heart failure, ventricular tachyarrhythmia, or cardiac arrest with successful resuscitation. CI, confidence interval.
See page 2069 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab130)
Introduction
Atrioventricular block (AVB) is the leading indication for pacemaker implantation.1 When not accompanied by other cardiac diseases, AVB is reported to have a good prognosis with survival rates equal to that of the general population.2 However, these findings apply to a typical pacemaker population, consisting mostly of elderly patients, in whom age-related fibrosis of the cardiac conduction system is considered the most frequent aetiology of AVB.3 , 4 In contrast, other aetiologies dominate among young patients.5 Prior studies have demonstrated that in approximately half of young patients, an underlying aetiology for AVB is not identified during the pre-implantation work-up.5 , 6 It is likely that separate pathological mechanisms may be present in young patients. Whether this affects the long-term outcome is unknown. Therefore, this study aimed to assess long-term morbidity and mortality in young patients with AVB of unknown aetiology.
Methods
Study population
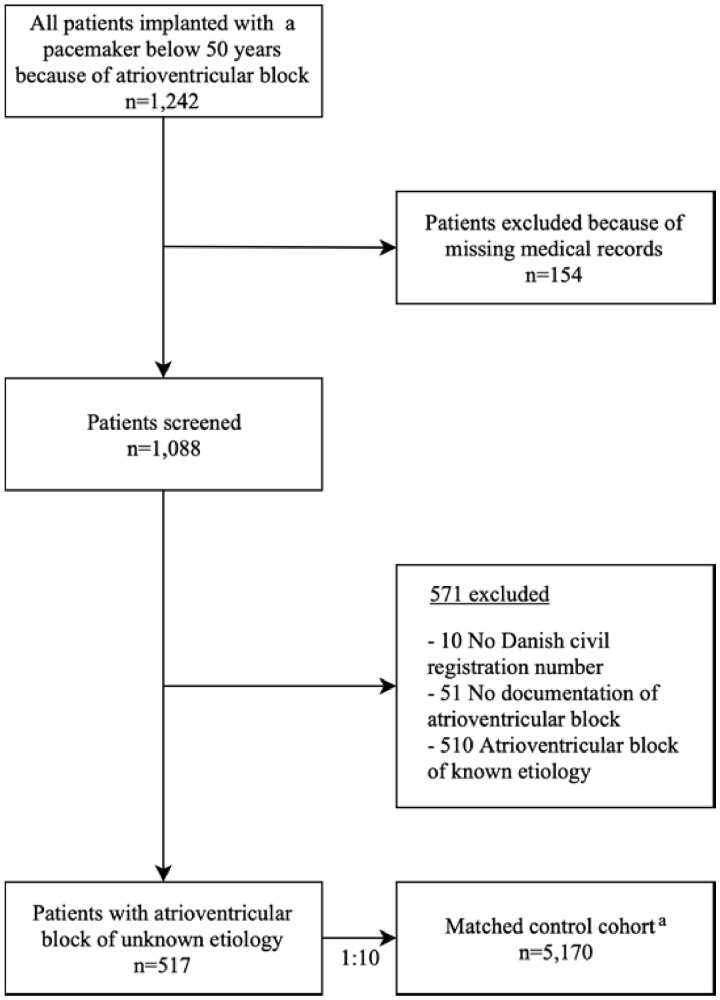
We conducted a nationwide retrospective cohort study of young patients with AVB of unknown aetiology treated on a clinical basis with pacemaker implantation. Consecutive patients who received their first pacemaker due to AVB before the age of 50 years between 1 January 1996 and 31 December 2015 were identified using the Danish Pacemaker and implantable cardioverter-defibrillator (ICD) Registry.1 By reviewing medical records, we included patients with AVB of unknown aetiology. Using the Danish Civil Registration Registry and the Danish National Patient Registry, a matched cohort consisting of 10 controls per case was generated from the Danish general population. The control cohort was matched based on age and gender. Moreover, controls also had to be alive and live in Denmark on the day of pacemaker implantation and could not have an ICD-10 code of pacemaker implantation (DZ950 or procedure code BFC) at any time prior to this date. Using national registries, we followed all patients and controls from the time of pacemaker implantation until a primary or secondary endpoint was reached, migration, or end of follow-up on 1 September 2018, whichever came first.
The study was approved by the Danish Data Protection Agency (record number: 2016-051-000001) and the Danish Patient Safety Authority (record number: 3-3013-1970/1/).
Registries
All people living in Denmark are given a unique and permanent civil registration number upon time of birth or immigration. This allows crosslink of information from large nationwide, population-based registries.7 , 8 In this study, we used data from the Danish Pacemaker and ICD Registry, the Danish Civil Registration Registry, the Danish National Patient Registry, the Danish Prescription Registry, and the Cause of Death Registry.
The Danish Pacemaker and ICD Registry is a national clinical database founded in 1982.1 The implanting physician enters technical and clinical details of all device-related procedures prospectively into the database. The Registry therefore holds information on all pacemaker implantations in Denmark in the study period.
Reviewing process
We retrieved all medical records from AVB patients identified in the Danish Pacemaker and ICD Registry fulfilling the study inclusion criteria. Atrioventricular block had to be documented by electrocardiogram (ECG), telemetric recording, loop recording, Holter monitoring, or a description of one of these modalities in the medical record. Patients with missing medical records were excluded. In order to select only patients with AVB of unknown aetiology, we further excluded patients with identified causes of AVB according to the medical record. This comprised patients with AVB due to congenital heart disease9 , 10 (congenitally corrected transposition, Steno-Fallot tetralogy, ventricular septal defect, or univentricular heart anatomy), iatrogenic causes (side-effects to antiarrhythmics or radiation therapy, planned His ablation, complications from cardiac surgery, radiofrequency ablation, or alcohol septal ablation), congenital AVB,11 cardioinhibitory reflex, cardiomyopathy,12 , 13 endocarditis, muscular dystrophy,14 ischaemic heart disease, sarcoidosis,15 borreliosis,16 hereditary causes, amyloidosis, myocarditis, cardiac tumour, or if any other aetiology was reported as cause of AVB in the medical record.5 To ensure a consistent review, all medical records were reviewed by the same physician (J.R.D., overseen by H.K.J.) and categorization of aetiologies was defined before reviewing patient data.
Baseline characteristics
Baseline data were defined as the day of pacemaker implantation. Information about persistent or intermittent AVB at baseline was obtained through review of medical records and ECGs. If data on echocardiography were available in the medical records, left ventricular ejection fraction (LVEF) was registered. The data were only registered, if echocardiography was performed within 30 days prior to pacemaker implantation. Information about the type of device was obtained from the Danish Pacemaker and ICD Registry. Information about cardiovascular comorbidity was obtained from the Danish National Patient Registry and the National Prescription Database. Drug classes and ICD codes used to define comorbidities are available in Supplementary material online, Table S1. Congestive heart failure was defined as a previous diagnosis of heart failure combined with treatment with loop diuretics. Patients were considered to suffer from hypertension if treated with a combination of at least two of the following antihypertensive drug classes prior to baseline: α-adrenergic blockers, non-loop diuretics, vasodilators, calcium channel blockers, and renin–angiotensin system inhibitors.17 Patients were considered to have diabetes mellitus if a prescription for glucose-lowering drugs was claimed prior to baseline. Hypercholesterolaemia was considered to be present if patients had either been registered with the diagnosis of hypercholesterolaemia or claimed a prescription for cholesterol-lowering drugs prior to baseline. Prior acute myocardial infarction (AMI) was considered to be present with a previous diagnosis of AMI. Patients were considered to have atrial fibrillation or atrial flutter with a previous diagnosis of atrial fibrillation or atrial flutter. Overall comorbidity of patients and controls was evaluated using the Charlson comorbidity index.18 The Charlson comorbidity index was calculated at baseline using the ICD-8 and ICD-10 codes from the 10-year period prior to baseline.19
Study outcomes
Information on all-cause death was obtained from the Danish Civil Registration Registry. Information on heart failure hospitalization, ventricular tachyarrhythmia, and cardiac arrest with successful resuscitation was collected from the Danish National Patient Registry and defined as the first admission to hospital, where an ICD-10 code of either heart failure (DI110, DI50), ventricular tachyarrhythmia (DI470, DI470C, DI472, DI472A, DI472B, DI472D, DI472H), or cardiac arrest with successful resuscitation (DI460) was given. Cardiovascular death was defined as code 23, 24, 25, 26, 27, 28, or 41 in the Cause of Death Registry.
The pre-specified primary outcome was a composite outcome consisting of (i) death from any cause, (ii) hospitalization due to heart failure, (iii) hospital admission for ventricular tachyarrhythmia, and (iv) hospital admission for cardiac arrest with successful resuscitation. Due to the long follow-up, we also computed the hazard ratio (HR) of the primary outcome in the follow-up period from 0 to 5 years, 5 to 10 years, and from 10 years onwards. This was done to investigate any change in risk over time. Also, we wanted to explore whether having intermittent or persistent AVB at baseline was associated with different risks. Therefore, the HR for the primary endpoint was also computed separately for these two groups. Secondary endpoints were death from any cause, cardiovascular death, hospitalization due to heart failure, and hospitalization for ventricular tachyarrhythmia.
As a post hoc analysis, we also investigated the association between AVB and atrial fibrillation or flutter.
Statistical analysis
Baseline variables are expressed as absolute number (proportion) and median [interquartile range (IQR)]. To minimize the risk of surveillance bias, a 30-day blinding period was introduced when generating cardiovascular comorbidity at baseline. The 30-day blinding period was introduced to minimize risk of bias due to increased medical attention in the pacemaker cohort. When diagnosed with AVB, comorbidities such as hypertension, diabetes, and hypercholesterolaemia that would otherwise not have been discovered, could be revealed during the pre-implantation work-up programme. This would make the control cohort look healthier solely because they were not examined for these diseases. Comparisons of baseline characteristics were performed using the χ2 test or the Mann–Whitney U test as appropriate.
Comparisons between groups were done using a stratified Cox regression model with stratification by the matching groups and thereby adjustment for the matching variables sex and age. Additional adjustment for Charlson comorbidity index groups (Table 1) was done by including the variable as covariate in the Cox regression model. For the secondary endpoints ‘cardiovascular death’ and ‘ventricular tachyarrhythmia’, the adjustments were done as Charlson score 0 or 1+ groups due to few events. Cumulative incidence proportions at 5, 10, and 15 years were calculated for the respective endpoints by the Aalen–Johansen method with death as a competing risk.20 For outcomes including death, the Kaplan–Meier method was used. Results are illustrated by graphics of cumulative incidence in which death was accounted for as a competing risk in the non-fatal endpoints. Comparison of patients with intermittent and persistent AVB at baseline was done as a post hoc analysis.
Table 1.
Baseline characteristics
| AVB cohorta, n (%) | General population cohortb, n (%) | P-value | |
|---|---|---|---|
| Total | 517 (100) | 5170 (100) | — |
| Male sex | 299 (57.8) | 2990 (57.8) | — |
| Median age | 41.3 (IQR 32.7–46.2) | 41.3 (IQR 32.7–46.2) | — |
| Age at baseline | |||
| 0–10 | 7 (1.6) | 70 (1.6) | — |
| 10–20 | 28 (5.4) | 280 (5.4) | — |
| 20–30 | 69 (13.3) | 690 (13.3) | — |
| 30–40 | 132 (25.5) | 1320 (25.5) | — |
| 40–50 | 281 (54.4) | 2810 (54.4) | — |
| Device type | |||
| Brady pacemaker | 513 (99.2) | — | — |
| Biventricular pacemaker | 4 (0.8) | — | — |
| LVEF | |||
| >50% | 351 (67.9) | — | — |
| 50–40% | 6 (1.2) | — | — |
| <40% | 4 (0.8) | — | — |
| Missing/older than 30 days | 156 (30.1) | — | — |
| Cardiovascular comorbidity | |||
| Congestive heart failure | 7 (1.4) | 4 (0.1) | <0.001 |
| Hypertension | 64 (12.4) | 300 (5.8) | <0.001 |
| Diabetes mellitus | 13 (2.5) | 86 (1.7) | 0.158 |
| Hypercholesterolaemia | 30 (5.8) | 138 (2.7) | <0.001 |
| Prior AMI | 7 (1.4) | 16 (0.3) | <0.001 |
| AF/AFL | 19 (3.7) | 13 (0.3) | <0.001 |
| Charlson comorbidity index | <0.001 | ||
| 0 | 421 (81.4) | 4793 (92.7) | — |
| 1 | 75 (14.5) | 339 (6.6) | — |
| 2–3 | 15 (2.9) | 25 (0.5) | — |
| 4+ | 6 (1.2) | 13 (0.2) | — |
AF, atrial fibrillation; AFL, atrial flutter; AMI, acute myocardial infarction; AVB, atrioventricular block; IQR, interquartile range; LVEF, left ventricular ejection fraction.
Patients <50 years when receiving their first pacemaker due to AVB.
Control cohort matched by age and gender.
Statistical analyses were performed using STATA version 15.1 software (Stata Corp., College Station, TX, USA).
Results
We included 517 patients receiving their first pacemaker before the age of 50 years because of AVB of unknown aetiology, representing 50.3% of the total number of pacemaker recipients below 50 years in the study period, and 5170 controls from the general population in the study.5 Since potential aetiologies of AVB could possibly have been diagnosed after the time of pacemaker implantation, we calculated the cumulative proportion of potential aetiologies of AVB (dilated or hypertrophic cardiomyopathy, sarcoidosis, muscular dystrophy, or amyloidosis) diagnosed during follow-up (Supplementary material online, Table S2). In total, 22 patients (4.3%) were diagnosed with one or more possible aetiology at a later point in time (Supplementary material online, Table S2).
Patient selection is shown in Figure 1. Median age at first pacemaker implantation was 41.3 years (IQR 32.7–46.2) and 299 (57.8%) were males. At baseline, the AVB cohort had more cardiovascular comorbidity and a higher Charlson comorbidity index than controls (Table 1). Data from pre-implantation echocardiography were available, and performed within the time window of 30 days prior to pacemaker implantation, in 361 (69.8%) patients. The data showed an LVEF >50% in 351 (97.2%) of these patients. A total of 513 (99.2%) patients received a conventional pacemaker and 4 (0.8%) received a biventricular pacemaker. In 438 (84.7%) patients, the AVB was intermittent, whereas 79 (15.3%) had persistent AVB. Besides a higher incidence of hypercholesterolaemia among patients with intermittent AVB, there was no difference in cardiovascular comorbidities between patients with intermittent and persistent AVB (Supplementary material online, Table S3). Right ventricular (RV) lead position was apical in 316 (61%) patients, septal in 197 (38%) patients, and epicardial in 4 (1%) patients. Cumulative incidence of reoperations due to lead malfunction was 0.9%, 3.3%, 6.5%, and 11.9% after 1, 5, 10, and 20 years, respectively. The cumulative incidence of reoperations due to infection was 0.4%, 0.9%, 1.7%, and 4.7% after 1, 5, 10, and 20 years, respectively.
Figure 1.
Patient inclusion in the period from 1 January 1996 until 31 December 2015. aThe control cohort was matched for age and gender.
Primary endpoints
After a median follow-up of 9.8 years (IQR 5.7–14.5 years), the primary outcome had occurred in 14.9% [95% confidence interval (CI) 11.9–18.6] of AVB patients and in 3.2% (95% CI 2.6–3.7) of controls (adjusted HR 3.8; 95% CI 2.9–5.1; P < 0.001) (Figure 2). The risk was relatively highest within the first 5 years after pacemaker implantation (adjusted HR 6.8; 95% CI 4.6–10.0; P < 0.001) as opposed to the period between 5 and 10 years after the procedure (HR 2.6; 95% CI 1.4–4.9; P = 0.004). After 10 years, there was no longer a significant difference in risk of the primary endpoint between the two groups (adjusted HR 1.8; 95% CI 1.0–3.3; P = 0.06). The cumulative incidences after 5, 10, and 15 years are listed in Table 2. There was no statistically significant difference in HRs of the primary endpoint when comparing patients aged 0–40 years (HR 5.25; 95% CI 3.1–9.0; P < 0.001) with patients aged 40–50 years (HR 3.45; 95% CI 2.5–4.8; P < 0.001) (P = 0.19).
Figure 2.
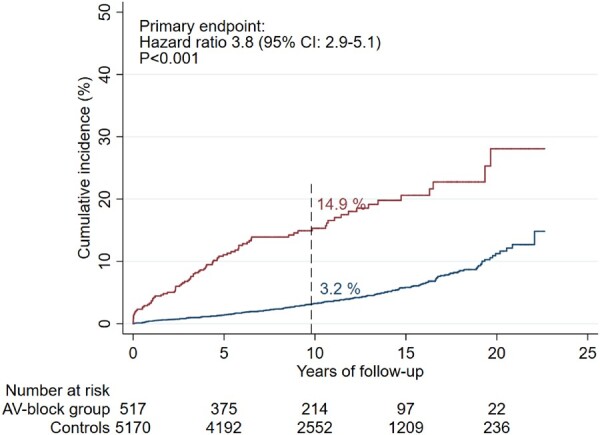
Cumulative incidence of the primary endpointa in patients <50 years when receiving their first pacemaker due to atrioventricular block compared with the general population.b CI, confidence interval. aComposite of death from any cause, heart failure hospitalization, ventricular tachyarrhythmia hospitalization, and cardiac arrest with successful resuscitation. bControls from the general population were matched 10:1 for age and gender. Dashed line represents median follow-up time.
Table 2.
Cumulative incidence and crude and adjusted hazard ratio for the primary and secondary endpoints in the general population and the atrioventricular block cohort
| Total number of events | 5-year cumulative incidence (95% CI) | 10-year cumulative incidence (95% CI) | 15-year cumulative incidence (95% CI) | Crude hazard ratio (95% CI) | Adjusted hazard ratioa (95% CI) | |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
| General population (reference) | 219 | 1.4% (1.1–1.8%) | 3.3% (2.7–3.9%) | 5.9% (5.0–6.9%) | 1.0 | 1.0 |
| AVB cohort | 84 | 11.1% (8.6–14.1%) | 15.3% (12.2–19.1%) | 20.6% (16.5–25.6%) | 4.9 (3.7–6.3) | 3.8 (2.9–5.1) |
| Death from any cause | ||||||
| General population (reference) | 170 | 1.0% (0.8–1.3%) | 2.4% (1.9–2.9%) | 4.4% (3.7–5.3%) | 1.0 | 1.0 |
| AVB cohort | 46 | 5.7% (3.8–7.9%) | 8.6% (6.2–11.5%) | 11.4% (8.1–15.3%) | 3.3 (2.3–4.6) | 2.2 (1.5–3.2) |
| Heart failure hospitalization | ||||||
| General population (reference) | 51 | 0.3% (0.2–0.5%) | 0.7% (0.5–1.0%) | 1.3% (0.9–1.8%) | 1.0 | 1.0 |
| AVB cohort | 45 | 5.0% (3.3–7.2%) | 7.7% (5.4–10.4%) | 11.1% (7.9–14.8%) | 10.2 (6.6–15.7) | 8.6 (5.4–13.8) |
| Cardiovascular death | ||||||
| General population (reference) | 15 | 0.1% (0.04–0.2%) | 0.2% (0.1–0.4%) | 0.4% (0–0.8%) | 1.0 | 1.0 |
| AVB cohort | 19 | 2.4% (1.3–4.1%) | 3.5% (2.1–5.5%) | 4.6% (2.7–7.2%) | 12.7 (6.4–24.9) | 11.7 (5.7–23.3) |
| Ventricular tachyarrhythmia hospitalization | ||||||
| General population (reference) | 9 | 0.1% (0.03–0.2%) | 0.2% (0.07–0.3%) | 0.2% (0.1–0.4%) | 1.0 | 1.0 |
| AVB cohort | 13 | 1.9% (0.9–3.4%) | 2.8% (1.5–4.7%) | 3.6% (1.8–6.3%) | 14.4 (6.1–33.6) | 14.6 (5.8–36.5) |
AVB, atrioventricular block; CI, confidence interval.
Hazard ratios were adjusted for the matching variable sex and age and for the Charlson comorbidity index.
When comparing patients with intermittent AVB with the corresponding controls, we found an HR of 2.9 (95% CI 2.1–4.1; P < 0.001) of the primary endpoint. When comparing patients with persistent AVB with their controls, we found an HR of 10.6 (95% CI 5.7–20.0; P < 0.001) of the primary endpoint (Figure 3).
Figure 3.
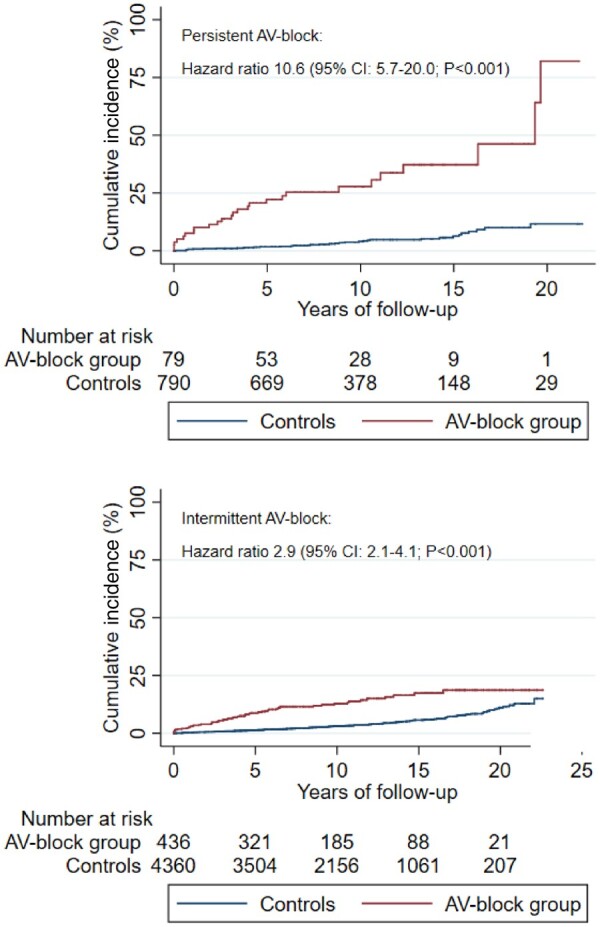
Cumulative incidence of the primary endpointa in patientsb with persistent or intermittent atrioventricular block compared with the general population.c AV, atrioventricular; CI, confidence interval. aComposite of death from any cause, heart failure hospitalization, ventricular tachyarrhythmia hospitalization, and cardiac arrest with successful resuscitation. bPatients <50 years when receiving their first pacemaker due to atrioventricular block. cControls from the general population were matched 10:1 for age and gender. Dashed line represents median follow-up time.
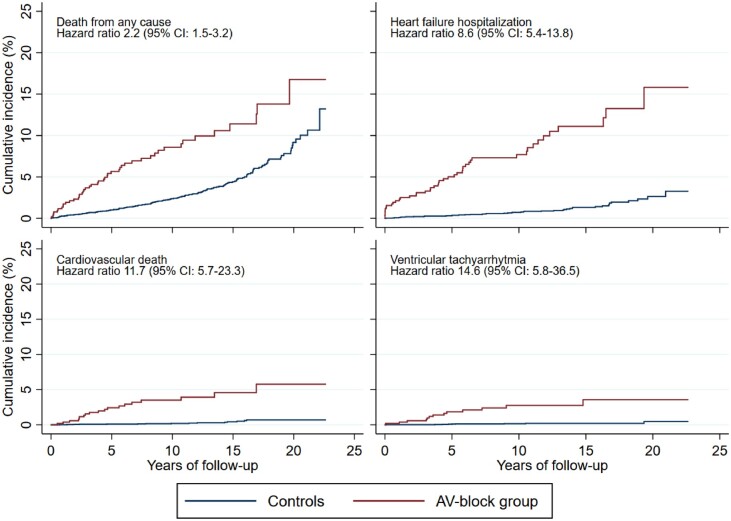
Secondary endpoints
Secondary endpoints are shown in Table 2 and cumulative incidences are graphically illustrated in Figure 4. After a median follow-up of 10.0 years (IQR 5.8–14.6), the cumulative incidence of death from any cause was 8.6% (95% CI 6.2–11.5%) in the AVB population compared with 2.4% (95% CI 1.9–2.9%) in controls (adjusted HR 2.2; 95% CI 1.5–3.2%). Cardiovascular deaths comprised 3.5% (95% CI 2.1–5.5%) in the AVB population and 0.2% (95% CI 0.1–0.4%) in the general population [median follow-up 10.0 (IQR 5.8–14.6) years] (adjusted HR 11.7; 95% CI 5.7–23.3). Heart failure hospitalization was observed in 7.7% (95% CI 5.4–10.4%) in the AVB population compared with 0.7% (95% CI 0.5–1.0%) in the general population [median follow-up 9.9 (IQR 5.7–14.5) years] (adjusted HR 8.6; 95% CI 5.4–13.8). When comparing patients with intermittent AVB with the corresponding controls, we found an HR of 8.0 (95% CI 4.9–13.0; P < 0.001) of heart failure hospitalization. When comparing patients with persistent AVB with their controls, we found an HR of 15.5 (95% CI 6.9–35.0; P < 0.001) of heart failure hospitalization (Supplementary material online, Figure S1). However, there was no statistically significant difference between the two HRs (P = 0.24).
Figure 4.
Cumulative incidence of secondary endpoints among patientsa and the general population.b AV, atrioventricular; CI, confidence interval. aPatients <50 years when receiving their first pacemaker due to atrioventricular block. bControls from the general population were matched 10:1 for age and gender. Deaths were considered competing risk in the non-fatal endpoints. Non-cardiovascular deaths were considered competing risk in the cardiovascular death endpoint.
Ventricular tachyarrhythmia was also more common in the AVB population (2.8%; 95% CI 1.5–4.7%) compared with the general population (0.2%; 95% CI 0.07–0.3%) [median follow-up 9.9 (IQR 5.8–14.6) years]; however, absolute event numbers were low.
Atrial fibrillation or flutter was seen in 8.8% (95% CI 6.3–12.3) of AVB patients and in 1.2% (95% CI 0.9–1.6) of controls (adjusted HR 7.3; 95% CI 4.8–11.0; P < 0.001) (Supplementary material online, Figure S2).
Discussion
In the present long-term follow-up study, we observed a three- to four-fold increase in risk of the composite endpoint of death or hospitalization for heart failure, ventricular tachyarrhythmia, or cardiac arrest with successful resuscitation in patients with AVB of unknown aetiology, receiving their first pacemaker before the age of 50 years. The risk was highest during the first 5 years after pacemaker implantation and in patients with persistent AVB at time of pacemaker implantation.
The increased risk was primarily driven by an increased risk of death from any cause and by hospitalization for heart failure. The increased risk persisted after adjustment for higher baseline comorbidity among AVB patients.
The increased risk in patients with persistent AVB may relate to the risk of developing heart failure due to high-burden ventricular pacing. This was observed in the MOST study in which patients with sinus node dysfunction who received >40% ventricular pacing had a 2.6-fold higher risk of heart failure hospitalization compared with patients who received ≤40% ventricular pacing.21 However, studies in patients with AVB have not demonstrated uniform results. While some studies demonstrate an even lower threshold, where pacing burden >20% was associated with an increased risk of pacing-induced cardiomyopathy,22 , 23 others did not show a difference in the risk of declining left ventricular systolic function when comparing high- and low-burden RV pacing in AVB patients with normal LVEF.24 It is important to bear in mind that these studies were conducted in typical pacemaker cohorts with a mean age of 69, 76, and 73 years, respectively, i.e. in considerable older patients than the patients in our study. It is likely that the pathogenesis of AVB differs among young and elderly AVB patients, which may also affect the response to ventricular pacing. This hypothesis may be supported by a cohort study of 6994 patients with AVB and suspected high-burden RV pacing in which the highest relative risk of heart failure hospitalization was observed among patients younger than 55 years.25 Furthermore, a single-centre study of 286 patients who underwent atrioventricular junction ablation with subsequent RV pacing showed that these patients did not experience significant change in LVEF after 20 months of follow-up.26 Nor did the DANPACE trial show any difference in heart failure hospitalizations between 1384 patients with sick sinus syndrome randomized to either AAIR or DDDR pacing.27 This finding may indicate that RV pacing alone is not the solitary factor contributing to the poor clinical outcome seen in younger AVB patients. Undiagnosed disease possibly plays a role in our findings and, in some cases, AVB may just be the initial manifestation of a more severe underlying disease. Previous studies of young and middle-aged patients with initially unexplained AVB demonstrated that subsequent myocardial biopsy or cardiac imaging revealed cardiac sarcoidosis in between 25% and 34% of patients.6 , 15 Patients with cardiac sarcoidosis have a high risk of adverse cardiac events,28 thus cardiac sarcoidosis should be acknowledged up front and primary defibrillator implantation considered. In recent guidelines, there has been an increased awareness that young patients with AVB may benefit from a more comprehensive pre-implantation work-up, and in the most recent American guidelines investigation for cardiac sarcoidosis is recommended in AVB patients <60 years.29 Due to the risk of immortal time bias, we did not use in the analysis a later diagnosis of sarcoidosis or other diseases that could be possible aetiologies of AVB.
Whether a more physiological pacing mode as an alternative to RV pacing may lead to better clinical outcomes is debated, and results are conflicting. The BLOCK HF trial30 showed that implantation of a biventricular pacemaker for patients with AVB and LVEF <50% reduced the incidence of the composite primary endpoint (all-cause mortality, urgent heart failure visits, or ≥15% increase in left ventricular end-systolic volume index) compared to conventional RV pacing. However, the PREVENT-HF31 did not demonstrate any clinical or echocardiographic benefits from biventricular pacing over RV pacing in AVB patients with preserved LVEF. His-bundle pacing (HBP) or left bundle branch (LBB) pacing may preserve LVEF better than RV pacing.32 , 33 However, with HBP battery drain is significantly higher and risk of lead failure is increased compared with RV pacing, and no randomized controlled data support that HBP is superior or even non-inferior to DDD pacing in AVB on long-term follow-up.33 , 34 Experience with LBB pacing is still very limited.
In our study, despite the vast majority of patients had LVEF >50%, the cumulative incidence of heart failure hospitalization was 7.7% (5.4–10.4%) after 10 years. Compared with the DANPACE trial,27 this is a relatively high incidence having the difference in age and comorbidity in mind. DANPACE patients had a mean age >70 years and much higher burden of comorbidity than our patients but only 12% experienced heart failure hospitalizations after a mean follow-up of 8.9 years. Moreover, our cohort had an increased risk of all-cause mortality. We found a more than two-fold increase in risk of all-cause death in our cohort, even after adjustment for a slightly higher Charlson comorbidity index at baseline. This finding supports the hypothesis that undiagnosed cardiac disease may play a role in our findings.
We observed that the risk of the primary endpoint was highest within the first 5 years after pacemaker implantation and declined thereafter. This pattern does not support a dose–response relationship, where the rate of complications rises with increasing duration of pacing. Instead, this may indicate that only some patients are vulnerable to pacing in which case these patients develop manifest disease within the first years after pacemaker implantation. The reason for this observation is unknown and there is no current knowledge on how to predict who will or will not deteriorate. This finding also adds to the hypothesis that existence of undiagnosed underlying cardiac disease may progress and lead to a poor clinical outcome if untreated. This is supported by the 5-year cumulative incidence of death from any cause of 5.7% (95% CI 3.8–7.9%), which is high in a young and healthy cohort with >95% having a Charlson comorbidity index of 1 or 0.
Although recent guidelines address the importance of a thorough pre-implantation work-up, our findings indicate that young AVB patients are not sufficiently addressed in current guidelines when it comes to follow-up strategy.29 , 35 In particular, our findings show that young pacemaker-treated AVB patients are at increased risk and may require special attention. A better follow-up strategy in patients with persistent AVB or perhaps increasing pace burden could potentially enable physicians to detect early stage heart failure or arrhythmogenic cardiomyopathy and initiate relevant treatment, which may in turn improve outcome.
Study limitations
The main strengths of this study include the large, nationwide cohort of well-characterized, consecutive, AVB patients and population-based controls as well as the almost complete long-term follow-up.36 However, there are a number of limitations. Patients were identified from the Danish Pacemaker and ICD Registry. Consequently, patients who died from bradyarrhythmia before receiving a pacemaker were not included in the study, which might have introduced selection bias. Likewise, 154 (12.4%) patients were excluded due to missing medical records. However, their age and sex distributions were similar to that of the included patients and they were evenly distributed among hospitals. Selection of patients with AVB of unknown aetiology was retrospective over a 20-year period and based on review of medical records. It is possible that a systematic, prospective pre-implantation work-up, where all patients underwent the same protocoled series of diagnostic tests, would have uncovered an underlying cardiac pathology in a higher proportion of patients. While today cardiac magnetic resonance is used in many Danish centres for young patients with AVB, this imaging modality was not part of a routine work-up of AVB patients in most of the study period. Also, genetic testing has only been used in a very limited proportion of the study population. Therefore, we cannot exclude that some patients with myocardial diseases like sarcoidosis or genetic mutations in LMNA or other genes were undiagnosed during the pre-implantation work-up. This is clearly a limitation to the study and should be considered when interpreting the results. However, during long-term follow-up, only a small proportion of patients were diagnosed with other potential aetiologies of AVB.
Furthermore, data on LVEF measured within 30 days prior to pacemaker implantation were only available in 361 (69.8%) patients. This is probably due to the retrospective, nationwide design and the long study period as echocardiography has been a standard procedure in patients with AVB throughout the study period. However, we observed no clustering of age groups, sex, calendar time, or hospitals, leading to believe that the LVEF available should differ from the missing LVEF data. We did not have information about RV pacing percentage. The higher risk of the primary endpoint observed in the subgroup of patients with persistent AVB may indicate that the percentage of RV pacing affects outcome in our cohort. However, this could not be evaluated in the present study since these data have not been collected in the Danish Pacemaker and ICD registry.
Additionally, LVEF was not available during follow-up, and therefore our study cannot elucidate whether the increased risk of the primary endpoint was driven by patients developing pacing-induced heart failure. Also, valid data on QRS width or morphology were not available from the Danish Registries during the study period, and therefore we cannot investigate whether these parameters were associated with outcome.
Lastly, there is a risk that an increased medical attention among pacemaker patients may have led to surveillance bias due to a higher probability of being diagnosed with heart failure, episodes ventricular tachyarrhythmia, or atrial fibrillation/flutter.
Conclusion
Atrioventricular block of unknown aetiology presenting before the age of 50 years and treated with pacemaker implantation was associated with a three- to four-fold higher rate of the composite endpoint of death or hospitalization for heart failure, ventricular tachyarrhythmia, or cardiac arrest with successful resuscitation. Patients with persistent AVB were at higher risk. These findings warrant improved follow-up strategies for young patients with AVB of unknown aetiology.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We greatly appreciate the biostatistical support given by Professor Erik Parner, Department of Biostatics’, Aarhus University. We thank our colleagues Michael Skov Hansen, Aabenraa Regional Hospital; Sam Riahi, Aalborg University Hospital; Søren Højberg, Bispebjerg Regional Hospital; Kristian Korsgaard Thomsen, Esbjerg Regional Hospital; Jim Hansen, Gentofte University Hospital; Troels Niemann, Herning Regional Hospital; Michael Dilou Jacobsen, Hillerød Regional Hospital; Gunnar Vagn Hagemann Jensen, Roskilde University Hospital; Anne Sejr Knudsen, Vejle Regional Hospital; and Jens Refsgaard, Viborg Regional Hospital for providing access to medical records.
Funding
This work was supported by unrestricted research grants from Skibsreder Per Henriksen, R. og hustrus Foundation, the Danish Heart Foundation (16-R107-A6707-22988), Novo Nordisk Foundation, Denmark (NNF18OC0031258), and A. P. Møller Foundation for the Advancement of Medical Science.
Conflict of interest: J.B.J. has received personal fees from Medtronic and Biotronik outside the submitted work. J.C.N. is supported by an unrestricted grant from the Novo Nordisk Foundation, Denmark (NNF16OC0018658 and NNF17OC0029148). H.K.J. is supported by an unrestricted grant from the Novo Nordisk Foundation, Denmark (NNF18OC0031258) and received lecture fees from Abbott, USA and Biosense Webster, USA. All other authors have declared no conflict of interest.
Contributor Information
Johnni Resdal Dideriksen, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus N, Denmark; Department of Clinical Medicine, Health, Aarhus University, Palle Juul-Jensens Boulevard 82, 8200 Aarhus N, Denmark.
Morten K Christiansen, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus N, Denmark.
Jens B Johansen, Department of Cardiology, Odense University Hospital, 5000 Odense C, Denmark.
Jens C Nielsen, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus N, Denmark; Department of Clinical Medicine, Health, Aarhus University, Palle Juul-Jensens Boulevard 82, 8200 Aarhus N, Denmark.
Henning Bundgaard, Department of Cardiology B2142, Unit for Inherited Cardiovascular Diseases, The Heart Centre, National University Hospital, 2100 Copenhagen, Denmark.
Henrik K Jensen, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus N, Denmark; Department of Clinical Medicine, Health, Aarhus University, Palle Juul-Jensens Boulevard 82, 8200 Aarhus N, Denmark.
References
- 1.Danish Pacemaker and ICD Register. Danish Pacemaker and ICD Register Annual report 2015. https://ssl.icddata.dk/download/Danish_Pacemaker_and_ICD_Register_Annual_Report_2015b.pdf (9 February 2021).
- 2. Udo EO, van Hemel NM, Zuithoff NP, Doevendans PA, Moons KG. Prognosis of the bradycardia pacemaker recipient assessed at first implantation: a nationwide cohort study. Heart 2013;99:1573–1578. [DOI] [PubMed] [Google Scholar]
- 3. Zoob M, Smith KS. The aetiology of complete heart-block. Br Med J 1963;2:1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lenegre J. Etiology and pathology of bilateral bundle branch block in relation to complete heart block. Prog Cardiovasc Dis 1964;6:409–444. [DOI] [PubMed] [Google Scholar]
- 5. Rudbeck-Resdal J, Christiansen MK, Johansen JB, Nielsen JC, Bundgaard H, Jensen HK. Aetiologies and temporal trends of atrioventricular block in young patients: a 20-year nationwide study. Europace 2019;21:1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol 2011;4:303–309. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 8. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlson SK, Patel AR, Chang PM. Bradyarrhythmias in congenital heart disease. Card Electrophysiol Clin 2017;9:177–187. [DOI] [PubMed] [Google Scholar]
- 10. Kasar T, Ayyildiz P, Tunca Sahin G, Ozturk E, Gokalp S, Haydin S, Guzeltas A, Ergul Y. Rhythm disturbances and treatment strategies in children with congenitally corrected transposition of the great arteries. Congenit Heart Dis 2018;13:450–457. [DOI] [PubMed] [Google Scholar]
- 11. Ambrosi A, Sonesson SE, Wahren-Herlenius M. Molecular mechanisms of congenital heart block. Exp Cell Res 2014;325:2–9. [DOI] [PubMed] [Google Scholar]
- 12. Strauss DG, Selvester RH, Lima JA, Arheden H, Miller JM, Gerstenblith G, Marbán E, Weiss RG, Tomaselli GF, Wagner GS, Wu KC. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol 2008;1:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res 2004;95:717–725. [DOI] [PubMed] [Google Scholar]
- 14. Groh WJ. Arrhythmias in the muscular dystrophies. Heart Rhythm 2012;9:1890–1895. [DOI] [PubMed] [Google Scholar]
- 15. Nery PB, Beanlands RS, Nair GM, Green M, Yang J, McArdle BA, Davis D, Ohira H, Gollob MH, Leung E, Healey JS, Birnie DH. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol 2014;25:875–881. [DOI] [PubMed] [Google Scholar]
- 16. Forrester JD, Mead P. Third-degree heart block associated with lyme carditis: review of published cases. Clin Infect Dis 2014;59:996–1000. [DOI] [PubMed] [Google Scholar]
- 17. Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124–d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 19. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer 2004;91:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA; MOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–2937. [DOI] [PubMed] [Google Scholar]
- 22. Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, Kanj M, Wazni OM, Saliba WI, Varma N, Wilkoff BL, Cantillon DJ. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm 2016;13:2272–2278. [DOI] [PubMed] [Google Scholar]
- 23. Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, Frankel DS. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm 2014;11:1619–1625. [DOI] [PubMed] [Google Scholar]
- 24. Ebert M, Jander N, Minners J, Blum T, Doering M, Bollmann A, Hindricks G, Arentz T, Kalusche D, Richter S. Long-term impact of right ventricular pacing on left ventricular systolic function in pacemaker recipients with preserved ejection fraction: results from a large single-center registry. J Am Heart Assoc 2016;5:e003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merchant FM, Hoskins MH, Musat DL, Prillinger JB, Roberts GJ, Nabutovsky Y, Mittal S. Incidence and time course for developing heart failure with high-burden right ventricular pacing. Circ Cardiovasc Qual Outcomes 2017;10:e003564. [DOI] [PubMed] [Google Scholar]
- 26. Chen L, Hodge D, Jahangir A, Ozcan C, Trusty J, Friedman P, Rea R, Bradley D, Brady P, Hammill S, Hayes D, Shen WK. Preserved left ventricular ejection fraction following atrioventricular junction ablation and pacing for atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:19–27. [DOI] [PubMed] [Google Scholar]
- 27. Brandt NH, Kirkfeldt RE, Nielsen JC, Mortensen LS, Jensen GVH, Johansen JB, Haugan K. Single lead atrial vs. dual chamber pacing in sick sinus syndrome: extended register-based follow-up in the DANPACE trial. Europace 2017;19:1981–1987. [DOI] [PubMed] [Google Scholar]
- 28. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol 2016;68:411–421. [DOI] [PubMed] [Google Scholar]
- 29. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:e51–e156. [DOI] [PubMed] [Google Scholar]
- 30. Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, Shinn T, Sutton MS; Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368:1585–1593. [DOI] [PubMed] [Google Scholar]
- 31. Stockburger M, Gómez-Doblas JJ, Lamas G, Alzueta J, Fernández-Lozano I, Cobo E, Wiegand U, Concha JF, Navarro X, Navarro-López F, de Teresa E. Preventing ventricular dysfunction in pacemaker patients without advanced heart failure: results from a multicentre international randomized trial (PREVENT-HF). Eur J Heart Fail 2011;13:633–641. [DOI] [PubMed] [Google Scholar]
- 32. Kronborg MB, Mortensen PT, Poulsen SH, Gerdes JC, Jensen HK, Nielsen JC. His or para-His pacing preserves left ventricular function in atrioventricular block: a double-blind, randomized, crossover study. Europace 2014;16:1189–1196. [DOI] [PubMed] [Google Scholar]
- 33. Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun H, Oren JW, Dandamudi G, Vijayaraman P. Clinical outcomes of his bundle pacing compared to right ventricular pacing. J Am Coll Cardiol 2018;71:2319–2330. [DOI] [PubMed] [Google Scholar]
- 34. Vijayaraman P, Naperkowski A, Subzposh FA, Abdelrahman M, Sharma PS, Oren JW, Dandamudi G, Ellenbogen KA. Permanent His-bundle pacing: long-term lead performance and clinical outcomes. Heart Rhythm 2018;15:696–702. [DOI] [PubMed] [Google Scholar]
- 35. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA,, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PEESC Committee for Practice Guidelines (CPG)Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker SDocument ReviwersKirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 36. Sundboll J, Adelborg K, Munch T, Froslev T, Sorensen HT, Botker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.