Keywords: brain, neuron, testis, sperm, molecular
Abstract
Several strands of evidence indicate the presence of marked similarities between human brain and testis. Understanding these similarities and their implications has become a topic of interest among the scientific community. Indeed, an association of intelligence with some semen quality parameters has been reported and a relation between dysfunctions of the human brain and testis has also been evident. Numerous common molecular features are evident when these tissues are compared, which is reflected in the huge number of common proteins. At the functional level, human neurons and sperm share a number of characteristics, including the importance of the exocytotic process and the presence of similar receptors and signalling pathways. The common proteins are mainly involved in exocytosis, tissue development and neuron/brain-associated biological processes. With this analysis, we conclude that human brain and testis share several biochemical characteristics which, in addition to their involvement in the speciation process, could, at least in part, be responsible for the expression of a huge number of common proteins. Nonetheless, this is an underexplored topic, and the connection between these tissues needs to be clarified, which could help to understand the dysfunctions affecting brain and testis, as well as to develop improved therapeutic strategies.
1. Introduction
The human body is an orchestrated set of different organs that work together, contributing to the maintenance of overall health and homeostasis. The human brain is the control center of the nervous system, playing a critical coordination role. It receives signals from sensory organs and translates them into functional information to multiple physiological compartments such as muscles and glands. In addition, the brain is also responsible for speech production, memory storage, and the elaboration of thought and emotion [1,2]. The human testis is the male gonad, and is of the utmost importance for reproduction and species evolution. It has two main functions: production of gametes (sperm) and synthesis/secretion of hormones (primarily, testosterone) [3,4].
Despite these clearly dissimilar functions and the apparent structural and morphological differences between human brain and testis, in the last four decades it has become increasingly evident that these tissues share several features. The similarity was further confirmed by analysis of gene expression, with evidence that human brain and testis, among all the organs of the body, share the highest number of genes [5,6]. More recently, authors found a positive correlation between general intelligence and three key measures of semen quality: sperm concentration, sperm count and sperm motility [7]. A possible association between male sexual dysfunction and neurological disorders was also proposed by several authors [8,9]. These findings raise some interesting questions. (i) Why do the human brain and testis share a similar gene expression profile? (ii) Have these tissues a similar cellular organization and cooperation between cell types? (iii) Are their functions related? (iv) What are the implications of the similarities between human brain and testis?
In this context, we review the similarities between human brain and testis, and between human neuron and sperm at the cellular and molecular levels. The proteomic profile of the two human tissues (brain and testis) and the two types of cells (neuron and sperm) were also compared and critically discussed.
2. Brain and testis
2.1. Cellular and molecular similarities
When human brain and testis, two apparently distinct tissues with very different functions, were compared, several similarities, spanning from molecular to cellular levels of organization, became evident. The main cellular and molecular similarities between these two organs are summarized in table 1.
Table 1.
Cellular and molecular similarities between human brain and testis.
| brain | testis |
|---|---|
| biochemical/physical support cells: astrocytes | biochemical/physical support cells: Sertoli cells |
| high energy demands | |
| metabolic cooperation: astrocytes produce lactate, which is used by neurons | metabolic cooperation: Sertoli cells produce lactate, which is used by germ cells |
| dependence on selenium metabolism | |
| high concentrations of polyunsaturated fatty acids | |
| highly susceptibility to oxidative damage | |
| blood–brain barrier | blood–testis barrier |
| neuroendocrine proprieties | |
| cytoskeleton motors (kinesins and dyneins): essential role in neuronal function | cytoskeleton motors (kinesins and dyneins): essential role in spermatogenesis |
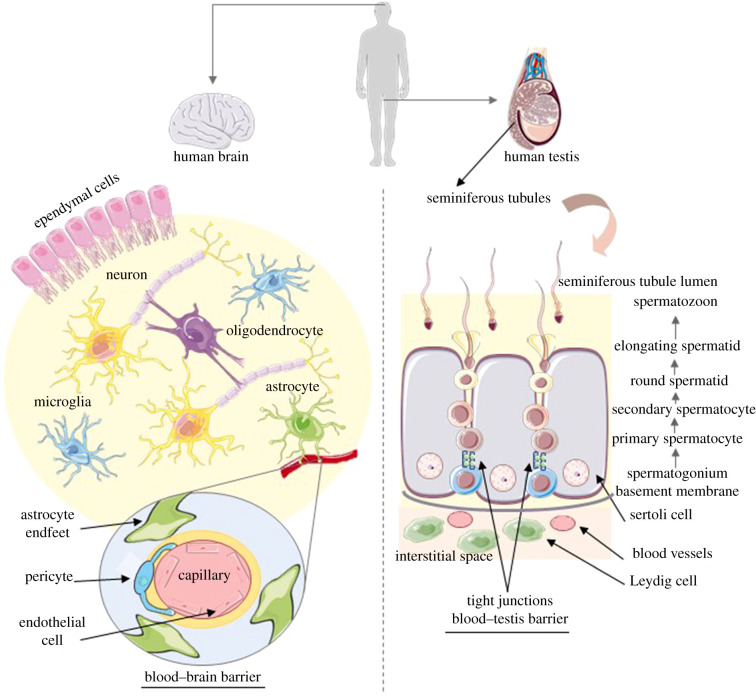
Human brain and testis are both constituted by different cell types that work together to maintain the integrity and function of the tissue. Human brain is a complex and organized tissue formed mainly by neurons and support cells named glia. Neurons are the most important cells in the brain, responsible for the transmission of information. To maintain their function, glia cells are in close relation with neurons. There are four different types of glia in human brain: astrocytes, oligodendrocytes, microglia and ependymal cells, each of them essential to maintain brain function [10,11]. Likewise, testis is a well-organized tissue, composed of seminiferous tubules, in which developing germ cells and Sertoli cells are in close interaction [12]. Adjacent to the seminiferous tubules and close to the blood vessels are the Leydig cells, which produce and secrete testosterone into blood vessels [13]. The cellular organization of these two tissues is summarized in figure 1.
Figure 1.
Summary of the cellular organization of human brain and testis.
Astrocytes and Sertoli cells are known as the biochemical support cells of brain and testis, respectively. Beyond their important role in the metabolism of these tissues, described below, they are responsible for the physical and nutritional support of neurons and germ cells, and essential for their development and survival [14,15].
Human brain and testis are high-energy-demand tissues, executing energy-demanding processes such as cognitive functions and spermatogenesis, respectively [16]. To support these energy requirements, a metabolic cooperation between the different cell types is clear in both tissues [17,18]. In the brain, astrocytes produce lactate as a glycogen-derived product, which is transported to the neurons that use it as a preferred energy source to maintain their synaptic activity. Thus, neuronal metabolic processes are highly dependent on the activity of astrocytes [17]. Similarly, a metabolic active cooperation between developing germ cells and Sertoli cells is evident. Sertoli cells convert glucose to lactate, which is transported to and used as a central energy metabolite by developing germ cells to maintain their metabolic activity [18,19]. In addition to a similar metabolic cooperation, brain and testis both depend on selenium metabolism. A selenium-deficient diet has been associated with increased susceptibility to neurotoxicity and impaired spermatogenesis [20]. In selenium-deficient conditions, brain and testis compete for selenium utilization so that castration was associated with attenuation of neurodegeneration, mainly by increasing selenium-dependent antioxidant activity in brain [20].
Compared to other tissues, the human brain and testis are particularly susceptible to oxidative damage, due to their high energy and oxygen demand, and abundance of polyunsaturated fatty acids (PUFAs). Indeed, Kabuto et al. [21] exposed mice to bisphenol A, an oxidative stress inducer, during embryonic/fetal life and infancy, and collected several tissues, finding a particular underdevelopment of brain and testis, caused by increased oxidative injury. Furthermore, brain and testis have the lowest transcriptional levels of oxidative stress-related genes (for example, the gene that encodes catalase), compared to other tissues [16]. To counteract their high susceptibility to oxidative stress, these two tissues have specific blood–tissue barriers, called the blood–brain and the blood–testis barrier [22,23]. An essential role of high concentrations of PUFAs in human brain and testis function and/or development has been reported [24,25]. In the brain, the most abundant PUFA is docosahexaenoic acid (DHA), which it is mainly located at the synaptic terminals of neurons, playing a central role in neurodevelopment, function and maintenance [24]. The human germ cell line has an active lipid metabolism and displays stage-specific differences in fatty acid pattern. The inverse correlation found between the percentage of abnormal sperm and the percentage of DHA suggests a role of PUFAs in sperm morphology and development [25].
In recent decades, the Leydig cells of the human testis have been recognized as members of the neuroendocrine system. The synthesis and release of a large number of biologically active substances that are typical for nerve and neuroendocrine cells has revealed that Leydig cells are neuroendocrine cells [26]. Indeed, several neuron-specific peptides and proteins, such as Substance-P [27], synaptophysin and neural cell-adhesion molecule, have been detected in human Leydig cells [28]. Some glial-cell-specific antigens—(glial fibrillary acidic protein (GFAP), galactocerebroside (GalC), cyclic 2′,3′-nucleotide-3′-phosphodiesterase (CNPase), A2B5-antigen and O4-antigen, which are considered to be marker molecules of astrocytes and oligodendrocytes—were also found in Leydig cells of human testis [26]. Besides Leydig cells, Sertoli cells also express some neuron- and glial cell-specific proteins. In fact, the three isoforms of neurofilament proteins, and GFAP, GalC and CNPase were found in both Leydig and Sertoli cells of human testis [26,29].
Cytoskeleton motors, including myosin, kinesins and dyneins, play essential roles in the brain, namely in neuron polarization, extension, shape and neurotransmission processes [30]. Motor proteins also play key roles in the formation of mature sperm [31]. Spermatogenesis includes several mitotic and meiotic divisions, for which the role of motor proteins in spindle organization, chromosome congression, chromatid separation, among others, are clear. Also in the final step of spermatogenesis, called spermiogenesis, kinesins seems to be crucial in acrosome biogenesis, nuclear shaping, tail formation, and spermatid maturation and transcription [31]. The vital role of these cytoskeleton motor proteins in brain and testis function is evident by several neurodegenerative and reproductive diseases that arise from mutations or other dysfunctions of these proteins in brain and testis, respectively [32,33].
2.2. Proteomic comparison
According to the apparent cellular and molecular similarities between human brain and testis, it has become clear that these tissues have a similar gene expression pattern. In a UniGene pilot investigation carried out by Guo et al. [5], the expression data of 760 human UniGenes in 17 tissues were retrieved and compared. Unexpectedly, among the 17 tissues compared, the highest similarity in gene expression patterns was between human brain and testis with a total of 364 shared expressed UniGenes [5]. According to this study, a large-scale analysis of the expression of 33 689 genes in 15 human tissues revealed that human brain and testis shared the greatest similarity in gene expression [6]. In addition, these authors demonstrated that the similarity of gene expression between brain and testis is not exclusive to humans and may be widely present in other mammals, including rodents [6]. Several authors have demonstrated that some genes are highly or selectively expressed in brain and testis of mice (Tb-rbp, Gpr37, Hst-1/Fgf-4) and rat (Ugt1a6, Glutx1, α4-b, Lancl1, Nep) [34–41]. Moreover, Danielsson et al. [42] found that human brain and testis share the highest number of group-enriched genes. Although transcriptomic profiling has become a standard approach to understand the (dys)function of tissues, it is also important to evaluate how gene expression relates to the proteins that are actually being expressed. To that purpose, it is possible to use proteomics, which gives information about protein composition of a cell, tissue or organism [43].
Herein, we compared the brain and testis proteome with that of 31 other tissues, representing all major tissues in the human body (heart, skeletal muscle, adrenal gland, parathyroid gland, thyroid gland, lung, gastrointestinal tract, salivary gland, oesophagus, stomach, duodenum, small intestine, colon, bone marrow, lymph node, spleen, appendix, pancreas, kidney, liver, gallbladder, epididymis, seminal vesicle, prostate, breast, cervix, endometrium, ovary, placenta, adipose tissue and skin), using the Human Protein Atlas (HPA) (available at www.proteinatlas.org) and the Jveen tool (available at http://jvenn.toulouse.inra.fr). The HPA is a programme that aims to map all the proteins in cells, tissues and organs using the integration of various technologies (e.g. antibody-based imaging, mass spectrometry-based proteomics, systems biology). Consistent with the gene expression analysis mentioned in the previous section [5,6], the highest number of common proteins was observed between brain and testis, suggesting that human brain and testis are the most similar tissues of the human body. The common proteins between these two tissues were retrieved and, to prevent redundancy, all proteins were annotated using the UniProtKB/Swiss-Prot accession number. From the total of 14 315 and 15 687 proteins that constitute the human brain and testis proteome, respectively, 13 442 are common to both tissues (figure 2; electronic supplementary material, table S1).
Figure 2.
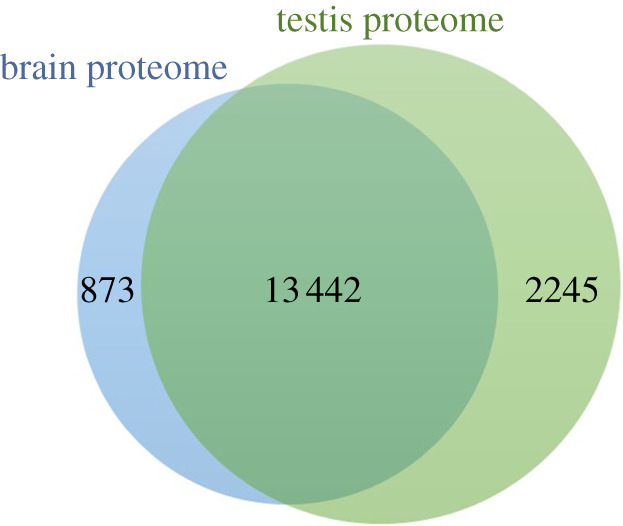
Veen diagram demonstrating the overlap between the human brain and testis proteome (based on the Jveen tool). The data of human brain and testis proteome were collected on 20 August 2019.
From the 13 442 common proteins between human brain and testis, we decided to highlight the proteins that are highly expressed in these two tissues, when compared with other human body tissues. To do that, we cross-checked the information from HPA with GeneCards (available at https://www.genecards.org/) and identified a total of 29 proteins highly expressed in brain and testis (table 2). To better understand the similarities between human brain and testis, we decided to explore the biological processes in which these 29 proteins are involved, using UniProt which is summarized in table 2. The analysis of protein-associated biological processes revealed specific roles of some proteins in brain and testis function and/or development. Since brain plays a key role in the control of testis function, particularly by the secretion of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by the hypothalamus and pituitary, we expected more common specific proteins involved in testis function/development. Counterintuitively, 31% of the proteins are involved in brain function development, as opposed to 7% of testis function/development-related proteins.
Table 2.
List of proteins highly expressed only in brain and testis, along with their UnitProt ID, gene name and the main biological process(es) associated (according to UnitProt). The biological processes associated with brain or testis function/development are bolded. CNS, central nervous system.
| UnitProt ID | gene name | protein name | biological processes |
|---|---|---|---|
| Q9H172 | ABCG4 | ATP-binding cassette sub-family G member 4 | cellular response to leukaemia inhibitory factor; cholesterol efflux; transmembrane transport |
| Q96M02 | C1ORF90 | (E2-independent) E3 ubiquitin-conjugating enzyme FATS | protein polyubiquitination and stabilization; regulation of centriole replication |
| Q13536 | C1ORF61 | protein CROC-4 (contingent replication of cDNA 4) | positive regulation of transcription by RNA polymerase II |
| Q5T035 | C9ORF129 | putative in characterized protein C9orf129 | — |
| P08912 | CHRM5 | muscarinic acetylcholine receptor M5 | chemical synaptic transport; dopamine transport; transmission of nerve impulse |
| Q12926 | ELAV2 | ELAV-like protein 2 | mRNA splicing, via spliceosome; regulation of transcription |
| Q49AJ0 | FAM135B | protein FAM135B | cellular lipid metabolic process |
| P43080 | GUCA1A | guanylyl cyclase-activating protein 1 | cellular response to calcium ion; signal transduction; visual perception |
| Q8NE63 | HIPK4 | homeodomain-interacting protein kinase 4 | histone phosphorylation; peptidyl-serine phosphorylation; protein autophosphorylation |
| A6NGN9 | IGLON5 | IgLON family member 5 | — |
| Q7Z553 | MDGA2 | MAM domain-containing glycosylphosphatidylinositol anchor protein 2 | spinal cord motor neuron differentiation |
| P60323 | NANOS3 | Nanos homolog 3 | germ cell development; multicellular organism development; regulation of cell cycle; spermatogenesis |
| O14594 | NCAN | neurocan core protein | cell adhesion; CNS development; chondroitin sulfate biosynthetic process |
| Q9NQ35 | NRIP3 | nuclear receptor-interacting protein 3 | — |
| Q9Y5K3 | PCYT1B | choline-phosphate cytidylyltransferase B | spermatogenesis; phosphatidylcholine biosynthetic process |
| P01213 | PDYN | proenkephalin-B | chemical synaptic transmission; G protein-coupled receptor signalling pathway; neuropeptide signalling pathway |
| Q96PV4 | PNMA5 | paraneoplastic antigen-like protein 5 | positive regulation of apoptotic process |
| Q8WY54 | PPM1E | protein phosphatase 1E | cellular response to drug; negative regulation of protein kinase activity |
| Q33E94 | RFX4 | transcription factor RFX4 | positive regulation of transcription by RNA polymerase II; cilium assembly |
| Q8N6R1 | SERP2 | stress-associated endoplasmic reticulum protein 2 | endoplasmic reticulum unfolded protein response; protein glycosylation; protein transport |
| Q6ZV89 | SH2D5 | SH2 domain-containing protein 5 | — |
| Q99963 | SH3GL3 | endophilin-A3 | CNS development; endocytosis; positive regulation of neuron differentiation |
| Q8TF17 | SH3TC2 | SH3 domain and tetratricopeptide repeat-containing protein 2 | peripheral nervous system myelin maintenance; regulation of intracellular protein transport |
| Q8N5S1 | SLC25A41 | solute carrier family 25 member 41 | — |
| Q99726 | SLC30A3 | zinc transporter 3 (ZnT-3) | regulation of sequestering zinc ion; response to zinc ion |
| O00570 | SOX1 | transcription factor SOX1 | cell differentiation; CNS development; chromatin organization; forebrain neuron development; neuron differentiation |
| Q16650 | TBR1 | T-box brain protein 1 | brain development; cell fate specification; regulation of axon guidance; regulation of transcription |
| O95409 | ZIC2 | zinc finger protein ZIC 2 | brain development; cell differentiation; positive regulation of transcription |
| O96T25 | ZIC5 | zinc finger protein ZIC 5 | cell differentiation; CNS development |
2.3. Why do brain and testis appear to have similar proteomes?
The increasing evidence for similarity between the human brain and testis gene expression and protein composition raises the question of the importance of these findings. It has been hypothesized that the testis could participate in human speciation along with the brain and placenta, which may contribute to the expression of the same set of genes in both tissues [44]. It has been suggested that evolutionary changes in gene expression contribute to most of the phenotypic differences between species, but how these gene expression patterns might be passed down to the offspring is a misunderstood topic [6,45]. The involvement of testis, along with the brain and placenta, in speciation was first suggested by Wilda et al. [44] and the hypothalamus–pituitary–testis axis was proposed to be implicated in maintaining the similar gene expression between brain and testis [6]. Indeed, testis has been proposed as the hotspot for the appearance of new genes, which are the raw material for the evolution of species [46]. Sperm seem to be the motor of speciation. On one hand, sperm competition, that is the competitive process between sperm of different males to fertilize the same egg, is important in the formation of new species. On the other hand, sperm is also crucial to maintain the integrity of a species, by acting as a reproductive isolation barrier that precludes gene flow between species. In fact, male hybrids, characterized by the combination of two different species, seem to produce significantly fewer mature spermatozoa due to incompatibilities in the last stages of sperm development [47]. The high and specific expression of fragile X mental retardation 1 gene (Fmr1) in brain and testis suggests that speciation recruits the same set of tissue-specific genes that are active in those organs that are important for speciation [44].
More recently, 60 new protein-coding genes that originated de novo in the human lineage since its divergence from chimpanzee (human-specific genes) were identified [48]. These proteins became fixed in the human population, and the high levels found in testis are also in agreement with the role of this organ in the transmission of gene expression patterns to the offspring [48]. The highest expression levels in cerebral cortex and testis suggested that these genes may contribute to phenotypic features that are exclusive of humans, such as the improved cognitive ability. Indeed, human-specific NOTCHNL2 genes were associated with a role in cortex development and neurogenesis, and have been proposed as a driving force in the evolution of human large brains [49]. Additional evidence seems to suggest that brain and testis function-associated genes are changing unusually quickly, becoming the most divergent genes between species [50].
The similarities between the human brain and testis proteome seems to be reflected in an apparent association between the (dys)function of these tissues. Indeed, an association was observed between the degenerative process in the central nervous system and testicular degeneration, without coexisting hypophyseal lesions [51]. In addition, mutations in X-linked aristaless-related homeobox gene (Arx) were associated with the X-linked lissencephaly with abnormal genitalia (XLAG) syndrome, a disease characterized by simultaneous microcephaly and hypogonadism [52]. Evidence in mouse suggested that alterations in the same protein may be simultaneously responsible for brain and testis dysfunction. Inactivation of Huntington disease gene (Hdh) in mouse brain and testis results in a progressive degenerative neuronal phenotype, along with sterility [53], while mutations in Arx caused abnormal development of forebrain and testis [52]. Moreover, a negative correlation was observed between testis volume and parental behaviour and nurturing-related brain activity [54].
3. Neuron and sperm
3.1. Cellular and molecular similarities
The morphology, genomic activity and function of human neuron and sperm are as different as any other two cells in the body [55]. Sperm is a very distinct cell, compared to other cells in the human body, mainly because it is a haploid cell and virtually devoid of transcription and translation [56]. However, beyond the similarities between brain and testis, several bodies of evidence of the similarities between human neuron and sperm, the fundamental units of these tissues, have been reported and are summarized in table 3.
Table 3.
Cellular and molecular similarities between human neuron and sperm.
| neuron | sperm |
|---|---|
| activate other cells: neurons or somatic effectors | activate other cells: oocyte |
| exocytosis of neurotransmitters in the synaptic space (essential for neuron function) | acrosomal exocytosis at the oocyte surface (essential for sperm function) |
| synaptic vesicles | acrosome |
| high concentrations of PUFAs | |
| presence of ‘neuronal’ receptors | |
| excitable cells | |
| presence of Ca2+ channels | |
| Ca2+ signalling involved in regulation of key functions | |
| common signalling pathways | |
Both neuron and sperm can activate other cells, though the activation mechanisms involved are different. After the plasma membrane interaction of sperm with oocyte, the sperm activates the oocyte and triggers a signal transduction cascade that ultimately results in the conversion of the oocyte to a diploid embryo [57]. Neurons also have the capacity to activate other cells, namely other neurons or somatic effector cells, through chemical synapses or gap junctions (electrical synapses), not requiring contact between cells [57].
Human neuron and sperm seem also to share similarities in exocytic process. Exocytosis is a central process to their individual abilities to carry out their functions. Several components of the neuronal synaptic vesicle exocytotic machinery have been found in sperm, notably including an intricate system of plasma membrane proteins, like synaptotagmins and SNARE complex [58–61]. Neurons use exocytosis for neurite outgrowth and to release neurotransmitters from synaptic vesicles, which is essential for communication between neurons [62]. The synaptic vesicles can be compared to the acrosome of sperm, which essentially contains hydrolytic enzymes and other important fertilization factors. These enzymes are released from the sperm through a specialized form of exocytosis. This process includes membrane loss and is necessary for zona pellucida breakdown and consequent sperm–oocyte fusion [55,63]. Despite the similarities of the exocytotic process in neurons and sperm, in sperm this event only occurs once, in contrast to the continuous exocytotic activity of a neuron [55].
After the release of neurotransmitters at the synaptic gap, they interact with post-synaptic receptors (‘neuronal’ receptors) to induce or inhibit neurotransmission. Several types of ‘neuronal’ receptors, like glutamate and gamma-aminobutyric (GABAA), glycine and nicotinic acetylcholine receptors, have been found in sperm [64–66]. Also in sperm, the ‘neuronal’ receptors play vital roles for its normal function, including in sperm acrosomal reaction, capacitation and motility [55,67]. Due to the presence of various voltage-gated ion channels and several ligand-gated receptor channels, involved in rapid membrane potential changes, neurons are considered excitable cells [68]. In sperm, diverse types of high- and low-voltage-activated channels have been reported, suggesting that sperm may, like neurons, be considered an excitable cell [69,70].
Calcium (Ca2+) signalling is central to the regulation of function in both neuron and sperm cells. These distinct cell types both need to generate precisely timed and localized [Ca2+]I signals. It appears that this has resulted in some striking similarities in the ways in which their Ca2+ signalling toolkits are employed [55]. Though all cells express a Ca2+ signalling toolkit, the types, locations and combinations of channels and pumps can vary significantly between cell types, because they are adapted to the requirements of the cell and its activities. Both neuron and sperm Ca2+ signalling toolkits involve a diverse range of components (including Ca2+-permeable channels and Ca2+ pumps) in both the plasma membrane and intracellular membranes, though the diversity in sperm is low in comparison to that of neurons [67,71]. In neurons, Ca2+ signalling is involved in the regulation of various key functions, including transmission, processing and storage of information [72]. For instance, synaptic neurotransmitter secretion, modulation of synaptic efficacy (underlying memory formation) and excitability of the neuronal membrane (the ease with which a nerve impulse can be induced) are all dependent on or modulated by [Ca2+]I [73]. In mature sperm cells, Ca2+ signalling is arguably at least as important as in neurons, playing central roles in the regulation of motility and capacitation (post-ejaculatory acquisition of fertilizing ability) [71]. The complex signalling pathway that leads to acrosome reaction also requires mobilization of Ca2+ stores within the acrosome [74]. The best-characterized Ca2+ channel in sperm is CatSper, which is a sperm-specific channel essential for hyperactivated motility [75,76]. The influx of extracellular Ca2+ is also required for acrosome reaction, though the involvement of CatSper here is unclear [69].
Several signalling pathways are common to neuron and sperm, and seem to play essential roles in both cell types. For instance, anandamide (AEA) signalling seems to modulate human sperm motility [77]. A role as a modulator of synaptic function was also described for AEA signalling pathway [78]. In addition, Wnt signalling occurs also in both cell types where it controls both sperm maturation and neuronal differentiation [79,80]. The mTOR signalling pathway was also associated with crucial events in both neuron and sperm. Indeed, mTOR signalling regulates sperm quality in older men and is important for normal neuronal growth [81,82].
3.2. Proteomic comparison
The sperm proteome was recently extracted, using the PubMed database, by Santiago et al. [83]. To avoid redundancy, from the total list of sperm proteins, we excluded duplicates and only reviewed proteins (annotated using the UniProtKB/Swiss-Prot accession number) were considered (electronic supplementary material, table S2). Based on the same criteria, the neuronal cells proteome was retrieved from HPA. To avoid redundancy, duplicates and unreviewed proteins (according to UnitProt) were excluded. A list of all neuron proteins (available at 31 March 2021) were obtained (electronic supplementary material, table S2). A total of 13 193 and 6653 reviewed proteins constitute the neuron and sperm proteomes, respectively. A Venn diagram analysis was conducted using the Jveen tool to recover the common proteins between these two cell types. From the total proteins, 5048 are common to both human sperm and neuron (electronic supplementary material, table S2; figure 3).
Figure 3.
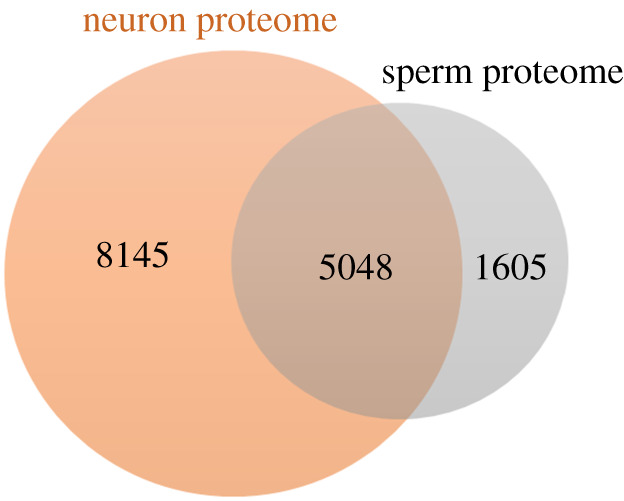
Venn diagram demonstrating the overlap between the neuron and sperm proteome (based on the Jveen tool).
From the 5048 common proteins in human neuron and sperm, a sublist was made considering the proteins with elevated expression in neuronal cells, according to HPA (www.proteinatlas.org). This analysis results in a total of 682 common proteins. Considering these common proteins, a GO analysis (using STRING: functional protein association networks) was performed and revealed a total of 328 GO terms significantly enriched, with an FDR < 0.05 (electronic supplementary material, table S2). In table 4, we summarize some of the most important biological processes in the context of the present study, together with the number of proteins associated with the GO term and the respective FDR of the annotation.
Table 4.
Main biological processes associated with the common proteins between human sperm and neuron. A list of all the associated biological processes are found in electronic supplementary material, table S2.
| GO term | description | count in gene set | FDR |
|---|---|---|---|
| development | |||
| GO:0032 502 | developmental process | 268/5401 | 4.08 × 10−9 |
| GO:0048869 | cellular developmental process | 175/3533 | 2.61 × 10−5 |
| GO:2000026 | regulation of multicellular organismal developmental | 90/1876 | 2.23 × 10−2 |
| GO:0021700 | developmental maturation | 17/216 | 3.35 × 10−2 |
| GO:0048639 | positive regulation of developmental growth | 14/165 | 3.93 × 10−2 |
| nervous system development | |||
| GO:0007399 | nervous system development | 181/2206 | 1.11 × 10−23 |
| GO:0022008 | neuron projection development | 68/616 | 6.03 × 10−13 |
| GO:0048666 | neuron development | 76/758 | 1.11 × 10−12 |
| GO:0061564 | axon development | 47/377 | 1.28 × 10−10 |
| GO:0007417 | central nervous system development | 60/861 | 3.14 × 10−5 |
| GO:0007420 | brain development | 47/650 | 1.80 × 10−4 |
| GO:0021695 | cerebellar cortex development | 7/49 | 3.63 × 10−2 |
| brain/neuron-associated processes | |||
| GO:0030182 | neuron differentiation | 86/940 | 1.64 × 10−12 |
| GO:0010975 | regulation of neuron projection development | 47/443 | 1.22 × 10−8 |
| GO:0007411 | axon guidance | 27/220 | 4.38 × 10−6 |
| GO:009893 | axonal transport | 9/43 | 1.50 × 10−3 |
| GO:0008038 | neuron recognition | 8/34 | 1.80 × 10−3 |
| GO:0001764 | neuron migration | 14/118 | 3.70 × 10−3 |
| GO:0007158 | neuron cell–cell adhesion | 5/14 | 7.50 × 10−3 |
| GO:0019228 | neuronal action potential | 6/31 | 2.17 × 10−2 |
| GO:0036514 | dopaminergic neuron axon guidance | 3/5 | 2.87 × 10−2 |
| exocytosis | |||
| GO:0017156 | calcium ion regulated exocytosis | 12/74 | 9.00 × 10−4 |
| GO:0016079 | synaptic vesicle exocytosis | 11/64 | 0.0011 |
| GO:0006904 | vesicle docking involved in exocytosis | 6/38 | 0.0439 |
| cell signalling | |||
| GO:0007267 | cell–cell signalling | 95/1073 | 4.86 × 10−13 |
| GO:0035637 | multicellular organismal signalling | 21/110 | 1.89 × 10−7 |
| GO:0023052 | signalling | 232/5108 | 1.10 × 10−4 |
| GO:0007215 | glutamate receptor signalling pathway | 9/43 | 1.50 × 10−3 |
| GO:1905114 | cell surface receptor signalling pathway | 30/383 | 1.80 × 10−3 |
| GO:1990034 | calcium-mediated signalling | 13/132 | 2.02 × 10−2 |
| GO:0035556 | intracellular signal transduction | 76/1528 | 2.17 × 10−2 |
| GO:0016055 | Wnt signalling pathway | 22/303 | 2.41 × 10−2 |
Among the common proteins between human neurons and sperm, several GO terms related to cell/tissue development were significantly enriched, suggesting that both cells play important roles in human tissue development. Also comparing neuron and sperm proteomes, it is observed that there are many common proteins involved in brain/neuron development and function. As expected by the important role of exocytosis in both sperm and neuron function as discussed above (table 4), both neuron and sperm express a huge number of proteins involved in exocytic process. Cell signalling-associated biological processes were also highlighted in this analysis, corroborating the idea that sperm and neuron share several important signalling pathways (table 4).
4. Concluding remarks
Human brain and testis share several molecular characteristics, which are reflected in a very similar proteomic profile. Our in silico analysis revealed that, surprisingly, human brain and testis have the highest number of common proteins, compared with other human body tissues. The common proteins are mainly involved in the function and/or development of brain, rather than in testis-associated processes. The human neuron and sperm are very distinct cells; however, they share several molecular features, and a huge number of proteins are common to both cells, mainly those involved in exocytotic and cell signalling processes, tissue development and brain/neuron-associated processes.
The similarity between human brain and testis may be explained by a biochemical convergence and by the involvement of these two tissues in the speciation process. The high similarity of proteins between human brain and testis may have clinical relevance. Indeed, the common proteins may be associated with the simultaneously impairment of brain and testis function. The identification of these proteins, along with the analysis of their role in brain and/or testis function, could help in better understanding the pathophysiology of these conditions, as well as in the development of new therapeutic strategies for treating brain or testis diseases.
Data accessibility
This article has no additional data.
Authors' contributions
B.M. and M.F. made a substantial contribution to the article conception and design, B.M. performed the literature search and drafted the manuscript. S.J.P., L.F.C.C., P.J.E. and M.F. critically revised the work. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Funding
We thank the Portuguese Foundation for Science and Technology (FCT), European Union, QREN, FEDER and COMPETE for funding iBiMED (UIDB/04501/2020, POCI-01-0145-FEDER-007628 and UID/BIM/04501/2019).
References
- 1.Raichle ME. 2010. Two views of brain function. Trends Cogn. Sci. 14, 180-190. ( 10.1016/j.tics.2010.01.008) [DOI] [PubMed] [Google Scholar]
- 2.Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner JT, Penny W.. 2004. Human brain function. Oxford, UK: Elsevier.
- 3.Amann RP. 1989. Structure and function of the normal testis and epididymis. J. Am. Coll. Toxicol. 8, 457-471. ( 10.3109/10915818909014532) [DOI] [Google Scholar]
- 4.Nieschlag E, Behre HM, Nieschlag S. 2010. Physiology of testicular function. In Andrology: male reproductive health and dysfunction (eds GF Weinbaver, CM Luetjens, M Simoni, E Nieschlag), pp. 1-629. Berlin, Germany: Springer. [Google Scholar]
- 5.Guo J, Zhu P, Wu C, Yu L, Zhao S, Gu X. 2003. In silico analysis indicates a similar gene expression pattern between human brain and testis. Cytogenet. Genome Res. 103, 58-62. ( 10.1159/000076290) [DOI] [PubMed] [Google Scholar]
- 6.Guo JH, Huang Q, Studholme DJ, Wu CQ, Zhao SY. 2005. Transcriptomic analyses support the similarity of gene expression between brain and testis in human as well as mouse. Cytogenet. Genome Res. 111, 107-109. ( 10.1159/000086378) [DOI] [PubMed] [Google Scholar]
- 7.Arden R, Gottfredson LS, Miller G, Pierce A. 2009. Intelligence and semen quality are positively correlated. Intelligence 37, 277-282. ( 10.1016/j.intell.2008.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glazer CH, et al. 2017. Male factor infertility and risk of multiple sclerosis: a register-based cohort study. Mult. Scler. J. 24, 1835-1842. ( 10.1177/1352458517734069) [DOI] [PubMed] [Google Scholar]
- 9.Fode M, Krogh-jespersen S, Brackett NL, Ohl DA, Lynne CM, Sønksen J. 2012. Male sexual dysfunction and infertility associated with neurological disorders. Asian J. Androl. 14, 61-68. ( 10.1038/aja.2011.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J. 2019. Basic neural units of the brain: neurons, synapses and action potential. arXiv. (http://arxiv.org/abs/1906.01703)
- 11.Jäkel S, Dimou L. 2017. Glial cells and their function in the adult brain: a journey through the history of their ablation. Front. Cell Neurosci. 11, 1-17. ( 10.3389/fncel.2017.00024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze W, Rehder U. 1984. Organization and morphogenesis of the human seminiferous epithelium. Cell Tissue Res. 237, 395-407. ( 10.1007/BF00228424) [DOI] [PubMed] [Google Scholar]
- 13.Svechnikov K, Landreh L, Weisser J, Izzo G, Colón E, Svechnikov I, Söder O. et al. 2010. Origin, development and regulation of human leydig cells. Horm. Res. Paediatr. 73, 93-101. ( 10.1159/000277141) [DOI] [PubMed] [Google Scholar]
- 14.Kıray H, Lindsay SL, Hosseinzadeh S, Barnett SC. 2016. The multifaceted role of astrocytes in regulating myelination. Exp. Neurol. 283, 541-549. ( 10.1016/j.expneurol.2016.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu C, Rojas T, Chin AC, Cheng W, Bernstein IA, Albacarys LK, Wright WW, Snyder SH. 2018. Multiple aspects of male germ cell development and interactions with Sertoli cells require inositol hexakisphosphate kinase-1. Sci. Rep. 8, 1-13. ( 10.1038/s41598-018-25468-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JM, Kim HG, Son CG. 2018. Tissue-specific profiling of oxidative stress-associated transcriptome in a healthy mouse model. Int. J. Mol. Sci. 19, 3174. ( 10.3390/ijms19103174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falkowska A, Gutowska I, Goschorska M, Nowacki P. 2015. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int. J. Mol. Sci. 16, 25 959-25 981. ( 10.3390/ijms161125939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boussouar F, Benahmed M. 2004. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. 15, 345-350. ( 10.1016/j.tem.2004.07.003) [DOI] [PubMed] [Google Scholar]
- 19.Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF. 2012. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 9, 330-338. ( 10.1038/nrurol.2012.77) [DOI] [PubMed] [Google Scholar]
- 20.Pitts MW, Kremer PM, Hashimoto AC, Torres DJ, Byrns CN, Williams CS, Berry MJ. 2015. Competition between the brain and testes under selenium-compromised conditions: insight into sex differences in selenium metabolism and risk of neurodevelopmental disease. J. Neurosci. 35, 15 326-15 338. ( 10.1523/JNEUROSCI.2724-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabuto H, Amakawa M, Shishibori T. 2004. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 74, 2931-2940. ( 10.1016/j.lfs.2003.07.060) [DOI] [PubMed] [Google Scholar]
- 22.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. 2015. Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064-1078. ( 10.1016/j.cell.2015.10.067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mital P, Hinton BT, Dufour JM. 2011. The blood-testis and blood-epididymis barriers are more than just their tight junctions1. Biol. Reprod. 84, 851-858. ( 10.1095/biolreprod.110.087452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford MA, Broadhurst CL, Ghebremeskel K, Sinclair AJ, Saugstad LF, Schmidt WF, Sinclair AJ, Cunnane SC. 2008. The role of docosahexaenoic and arachidonic acids as determinants of evolution and hominid brain development. In Fish Glob Welf Environ 5th World Fish Congr, pp. 57-76. Tokyo, Japan: JSFS. [Google Scholar]
- 25.Lenzi A, Gandini L, Maresca V, Rago R, Sgrò P, Dondero F, Picardo M. 2000. Fatty acid composition of spermatozoa and immature germ cells. Mol. Hum. Reprod. 6, 226-231. ( 10.1093/molehr/6.3.226) [DOI] [PubMed] [Google Scholar]
- 26.Davidoff MS, Middendorff R, Köfüncü E, Müller D, Ježek D, Holstein AF. 2002. Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules. Acta Histochem. 104, 39-49. ( 10.1078/0065-1281-00630) [DOI] [PubMed] [Google Scholar]
- 27.Schulze W, Davidoff MS, Holstein AF. 1987. Are Leydig cells of neural origin? Substance P-like immunoreactivity in human testicular tissue. Acta Endocrinol (Copenh). 115, 373-377. ( 10.1530/acta.0.1150373) [DOI] [PubMed] [Google Scholar]
- 28.Davidoff MS, Schulze W, Middendorff R, Holstein AF. 1993. The Leydig cell of the human testis: a new member of the diffuse neuroendocrine system. Cell Tissue Res. 271, 429-439. ( 10.1007/BF02913725) [DOI] [PubMed] [Google Scholar]
- 29.Davidoff MS, Middendorff R, Pusch W, Müller D, Wichers S, Holstein AF. 1999. Sertoli and Leydig cells of the human testis express neurofilament triplet proteins. Histochem. Cell Biol. 111, 173-187. ( 10.1007/s004180050347) [DOI] [PubMed] [Google Scholar]
- 30.Xiao Q, Hu X, Wei Z, Tam KY. 2016. Cytoskeleton molecular motors: structures and their functions in neuron. Int. J. Biol. Sci. 12, 1083-1092. ( 10.7150/ijbs.15633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma D-D, Wang D-H, Yang W-X. 2017. Kinesins in spermatogenesis†. Biol. Reprod. 96, 267-276. ( 10.1095/biolreprod.116.144113) [DOI] [PubMed] [Google Scholar]
- 32.Liu XA, Rizzo V, Puthanveettil SV. 2012. Pathologies of axonal transport in neurodegenerative diseases. Transl. Neurosci. 3, 355-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Ou Y, Cheng M, Shojaei Saadi H, Thundathil JC, van der Hoorn FA. 2012. KLC3 is involved in sperm tail midpiece formation and sperm function. Dev. Biol. 366, 101-110. ( 10.1016/j.ydbio.2012.04.026) [DOI] [PubMed] [Google Scholar]
- 34.Brands A, Münzel PA, Bock KW. 2000. In situ hybridization studies of UDP-glucuronosyltransferase UGT1A6 expression in rat testis and brain. Biochem. Pharmacol. 59, 1441-1444. ( 10.1016/S0006-2952(00)00274-4) [DOI] [PubMed] [Google Scholar]
- 35.Han J, Gu W, Hecht NB. 1995. Testis-brain RNA-binding protein, a testicular translational regulatory RNA-binding protein, is present in the brain and binds to the 3′ untranslated regions of transported brain mRNAs1. Biol. Reprod. 53, 707-717. ( 10.1095/biolreprod53.3.707) [DOI] [PubMed] [Google Scholar]
- 36.Ibberson M, Riederer BM, Uldry M, Guhl B, Roth J, Thorens B. 2002. Immunolocalization of GLUTX1 in the testis and to specific brain areas and vasopressin-containing neurons. Endocrinology 143, 276-284. ( 10.1210/endo.143.1.8587) [DOI] [PubMed] [Google Scholar]
- 37.Maeda K, Inui S, Tanaka H, Sakaguchi N. 1999. A new member of the α4-related molecule (α4-b) that binds to the protein phosphatase 2A is expressed selectively in the brain and testis. Eur. J. Biochem. 264, 702-706. ( 10.1046/j.1432-1327.1999.00571.x) [DOI] [PubMed] [Google Scholar]
- 38.Marazziti D, Gallo A, Golini E, Matteoni R, Tocchini-Valentini GP. 1998. Molecular cloning and chromosomal localization of the mouse Gpr37 gene encoding an orphan G-protein-coupled peptide receptor expressed in brain and testis. Genomics 53, 315-324. ( 10.1006/geno.1998.5433) [DOI] [PubMed] [Google Scholar]
- 39.Mayer H, Bauer H, Breuss J, Ziegler S, Prohaska R. 2001. Characterization of rat LANCL1, a novel member of the lanthionine synthetase C-like protein family, highly expressed in testis and brain. Gene 269, 73-80. ( 10.1016/S0378-1119(01)00463-2) [DOI] [PubMed] [Google Scholar]
- 40.Tanja O, Facchinetti P, Rose C, Bonhomme MC, Gros C, Schwartz JC. 2000. Neprilysin II: a putative novel metalloprotease and its isoforms in CNS and testis. Biochem. Biophys. Res. Commun. 271, 565-570. ( 10.1006/bbrc.2000.2664) [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto H, Ochiya T, Takahama Y, Ishii Y, Osumi N, Sakamoto H, Terada M. 2000. Detection of spatial localization of Hst-1/Fgf-4 gene expression in brain and testis from adult mice. Oncogene 19, 3805-3810. ( 10.1038/sj.onc.1203752) [DOI] [PubMed] [Google Scholar]
- 42.Danielsson A, Djureinovic D, Fagerberg L, Hallstro B, Ponte F, Lindskog C, Uhlén M, Pontén F. 2014. The human testis-specific proteome defined by transcriptomics and antibody-based profiling. Mol. Hum. Reprod. 20, 476-488. ( 10.1093/molehr/gau018) [DOI] [PubMed] [Google Scholar]
- 43.Liu T-Y, Huang HH, Wheeler D, Xu Y, Wells JA, Song YS, Wiita AP. 2017. Time-resolved proteomics extends ribosome profiling-based measurements of protein synthesis dynamics. Cell Syst. 4, 636-644. e9. ( 10.1016/j.cels.2017.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilda M, Bächner D, Zechner U, Kehrer-Sawatzki H, Vogel W, Hameister H. 2000. Do the constraints of human speciation cause expression of the same set of genes in brain, testis, and placenta? Cytogenet. Cell Genet. 91, 300-302. ( 10.1159/000056861) [DOI] [PubMed] [Google Scholar]
- 45.Khaitovich P, Enard W, Lachmann M, Pääbo S. 2006. Evolution of primate gene expression. Nat. Rev. Genet. 7, 693-702. ( 10.1038/nrg1940) [DOI] [PubMed] [Google Scholar]
- 46.Dion-Côté A-M. 2019. A hotspot for new genes. Elife 8, 8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wünsch LK, Pfennig KS. 2013. Failed sperm development as a reproductive isolating barrier between species. Evol. Dev. 15, 458-465. ( 10.1111/ede.12054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu DD, Irwin DM, Zhang YP. 2011. De novo origin of human protein-coding genes. PLoS Genet. 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki IK, et al. 2018. Human-specific NOTCH2NL genes expand cortical neurogenesis through delta/notch regulation. Cell 173, 1370-1384.e16. ( 10.1016/j.cell.2018.03.067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varki A, Altheide TK. 2005. Comparing the human and chimpanzee genomes: searching for needles in a haystack. Genome Res. 15, 1746-1758. ( 10.1101/gr.3737405) [DOI] [PubMed] [Google Scholar]
- 51.Boström K, Brun A. 1971. Testicular changes in association with malformation of TFIE central nervous system and mental retardation. Acta Pathol. Microbiol. Scand. Pathol. 79A, 249-256. ( 10.1111/j.1699-0463.1971.tb01816.x) [DOI] [PubMed] [Google Scholar]
- 52.Kitamura K, et al. 2002. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat. Genet. 32, 359-369. ( 10.1038/ng1009) [DOI] [PubMed] [Google Scholar]
- 53.Dragatsis I, Levine MS, Zeitlin S. 2000. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nat. Genet. 26, 300-306. ( 10.1038/81593) [DOI] [PubMed] [Google Scholar]
- 54.Mascaro JS, Hackett PD, Rilling JK. 2013. Testicular volume is inversely correlated with nurturing-related brain activity in human fathers. Proc. Natl Acad.Sci. USA 110, 15 746-15 751. ( 10.1073/pnas.1305579110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meizel S. 2004. The sperm, a neuron with a tail: ‘neuronal’ receptors in mammalian sperm. Biol. Rev. Camb. Philos. Soc. 79, 713-732. ( 10.1017/S1464793103006407) [DOI] [PubMed] [Google Scholar]
- 56.Ren X, Chen X, Wang Z, Wang D. 2017. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 63, 439-443. ( 10.1262/jrd.2016-093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belousov AB, Fontes JD. 2013. Neuronal gap junctions: making and breaking connections during development and injury. Trends Neurosci. 36, 227-236. ( 10.1016/j.tins.2012.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rizo J, Südhof TC. 2002. Snares and munc18 in synaptic vesicle fusion. Nat. Rev. Neurosci. 3, 641-653. ( 10.1038/nrn898) [DOI] [PubMed] [Google Scholar]
- 59.Michaut M, De Blas G, Tomes CN, Yunes R, Fukuda M, Mayorga LS. 2001. Synaptotagmin VI participates in the acrosome reaction of human spermatozoa. Dev. Biol. 235, 521-529. ( 10.1006/dbio.2001.0316) [DOI] [PubMed] [Google Scholar]
- 60.Tomes CN, Michaut M, De BG, Visconti P, Matti U, Mayorga LS. 2002. SNARE complex assembly is required for human sperm acrosome reaction. Dev. Biol. 243, 326-338. ( 10.1006/dbio.2002.0567) [DOI] [PubMed] [Google Scholar]
- 61.Hutt DM, Cardullo RA, Baltz JM, Ngsee JK. 2002. Synaptotagmin VIII is localized to the mouse sperm head and may function in acrosomal exocytosis1. Biol. Reprod. 66, 50-56. ( 10.1095/biolreprod66.1.50) [DOI] [PubMed] [Google Scholar]
- 62.Pierce A, Miller G, Arden R, Gottfredson LS. 2009. Why is intelligence correlated with semen quality? Commun. Integr. Biol. 2, 1-3. ( 10.4161/cib.2.5.8716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harper CV, Cummerson JA, White MRH, Publicover SJ, Johnson PM. 2008. Dynamic resolution of acrosomal exocytosis in human sperm. J. Cell Sci. 121, 2130-2135. ( 10.1242/jcs.030379) [DOI] [PubMed] [Google Scholar]
- 64.Ritta MN, Calamera JC, Bas DE. 1998. Occurrence of GABA and GABA receptors in human spermatozoa. Mol. Hum. Reprod. 4, 769-773. ( 10.1093/molehr/4.8.769) [DOI] [PubMed] [Google Scholar]
- 65.Bray C, Son J-H, Kumar P, Harris JD, Meizel S. 2002. A role for the human sperm glycine receptor/Cl− channel in the acrosome reaction initiated by recombinant ZP31. Biol. Reprod. 66, 91-97. ( 10.1095/biolreprod66.1.91) [DOI] [PubMed] [Google Scholar]
- 66.Baccetti B, Burrini AG, Collodel GC, Falugi C, Moretti E, Piomboni P. 1995. Localisation of two classes of acetylcholine receptor-like molecules in sperms of different animal species. Zygote 3, 207-217. ( 10.1017/S0967199400002604) [DOI] [PubMed] [Google Scholar]
- 67.Ramírez-Reveco A, Villarroel-Espíndola F, Rodríguez-Gil JE, Concha II. 2017. Neuronal signaling repertoire in the mammalian sperm functionality. Biol. Reprod. 96, 505-524. ( 10.1095/biolreprod.116.144154) [DOI] [PubMed] [Google Scholar]
- 68.Schulz DJ, Baines RA, Hempel CM, Li L, Liss B, Misonou H. 2006. Cellular excitability and the regulation of functional neuronal identity: from gene expression to neuromodulation. J. Neurosci. 26, 10 362-10 367. ( 10.1523/JNEUROSCI.3194-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jagannathan S, Publicover SJ, Barratt CLR. 2002. Voltage-operated calcium channels in male germ cells. Reproduction 123, 203-215. ( 10.1530/rep.0.1230203) [DOI] [PubMed] [Google Scholar]
- 70.Darszon A, Labarca P, Nishigaki T, Espinosa F. 1999. Ion channels in sperm physiology. Physiol. Rev. 79, 481-510. ( 10.1152/physrev.1999.79.2.481) [DOI] [PubMed] [Google Scholar]
- 71.Darszon A, Nishigaki T, Beltran C, Treviño CL. 2011. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 91, 1305-1355. ( 10.1152/physrev.00028.2010) [DOI] [PubMed] [Google Scholar]
- 72.Kostyuk PG. 2007. Key role of calcium signaling in synaptic transmission. Neurophysiology 39, 248-250. ( 10.1007/s11062-007-0034-5) [DOI] [Google Scholar]
- 73.Brini M, Calì T, Ottolini D, Carafoli E. 2014. Neuronal calcium signaling: function and dysfunction. Cell. Mol. Life Sci. 71, 2787-2814. ( 10.1007/s00018-013-1550-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia J, Ren D. 2009. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels1. Biol. Reprod. 80, 1092-1098. ( 10.1095/biolreprod.108.074039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lishko PV, Mannowetz N. 2018. CatSper: a unique calcium channel of the sperm flagellum. Curr. Opin. Physiol. 2, 109-113. ( 10.1016/j.cophys.2018.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Publicover S, Harper CV, Barratt C. 2007. [Ca2+]i signalling in sperm: making the most of what you've got. Nat. Cell. Biol. 9, 235-242. ( 10.1038/ncb0307-235) [DOI] [PubMed] [Google Scholar]
- 77.Amoako AA, Marczylo TH, Marczylo EL, Elson J, Willets JM, Taylor AH, Konje JC. 2013. Anandamide modulates human sperm motility: implications for men with asthenozoospermia and oligoasthenoteratozoospermia. Hum. Reprod. 28, 2058-2066. ( 10.1093/humrep/det232) [DOI] [PubMed] [Google Scholar]
- 78.Castillo P, Younts T, Chávez A, Hashimotodani Y. 2013. Endocannabinoid signaling and synaptic function. Neuron 76, 70-81. ( 10.1016/j.neuron.2012.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koch S, Acebron SP, Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. 2015. Post-transcriptional Wnt signaling governs epididymal sperm maturation post-transcriptional Wnt signaling. Cell 163, 1225-1236. ( 10.1016/j.cell.2015.10.029) [DOI] [PubMed] [Google Scholar]
- 80.Rosso SB, Inestrosa NC. 2013. WNT signaling in neuronal maturation and synaptogenesis. Front. Cell. Neurosci. 7, 1-11. ( 10.3389/fncel.2013.00103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silva JV, Cabral M, Correia R, Carvalho P, Sousa M, Oliveira PF, Fardilha M. 2019. mTOR signaling pathway regulates sperm quality in older men. Cell 8, 1-13. ( 10.3390/cells8060629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takei N, Nawa H. 2014. mTOR signaling and its roles in normal and abnormal brain development. Front. Mol. Neurosci. 7, 1-12. ( 10.3389/fnmol.2014.00028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santiago J, Vieira Silva J, Fardilha M. 2019. First insights on the presence of the unfolded protein response in human spermatozoa. Int. J. Mol. Sci. 20, 1-16. ( 10.3390/ijms20215518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaerkady R, Kerr CL, Marimuthu A, Kelkar DS, Kashyap MK, Gucek M, Gearhart JD, Pandey A. 2009. Temporal analysis of neural differentiation using quantitative proteomics. J. Proteome Res. 8, 1315-1326. ( 10.1021/pr8006667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dammer EB, Duong DM, Diner I, Gearing M, Feng Y, Lah JJ, Levey AI, Seyfried NT. 2013. Neuron enriched nuclear proteome isolated from human brain. J. Proteome Res. 12, 3193-3206. ( 10.1021/pr400246t) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Djuric U, Rodrigues DC, Batruch I, Ellis J, Shannon P, Diamandis P. 2017. Spatiotemporal proteomic profiling of human cerebral development. Mol. Cell. Proteom. 16, 1558-1562. ( 10.1074/mcp.M116.066274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drummond ES, Nayak S, Ueberheide B, Wisniewski T. 2015. Proteomic analysis of neurons microdissected from formalin-fixed, paraffin-embedded Alzheimer's disease brain tissue. Sci. Rep. 5, 1-8. ( 10.1038/srep15456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fathi A, Hatami M, Vakilian H, Han CL, Chen YJ, Baharvand H, Salekdeh GH. 2014. Quantitative proteomics analysis highlights the role of redox hemostasis and energy metabolism in human embryonic stem cell differentiation to neural cells. J. Proteomics 101, 1-16. ( 10.1016/j.jprot.2014.02.002) [DOI] [PubMed] [Google Scholar]
- 89.Ramachandran U, Manavalan A, Sundaramurthi H, Sze SK, Feng ZW, Hu JM, Heese K. 2012. Tianma modulates proteins with various neuro-regenerative modalities in differentiated human neuronal SH-SY5Y cells. Neurochem. Int. 60, 827-836. ( 10.1016/j.neuint.2012.03.012) [DOI] [PubMed] [Google Scholar]
- 90.Villeneuve L, Tiede LM, Morsey B, Fox HS. 2013. Quantitative proteomics reveals oxygen-dependent changes in neuronal mitochondria affecting function and sensitivity to rotenone. J. Proteome Res. 12, 4599-4606. ( 10.1021/pr400758d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu G, Stevens SM, Kobiessy F, Brown H, McClung S, Gold MS, Borchelt DR. 2012. Identification of proteins sensitive to thermal stress in human neuroblastoma and glioma cell lines. PLoS ONE 7, 1-13. ( 10.1371/journal.pone.0049021) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.