Levosimendan is a new inodilator which has three main mechanisms of action: increases the calcium sensitivity of cardiomyocytes by binding to cardiac troponin C, acts as a vasodilator due to the opening of potassium channels, thus exerting cardio-protective effects [1]. Due to the unique mechanism of action, levosimendan has multifaceted cardio-protective effects, as demonstrated previously [2]. Levosimendan is indicated for inotropic support in acute decompensated heart failure (HF) in situations where conventional therapy is not sufficient, and in cases where inotropic support is considered appropriate (class IIb recommendation according to the European Society of Cardiology guidelines) [3]. In addition, levosimendan was showed to accelerate the recovery in patients with takotsubo cardiomyopathy [4]. Dobutamine, in turn, remains the most widely used therapy in patients with acute decompensated HF. Although dobutamine improves hemodynamics and symptoms in these patients, it has been associated with an increased risk of death and other cardiovascular events [5]. Hence, there is a greatly unmet need for agents that improve hemodynamics and relieve symptoms without adversely affecting survival. In contrast to dobutamine, levosimendan has a safe and predictable profile of action and does not induce tolerance, facilitating its administration in HF patients [6].
Because the results of previous clinical studies are inconclusive, herein was performed a systematic review and meta-analysis to verify the efficacy and safety of levosimendan and dobutamine in patients with acute HF. Two authors (L.S. and A.G.) independently searched PubMed, the Cochrane Library and the Google Scholar for articles written in English (last update January 7th, 2021). The key search words were: “levosimendan” AND “dobutamine” AND “heart failure” OR “HF”. All statistical analyses were performed with Review Manager Software 5.4 (The Cochrane Collaboration, Oxford, Copenhagen, Denmark). All results are presented as mean difference (MD) or odds ratio (OR) with 95% confidence interval (CI). When the continuous outcome was reported in a study as median, range, and interquartile range, means and standard deviations were estimated using the formula described by Hozo et al. [7]. The random-effects model was used for I2 > 50%. Statistical testing was two-tailed. P < 0.05 was considered statistically significant.
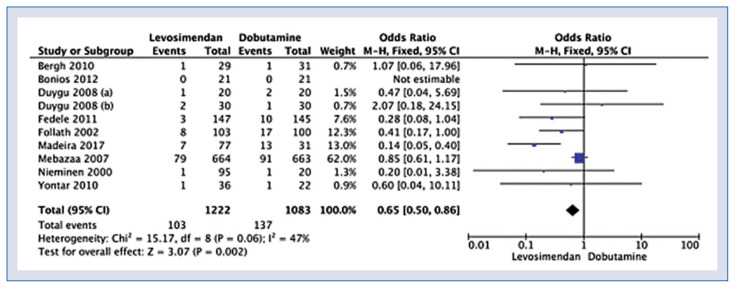
Ten studies including 2305 were eligible for quantitative analysis. The full list of the included publications is presented in Supplementary Digital Content. Characteristics of included studies was presented in Supplementary Table S1. In-hospital mortality (or 30-day mortality) group was reported in 10 studies and occurred in 8.4% patients treated with levosimendan and 12.7% patients treated with dobutamine (OR = 0.65; 95% CI: 0.50–0.86; p = 0.002; I2 = 47%; Fig. 1). In contrast, 6-month mortality was reported in 3 studies and was 25.8% for levosimendan compared with 29.5% for dobutamine (OR = 0.84; 95% CI: 0.67–1.04; p = 0.11; I2 = 36%; Suppl. Fig. S1).
Figure 1.
Forest plot of in-hospital mortality in the levosimendan versus the dobutamine group. The center of each square represents the weighted odds ratio for individual trials, and the corresponding horizontal line stands for 95% confidence interval (CI). The diamonds represent pooled results. Procedure time presented in seconds.
The use of levosimendan compared to dobutamine was associated with a lower frequency of complications including acute decompensated cardiac failure (12.2% vs. 16.8%, respectively; OR = 69; 95% CI: 0.51–0.93; p = 0.02; I2 = 0%), and a higher risk of atrial fibrillation (8.1% vs. 5.4%; OR = 1.56; 95% CI: 1.04–2.35; p = 0.03; I2 = 0%). A detailed overview of adverse events is presented in Supplementary Table S2.
Length of hospital stay was 10.7 ± 7.0 days in the levosimendan group compared to 12.4 ± 6.6 days in the dobutamine group (MD = −1.92; 95% CI: −2.47 to −1.36; p < 0.001; I2 = 0%; Suppl. Fig. S2).
In conclusion, the present study demonstrated that levosimendan decreased in-hospital (or 30-days) mortality and length of hospital stay, compared to dobutamine. In addition, there was a trend towards lower 6-month mortality on levosimendan. Taking into account the promising results of our meta-analysis and the cardioprotective effects of levosimendan demonstrated in multiple studies, there is a need for a well-designed multicenter randomized placebo-controlled study, including an adequately large group of outpatients with acute HF to ultimately determine the effect of levosimendan on long-term prognosis [8].
Supplementary Information
Footnotes
This paper was guest edited by Prof. Togay Evrin
Conflict of interest: None declared
References
- 1.Pathak A, Lebrin M, Vaccaro A, et al. Pharmacology of levosimendan: inotropic, vasodilatory and cardioprotective effects. J Clin Pharm Ther. 2013;38(5):341–349. doi: 10.1111/jcpt.12067. [DOI] [PubMed] [Google Scholar]
- 2.Pollesello P, Papp Z. The cardioprotective effects of levosimendan: preclinical and clinical evidence. J Cardiovasc Pharmacol. 2007;50(3):257–263. doi: 10.1097/FJC.0b013e3180986230. [DOI] [PubMed] [Google Scholar]
- 3.Harjola VP, Giannakoulas G, von Lewinski D, et al. Use of levosimendan in acute heart failure. Eur Heart J Suppl. 2018;20:I2–II10. doi: 10.1093/eurheartj/suy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaman M, Arslan U, Kaya A, et al. Levosimendan accelerates recovery in patients with takotsubo cardiomyopathy. Cardiol J. 2016;23(6):610–615. doi: 10.5603/CJ.a2016.0100. [DOI] [PubMed] [Google Scholar]
- 5.Bayram M, De Luca L, Massie MB, et al. Reassessment of dobutamine, dopamine, and milrinone in the management of acute heart failure syndromes. Am J Cardiol. 2005;96(6A):47G–58G. doi: 10.1016/j.amjcard.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Slawsky MT, Colucci WS, Gottlieb SS, et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Study Investigators Circulation. 2000;102(18):2222–2227. doi: 10.1161/01.cir.102.18.2222. [DOI] [PubMed] [Google Scholar]
- 7.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tycińska A, Gierlotka M, Bugajski J, et al. Levosimendan in the treatment of patients with acute cardiac conditions: an expert opinion of the Association of Intensive Cardiac Care of the Polish Cardiac Society. Kardiol Pol. 2020;78(7–8):825–834. doi: 10.33963/KP.15551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.