Abstract
Aims
Whether isolated diastolic hypertension (IDH), as defined by the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) guideline, is associated with cardiovascular disease (CVD) has been disputed. We aimed to further study the associations of IDH with (i) subclinical CVD in the form of coronary artery calcium (CAC), (ii) incident systolic hypertension, and (iii) CVD events.
Methods and results
We used multivariable-adjusted logistic and Cox regression to test whether IDH by 2017 ACC/AHA criteria (i.e. systolic blood pressure <130 mmHg and diastolic blood pressure ≥80 mmHg) was associated with the above outcomes in the Multi-Ethnic Study of Atherosclerosis (MESA). In a random-effects meta-analysis of the association between IDH and CVD events, we combined the MESA results with those from seven other previously published cohort studies. Among the 5104 MESA participants studied, 7.5% had IDH by the 2017 ACC/AHA criteria. There was no association between IDH and CAC [e.g. adjusted prevalence odds ratio for CAC >0 of 0.88 (95% CI 0.66, 1.17)]. Similarly, while IDH was associated with incident systolic hypertension, there was no statistically significant associations with incident CVD [hazard ratio 1.19 (95% CI 0.77, 1.84)] or death [hazard ratio 0.94 (95% CI 0.65, 1.36)] over 13 years in MESA. In a stratified meta-analysis of eight cohort studies (10 037 843 participants), the 2017 IDH definition was also not consistently associated with CVD and the relative magnitude of any potential association was noted to be numerically small [e.g. depending on inclusion criteria applied in the stratification, the adjusted hazard ratios ranged from 1.04 (95% CI 0.98, 1.10) to 1.09 (95% 1.03, 1.15)].
Conclusion
The lack of consistent excess in CAC or CVD suggests that emphasis on healthy lifestyle rather than drug therapy is sufficient among the millions of middle-aged or older adults who now meet the 2017 ACC/AHA criteria for IDH, though they require follow-up for incident systolic hypertension. These findings may not extrapolate to adults younger than 40 years, motivating further study in this age group.
Keywords: Isolated diastolic hypertension, Coronary artery calcium, Cardiovascular disease
Graphical Abstract
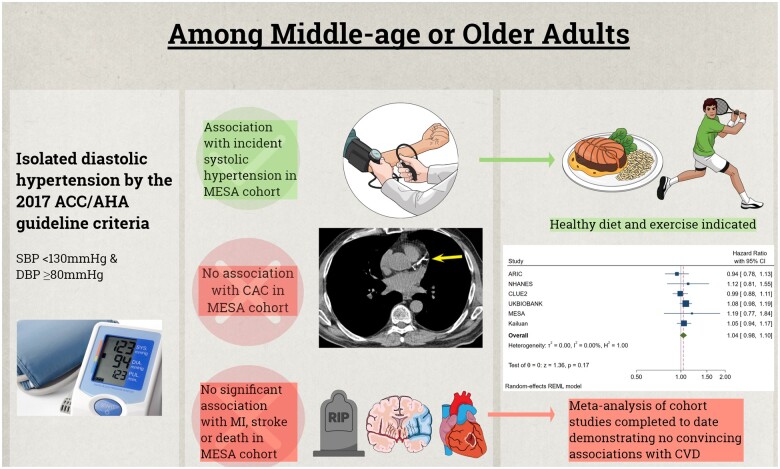
In the Multi-Ethnic Study of Atherosclerosis (MESA), isolated diastolic hypertension (IDH) by the 2014 ACC/AHA definition is associated with future development of systolic hypertension but is not significantly associated with coronary artery calcification (CAC) or cardiovascular disease (CVD) events. These MESA results were added to a meta-analysis of prior reports on the association between IDH by the 2017 ACC/AHA definition and CVD events, finding that no consistent association was found, particularly when studies were stratified by the quality of blood pressure measurement.
See page 2130 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab109)
Introduction
Observational studies have shown that elevations in both systolic blood pressure (BP) and diastolic BP are associated with increased risk for cardiovascular disease (CVD).1 Randomized clinical trial data support therapeutically reducing diastolic BP to <90 mmHg2 and systolic BP to <120 mmHg in appropriately selected adults.3 Although current European Society of Cardiology (ESC) guidelines continue to define hypertension as BP ≥140/90 mmHg,4 the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) guideline for the management of hypertension revised the definition to ≥130/80 mmHg.5 While the lower systolic BP threshold is based on a number of lines of compelling evidence,3 the lower diastolic BP threshold in US guidelines (i.e., 80 mmHg) was recommended based on expert opinion. This lower diastolic BP threshold increases the proportion of adults (e.g. an increase of >10 million in America6) now eligible for a diagnosis of hypertension based solely on the presence of isolated diastolic hypertension (IDH), despite having systolic BP <130 mmHg. Specifically, IDH is now defined as a systolic BP <130 mmHg with a diastolic BP ≥80 mmHg in America but continues to be defined as a systolic BP <140 with a diastolic BP ≥90 mmHg in Europe.
There is uncertainty about whether IDH is independently associated with adverse clinical outcomes. While several analyses of the ESC definition of IDH have not found any association with CVD,7–12 a number of others have.13–16 Complicating matters further, relatively few analyses of the 2017 ACC/AHA definition of IDH have been undertaken to date, with five cohorts demonstrating no increased CVD risk after adjustment6 , 16 , 17 and three cohorts suggesting a small-to-modest association.15 , 18–20 There has also been heterogeneity in the conduct of these studies, with the studies suggesting an association all using BP obtained during usual clinical practice but the studies finding no association using BP readings obtained under more reliable and valid research conditions.21 This is relevant because diastolic BP is difficult to measure accurately.22 As such, some controversy exists as to whether IDH (particularly by the 2017 ACC/AHA definition) increases risk for CVD, a question that is important to clarify given it has implications for millions of adults.
Because IDH is a relatively small subset of hypertension and because persons with IDH are often middle-aged or younger (and less likely to suffer events), a criticism of the prior null observational studies is that they may have been underpowered to detect associations between IDH and CVD events.15 Consequently, studies evaluating the association between IDH and subclinical markers for CVD may provide the additional power necessary to further probe the clinical implications of IDH; information that is especially needed for the new 2017 ACC/AHA definition of IDH. One such subclinical marker is coronary artery calcium (CAC), which is established as the strongest subclinical marker of risk for incident CVD events in a number of primary prevention populations.23–25 Therefore, we conducted analyses in the Multi-Ethnic Study of Atherosclerosis (MESA) to test the association of IDH with CAC. We then tested the association of IDH with clinical CVD events in this contemporary multi-ethnic sample. We augmented this MESA analysis of CVD outcomes by conducting a meta-analysis of all the observational studies to date reporting on the association of IDH by the 2017 ACC/AHA definition and CVD events. Finally, because IDH by the more traditional ESC definition has been shown to be associated with incident systolic hypertension,26 we studied whether the same was true for IDH by the 2017 ACC/AHA definition.
Methods
Study participants
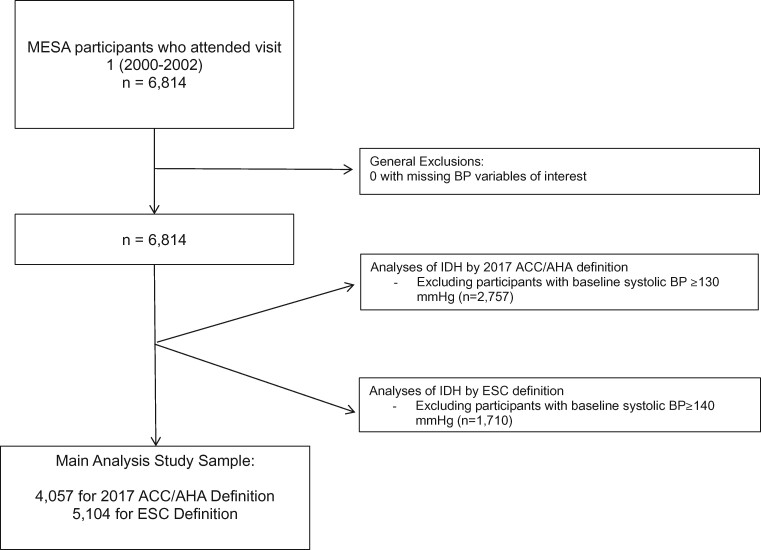
Multi-Ethnic Study of Atherosclerosis is a community-based cohort study of the prevalence, correlates, and progression of subclinical and clinical CVD. Comprehensive details of the MESA study are reported elsewhere.27 Briefly, 6814 participants between the ages of 45 and 84 without clinical CVD at baseline were enrolled between 2000 and 2002 (i.e. MESA visit 1) at six US field centres (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St Paul, MN). Five further follow-up visits were conducted between 2002 and 2018. For this analysis, we excluded participants with systolic hypertension at baseline (defined as systolic BP ≥140 mmHg in analyses of the ESC definition of IDH and systolic BP ≥130 mmHg in analyses of the 2017 ACC/AHA definition) (Figure 1). This study was approved by the institutional review boards at each centre and all participants provided written informed consent.
Figure 1.
Flow chart of the exclusion process used to construct the main analytic samples in Multi-Ethnic Study of Atherosclerosis. BP, blood pressure.
Baseline demographic and clinical variables
At the baseline examination, self-reported information was collected pertaining to demographics, lipid-lowering and anti-hypertensive medication use, cigarette smoking, and alcohol use using standardized questionnaires. Information on race was self-reported (selected from several fixed categories defined by the investigators). Diabetes mellitus was defined as a fasting blood glucose concentration of ≥126 mg/dL, self-report, or the use of insulin or oral hypoglycaemic medications. Systolic and diastolic BPs were measured three times after 5 min of seated rest using a Dinamap Pro-10028 automated oscillometric sphygmomanometer (Dinamap, Critikon, Tampa, FL, USA), and the mean of the last two measurements was used for analyses. Body mass index, fasting lipid panel, and estimated glomerular filtration rate (eGFR) calculated from creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, were also measured.
Coronary artery calcium
Coronary artery calcium was measured in all participants at Exam 1. Each of the six MESA centres evaluated CAC with the use of an electron-beam computed tomography (CT) scanner or a multi-detector CT system following standard protocols and all images were interpreted at a single CT reading centre (LA Biomedical Research Institute at Harbor–UCLA Medical Center, Torrance, CA, USA). Follow-up CAC measurements were performed on half the cohort (randomly selected) at a second examination (September 2002 through January 2004) and the other half of the cohort at a third examination (March 2004 through July 2005) at an average of 1.6 and 3.2 years after the participant’s first examination, respectively. For the cardiac CTs performed during MESA examinations, each participant was scanned twice and the mean CAC score of the two scans was used. Intra-observer and inter-observer agreements were excellent (κ statistics, 0.93 and 0.90, respectively), and the intra-class correlation coefficient for the Agatston score was 0.99.
Incident systolic or treated hypertension
We defined this outcome as new anti-hypertensive drug therapy over follow-up or new systolic hypertension (i.e. systolic BP ≥140 mmHg in analyses of the ESC definition of IDH and systolic BP ≥130 mmHg in analyses of the 2017 ACC/AHA definition). Because this outcome required the physical measurement of BP using the Dinamap Pro-100 device as part of follow-up MESA study visits, follow-up for this outcome ended at the last MESA study visit a given participant attended. Persons on baseline anti-hypertensive medication (numbers available in Table 1) were excluded from analyses of this particular outcome.
Table 1.
Baseline demographics according to blood pressure status
| Normotension by ESC | IDH by ESC | P-value | Normotension by ACC/AHA (2017) | IDH by ACC/AHA (2017) | P-value | P-value * | |
|---|---|---|---|---|---|---|---|
| Number (%) | 5059 (99.1) | 45 (0.9) | 3752 (92.5) | 305 (7.5) | |||
| Age, years | 60.5 (10.0) | 56.3 (8.2) | 0.004 | 59.7 | 56.7 | <0.001 | <0.001 |
| Sex, N (%) | <0.001 | <0.001 | <0.001 | ||||
| Female | 2622 (51.8) | 6 (13.3) | 2004 (53.4) | 75 (24.6) | |||
| Male | 2437 (48.2) | 39 (86.7) | 1748 (46.6) | 230 (75.4) | |||
| Race/ethnicity, N (%) | 0.36 | 0.03 | 0.047 | ||||
| White (non-Hispanic) | 2071 (40.9) | 18 (40.0) | 1602 (42.7) | 109 (35.7) | |||
| Chinese, American | 612 (12.1) | 4 (8.9) | 464 (12.4) | 36 (12.3) | |||
| Black | 1266 (25.0) | 16 (35.6) | 856 (22.8) | 90 (29.5) | |||
| Hispanic | 1110 (21.0) | 7 (15.6) | 830 (22.1) | 70 (23.0) | |||
| Smoking, N (%) | 0.46 | 0.82 | 0.80 | ||||
| Never | 2519 (49.9) | 18 (40.9) | 1879 (50.2) | 148 (48.5) | |||
| Former | 1829 (36.3) | 18 (40.9) | 1326 (35.4) | 110 (36.1) | |||
| Current | 698 (13.8) | 8 (18.2) | 539 (14.4) | 47 (15.4) | |||
| Alcohol use, N (%) | 0.11 | 0.44 | 0.49 | ||||
| Never | 951 (18.9) | 4 (9.1) | 697 (18.7) | 48 (15.8) | |||
| Former | 1176 (23.4) | 8 (18.2) | 854 (22.9) | 70 (23.0) | |||
| Current | 2901 (57.7) | 32 (72.7) | 2180 (58.4) | 186 (61.2) | |||
| LDL cholesterol, mg/dL | 117.2 (31.7) | 115.7 (25.4) | 0.74 | 117.0 (31.8) | 119.2 (29.5) | 0.24 | 0.27 |
| HDL cholesterol, mg/dL | 50.7 (14.8) | 50.5 (12.6) | 0.94 | 50.9 (15.0) | 47.7 (12.4) | <0.001 | <0.001 |
| Triglycerides, mg/dL | 130.0 (89.7) | 143.1 (88.2) | 0.33 | 126.74 (78.6) | 138.0 (87.4) | 0.02 | 0.02 |
| Any lipid-lowering medication, N (%) | 759 (15.0) | 4 (8.9) | 0.25 | 534 (14.2) | 34 (11.2) | 0.13 | 0.17 |
| Diabetes, N (%) | 550 (10.9) | 3 (6.7) | 0.36 | 383 (10.2) | 22 (7.2) | 0.09 | 0.11 |
| BMI, kg/m2 | 28.1 (5.37) | 28.1 (4.90) | 0.94 | 27.8 (5.4) | 28.3 (4.4) | 0.18 | 0.19 |
| eGFR, mL/min/1.73 m2 | 79.4 (15.8) | 80.6 (15.1) | 0.60 | 79.7 (15.6) | 83.5 (14.9) | <0.001 | <0.001 |
| 10-year ASCVD risk estimate (pooled cohort equations) | 6.7 (2.8–14.5) | 8.9 (5.6–13.7) | <0.001 | 5.5 (2.3–12.4) | 7.0 (3.8–11.2) | <0.001 | 0.002 |
| Anti-hypertensive medication, N (%) | 1547 (30.6) | 23 (51.1) | 0.003 | 1002 (26.7) | 85 (27.9) | 0.66 | <0.001 |
| Systolic BP, mmHg | 116.8 (13.1) | 134.8 (3.9) | <0.001 | 111.5 (10.5) | 122.7 (4.8) | <0.001 | <0.001 |
Results are mean (SD) for normally distributed continuous variables and median (quartile 1-quartile 3) otherwise.
ACC, American College of Cardiology; AHA, American Heart Association; BMI, Body Mass Index; BP, blood pressure; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; HDL, high-density lipoprotein; IDH, isolated diastolic hypertension; LDL, low-density lipoprotein.
P-value comparing those with IDH by ESC to those with IDH by ACC/AHA.
Follow-up for incident clinical outcomes in Multi-Ethnic Study of Atherosclerosis
At 9- to 12-month intervals, participants or a family member were contacted to inquire about outpatient visits, hospital admissions, and deaths. Self-reported diagnoses were verified using medical records of outpatient visits and hospitalizations. For deaths, interviews with the next of kin and death certificates were used for verification. Two physicians from the MESA mortality and morbidity review committee independently classified events.
Both myocardial infarction and stroke were adjudicated. A diagnosis of myocardial infarction was based on symptoms, electrocardiogram, and levels of cardiac biomarkers. If a death occurred within 28 days of a myocardial infarction, if the participant had chest pain 72 h before death, or if the participant had known coronary heart disease and no other non-cardiac cause of death; then a death was considered to be due to coronary heart disease.
A stroke was considered to be present if there was a focal neurologic deficit lasting 24 h or until death, or if <24 h, there was a clinically relevant lesion on brain imaging and no non-vascular cause. Self-reported diagnoses of stroke were verified with medical records of outpatient visits, hospital records, and death certificates. For fatal events, International Classification of Diseases revision codes of all deaths were reviewed by study staff. We used the composite outcome of hard CVD, defined as myocardial infarction, coronary heart disease death, cerebrovascular accident (CVA, transient ischaemic attack or ischaemic or haemorrhagic stroke), death from CVA, and other CVD death.
In post hoc sensitivity analyses, we also evaluated other incident outcomes pertinent to known complications of hypertension: chronic kidney disease, heart failure, and atrial fibrillation. A full description of the adjudication process of events is described elsewhere.27
Statistical analyses
For the MESA analysis, IDH was defined by both the traditional (ESC) BP cut-off (SBP <140 mmHg and DBP ≥90 mmHg) and the new ACC/AHA 2017 definition (SBP <130 mmHg and DBP ≥80 mmHg). For analyses of IDH by the ESC definition, the reference group was defined as BP <140/90 mmHg. For analyses of IDH by the 2017 ACC/AHA definition, the reference group was BP <130/80 mmHg. The prevalence of IDH at visit 1, using both definitions, was described. Baseline characteristics were compared across the various BP categories using analysis of variance for continuous variables and chi-squared testing for proportions.
Next, the cross-sectional categorical association of IDH with baseline CAC >0 (assessed at visit 1) was characterized in MESA with logistic regression. The first model was unadjusted. The second model was adjusted for age, sex, race/ethnicity, body mass index (kg/m2), smoking (current, former, never), alcohol consumption (current, former, or never), low-density lipoprotein cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), triglycerides (mg/dL), eGFR, BP-lowering medication (yes/no), and history of diagnosed diabetes (yes/no). The third model included all variables in Model 2 plus baseline (visit 1) systolic BP. Using linear regression, we repeated cross-sectional analyses testing for any continuous association between IDH and log(CAC + 1). This log(CAC + 1) transformation is standard when using CAC as a continuous dependent variable in linear regression, given the high proportion of CAC = 0 in any sample and the positive skew of values for those with CAC >0. Using log-binomial regression, with adjustments as described above, we also tested the association of IDH with incident CAC >0 (detected at either MESA visit 2 or visit 3) among those with CAC = 0 at visit 1.
We then characterized the prospective association of baseline IDH (both definitions) with incident systolic or treated hypertension (assessed at each MESA follow-up visit) using Cox models adjusted for covariates as described above; except in this analysis we also excluded persons on baseline BP therapy and, therefore, this variable was not included in Model 2. To examine associations with clinical events, Kaplan–Meier plots were generated to visually demonstrate the survival functions by categories of IDH vs. normotension. Furthermore, we conducted analyses of incident CVD, death, and other outcomes using Cox models that were also adjusted for the covariates described above in models 1 through 3. The proportional hazards assumption was examined using log-(-log) plots and by testing risk factor-by-time interactions. Each analysis was then repeated after stratification by age, sex, race/ethnicity, and hypertension treatment status (with testing also for statistical interaction). We also conducted a sensitivity analysis analysing incident CVD or death in Model 3, but with updated anti-hypertensive and lipid-lowering therapies as time-varying covariables in the model.
Finally, using data from the most completely adjusted models reported, we conducted a stratified meta-analysis of the adjusted association between IDH when defined by the 2017 ACC/AHA definition and CVD events, in MESA and in the seven previously published cohort studies (eight cohorts in total). To inform this meta-analysis, systematic review was performed in accordance with the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines.29 We used I 2 statistics to assess for heterogeneity and I 2 >25% were considered significant. We used a random-effects model with restricted maximum likelihood in collating the adjusted associations reported in prior analyses and we stratified our meta-analysis according to the BP assessment method used in the studies included (i.e. either using research-grade BP measurement or including routine BP recordings clinically collected for electronic health databases). All analyses were conducted with Stata 16 (StataCorp., College Station, TX, USA). P-values <0.05 (two-sided) were considered statistically significant.
Results
Baseline characteristics
Of the 5104 MESA participants analysed, only 45 (0.9%) met criteria for IDH by the ESC definition, compared with 305 (7.5%) by the 2017 ACC/AHA definition. Of the 305 with IDH by the new ACC/AHA criteria, 6 participants also had IDH by the ESC definition whereas 299 were newly diagnosed. Baseline CAC strata had nearly identical proportions of participants with IDH by the 2017 ACC/AHA definition: 7.7% among those with CAC = 0, 7.1% among those with CAC 1–100, and 7.5% of those with CAC >100. Individuals with IDH by both criteria were more likely to be younger and male than those with normal BP (Table 1). By the 2017 ACC/AHA definition alone, those with IDH had higher mean high-density lipoprotein cholesterol, triglycerides, and eGFR. The prevalence of IDH using the 2017 ACC/AHA definition compared with normotension varied by ethnicity (P = 0.032) with highest rates amongst the Black, African American group.
Coronary artery calcium
In adjusted logistic models of the full study sample (n = 5104), there was no statistically significant cross-sectional association between IDH using the ESC definition and CAC >0 at baseline [odds ratio 1.23; 95% confidence interval (CI) 0.63, 2.42; P = 0.54, Model 3] (Table 2). However, the point estimate suggests a trend for increased risk with this definition. In contrast, the lack of a significant cross-sectional association between the 2017 ACC/AHA definition of IDH and baseline CAC >0 was more robust statistically (odds ratio 0.88; 95% CI 0.66, 1.17; P = 0.37). Similarly, comparing persons with and without IDH using the 2017 ACC/AHA definition, adjusted linear regression demonstrated no difference in mean CAC when modelled as a continuous variable (beta-coefficient −0.08; 95% CI −0.33, 0.17). Furthermore, no prospective association between the 2017 IDH definition at baseline and incident CAC >0 at either visit 2 or 3 was found in adjusted models (relative risk ratio 0.91; 95% CI 0.63, 1.31; P = 0.16).
Table 2.
Crude and adjusted regression of the association between isolated diastolic hypertension, by both European Society of Cardiology and American College of Cardiology/American College of Cardiology definitions, and coronary artery calcium
| Mean difference in baseline log (CAC + 1) |
Prevalence of baseline CAC >0 |
Incident development of CAC >0a
|
||||
|---|---|---|---|---|---|---|
| Beta-coefficient (95% CI) | Ratio of geometric means (95% CI) | n/N | Odds ratio (95% CI) | n/N | Relative risk (95% CI) | |
| IDH (ESC definition) | ||||||
| Model 1 |
0.44 (−0.27, 1.16) |
1.55 (0.76, 3.19) |
2326/5104 |
1.37 (0.76, 2.46) |
867/2778 |
2.02 (0.85, 4.76) |
| Model 2 |
0.41 (−0.20, 1.02) |
1.51 (0.82, 2.77) |
1.40 (0.72, 2.74) |
1.72 (0.69, 4.30) |
||
| Model 3 |
0.32 (−0.30, 0.93) |
1.38 (0.74, 2.53) |
1.23 (0.63, 2.42) |
1.47 (0.58, 3.72) |
||
| IDH (2017 ACC/AHA definition) | ||||||
| Model 1 |
−0.03 (−0.31, 0.24) |
0.97 (0.73, 1.27) |
1727/4057 |
0.95 (0.75, 1.20) |
706/2330 |
1.11 (0.80, 1.54) |
| Model 2 |
−0.04 (−0.28, 0.20) |
0.96 (0.76, 1.22) |
0.92 (0.70, 1.22) |
1.02 (0.72, 1.44) |
||
| Model 3 |
−0.08 (−0.33, 0.17) |
0.92 (0.71, 1.18) |
0.88 (0.66, 1.17) |
0.91 (0.63, 1.31) |
||
‘n’ refers to the number of events, ‘N’ refers to the number of participants at risk for events.
Beta-coefficients and odds ratios were for the comparison to participants without IDH. Model 1 was unadjusted. The second model adjusted for age, sex, race/ethnicity, body mass index (kg/m2), smoking (current, former, never), alcohol consumption (current, former, or never), low-density lipoprotein cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate, blood pressure-lowering medication (yes/no), and history of diagnosed diabetes (yes/no). The third model included all variables in Model 2 and visit 1 systolic blood pressure.
ACC, American College of Cardiology; AHA, American Heart Association; CAC, coronary artery calcium; CI, confidence interval; ESC, European Society of Cardiology; IDH, isolated diastolic hypertension.
For the analysis of incident CAC developing during follow-up, we excluded persons with CAC >0 at the baseline visit and derived relative risk ratios (compared with persons without IDH) using log-binomial regression.
Incident systolic or treated hypertension
Excluding the subgroup of participants taking anti-hypertensive medication at baseline and using the ESC definition, IDH was associated with incident development of systolic hypertension or anti-hypertensive drug treatment [hazard ratio (HR) 3.76; 95% CI 2.38, 5.94; P < 0.001 in Model 2]. This association remained significant even after adjustment for visit 1 systolic BP in Model 3 (Table 3). Although similar findings were seen using the ACC/AHA 2017 definition (HR 1.71; 95% CI 1.45, 2.03; P < 0.001 in Model 2) they were no longer significant when adjusted for visit 1 systolic BP.
Table 3.
Crude and adjusted cox regression of the prospective association between isolated diastolic hypertension at baseline, by both European Society of Cardiology and American College of Cardiology/American College of Cardiology definitions, and incident systolic hypertension or new anti-hypertensive drug therapy at a follow-up Multi-Ethnic Study of Atherosclerosis visit
| Incident development of systolic hypertension or drug therapy |
||
|---|---|---|
| n/N | HR (95% CI) | |
| IDH (ESC definition) | ||
| Model 1a | 2136/3532 | 2.95 (1.90–4.59) |
| Model 2a | 3.76 (2.38–5.94) | |
| Model 3a | 1.77 (1.12–2.81) | |
| IDH (2017 ACC/AHA definition) | ||
| Model 1a | 1645/2968 | 1.86 (1.59–2.19) |
| Model 2a | 1.71 (1.45–2.03) | |
| Model 3a | 1.02 (0.85–1.21) | |
Persons taking anti-hypertensive medication at baseline were excluded from this analysis and incident systolic hypertension was defined as a new systolic blood pressure ≥130 mmHg.
‘n’ refers to the number of events, ‘N’ refers to the number of participants at risk for events.
HRs compared with participants without IDH.
ACC, American College of Cardiology; AHA, American Heart Association; CI, confidence interval; HR, hazard ratio; ESC, European Society of Cardiology; IDH, isolated diastolic hypertension.
Models as in Table 2.
Incident cardiovascular disease events and mortality
When the full MESA study sample (n = 5104) was again analysed, the mean duration of follow-up for incident events was 12.1 years for CVD events (n = 404 events) and 13 years for all-cause mortality (n = 789 events). The absolute incidence rate for CVD was 6.4 (per 1000 patient-years) among persons with normal BP and 5.1 among those with IDH, when the ESC definition was applied. Consistent with this, IDH was not independently associated with CVD events by the ESC definition (HR 0.83; 95% CI 0.26, 2.64; P = 0.75, Model 3, Table 4). Similar results were found for all-cause mortality (e.g. incidence rate of 11.7 for normotension vs. 6.5 for IDH by the ESC criteria). However, because of the low number participants with IDH by this definition in MESA, the level of uncertainty in these estimates is high.
Table 4.
Crude and adjusted cox regression of the prospective association of isolated diastolic hypertension, by both European Society of Cardiology and American College of Cardiology/American College of Cardiology definitions, with incident cardiovascular disease, the composite outcome, and all-cause death over 13 years follow-up
| CVD |
Composite (CVD, CKD, atrial fibrillation, and HF) |
All-cause death |
||||
|---|---|---|---|---|---|---|
| n/N | HR (95% CI) | n/N | HR (95% CI) | n/N | HR (95% CI) | |
| IDH (ESC definition) | ||||||
| Model 1a | 404/5099 | 0.79 (0.25–2.46) | 844/5100 | 0.75 (0.34, 1.67) | 789/5099 | 0.55 (0.21–1.47) |
| Model 2a | 1.00 (0.32–3.15) | 0.80 (0.36, 1.80) | 0.80 (0.30–2.16) | |||
| Model 3a | 0.83 (0.26–2.64) | 0.73 (0.33, 1.66) | 0.85 (0.31–2.29) | |||
| IDH (2017 ACC/AHA definition) | ||||||
| Model 1a | 287/4053 | 1.15 (0.76–1.73) | 614/4053 | 1.10 (0.82, 1.46) | 574/4053 | 0.70 (0.49–1.00) |
| Model 2a | 1.37 (0.90–2.08) | 1.37 (1.00, 1.83) | 0.91 (0.63–1.30) | |||
| Model 3a | 1.19 (0.77–1.84) | 1.28 (0.94, 1.74) | 0.94 (0.65–1.36) | |||
‘n’ refers to the number of events, ‘N’ refers to the number of participants at risk for events.
HRs compared with participants without IDH.
ACC, American College of Cardiology; AHA, American Heart Association; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; ESC, European Society of Cardiology; HF, heart failure; HR, hazard ratio; IDH, isolated diastolic hypertension.
Models as in Table 2.
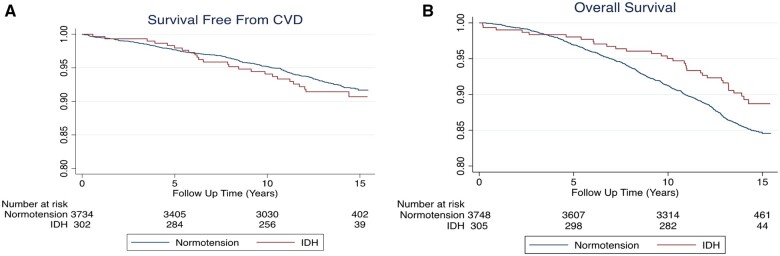
Participants with IDH by the 2017 ACC/AHA definition had a 6.4 per 1000 patient-year incidence rate of CVD and 6.5 incidence rate of death. This compared with rates of 5.6 and 10.9, respectively, for persons with normotension by 2017 criteria (Figure 2). In multivariable-adjusted models, compared with normotension, persons with IDH were found to have no statistically significant evidence for an excess risk in either CVD (HR 1.19; 95% CI 0.77, 1.84; Model 3) or all-cause death (HR 0.94; 95% CI 0.65, 1.36; Model 3).
Figure 2.
Kaplan–Meier Cumulative Survival Graphs according to isolated diastolic hypertension by the 2017 American College of Cardiology/American Heart Association definition. Survival free from CVD (panel A) and overall survival (panel B) among persons with isolated diastolic hypertension is similar to those without isolated diastolic hypertension. CVD, cardiovascular disease.
Sensitivity analyses
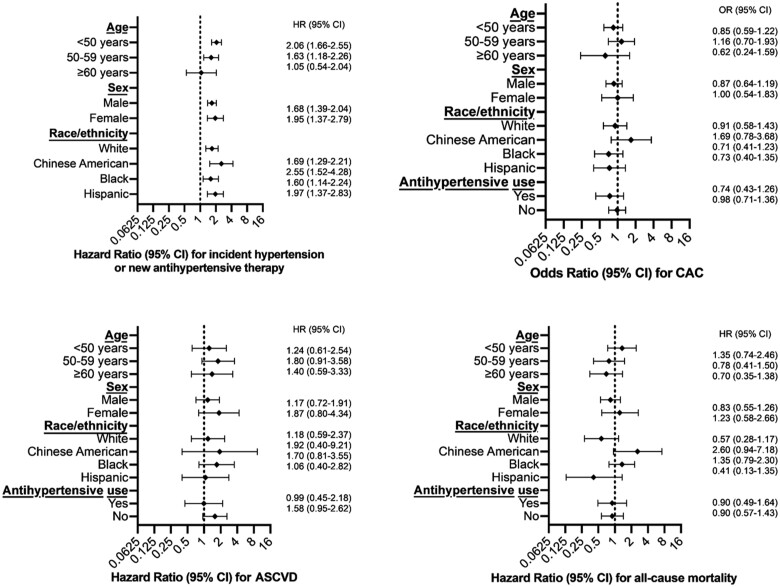
Results using both definitions of IDH and for all outcomes tested were similarly null when stratified by age, race/ethnicity, sex, and current anti-hypertensive medication use (Figure 3), again with the exception of the outcome of incident systolic or treated hypertension. No interaction P-value was <0.05. Of note, although IDH by the 2017 ACC/AHA definition was associated with a lower risk of non-zero CAC in those on baseline hypertension treatment using the crude model (odds ratio 0.55; 95% CI 0.35, 0.86; P = 0.008), this did not persist in the adjusted models. We also found no statistically significant association of IDH by the 2017 ACC/AHA definition with CVD (HR 1.21; 95% CI 0.77,1.92) or with death (HR 0.72; 95% CI 0.46, 1.12) in the sensitivity analysis that included statin and anti-hypertensive medications as time-varying covariables in Model 3. Finally, in post hoc sensitivity analyses testing the additional outcomes of chronic kidney disease, heart failure, and atrial fibrillation, IDH was also not associated with significant excesses in risk (Table 4).
Figure 3.
Subgroup analyses of isolated diastolic hypertension by the 2017 American College of Cardiology/American Heart Association Definition. Odds ratios (OR) and hazard ratios (HR) for outcomes, comparing persons with isolated diastolic hypertension to those without isolated diastolic hypertension, among various subgroups show results consistent with the main analysis. All comparisons are to Multi-Ethnic Study of Atherosclerosis participants with normal blood pressure (<130/80 mmHg for this analysis). The model is adjusted for age, sex, race/ethnicity, body mass index (kg/m2), smoking (current, former, never), alcohol consumption (current, former, or never), low-density lipoprotein cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate, and history of diagnosed diabetes (yes/no). In these subgroup analyses, the model did not include the variable to which the sample was sub-grouped (e.g. the analyses of race/ethnicity subgroups did not include race/ethnicity in the model). ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium
Meta-analysis of the association between isolated diastolic hypertension and incident cardiovascular disease
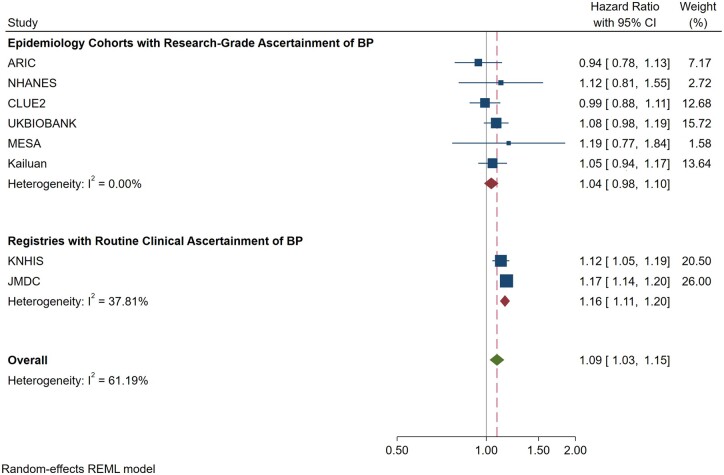
Because MESA may have been underpowered to completely rule out an association between IDH by the 2017 ACC/AHA definition and incident CVD events, we conducted a meta-analysis pooling our results from MESA with those from the seven other cohorts that have previously tested this association after adjustment for potential confounders (Figure 4 and Graphical abstract). This meta-analysis is stratified in that it is reported both with and without inclusion of two large cohorts that used BP measurements obtained during routine clinical practice (with the other six studies using BP measurements from standardized research protocols). In this stratified meta-analysis, the 2017 IDH definition was also not consistently associated with CVD and the magnitude of any potential relative association was also noted to be numerically small [e.g. depending on meta-analysis inclusion criteria, the adjusted HR ranged from 1.04 (95% CI 0.98–1.10) to 1.09 (95% 1.03–1.15)].
Figure 4.
Random-effects Stratified Meta-analysis of the Adjusted Association between isolated diastolic hypertension by the 2017 American College of Cardiology/American Heart Association definition and cardiovascular disease events in studies published to date (N = 10 037 843 participants). Stratification is according to epidemiologic studies reporting research-grade measurement of blood pressure vs. epidemiologic studies and registry studies that included blood pressure measurements obtained as part of routine clinical care. CI, confidence interval.
Discussion
This analysis of an ethnically diverse and contemporary US cohort found that IDH was associated with incident development of systolic or treated hypertension, both when the ESC and 2017 ACC/AHA definitions were used. However, the lack of any statistically significant association between IDH by the 2017 ACC/AHA definition and either subclinical CVD in the form of CAC or clinical CVD in MESA is noteworthy. These findings are consequential because, if applied, the new 2017 ACC/AHA definition results in an estimated 5% increase in the number of adults eligible to be diagnosed with hypertension based on the presence of IDH alone (in America this translates into ∼12 million adults).6 Because our CVD event results from MESA may have been underpowered, we also conducted a meta-analysis of IDH defined by the 2017 ACC/AHA criteria and found either (i) no excess in adjusted risk for CVD in collated cohorts using research-grade BP measures; or (ii) a numerically small excess in relative risk (i.e. HR 1.09) when all cohorts were combined (i.e. including cohorts with less reliable measures of BP). Taken together, our data question whether middle-aged or older adults with IDH by the 2017 ACC/AHA definition need to be labelled as hypertensive or considered for drug therapy.
For the most part, adequately powered studies testing the association between IDH by the ESC definition and CVD events have demonstrated increased risk among these adults who have well-controlled systolic BP but diastolic BP ≥90 mmHg.16 , 21 In contrast, most of the previous studies testing the association between IDH by the 2017 ACC/AHA definition and CVD events have been null. However, these latter null studies have been hampered by concerns for inadequate statistical power (both because IDH by this definition is relatively uncommon and because approximately half the adults newly diagnosed with IDH by the 2017 ACC/AHA definition are younger than 45 years6 and are, consequently, less likely to develop events). The current analysis aimed to address this power concern. First, the use of MESA data allows an investigation into the association between IDH and subclinical CVD, as defined by CAC. Because CAC is a continuous outcome and not a binary one, linear regression analyses have improved power to test for any association with IDH. While there was a trend for increased CAC among persons with IDH by the ESC definition, the statistically conclusive lack of association between the 2017 ACC/AHA definition of IDH and increased CAC, either in cross-section or prospectively, provides further evidence that IDH, when defined by the 2017 ACC/AHA criteria, does not appear to be associated with excess CVD risk.6 Second, we pooled MESA event data with those from published cohorts in a meta-analysis and found a persistent lack of consistent association between IDH by the 2017 ACC/AHA definition and CVD events.
The few individual studies to date that do suggest an association between IDH by the 2017 ACC/AHA definition and CVD outcomes used data from administrative datasets where BP values were taken from a range of clinical settings and measured and recorded under normal practice conditions.15 , 18–20 However, it is known that diastolic BP can be challenging to measure accurately on a consistent basis in usual clinical care.22 We believe that the current MESA data, as well as the stratified meta-analysis reported above, showing no statistically significant adjusted association of IDH with CVD events among the cohorts of middle-aged to older adults who had BP measured using rigorous research methodologies, are more likely to be valid.
However, we cannot conclude from this analysis that the 2017 ACC/AHA definition of IDH is completely benign. Specifically, and as it has also been previously described for IDH by the traditional (and current ESC) definition,26 the present analysis from MESA does demonstrate that the 2017 ACC/AHA definition of IDH identifies adults at risk for future systolic hypertension. Therefore, while our study does not support the initiation of anti-hypertensive therapies for middle-aged to older adults with baseline IDH, it nonetheless highlights the importance of serially evaluating these patients for the development of systolic hypertension over time, with continued emphasis on lifestyle improvements. Furthermore, our results may not apply to young adults. Indeed, the only report to date testing the association between the 2017 ACC/AHA definition of IDH and CVD events among young adults specifically suggests that there may be risk among Korean adults younger than those included in our current study.18 , 30 This Korean study comprised of data from a large insurance database, which used routine clinical measures of BP. Therefore, additional research from rigorous cohorts of young adults with research-grade BP measurement will be necessary to probe the possible age interaction further. Furthermore, the possibility of an interaction based on different ethnicities warrants future study.
Limitations
First, as an observational study, residual confounding cannot be excluded. Second, the meta-analysis stratum that included registry data comprising of routine clinical BP measurements suggested a small though statistically significant association between IDH by the 2017 ACC/AHA definition and CVD. However, heterogeneity was introduced (I 2 statistic = 61%) to the meta-analysis when these registry data were added to the cohorts with more rigorous measurement of BP and, even if these less reliable estimates are valid, the relative increase in risk of just 9% is numerically small (noting also that there was a low absolute risk for CVD among persons with normotension and IDH, both in the previously reported studies and in MESA). Third, the minimum participant age in MESA was 45 at baseline and our meta-analysis contained few participants below 50 years; therefore, as mentioned above, the results presented here may not be generalizable to younger adults.18 , 30 Fourth, we included persons with and without anti-hypertensive therapy in our analyses, though BP guideline targets pertain to both initiation and titration of therapy. Results stratifying participants with and without baseline use of anti-hypertensive therapies were concordant. Fifth, variation between studies in the BP measurement method (i.e. manual vs. automatic oscillometric) might partially influence analyses of IDH. Specifically, direct measurement of diastolic BP is only possible with intra-arterial assessment and the mechanism of indirect measurement used by manual vs. oscillometric devices differs slightly for diastolic BP (manual being based on human auscultation and automatic-oscillometric devices being based on algorithms that detect vibrations in the arterial wall). Prior data indicate that the automatic BP device used in MESA may underestimate diastolic BP (compared with mercury sphygmomanometry) by up to 3 mmHg.28 Sixth, the power in MESA to test associations between IDH and CVD events was low, though our results are augmented by a well-powered meta-analysis. Seventh, we were unable to include one report15 in our meta-analysis because it modelled the 2017 ACC/AHA definition of IDH using diastolic BP as a Z-score continuous exposure rather than a categorical exposure. Finally, we included the most fully adjusted HRs available in each cohort for our meta-analysis, but we note there was heterogeneity in the number and type of variables adjusted for and that meta-analyses of cohort studies are challenging and should be considered hypothesis generating.
Conclusion
There are no randomized trials proving that diastolic BP should be treated to below 90 mmHg and the preponderance of observational research demonstrates higher risk for CVD among persons with controlled systolic BP only when diastolic BP ≥90 mmHg. In our analyses, the 2017 ACC/AHA definition of IDH (i.e. diastolic BP ≥80 mmHg) was not consistently associated with statistically significant increases in risk for either coronary calcification or incident cardiovascular events in middle-aged or older adults. These findings collectively indicate that the decision by the ESC to maintain a diastolic BP threshold of ≥90 mmHg for the diagnosis of hypertension was reasonable. However, more research is needed in high-quality cohorts of younger adults, and randomized trials of anti-hypertensive therapy among persons with IDH would also be valuable to fully understand the implications of this BP phenotype on CVD.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Limited MESA results can be accessed in the form of an open dataset available at https://biolincc.nhlbi.nih.gov/studies/
Funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Dr M.J.B. is supported by RO1-HL-46666.
Conflict of interest: Dr B.M.P. serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. The other authors declare that there is no conflict of interest.
Contributor Information
Alan P Jacobsen, Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Mahmoud Al Rifai, Department of Medicine, Section of Cardiology, Baylor College of Medicine, Houston, TX, USA.
Kelly Arps, Department of Medicine, Duke University School of Medicine, Durham, NC, USA.
Seamus P Whelton, Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Matthew J Budoff, Division of Cardiology, Harbor-UCLA Medical Center, Torrance, CA, USA.
Khurram Nasir, Division of Cardiovascular Prevention and Wellness, Department of Cardiology, Houston Methodist DeBakey Heart & Vascular Center, Houston, TX, USA.
Michael J Blaha, Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Bruce M Psaty, Cardiovascular Health Research Unit, Departments of Medicine, Epidemiology, and Health Services, University of Washington and Kaiser Permanente Health Research Institute, Seattle, WA, USA.
Roger S Blumenthal, Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Wendy S Post, Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
John W McEvoy, Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA; National Institute for Prevention and Cardiovascular Health, National University of Ireland Galway School of Medicine, Moyola Lane, Newcastle, Galway, H91 FF68, Ireland.
References
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2. Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 3.SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:2199–2269.29146533 [Google Scholar]
- 6. McEvoy JW, Daya N, Rahman F, Hoogeveen RC, Blumenthal RS, Shah AM, Ballantyne CM, Coresh J, Selvin E. Association of isolated diastolic hypertension as defined by the 2017 ACC/AHA blood pressure guideline with incident cardiovascular outcomes. JAMA 2020;323:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fang J, Madhavan S, Cohen H, Alderman MH. Isolated diastolic hypertension. A favorable finding among young and middle-aged hypertensive subjects. Hypertension 1995;26:377–382. [DOI] [PubMed] [Google Scholar]
- 8. Pickering TG. Isolated diastolic hypertension. J Clin Hypertens 2003;5:411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nielsen WB, Lindenstrom E, Vestbo J, Jensen GB. Is diastolic hypertension an independent risk factor for stroke in the presence of normal systolic blood pressure in the middle-aged and elderly? Am J Hypertens 1997;10:634–639. [DOI] [PubMed] [Google Scholar]
- 10. Strandberg TE, Salomaa VV, Vanhanen HT, Pitkala K, Miettinen TA. Isolated diastolic hypertension, pulse pressure, and mean arterial pressure as predictors of mortality during a follow-up of up to 32 years. J Hypertens 2002;20:399–404. [DOI] [PubMed] [Google Scholar]
- 11. Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002;162:577–581. [DOI] [PubMed] [Google Scholar]
- 12. Lindenstrøm E, Boysen G, Nyboe J. Influence of systolic and diastolic blood pressure on stroke risk: a prospective observational study. Am J Epidemiol 1995;142:1279–1290. [DOI] [PubMed] [Google Scholar]
- 13. Li Y, Wei F-F, Thijs L, Boggia J, Asayama K, Hansen TW, Kikuya M, Björklund-Bodegård K, Ohkubo T, Jeppesen J, Gu Y-M, Torp-Pedersen C, Dolan E, Liu Y-P, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Mena L, Maestre GE, Filipovský J, Imai Y, O’Brien E, Wang J-G, Staessen JA, International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes Investigators. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation 2014;130:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, Liu K, Greenland P, Lloyd-Jones DM. Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. J Am Coll Cardiol 2015;65:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med 2019;381:243–251. [DOI] [PubMed] [Google Scholar]
- 16. McGrath BP, Kundu P, Daya N, Coresh J, Selvin E, McEvoy JW, Chatterjee N. Isolated diastolic hypertension in the UK Biobank: comparison of ACC/AHA and ESC/NICE guideline definitions. Hypertension 2020;76:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu S, Ji C, Shi J, Chen S, Huang Z, Jonas JB. Isolated diastolic hypertension as defined by the 2017 American College of Cardiology/American Heart Association blood pressure guideline and incident cardiovascular events in Chinese. J Hypertens 2021;39:519–525. [DOI] [PubMed] [Google Scholar]
- 18. Lee H, Yano Y, Cho SMJ, Park S, Lloyd-Jones DM, Kim HC. Cardiovascular risk of isolated diastolic hypertension defined by the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline: a nationwide age-stratified cohort study. Hypertension 2020;76:e44–e46. [DOI] [PubMed] [Google Scholar]
- 19. Choi YJ, Kim SH, Kang SH, Yoon CH, Lee HY, Youn TJ, Chae IH, Kim CH. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: a nationwide population-based study from Korea. Eur Heart J 2019;40:724–731. [DOI] [PubMed] [Google Scholar]
- 20. Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Takeda JT, Morita N, Yasunaga H, Komuro I. Association of isolated diastolic hypertension based on the cutoff value in the 2017 American College of Cardiology/American Heart Association blood pressure guidelines with subsequent cardiovascular events in the general population. J Am Heart Assoc 2020;9:e017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGrath BP, McEvoy JW. Did the 2017 ACC/AHA blood pressure guideline get it wrong in reducing the diastolic threshold to define hypertension from 90 to 80 mmHg? J Clin Hypertens 2020;22:1200–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blank SG, Mann SJ, James GD, West JE, Pickering TG. Isolated elevation of diastolic blood pressure. Real or artifactual? Hypertension 1995;26:383–389. [DOI] [PubMed] [Google Scholar]
- 23. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol 2018;72:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, Kronmal R, Lima JAC, Liu KJ, McClelland RL, Michos E, Post WS, Shea S, Watson KE, Wong ND. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 2018;39:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franklin SS, Pio JR, Wong ND, Larson MG, Leip EP, Vasan RS, Levy D. Predictors of new-onset diastolic and systolic hypertension: the Framingham Heart Study. Circulation 2005;111:1121–1127. [DOI] [PubMed] [Google Scholar]
- 27. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez RA, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 28. Ni H, Wu C, Prineas R, Shea S, Liu K, Kronmal R, Bild D. Comparison of Dinamap PRO-100 and mercury sphygmomanometer blood pressure measurements in a population-based study. Am J Hypertens 2006;19:353–360. [DOI] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee H, Yano Y, Cho SMJ, Park JH, Park S, Lloyd-Jones DM, Kim HC. Cardiovascular risk of isolated systolic or diastolic hypertension in young adults. Circulation 2020;141:1778–1786. [DOI] [PubMed] [Google Scholar]