Abstract
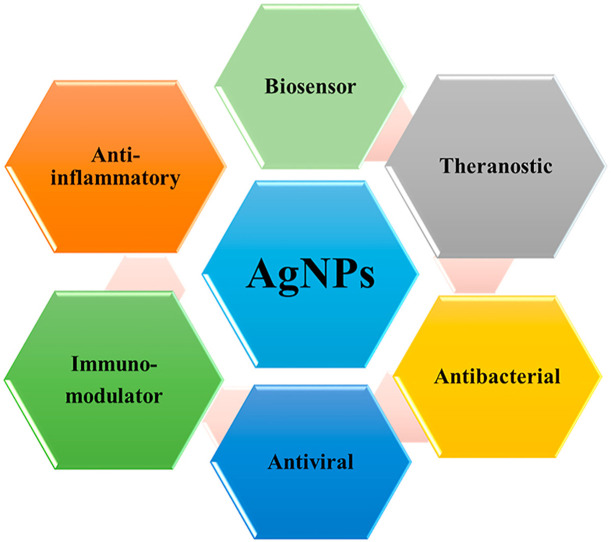
COVID-19 is a highly contagious and widespread disease that has strained the global healthcare system to the hilt. Silver nanoparticles (AgNPs) are well known for their potent antimicrobial, antiviral, immunomodulatory and biosensing properties. AgNPs have been found to be potential antiviral agent that act against many deadly viruses and is presumed to be effective against COVID-19. AgNPs can generate free radicals and reactive oxygen species (ROS) leading to apoptosis mediated cell death thereby inhibiting viral infection. The shape and size of AgNPs play an important role in its biomedical applications as alterations may result in variable biological interaction and activity. Herein, we propose that AgNPs can be utilized for effective management of the ongoing COVID-19 pandemic by highlighting the current status of AgNPs in the fight against COVID-19.
Keywords: Silver nanoparticles, COVID-19, Antiviral activity, Antimicrobial, Cytokine storm
Graphical abstract
1. Introduction
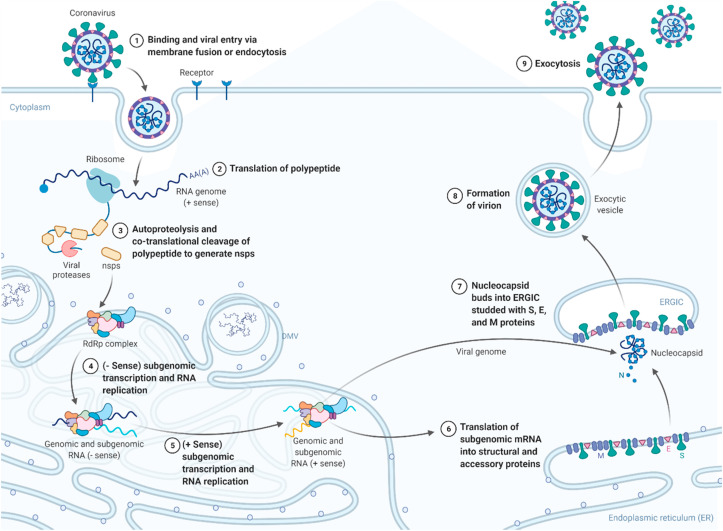
In December 2019, in Wuhan city of China, clusters of pneumonia cases similar to the severe acute respiratory syndrome (SARS) were reported, which was caused by a newly discovered strain of coronavirus (CoV) [1]. Subsequently, the disease was named COVID-19, and the virus involved in this disease was designated as SARS-CoV-2 [2]. Coronaviruses comprise of a big class of viruses that consists of a core of genetic material (single-stranded RNA genome) enveloped by a spherical lipid layer with spikes like protein structure attached to the surface. This structure gives it a crown-like appearance, and in Latin, corona means crown, so this is why, these viruses are labelled as coronaviruses [3,4]. The genomic sequence of this new virus is 78.5% similar to that of the previously known SARS-CoV. However, SARS-CoV-2 transmits faster than SARS-CoV [[5], [6], [7]]. The SARS-CoV-2 virion contains structural proteins namely spike, envelope, membrane, and nucleocapsid. The RNA genome of the virion is encapsulated by the nucleocapsid, while membrane and envelop protein in assembly process confirm its incorporation into the viral particle. Spike protein has specificity towards the host entry receptors i.e., the spike protein interacts with the host angiotensin-converting enzyme 2 (ACE2), alongwith host factors like cell surface serine protease namely transmembrane protease, serine 2 (TMPRSS2) which stimulates the uptake and fusion of the virus at host cellular membrane [8]. The positive sense RNA genome has two open reading frames (ORFs) i.e., ORF1a and ORF1b. The translation of ORF1a results in the formation of polyproteins (pp) pp1a and pp1ab which are processed into sixteen non-structural proteins (nsps) by post-translational and co-translational modifications. The non-structural proteins are responsible for forming viral replication and transcription complex. The translated structural proteins are translocated to the membranes of endoplasmic reticulum (ER) followed by transit via ER to the golgi complex intermediatory compartment (ERGIC) where production of new viral genomic RNA takes place leading into the budding of virions. Lastly, virions are released from the host via exocytosis as shown in Fig. 1 [9,10]. Currently, most of the treatments for COVID-19 management are given for symptomatic relief and support the respiratory system of seriously ill patients [11,12]. Though, the many vaccines including Sputnik-V and BNT162b2 have been granted emergency use authorization, their safety remains unclear and the recent complications including the disputed cases of Bell's palsy with BNT162b2, indicate the enormity of risk involved in approving vaccines in a haste [[13], [14], [15]]. The need of the present time is to develop and design potent antiviral agents that may aid in fighting and overcoming the COVID-19 pandemic [[16], [17], [18]].
Fig. 1.
The life cycle of SARS CoV 2 inside the host. The figure was created with BioRender.com.
Metals including calcium, iron, selenium and zinc play crucial role in a number of vital biochemical reactions which are essential for growth and survival [[19], [20], [21]]. Though, silver (Ag) is not an essential element, it is an important element of biomedical importance. Nanoparticles (NPs) are well known for their numerous advantages including increased surface area, tailored release profile of the cargo, modulation of drug pharmacokinetics, reduced toxicity and improved biological response [22,23]. AgNPs are considered as one of the potential therapeutic nanoparticles and are included in most commercialized NPs considering their distinctive catalytic, optical and clinical applications. AgNPs can induce immunologic response and cause inflammatory cell apoptosis in the host. Silver is used in a significant number of Ayurvedic formulations including fortified Chyavanprash and Bhasmas (fine metal powders claimed to be of nano size). The silver Bhasma has been traditionally used for the treatment of inflammation, pain, memory improvement and other diseases. Further, silver has been reported to be beneficial against a variety of inflammatory, cardiovascular and other non-communicable disorders [19,[24], [25], [26], [27]]. Silver possesses potent antimicrobial and immunomodulatory effects and its nano form has been reported to attenuate the progression of multiple diseases including diabetes [28]. Some of the marketed products based on silver are PolyMem Silver™ (Aspen), Acticoat™ and Bactigras™ (Smith & Nephew), Tegaderm™ (3 M) and Aquacel™ (ConvaTec) [29].
2. AgNPs as an antimicrobial and antiviral agent
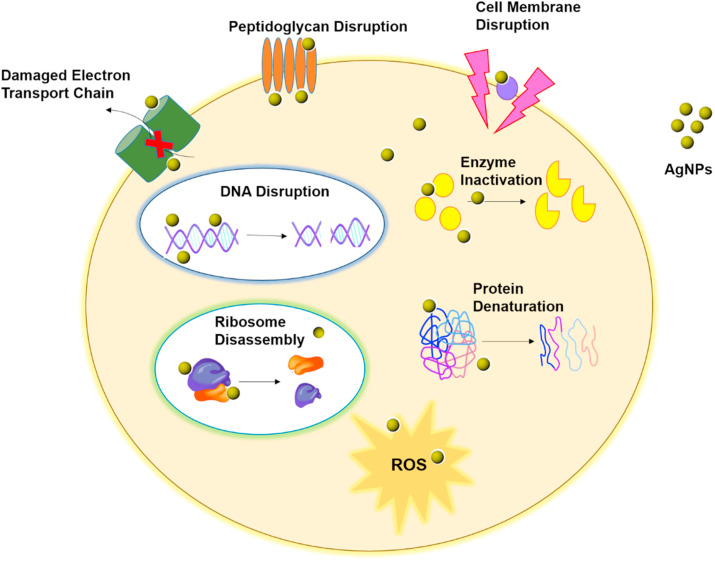
It is need of the hour to halt the advancement in SARS-CoV-2 virus and in this direction AgNPs could be an attractive approach [30]. In various forms silver based formulations are used in the clinical settings such as in burn injury, wound dressings and as antibiotic coating material on the medical devices [31,32]. AgNPs show efficient antimicrobial action in comparison to its elemental salts owing to their significantly larger surface area that offers superior contact with microorganisms [33]. The antibacterial mechanism of AgNPs is achieved because the silver ions are continuously released which leads to death of bacteria. AgNPs make attachment with the cell membrane and invade the bacteria. The cell membrane of bacteria is rich in phosphorus and sulfur-containing proteins. AgNPs interact with these proteins and DNA in the bacterial cell. Once AgNPs enter inside the bacterial cell, it creates a low molecular weight area in the middle of the bacteria. AgNPs interact with ribosomes resulting in disassembly and denaturation in the cytoplasm and inactivation of various enzymes, causing disturbance in the respiratory chain and cell division which finally leads to microbial cell death [34]. Additionally, the antimicrobial activity of AgNPs was probed against different microbial species like Escherichia coli, Staphylococcus aureus and others [35,36]. AgNPs act by generating free radicals in microbial surroundings and inhibit their growth which was confirmed by electron spin resonance spectroscopy. Silver sulfadiazine which is a blend of silver and sulfadiazine and is used as 1% water-soluble cream, acts as a wide-spectrum antibiotic against different microbes [37]. The mechanism of antibacterial activity of AgNPs is shown in Fig. 2 .
Fig. 2.
The mechanism of antibacterial activity of silver nanoparticles.
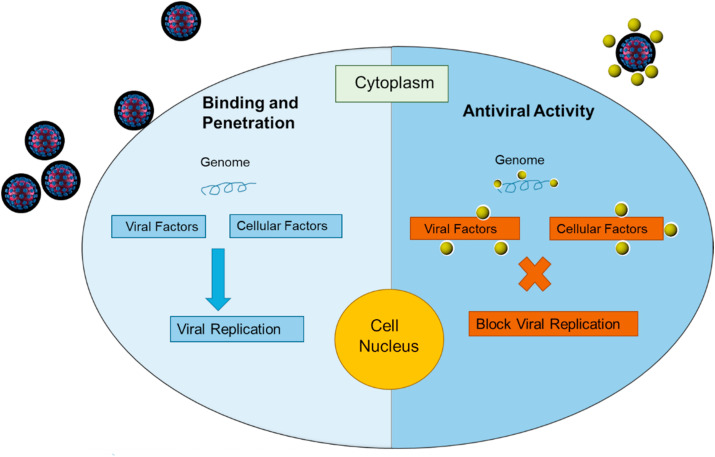
AgNPs possess potent antiviral activity. AgNPs attach to the viral genome thereby blocking the activity and interaction of various viral and cellular factors responsible for replication resulting in the inhibition of viral replication and release of progeny virions [38]. The antiviral mechanism of AgNPs is shown in Fig. 3 . Moreover, the interaction of AgNPs with viruses can be enhanced by evaluating various physiochemical properties like size, shape, surface charge, dispersity, and protein corona effects. Additionally, several proteins with distinct quantities and identities over the NPs can modulate their physicochemical features, targeting, cellular uptake, circulation lifetime in the blood, toxicity and influence the physiological response. In this regard, various molecules can be utilized to maintain the stability and integrity of NPs in biological fluids [39]. Moreover, some interacting biomolecules, copolymers, cells can aid in enhancing the interaction. Xia et al., reported the enhanced targeting ability of AgNPs by conjugating with copolymers [40]. Jose et al., demonstrated the antiviral effect of AgNPs (1-10 nm) against HIV-1 virus at doses of 0.1-100 μg/mL via preferentially binding to the gp120 glycoprotein knobs that are present in the close vicinity of CD4 binding domain. Interaction of AgNPs with gp120 glycoprotein knob resulted in the blockage of virus attachments to the host cells rendering them incapable to invade host cells [41]. Yasutaka et al., examined the antiviral activity of AgNPs and chitosan AgNPs composite against H1N1 influenza A virus in vitro in H1N1 infected Madin-Darby canine kidney (MDCK) cells. Smaller size AgNPs were reported more effective [42]. Further, AgNPs decorated with silica elicit potent antiviral effects against influenza A virus (IFV-A) by modulating the viral membrane protein that inactivates the IFV-A [43]. AgNPs show antiviral effects against hepatitis B virus by interacting with dsDNA or by attaching to viral bodies and halt their pathogenic characteristics [44,45]. In a recent work, AgNPs (2-15 nm) were reported to inhibit extracellular SARS-CoV-2 at a concentration as low as 1 ppm. It was reported via a luciferase based pseudovirus entry assay where AgNPs prevented the virus entry inside the host cell by disturbing viral integrity [36]. AgNPs blocked the binding and stopped the penetration of monkey pox virus in host cells, making them inefficient to replicate [46]. Furthermore, AgNPs coated with polyvinylpyrrolidone (PVP) interfere with respiratory syncytial virus and herpes simplex virus to inhibit their attachment to the cellular membrane rendering them incapable of infection [47,48]. Table 1 enlists the antiviral effects of AgNPs. Taken together, these reported findings demonstrate that AgNPs can contribute a considerable role in confronting COVID-19 pandemic.
Fig. 3.
The mechanism of antiviral activity of silver nanoparticles.
Table 1.
The table enlists the potent antiviral effects of silver nanoparticles (AgNPs) against various types of virus.
| Virus | Composition of AgNPs based formulation | Mechanism of Action | References |
|---|---|---|---|
| Human immunodeficiency virus type 1 (HIV-1) | PVP-coated AgNPs | Association with gp120 | [41] |
| H1N1, Influenza virus A (IFA) | AgNPs and chitosan-AgNPs composite, AgNPs decored with silica | Prevent binding of virus to the plasma membrane, modulating the viral membrane protein that inactivates the IFV-A | [42,75] |
| Hepatitis B virus (HBV) | AgNPs | Interaction with DNA and/or by attaching to viral particles | [44] |
| SARS-CoV-2 | AgNPs | Inhibits the virion entry inside the host | [36] |
| Monkeypox virus (MPV) | Polysaccharide-coated AgNPs and AgNPs | Inhibition of virus-host cell attachment and entry | [46] |
| Respiratory syncytial virus | AgNPs coated with PVP | Interference with viral attachment | [48] |
| Herpes simplex virus type 1 (HSV-1) | AgNPs coated with MES | Competition with binding site of virus | [47] |
3. AgNPs may restrain severe inflammatory response, cytokine storm and lung fibrosis in COVID-19
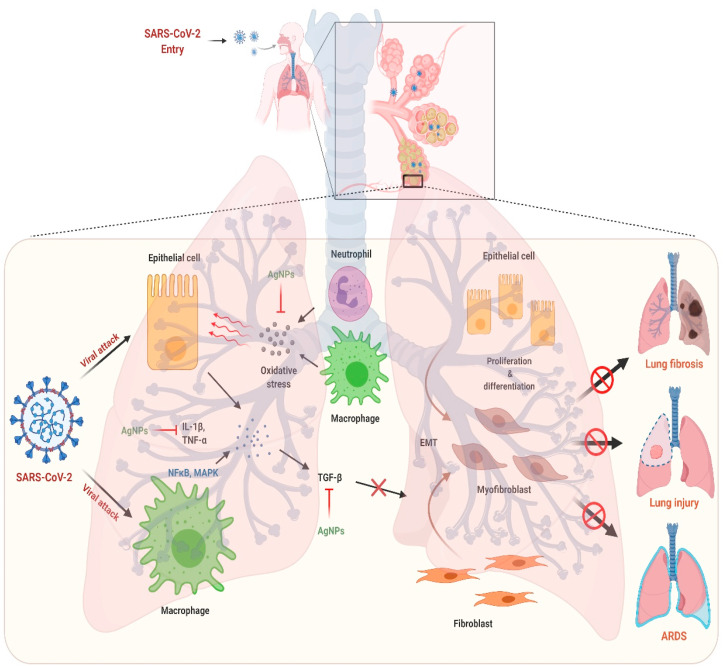
Severe inflammation and lung fibrosis are the typical hallmarks of the patient suffering from COVID-19. Cytokine storm causes the induction of systemic as well as pulmonary inflammation by driving the pro-inflammatory signaling which is also involved in lung fibrosis [17,49,50]. In this context, AgNPs can be a promising therapeutic candidate having potential anti-inflammatory and anti-fibrotic properties by virtue of its capability to abrogate the inflammatory cytokines by modulating their transcriptional activity. In different pre-clinical animal models, AgNPs reduced the concentrations of inflammatory cytokines like interleukin (IL)-1β, IL-6, IL-17, transforming growth factor beta (TGF-β), and tumor necrosis factor alpha (TNF-α) resulting in the reduction of inflammation and diminished fibrotic cascade by modulation of NFκB and MAPKinase pathways [[51], [52], [53]]. In a recent study, AgNPs showed anti-fibrotic effects in a dextran sodium sulphate (DSS) induced colitis model. AgNPs decreased fibrosis and collagen deposition by reducing the expression of profibrotic genes like Col 1a1 and Col 1a2 as evident from histological findings and confirmed by mRNA expression studies. Thus, AgNPs may halt the elevated levels of cytokines and cytokine mediated inflammation and fibrosis in the COVID-19 as shown in Fig. 4 [54].
Fig. 4.
The proposed mechanism of action of silver nanoparticles against COVID-19 induced lung injury and lung fibrosis. The figure was created with BioRender.com.
4. AgNPs as an immunomodulator
AgNPs have been referred to as a convincing therapeutic NPs possessing potent immunomodulatory features. Since ages, silver is used as a health supplement in Ayurvedic system of medicine to boost immune system and to strengthen the vital functions of body. AgNPs are known to induce immunogenic response within the biological hosts, together with the immune cells [55]. Bhol et al., reported that AgNPs induce the downregulation of IL-12 and TNF-α by promoting apoptosis in inflammatory cells [56]. Further, Morris et al., demonstrated marked decline in pro-inflammatory chemokines such as CCL2, CCL3, CCL5 and pro-inflammatory cytokines viz., IL-1α, IL-6, TNF-α upon AgNPs treatment [57]. Similarly, Greulich et al., reported that AgNPs treated peripheral blood mononuclear and mesenchymal stem cells (MSCs) exhibit decreased generation of cytokines like TNF-α, IL-6, IL-8, IL-11, interferon gamma (IFN-γ) and more weakly, IL-5 [58,59]. It has also been reported that AgNPs can remarkably reduce the load of viral infection by inhibiting nuclear translocation of NFκB [60]. Moreover, the extended intravenous administration of AgNPs of size 20 nm or 100 nm in rats exhibit several immunomodulatory effects such as declining body weight, enhanced spleen weight and size, and an inclination in both B cell and T cell count in spleen. However, high doses of AgNPs result in overall repression of natural killer (NK) cells activity, halting IL-10 and IFN-γ synthesis by spleen cells, enhanced IL-1β and decline in IL-6, IL-10 and TNF-α by spleen cells, upregulation of IgM and IgE in serum and an enhancement in blood neutrophilic granulocytes [61]. Additionally, the administration of AgNPs orally leads to suppression of proliferation of lymphocytes while promotes phagocytosis alongwith causing respiratory burst of monocytes and granulocytes [62]. Further, the intratracheal delivery of AgNPs in mice subsequently results in enhanced neutrophil counts and IL-1β levels. The exposure for 24, 48 or 72 h with AgNPs led to decline in the murine macrophage cell viability and a remarkable decrease in nitric oxide (NO) production was also observed [63]. It has been observed that murine macrophage exposure to AgNPs in vitro leads to significant decrease in cell viability with an observation of intracellular localization disclosing the distribution of Ag generally to high molecular weight proteins, and AgNPs colocalization with lysosomes. This study suggests that AgNPs are internalized by macrophages and transferred to the lysosomes where they induce inflammatory response [64]. The immunomodulatory effect of AgNPs is also dependent on size as seen in Raw 264.7 murine macrophages, where NPs of 20 nm diameter were found to stimulate ROS production, apoptotic cell death, decline in metabolic activity and generation of IL-6, IL-1β, IL-1α, G-CSF, TNF-α, MIP-2, MIP-1a and MIP-1b, then 80 nm or 113 nm diameter AgNPs [65]. Additionally, the size dependent effect of AgNPs was shown in human macrophages utilizing cDNA microarray analysis where 5 nm AgNPs leads to more potent proinflammatory cytokines and stress gene expression compared to the 100 nm AgNPs [66]. Similarly, the effect of 20 nm and 110 nm AgNPs has been examined in several epithelial cell lines and macrophages. It was found that 20 nm NPs were more destructive to both the cell lines because the smaller particles are easily dissolved in acidic phagolysosomes, that is accordant with silver induced toxicity [67]. Thus, AgNPs can be utilized as an adjuvant therapy in current treatment regimen to boost the immunity of COVID-19 patients.
5. AgNPs as a biosensor and theranostic agent
To combat the battle against COVID-19, biosensors are playing a pivotal role [68]. Currently, many novel biosensors are being developed for COVID-19 detection. Most of them are based on the antibodies detection by different types of assays, such as enzyme-linked immunosorbent assay (ELISA) and real time - polymerase chain reaction (RT-PCR). But these kits are expensive and time consuming. Further, in some cases, these kits give false-positive results. So, it is required to develop highly accurate, sensitive, and affordable sensors for early-stage diagnosis of COVID-19 [69]. AgNPs-based biosensors could be utilized in diagnostics and detection of viral infections precisely [70]. AgNPs can also be useful to reduce the impact and burden of disease by providing simple, faster and ready to use systems that do not need specific equipment and trained manpower [71]. AgNPs containing paper-based colorimetric DNA sensors for the detection of the middle east respiratory syndrome (MERS) have been reported earlier. A cationic pyrrolidinyl peptide nucleic acid (acpcPNA) probe was used which can easily detect the MERS-CoV DNA. The probe was positively charged with lysine moiety present at the carboxyl terminus so it can interact with the negatively charged AgNPs or targeted DNA. In case of binding to the AgNPs, it leads to NP aggregation and finally significant colour changes, while in case of the DNA binding, it prevents aggregation, without any colour change. Thus, it serves as a point-of-care diagnostic tool for viral DNA detection. Similarly, AgNPs could be used to detect SARS-CoV-2 DNA. AgNPs have Surface-enhanced Raman scattering (SERS) properties and can produce up to 1014 times enhancement in the signal as compared to the conventional Raman scattering. Additionally, the use of molecular beacons and aptamers along with AgNPs could also be a potential alternative to enhance the sensitivity and specificity of the detection [72]. We have summarized some of the AgNPs based biosensors for viral detection in Table 2 .
Table 2.
Summary of some of the AgNPs based biosensors.
| S.No | Organism | Disease | Detection | Sensor type | Ref |
|---|---|---|---|---|---|
| 1. | Hepatitis B | Liver infection | DNA | Electrochemical | [76] |
| 2. | WNV | Neurological disease | Antibody | Optical | [77] |
| 3. | Hepatitis B | Liver infection | Aptamer | Electrochemical | [78] |
| 4. | Influenza (H5N1) | Breathing problems and pneumonia | Aptamer | Optical | [79] |
| 5. | HIV-1 | Attacks immune system | DNA | Surface-enhanced Raman scattering (SERS) | [80] |
| 6. | Hepatitis C | Liver cancer and lymphomas in humans | Antigen | Immunosensor | [81] |
| 7. | Hepatitis B | Liver infection | DNA | Fluorescent microarray | [82] |
| 8. | Avian influenza virus H7 | Bird flu | Antibody | Electrochemical | [83] |
| 9. | Bacteria | Infectious diseases caused by contaminated water | Bacteria | Fluorescence | [84] |
| 10. | Pseudomonas aeruginosa | Opportunistic infections such as pneumonia etc | Antibody | Fluorescence immunoassay | [85] |
AgNPs also exhibit theranostic applications as they possess distinctive electronic, catalytic, photonic, and therapeutic features. They bear huge variations in their synthesis, specially varied shape and size along with surface functionalization via direct chemisorption, physisorption, covalent binding or affinity interactions, elicit their extensive and escalated usage at various platforms for theranostic purposes. Mukherjee et al., showed multifunctional biological features of AgNPs such as anti-bacterial, anti-viral, anti-cancer, drug delivery vehicle, and bioimaging. They demonstrated that AgNPs exhibit bright red fluorescence inside the cancer cells which can be used to examine the drug localization. In this way, AgNPs simultaneously deliver the drug, act as anti-cancer agent and perform diagnosis [73]. Similarly, Kim et al., showed AgNPs as theranostic antibacterial agents as they kill Pseudomonas aeruginosa and Staphylococcus aureus and simultaneously exhibit photoluminiscent monitoring [74].
6. Conclusions
AgNPs provide unique means to inhibit the SARS-CoV-2 virus growth and at the same time prevent the secondary microbial infections owing to its potent antimicrobial effects. Antiviral effects of AgNPs have been demonstrated against SARS-CoV-2 with NPs of 2–15 nm size. Further, AgNPs might curb the progression of cytokine storm, inflammatory signaling, reduction in pulmonary insufficiency, and modulation of the EMT signaling cascade. Moreover, AgNPs provides versatile avenues for development of highly sensitive biosensors. We sincerely hope that AgNPs, may play an extremely vital role in winning the battle against the COVID-19 pandemic in the near future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are grateful to the Dean, Faculty of Veterinary Science, P. V. N. R. Telangana Veterinary University (PVNRTVU), Hyderabad.
References
- 1.Li Q., et al. Early transmission dynamics in wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab Med. 2020;144(7):799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 3.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belouzard S., et al. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sivasankarapilai V.S., Madaswamy S.L., Dhanusuraman R. Role of nanotechnology in facing SARS-CoV-2 pandemic: solving crux of the matter with a hopeful arrow in the quiver. Sensors International. 2021:100096. doi: 10.1016/j.sintl.2021.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh V., et al. Critical neurological features of COVID-19: role of imaging methods and biosensors for effective diagnosis. Sensors International. 2021:100098. doi: 10.1016/j.sintl.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhaundiyal A., et al. Life Sciences; 2020. Is Highly Expressed ACE 2 in Pregnant Women “A Curse” in Times of COVID-19 Pandemic? p. 118676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal K.M., et al. Study and overview of the novel corona virus disease (COVID-19) Sensors International. 2020;1:100037. doi: 10.1016/j.sintl.2020.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang J., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zumla A., et al. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395(10224):e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrahari R., et al. Update vision on COVID-19: structure, immune pathogenesis, treatment and safety assessment. Sensors International. 2021;2:100073. doi: 10.1016/j.sintl.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burki T.K. The Russian vaccine for COVID-19. The Lancet Respiratory Medicine. 2020;8(11):e85–e86. doi: 10.1016/S2213-2600(20)30402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorp, H.H., A Dangerous Rush for Vaccines, 2020, American Association for the Advancement of Science. [DOI] [PubMed]
- 15.Codeluppi L., et al. Facial palsy during the COVID-19 pandemic. Brain and behavior. 2020 doi: 10.1002/brb3.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana I., et al. Can bilirubin nanomedicine become a hope for the management of COVID-19? Med. Hypotheses. 2021;149:110534. doi: 10.1016/j.mehy.2021.110534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allawadhi P., et al. Nanoceria as a possible agent for the management of COVID-19. Nano Today. 2020;35:100982. doi: 10.1016/j.nantod.2020.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khurana A., et al. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 19.Khurana A., et al. Superoxide dismutase mimetic nanoceria restrains cerulein induced acute pancreatitis. Nanomedicine. 2019;14(14):1805–1825. doi: 10.2217/nnm-2018-0318. [DOI] [PubMed] [Google Scholar]
- 20.Kumar G.S., et al. Selenium nanoparticles involve HSP-70 and SIRT1 in preventing the progression of type 1 diabetic nephropathy. Chem. Biol. Interact. 2014;223:125–133. doi: 10.1016/j.cbi.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Kirwale S., et al. Selenium nanoparticles induce autophagy mediated cell death in human keratinocytes. Nanomedicine. 2019;14(15):1991–2010. doi: 10.2217/nnm-2018-0397. [DOI] [PubMed] [Google Scholar]
- 22.Khurana A., et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021:101142. doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De M., Ghosh P.S., Rotello V.M. Applications of nanoparticles in biology. Adv. Mater. 2008;20(22):4225–4241. [Google Scholar]
- 24.Allawadhi P., et al. Isoproterenol-induced cardiac ischemia and fibrosis: plant-based approaches for intervention. Phytother Res. 2018;32(10):1908–1932. doi: 10.1002/ptr.6152. [DOI] [PubMed] [Google Scholar]
- 25.Mansour H., Eid M., El-Arnaouty M. Effect of silver nanoparticles synthesized by gamma radiation on the cytotoxicity of doxorubicin in human cancer cell lines and experimental animals. Hum. Exp. Toxicol. 2018;37(1):38–50. doi: 10.1177/0960327116689717. [DOI] [PubMed] [Google Scholar]
- 26.Hebeish A., et al. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int. J. Biol. Macromol. 2014;65:509–515. doi: 10.1016/j.ijbiomac.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 27.Rawat P.S., et al. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021;139:111708. doi: 10.1016/j.biopha.2021.111708. [DOI] [PubMed] [Google Scholar]
- 28.Alkaladi A., Abdelazim A.M., Afifi M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2014;15(2):2015–2023. doi: 10.3390/ijms15022015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burdușel A.-C., et al. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials. 2018;8(9):681. doi: 10.3390/nano8090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drake P.L., Hazelwood K.J. Exposure-Related health effects of silver and silver compounds: a review. Ann. Occup. Hyg. 2005;49(7):575–585. doi: 10.1093/annhyg/mei019. [DOI] [PubMed] [Google Scholar]
- 31.Zilberman M., Elsner J.J. Antibiotic-eluting medical devices for various applications. J. Contr. Release. 2008;130(3):202–215. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Atiyeh B.S., et al. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33(2):139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Use of silver in the prevention and treatment of infections: silver review. Surg. Infect. 2013;14(1):8–20. doi: 10.1089/sur.2011.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy A., et al. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5):2673–2702. doi: 10.1039/c8ra08982e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.S., et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Jeremiah S.S., et al. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020;533(1):195–200. doi: 10.1016/j.bbrc.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser J., et al. An in vitro study of the anti-microbial efficacy of a 1% silver sulphadiazine and 0.2% chlorhexidine digluconate cream Silvazine (TM), 1% silver sulphadiazine cream Flamazine (TM) and a silver coated dressing Acticoat (TM). Burns : journal of the International Society for Burn Injuries. 2004;30:35–41. doi: 10.1016/j.burns.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Salleh A., et al. The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials. 2020;10(8):1566. doi: 10.3390/nano10081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auría-Soro C., et al. Interactions of nanoparticles and biosystems: microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials. 2019;9(10):1365. doi: 10.3390/nano9101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Q.-s., et al. Enhancing the targeting ability of nanoparticles via protected copolymers. Nanoscale. 2020;12(14):7804–7813. doi: 10.1039/d0nr01176b. [DOI] [PubMed] [Google Scholar]
- 41.Elechiguerra J.L., et al. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005;3(1):6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori Y., et al. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res Lett. 2013;8(1):93. doi: 10.1186/1556-276X-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park S., et al. Inactivation of influenza A virus via exposure to silver nanoparticle-decorated silica hybrid composites. Environ. Sci. Pollut. Control Ser. 2018;25(27):27021–27030. doi: 10.1007/s11356-018-2620-z. [DOI] [PubMed] [Google Scholar]
- 44.Speshock J.L., et al. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010;8:19. doi: 10.1186/1477-3155-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangayasami A., et al. Influence of nanotechnology to combat against COVID-19 for global health emergency: a review. Sensors International. 2021;2:100079. doi: 10.1016/j.sintl.2020.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers J.V., et al. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Research Letters. 2008;3(4):129. [Google Scholar]
- 47.Baram-Pinto D., et al. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjugate Chem. 2009;20(8):1497–1502. doi: 10.1021/bc900215b. [DOI] [PubMed] [Google Scholar]
- 48.Sun L., et al. Silver nanoparticles inhibit replication of respiratory syncytial virus. J. Biomed. Nanotechnol. 2008;4(2):149–158. [Google Scholar]
- 49.Allawadhi P., et al. Potential of electric stimulation for the management of COVID-19. Med. Hypotheses. 2020 Nov;144:110259. doi: 10.1016/j.mehy.2020.110259. Epub 2020 Sep. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khurana A., et al. Yttrium oxide nanoparticles reduce the severity of acute pancreatitis caused by cerulein hyperstimulation. Nanomed. Nanotechnol. Biol. Med. 2019;18:54–65. doi: 10.1016/j.nano.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Wong K.K., et al. Further evidence of the anti-inflammatory effects of silver nanoparticles. ChemMedChem. 2009;4(7):1129–1135. doi: 10.1002/cmdc.200900049. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Carter D.A., et al. Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Sci. Rep. 2017;7(1):42871. doi: 10.1038/srep42871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hebeish A., et al. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int. J. Biol. Macromol. 2014;65:509–515. doi: 10.1016/j.ijbiomac.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 54.Asgharzadeh F., et al. Therapeutic effects of silver nanoparticle containing sulfasalazine on DSS-induced colitis model. J. Drug Deliv. Sci. Technol. 2020:102133. [Google Scholar]
- 55.Lansdown, A., Silver in Health Care: Antimicrobial Effects and Safety in Use. [DOI] [PubMed]
- 56.Bhol K.C., Alroy J., Schechter P.J. Anti-inflammatory effect of topical nanocrystalline silver cream on allergic contact dermatitis in a Guinea pig model. Clin. Exp. Dermatol. 2004;29(3):282–287. doi: 10.1111/j.1365-2230.2004.01515.x. [DOI] [PubMed] [Google Scholar]
- 57.Morris D., et al. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses. 2019;11(8) doi: 10.3390/v11080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greulich C., et al. Cell type-specific responses of peripheral blood mononuclear cells to silver nanoparticles. Acta Biomater. 2011;7(9):3505–3514. doi: 10.1016/j.actbio.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 59.Greulich C., et al. Studies on the biocompatibility and the interaction of silver nanoparticles with human mesenchymal stem cells (hMSCs) Langenbeck's Arch. Surg. 2009;394(3):495–502. doi: 10.1007/s00423-009-0472-1. [DOI] [PubMed] [Google Scholar]
- 60.Abdellatif A.A.H., Rasheed Z. Silver citrate nanoparticles inhibit PMA-induced TNFα expression via deactivation of NF-κB activity in human cancer cell-lines. MCF-7. 2020;15:8479–8493. doi: 10.2147/IJN.S274098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Jong W.H., et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34(33):8333–8343. doi: 10.1016/j.biomaterials.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 62.Malaczewska J. The in vitro effect of commercially available noble metal nanocolloids on the splenocyte proliferative response and cytokine production in mice. Pol. J. Vet. Sci. 2014;17(1) doi: 10.2478/pjvs-2014-0005. [DOI] [PubMed] [Google Scholar]
- 63.Shavandi Z., Ghazanfari T., Moghaddam k.N. In vitro toxicity of silver nanoparticles on murine peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011;33(1):135–140. doi: 10.3109/08923973.2010.487489. [DOI] [PubMed] [Google Scholar]
- 64.Arai Y., Miyayama T., Hirano S. Difference in the toxicity mechanism between ion and nanoparticle forms of silver in the mouse lung and in macrophages. Toxicology. 2015;328:84–92. doi: 10.1016/j.tox.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Giovanni M., et al. Pro-inflammatory responses of RAW264. 7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. J. Hazard Mater. 2015;297:146–152. doi: 10.1016/j.jhazmat.2015.04.081. [DOI] [PubMed] [Google Scholar]
- 66.Lim D.-H., et al. The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress genes in human macrophages using cDNA microarray analysis. Biomaterials. 2012;33(18):4690–4699. doi: 10.1016/j.biomaterials.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Hamilton R.F., Buckingham S., Holian A. The effect of size on Ag nanosphere toxicity in macrophage cell models and lung epithelial cell lines is dependent on particle dissolution. Int. J. Mol. Sci. 2014;15(4):6815–6830. doi: 10.3390/ijms15046815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Della Ventura B., et al. medRxiv; 2020. Colorimetric Test for Fast Detection of SARS-CoV-2 in Nasal and Throat Swabs; p. 2020. 08.15.20175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagraik R., et al. Amalgamation of biosensors and nanotechnology in disease diagnosis: mini-review. Sensors International. 2021;2:100089. [Google Scholar]
- 70.Udugama B., et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 71.Cohen J., Kupferschmidt K. Labs scramble to produce new coronavirus diagnostics. Science. 2020;367(6479):727. doi: 10.1126/science.367.6479.727. [DOI] [PubMed] [Google Scholar]
- 72.Teengam P., et al. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 2017;89(10):5428–5435. doi: 10.1021/acs.analchem.7b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjee S., et al. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system) Theranostics. 2014;4(3):316–335. doi: 10.7150/thno.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim T., et al. Composite porous silicon–silver nanoparticles as theranostic antibacterial agents. ACS Appl. Mater. Interfaces. 2016;8(44):30449–30457. doi: 10.1021/acsami.6b09518. [DOI] [PubMed] [Google Scholar]
- 75.Lu L., et al. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008;13(2):253–262. [PubMed] [Google Scholar]
- 76.Li X., Scida K., Crooks R.M. Detection of hepatitis B virus DNA with a paper electrochemical sensor. Anal. Chem. 2015;87(17):9009–9015. doi: 10.1021/acs.analchem.5b02210. [DOI] [PubMed] [Google Scholar]
- 77.Neng J., et al. Surface-enhanced Raman scattering (SERS) detection of multiple viral antigens using magnetic capture of SERS-active nanoparticles. Biosens. Bioelectron. 2013;41:316–321. doi: 10.1016/j.bios.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 78.Niu S., et al. Sensitive DNA biosensor improved by Luteolin copper (II) as indicator based on silver nanoparticles and carbon nanotubes modified electrode. Anal. Chim. Acta. 2009;651(1):42–47. doi: 10.1016/j.aca.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 79.Pang Y., et al. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core–shell nanoparticles metal-enhanced fluorescence (MEF) Biosens. Bioelectron. 2015;66:527–532. doi: 10.1016/j.bios.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 80.Wabuyele M.B., Vo-Dinh T. Detection of human immunodeficiency virus type 1 DNA sequence using plasmonics nanoprobes. Anal. Chem. 2005;77(23):7810–7815. doi: 10.1021/ac0514671. [DOI] [PubMed] [Google Scholar]
- 81.Valipour A., Roushani M. Using silver nanoparticle and thiol graphene quantum dots nanocomposite as a substratum to load antibody for detection of hepatitis C virus core antigen: electrochemical oxidation of riboflavin was used as redox probe. Biosens. Bioelectron. 2017;89:946–951. doi: 10.1016/j.bios.2016.09.086. [DOI] [PubMed] [Google Scholar]
- 82.Jin F., Li H., Xu D. Enzyme-free fluorescence microarray for determination of hepatitis B virus DNA based on silver nanoparticle aggregates-assisted signal amplification. Anal. Chim. Acta. 2019;1077:297–304. doi: 10.1016/j.aca.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 83.Huang J., et al. Silver nanoparticles coated graphene electrochemical sensor for the ultrasensitive analysis of avian influenza virus H7. Anal. Chim. Acta. 2016;913:121–127. doi: 10.1016/j.aca.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 84.Roh S.G., et al. Photoluminescence-tunable fluorescent carbon dots-deposited silver nanoparticle for detection and killing of bacteria. Mater. Sci. Eng. C. 2019;97:613–623. doi: 10.1016/j.msec.2018.12.070. [DOI] [PubMed] [Google Scholar]
- 85.Ellairaja S., et al. Novel pyrimidine tagged silver nanoparticle based fluorescent immunoassay for the detection of Pseudomonas aeruginosa. J. Agric. Food Chem. 2017;65(8):1802–1812. doi: 10.1021/acs.jafc.6b04790. [DOI] [PubMed] [Google Scholar]