Abstract
Neuromyelitis optica spectrum disorders (NMOSDs) are autoimmune demyelinating diseases involving the central nervous system, affecting the spinal cord and optic nerves. There are few reports of paraneoplastic NMOSD associated with malignant melanoma. Here, we report a rare case of anti‐aquaporin 4 (AQP4) antibody‐positive NMOSD associated with malignant melanoma. A 61‐year‐old Japanese woman was diagnosed with malignant melanoma and lung metastasis four years after a diagnosis of anti‐AQP4 antibody‐positive NMOSD. When diagnosing and treating patients with NMOSD, physicians should be aware of the development of malignancy for at least several years.
Keywords: anti‐aquaporin‐4 antibody, malignant melanoma, neuromyelitis optica spectrum disorders, paraneoplastic neurological syndrome
This is only the second reported case of anti‐AQP4 antibody‐positive NMOSD with malignant melanoma. Physicians should be aware of development of malignancy for at least several years after diagnosing and treating patients with NMOSD.
INTRODUCTION
Neuromyelitis optica spectrum disorders (NMOSDs) are autoimmune demyelinating diseases involving the central nervous system (CNS), including optic neuritis and transverse myelitis. NMOSD has been associated with anti‐aquaporin‐4 (AQP4) antibodies. 1 The prevalence of anti‐AQP4 antibody‐positive NMOSD is 3.42 in 100 000 individuals in Japan. 2 The mean age of onset is 30–40 years, but elderly patients are often seen. 3 Several reports have associated the CNS with malignant melanoma, such as in cerebellar degeneration, 4 limbic encephalitis, 5 Guillain‐Barré syndrome, 6 and chronic inflammatory demyelinating polyneuropathy, 7 but there are few reports of paraneoplastic neurological syndrome (PNS) associated with malignant melanoma. We present a 65‐year‐old Japanese female patient with a rare case of anti‐AQP4 antibody‐positive NMOSD complicated with malignant melanoma.
CASE REPORT
A 61‐year‐old Japanese woman with no specific medical, smoking, or family history was admitted to our hospital because of palsy of the left upper extremity and bilateral lower extremities and urinary disorder. Brain magnetic resonance imaging (MRI) revealed multiple hyperintense T2 lesions (Figure 1). Spinal MRI showed signal abnormalities from the medulla oblongata and the postrema to the 10th thoracic level. Serum anti‐AQP4 antibodies were positive; therefore, she was diagnosed with NMOSD. 8 She had no complications of collagen vascular disease and no findings of malignancies on abdominal computed tomography (CT). Improvement of manual muscle testing of the left upper extremity (from 3 to 4) and bilateral lower extremities (from 0 to 3) was achieved via combined treatment with high‐dose intravenous methylprednisolone (1 g/day for three days) and immunoadsorption therapy for five cycles, but neither the total sensory disturbance below the fourth thoracic level nor urinary disturbance improved. After these treatments, combined treatment with oral prednisolone and an immunosuppressant was subsequently continued at an initial dose of 40 mg/day with 2 mg of tacrolimus, tapered down to 10 mg, resulting in a relapse‐free status for four years.
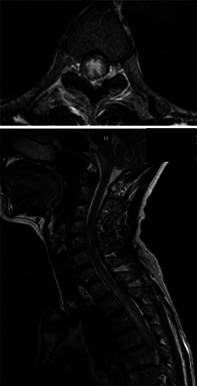
FIGURE 1.
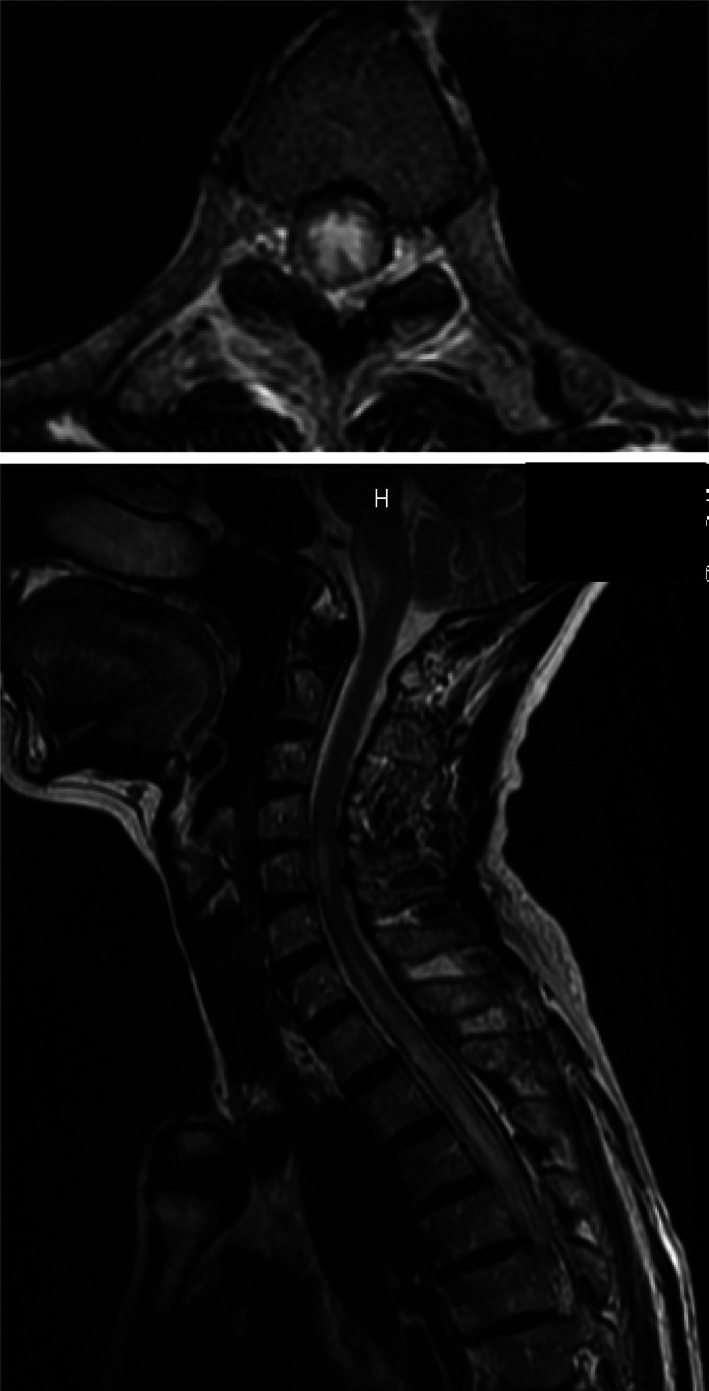
Spinal magnetic resonance imaging (MRI) scan of the patient at her first visit showing signal abnormalities and the postrema from the medulla oblongata to the 10th thoracic level
Four years after the onset of NMOSD, she experienced anorexia, nausea, general fatigue, and dry cough two weeks before admission to our hospital. Physical examination on admission revealed the following: height, 154.0 cm; bodyweight, 61.5 kg; body temperature, 37.0°C; heart rate, 120 bpm; blood pressure, 151/84 mmHg; and oxygen saturation, 94% (nasal oxygen 3 L/min, at rest). Right respiratory sounds were attenuated on chest auscultation, but there was no exacerbation of neurological findings. Laboratory results (Table 1) showed a high titer of cancer antigen 125 (246.3 U/ml) and neuron‐specific enolase (43.9 ng/ml). Chest and abdominal CT findings revealed a 110 mm pulmonary mass in the right lower lobe, multiple irregular pulmonary nodules, right pleural effusion, and multiple liver, skin, left adrenal, right renal, and bone metastases (Figure 2). Brain and spinal MRI revealed no metastases or new NMOSD lesions. Cerebrospinal fluid findings including cytology were normal (Table 1). Cytological findings of the right pleural effusion revealed no evidence of malignancy. There were several metastatic lesions of malignant melanoma in the chest and abdomen in our patient; thus, the primary lesion was unclear. A biopsy was performed of the metastatic lesions in the right chest and liver. Immunocytochemistry of skin and liver needle biopsies (Figure 3(a)–(d)) revealed tumor cells positive for S‐100, HMB45, and SOX10, and negative results for BRAF, which indicated a diagnosis of malignant melanoma. The PD‐L1 tumor proportion score was less than 1% (Figure 3(e)). Malignant disease was diagnosed four years after the onset of encephalomyelitis (within five years) in the present patient, fulfilling the criteria for classical PNS. 9 Therefore, she was diagnosed with paraneoplastic NMOSD associated with malignant melanoma. At that time, serum anti‐AQP4 antibodies were still positive. Serum onconeural antibodies, such as anti‐Hu, SOX1, CV2,Yo, Ri, MOG, recoverin, titin, ZIC4, GAD65 were all negative.
TABLE 1.
Laboratory data on admission
| <Blood cell counts> | T‐bil | 0.55 | mg/dl | CYFRA21‐1 | 1.7 | ng/ml | ||
|---|---|---|---|---|---|---|---|---|
| WBC | 9200 | /μl | AST | 54 | IU/L | Pro‐GRP | 44.0 | pg/ml |
| Neutrophils | 88.8 | % | ALT | 52 | IU/L | CA125 | 246.3 | U/ml |
| Lymphocytes | 5.0 | % | LDH | 816 | IU/L | SLX | 30 | U/ml |
| Eosinophils | 0.2 | % | BUN | 9.0 | mg/dl | NSE | 43.9 | ng/ml |
| Monocytes | 5.7 | % | Cre | 0.55 | mg/dl | AFP | 4.5 | ng/mLl |
| RBC | 4.25 × 106 | /μl | Na | 134 | mEq/L | PIVKA‐II | 26.94 | mAU/ml |
| Hb | 13.7 | g/dl | K | 4.2 | mEq/L | sIL‐2R | 771 | U/ml |
| Ht | 40.7 | % | Cl | 101 | mEq/L | <cerebrospinal fluid> | ||
| Platelets | 22.8 × 104 | /μl | Glucose | 107 | mg/dl | Cell counts | 1 | /μl |
| <Blood chemistry> | HbA1c | 5.4 | % | Protein | 16 | mg/dl | ||
| TP | 5.8 | g/dl | CRP | 0.63 | mg/dl | Cytology | No malignancy | |
| Alb | 3.4 | g/dl | CEA | 2.5 | ng/ml | IgG index | 0.47 | |
| CA19‐9 | 5.1 | U/ml | Oligoclonal bands | Absent | ||||
Abbreviations: AFP, α‐fetoprotein; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CA125, cancer antigen 125; CA19‐9, carbohydrate antigen 19–9; CEA, carcinoembryonic antigen; CRP, C‐reactive protein; CYFRA 21–1, cytokeratin fragment 21–1; Hb, hemoglobin; Ht, hematocrit; LDH, lactate dehydrogenase; NSE, neuron‐specific enolase; PIVKA‐II, protein induced by vitamin K absence‐II; Pro‐GRP, Pro‐gastrin‐releasing peptide; RBC, red blood cell; sIL‐2R, soluble interleukin‐2 receptor; SLX, sialyl Lewis X‐i antigen; T‐bil, total bilirubin; TP, total protein; WBC, white blood cell; γ‐GTP, gamma‐glutamyl transferase.
FIGURE 2.
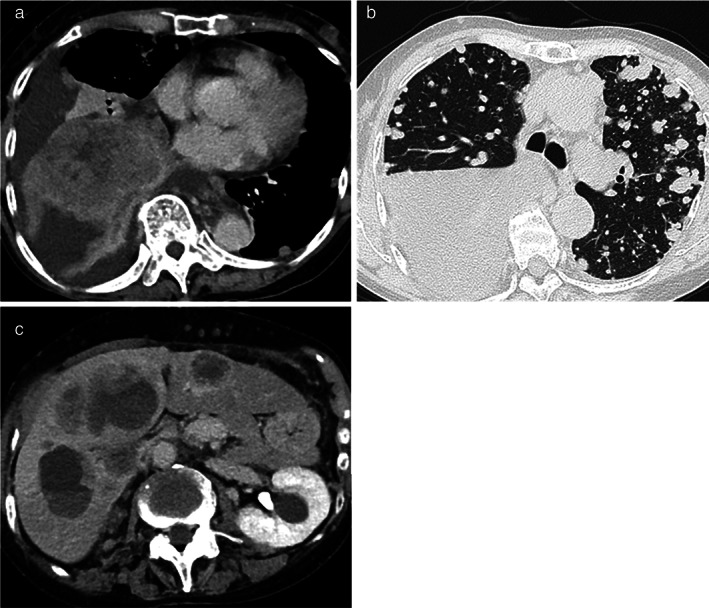
Chest and abdominal computed tomography (CT) scan of the patient. A 110 mm mass was seen in the right lower lobe (a), and multiple irregular pulmonary nodules, right pleural effusion (b), multiple liver metastases, and multiple skin metastases (c) were observed
FIGURE 3.
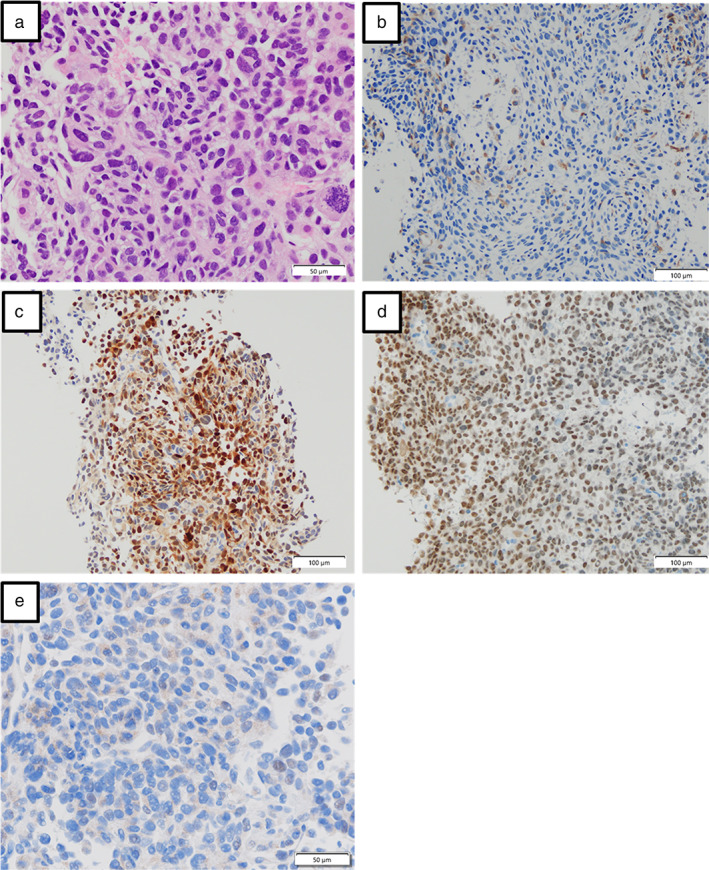
(a) Histopathological findings of the liver biopsy specimen showing atypical cells with prominent nucleoli. Images of immunohistochemical staining, wherein the cytoplasm of malignant cells is stained positive with HMB45 (b) and antibodies against S‐100 (c) and SOX10 (d) and PD‐L1 (e)
Combined systemic chemotherapy with carboplatin (area under the concentration‐time curve, 5), paclitaxel (200 mg/m2), and bevacizumab (15 mg/kg) was administered as first‐line treatment for malignant melanoma, but disease progression was observed after two courses. As such, nivolumab (240 mg) was administered as second‐line treatment. However, pulmonary and liver metastases progressed after two courses of nivolumab with worsening of her respiratory condition and performance status. She died 120 days after the diagnosis of malignant melanoma.
DISCUSSION
We present a rare case of paraneoplastic anti‐AQP4 antibody‐positive NMOSD associated with malignant melanoma that completely fulfilled the diagnostic criteria for both NMOSD and PNS. Our patient was diagnosed with anti‐AQP4 antibody‐positive NMOSD four years before the diagnosis of malignant melanoma.
The prevalence of paraneoplastic neuromyelitis optica (NMO) with positive results for anti‐AQP4 antibodies is 3%–5% in NMO patients and 0.017% in patients suspected to have PNS. 10 , 11 Some cases were also identified as paraneoplastic NMOSD, including various cancers. 10 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Only one case of malignant melanoma complicated with anti‐AQP4 antibody‐positive NMOSD has been reported. 19 However, that patient developed NMOSD while under interferon treatment, and the authors ruled out paraneoplastic NMOSD due to no recurrence of melanoma and a 48‐month interval between melanoma excision and the development of NMOSD. 19 In contrast, our patient developed malignant melanoma after NMOSD. Classical PNS is defined as “a classical syndrome and cancer that develops within five years of the diagnosis of the neurological disorder;” hence, only our patient fulfilled the criteria for PNS. 9
“Paraneoplastic NMOSD” has, in part, been controversial because excluding the possibility of accidental complications might be difficult. Anti‐AQP4 antibodies are thought to be the putative mechanism for the development of NMOSD when cancer cells are positive for anti‐AQP4 antibodies. 10 A case report detailing the development of anti‐AQP4 antibody‐positive NMOSD immediately after the relapse of stomach cancer might support this concept, although there was no immunohistochemical confirmation of AQP4 in cancer cells. 18 Furthermore, paraneoplastic NMOSD has previously been reported in a patient with adenocarcinoma of the esophagogastric (EG) junction that exhibited immunoreactivity to AQP4 and an elevated serum AQP4‐IgG level that turned to be undetectable after surgical resection of the tumor, 19 suggestive of an induction of anti‐AQP4 antibodies by the tumor cells that lead to NNMOSD. In our patient, although the onset of NMOSD could be coincidental, it was also speculated that occult malignant melanoma for four years led to the development of NMOSD.
This case report had some limitations. First, NMOSD treatment, like tacrolimus, may promote carcinogenesis. 20 Second, the biopsy tissues were not stained for AQP4. Third, the detailed causal relationship between NMOSD and malignant melanoma mediated by anti‐AQP4 antibodies remains unknown.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Morimoto T, Hayashida S, Yamasaki K, Sasahara Y, Takaki T, Yatera K. Paraneoplastic neuromyelitis optica spectrum disorder associated with malignant melanoma: A case report. Thorac Cancer. 2021;12:1775–1779. 10.1111/1759-7714.13965
REFERENCES
- 1. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic‐spinal multiple sclerosis binds to the aquaporin‐4 water channel. J Exp Med. 2005;202:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miyamoto K, Fujihara K, Kira JI, Kuriyama N, Matsui M, Tamakoshi A, et al. Nationwide epidemiological study of neuromyelitis optica in Japan. J Neurol Neurosurg Psychiatry. 2018;89:667–8. [DOI] [PubMed] [Google Scholar]
- 3. Nagaishi A, Takagi M, Umemura A, Tanaka M, Kitagawa Y, Masui M, et al. Clinical features of neuromyelitis optica in a large Japanese cohort: comparison between phenotypes. J Neurol Neurosurg Psychiatry. 2011;82:1360–4. [DOI] [PubMed] [Google Scholar]
- 4. Jarius S, Steinmeyer F, Knobel A, Streitberger K, Hotter B, Horn S, et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol. 2013;256:94–6. [DOI] [PubMed] [Google Scholar]
- 5. Becquart C, Ryckewaert G, Desmedt E, Defebvre L, Le Rhun E, Mortier L. Limbic encephalitis: a new paraneoplastic auto‐immune manifestation associated with metastatic melanoma? Ann Dermatol Venereol. 2013;140:278–81.[in French]. [DOI] [PubMed] [Google Scholar]
- 6. Kraft Rovere R, Pires de Souza ME, Fernanda Hilgert S, Rodrigues Chamse Ddine Y, Silva de Lima A. Melanoma metastasis to the gastric mucosa preceded by Guillain‐Barré as a paraneoplastic syndrome. Gastrointest Cancer Res. 2013;6:150–1. [PMC free article] [PubMed] [Google Scholar]
- 7. Bird SJ, Brown MJ, Shy ME, Scherer SS. Chronic inflammatory demyelinating polyneuropathy associated with malignant melanoma. Neurology. 1996;46:822–4. [DOI] [PubMed] [Google Scholar]
- 8. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chinis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pittock SJ, Lennon VA. Aquaporin‐4 autoantibodies in a paraneoplastic context. Arch Neurol. 2008;65:629–32. [DOI] [PubMed] [Google Scholar]
- 11. Sepúlveda M, Sola‐Valls N, Escudero D, Rojc B, Barón M, Hernández‐Echebarria L, et al. Clinical profile of patients with paraneoplastic neuromyelitis optica spectrum disorder and aquaporin‐4 antibodies. Mult Scler. 2018;24:1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mueller S, Dubal DB, Josephson SA. A case of paraneoplastic myelopathy associated with the neuromyelitis optica antibody. Nat Clin Pract Neurol. 2008;4:284–8. [DOI] [PubMed] [Google Scholar]
- 13. De Santis G, Caniatti L, De Vito A, De Gennaro R, Granieri E, Tola MR. A possible paraneoplastic neuromyelitis optica associated with lung cancer. Neurol Sci. 2009;30:397–400. [DOI] [PubMed] [Google Scholar]
- 14. Al‐Harbi T, Al‐Sarawi A, Binfalah M, Dermime S. Paraneoplastic neuromyelitis optica spectrum disorder associated with stomach carcinoid tumor. Hematol Oncol Stem Cell Ther. 2014;7:116–9. [DOI] [PubMed] [Google Scholar]
- 15. Yang HK, Woo SJ, Park WY, Hwang JM. Paraneoplastic neuromyelitis optica associated with ANNA‐1 antibodies in invasive thymoma. BMC Ophthalmol. 2014;14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deuel LM, Bunch ME. A case of paraneoplastic neuromyelitis optica associated with small cell lung carcinoma. J Neuroimmunol. 2018;316:130–2. [DOI] [PubMed] [Google Scholar]
- 17. Kon T, Ueno T, Suzuki C, Nunomura J, Igarashi S, Sato T, et al. Aquaporin‐4 antibody positive neuromyelitis optica spectrum disorder associated with esophageal cancer. J Neuroimmunol. 2017;309:38–40. [DOI] [PubMed] [Google Scholar]
- 18. Takewaki D, Kasai T, Itoh K, Shiga K, Tokui M, Tanaka A, et al. Case of neuromyelitis optica with recurrent stomach carcinoma. Clin Exp Neuroimmunol. 2017;8:327–30. [Google Scholar]
- 19. Sudo A, Chihara N, Takenaka Y, Nakamura T, Ueda T, Sekiguchi K, et al. Paraneoplastic NMOSD associated with EG junction adenocarcinoma expressing unprotected AQP4. Neurol Neuroimmunol Neuroinflamm. 2018;9(5):e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lebrun C, Debouverie M, Vermersch P, Clavelou P, Rumbach L, de Seze J, et al. Cancer risk and impact of disease‐modifying treatments in patients with multiple sclerosis. Mult Scler. 2008;14:399–405. [DOI] [PubMed] [Google Scholar]


