Abstract
Aims
The aim of this study was to investigate the cross-sectional associations of modelled residential road traffic noise with cardiovascular disease risk factors [systolic (SBP) and diastolic blood pressure (DBP), C-reactive protein, triglycerides, glycated haemoglobin, and self-reported hypertension] in UK Biobank.
Methods and results
The UK Biobank recruited 502 651 individuals aged 40–69 years across the UK during 2006–10. Road traffic noise (Lden and Lnight) exposure for 2009 was estimated at baseline address using a simplified version of the Common Noise Assessment Methods model. We used multivariable linear and logistic regression models, adjusting for age, sex, body mass index (BMI), smoking, alcohol intake, area- and individual-level deprivation, season of blood draw, length of time at residence, and nitrogen dioxide (main model), in an analytical sample size of over 370 000 participants. Exposure to road-traffic Lden >65 dB[A], as compared to ≤55 dB[A], was associated with 0.77% [95% confidence interval (CI) 0.60%, 0.95%], 0.49% (95% CI 0.32%, 0.65%), 0.79% (95% CI 0.11%, 1.47%), and 0.12% (95% CI −0.04%, 0.28%) higher SBP, DBP, triglycerides, and glycated haemoglobin, respectively. Removing BMI from the main model yielded significant positive associations with all five markers with elevated percent changes. The associations with SBP or DBP did not appear to be impacted by hypertension medication while a positive association with prevalent self-reported hypertension was seen in the non-medicated group who exposed to a Lden level of 60–65 dB[A] (odds ratio 1.07, 95% CI 1.00, 1.15).
Conclusion
Exposure to road traffic noise >65 dB[A], independent of nitrogen dioxide, was associated with small but adverse changes in blood pressure and cardiovascular biochemistry.
Keywords: Transportation, Air pollution, Blood pressure, Blood lipids, Blood glucose, Inflammation
Graphical Abstract
In the largest analysis to date of over 370,000 UK Biobank participants, exposure to high road traffic noise levels greater than 65dB, as compared to less than 55dB, was associated with 0.77%, 0.49%, 0.79% and 0.12% changes in SBP, DBP, triglycerides and glycated haemoglobin respectively, independent in nitrogen dioxide.
See page 2085 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab104)
Introduction
Road traffic noise is an important environmental risk factor for cardiovascular disease (CVD), as increasingly reported in both observational and experimental studies.1 However, the biological mechanisms underlying the association remain to be thoroughly elucidated. A proposed hypothesis suggests that chronic exposure to noise leads to activation of the autonomic and endocrine system, generating unfavourable changes in traditional risk factors such as blood pressure, blood lipids, and blood glucose which, if left untreated, will manifest in CVD.2 Long-term sleep disturbance as a result of night-time noise exposure can also impact cardiovascular health due to repeated arousal and activation of the stress cascade via autonomic and endocrine systems.3
Of all the cardiovascular outcomes examined to date, the relationship between road traffic noise and hypertension is the most studied. A meta-analysis by the World Health Organisation reported a 5% increase in prevalence of hypertension [95% confidence interval (CI) 2%, 8%] per 10 dB of road traffic noise based on 26 cross-sectional studies published up to 2014, for which the overall quality of evidence was rated as very low.4 Another meta-analysis of 14 cohort and case–control studies published between 2011 and 2017 reported a relative risk of 1.02 (95% CI 0.98, 1.05), for which the overall quality of evidence was rated as low.5 Both reviews indicated that further research is very likely to have an important impact on the estimated risk. Only a few studies have investigated the relationship with continuous blood pressure traits in adults and reported heterogeneous results. Some only observed a positive association with either systolic blood pressure (SBP)6–8 or diastolic blood pressure (DBP).9 One study found a null association with either measure.10 The pooled analysis of over 88 000 participants from three European cohorts was the largest study but reported negative associations with both measures.11 Studies of C-reactive protein (CRP), blood lipids and glucose are still very limited.12–14 Traffic-related air pollution, the impact of which on cardiovascular health is well documented,15 has the potential to confound associations between road traffic noise exposure and cardiovascular outcomes and, therefore, should be considered when attempting to disentangle road traffic noise effects.
Here, we examined cross-sectional associations of long-term residential road traffic noise with SBP, DBP, triglycerides, glycated haemoglobin, CRP, and self-reported hypertension, accounting for individual-level confounders including traffic-related air pollution in the largest study to date involving over 370 000 participants in UK Biobank.
Methods
Study population
A total of 502 651 individuals aged 40–69 years living within 25 miles of one of the 22 study assessment centres across the UK were recruited into UK Biobank during baseline assessment from 2006 to 2010.16 A comprehensive set of individual-level data was provided by participants using touchscreen questionnaires while biological and physical measurements were also collected. Despite a relatively low response rate (5.5%), risk factor associations in UK Biobank are likely generalizable.17 All participants provided written consent and ethical approval was obtained from the North West Multi-Centre Research Ethical Committee and Patient Information Advisory Group.
Cardiovascular risk factors
Non-fasting blood samples were collected and transported in temperature-controlled boxes for storage. Serum concentrations of high-sensitivity CRP (mg/L), triglycerides (mmol/L), and glycated haemoglobin (mmol/mol) were analysed using immunoturbidimetric, glycerol phosphate oxidase peroxidase and high-performance liquid chromatography, respectively.18 SBP and DBP (mmHg) were measured twice using a digital Omron HEM-7015IT monitor, following a standard protocol.19 The mean of two measures of SBP and DBP was obtained to account for random fluctuations.
Noise exposure assessment
Address-level annual mean road traffic noise estimates were modelled using a simplified version of the Common Noise Assessment Methods in Europe model, developed and validated for epidemiological studies.20 , 21 This simplified model has relatively good performance on exposure ranking (Spearman ratio: 0.75)21 and has been used in previous analyses.14 Annual mean A-weighted sounds pressure level in decibels (dB[A]) for 2009 was estimated based on all road sources within 500 m of residential address. The model considered detailed information on noise propagation (refraction and diffraction), absorption from buildings and land use, distance between receptor and source and angle of view, meteorology, building heights, land cover, road network geography, and calculated hourly vehicle flows using a daily average traffic profile. We used the noise indicator L den (weighted average 24-h noise sound level, with a penalty of 5 and 10 dB added to the evening hours and night hours, respectively) and L night (average sound pressure level during night-time hours 23:00–07:00), to be comparable to previous studies.
Covariates
Age (in years continuous), sex (female, male), smoking status (current, past, never), alcohol intake frequency (daily or almost daily, 3–4 times a week, 1–2 times a week, occasional drinker, never), use of antihypertensive medication, and self-reported hypertension and diabetes (‘ever-had’) were obtained from questionnaires. Body mass index (BMI, kg/m2) was calculated using height (cm) and weight (kg) measured after removal of heavy clothes and shoes. Season of blood draw (spring, summer, autumn, winter) was recorded during clinical measurements. Time at residence of recruitment in years was obtained. Household income before tax (<£18 000, £18 000–£30 999, £31 000–51 999, £52 000–£100 000, >£100 000) and economic activity (economically active (paid employment), economically inactive (unpaid employment, unemployment, housework, retired, etc.) was used as proxies for individual socioeconomic status. Townsend deprivation index (quintiles: most deprived to least deprived) is a composite area-level indicator of material deprivation based on unemployment, non-car ownership, non-house ownership, and household overcrowding using information from the UK 2011 Census. Address-level annual average concentrations for nitrogen dioxide (NO2), a primary indicator of roadside air pollution, and particulate matter with a diameter of <2.5 μm (PM2.5), for which road vehicles are important emission sources, were integrated into UK Biobank as part of a previous study.22 , 23 Land-use regression models were used to predict annual average NO2 and PM2.5 exposure at address for year 2010. The models used AirBase routine monitoring data with geospatial variables on road network (road class, road length), land use (residential, natural, industry, urban green), population density and altitude. The model performance [explained variance (R 2) between modelled and measured exposures] was 89% and 82%, for NO2 and PM2.5 respectively.
Statistical analysis
Descriptive analysis was conducted for covariates, exposures, and outcomes in the whole population. Spearman correlations between road traffic noise metrics and air pollutants were calculated.
The distribution of noise metric L den was right skewed and therefore categorized as ≤55, >55 to ≤60, >60 to ≤65, and >65 dB[A] in the analysis. The reference value was set at 55 dB[A] as it is close to the median of L den and a suggested health effect threshold set by European Union. Categorization was also applied to L night as ≤45, >45 to ≤ 50, >50 to ≤55, and >55 dB[A]. The reference value was set at 45 dB[A] following the 2018 European noise guideline.24 Each risk factor was examined as a log-transformed continuous outcome to address skewness of data. CRP levels >10 mg/L were encoded as missing as levels above this value may indicate a current infection.14
Association between residential annual mean road traffic noise (L den or L night) and each risk factor was analysed using multivariable linear regression. For the binary outcome of self-reported hypertension, multivariable logistic regression was used. Results for SBP, DBP, triglycerides, glycated haemoglobin and CRP were presented as percent change and 95% CI in mean differences between the reference group and other groups while results for self-reported hypertension were presented as odds ratios (ORs) and 95% CI.
The models were as follows: Model 1: unadjusted; Model 2: fully adjusted model with adjustment for age, sex, BMI, smoking status, alcohol intake frequency, household income, Townsend deprivation index, time at residence, season of blood draw, economic activity; Model 3: Model 2 further adjusted for NO2; and Model 4: Model 2 further adjusted for PM2.5. Most previous studies have accounted for NO2 effects; to facilitate comparisons, we set a priori Model 3 as the main model in our study.
Sensitivity analyses were performed to test the robustness of Model 3: (i) adjustment without BMI as BMI may be on the causal pathway; (ii) further adjustment for ever-had hypertension and diabetes to capture high-risk individuals; (iii) to address the potential issue of missing data, we used multiple imputation (m = 20) using chained equations with fully conditional specification of prediction equations. All covariates were included in the imputation equation; (iv) to repeat the analysis of categorical L den by lowering the reference value to 52 dB[A], which is close to the 5th percentile of L den distribution. The corresponding categories were ≤52, >52 to ≤55, >55 to ≤58, >58 to ≤61, >61 to ≤64 and >64 dB[A]. An increment of 3 dB was chosen as it represents a doubling of sound energy levels, which is audible to human ear as a small change in loudness.25
For the analyses on SBP, DBP and self-reported hypertension in Model 3, we further investigated the role of antihypertensive medication. As with a previous study,26 we tested different approaches: (i) to further adjust for medication; (ii) to restrict analyses to participants on medication; and (iii) to restrict analyses to non-medicated participants. To examine the assumption of linearity between L den and SBP or DBP, we conducted restricted cubic splines analyses by placing three knots at 55, 60 and 65 dB[A] of the L den distribution.
We explored effect modification in Model 3 a priori by sex, age (≥65 vs. <65 years), household income, area Townsend index, and time at current residence (≥10 vs. <10 years). All statistical analysis was performed using STATA/IC v 15.1.
Results
The analytical sample included 502 521 participants, 54.4% were female and mean age was 56.5 years (Table 1). The mean L den exposure was 56.1 dB[A], ranging from 51.5 to 93.4 dB[A]. About 12% of the study participants were exposed to a residential L den >60 dB[A]. The mean NO2 and PM2.5 exposure levels were 26.7 and 9.9 μg/m3. Spearman’s correlation coefficients between L den and NO2 (0.23) or PM2.5 (0.24) were low but were high between L den and L night (0.99) and between NO2 and PM2.5 (0.85).
Table 1.
Descriptive characteristics of UK Biobank participants
| General characteristics | |
|---|---|
| Total (N) | 502 521 |
| Sex | |
| Female | 273 391 (54.4%) |
| Male | 229 130 (45.6%) |
| Age at recruitment (years), mean ± SD | 56.5 ± 8.1 |
| Health characteristics | |
| BMIa (n = 491 283) | |
| Underweight (<18.5 kg/m2) | 2623 (1.0%) |
| Healthy (18.5–24.9 kg/m2) | 157 409 (31.0%) |
| Overweight (25–29.9 kg/m2) | 209 092 (42.6%) |
| Obese (>30 kg/m2) | 122 159 (24.9%) |
| Smoking status (n = 499 571) | |
| Never smoker | 273 527 (54.8%) |
| Former smoker | 173 067 (34.6%) |
| Current smoker | 52 977 (10.6%) |
| Alcohol intake frequency (n = 501 019) | |
| Daily or almost daily | 101 774 (20.3%) |
| Three or four times a week | 115 441 (23.0%) |
| Once or twice a week | 129 292 (25.8%) |
| Occasional drinkersb | 113 865 (22.7%) |
| Never drinker | 40 647 (8.2%) |
| High blood pressure medication (n = 224 545) | |
| Yes | 56 085 (25.0%) |
| No | 168 460 (75.0%) |
| Socioeconomic status characteristics | |
| Economic statusc (n = 496 767) | |
| Economically active | 287 162 (57.7%) |
| Economically inactive | 209 605 (42.3%) |
| Townsend deprivation index at recruitment, quintiles (n = 501 898) | |
| 1 (least deprived) | 100 679 (20.3%) |
| 2 | 100 127 (20.2%) |
| 3 | 100 346 (19.2%) |
| 4 | 100 372 (20.3%) |
| 5 (most deprived) | 100 374 (20.0%) |
| Average total household income before tax (n = 425 350) | |
| <£18 000 | 97 200 (22.9%) |
| £18 000 to £30 999 | 108 178 (25.4%) |
| £31 000 to £51 999 | 110 773 (26.0%) |
| £52 000 to £100 000 | 86 267 (20.3%) |
| >£100 000 | 22 932 (5.4%) |
| Season of blood drawd | |
| Spring | 146 470 (29.2%) |
| Summer | 131 999 (26.3%) |
| Autumn | 119 389 (23.7%) |
| Winter | 104 663 (20.8%) |
| Length of time at residence (years), mean ± SD (n = 499 846) | 17.4 ± 12.1 |
| Exposure characteristics | |
| L den, dB[A], mean ± SD, range (n = 495 155) | 56.1 ± 4.3 (51.5–93.4) |
| Low (≤55 dB[A]) | 254 874 (51.4%) |
| Low–medium (>55 to ≤60 dB[A]) | 179 765 (36.3%) |
| Medium–high (>60 to ≤65 dB[A]) | 29 024 (5.9%) |
| High (>65 dB[A]) | 31 492 (6.4%) |
| L night, dB[A], mean ± SD, range (n = 495 155) | 46.6 ± 4.3 (42.1–93.9) |
| Low (≤45 dB[A]) | 205 930 (41.6%) |
| Low–medium (>45 to ≤50 dB[A]) | 222 522 (44.9%) |
| Medium–high (>50 to ≤55 dB[A]) | 33 073 (6.7%) |
| High (>55 dB[A]) | 33 630 (6.8%) |
| NO2 (μg/m3), mean ± SD, range (n = 495 155) | 26.7 ± 7.6 (12.9–108.5) |
| PM2.5 (μg/m3), mean ± SD, range (n = 461 228) | 9.99 ± 1.1 (8.2–21.3) |
| L den, NO2, r s | 0.23 |
| L den, PM2.5, r s | 0.24 |
| Outcome characteristics,e | |
| Systolic blood pressure (mmHg), mean ± SD, range (n = 456 977) | 137.8 ± 18.6, (65.0–253.5) |
| Diastolic blood pressure (mmHg), mean ± SD, range (n = 456 989) | 82.2 ±10.1 (36.5–120.0) |
| C-reactive protein (mg/L), median ± IQR, range (n = 449 139) | 1.3 ± 1.9 (0.1–10.0) |
| Triglycerides (mmol/L), median ± IQR, range (n = 469 226) | 1.8 ± 1.0 (0.2–11.3) |
| Glycated haemoglobin (mmol/mol), mean ± SD, range (n = 455 865) | 35.4 ± 4.3 (15.0–54.0) |
| Self-reported hypertension (n = 500 298) | |
| Yes | 135 539 (27.1%) |
| No | 364 539 (72.9%) |
BMI, body mass index; IQR, interquartile range; NO2, nitrogen dioxide; PM2.5, particulate matter with a diameter <2.5 μm; SD, standard deviation; WHO, World Health Organisation.
BMI categorized according to WHO and UK classifications.
Includes individuals who drink on special occasions only and individuals who drink ∼1–2 times a month.
Economic status refers to paid employment and unpaid employment.
Based on date attending assessment centre: winter (December, January, February), spring (March, April, May), summer (June, July August), autumn (September, October, November).
Median ± IQR used where mean differed from median by >10%.
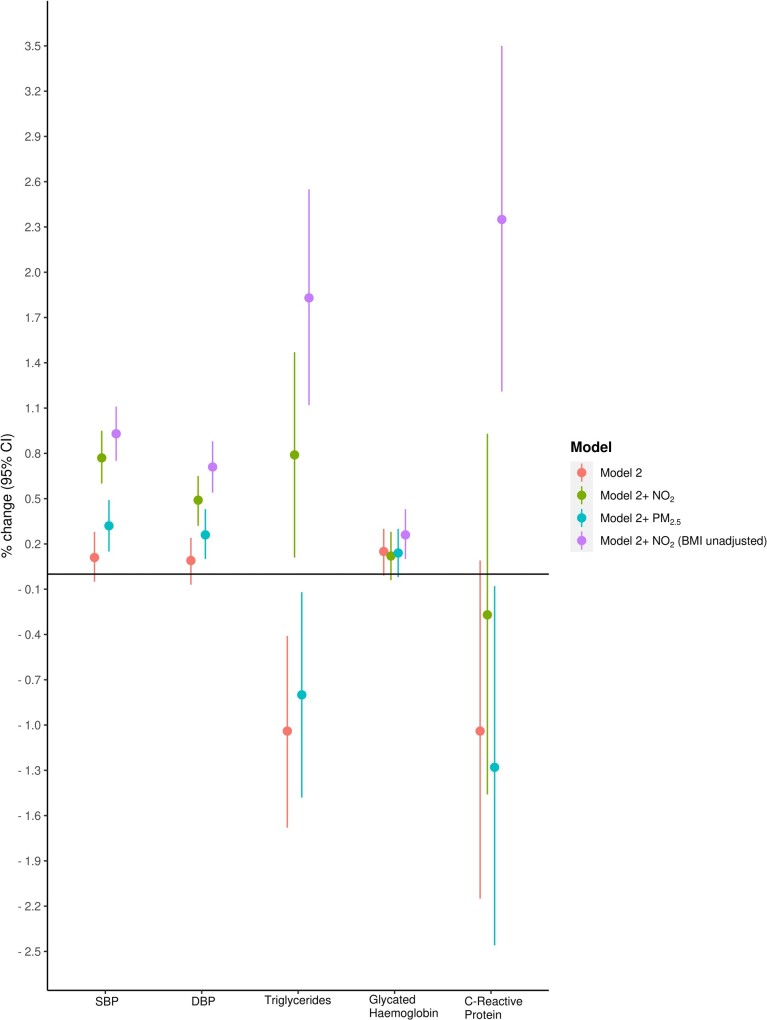
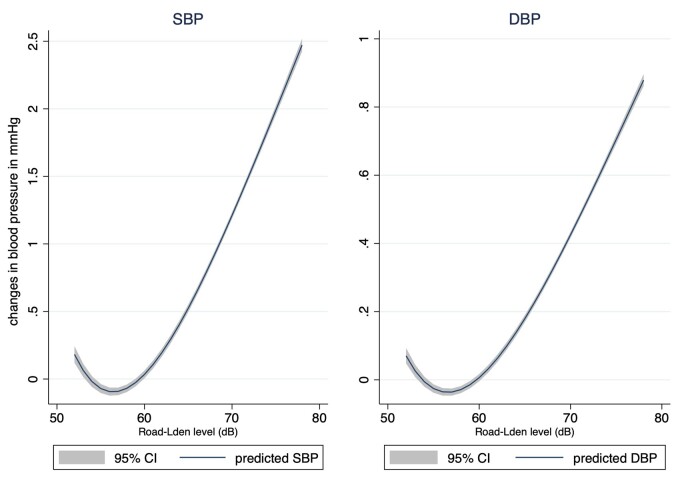
In the fully adjusted Model 2, exposure to road-L den >65 dB[A], as compared to those exposed ≤55 dB[A], was positively associated with SBP [0.11% (95% CI −0.05, 0.28)]; after further adjustment for NO2 or PM2.5 exposure, the effect estimate changed to 0.77% (95% CI 0.60, 0.95) (Model 3) and 0.32% (95% CI 0.15, 0.49) (Model 4), respectively (Table 2 and Figure 1). Similarly, for DBP, after further adjustment for NO2 or PM2.5, the effect estimate changed from 0.09% (95% CI −0.07, 0.24) to 0.49% (95% CI 0.32, 0.65) and 0.26% (95% CI 0.10, 0.43), respectively. The restricted cubic spline analysis showed that the relationship between road-L den and SBP or DBP only seems to be linear at levels >60 dB (Figure 2). No positive association between road-L den and prevalence of self-reported hypertension was observed in the adjusted models (Table 2).
Table 2.
The association between exposure to road traffic noise (L den) and cardiovascular disease risk factors
| Noise exposure (L den) | N | Model 1, % change (95% CI) | N | Model 2, % change (95% CI) | N | Model 3, % change (95% CI) | N | Model 4, % change (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 451 132 | 378 302 | 378 302 | 365 675 | ||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | Reference | ||||
| Low–medium (>55 to ≤60 dB[A]) | −0.31 (−0.40, −0.23) | −0.10 (−0.19, −0.02) | −−0.04 (−0.12, 0.05) | −−0.06 (−0.15, 0.03) | ||||
| Medium–high (>60 to ≤65 dB[A]) | −0.53 (−0.70, −0.36) | −0.11 (−0.28, 0.05) | −−0.03 (−0.20, 0.13) | −−0.04 (−0.21, 0.13) | ||||
| High (>65 dB[A]) | −0.25 (−0.41, −0.09) | 0.11 (−0.05, 0.28) | 0.77 (0.60, 0.95) | 0.32 (0.15, 0.49) | ||||
| Diastolic blood pressure (mmHg) | 450 843 | 378 073 | 378 073 | 365 454 | ||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | Reference | ||||
| Low–medium (>55 to ≤60 dB[A]) | −−0.04 (−0.12, 0.04) | −0.06 (−0.14, 0.02) | −−0.02 (−0.10, 0.06) | 0.04 (−0.12, 0.05) | ||||
| Medium–high (>60 to ≤65 dB[A]) | −0.24 (−0.40, −0.09) | −0.16 (−0.32, 0.00) | −−0.11 (−0.27, 0.05) | −−0.11 (−0.27, 0.05) | ||||
| High (>65 dB[A]) | 0.11 (−−0.04, 0.27) | 0.09 (−0.07, 0.24) | 0.49 (0.32, 0.65) | 0.26 (0.10, 0.43) | ||||
| Triglycerides (mmol/L) | 462 342 | 389 392 | 389 392 | 361 072 | ||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | Reference | ||||
| Low–medium (>55 to ≤60 dB[A]) | −−0.29 (−−0.62, 0.03) | −0.28 (−0.61, 0.04) | −−0.10 (−0.43, 0.22) | −−0.16 (−0.50, 0.18) | ||||
| Medium–high (>60 to ≤65 dB[A]) | −0.89 (−1.53, −0.24) | −0.79 (−1.43, −0.14) | −−0.57 (−1.22, 0.08) | −0.81 (−1.48, −0.14) | ||||
| High (>65 dB[A]) | −−0.31 (−−0.94, 0.32) | −1.04 (−1.68, −0.41) | 0.79 (0.11, 1.47) | −0.80 (−1.48, −0.12) | ||||
| Glycated haemoglobin (mmol/mol) | 449 135 | 380 510 | 378 890 | 351 368 | ||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | Reference | ||||
| Low–medium (>55 to ≤60 dB[A]) | 0.03 (−−0.04, 0.11) | 0.05 (−0.03, 0.13) | 0.05 (−0.03, 0.13) | 0.06 (−0.02, 0.14) | ||||
| Medium–high (>60 to ≤65 dB[A]) | −−0.08 (−−0.23, 0.08) | −0.04 (−0.20, 0.11) | −−0.05 (−0.20, 0.11) | −−0.10 (−0.26, 0.06) | ||||
| High (>65 dB[A]) | 0.36 (0.21, 0.51) | 0.15 (−0.01, 0.30) | 0.12 (−0.04, 0.28) | 0.14 (−0.02, 0.30) | ||||
| C-reactive protein (mg/L) | 442 544 | 373 261 | 373 261 | 346 188 | ||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | Reference | ||||
| Low–medium (>55 to ≤60 dB[A]) | 0.27 (−−0.34, 0.87) | −0.44 (−1.02, 0.14) | −−0.36 (−0.94, 0.22) | −−0.60 (−1.20, 0.01) | ||||
| Medium–high (>60 to ≤65 dB[A]) | −0.48 (−−1.68, 0.74) | −1.12 (−2.26, 0.04) | −−1.03 (−2.18, 0.13) | −−1.17 (−2.36, 0.03) | ||||
| High (>65 dB[A]) | 1.68 (0.50, 2.88) | −1.04 (−2.15, 0.09) | −−0.27 (−1.46, 0.93) | −1.28 (−2.46, −0.08) | ||||
|
| ||||||||
| Noise exposure (L den) | N | Model 1, odds ratio (95% CI) | N | Model 2, odds ratio (95% CI) | N | Model 3, odds ratio (95% CI) | N | Model 4, odds ratio (95% CI) |
|
| ||||||||
| Self-reported hypertension | 492 993 | 413 845 | 413 845 | 384 204 | ||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | Reference | ||||
| Low–medium (>55 to ≤60 dB[A]) | 1.00 (0.99, 1.01) | 1.00 (0.98, 1.01) | 0.99 (0.98, 1.01) | 0.99 (0.97, 1.00) | ||||
| Medium–high (>60 to ≤65 dB[A]) | 1.00 (0.97, 1.03) | 0.98 (0.95, 1.02) | 0.98 (0.95, 1.01) | 0.97 (0.94, 1.00) | ||||
| High (>65 dB[A]) | 1.03 (1.00, 1.05) | 0.99 (0.96, 1.02) | 0. 95 (0.92, 0.98) | 0.97 (0.94, 1.00) | ||||
| Continuous L den, per 1 dB[A] | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (0.99, 1.00) | 1.00 (0.99, 1.00) | ||||
Model 1: unadjusted crude model. Model 2: fully adjusted model. Adjusted for sex, age, BMI, smoking status, alcohol intake frequency, Townsend deprivation index, household income, economic status, season of blood draw, length of time at residence. Model 3: fully adjusted model (Model 2) + adjusted for NO2. Model 4: fully adjusted model (Model 2) + adjusted for PM2.5. Bold represents significance at P < 0.05.
BMI, body mass index; CI, confidence interval.
Figure 1.
A summary of cross-sectional associations between road-L den and percent changes in cardiovascular risk factors, comparing >65 to ≤55 dB. Model 2: fully adjusted model. Adjusted for sex, age, BMI, smoking status, alcohol intake frequency, Townsend deprivation index, household income, economic status, season of blood draw, length of time at residence.
Figure 2.
Cross-sectional changes (mmHg) in systolic and diastolic blood pressures in relation to road traffic noise exposure in Model 3 (fully adjusted model + NO2) based on the restricted cubic spline analysis. Model 3: adjusted for sex, age, BMI, smoking status, alcohol intake frequency, Townsend deprivation index, household income, economic status, season of blood draw, length of time at residence, and nitrogen dioxide.
The significant positive associations with both SBP and DBP found in the highest noise group in Model 3 did not appear to be affected by further adjusting for antihypertensive medication use or restricting analyses to those with or without medication (Table 3). In contrast, higher positive ORs were observed for self-reported hypertension among the non-medicated participants who exposed to road-L den of 55–60 dB (1.03, 95% CI 0.99, 1.06) and of 60–65 dB (1.07, 95% CI 1.00, 1.15), but the association was null in the highest noise group.
Table 3.
The association between road traffic noise and systolic blood pressure, diastolic blood pressure, and self-reported hypertension, stratified by high blood pressure medication intake
| Noise exposure, L den | N | Model 3—further adjusted for antihypertensive medication, % change (95% CI) | N | Model 3—restricted to subjects with antihypertensive medication, % change (95% CI) | N | Model 3—restricted to subjects without antihypertensive medication, % change (95% CI) | P Interaction* | |
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 178 409 | 43 384 | 135 025 | |||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | |||||
| Low–medium (>55 to ≤60 dB[A]) | 0.09 (−0.02, 0.21) | 0.16 (−0.08, 0.41) | 0.09 (−0.04, 0.22) | 0.00 (+) | ||||
| Medium–high (>60 to ≤65 dB[A]) | 0.25 (0.02, 0.15) | 0.60 (0.11, 1.11) | 0.13 (−0.13, 0.39) | 0.00 (+) | ||||
| High (>65 dB[A]) | 0.75 (0.51, 0.99) | 0.75 (0.23, 1.26) | 0.73 (0.47, 1.00) | 0.00 (+) | ||||
| Diastolic blood pressure (mmHg) | 178 238 | 43 346 | 134 892 | |||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | |||||
| Low–medium (>55 to ≤60 dB[A]) | 0.00 (−0.11, 0.11) | 0.03 (−0.21, 0.27) | 0.01 (−0.12, 0.15) | 0.10 (−) | ||||
| Medium–high (>60 to ≤65 dB[A]) | −0.02 (−0.25, 0.20) | 0.00 (−0.59, 0.49) | −0.06 (−0.31, 0.20) | 0.87 (−) | ||||
| High (>65 dB[A]) | 0.51 (0.27, 0.75) | 0.64 (0.14, 1.16) | 0.44 (0.17, 0.71) | 0.64 (−) | ||||
|
| ||||||||
| Noise exposure, L den | Model 3—further adjusted for antihypertensive medication, odds ratio (95% CI) | Model 3—restricted to subjects with antihypertensive medication, odds ratio (95% CI) | Model 3—restricted to subjects without antihypertensive medication, odds ratio (95% CI) | P Interaction | ||||
|
| ||||||||
| Self-reported hypertension | 193 911 | 47 284 | 146 625 | |||||
| Low (≤55 dB[A]) | Reference | Reference | Reference | |||||
| Low–medium (>55 to ≤60 dB[A]) | 1.02 (0.98, 1.05) | 0.99 (0.92, 1.05) | 1.03 (0.99, 1.06) | 0.00 (+) | ||||
| Medium–high (>60 to ≤65 dB[A]) | 1.06 (0.99, 1.13) | 1.00 (0.88, 1.14) | 1.07 (1.00, 1.15) | 0.00 (+) | ||||
| High (>65 dB[A]) | 0.97 (0.91, 1.04) | 0.89 (0.78, 1.02) | 0.99 (0.93, 1.07) | 0.00 (+) | ||||
| Continuous L den, per 1 dB[A] | 1.00 (1.00, 1.00) | 0.99 (0.99, 1.00) | 1.00 (1.00, 1.00) | 0.00 (+) | ||||
Model 3: fully adjusted model (Model 2) + adjusted for NO2. P interaction*: interaction between road traffic noise (L den) and high blood pressure medication; sign in the bracket indicates the direction of the interaction term. Bold represents significance at P < 0.05.
CI, confidence interval.
In the fully adjusted Model 2, exposure to road-L den >65 dB[A], as opposed to those exposed ≤55 dB[A], was negatively associated with triglycerides [−1.04% (95% CI −1.68, −0.41)]; after further adjustment for NO2 exposure, there was a significant positive association [0.79% (95% CI 0.11, 1.47)] (Table 2 and Figure 1). However, when PM2.5 was further adjusted, the direction and strength of the association was opposite to those in which adjustment of NO2 was made.
Comparing road-L den >65 vs. ≤55 dB[A], a positive association was found with glycated haemoglobin [0.15% (95% CI −0.01, 0.30)] in Model 2; after further adjustment for either air pollutant did not change the effect estimate materially (Table 2 and Figure 1). Associations with CRP were largely negative and non-significant, except in the model when PM2.5 was further considered [−1.28% (95% CI −2.46, −0.08)].
Similar significant positive associations were observed for SBP, DBP and triglycerides when comparing L night >55 vs. ≤ 45 dB[A] in the models further adjusted for NO2 (Supplementary material online, Appendix SA).
Sensitivity analyses on our main model, Model 3 (Model 2 further adjusted for NO2), by not adjusting for BMI, altered the results (Figure 1 and Supplementary material online, Appendix SB). The effect estimates for the significant positive associations between L den and SBP, DBP, and triglycerides in the highest exposure group (>65 dB) increased by 20%, 45%, and 131%, respectively, while significant positive associations were also seen for glycated haemoglobin [0.26% (95% CI 0.10, 0.43)] and CRP [2.35% (95% CI 1.21, 3.50)]. Effect estimates did not change materially by further adjusting for ever-had hypertension or diabetes in Model 3 (Supplementary material online, Appendix SC). Significant positive associations were still observed for SBP, DBP, and triglycerides in the >65 dB group based on the analyses of the imputed datasets in Model 3 (Supplementary material online, Appendix SD), with similar effect estimates as those in Table 2. Results also remained similar when the reference value was lowered to L den to ≤52 dB[A] in the analyses (Supplementary material online, Appendix SE).
Effect modification by sex was observed for SBP, glycated haemoglobin, and CRP (Supplementary material online, Table SF1) and by household income was observed for SBP and DBP (Supplementary material online, Table SF2). In the highest noise group >65 dB[A], positive associations with higher estimates were seen for CRP for females and glycated haemoglobin for males, but neither were statistically significant (Supplementary material online, Table SF3). Individuals aged ≥65 years, exposed to road-L den of 55–60 dB[A], had a higher estimated change (0.13%) in haemoglobin as compared to that (−0.01%) of individuals aged <65 years (Supplementary material online, Table SF4). A significant positive association (0.21%, 95% CI 0.03, 0.39) was found with haemoglobin in the highest noise group among individuals aged <65 years. No effect modification by either time at residence or area Townsend index was observed (Supplementary material online, Table SF5). Stronger associations with both SBP and DBP were seen in the higher household income groups comparing >65 vs. ≤ 55 dB[A] (Supplementary material online, Table SF6).
Discussion
In the UK Biobank, comparing annual average residential road traffic L den >65 vs. ≤55 dB[A], the positive estimates of SBP and DBP became significantly higher after further adjusting for either NO2 or PM2.5. Current use of antihypertensive medication did not appear to affect these associations. The association with glycated haemoglobin was positive and not confounded by either pollutant; in contrast, the associations with both triglycerides and CRP were less stable, depending on whether NO2 or PM2.5 was further adjusted. Another key finding suggests that BMI may be on the causal pathway between road traffic noise and these traditional CVD markers as excluding BMI from the analyses yielded significant positive associations in the highest noise group (>65 dB[A]) among all five markers.
Blood pressure and hypertension
To date, only a handful of studies have investigated the association between long-term road traffic noise exposure and changes in blood pressure among adults. The estimated associations across these previous studies are not consistent. Three studies from Spain,7 Switzerland,8 and Denmark6 reported a significant positive association with SBP but not DBP, and only among subgroups (men, older participants, or diabetic individuals),6 , 8 or when night-time indoor bedroom noise exposure (i.e. with reduced exposure misclassification) was used in the analysis.7 In contrast, a German study only observed a significant positive association with DBP but not SBP, and the association was stronger among men or diabetic individuals.9 A London study analysed night-time road traffic noise at both continuous and categorical scales but found no association with either blood pressure measure.10 These studies had a sample size ranging from 2500 to 44 000 participants. A pooled analysis of individual-level data from three European cohorts (Lifelines, HUNT3, and EPIC-Oxford) was the largest previous study with a sample size of over 88 000 participants.11 Unexpectedly, this study reported a significant negative association with DBP, which may be mainly driven by the Lifelines cohort, the largest and youngest cohort (mean age of 44 years) among the three. When categorical noise levels and blood pressure associations were analysed by cohort, significant positive associations were found in the >60 dB group vs. ≤50 dB, for both SBP and DBP in HUNT3, while in the EPIC-Oxford cohort, the association with SBP was positive in the highest >65 dB group vs. ≤55 dB. These findings, however, should be interpreted with caution as the number of participants exposed to the highest noise group in each cohort was relatively small (N = 230 for HUNT3 and N = 580 for EPIC-Oxford). Most of these previous studies analysed the relationship assuming a linear relationship and some found that the relationship was not confounded by air pollution7 , 11 by analysing models without and with air pollution adjustment.
Based on the largest study sample to date, in which over 30 000 participants were classified as highly exposed to residential road traffic noise (i.e. >65 dB), our study offers some new insights into this relationship. First, we found significant positive associations with both SBP and DBP only among highly exposed participants, suggesting that the relationship is likely non-linear. The restricted cubic spline analyses further revealed that the relationship only seems to be linear at levels above 60 dB. Second, the positive effect estimates were significantly amplified upon inclusion of either NO2 or PM2.5 into the model, suggesting that the effect estimates of road traffic noise on both SBP and DBP may be underestimated without accounting for air pollution effect, particularly that from near-road traffic as indicated by NO2 in our analysis. It may be possible that NO2 mainly originates from road traffic while PM2.5 could have many sources other than road traffic, thereby contributing in part to a stronger confounding effect by NO2. NO2 could also be a proxy of ultrafine particles, a pollutant sharing very similar propagation behaviour to noise27 and was recently linked to short-term adverse changes in blood pressure.28 As recently pointed out, it is still not completely clear whether traffic noise and air pollution have differing, additive, synergistic and/or antagonistic effects on cardiovascular outcomes.2 Third, we tested different approaches to accounting for antihypertensive medication use but the results remained robust.
The overall quality of evidence on the association between road traffic noise and prevalent or incident hypertension is rated as very low to low.4 , 5 We observed a null cross-sectional association with self-reported hypertension in the UK Biobank cohort. This is consistent with two recent London-based studies on both prevalent or incident hypertension.10 , 29 The ESCAPE study found that the pooled positive estimate, from six European cohorts, on incident self-reported hypertension was attenuated to null after adjusting for PM2.5.30 More recently, a Danish study reported no association between road traffic noise exposure and prescriptions for hypertension medication after a 14-year follow-up31 while a Ontario study found a significant 2% increase in incident hypertension with a 15-year follow-up.32 The latter study was based on a health insurance database covering over 700 000 participants with the assignment of noise estimates at postcode level and a lack of adjustment for lifestyle factors. Despite we found a null association among all participants, our analyses did show that the associations with self-reported hypertension tended to be positive and stronger among those who were currently not using any antihypertensive medication and exposed to an L den level between 55 and 65 dB. This finding suggests that long-term exposure to moderate/high levels of road traffic noise may be harmful on potentially uncontrolled hypertension.
Glycated haemoglobin, triglycerides, and C-reactive protein
The positive associations with glycated haemoglobin were consistent across all adjusted models, without or with air pollution adjustment. In a recent analysis of the Lifelines cohort, a significant positive association was found with blood glucose but not glycated haemoglobin, independent of air pollution adjustment.14 While studies of road traffic noise on blood glucose levels remain scarce, studies evaluating associations with diabetes generally reported an increased risk, for example a meta-analysis showed a 7% (95% CI 2%, 12%) increased risk for every 5 dB higher road traffic noise exposure.33
We found opposite associations with triglycerides upon adjustment of NO2 or PM2.5. Two studies, of young34 or middle-aged35 adults, found that the estimated changes in triglycerides of long-term exposure to NO2 were much higher as compared to PM2.5 while studies on long-term traffic noise exposure remain few. In our previous work, the positive association with triglycerides no longer remained significant after adjustment for NO2 or PM10 in the cohorts of Lifelines and HUNT3.14 The relative importance of gaseous and particulate pollutants on blood lipid profiles, and their respective interactions with traffic noise, is unclear. Future experimental and epidemiological studies are warranted to investigate both traffic noise and air pollution on lipid metabolism.
In a previous analysis of the Lifelines and HUNT3 cohorts, a positive association between long-term road traffic noise exposure and CRP was observed; however, the estimate was slightly attenuated but remained positive after controlling for air pollution.14 This is in contrast with our findings of negative associations in air pollution-adjusted models. The inconsistency between the two studies may be because our model had also adjusted for both BMI and area-level socioeconomic status. Recently, the population-based SAPALDIA cohort in Switzerland found significant enrichment of DNA methylation relating to CRP, independent of other noise sources and air pollution, with road L den exposure.36 This is in line with findings from novel experimental models, which found that traffic noise could induce oxidative stress and inflammation in the blood and the vasculature via an increased level of angiotensin II.1 More evidence is needed to support this novel mechanism through systemic inflammation for the association between traffic noise and health outcomes.
Role of body mass index
Notably, we observed stronger significant positive associations for all five risk factors after un-adjusting for BMI. This raises the possibility that BMI may be on the causal pathway on the investigated associations between traffic noise and cardiovascular health. A handful of studies in Europe, including the UK Biobank study, have suggested a positive association between long-term exposure to traffic noise and adiposity markers.37 We suspect that BMI may likely serve as a mediator rather than a confounder in our investigated associations and this speculation should necessitate a formal mediation analysis in future works. In particular, exploring the potential mediating role of the so-called metabolically healthy obesity status in the associations between traffic noise and cardiovascular outcomes represents an important knowledge gap.
Mechanisms
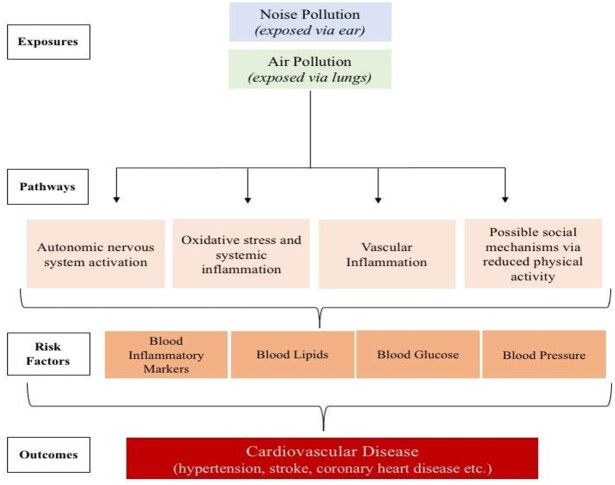
The exact mechanisms between noise exposure and cardiovascular health are not completely understood. The most frequently mentioned mechanism is that chronic exposure to noise leads to activation of the autonomic and endocrine systems, and a subsequent cascade of stress hormones (i.e. catecholamines), which will be causing adverse changes in blood pressure, blood lipids and blood glucose.2 As discussed earlier, a novel pathway via adverse changes in systemic inflammation or vascular inflammation only came to light recently38 , 39 (Graphical Abstract). A study recently reported that the amygdala may be involved in processing the stress response via heightened arterial inflammation.40 There may also exist a potential social mechanism in light of some recent studies linking road traffic noise41 or noise annoyance42 with reduced levels of physical activity, the consequences of which may cause unfavourable changes in CVD biochemistry profiles.
Strengths and limitations
The main strength of this study is the large study sample of >370 000 individuals in the main adjusted models, with detailed information on a variety of demographic, lifestyle, individual, and area-level socioeconomic variables. The study has limitations. The cross-sectional study design offers little support on causality and longitudinal studies are needed to strengthen the findings. Residual confounding from diet, physical activity, dyslipidaemia, family history, and other unmeasured factors may potentially bias our results. Light exposure at night, for which we did not have data, may potentially be another important confounding factor as it has been associated with the progression of carotid atherosclerosis43 and CVD hospitalization and death.44
Furthermore, as with all other studies of this type, exposure assignment of road traffic noise bears some uncertainty as time spent outside of home, layout of rooms in the house, window opening habits, noise sensitivity and indoor noise levels were not taken into account. Such exposure misclassification may have diluted our observed associations. L night exposure likely has reduced misclassification as most people stay at home during night-time hours. However, our modelled estimates from using categorical L night were similar as using L den. Our noise model at residential address did not specify a particular façade point, and therefore, it is likely that our estimated effects may have been underestimated. For instance, Foraster et al. 7 estimated that change in SBP was −0.20 mmHg (95% CI −1.25, 0.84) per 5 dB[A] of outdoor road traffic L night. The estimate increased to 0.36 mmHg (95% CI −0.06, 0.77) when outdoor road traffic L night at bedroom façade was analysed and 0.72 mmHg (95% CI 0.29, 1.15) when indoor road traffic L night in the bedroom was analysed. These findings highlight the importance of applying façade modelling in estimating noise exposures, which likely improves health effects estimation to a greater accuracy. Another limitation of our noise model is that it tended to over-estimate noise exposure at low levels due to the assumed national traffic flow baseline value but to under-estimate exposure for those heavily trafficked minor roads.2 Because of this, continuous noise estimates may be subject to more uncertainty and therefore we opted for categorical noise analyses, which may have relatively reduced misclassification.
The respective 0.77%, 0.49% and 0.79% increase in SBP, DBP and triglycerides observed in the main model equates to an approximate increase of 1.06 mmHg, 0.40 mmHg and 0.014 mmol/L for every 10 dB higher of road traffic noise. These effect sizes are small and potentially within the precision of instrumentation and random variation for an individual. Measurements were only taken at baseline visit, which did not truly reflect longer-term levels of these markers as measurement errors and intra-individual biological variability over time cannot be reliably accounted for without repeated measurements. A recent report from UK Biobank showed consistent mean values and a high self-correlation between baseline and repeated measurements among 20 000 participants for blood lipids and glycated haemoglobin.45 For an individual, clinical impact of such magnitude would be very small, if not negligible, despite statistical significance. At the population level, this may be particularly alarming given that currently across Europe 32 million people (nearly five million in UK) are exposed to road traffic noise >65 dB.46
In conclusion, this study provides evidence of long-term exposure to road traffic noise over 65 dB and elevated levels of CVD risk factors, particularly SBP and DBP. Future research with the consideration of both traffic noise and air pollution exposures is needed to provide further clarification on the multiple mechanisms between road traffic noise and CVD manifestation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data underlying this article can be requested from UK Biobank (https://www.ukbiobank.ac.uk/).
Supplementary Material
Acknowledgements
This study was conducted using the UK Biobank resources (project number: 5179). Z.K. would like to acknowledge the support of Acoustics at Ove Arup and Partners for providing time for the editing of this paper.
Funding
The MRC Centre for Environment and Health is funded by the UK Medical Research Council (Grant Number: MR/S019669/1). Z.K. and D.F. are supported by the MRC Centre for Environment and Health (Grant Number: MR/M501669/1). Y.C. is supported by an Early-Career Research Fellowship through the MRC Centre for Environment and Health (Grant Number: MR/M501669/1). Y.C. is supported by the PEAK Urban programme, funded by UKRI’s Global Challenge Research Fund (Grant Number: ES/P011055/1).
Conflict of interest: none declared.
Contributor Information
Zuzana Kupcikova, Acoustics, Ove Arup & Partners, 13 Fitzroy Street, London W1T 4BQ, UK; MRC Centre for Environment and Health, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, St Mary's Campus, Norfolk Place, London W2 1PG, UK.
Daniela Fecht, MRC Centre for Environment and Health, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, St Mary's Campus, Norfolk Place, London W2 1PG, UK.
Rema Ramakrishnan, Nuffield Department of Women’s & Reproductive Health, Women's Centre (Level 3), John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, UK; Deep Medicine Programme, Oxford Martin School, University of Oxford, 34 Broad St, Oxford OX1 3BD, UK.
Charlotte Clark, Acoustics, Ove Arup & Partners, 13 Fitzroy Street, London W1T 4BQ, UK.
Yutong Samuel Cai, MRC Centre for Environment and Health, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, St Mary's Campus, Norfolk Place, London W2 1PG, UK; Nuffield Department of Women’s & Reproductive Health, Women's Centre (Level 3), John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, UK; Deep Medicine Programme, Oxford Martin School, University of Oxford, 34 Broad St, Oxford OX1 3BD, UK.
References
- 1. Münzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sørensen M. Environmental noise and the cardiovascular system. J Am Coll Cardiol 2018;71:688–697. [DOI] [PubMed] [Google Scholar]
- 2. Münzel T, Sørensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017;38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Münzel T, Kröller-Schön S, Oelze M, Gori T, Schmidt FP, Steven S, Hahad O, Röösli M, Wunderli J-M, Daiber A, Sørensen M. Adverse cardiovascular effects of traffic noise with a focus on nighttime noise and the new WHO noise guidelines. Annu Rev Public Health 2020;41:309–328. [DOI] [PubMed] [Google Scholar]
- 4. Kempen E, Casas M, Pershagen G, Foraster M. WHO environmental noise guidelines for the European region: a systematic review on environmental noise and cardiovascular and metabolic effects: a summary. Int J Environ Res Public Health 2018;15:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dzhambov AM, Dimitrova DD. Residential road traffic noise as a risk factor for hypertension in adults: systematic review and meta-analysis of analytic studies published in the period 2011–2017. Environ Pollut 2018;240:306–318. [DOI] [PubMed] [Google Scholar]
- 6. Sørensen M, Hvidberg M, Hoffmann B, Andersen ZJ, Nordsborg RB, Lillelund KG, Jakobsen J, Tjønneland A, Overvad K, Raaschou-Nielsen O. Exposure to road traffic and railway noise and associations with blood pressure and self-reported hypertension: a cohort study. Environ Health 2011;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foraster M, Künzli N, Aguilera I, Rivera M, Agis D, Vila J, Bouso L, Deltell A, Marrugat J, Ramos R, Sunyer J, Elosua R, Basagaña X. High blood pressure and long-term exposure to indoor noise and air pollution from road traffic. Environ Health Perspect 2014;122:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dratva J, Phuleria HC, Foraster M, Gaspoz JM, Keidel D, Künzli N, Liu LJS, Pons M, Zemp E, Gerbase MW, Schindler C. Transportation noise and blood pressure in a population-based sample of adults. Environ Health Perspect 2012;120:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pitchika A, Hampel R, Wolf K, Kraus U, Cyrys J, Babisch W, Peters A, Schneider A. Long-term associations of modeled and self-reported measures of exposure to air pollution and noise at residence on prevalent hypertension and blood pressure. Sci Total Environ 2017;593–594:337–346. [DOI] [PubMed] [Google Scholar]
- 10. Halonen JI, Dehbi HM, Hansell AL, Gulliver J, Fecht D, Blangiardo M, Kelly FJ, Chaturvedi N, Kivimäki M, Tonne C. Associations of night-time road traffic noise with carotid intima-media thickness and blood pressure: the Whitehall II and SABRE study cohorts. Environ Int 2017;98:54–61. [DOI] [PubMed] [Google Scholar]
- 11. Zijlema W, Cai Y, Doiron D, Mbatchou S, Fortier I, Gulliver J, de Hoogh K, Morley D, Hodgson S, Elliott P, Key T, Kongsgard H, Hveem K, Gaye A, Burton P, Hansell A, Stolk R, Rosmalen J. Road traffic noise, blood pressure and heart rate: pooled analyses of harmonized data from 88,336 participants. Environ Res 2016;151:804–813. [DOI] [PubMed] [Google Scholar]
- 12. Eze IC, Foraster M, Schaffner E, Vienneau D, Héritier H, Rudzik F, Thiesse L, Pieren R, Imboden M, von Eckardstein A, Schindler C, Brink M, Cajochen C, Wunderli JM, Röösli M, Probst-Hensch N. Long-term exposure to transportation noise and air pollution in relation to incident diabetes in the SAPALDIA study. Int J Epidemiol 2017;46:1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sørensen M, Hjortebjerg D, Eriksen KT, Ketzel M, Tjønneland A, Overvad K, Raaschou-Nielsen O. Exposure to long-term air pollution and road traffic noise in relation to cholesterol: a cross-sectional study. Environ Int 2015;85:238–243. [DOI] [PubMed] [Google Scholar]
- 14. Cai Y, Hansell AL, Blangiardo M, Burton PR, de Hoogh K, Doiron D, Fortier I, Gulliver J, Hveem K, Mbatchou S, Morley DW, Stolk RP, Zijlema WL, Elliott P, Hodgson S; BioSHaRE. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur Heart J 2017;38:2290–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lelieveld J, Klingmüller K, Pozzer A, Pöschl U, Fnais M, Daiber A, Münzel T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 2019;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ 2020;368:m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UK Biobank. Biomarker assay quality procedures: approaches used to minimise systematic and random errors (and the wider epidemiological implications). https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/biomarker_issues.pdf (24 February 2021).
- 19.UK Biobank. Blood Pressure. http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/Bloodpressure.pdf (24 February 2021).
- 20. Kephalopoulos S, Paviotti M, Anfosso-Lédée F, Van Maercke D, Shilton S, Jones N. Advances in the development of common noise assessment methods in Europe: the CNOSSOS-EU framework for strategic environmental noise mapping. Sci Total Environ 2014;482–483:400–410. [DOI] [PubMed] [Google Scholar]
- 21. Morley DW, de Hoogh K, Fecht D, Fabbri F, Bell M, Goodman PS, Elliott P, Hodgson S, Hansell AL, Gulliver J. International scale implementation of the CNOSSOS-EU road traffic noise prediction model for epidemiological studies. Environ Pollut 2015;206:332–341. [DOI] [PubMed] [Google Scholar]
- 22. Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, Tsai M-Y, Künzli N, Schikowski T, Marcon A, Eriksen KT, Raaschou-Nielsen O, Stephanou E, Patelarou E, Lanki T, Yli-Tuomi T, Declercq C, Falq G, Stempfelet M, Birk M, Cyrys J, von Klot S, Nádor G, Varró MJ, Dėdelė A, Gražulevičienė R, Mölter A, Lindley S, Madsen C, Cesaroni G, Ranzi A, Badaloni C, Hoffmann B, Nonnemacher M, Krämer U, Kuhlbusch T, Cirach M, de Nazelle A, Nieuwenhuijsen M, Bellander T, Korek M, Olsson D, Strömgren M, Dons E, Jerrett M, Fischer P, Wang M, Brunekreef B, de Hoogh K. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—the ESCAPE project. Atmos Environ 2013;72:10–23. [Google Scholar]
- 23. Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, Declercq C, Dėdelė A, Dons E, de Nazelle A, Dimakopoulou K, Eriksen K, Falq G, Fischer P, Galassi C, Gražulevičienė R, Heinrich J, Hoffmann B, Jerrett M, Keidel D, Korek M, Lanki T, Lindley S, Madsen C, Mölter A, Nádor G, Nieuwenhuijsen M, Nonnemacher M, Pedeli X, Raaschou-Nielsen O, Patelarou E, Quass U, Ranzi A, Schindler C, Stempfelet M, Stephanou E, Sugiri D, Tsai MY, Yli-Tuomi T, Varró MJ, Vienneau D, Klot S, Wolf K, Brunekreef B, Hoek G. Development of land use regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 2012;46:11195–11205. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Environmental Noise Guidelines for the European Region. Geneva: WHO; 2018. https://www.euro.who.int/en/publications/abstracts/environmental-noise-guidelines-for-the-european-region-2018 (24 February 2021). [Google Scholar]
- 25. Hansell AL, Blangiardo M, Fortunato L, Floud S, de Hoogh K, Fecht D, Ghosh RE, Laszlo HE, Pearson C, Beale L, Beevers S, Gulliver J, Best N, Richardson S, Elliott P. Aircraft noise and cardiovascular disease near Heathrow airport in London: small area study. BMJ 2013;347:f5432–f5432. [DOI] [PubMed] [Google Scholar]
- 26. Foraster M, Basagaña X, Aguilera I, Rivera M, Agis D, Bouso L, Deltell A, Marrugat J, Ramos R, Sunyer J, Vila J, Elosua R, Künzli N. Association of long-term exposure to traffic-related air pollution with blood pressure and hypertension in an adult population-based cohort in Spain (the REGICOR study). Environ Health Perspect 2014;122:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Héritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, de Hoogh K, Thiesse L, Rudzik F, Habermacher M, Köpfli M, Pieren R, Brink M, Cajochen C, Wunderli JM, Probst-Hensch N, Röösli M. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: a nationwide cohort study in Switzerland. Eur Heart J 2019;40:598–603. [DOI] [PubMed] [Google Scholar]
- 28. Soldevila N, Vinyoles E, Tobias A, Banegas JR, De La SA, Gorostidi M, Segura J, De La CJJ, Muñoz-Pérez MA, Querol X, Ruilope LM. How do ultrafine particles in urban air affect ambulatory blood pressure? J Hypertens 2020;38:845–849. [DOI] [PubMed] [Google Scholar]
- 29. Carey IM, Anderson HR, Atkinson RW, Beevers S, Cook DG, Dajnak D, Gulliver J, Kelly FJ. Traffic pollution and the incidence of cardiorespiratory outcomes in an adult cohort in London. Occup Environ Med 2016;73:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuks KB, Weinmayr G, Basagaña X, Gruzieva O, Hampel R, Oftedal B, Sørensen M, Wolf K, Aamodt G, Aasvang GM, Aguilera I, Becker T, Beelen R, Brunekreef B, Caracciolo B, Cyrys J, Elosua R, Eriksen KT, Foraster M, Fratiglioni L, Hilding A, Houthuijs D, Korek M, Künzli N, Marrugat J, Nieuwenhuijsen M, Östenson CG, Penell J, Pershagen G, Raaschou-Nielsen O, Swart WJR, Peters A, Hoffmann B. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). Eur Heart J 2017;38:983–990. [DOI] [PubMed] [Google Scholar]
- 31. Thacher JD, Poulsen AH, Roswall N, Hvidtfeldt U, Raaschou-Nielsen O, Jensen SS, Ketzel M, Brandt J, Overvad K, Tjønneland A, Münzel T, Sørensen M. Road traffic noise exposure and filled prescriptions for antihypertensive medication: a Danish cohort study. Environ Health Perspect 2020;128:057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shin S, Bai L, Oiamo TH, Burnett RT, Weichenthal S, Jerrett M, Kwong JC, Goldberg MS, Copes R, Kopp A, Chen H. Association between road traffic noise and incidence of diabetes mellitus and hypertension in Toronto, Canada: a population-based cohort study. J Am Heart Assoc 2020;9:e013021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zare Sakhvidi MJ, Zare Sakhvidi F, Mehrparvar AH, Foraster M, Dadvand P. Association between noise exposure and diabetes: a systematic review and meta-analysis. Environ Res 2018;166:647–657. [DOI] [PubMed] [Google Scholar]
- 34. Kim JS, Chen Z, Alderete TL, Toledo-Corral C, Lurmann F, Berhane K, Gilliland FD. Associations of air pollution, obesity and cardiometabolic health in young adults: the meta-AIR study. Environ Int 2019;133:105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang BY, Bloom MS, Markevych I, Qian ZM, Vaughn MG, Cummings-Vaughn LA, Li S, Chen G, Bowatte G, Perret JL, Dharmage SC, Heinrich J, Yim SH, Lin S, Tian L, Yang M, Liu KK, Zeng XW, Hu LW, Guo Y, Dong GH. Exposure to ambient air pollution and blood lipids in adults: the 33 Communities Chinese Health Study. Environ Int 2018;119:485–492. [DOI] [PubMed] [Google Scholar]
- 36. Eze IC, Foraster M, Schaffner E, Vienneau D, Pieren R, Imboden M, Wunderli J-M, Cajochen C, Brink M, Röösli M, Probst-Hensch N. Incidence of depression in relation to transportation noise exposure and noise annoyance in the SAPALDIA study. Environ Int 2020;144:106014. [DOI] [PubMed] [Google Scholar]
- 37. Cai Y, Zijlema WL, Sørgjerd EP, Doiron D, de Hoogh K, Hodgson S, Wolffenbuttel B, Gulliver J, Hansell AL, Nieuwenhuijsen M, Rahimi K, Kvaløy K. Impact of road traffic noise on obesity measures: observational study of three European cohorts. Environ Res 2020;191:110013. [DOI] [PubMed] [Google Scholar]
- 38. Münzel T, Miller MR, Sørensen M, Lelieveld J, Daiber A, Rajagopalan S. Reduction of environmental pollutants for prevention of cardiovascular disease: it’s time to act. Eur Heart J 2020;41:3989–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eze IC, Jeong A, Schaffner E, Rezwan FI, Ghantous A, Foraster M, Vienneau D, Kronenberg F, Herceg Z, Vineis P, Brink M, Wunderli JM, Schindler C, Cajochen C, Röösli M, Holloway JW, Imboden M, Probst-Hensch N. Genome-wide DNA methylation in peripheral blood and long-term exposure to source-specific transportation noise and air pollution: the SAPALDIA study. Environ Health Perspect 2020;128:067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, Tung B, Wang Y, Ishai A, Scott JA, Shin LM, Fayad ZA, Koenen KC, Rajagopalan S, Pitman RK, Tawakol A. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. Eur Heart J 2020;41:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roswall N, Ammitzbøll G, Christensen J, Raaschou-Nielsen O, Jensen S, Tjønneland A, Sørensen M. Residential exposure to traffic noise and leisure-time sports—a population-based study. Int J Hyg Environ Health 2017;220:1006–1013. [DOI] [PubMed] [Google Scholar]
- 42. Foraster M, Eze IC, Vienneau D, Brink M, Cajochen C, Caviezel S, Héritier H, Schaffner E, Schindler C, Wanner M, Wunderli JM, Röösli M, Probst-Hensch N. Long-term transportation noise annoyance is associated with subsequent lower levels of physical activity. Environ Int 2016;91:341–349. [DOI] [PubMed] [Google Scholar]
- 43. Obayashi K, Yamagami Y, Tatsumi S, Kurumatani N, Saeki K. Indoor light pollution and progression of carotid atherosclerosis: a longitudinal study of the HEIJO-KYO cohort. Environ Int 2019;133:105184. [DOI] [PubMed] [Google Scholar]
- 44. Sun S, Cao W, Ge Y, Ran J, Sun F, Zeng Q, Guo M, Huang J, Lee RS-Y, Tian L, Wellenius GA. Outdoor light at night and risk of coronary heart disease among older adults: a prospective cohort study. Eur Heart J 2020;doi:10.1093/eurheartj/ehaa846. [DOI] [PubMed] [Google Scholar]
- 45. Allen NE, Arnold M, Parish S, Hill M, Sheard S, Callen H, Fry D, Moffat S, Gordon M, Welsh S, Elliott P, Collins R. Approaches to minimising the epidemiological impact of sources of systematic and random variation that may affect biochemistry assay data in UK Biobank. Wellcome Open Res 2020;5:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.European Environment Agency. Managing Exposure to Noise in Europe. Luxembourg: EEA; 2017. https://www.eea.europa.eu/themes/human/noise/sub-sections/noise-in-europe-updated-population-exposure (24 February 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article can be requested from UK Biobank (https://www.ukbiobank.ac.uk/).