Abstract
Background
Many studies confirmed an association between COVID-19 and venous thromboembolism (VTE). Whether the risk of VTE significantly differed between COVID-19 cohorts and non-COVID-19 cohorts with similar disease severity remains unknown.
Objectives
The aim of this systematic review with meta-analysis was to compare the rate of VTE between COVID-19 and non-COVID-19 cohorts with similar disease severity.
Methods
A systematic literature search (MEDLINE, Embase and Google Scholar) was conducted from January 1, 2020 to March 31, 2021 to identify studies reporting VTE in COVID-19. Relative risks (RR) were estimated for the effect measure with 95% confidence intervals.
Results
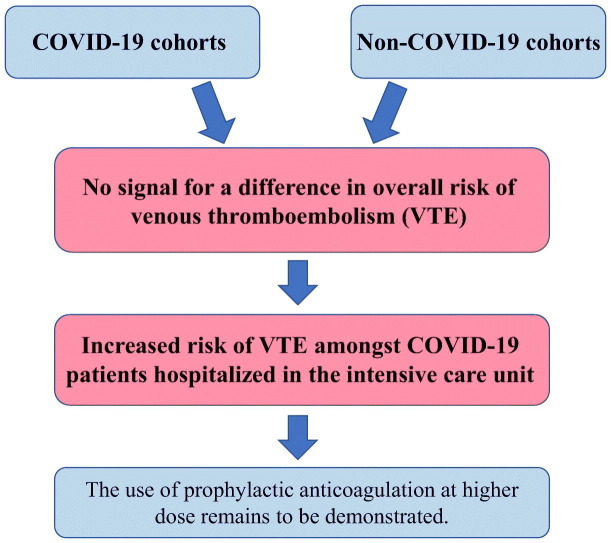
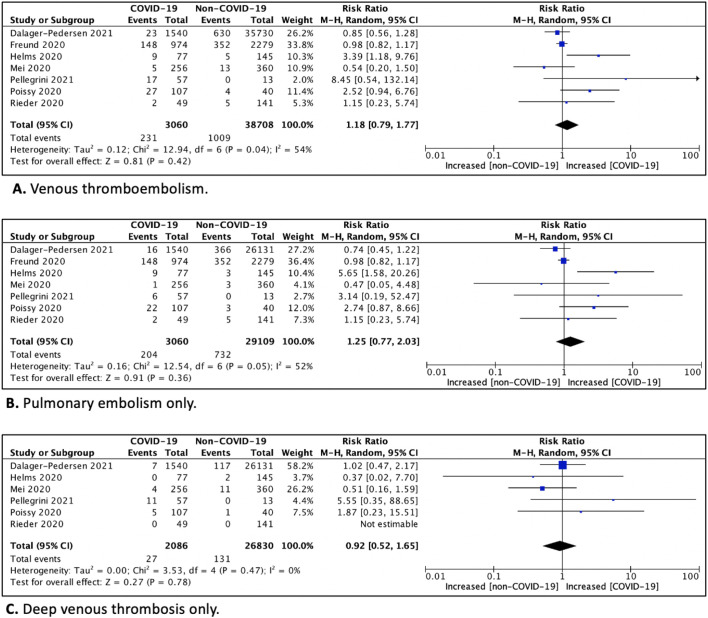
Seven studies (41,768 patients) evaluated VTE in COVID-19 cohorts compared to non-COVID-19 cohorts. The overall risk of VTE (RR 1.18; 95%CI 0.79–1.77; p = 0.42; I2 = 54%), pulmonary embolism (RR 1.25; 95%CI 0.77–2.03; p = 0.36; I2 = 52%) and deep venous thrombosis (RR 0.92; 95%CI 0.52–1.65; p = 0.78; I2 = 0%) did not significantly differ between COVID-19 and non-COVID-19 cohorts. However, subgroup analyses suggested an increased risk of VTE amongst CODID-19 versus non COVID-19 cohorts when only patients hospitalized within the intensive care unit (ICU) were considered (RR 3.10; 95%CI 1.54–6.23), which was not observed in cohorts of predominantly non-ICU patients (RR 0.95; 95%CI 0.81–1.11) (Pinteraction = 0.001).
Conclusion
There was no signal for a difference in VTE in COVID-19 cohorts compared to non-COVID-19 cohorts, except for the subgroup of patients hospitalized in the ICU. These results should be viewed as exploratory and further studies are needed to confirm these results.
Keywords: COVID-19, Venous thromboembolism, Anticoagulation, Pulmonary embolism
Graphical abstract
1. Introduction
In the early stages of the coronavirus disease 2019 (COVID-19) pandemic, pulmonary vascular abnormalities were reported on chest computed tomography (CT) of patients infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Subsequent reports suggested a high incidence of venous thromboembolism (VTE), reaching up to 6–69% in cohorts of critically ill patients [2,3]. This amazingly high risk of COVID-19-associated VTE was attributed to the cytokine storm and hyperinflammation frequently observed in these patients, resulting in sepsis-induced coagulopathy, disseminated intravascular coagulation [4], platelets dysfunction [5], endothelialitis, in-situ thrombosis and micro-thrombosis [6]. Since then, many studies confirmed an association between COVID-19 and VTE [7]. In an attempt to prevent VTE, several studies evaluated high-dose prophylaxis or even therapeutic anticoagulant doses in patients hospitalized for COVID-19 [[8], [9], [10]]. Despite the lack of quality published evidence, many institutional protocols even adopted intermediate- or therapeutic intensity dose strategy for thromboprophylaxis based on local experience and the assumption of a significant risk for VTE [11,12].
It is noteworthy, however, that both human and animal studies have shown that pulmonary thrombosis is common in sepsis-induced acute respiratory distress syndrome (ARDS) [13], pneumonia [14,15] and severe influenza infections [16], with the complex interplay of inflammatory and coagulation abnormalities contributing to the phenomenon of immunothrombosis [17]. Whether the high-risk of VTE reported in these COVID-19 series is specifically related to the SARS-CoV-2 tropism for the endothelium and subsequent coagulopathy, or is partly explained by inherent bias remains elusive. The aim of this systematic review with meta-analysis was thus to assess whether the risk of VTE significantly differed between COVID-19 cohorts and non-COVID-19 cohorts with similar disease severity.
2. Methods
The methods of this systematic review and meta-analysis are in accordance with “Cochrane Handbook for Systematic Reviews of Interventions” [18]. We wrote this report according to the recommendations of the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) [19] and the referred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [20].
2.1. Search strategy
This is a sub-analysis of our protocol registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO, registration No. CRD42020183842) that aimed to determine the prevalence of thrombotic events in patients with COVID-19 [7]. We initially searched MEDLINE, Embase and Google Scholar between January 1, 2020 and September 30, 2020. The grey literature was explored. There was no restriction on the language and the type of publication. Literature search was updated for this sub-analysis up to March 31, 2021.
2.2. Study selection
In the primary analysis, we included studies presenting these criteria: i) cohort of >10 patients, ii) patients with COVID-19 (positive reverse transcription polymerase chain reaction or positive CT-scan in patients with suggestive gestalt); iii) data reporting one of the outcomes of interest. For this subanalysis, we identified case-control studies comparing the rate of VTE in COVID-19 and non-COVID-19 patients. Two reviewers (B.K.T and J.C.L.) independently reviewed titles and abstracts of all articles related to the subject, as well as full papers when pertinent for a final decision concerning its inclusion in the meta-analysis. Two reviewers (V.M. and J.C.L.) independently extracted relevant information from all selected papers. Disagreements were resolved by consensus or by consulting a third reviewer (S.P.).
2.3. Outcomes
The outcome for the sub-analysis was the rate of VTE in COVID-19 cohorts compared to non-COVID-19 cohorts. VTE included pulmonary embolism (PE) and/or deep venous thrombosis (DVT). VTE could be diagnosed by symptomatic or systematic testing. The rate of PE only and DVT only were also individually assessed.
2.4. Assessment of methodological quality
The risk of bias of the selected studies was evaluated independently by two reviewers (V.M. and S.P.) using the methodological index for non-randomized studies (MINORS) for observational studies [21]. Publication bias was evaluated visually by a funnel plot. The strength of the body evidence was evaluated independently by two reviewers (V.M. and S.P.) for each outcome according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system [22].
2.5. Statistical analysis
Analyses were performed using the Review Manager (Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The meta-analysis comparing the rate of VTE in COVID-19 cohorts to non-COVID-19 cohorts was conducted using the Mantel-Haenszel method based on a random-effects model. Relative risks (RR) were estimated with a 95% confidence intervals (CI) and a p value. A p value <0.05 was considered statistically significant. Forest plots were created. Statistical heterogeneity was identified by using I2. I2 > 50% was considered as substantial statistically heterogeneity. We also planned subgroup analyses to investigate sources of heterogeneity in the main analysis according to hospitalization settings (cohorts of ICU patients only vs predominantly non-ICU patients), presence or absence of ventilator support, study types (prospective vs retrospective) and presence or absence of thromboprophylaxis.
3. Results
3.1. Characteristics of the selected studies
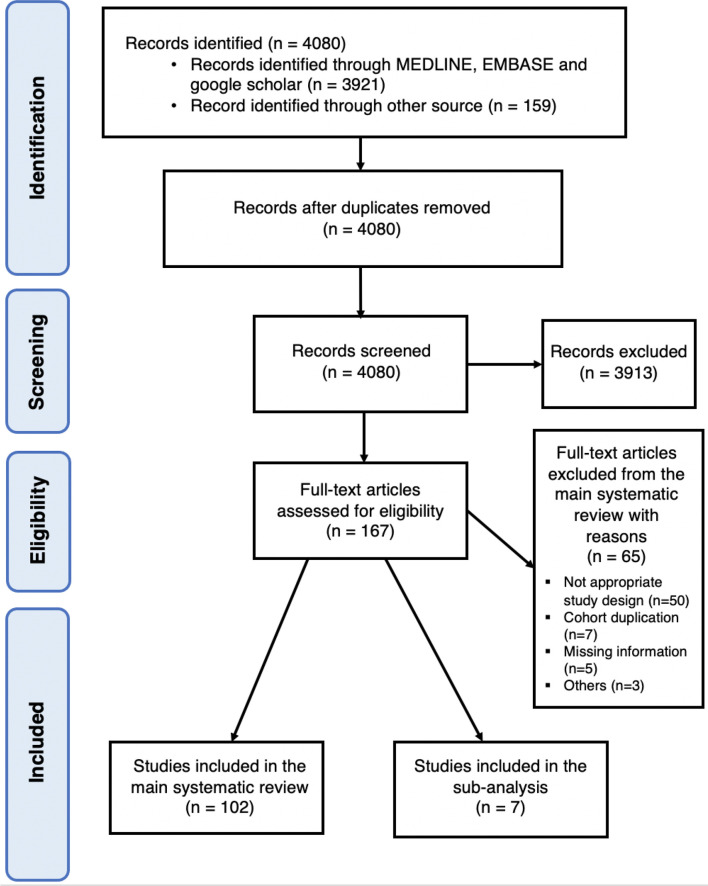
We identified 4080 citations of which 167 were retained for full-text evaluation. Seven articles [[23], [24], [25], [26], [27], [28], [29]] (41,768 patients) reported the rate of VTE in COVID-19 cohorts compared to non-COVID-19 cohorts (Fig. 1 ). The characteristics of the studies are reported in Table 1 . Three were prospective studies [23,27,29] and 4 were retrospective studies [[24], [25], [26],28]. One study matched COVID-19 and non-COVID-19 patients using a 1:3 ratio [23]. Three studies included 100% patients from the intensive care unit (ICU) [23,25,29] whereas 2 studies enrolled the patients from the emergency department [26,27] and 2 studies did not reported it [24,28]. One study compared patients with ARDS related or not to COVID-19 [23], whereas COVID-19 patients were compared to patients with community-acquired pneumonia [24], influenza [25,28] and non-COVID-19 infections [[26], [27], [28], [29]]. Within each study, groups were comparable in terms of ICU admission rates, sex, age and methods for VTE diagnosis. Patients were all on prophylactic anticoagulation in 2 studies [24,25], 22–38% of the patients were on therapeutic anticoagulation in 2 studies [23,27], 1 study [29] included patients on prophylactic, intermediate or therapeutic dose anticoagulation and 2 studies [26,28] did not report this data. VTE was diagnosed based on symptomatic testing in 4 studies [23,[25], [26], [27]], in selected patients considered at high-risk based on institutional standards in 1 study [24], in symptomatic testing for PE and systematic screening for DVT in 1 study [29] and was not reported in 1 study [28].
Fig. 1.
Study selection.
Table 1.
Characteristics of studies evaluating VTE in COVID-19 cohorts.
| Study | Country | Design | COVID-19 status | Patients in ICU during the study (%) | Number of patients | Mean follow-up (days) | Male sex (%) | Median age (Q1;Q3) | Diagnosis of thrombotic event | Prophy-lactic dose A/C (%) | Interme-diate dose A/C (%) | Thera-peutic dose A/C (%) | D-dimer (mg/L) | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dalager-Pedersen et al. (2021) [28] | Denmark | Retrospective cohort study | COVID-19 | NR | 1540 | 30 | 56.6 | 72.0 (58.0;81.0) | NR | NR | NR | NR | NR | NR |
| Non-COVID-19 | NR | 26,131 | 30 | 50.1 | 68.0 (48.0;78.0) | NR | NR | NR | NR | NR | NR | |||
| Influenza | NR | 9599 | 30 | 48.8 | 70.0 (59.0;80.0) | NR | NR | NR | NR | NR | NR | |||
| Freund et al. (2020) [26] | France, Spain, Belgium, Italy, Chile, Canada | Retrospective cohort study | COVID-19 | 0 | 974 | NR | 48.0a | 61.0 ± 19.0a, b | Symptomatic testing | NR | NR | NR | NR | NR |
| Non-COVID-19 | 0 | 2279 | NR | 48.0a | 61.0 ± 19.0 a, b | Symptomatic testing | NR | NR | NR | NR | NR | |||
| Helms et al. (2020) [23] | France | Prospective (COVID-19) and “historical prospective” (non-COVID-19) cohort study | COVID-19 ARDS | 100 | 77c | 7d | 81.8 | 68.0 (61.0;75.0) | Symptomatic testing | 77.9 | 0.0 | 22.1 | NR | NR |
| Non-COVID-19 ARDS | 100 | 145c | NR | 77.2 | 72.0 (61.0;80.0) | Symptomatic testing | 75.9 | 0.0 | 24.1 | NR | NR | |||
| Mei et al. (2020) [24] | China | Retrospective cohort study | COVID-19 | NR | 256 | NR | 51.2 | 55.5 (range 0.5–87.0) | Testing when Padua score > 4 | 100e | 0.0 | 0.0 | 0.510 | 16 |
| Community acquired pneumonia | NR | 360 | NR | 58.6 | 61.0 (range 15.0–95.0) | Testing when Padua score > 4 | 100e | 0.0 | 0.0 | 0.590 | 14 | |||
| Pellegrini et al. (2021) [29] | Brazil | Prospective cohort study | COVID-19 | 100 | 57 | NRf | 52.6 | 56.0 ± 13.0b | Symptomatic testing for PE and systematic screening for DVT | 19.3 | 77.2 | 3.5 | 0.001 | 25 |
| Non-COVID-19 | 100 | 13 | NRf | 53.8 | 57.0 ± 20.0b | Symptomatic testing for PE and systematic screening for DVT | 53.8 | 23.1 | 2.7 | 0.002 | 3 | |||
| Poissy et al. (2020) [25] | France | Retrospective cohort study | COVID-19 | 100 | 107 | NR | NR | NR | Symptomatic testing | 100g | 0.0 | NR | NR | NR |
| Influenza | 100 | 40 | NR | NR | NR | Symptomatic testing | 100g | 0.0 | NR | NR | NR | |||
| Rieder et al. (2020) [27] | Germany | Prospective cohort study | COVID-19 | 16.3 | 49 | 30 | 61.2 | 60.0 (48.5;71.5) | Symptomatic testing | NR | NR | 24.3 | 1.100 | 3 |
| Non-COVID-19 | 7.1 | 141 | 30 | 50.4 | 60.0 (43.5;76.5) | Symptomatic testing | NR | NR | 37.6 | 0.800 | 7 |
A/C: anticoagulation; COVID-19: coronavirus disease 2019; DVT: deep venous thrombosis; ICU: intensive care unit; NR: not reported; PE: pulmonary embolism; VTE: venous thromboembolism.
Data for the whole studied population.
Mean ± standard deviation.
COVID-19 and non-COVID-19 patients were matched in a ratio 1:3 based on the propensity scores generated by the multivariable logistic regression model. Independent variables used in the multivariable logistic regression are age, sex, medical history of malignancies, cardiovascular diseases, cerebrovascular diseases, venous thrombo-embolic event, immune diseases, chronic liver diseases, chronic renal diseases, respiratory diseases, Simplified Acute Physiology Score II, Sequential Organ Failure Assessment Score, PaO2/FiO2 on ICU admission, anticoagulant treatment and extracorporeal membrane oxygenation.
At least.
Mechanical intermittent pneumatic compression device if contraindicated to anticoagulants.
Follow-up until hospital discharge or death.
2 patients were on therapeutic anticoagulation for a history of DVT and atrial fibrillation at the time of PE diagnosis, not mentioned the percentage of therapeutic anticoagulation in patients without PE diagnosis.
3.2. VTE occurrence
The cumulative relative risk (RR) estimate for VTE amongst COVID-19 patients was 1.18 (95%CI 0.79–1.77; p = 0.42; I2 = 54%) compared to non-COVID-19 patients. (Fig. 2A). Consistently, no statistically significant results were seen in patients with COVID-19 compared to non-COVID-19 patients in regards of PE only (RR 1.25; 95%CI 0.77–2.03; p = 0.36; I2 = 52%) (Fig. 2B) and DVT only (RR 0.92; 95%CI 0.52–1.65; p = 0.78; I2 = 0%) (Fig. 2C). Subgroup analyses, suggested an increased risk of VTE amongst COVID-19 versus non-COVID-19 patients hospitalized within the ICU (RR 3.10; 95%CI 1.54–6.23), which was not observed in cohorts of predominantly non-ICU patients (RR 0.95; 95%CI 0.81–1.11) (Pinteraction = 0.001). The risk of VTE in COVID-19 versus non-COVID-19 patients was also increased in prospective studies only (RR 2.74; 95%CI 1.18–6.40) (Table 2 ). However, no difference was seen in regards of the presence or absence of ventilator support and presence or absence of thromboprophylaxis.
Fig. 2.
Forest plot and relative risk (RR) for venous thromboembolism (A), pulmonary embolism only (B) and deep venous thrombosis only (C) in COVID-19 cohorts compared to non-COVID-19 cohorts.
Table 2.
Subgroup analysis to explore sources of heterogeneity in estimate of venous thromboembolism occurrence.
| Number of studies | Number of patients | Risk relative (95% confidence interval) | I2 | p-value (pinteraction) | |
|---|---|---|---|---|---|
| Study settings | |||||
| ICU only | 3 | 439 | 3.10 (1.54–6.23) | 0% | 0.001 |
| Predominantly non-ICU | 4 | 41,329 | 0.95 (0.81–1.11) | 0% | 0.26 |
| Ventilator support | |||||
| Presence | 4 | 475 | 1.72 (0.63–4.67) | 53% | |
| Absence | 5 | 41,293 | 0.96 (0.78–1.18) | 7% | |
| Type of studies | 0.03 | ||||
| Prospective | 3 | 482 | 2.74 (1.18–6.40) | 1% | |
| Retrospective | 4 | 41,286 | 0.97 (0.70–1.35) | 44% | |
| Anticoagulation | 0.06 | ||||
| Prophylactic, intermediate and/or therapeutic dose | 3 | 482 | 2.74 (1.18–6.40) | 1% | |
| Prophylactic dose only | 2 | 763 | 1.17 (0.26–5.32) | 78% | |
| Not reported | 2 | 40,523 | 0.96 (0.82–1.13) | 0% |
3.3. Risk of bias within studies, publication bias and quality evidence
The median MINORS score was 12 (range 9–19) for the studies comparing VTE in COVID-19 cohorts compared to non-COVID-19 cohorts (Table S1). Visual inspection of the funnel plots of VTE occurrence were exploratory given the limited number of studies and yielded no publication bias (Fig. S1). The strength of evidence based on the GRADE methodology was considered very low for VTE occurrence (Table S2).
4. Discussion
The present meta-analysis suggests that while VTE are frequent amongst patients hospitalized for COVID-19, the VTE occurrence appears comparable between COVID-19 patients and those with non-COVID-19 infections. However, patients with COVID-19 hospitalized in the ICU may to be at the highest risk of developing VTE, being significantly higher than non-COVID-19 patients hospitalized in the ICU. Severe COVID-19 requiring ICU admission may thus be a risk factor for developing VTE.
In the early stages of the COVID-19 pandemics, anecdotal reports described cases of PE diagnosed concomitantly with COVID-19 [1,30]. Disseminated intravascular coagulation, microthrombi in different organs including the pulmonary circulation [31] and VTE were subsequently documented in a significant proportion of critically ill patients. High levels of D-dimers were repeatedly shown to be associated with the need for ICU admission and mortality amongst COVID-19 patients [32]. This is not surprising since lung coagulopathy is relevant in the pathogenesis of ARDS [33]. The cytokines storm and pulmonary microthrombi observed with COVID-19 are thus consistent with the immunothrombosis model, which highlights the bidirectional relationship between the immune system, inflammation and thrombin generation during severe infection [17]. Importantly, these phenomena may be enhanced in COVID-19 patients in whom the occurrence of VTE appeared to occur at a higher rate when compared to patients with similarly severe ARDS and infections. This finding thus supports early reports suggesting extremely high incidence of VTE in critically ill patients with COVID-19 [2,[34], [35], [36]].
Severe COVID-19 commonly presents an atypical form of ARDS with significant dissociation between relatively well-preserved lung mechanics and severe hypoxemia, for which the loss of lung perfusion regulation and hypoxic vasoconstriction has been proposed as potential explanations [37]. However, since the risk of VTE gained significant attention of the medical community, this may have led to an increased reliance on chest CT to search for other mechanisms. Moreover, preliminary reports described pulmonary vascular abnormalities on chest CT [1], where vascular thickening was significantly associated with COVID-19 compared to non-COVID-19 pneumonia [38]. It is therefore possible that chest CT being more routinely performed in COVID-19 patients may have led to VTE overdiagnosis or distal PE for which the clinical relevance remains uncertain. In addition to confirmation bias, selective reporting is also plausible in the context of the rapidly evolving publications on COVID-19 over the last year. On the other side, an underreporting of VTE amongst patients with COVID-19 infection hospitalized in the non-ICU setting cannot be excluded, which could have explained that overall VTE rates in predominantly non-ICU patients were comparable between COVID-19 and non-COVID-19 patients.
Importantly, many institutional protocols adopted aggressive thromboprophylaxis for severe COVID-19 patients. Heparins also have non-anticoagulant properties, including anti-inflammatory [39] antiviral [40] and protective effects on the pulmonary endothelium [41]. Nonetheless, current guidelines recommend the use of thromboprophylaxis at a prophylactic dose in all COVID-19 patients admitted to the hospital [[42], [43], [44], [45]] while awaiting for results of ongoing studies evaluating intermediate and therapeutic anticoagulants dose for the prevention of VTE [46]. This approach appears reasonable in the context of a significant proportion of COVID-19 patients, both on prophylactic and therapeutic anticoagulation, exhibiting major bleeding [47]. The recruitment of critically ill COVID-19 patients were also prematurely stopped for futility reasons in ongoing anticoagulation trials. More importantly, while immunothrombosis is incontestably implicated in ARDS pathophysiology and VTE are quite prevalent in patients with severe COVID-19 and non-COVID-19-related ARDS, previous attempts to translate the beneficial effects of anticoagulation documented in animal models of ARDS and severe sepsis failed to demonstrate survival benefit in humans [48,49]. These results may reflect the complex interplay between inflammation, immune factors and endothelialitis that contribute to immunothrombosis and VTE in severe COVID-19 [6,50].
We acknowledge that our meta-analysis presents some limitations. First, all the included studies were observational studies and case series resulting in a certain risk of bias evaluated by the MINORS tools [21] and a very low strength of evidence based on the GRADE methodology [22]. The different cohorts were also heterogenous. Third, only seven studies were included in the evaluation of VTE in COVID-19 cohorts compared to non-COVID-19 cohorts although more than 100 publications have been published on VTE occurrence in COVID-19 patients. Despite the fact that the present meta-analysis incorporated data from a large study population, this finding confirms that a minority of studies assessed whether COVID-19 was specifically associated with VTE in comparison to non-COVID infections of similar severity. Fourth, VTE rates amongst ICU (22.0%) in the present meta-analysis were comparable to those previously reported in recent meta-analyses on COVID-19, occurring in 23.2% (95%CI 17.5–29.6%) [7]. Conversely, the rate of VTE observed amongst non-ICU patients (6.2%) was were slightly lower compared to recent meta-analysis (9.0%; 95%CI 6.9–11.4%) [7]. Accordingly, an increased risk of VTE in non-ICU patients with COVID-19 compared to non-COVID infections could not be excluded. Finally, the follow-up duration was short for most included studies. Whether these results are representative of the VTE risk for patients hospitalized for longer periods remains to be determined.
5. Conclusion
The present meta-analysis documented no signal for a difference in overall risk of VTE occurrence in COVID-19 cohorts compared to non-COVID-19 cohorts. However, subgroup analyses suggest an increased risk of VTE amongst COVID-19 patients hospitalized in the ICU. However, these results should be viewed as exploratory and further studies are needed to confirm our results and to evaluate the most appropriate thromboprophylaxis dosing in patients with COVID-19.
Author contributions
SP had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. VM, BKT, SM, FP, MC and JCL contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. Specifically: V. Mai contributed to study design, completed the literature search, data collection, data analysis, data interpretation, and drafted the first version of the manuscript. B. K. Tan completed the literature search and data collection. S. Mainbourg contributed to data interpretation and revised the manuscript. F. Potus contributed to data interpretation and revised the manuscript. M. Cucherat contributed to data interpretation and revised the manuscript. J. C. Lega contributed to data collection, data interpretation and revised the manuscript. S. Provencher contributed to study design, literature search, data analysis, data interpretation and wrote and revised the manuscript.
Financial/nonfinancial disclosures
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
No funding was received to perform this meta-analysis.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vph.2021.106882.
Appendix A. Supplementary data
Supplementary material
References
- 1.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;200343 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.-D., Sacco C., Bertuzzi A., Sandri M.T., Barco S., Humanitas C.-T.F. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M., Weyrich A.S., Yost C.C., Rondina M.T., Campbell R.A. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan B.K., Mainbourg S., Frigerri A., Bertoletti L., Douplat M., Dargaud Y., Grange C., Lobbes H., Provencher S., Lega J.-C. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax. 2021 doi: 10.1136/thoraxjnl-2020-215383. thoraxjnl-2020-215383. [DOI] [PubMed] [Google Scholar]
- 8.Pavoni V., Gianesello L., Pazzi M., Stera C., Meconi T., Frigieri F.C. Venous thromboembolism and bleeding in critically ill COVID-19 patients treated with higher than standard low molecular weight heparin doses and aspirin: a call to action. Thromb. Res. 2020;196:313–317. doi: 10.1016/j.thromres.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stessel B., Vanvuchelen C., Bruckers L., Geebelen L., Callebaut I., Vandenbrande J., Pellens B., Van Tornout M., Ory J.-P., van Halem K., Messiaen P., Herbots L., Ramaekers D., Dubois J. Impact of implementation of an individualised thromboprophylaxis protocol in critically ill ICU patients with COVID-19: a longitudinal controlled before-after study. Thromb. Res. 2020;194:209–215. doi: 10.1016/j.thromres.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattioli M., Benfaremo D., Mancini M., Mucci L., Mainquà P., Polenta A., Baldini P.M., Fulgenzi F., Dennetta D., Bedetta S., Gasperoni L., Caraffa A., Frausini G. Safety of intermediate dose of low molecular weight heparin in COVID-19 patients. J. Thromb. Thrombolysis. 2020:1–7. doi: 10.1007/s11239-020-02243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;27327 doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., Goodarzi K., Bendapudi P.K., Bornikova L., Gupta S., Leaf D.E., Kuter D.J., Rosovsky R.P. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93(3):212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro D.D., Lijfering W.M., Vlieg A. Van Hylckama, Rosendaal F.R., Cannegieter S.C. Pneumonia and risk of venous thrombosis: results from the MEGA study. J. Thromb. Haemost. 2012;10(6):1179–1182. doi: 10.1111/j.1538-7836.2012.04732.x. [DOI] [PubMed] [Google Scholar]
- 15.Clayton T.C., Gaskin M., Meade T.W. Recent respiratory infection and risk of venous thromboembolism: case-control study through a general practice database. Int. J. Epidemiol. 2011;40(3):819–827. doi: 10.1093/ije/dyr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y., Shen Y., Li J., Liu J., Liu S., Song H. Viral infection related venous thromboembolism: potential mechanism and therapeutic targets. Ann. Palliat. Med. 2020;9(3):1257–1263. doi: 10.21037/apm.2020.04.05. [DOI] [PubMed] [Google Scholar]
- 17.Gaertner F., Massberg S. Blood coagulation in immunothrombosis-at the frontline of intravascular immunity. Semin. Immunol. 2016;28(6):561–569. doi: 10.1016/j.smim.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 22.Atkins D., Best D., Briss P.A., Eccles M., Falck-Ytter Y., Flottorp S., Guyatt G.H., Harbour R.T., Haugh M.C., Henry D., Hill S., Jaeschke R., Leng G., Liberati A., Magrini N., Mason J., Middleton P., Mrukowicz J., O'Connell D., Oxman A.D., Phillips B., Schunemann H.J., Edejer T., Varonen H., Vist G.E., Williams J.W., Jr., Zaza S. Grading quality of evidence and strength of recommendations. Bmj. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.-M., Meziani F., Group C.T. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei F., Fan J., Yuan J., Liang Z., Wang K., Sun J., Guan W., Huang M., Li Y., Zhang W.W. Comparison of venous thromboembolism risks between COVID-19 pneumonia and community-acquired pneumonia patients. Arterioscler. Thromb. Vasc. Biol. 2020;40(9):2332–2337. doi: 10.1161/ATVBAHA.120.314779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S., Cousin N., Durand A., Kalioubie A.E., Favory R., Girardie P., Houard M., Jaillette E., Jourdain M., Ledoux G., Mathieu D., Moreau A.-S., Niles C., Nseir S., Onimus T., Préau S., Robriquet L., Rouzé A., Simonnet A., Six S., Toussaint A., Dupont A., Bauters A., Zawadzki C., Paris C., Trillot N., Wibaut B., Hochart A., Marichez C., Dalibard V., Vanderziepe S., Bourgeois L., Gaul A., Jospin A., Stepina N., Pradines B., Tournoys A., Brousseau T., Rémy M., Hutt A. Pulmonary embolism in patients with COVID-19. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 26.Freund Y., Drogrey M., Miró Ò., Marra A., Féral-Pierssens A.L., Penaloza A., Hernandez B.A.L., Beaune S., Gorlicki J., Ayar P. Vaittinada, Truchot J., Pena B., Aguirre A., Fémy F., Javaud N., Chauvin A., Chouihed T., Montassier E., Claret P.G., Occelli C., Roussel M., Brigant F., Ellouze S., Le Borgne P., Laribi S., Simon T., Lucidarme O., Cachanado M., Bloom B. Association between pulmonary embolism and COVID-19 in emergency department patients undergoing computed tomography pulmonary angiogram: the PEPCOV international retrospective study. Acad. Emerg. Med. 2020;27(9):811–820. doi: 10.1111/acem.14096. [DOI] [PubMed] [Google Scholar]
- 27.Rieder M., Goller I., Jeserich M., Baldus N., Pollmeier L., Wirth L., Supady A., Bode C., Busch H.-J., Schmid B., Duerschmied D., Gauchel N., Lother A. Rate of venous thromboembolism in a prospective all-comers cohort with COVID-19. J. Thromb. Thrombolysis. 2020;50(3):558–566. doi: 10.1007/s11239-020-02202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalager-Pedersen M., Lund L.C., Mariager T., Winther R., Hellfritzsch M., Larsen T.B., Thomsen R.W., Johansen N.B., Søgaard O.S., Nielsen S.L., Omland L., Lundbo L.F., Israelsen S.B., Harboe Z.B., Pottegård A., Nielsen H., Bodilsen J. Venous thromboembolism and major bleeding in patients with COVID-19: a nationwide population-based cohort study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellegrini J.A.S., Rech T.H., Schwarz P., de Oliveira A.C.T., Vieceli T., Moraes R.B., Sekine L., Viana M.V. Incidence of venous thromboembolism among patients with severe COVID-19 requiring mechanical ventilation compared to other causes of respiratory failure: a prospective cohort study. J. Thromb. Thrombolysis. 2021:1–11. doi: 10.1007/s11239-021-02395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020;41 doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Weiren, Yu Hong, Gou J., Li X., Sun Y., Li J., Liu L. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19) Preprints. 2020 [Google Scholar]
- 32.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb. Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozolina A., Sarkele M., Sabelnikovs O., Skesters A., Jaunalksne I., Serova J., Ievins T., Bjertnaes L.J., Vanags I. Activation of coagulation and fibrinolysis in acute respiratory distress syndrome: a prospective pilot study. Front. Med. (Lausanne) 2016;3:64. doi: 10.3389/fmed.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wichmann Dominic, Sperhake Jan-Peter, Lütgehetmann Marc, Steurer Stefan, Edler Carolin, Heinemann Axel, Heinrich Fabian, Mushumba Herbert, Kniep Inga, Schröder Ann Sophie, Burdelski Christoph, de Heer Geraldine, Nierhaus Axel, Frings Daniel, Pfefferle Susanne, Becker Heinrich, Bredereke-Wiedling Hanns, de Weerth Andreas, Paschen Hans-Richard, Sheikhzadeh-Eggers Sara, Stang Axel, Schmiedel Stefan, Bokemeyer Carsten, Addo Marylyn M., Aepfelbacher Martin, Püschel Klaus, Kluge Stefan. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020;0(0) doi: 10.7326/M20-2003. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not Lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai H.X., Hsieh B., Xiong Z., Halsey K., Choi J.W., Tran T.M.L., Pan I., Shi L.B., Wang D.C., Mei J., Jiang X.L., Zeng Q.H., Egglin T.K., Hu P.F., Agarwal S., Xie F., Li S., Healey T., Atalay M.K., Liao W.H. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020;200823 doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2008;122(6):743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Shukla D., Spear P.G. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Invest. 2001;108(4):503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iba T., Hashiguchi N., Nagaoka I., Tabe Y., Kadota K., Sato K. Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intensive Care Med. Exp. 2015;3(1):36. doi: 10.1186/s40635-015-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., Holley A.B., Jimenez D., Le Gal G., Rali P., Wells P. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. CHEST. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., Levi M., Samama C.M., Thachil J., Giannis D., Douketis J.D., C.C.T. the Subcommittee on Perioperative, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis, Haemostasis Scientific and Standardization Committee Communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C.D., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Caprini J.A., Tafur A.J., Burton J.R., Francese D.P., Wang E.Y., Falanga A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Steg P.G., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., Lip G.Y.H. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ASH ASH Guidelines on Use of Anticoagulation in Patients With COVID-19. 2020. https://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19 (Accessed October 23rd, 2020 2020)
- 46.Tritschler T., Mathieu M.-E., Skeith L., Rodger M., Middeldorp S., Brighton T., Sandset P.M., Kahn S.R., Angus D.C., Blondon M., Bonten M.J., Cattaneo M., Cushman M., Derde L.P.G., DeSancho M.T., Diehl J.-L., Goligher E., Jilma B., Jüni P., Lawler P.R., Marietta M., Marshall J.C., McArthur C., Miranda C.H., Mirault T., Morici N., Perepu U., Schörgenhofer C., Sholzberg M., Spyropoulos A.C., Webb S.A., Zarychanski R., Zuily S., Le Gal G., f.t.I.N.o.V.T.C.R.N. INVENT-VTE Anticoagulant interventions in hospitalized patients with COVID-19: A scoping review of randomized controlled trials and call for international collaboration. J. Thromb. Haemost. 2020;18(11):2958–2967. doi: 10.1111/jth.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D. Jiménez, A. García-Sanchez, P. Rali, A. Muriel, B. Bikdeli, P. Ruiz-Artacho, R. Le Mao, C. Rodríguez, B.J. Hunt, M. Monreal, Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis, Chest. [DOI] [PMC free article] [PubMed]
- 48.Obi A.T., Tignanelli C.J., Jacobs B.N., Arya S., Park P.K., Wakefield T.W., Henke P.K., Napolitano L.M. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J. Vasc. Surg. Venous Lymph. Disord. 2019;7(3):317–324. doi: 10.1016/j.jvsv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Hofstra J.J., Vlaar A.P., Prins D.J., Koh G., Levi M., Schultz M.J., Binnekade J.M., Juffermans N.P. Early intravenous unfractionated heparin and outcome in acute lung injury and acute respiratory distress syndrome: a retrospective propensity matched cohort study. BMC Pulm. Med. 2012;12:43. doi: 10.1186/1471-2466-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potus F., Mai V., Lebret M., Malenfant S., Breton-Gagnon E., Lajoie A.C., Boucherat O., Bonnet S., Provencher S. Novel insights on the pulmonary vascular consequences of COVID-19. Am. J. Phys. Lung Cell. Mol. Phys. 2020;319(2):L277–l288. doi: 10.1152/ajplung.00195.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material