Abstract
Backgroud
Recent studies have indicated that programmed cell death‐ligand 1 (PD‐L1) and cluster of differentiation 47 (CD47) play an essential role in tumor immune evasion and may serve as potential targets for combined immunotherapy. The aim of our study was to evaluate the PD‐L1/CD47 expression status in lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD), and explore its survival impact and relevance with the immune microenvironment.
Methods
The specimens from 190 LUSC and 240 LUAD patients who underwent intent‐to‐treat surgeries were retrospectively collected for immunohistochemistry assays of PD‐L1, CD47, cluster of differentiation 8 (CD8), and cluster of differentiation 68 (CD68).
Results
A total of 96 (22.3%) and 296 (68.8%) cases were positive for PD‐L1 and CD47 expression, respectively, and 80 (18.6%) of them demonstrated the co‐expression of PD‐L1/CD47. The rate of PD‐L1/CD47 co‐expression was 23.7% in LUSC, significantly higher than the 14.6% in LUAD (p = 0.018). The median overall survival (OS) for all patients was 55.9 months (range 2.0–146.0 months). The univariate analysis showed that patients with positive CD47 expression (LUSC p = 0.003, LUAD p = 0.036) and PD‐L1/CD47 co‐expression (LUSC p = 0.023, LUAD p = 0.004) exhibited significantly worse prognosis. The multivariate analysis demonstrated that PD‐L1/CD47 co‐expression was an independent prognostic factor for OS (LUSC hazard ratio [HR] 1.922, 95% CI 1.245–2.969, p = 0.003; LUAD HR 1.549, 95% CI 1.015–2.364, p = 0.043). PD‐L1/CD47 co‐expression was associated with high CD8‐positive T‐lymphocyte density in LUSC (p = 0.004) and LUAD (p = 0.043), and with high CD68‐positive macrophage density in LUSC (p = 0.026).
Conclusions
PD‐L1/CD47 co‐expression was an independent prognostic factor for LUSC and LUAD patients and may serve as a potential predictive biomarker for combined dual‐targeting immunotherapy.
Keywords: CD47, immunotherapy, non‐small cell lung cancer, PD‐L1, prognosis
Programmed cell death‐ligand 1 (PD‐L1)/cluster of differentiation 47 (CD47) co‐expression was an independent prognostic factor for non‐small cell lung cancer patients and was associated with high cluster of differentiation 8‐positive T‐lymphocyte density in lung squamous cell carcinoma (LUSC) and lung adenocarcinoma, and high cluster of differentiation 68‐positive macrophage density in LUSC. PD‐L1/CD47 co‐expression may serve as a potential predictive biomarker for future combined dual‐targeting immunotherapy.

INTRODUCTION
Lung cancer, which is the leading cause of cancer deaths, results in more than 25% of cancer‐related mortality worldwide. 1 Approximately 2.5 million people are newly diagnosed with lung cancer per year, 85% of them with non‐small cell lung cancer (NSCLC). Adenocarcinoma and squamous cell carcinoma are the two most common subtypes. 2 Targeted therapy has been applied to clinical practice and has revolutionized NSCLC treatment. However, only a small percentage of patients could benefit from tyrosine kinase inhibitors (TKI). 3
Immune checkpoints can prevent autoimmunity in our bodies. 4 However, overexpression of some immune checkpoint molecules in tumors can generate an immunosuppressive microenvironment, making tumor cells evade the recognition of T cells and macrophages, 5 leading to the rapid growth of tumors. Blockade of the immune checkpoints has become one of the most promising therapeutic strategies for malignant tumors. programmed cell death 1 (PD‐1)/programmed cell death‐ligand (PD‐L1) is the most widely recognized immune checkpoint, 4 , 6 and PD‐1/PD‐L1 inhibitors have showed a response rate of 30%, greatly improving the survival of NSCLC patients. 7 However, a large number of patients still cannot benefit from PD‐1/PD‐L1 monotherapy. Cluster of differentiation 8 (CD8) T cells, a subpopulation of adaptive lymphocytes, play a crucial role in the PD‐1/PD‐L1 inhibitor‐mediated antitumor effect. They attack tumor cells presenting tumor‐associated antigen with major histocompatibility complex class I (MHC I) on their surface, and then lead to tumor cell cytostasis and killing by producing interferon gamma. 8
Recently, macrophages have emerged as a new potential target of immunotherapy. Tumor‐associated macrophages (TAMs) can eliminate tumor cells through phagocytosis, maintaining a suppressive tumor microenvironment (TMA). 1 Cluster of differentiation 47 (CD47) is a transmembrane glycoprotein ubiquitously expressed in normal cells and serves as a cellular receptor regulating phagocytosis. CD47 binding to the signal‐regulated protein (SIRPα) expressed on the surface of macrophages can protect cells from being “eaten” by macrophages. 4 Various malignancies such as gastric, bladder, colorectal, and breast cancer were found to be associated with CD47 overexpression. 9 However, previous studies focusing on the relationship between CD47 expression and NSCLC remain limited.
Several studies have confirmed that the combination of two or three immune checkpoint inhibitors could show better antitumor effects. 10 For instance, the combination of PD‐1/PD‐L1 inhibitor and CD47 blockade could generate synergistic antitumor efficacy. 11 , 12 The aim of this study was to evaluate the expression level of PD‐L1 and CD47 in lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) patients, explore the relationship between this expression status and clinical characteristics and prognosis of patients, and assess cytotoxic T‐lymphocyte and macrophage infiltration to depict the microenvironment.
METHODS
Patients
A total of 430 patients diagnosed with NSCLC, including 190 patients with LUSC and 240 with LUAD according to the 2015 World Health Organization (WHO) classification, were enrolled in this study. They all underwent intent‐to‐treat surgeries in the Cancer Hospital, Chinese Academy of Medical Sciences from April 2006 to November 2013. The surgeries included sublobectomy, lobectomy or pneumonectomy if the clinical condition permitted, and all the patients underwent systematic or lobe‐specific lymph node dissection. Standard clinicopathological characteristics and tumor tissues were retrospectively obtained. Clinical characteristics we extracted included age, gender, smoking status, tumor differentiation, T staging, N staging, M staging, TNM staging, surgical procedures, completeness of resection, and overall survival. The tumors were staged according to the American Joint Committee on Cancer staging system (the 8th version). All the 430 patients were followed up until 20 September 2018 or until the date of death. The follow‐up information was obtained from their medical records or by getting in contact with their families. This study was approved by the ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Evaluation of the tumor immune microenvironment
Immunohistochemistry (IHC) of PD‐L1, CD47, CD8 and cluster of differentiation 68 (CD68) was performed on tissue microarrays (TMAs). The tissues were obtained by surgery and embedded in paraffin. We took two 2‐mm cores from each sample to constitute the TMAs, and then 4‐μm thick TMA sections were manufactured. We incubated the TMAs with the primary antibodies against CD47 (EPR21794, Abcam), PD‐L1 (28‐8, Abcam), CD68 (KP1, Abcam) and CD8 (D8A8Y, CST), and then with the secondary antibodies and 3, 3′‐diaminobenzidine (DAB). Two independent pathologists, who were blinded to the clinical information, examined the results of the IHC. Membranous tumor proportion score (TPS) was applied to score the PD‐L1 and CD47 expression. PD‐L1 and CD47 positive were defined as TPS ≥ 1% and TPS ≥ 5%, respectively. PD‐L1/CD47 co‐expression was defined as PD‐L1 positive and CD47 positive. We counted the number of CD8‐positive tumor‐infiltrating lymphocytes (TILs) and CD68‐positive macrophages in six high‐power fields and calculated the average for each case. With the median count as the cut‐off value, we divided the density of CD8‐positive TILs and the density of CD68‐positive macrophages into high and low groups.
Statistical analysis
Overall survival (OS) was defined as the time from initial surgery to death or the last follow‐up. All data analyses were performed using SPSS version 26. The Kaplan–Meier method was used to evaluate survival and the log‐rank test was used to determine significance. The Cox proportional hazard model was used to assess the prognosis with adjustment for clinicopathological factors. p < 0.05 was considered statistically significant.
RESULTS
Patients and clinicopathological characteristics
A total of 430 patients with NSCLC (190 with LUSC and 240 with LUAD) were involved. The clinicopathological characteristics are shown in Tables 1 and 2. The median age of all patients was 60.4 years (range 35–80 years), 326 (75.8%) were male, and 284 (66.0%) were smokers. At diagnosis, 62.6% of these patients had regional lymph node metastasis, and there were 97 (22.6%) stage I patients, 136 (31.6%) stage II patients, 195 (45.3%) stage III patients, and 2 (0.4%) stage IV patients. All patients underwent intent‐to‐treat surgeries including sublobectomy, lobectomy, bilobectomy or pneumonectomy. A total of 96 (22.3%) cases were positive for PD‐L1 expression and 296 (68.8%) were positive for CD47 expression, respectively, and 80 (18.6%) of them demonstrated co‐expression of PD‐L1/CD47. The representative IHC images for PD‐L1 and CD47 are shown in Figure 1. The rates of PD‐L1 positive and co‐expression of PD‐L1/CD47 were 26.8% and 23.7% in LUSC cases, respectively, which were significantly higher than the 18.8% and 14.6% in LUAD cases (PD‐L1 positive: p = 0.048; co‐expression of PD‐L1/CD47: p = 0.018). However, there was no significant difference in the CD47‐positive rate between LUSC and LUAD cases (LUSC 65.3%, LUAD 71.7%).
TABLE 1.
The univariate and multivariate analyses of factors associated with overall survival in LUSC cases
| Variable | N (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |||
| Age (years) | <60 | 88 (46.3) | 0.743 (0.510–1.083) | 0.122 | 0.684 (0.466–1.005) | 0.053 |
| ≥60 | 102 (53.7) | |||||
| Gender | Male | 183 (96.3) | 1.435 (0.455–4.521) | 0.537 | 1.101 (0.345–3.517) | 0.871 |
| Female | 7 (3.7) | |||||
| Smoking history | Smokers | 176 (92.6) | 0.852 (0.396–1.830) | 0.681 | ||
| Nonsmokers | 14 (7.4) | |||||
| Differentiation | Poor | 95 (50.0) | 1.415 (0.973–2.059) | 0.069 | 1.590 (1.073–2.357) | 0.021 |
| Moderate and high | 95 (50.0) | |||||
| T | 1–2 | 124 (65.3) | 0.364 (0.250–0.531) | <0.001 | 0.437 (0.297–0.643) | <0.001 |
| 3–4 | 66 (34.7) | |||||
| N | 0–1 | 131 (68.9) | 0.421 (0.282–0.602) | <0.001 | 0.399 (0.268–0.595) | <0.001 |
| 2 | 59 (33.1) | |||||
| TNM stage | 1–2 | 104 (54.7) | 0.344 (0.235–0.504) | <0.001 | ||
| 3 | 86 (45.3) | |||||
| PD‐L1 | Positive | 51 (26.8) | 1.504 (1.007–2.248) | 0.046 | ||
| Negative | 139 (73.2) | |||||
| CD47 | Positive | 124 (65.3) | 1.866 (1.232–2.826) | 0.003 | ||
| Negative | 66 (34.7) | |||||
| PD‐L1/CD47 | Co‐expression | 45 (23.7) | 1.613 (1.069–2.435) | 0.023 | 1.922 (1.245–2.969) | 0.003 |
| Others | 145 (76.3) | |||||
Abbreviations: CD47, cluster of differentiation 47; CI, confidence interval; HR hazard ratio; LUSC, lung squamous cell carcinoma; PD‐L1, programmed cell death‐ligand 1.
TABLE 2.
The univariate and multivariate analyses of factors associated with overall survival in LUAD cases
| Variable | N (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |||
| Age (years) | <60 | 122 (50.8) | 0.548 (0.399–0.753) | <0.001 | 0.535 (0.387–0.739) | <0.001 |
| ≥60 | 118 (49.2) | |||||
| Gender | Male | 143 (59.6) | 1.223 (0.887–1.685) | 0.220 | 1.279 (0.915–1.789) | 0.150 |
| Female | 97 (40.4) | |||||
| Smoking history | Smokers | 108 (45.0) | 0.784 (0.574–1.072) | 0.127 | ||
| Nonsmokers | 132 (55.0) | |||||
| Differentiation | Poor | 133 (55.4) | 1.248 (1.063–1.465) | 0.007 | 1.094 (0.921–1.299) | 0.306 |
| Moderate and high | 107 (44.6) | |||||
| T | 1–2 | 176 (73.3) | 0.737 (0.623–0.872) | <0.001 | 0.625 (0.440–0.887) | 0.009 |
| 3–4 | 64 (36.7) | |||||
| N | 0–1 | 148 (61.7) | 0.483 (0.353–0.661) | <0.001 | 0.492 (0.354–0.684) | <0.001 |
| 2 | 92 (38.3) | |||||
| TNM stage | 1–2 | 129 (53.8) | 0.494 (0.361–0.677) | <0.001 | ||
| 3–4 | 111 (46.2) | |||||
| PD‐L1 | Positive | 45 (18.8) | 1.517 (1.041–2.210) | 0.030 | ||
| Negative | 195 (81.3) | |||||
| CD47 | Positive | 172 (71.7) | 1.470 (1.026–2.106) | 0.036 | ||
| Negative | 68 (28.3) | |||||
| PD‐L1/CD47 | Co‐expression | 35 (14.6) | 1.815 (1.204–2.736) | 0.004 | 1.549 (1.015–2.364) | 0.043 |
| Others | 205 (85.4) | |||||
Abbreviations: CD47, cluster of differentiation 47; CI, confidence interval; HR hazard ratio; LUAD, lung adenocarcinoma; PD‐L1, programmed cell death‐ligand 1.
FIGURE 1.
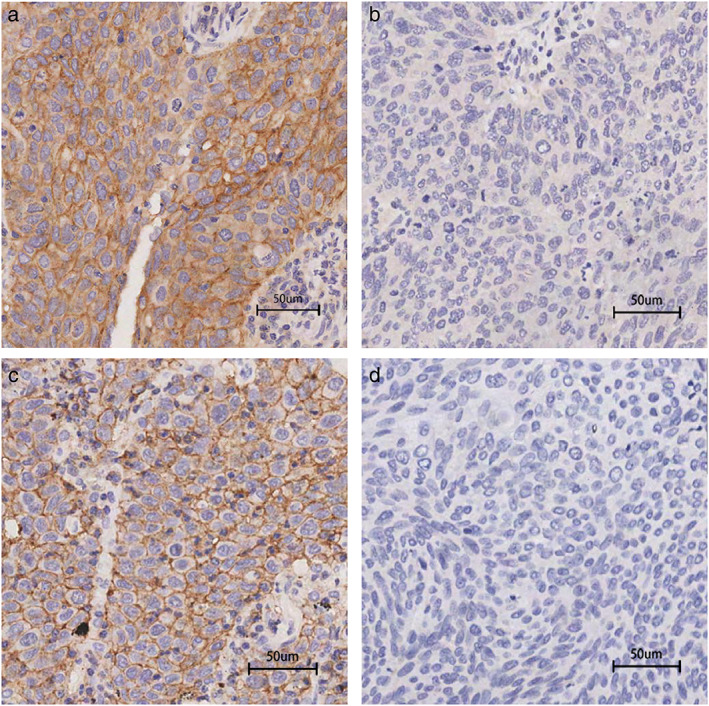
Representative images of immunohistochemical staining for PD‐L1 and CD47. (a) Positive membrane staining for PD‐L1. (b) Negative membrane staining for PD‐L1. (c) Positive membrane staining for CD47. (d) Negative membrane staining for CD47. Scale bar: 50 μm. PD‐L1, programmed cell death‐ligand 1; CD47, cluster of differentiation 47
Analysis of survival
Follow‐up was performed for all patients and 270 (62.8%) patients had died at the time of data extraction. The median OS for all patients was 55.9 months (range 2.0–146.0 months) and the 5‐year survival rate was 46.3%. We performed survival analysis using the Kaplan–Meier curve and found that in both the LUSC and LUAD cohorts patients with positive CD47 expression (LUSC: p = 0.003; LUAD: p = 0.036), positive PD‐L1 expression (LUSC: p = 0.046; LUAD: p = 0.030) and PD‐L1/CD47 co‐expression (LUSC: p = 0.023; LUAD: p = 0.004) exhibited a significantly worse prognosis (Tables 1 and 2, and Figure 2). In addition, advanced T stage, N stage, and TNM stage were associated with poor prognosis in LUSC patients (T stage: p < 0.001; N stage: p < 0.001; TNM stage: p < 0.001) (Table 1), and older age, poor tumor differentiation, and advanced T stage, N stage, and TNM stage were associated with poor prognosis in LUAD patients (age: p < 0.001; differentiation: p = 0.007; T stage: p < 0.001; N stage: p < 0.001; TNM stage: p < 0.001) (Table 2). In multivariate analysis, we identified that tumor differentiation, T stage, N stage, and PD‐L1/CD47 co‐expression were independent prognostic factors for LUSC patients (differentiation: HR 1.590, 95% CI 1.073–2.357, p = 0.021; T stage: HR 0.437, 95% CI 0.297–0.643, p < 0.001; N stage: HR 0.399, 95% CI 0.268–0.595, p < 0.001; PD‐L1/CD47 co‐expression: HR 1.922, 95% CI 1.245–2.969, p = 0.003) (Table 1), and age, T stage, N stage, and PD‐L1/CD47 co‐expression were independent prognostic factors for LUAD patients (age: HR 0.535, 95% CI 0.387–0.739, p < 0.001; T stage: HR 0.625, 95% CI 0.440–0.887, p = 0.009; N stage: HR 0.492, 95% CI 0.354–0.684, p < 0.001; PD‐L1/CD47 co‐expression: HR 1.549, 95% CI 1.015–2.364, p = 0.043) (Table 2).
FIGURE 2.
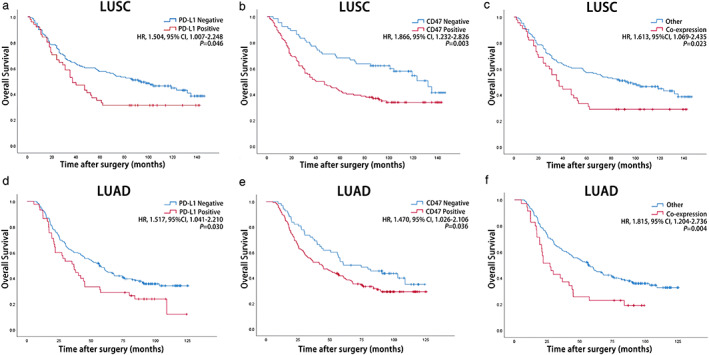
Kaplan–Meier curves demonstrating overall survival of patients with LUSC and LUAD according to PD‐L1 expression status (LUSC (a), LUAD (d)), CD47 expression status (LUSC (b), LUAD (e)), and PD‐L1/CD47 co‐expression status (LUSC (c), LUAD, (f)). CD47, cluster of differentiation 47; CI, confidence interval; HR, hazard ratio; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PD‐L1, programmed cell death‐ligand 1
Association between PD‐L1/CD47 expression and CD8‐positive TIL density and CD68‐positive macrophage density
IHC for CD8 and CD68 was performed in this study. The median CD8 densities were 23 (range 0–231) and 18 (range 0–110), and the median CD68 densities were 3 (range 0–22) and 5 (range 0–30) in the LUSC and LUAD cohorts, respectively. All patients were divided into CD8‐positive TILs or CD68‐positive macrophage high and low groups according to the median density as the boundary. The representative IHC images for CD8 and CD68 are shown in Figure 3. The percentage of patients with high CD8‐positive TIL density was significantly higher in the PD‐L1 positive group (LUSC: p = 0.005; LUAD: p = 0.008) and the PD‐L1/CD47 co‐expression group (LUSC: p = 0.004; LUAD: p = 0.043) in both cohorts (Tables 3 and 4). The percentage of patients with high CD68‐positive macrophage density was significantly higher in the CD47‐positive group (p < 0.001) and the PD‐L1/CD47 co‐expression group (p = 0.026) in the LUSC cohort but not in the LUAD cohort (p = 0.199 and 0.365) (Tables 3 and 4).
FIGURE 3.
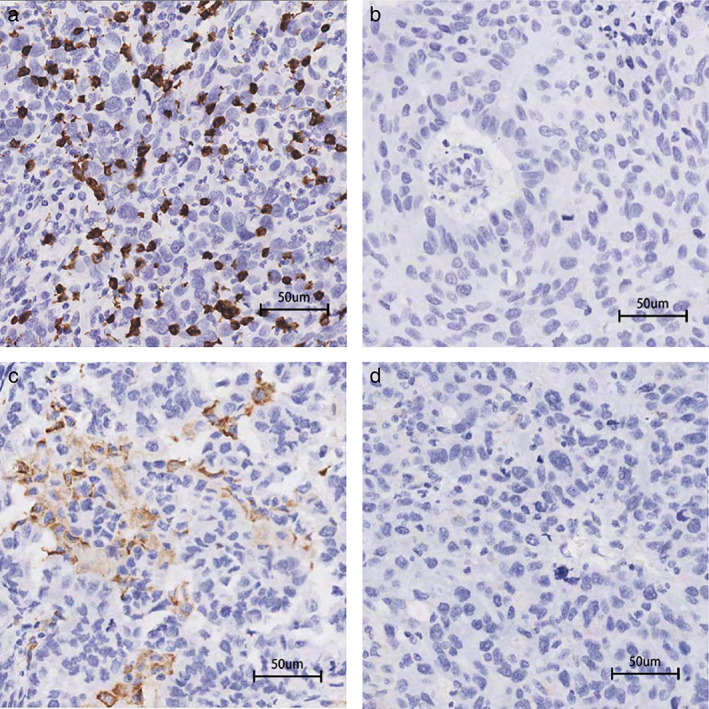
Representative images of immunohistochemical staining for CD8 and CD68. (a) High density of CD8‐positive lymphocyte infiltration. (b) Low density of CD8‐positive lymphocyte infiltration. (c) High density of CD68‐positive macrophage infiltration. (d) Low density of CD68‐positive macrophage infiltration. Scale bar: 50 μm. CD8, cluster of differentiation 8; CD68, cluster of differentiation 68
TABLE 3.
Associations between CD8‐positive T cells and CD68‐positive macrophage density with PD‐L1 expression, CD47 expression, and PD‐L1/CD47 co‐expression in LUSC cases
| Factors | PD‐L1, N% | CD47 | PDL1/CD47 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | p | Positive | Negative | p | Positive | Negative | p | ||
| CD8 | Low | 17 | 79 | 0.005 | 57 | 39 | 0.095 | 14 | 82 | 0.004 |
| High | 34 | 60 | 67 | 27 | 31 | 63 | ||||
| CD68 | Low | 20 | 77 | 0.051 | 48 | 49 | <0.001 | 16 | 96 | 0.026 |
| High | 31 | 62 | 76 | 17 | 29 | 64 | ||||
Abbreviations: CD8, cluster of differentiation 8; CD47, cluster of differentiation 47; CD68, cluster of differentiation 68; CI, confidence interval; HR hazard ratio; LUSC, lung squamous cell carcinoma; PD‐L1, programmed cell death‐ligand 1.
TABLE 4.
Associations between CD8‐positive T cells and CD68‐positive macrophage density with PD‐L1 expression, CD47 expression, and PD‐L1/CD47 co‐expression in LUAD cases
| Factors | PD‐L1, N% | CD47 | PDL1/CD47 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | p | Positive | Negative | p | Positive | Negative | p | ||
| CD8 | Low | 15 | 108 | 0.008 | 84 | 39 | 0.254 | 12 | 111 | 0.043 |
| High | 30 | 87 | 88 | 29 | 23 | 94 | ||||
| CD68 | Low | 17 | 104 | 0.070 | 82 | 39 | 0.199 | 15 | 106 | 0.365 |
| High | 28 | 91 | 90 | 29 | 20 | 99 | ||||
Abbreviations: CD8, cluster of differentiation 8; CD47, cluster of differentiation 47; CD68, cluster of differentiation 68; CI, confidence interval; HR hazard ratio; LUAD, lung adenocarcinoma; PD‐L1, programmed cell death‐ligand 1.
DISCUSSION
All the patients in our cohort underwent intent‐to‐treat surgeries. A total of 246 (56.9%) patients were stage III at diagnosis. The median OS of our cohort was 55.9 months (range 2.0–146.0 months), which was slightly longer than that reported by previous studies, 13 and the 5‐year survival rate of our cohort was 46.3% (stage I 67.0%, stage II 53.7%, stage III 31.3%, stage IV 0%). In the separate LUSC or LUAD cohort, patients with advanced T/N/TNM stage exhibited significantly worse prognosis, and T stage and N stage were demonstrated to be independent prognostic factors, which was consistent with reports by previous studies. 13 Inhibition of the PD‐1/PD‐L1 immune checkpoint is a promising anticancer therapy, especially for metastatic NSCLC, and PD‐L1 expression is a predictive biomarker for immunotherapy. The PD‐L1‐positive rate in our study was 22.3%, which is lower than that reported by previous studies, ranging from 24% to 60%. 14 , 15 , 16 The difference might be due to the limited sample size, different definition of PD‐L1 positivity, different tumor stages, and different percentages of histologic subtypes. We found that the PD‐L1‐positive rate was 26.8% in our LUSC cohort and more than 18.8% in our LUAD cohort, which was consistent with previous studies. 17 , 18 , 19 PD‐L1 expression was correlated with poor prognosis in our LUSC and LUAD cohorts. Although PD‐1/PD‐L1 inhibitors have provided new treatment options for NSCLC patients and greatly improved the prognosis, the response rate was approximately 20% in unselected patients and nearly 50% in selected patients, 20 and a large proportion of patients still failed to benefit from PD‐1/PD‐L1 inhibitor treatment, indicating that PD‐1/PD‐L1 blockade monotherapy was not potent enough.
Previous studies demonstrated that CD47 is an immune checkpoint molecule that is ubiquitously expressed in normal tissues. CD47 mediates a “do not eat me” signal to ensure that healthy autologous cells will not be eaten. For instance, CD47 expressed on the surface of immature red blood cells transmits a “do not eat me” signal by binding to SIRPα on macrophages to prevent red blood cells being inappropriately phagocytosed. 21 Meanwhile, the old red blood cells do not express CD47 on their surface membranes, facilitating elimination from the circulation and similarly contributing to blood hemostasis. Various studies have shown that tumor cells also express CD47 on their surface, thereby protecting them from phagocytosis by macrophages. CD47 blockade can promote macrophage‐ and dendritic cell‐mediated phagocytosis of tumor cells. Macrophages submit cancer cell antigens to T cells through MHC I to activate CD8+ T cells. Then CD8+ T cells target the tumor cells and destroyed them. 22 Yoshida et al. have showed that the CD47‐positive rate is 49.5% in early stage gastric cancers. They found that the overall 5‐year survival rate for the CD47‐positive subgroup was significantly lower than that for the CD47‐negative subgroup. 23 Similar findings in other tumors have also been reported, including acute myeloid leukemia, non‐Hodgkin's lymphoma, breast cancer, liver cancer, and small‐cell lung cancer. 24 , 25 , 26 , 27 , 28 , 29 Several studies have focused on CD47 expression in NSCLC. Hui Zhao et al. constructed an assay system in vitro based on three‐dimensional microfluidic chip to culture NSCLC cells and found CD47 downregulation in NSCLC cells in vivo conspicuously inhibited the development of tumors. In recent studies of NSCLC, the CD47 positive rates ranged from 53% to 84%, while the CD47 positive rates were 65.3% and 71.7% in LUSC and LUAD, respectively, in our study. 1 , 11 As far as we know, this is the largest cohort of LUSC and LUAD cases focusing on PD‐L1 and CD47 co‐expression. We observed that high CD47 expression was associated with poor prognosis, indicating the therapeutic potential of targeting CD47 for NSCLC patients.
Formerly, CD47 and PD‐L1 inhibitors were believed to arouse therapeutic benefit through respective mechanisms. However, Sockolosky et al. found that when CD47‐mediated SIRPα signaling was blocked, anti‐PD‐L1 antibodies intensified macrophage phagocytosis of B16F10 cells in vitro. 30 , 31 When the PD‐1/PD‐L1 axis is blocked, phagocytosed tumor cell antigens submitted by macrophages can potentiate T‐cell responses. Although blocking CD47 alone can produce antitumor effects, blocking CD47 and PD‐L1 at the same time will produce better synergistic effects. Therefore, PD‐L1 and CD47 dual‐targeting might demonstrate a preliminary promising therapeutic effect, and exploring the co‐expression status of PD‐L1 and CD47 in NSCLC could provide clues for refined combined immunotherapy. Through univariate and multivariate analysis, our study showed PD‐L1 and CD47 co‐expression was an independent prognostic factor in both the LUAD and LUSC cohorts. A total of 18.6% patients had co‐expression of PD‐L1 and CD47, and there was a significant correlation between the expression levels of PD‐L1 and CD47, which might be due to an essential oncogenic transcription factor MYC. The expression levels of PD‐L1 and CD47 on the surface of tumor cells were simultaneously regulated by MYC, further indicating the theoretically feasible application prospect of PD‐L1 and CD47 dual‐targeting therapy. 32 Advancement in gene and protein engineering technology has developed multiple bispecific antibodies (BsAb), which can block multiple immune checkpoints as a single agent. “BsAb or multispecific antibodies (MsAb)” as next‐generation therapeutic agents have promoted multiple candidates into ongoing clinical trials. Recently, several anti‐CD47 bispecific antibodies have been under investigation in patients with leukemia and advanced solid tumors. 33
Furthermore, we also analyzed the expression status of CD8 and CD68, the specific markers of killer T cells and macrophages. CD8 is a transmembrane glycoprotein that serves as a coreceptor for the T‐cell receptor. CD8 is predominantly expressed on cytotoxic T cells, which play central roles in cell‐mediated immune attack, whereas it can be expressed on natural killer and dendritic cells. The percentage of patients with high CD8‐positive TIL density was higher in the PD‐L1 positive group and the PD‐L1/CD47 co‐expression group in both LUSC and LUAD, and the percentage of patients with high CD68‐positive macrophage density was higher in the CD47 positive group and the PD‐L1/CD47 co‐expression group in LUSC. The association between CD8 T cells and PD‐L1 expression on cancer cells was observed in many types of cancers, 34 , 35 which could be explained by an underlying mechanism that PD‐L1 expression could be induced by CD8 T lymphocytes via interferon‐γ. 36 The existence of CD8 T cells and macrophages forces tumor cells to develop an adaptive immune escape mechanism. PD‐L1 and CD47 expression was closely connected to the tumor immune microenvironment in NSCLC, indicating that they could be potential predictive biomarkers for future combined immunotherapy.
In conclusion, we summarized the status of PD‐L1 and CD47 expression in LUSC and LUAD, and found that PD‐L1/CD47 co‐expression is an independent prognostic factor and is associated with CD8‐positive TILs density. PD‐L1/CD47 co‐expression might serve as a predictive biomarker for future combined dual‐targeting immunotherapy.
CONFLICT OF INTEREST
No conflict of interest was declared.
ACKNOWLEDGMENTS
This study was supported by the Beijing Hope Run Special Fund of Cancer Foundation of China (LC2020B09, LC2020A33, LC2018B14, LC2019B15), the National Key R&D Program of China (2018YFC1315000, 2017YFC1311000), the National Natural Science Foundaion of China (81871885), the CAMS Initiative for Innovative Medicine (2017‐I2M‐1‐005, 2017‐I2M‐2‐003), and Guangdong Association of Clinical Trials (GACT)/Chinese Thoracic Oncology Group (CTONG), the Beijing Science and Technology Project (Z181100001718212), the Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (2017B030314120), the Training Programme Foundation for the Talents in Beijing City (2017000021223TD05).
Yang Z, Peng Y, Guo W, Xu J, Li L, Tian H, et al. PD‐L1 and CD47 co‐expression predicts survival and enlightens future dual‐targeting immunotherapy in non‐small cell lung cancer. Thorac Cancer. 2021;12:1743–1751. 10.1111/1759-7714.13989
Funding information National Key R&D Program of China, Grant/Award Numbers: 2018YFC1315000, 2017YFC1311000; Beijing Hope Run Special Fund of Cancer Foundation of China, Grant/Award Numbers: LC2020B09, LC2020A33, LC2018B14, LC2019B15; National Natural Science Foundaion of China, Grant/Award Number: 81871885; CAMS Initiative for Innovative Medicine, Grant/Award Numbers: 2017‐I2M‐1‐005, 2017‐I2M‐2‐003; Beijing Science and Technology Project, Grant/Award Number: Z181100001718212; Guangdong Association of Clinical Trials (GACT) /Chinese Thoracic Oncology Group (CTONG) and Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer, Grant/Award Number: 2017B030314120; Training Programme Foundation for the Talents in Beijing City, Grant/Award Number: 2017000021223TD05
Contributor Information
Jiachen Xu, Email: xjcwelcome@126.com.
Fengwei Tan, Email: tanfengwei@cicams.ac.cn.
Shugeng Gao, Email: gaoshugeng@cicams.ac.cn.
Jie He, Email: prof.jiehe@gmail.com.
REFERENCES
- 1. Arrieta O, Aviles‐Salas A, Orozco‐Morales M, Hernandez‐Pedro N, Cardona AF, Cabrera‐Miranda L, et al. Association between CD47 expression, clinical characteristics and prognosis in patients with advanced non‐small cell lung cancer. Cancer Med. 2020;9:2390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu T, Yang X, Huang Y, Zhao M, Li M, Ma K, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santini FC, Hellmann MD. PD‐1/PD‐L1 axis in lung cancer. Cancer J. 2018;24:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tong B, Wang M. CD47 is a novel potent immunotherapy target in human malignancies: Current studies and future promises. Future Oncol. 2018;14:2179–88. [DOI] [PubMed] [Google Scholar]
- 5. Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci. 2015;106:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD‐1/PD‐L1 blockade in cancer treatment: Perspectives and issues. Int J Clin Oncol. 2016;21:462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu PC, Jablons DM, Yang CT, You L. Epidermal growth factor receptor (EGFR) pathway, Yes‐associated protein (YAP) and the regulation of programmed death‐ligand 1 (PD‐L1) in non‐small cell lung cancer (NSCLC). Int J Mol Sci. 2019;20:3821–39. 10.3390/ijms20153821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. [DOI] [PubMed] [Google Scholar]
- 9. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mullard A. Trispecific antibodies take to the clinic. Nat Rev Drug Discov. 2020;19:657–8. [DOI] [PubMed] [Google Scholar]
- 11. Yang Z, Xu J, Li R, Gao Y, He J. PD‐L1 and CD47 co‐expression in pulmonary sarcomatoid carcinoma: A predictor of poor prognosis and potential targets of future combined immunotherapy. J Cancer Res Clin Oncol. 2019;145:3055–65. [DOI] [PubMed] [Google Scholar]
- 12. Zhao H, Wang J, Kong X, Li E, Liu Y, Du X, et al. CD47 promotes tumor invasion and metastasis in non‐small cell lung cancer. Sci Rep. 2016;6:29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang BY, Huang JY, Chen HC, Lin CH, Lin SH, Hung WH, et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol. 2020;146:43–52. [DOI] [PubMed] [Google Scholar]
- 14. Yoneshima Y, Ijichi K, Anai S, Ota K, Otsubo K, Iwama E, et al. PD‐L1 expression in lung adenocarcinoma harboring EGFR mutations or ALK rearrangements. Lung Cancer. 2018;118:36–40. [DOI] [PubMed] [Google Scholar]
- 15. Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD‐L1 expression in lung cancer. J Thorac Oncol. 2016;11:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyazawa T, Marushima H, Saji H, Kojima K, Hoshikawa M, Takagi M, et al. PD‐L1 expression in non‐small‐cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surg. 2019;25:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen‐Corish M, et al. PD‐L1 expression is a favorable prognostic factor in early stage non‐small cell carcinoma. Lung Cancer. 2015;89:181–8. [DOI] [PubMed] [Google Scholar]
- 18. Janzic U, Kern I, Janzic A, Cavka L, Cufer T. PD‐L1 expression in squamous‐cell carcinoma and adenocarcinoma of the lung. Radiol Oncol. 2017;51:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin G, Fan X, Zhu W, Huang C, Zhuang W, Xu H, et al. Prognostic significance of PD‐L1 expression and tumor infiltrating lymphocyte in surgically resectable non‐small cell lung cancer. Oncotarget. 2017;8:83986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gniadek TJ, Li QK, Tully E, Chatterjee S, Nimmagadda S, Gabrielson E. Heterogeneous expression of PD‐L1 in pulmonary squamous cell carcinoma and adenocarcinoma: Implications for assessment by small biopsy. Mod Pathol. 2017;30:530–8. [DOI] [PubMed] [Google Scholar]
- 21. Hayat SMG, Bianconi V, Pirro M, Jaafari MR, Hatamipour M, Sahebkar A. CD47: Role in the immune system and application to cancer therapy. Cell Oncol (Dordr). 2020;43:19–30. [DOI] [PubMed] [Google Scholar]
- 22. Mittrücker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch Immunol Ther Exp (Warsz). 2014;62:449–58. [DOI] [PubMed] [Google Scholar]
- 23. Yoshida K, Tsujimoto H, Matsumura K, Kinoshita M, Takahata R, Matsumoto Y, et al. CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med. 2015;4:1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Starr JS, Jiang L, Li Z, Qiu Y, Menke DM, Tun HW. CD47 and osteopontin expression in diffuse large B‐cell lymphoma with nodal and intravascular involvement. Clin Lymphoma Myeloma Leuk. 2013;13:597–601. [DOI] [PubMed] [Google Scholar]
- 25. Galli S, Zlobec I, Schurch C, Perren A, Ochsenbein AF, Banz Y. CD47 protein expression in acute myeloid leukemia: A tissue microarray‐based analysis. Leuk Res. 2015;39:749–56. [DOI] [PubMed] [Google Scholar]
- 26. Xiao Z, Chung H, Banan B, Manning PT, Ott KC, Lin S, et al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. 2015;360:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, et al. CD47‐blocking immunotherapies stimulate macrophage‐mediated destruction of small‐cell lung cancer. J Clin Invest. 2016;126:2610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaur S, Elkahloun AG, Singh SP, Chen QR, Meerzaman DM, Song T, et al. A function‐blocking CD47 antibody suppresses stem cell and EGF signaling in triple‐negative breast cancer. Oncotarget. 2016;7:10133–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sudo T, Takahashi Y, Sawada G, Uchi R, Mimori K, Akagi Y. Significance of CD47 expression in gastric cancer. Oncol Lett. 2017;14:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci USA. 2016;113:E2646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tseng D, Volkmer JP, Willingham SB, Contreras‐Trujillo H, Fathman JW, Fernhoff NB, et al. Anti‐CD47 antibody‐mediated phagocytosis of cancer by macrophages primes an effective antitumor T‐cell response. Proc Natl Acad Sci USA. 2013;110:11103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, et al. MYC regulates the antitumor immune response through CD47 and PD‐L1. Science. 2016;352:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thakur A, Huang M, Lum LG. Bispecific antibody based therapeutics: Strengths and challenges. Blood Rev. 2018;32:339–47. [DOI] [PubMed] [Google Scholar]
- 34. Huang CY, Wang Y, Luo GY, Han F, Li YQ, Zhou ZG, et al. Relationship between PD‐L1 expression and CD8+ T‐cell immune responses in hepatocellular carcinoma. J Immunother. 2017;40:323–33. [DOI] [PubMed] [Google Scholar]
- 35. Ock CY, Keam B, Kim S, Lee JS, Kim M, Kim TM, et al. Pan‐cancer immunogenomic perspective on the tumor microenvironment based on PD‐L1 and CD8 T‐cell infiltration. Clin Cancer Res. 2016;22:2261–70. [DOI] [PubMed] [Google Scholar]
- 36. Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up‐regulation of PD‐L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]


