Abstract
Aims/Introduction
The association between gestational diabetes mellitus (GDM) and adverse maternal and perinatal outcomes in twin pregnancies remains unclear. This study was undertaken to highlight risk factors for GDM in women with dichorionic (DC) twins, and to determine the association between GDM DC twins and adverse maternal and perinatal outcomes in a large homogeneous Taiwanese population.
Materials and Methods
A retrospective cross‐sectional study was carried out on 645 women with DC twins, excluding pregnancies complicated by one or both fetuses with demise (n = 22) or congenital anomalies (n = 9), who gave birth after 28 complete gestational weeks between 1 January 2001 and 31 December 2018. Univariable and multiple logistic regression analyses were carried out.
Results
Maternal age >34 years (adjusted odds ratio 2.52; 95% confidence interval 1.25–5.07) and pre‐pregnancy body mass index >24.9 kg/m2 (adjusted odds ratio 2.83, 95% confidence interval 1.47–5.46) were independent risk factors for GDM in women with DC twins. Newborns from women with GDM DC twins were more likely to be admitted to the neonatal intensive care unit (adjusted odds ratio 1.70, 95% confidence interval 1.06–2.72) than newborns from women with non‐GDM DC twins. Other pregnancy and neonatal outcomes were similar between the two groups.
Conclusions
Advanced maternal age and pre‐pregnancy overweight or obesity are risk factors for GDM in women with DC twins. Except for a nearly twofold increased risk of neonatal intensive care unit admission of newborns, the pregnancy and neonatal outcomes for women with GDM DC twins are similar to those for women with non‐GDM DC twins.
Keywords: Dichorionic twins, Gestational diabetes mellitus, Perinatal outcomes
Advanced maternal age and pre‐pregnancy overweight or obesity are risk factors for gestational diabetes mellitus in women with dichorionic twins. Except for a nearly two‐fold increased risk of neonatal intensive care unit admission of newborns, the pregnancy and neonatal outcomes for women with gestational diabetes mellitus with dichorionic twins are similar to those for women with non‐gestational diabetes mellitus with dichorionic twins.
Introduction
It is well recognized that a high glucose level in the diabetic range confers risk to the fetus in singleton pregnancies. Late complications include pre‐eclampsia and large‐for‐gestational age (LGA) infants, consequentially resulting in shoulder dystocia and birth injury 1 , 2 . Neonates from singleton pregnancies with gestational diabetes mellitus (GDM) are also more likely to have hypoglycemia, respiratory distress and hyperbilirubinemia compared with newborns from women with a normal pregnancy 1 . However, the association between GDM and adverse maternal and perinatal outcomes in women with twin pregnancies remains unclear. Several studies concluded that women with twin pregnancies who were complicated by GDM are at an increased risk for gestational hypertension and pre‐eclampsia compared with women with twin pregnancies, but without GDM 3 , 4 , 5 , 6 , 7 , 8 , 9 . Some studies further found that these women also had higher rates of cesarean deliveries (CS) 8 , 10 and induction of labor 6 , whereas others did not show similar findings 7 , 9 , 11 . Studies that focused on the effects of GDM in twin pregnancies on perinatal outcomes presented even more conflicting results. In some studies, the rates of preterm birth 4 , 7 , 10 , LGA 7 , 8 , 10 , 12 , macrosomia 4 , 7 , birth trauma 7 , perinatal death 7 , neonatal jaundice 10 , respiratory distress 13 , 14 and neonatal intensive care unit (NICU) admission 3 , 9 , 14 were higher in women with GDM with twin pregnancies than in those without GDM; on the contrary, other studies found no differences in most major perinatal outcomes, such as preterm birth 6 , 9 , 15 , LGA 5 , 9 , 11 , 15 , low 5‐min Apgar score (<7) 9 , 15 and perinatal death 9 , 15 , 16 between GDM and non‐GDM twins. Some studies even revealed better outcomes, along with reducing risk for small‐for‐gestational age (SGA) infants, preterm birth <32 gestational weeks, low 5‐min Apgar score and perinatal mortality 6 , 17 .
Besides conflicting evidence, re‐evaluation of the association between twin pregnancies with GDM and adverse perinatal outcomes is stipulated for several reasons. First, the rates of GDM and twin pregnancy are progressively growing 2 , 18 , 19 , and their simultaneous presentation is likely to increase. Therefore, clinicians require not only updated, but consistent information to counsel and manage these patients. Second, prior studies were mainly carried out on American and European populations 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 16 , 17 , 20 , 21 , 22 , whereas only one was on Asian women 15 . Third, our recent study showed that risk factors for GDM women with singleton pregnancies included maternal age >34 years, pre‐pregnancy body mass index (BMI) >24.9 kg/m2, a prior history of fetal death, chronic hypertension, genetic amniocentesis and artificial reproductive technology 23 . It is unclear whether a similar risk profile is present in women with twin pregnancy. Finally, many prior studies that utilized birth registries were often with incomplete information, coding errors and misclassification 8 , 14 , 17 , 20 . Furthermore, some studies failed to control confounding variables, such as conception methods, reproductive history, maternal demographics and concurrent pregnancy complications, because this information was absent or unavailable from their birth registries or databases 3 , 11 , 13 , 15 , 22 .
With the aforementioned reasons, the present study was undertaken to investigate the risk factors associated with the development of GDM in women with twin pregnancies, and to determine the association between GDM twin pregnancies and adverse maternal and perinatal outcomes within Taiwanese women. We particularly focused on dichorionic (DC) twins, as this type of twin pregnancy accounts for the majority of twin pregnancies, and has a different antenatal management plan from monochorionic (MC) twins 24 .
Methods
Data collection
The present retrospective cross‐sectional study was carried out to evaluate women who gave birth between 1 January 2001 and 31 December 2018 at Taipei Chang Gung Memorial Hospital, Taipei, Taiwan. Study data were obtained from a computerized obstetrics database, which includes maternal demographics, medical and obstetric histories, and information on the course of the index pregnancy and perinatal outcomes. Details of the organization of the database have been reported previously 23 , 25 , 26 . The institutional review board of the hospital approved the study (No. 201800894B0). Informed consent was not required given the retrospective nature of the study and the anonymity of participant information. All deliveries after 28 gestational weeks by women with DC twin pregnancies were analyzed. Pregnancies complicated by pre‐pregnancy overt diabetes mellitus, fetal chromosomal or structural anomalies and fetal death were excluded. Figure 1 shows the sample selection process. Only the first pregnancy of the participant during the study period was analyzed to avoid concerns regarding the correlated nature of pregnancy outcomes.
Figure 1.
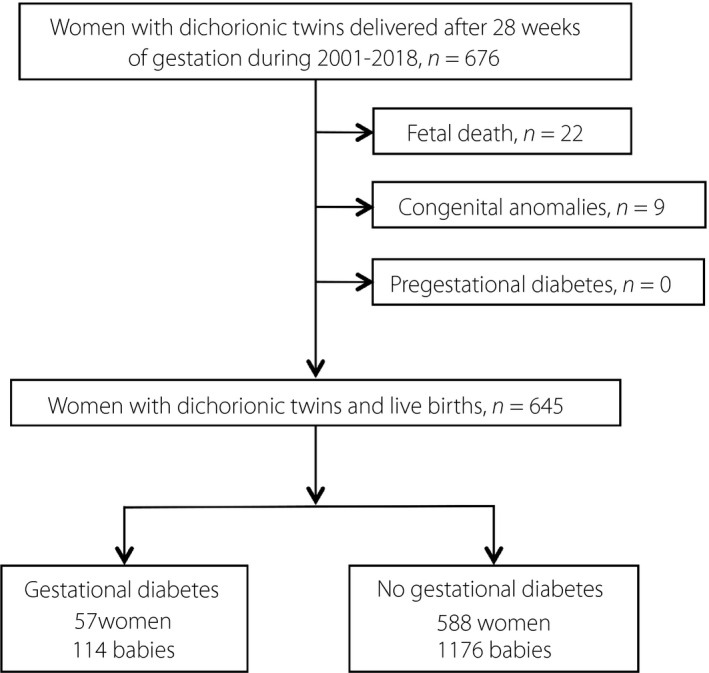
Flow chart of selection of the study population.
Determination of chorionicity
In this hospital, all women with twin pregnancies undergo ultrasonography to determine the chorionicity before 14 complete gestational weeks. A diagnosis of DC twin is made if there are two separate placentas, or one placenta with a positive twin peak sign at the intertwin membrane junction 27 . In contrast, MC twin is defined as one placental mass without the twin peak sign. Chorionicity is further confirmed by neonatal sex at delivery or by postpartum examination of the placenta if the neonatal sexes are the same.
Diagnosis of GDM
In our hospital, all pregnant women are universally screened for GDM between 24 and 28 weeks of gestation. Women with a pre‐pregnancy BMI >24.9 kg/m2 and one of the following risk factors had a 75‐g, 2‐h oral glucose tolerance test (OGTT) to screen for overt diabetes mellitus at their first antenatal visit: (i) known impaired glucose metabolism; (ii) history of GDM, macrosomia and stillbirth; (iii) first‐degree relative with diabetes mellitus; and (iv) hypertension. Overt diabetes mellitus was diagnosed when the fasting glucose level was ≥126 mg/dL or 2‐h glucose level ≥200 mg/dL. If early screening results were negative, GDM screening was repeated at 24–28 weeks of gestation.
Before July 2011, we used a two‐step approach to screen and diagnose GDM, as recommended by the American College of Obstetricians and Gynecologists 28 . Pregnant women underwent a non‐fasting 50‐g, 1‐h glucose challenge test, and if the result was ≥140 mg/dL, a 100‐g, 3‐h OGTT was then carried out. The OGTT thresholds were as follows: fasting glucose, 95 mg/dL; 1‐h, 180 mg/dL; 2‐h, 155 mg/dL; and 3‐h, 140 mg/dL. The GDM diagnosis was established by two or more abnormal values on the OGTT. In January 2011, a one‐step screening strategy recommended by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) was introduced and endorsed by the Taiwan Society of Perinatology. Based on the results of a 75‐g, 2‐h OGTT, a woman was diagnosed to have GDM when one or more of her glucose measurements equaled or exceeded the following levels: fasting, 92 mg/dL; 1 h, 180 mg/dL; or 2 h, 153 mg/dL 29 , 30 . By consensus, the department decided to replace the two‐step method with the one‐step method and IADPSG criteria as our routine method of GDM screening and diagnosis, formally beginning on 1 July 2011 2 , 23 .
Estimation of minimum sample size
One of the main objectives of the present study was to investigate the risk factors for GDM in women with DC twin pregnancies. Advanced maternal age (>34 years) and high pre‐pregnancy BMI (>24.9 kg/m2) are the two most recognized risk factors for GDM 20 , 23 , 31 . According to our previous study of women with singleton pregnancies 23 , the rates of maternal age >34 years, GDM in women aged ≤34 years and GDM in women aged >34 years were 45%, 6% and 13%, respectively. Based on these data, we estimated that a sample size of at least 541 women, including 246 women aged >34 years and 295 women aged ≤34 years, was required to have 80% power to detect the difference with a 95% two‐sided significance level. Furthermore, the rates of women with a high pre‐pregnancy BMI (>24.9 kg/m2), GDM in women with a low or normal pre‐pregnancy BMI (≤24.9 kg/m2), and GDM in women with a high pre‐pregnancy BMI (>24.9 kg/m2) were 11%, 7% and 17%, respectively. Based on these data, we estimated that a sample size of at least 594 women, including 66 women with a high pre‐pregnancy BMI and 528 women with a low or normal pre‐pregnancy BMI, were required to have 80% power to detect the difference with a 95% two‐sided significance level.
Definitions of variables
Variables considered as potential risk factors for GDM included: maternal age at delivery (stratified as <20, 20–34 and >34 years); pre‐pregnancy BMI (stratified as <18.5, 18.5–24.9 and >24.9 kg/m2); primiparity (yes or no); genetic amniocentesis (yes or no); conception assisted by reproductive technology (yes or no); prior histories of induced abortions (yes or no), fetal death (yes or no) and preterm birth (before 37 complete weeks of gestation; yes or no); cigarette smoking during pregnancy (yes or no); medical diseases, such as chronic hypertension (yes or no), hypo‐ and hyperthyroidism (yes or no), uterine fibroids (yes or no), and colonization of group B streptococcus (GBS) at the genito‐rectal tract (yes or no); and male fetus (yes or no).
In our hospital, the height of each pregnant woman was measured and her self‐reported pre‐pregnancy weight was recorded at the first antenatal visit. Height and the self‐reported pre‐pregnancy weight were used to calculate the pre‐pregnancy BMI (calculated as weight [kg] / height [m]2). Furthermore, regardless of the planned mode of birth, we offer universal screening to all pregnant women between 35 and 37 weeks of gestation with a vaginal–rectal swab for GBS culture. The screening is usually carried out 4 weeks earlier in women with twin gestation, and repeated at 35–37 weeks if the initial screening result is negative. When a woman presents with either preterm labor or preterm premature rupture of membranes (PROM), a vaginal–rectal swab for GBS culture is routinely obtained at the time of initial presentation. In addition, women with GBS bacteriuria at any time in pregnancy are regarded as having heavy maternal vaginal–rectal colonization.
The following adverse pregnancy and neonatal outcomes were examined: CS (either elective or emergent); preterm birth before 34 or 37 complete weeks of gestation; pre‐eclampsia, defined as gestational hypertension (two recordings of systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg at least 4 h apart after 20 weeks of gestation) with proteinuria (excretion of urinary protein ≥300 mg in 24 h or a dipstick reading of at least 1+ for midstream urine specimens if 24‐h collection was not available) in previously normotensive women; PROM, defined as rupture of membranes before onset of labor; placental abruption, defined as premature separation of a normally implanted placenta before delivery of the fetus; placenta previa, defined as complete or partial covering of the internal cervical os by placental tissues; placenta accreta, defined as the presence of one of the following criteria: (i) difficult manual, piecemeal removal of placenta, carried out if there was no evidence of placental separation 20 min after parturition, despite active management in the third‐stage labor, (ii) sonographic evidence of retained placental fragments requiring curettage after a vaginal delivery, (iii) heavy bleeding from implantation site after placental removal during CS, managed conservatively with excision of the part of the uterine wall and the attached placenta, or oversewing the bleeding defects, and (iv) histological confirmation of a hysterectomy specimen; postpartum hemorrhage, defined as a blood loss >500 mL for vaginal delivery, 1,000 mL for CS or excessive bleeding that results in signs of hypovolemia, such as hypotension or tachycardia; meconium‐stained amniotic fluid; oligohydramnios, defined as the deepest single maximal vertical pocket measurement <2 cm; polyhydramnios, defined as the deepest single maximal vertical pocket measurement >8 cm; acute chorioamnionitis, defined as maternal body temperature >38°C, rupture of membranes and presence of one of the following conditions: leukocytosis (white blood cell counts >12,000/μL), elevated serum levels of C‐reactive protein (>5 mg/dL) and fetal tachycardia (a baseline rate >160 beats/min) followed by histological confirmation; SGA infants, defined as birthweight <10th percentile of mean weight corrected for fetal sex and gestational age 19 ; LGA infants, defined as birthweight >90th percentile of mean weight corrected for fetal sex and gestational age 19 ; birthweight <1,500 g or 2,500 g; 1‐min and 5‐min Apgar score <7; admission to the NICU; and neonatal death, defined as a death during the first 28 days of life.
Statistical analysis
We first evaluated maternal and pregnancy characteristics, and neonatal outcomes between women with DC twins complicated by GDM and those without GDM. The differences were compared using Student’s t‐test, χ2‐test or Fisher’s exact test when appropriate. We then carried out multiple logistic regression to investigate independent risk factors for GDM in women with DC twins. Variables that were considered as potential risk factors for GDM included: maternal age at delivery; pre‐pregnancy BMI; primiparity; genetic amniocentesis; conception assisted by reproductive technology; histories of induced abortions, fetal death and preterm birth; cigarette smoking during pregnancy; medical diseases, such as chronic hypertension, hypothyroidism and hyperthyroidism; uterine fibroids; colonization of group B streptococcus at the genito‐rectal tract; and male fetus.
To study the association between GDM and pre‐eclampsia, the aforementioned variables and GDM were included in the multiple logistic regression to adjust their confounding effects. Furthermore, to investigate the association between GDM and adverse neonatal outcomes, such as admission to the NICU, the same variables – GDM, PROM and pre‐eclampsia – were included in the multiple logistic regression analysis.
All statistical analyses were carried out using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). Categorical variables are presented as the number (percentage), and continuous variables as mean ± standard deviation. A P‐value <0.05 was considered statistically significant. In the multiple logistic regression, adjusted odds ratios (OR) and associated 95% confidence intervals (CI) were calculated to assess the associations between various risk factors and the development of GDM, and between GDM and adverse pregnancy and neonatal outcomes in women with DC twin pregnancies.
Results
Maternal characteristics and pregnancy outcomes of the study population
During the study period, a total of 676 women with DC twin pregnancies delivered after 28 complete weeks of gestation. After excluding women who had either one or both fetuses with demise (n = 22) or congenital anomalies (n = 9), data of 645 women and their babies were analyzed. Among the 645 women, 57 women (8.8%) were diagnosed with GDM. All 57 GDM women were given lifestyle modification advice, had nutritional therapy and underwent regular monitoring of blood glucose levels. None of them had pharmaceutical treatment for GDM with metformin or insulin.
Maternal characteristics and pregnancy outcomes of the study population are presented in Table 1. The rates of maternal age at delivery >34 years, pre‐pregnancy BMI >24.9 kg/m2 and genetic amniocentesis were higher in women with twin pregnancies complicated by GDM than in women with twin pregnancies, but without GDM. Furthermore, no significant differences were found in the rates of various pregnancy complications, including elective or emergent CS, preterm birth before 34 or 37 weeks of gestation, PROM, placental abruption, placenta previa, placenta accreta, postpartum hemorrhage, meconium‐stained amniotic fluid, oligohydramnios, polyhydramnios and acute chorioamnionitis, between women with GDM twin pregnancies and women with non‐GDM twin pregnancies. Women with twin pregnancies complicated by GDM had a higher rate of pre‐eclampsia (10.5% vs 4.6%) compared with women with twin pregnancies, but without GDM. However, the difference did not reach statistical significance in the univariable analysis (P = 0.061).
Table 1.
Maternal characteristics and pregnancy outcomes of the study population
| No GDM (n = 588) | GDM (n = 57) | P | |
|---|---|---|---|
| Maternal characteristics | |||
| Age (years) | |||
| 20–34 | 308 (52.4%) | 15 (26.3%) | <0.001 |
| >34 | 280 (47.6%) | 42 (73.7%) | <0.001 |
| Pre‐pregnancy body mass index (kg/m2) | |||
| <18.5 | 51 (8.7%) | 2 (3.5%) | 0.175 |
| 18.5–24.9 | 467 (79.4%) | 38 (66.7%) | 0.026 |
| >24.9 | 70 (11.9%) | 17 (29.8%) | <0.001 |
| Weight gain during pregnancy (kg) | 16.0 ± 5.1 | 12.7 ± 5.2 | <0.001 |
| Primiparity | 410 (69.7%) | 43 (75.4%) | 0.449 |
| Prior induced or spontaneous abortions | 179 (30.4%) | 16 (28.1%) | 0.765 |
| Prior fetal death | 9 (1.5%) | 1 (1.8%) | 0.606 |
| Prior preterm birth | 3 (0.5%) | 1 (1.8%) | 0.310 |
| Conception by reproductive technology | 286 (48.6%) | 34 (59.6%) | 0.128 |
| Cigarette smoking during pregnancy | 3 (0.5%) | 0 | 1.000 |
| Genetic amniocentesis | 239 (40.6%) | 32 (56.1%) | 0.025 |
| Uterine fibroids | 8 (1.4%) | 2 (3.5%) | 0.219 |
| Chronic hypertension | 1 (0.2%) | 0 | 1.000 |
| Hyperthyroidism | 4 (0.7%) | 1 (1.8%) | 0.371 |
| Hypothyroidism | 4 (0.7%) | 0 | 1.000 |
| Group B streptococcal colonization | 14 (2.4%) | 3 (5.3%) | 0.184 |
| Pregnancy outcomes | |||
| Cesarean delivery | 528 (89.8%) | 47 (82.5%) | 0.114 |
| Elective | 517 (97.9%) † | 46 (97.9%) ‡ | 0.983 |
| Emergent | 11 (2.1%) § | 1 (2.1%) ¶ | 0.983 |
| Preterm birth <34 weeks | 77 (13.1%) | 8 (14.0%) | 0.838 |
| Preterm birth <37 weeks | 362 (61.6%) | 41 (71.9%) | 0.152 |
| Pre‐eclampsia | 27 (4.6%) | 6 (10.5%) | 0.061 |
| Premature rupture of membranes | 51 (8.7%) | 4 (7.0) | 0.808 |
| Placental abruption | 6 (1.0%) | 0 | 1.000 |
| Placenta previa | 7 (1.2%) | 2 (3.5%) | 0.185 |
| Placenta accreta | 3 (0.5%) | 1 (1.8%) | 0.310 |
| Postpartum hemorrhage | 14 (2.4%) | 0 | 0.625 |
| Meconium‐stained amniotic fluid | 8 (1.4%) | 2 (3.5%) | 0.219 |
| Oligohydramnios | 18 (3.1%) | 0 | 0.392 |
| Polyhydramnios | 4 (0.7%) | 0 | 1.000 |
| Acute chorioamnionitis | 3 (0.5%) | 1 (1.8%) | 0.310 |
Data are presented as a number (%) or mean ± standard deviation. P‐values are based on the χ2‐test, Fisher’s exact test or Student’s t‐test.
GDM, gestational diabetes mellitus.
Including 401 women with maternal request, seven with placenta previa, 33 with a prior history of cesarian deliveries or myomectomy and 76 with malpresentation of the presenting twin.
Including 34 women with maternal request, two with placenta previa, three with a prior history of cesarian deliveries or myomectomy and seven with malpresentation of the presenting twin.
Including three women with acute chorioamnionitis, five with placental abruption and three with unreassuringly fetal heart rate tracing.
One woman with acute chorioamnionitis.
Neonatal characteristics and outcomes of the study population
Neonatal characteristics and outcomes of the study population are presented in Table 2. Newborns of women with GDM twin pregnancies are more likely to be admitted to the NICU than those of women with non‐GDM twin pregnancies (28.9% vs 19.6%, P = 0.028). Besides the aforementioned findings, no significant differences were found in the mean gestational age and birthweight, and rates of other adverse neonatal outcomes, including SGA, LGA, birth weight <1,500 g or <2,500 g, low 1‐min and 5‐min Apgar scores, and neonatal death.
Table 2.
Neonatal outcomes of the study population
| Variable | No GDM (n = 1,176) | GDM (n = 114) | P |
|---|---|---|---|
| Gestational age (weeks) | 35.6 ± 2.0 | 35.4 ± 1.9 | 0.175 |
| Birthweight (g) | 2,317 ± 452 | 2,301 ± 450 | 0.706 |
| Male fetus | 599 (50.9%) | 59 (51.8%) | 0.922 |
| Small‐for‐gestational age infants | 106 (9.0%) | 10 (8.8%) | 1.000 |
| Large‐for‐gestational age infants | 126 (10.7%) | 16 (14.0%) | 0.274 |
| Birthweight <1,500 g | 62 (5.3%) | 3 (2.6%) | 0.268 |
| Birthweight <2,500 g | 743 (63.2%) | 79 (69.3%) | 0.221 |
| 1‐min Apgar score <7 | 52 (4.4%) | 4 (3.5%) | 0.812 |
| 5‐min Apgar score <7 | 5 (0.4%) | 2 (1.8%) | 0.121 |
| Neonatal intensive care unit admission | 231 (19.6%) | 33 (28.9%) | 0.028 |
| Neonatal death | 4 (0.3%) | 0 | 1.000 |
Data are presented as mean ± standard deviation or a number (%). P‐values are based on Student’s t‐test, χ2‐test or Fisher’s exact test.
GDM, gestational diabetes mellitus.
Results of multiple logistic regression
After adjusting for confounding effects among various maternal characteristics, maternal age at delivery >34 years (adjusted OR 2.52; 95% CI 1.25–5.07) and pre‐pregnancy BMI >24.9 kg/m2 (adjusted OR 2.83, 95% CI 1.47–5.46) remained as significant risk factors for GDM in women with DC twin pregnancies (Table 3).
Table 3.
Results of the multiple logistic regression analysis on the risk factors for gestational diabetes in women with dichorionic twin pregnancies †
| Adjusted odds ratio | 95% confidence interval | P | |
|---|---|---|---|
| Maternal age >34 years | 2.52 | 1.25–5.07 | 0.010 |
| Pre‐pregnancy body mass index >24.9 kg/m2 | 2.83 | 1.47–5.46 | 0.002 |
| Primiparity | 1.59 | 0.77–3.26 | 0.208 |
| Prior induced or spontaneous abortions | 0.78 | 0.41–1.51 | 0.465 |
| Prior fetal death | 0.88 | 0.04–19.10 | 0.937 |
| Prior preterm birth | 6.40 | 0.26–159.01 | 0.257 |
| Conception by reproductive technology | 1.10 | 0.59–2.05 | 0.764 |
| Genetic amniocentesis | 1.29 | 0.70–2.38 | 0.415 |
| Uterine fibroids | 1.86 | 0.35–9.78 | 0.463 |
| Hyperthyroidism | 2.90 | 0.29–28.93 | 0.363 |
| Group B streptococcal colonization | 3.16 | 0.81–12.36 | 0.099 |
Cigarette smoking during pregnancy, chronic hypertension and hypothyroidism were not quantifiable, as none of the 57 women with gestational diabetes mellitus had these conditions.
Although a higher rate of pre‐eclampsia was noted in women with twin pregnancies complicated by GDM than that in women with non‐GDM twin pregnancies, the difference was not significant after adjustment for confounding effects of maternal characteristics in the multiple logistic regression (adjusted OR 1.08, 95% CI 0.35–3.36; Table 4). In contrast, the association between GDM and NICU admission remains significant after adjusting for the confounding effects of maternal characteristics and pregnancy complications, including PROM and pre‐eclampsia (adjusted OR 1.70, 95% CI 1.06–2.72; Table 5).
Table 4.
Results of the multiple logistic regression analysis on the association between gestational diabetes and pre‐eclampsia in women with dichorionic twin pregnancies †
| Adjusted odds ratio | 95% confidence interval | P | |
|---|---|---|---|
| Maternal age >34 years | 1.18 | 0.47–2.92 | 0.725 |
| Pre‐pregnancy body mass index >24.9 kg/m2 | 3.07 | 1.28–7.36 | 0.012 |
| Pre‐pregnancy body mass index <18.5 kg/m2 | 0.46 | 0.06–3.63 | 0.464 |
| Primiparity | 13.55 | 1.78–103.46 | 0.012 |
| Prior induced or spontaneous abortions | 1.50 | 0.66–3.40 | 0.337 |
| Conception by reproductive technology | 1.28 | 0.54–3.02 | 0.572 |
| Genetic amniocentesis | 2.43 | 1.04–5.68 | 0.041 |
| Uterine fibroids | 1.81 | 0.19–17.65 | 0.611 |
| Gestational diabetes mellitus | 1.08 | 0.35–3.36 | 0.805 |
History of fetal death, history of preterm birth, cigarette smoking during pregnancy, chronic hypertension, hyperthyroidism, hypothyroidism and group B streptococcal colonization at the genito‐rectal tract were not quantifiable, as none of the 33 women with pre‐eclampsia had these conditions.
Table 5.
Results of the multiple logistic regression analysis on the association between gestational diabetes and admission to the neonatal intensive care unit in women with dichorionic twin pregnancies †
| Adjusted odds ratio | 95% confidence interval | P | |
|---|---|---|---|
| Maternal age >34 years | 1.09 | 0.79–1.51 | 0.612 |
| Pre‐pregnancy body mass index >24.9 kg/m2 | 1.01 | 0.66–1.54 | 0.965 |
| Pre‐pregnancy body mass index <18.5 kg/m2 | 1.34 | 0.81–2.20 | 0.254 |
| Primiparity | 1.29 | 0.90–1.85 | 0.161 |
| Prior induced or spontaneous abortions | 0.93 | 0.78–1.11 | 0.433 |
| Prior fetal death | 2.16 | 0.53–8.78 | 0.281 |
| Conception by reproductive technology | 1.00 | 0.99–1.01 | 0.714 |
| Genetic amniocentesis | 1.67 | 1.22–2.28 | 0.001 |
| Cigarette smoking during pregnancy | 26.91 | 2.96–244.85 | 0.003 |
| Uterine fibroids | 0.89 | 0.29–2.81 | 0.848 |
| Group B streptococcal colonization | 0.36 | 0.11–1.20 | 0.097 |
| Premature rupture of membranes | 3.11 | 1.99–4.86 | <0.001 |
| Gestational diabetes mellitus | 1.70 | 1.06–2.72 | 0.028 |
| Pre‐eclampsia | 2.70 | 1.50–4.84 | 0.001 |
| Hyperthyroidism | 1.40 | 0.33–5.99 | 0.654 |
| Hypothyroidism | 1.83 | 0.35–9.62 | 0.478 |
History of preterm birth and chronic hypertension were not quantifiable, as none of the 132 women with newborns admitted to the neonatal intensive care unit had these conditions.
Discussion
Similar to prior studies of women with singleton and twin pregnancies 20 , 23 , 31 , we found that maternal age at delivery >34 years and pre‐pregnancy BMI >24.9 kg/m2 are major risk factors for GDM in women with DC twin pregnancies. Furthermore, women with GDM twins have similar pregnancy and neonatal outcomes compared with women with non‐GDM twins, except for a nearly twofold increased risk of NICU admission for newborns from women with GDM twins.
GDM has been shown to be a risk factor for pre‐eclampsia in women with singleton 32 and twin pregnancies 3 , 4 , 5 , 6 , 7 , 8 , 9 . In the present study, the rate of pre‐eclampsia was higher in women with GDM twins than in women with non‐GDM twins; the difference was close to being statistically significant in the univariable analysis (P = 0.061). However, the association between GDM and pre‐eclampsia was found to be insignificant in the multiple logistic regression. This indicates that the association between GDM and pre‐eclampsia in women with twin pregnancies is likely caused by confounding factors, such as a high pre‐pregnancy BMI and primiparity.
Other than an increased risk for NICU admission, we found that newborns from women with GDM twins had similar neonatal outcomes to those from women with non‐GDM twins. This was in contrast to several previous reports 4 , 7 , 8 , 10 , 12 , but consistent with a recent meta‐analysis of 13 observational studies 21 . The meta‐analysis found no differences in the incidence of LGA or SGA neonates, respiratory distress, neonatal hypoglycemic, or low Apgar scores between twin pregnancies with and without GDM. We also found that newborns of GDM twins had a higher rate of NICU admission in comparison with newborns of non‐GDM twins, which was consistent with a previous study 21 . The association between maternal GDM and increased risk of neonatal respiratory distress syndrome is well established, and transient respiratory distress is one of the most common cause for admission to the NICU in newborns 33 . Possible mechanisms underlying the association between maternal GDM and increased risk of neonatal respiratory distress syndrome include a delayed secretion of phosphatidylglycerol, and reduced levels of surfactant proteins A and B in fetal lung epithelial cells in response to hyperglycemia in GDM 34 , 35 .
Explanations for the non‐significant differences of other adverse neonatal outcomes between infants of GDM and non‐GDM twins in the present study are not clear. It is likely that previous studies included both MC and DC twins, whereas we only included DC twins for analysis. MC twins are vulnerable to complications of interdependent placental circulations in a way that DC twins are not. Therefore, MC twins are more susceptible to adverse obstetric and perinatal outcomes, such as a lower gestational age, a lower birth weight, and a higher incidence of NICU admission and neonatal morbidity 36 , 37 . Another possibility for the conflicting results could be due to the mode of delivery. Nearly all twin deliveries at our hospital are carried out by an elective CS, thus reducing the rates of labor‐related complications.
In the present study, pregnancies complicated by fetal congenital anomalies or death were excluded from the analysis. It is arguable that fetal congenital anomalies or death are potentially associated with GDM and should be considered as adverse pregnancy outcomes. However, we had several reasons to exclude women with fetal congenital anomalies or demise from the analysis. First, elective termination of pregnancy is permitted in Taiwan in cases of major structural or lethal anomalies, provided that the parents undergo comprehensive counseling with specialists of maternal–fetal medicine and neonatology. Second, we thought that the presence of congenital anomalies or death of either one or both fetuses could have an influence on the decision regarding the timing and modes of delivery. This might cause a bias in the results of our analysis. Third, among 22 women with death of either one or both fetuses, only one had GDM, whereas the remaining 21 women did not. The rates of fetal death in women with and without GDM were 1.7% (1/58) and 3.4% (21/618), respectively. No statistical difference was noted between these two groups of women based on the χ2‐test (P = 0.49). After excluding 22 women with death of either one or both fetuses, among nine women with fetal congenital anomalies, one had GDM, whereas the remaining eight women had no GDM. The rates of fetal anomalies in women with and without GDM were 1.8% (1/57) and 1.3% (8/598), respectively. Again, no statistical difference was noted between these two groups of women (P = 0.80). As a result, we believed that it was more appropriate to exclude women with pregnancies complicated by fetal congenital anomalies and fetal death from the analysis.
The present study was rigorous with regard to the use of data from medical and delivery records, and patient interview, rather than from birth registries, and application of multivariable logistic regression to adjust for confounders. Therefore, the association between GDM and adverse maternal and perinatal outcomes in women with twin pregnancies was objectively investigated. Nevertheless, we acknowledge some limitations of the study. First, it was based on a single tertiary care hospital in Taiwan, thus, limiting the generalizability of the conclusions. Second, due to its observational and retrospective design, some important factors for the development of GDM were not analyzed, because such information was not available in our database. These factors include a prior history of GDM, family history of diabetes mellitus and weight gain before GDM screening. Finally, we did not examine maternal and neonatal metabolomic profiles, neonatal morbidities (i.e., hypoglycemia, hyperbilirubinemia, respiratory distress syndrome and need for phototherapy), and long‐term outcomes of the mothers and their infants. Further studies are required to clarify not only the differences in maternal and neonatal metabolomic profiles between GDM twins and non‐GDM twins, but also whether GDM increases the risks of aforementioned neonatal adverse outcomes. Additionally, more studies are required to provide information on risks of cardiovascular or metabolic diseases later in life in women and children of DC twin pregnancies complicated by GDM.
Furthermore, it might be argued that diagnostic criteria for GDM and some obstetric practice policies that changed during the course of the study might have contributed to the differential changes in outcomes; however, this scenario is unlikely. With the exception of the implementation of the IADPSG criteria for the diagnosis of GDM and a protocol of antenatal magnesium sulfate therapy for neuroprotection in preterm delivery <32 weeks of gestation in 2011, the clinical structure of the department remained unchanged and there were no additional changes in obstetric care during the time period that would be expected to confound the study results. Furthermore, the present recent study showed that GDM women diagnosed by the IADPSG criteria had essentially similar risk factor profile to those GDM women diagnosed by the two‐step method recommended by the American College of Obstetricians and Gynecologists. The only difference was that additional risk factors, such as artificial reproductive technology and genetic amniocentesis, were noted with IADPSG‐defined GDM 23 . Furthermore, we used multiple logistic regression to adjust for the effects of potential confounders (maternal characteristics, obstetric history and conception methods) when we studied and assessed the independent risk factors for GDM, and the association between GDM and adverse pregnancy and neonatal outcomes in women with DC twins. For the aforementioned reasons, we believe that the possible biases relating to a long study period and different GDM diagnostic criteria was negligible.
In summary, we conclude that advanced maternal age and pre‐pregnancy overweight or obesity are significant risk factors for GDM in women with DC twins. Women with GDM DC twins have similar pregnancy and neonatal outcomes to women with non‐GDM DC twins, except for a nearly twofold increased risk of NICU admission for their newborns. The present findings provide useful information for clinicians to identify, counsel and provide timely management for women with twin pregnancies at risk for GDM.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology, Taiwan (109‐2314‐B‐182A‐097‐MY3) and Chang Gung Memorial Hospital (CMRPG1J0071). The authors are grateful to the Taipei Common Laboratory of Chang Gung Memorial Hospital for providing statistical assistance, and to Dr Lulu Huang for English editorial assistance.
J Diabetes Investig 2021; 12: 1083–1091
References
- 1. Landon MB, Gabbe SG. Gestational diabetes mellitus. Obstet Gynecol 2011; 118: 1379–1393. [DOI] [PubMed] [Google Scholar]
- 2. Hung TH, Hsieh TT. The effects of implementing the International Association of Diabetes and Pregnancy Study Groups criteria for diagnosing gestational diabetes on maternal and neonatal outcomes. PLoS One 2015; 10: e0122261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buhling KJ, Henrich W, Starr E, et al. Risk for gestational diabetes and hypertension for women with twin pregnancy compared to singleton pregnancy. Arch Gynecol Obstet 2003; 269: 33–36. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez Gonzalez NL, Goya M, Bellart J, et al. Obstetric and perinatal outcome in women with twin pregnancy and gestational diabetes. J Matern Fetal Neonatal Med 2012; 25: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 5. Guillen MA, Herranz L, Barquiel B, et al. Influence of gestational diabetes mellitus on neonatal weight outcome in twin pregnancies. Diabet Med 2014; 31: 1651–1656. [DOI] [PubMed] [Google Scholar]
- 6. Okby R, Weintraub AY, Sergienko R, et al. Gestational diabetes mellitus in twin pregnancies is not associated with adverse perinatal outcomes. Arch Gynecol Obstet 2014; 290: 649–654. [DOI] [PubMed] [Google Scholar]
- 7. Dinham GK, Henry A, Lowe SA, et al. Twin pregnancies complicated by gestational diabetes mellitus: a single centre cohort study. Diabet Med 2016; 33: 1659–1667. [DOI] [PubMed] [Google Scholar]
- 8. Lai FY, Johnson JA, Dover D, et al. Outcomes of singleton and twin pregnancies complicated by pre‐existing diabetes and gestational diabetes: a population‐based study in Alberta, Canada, 2005–11. J Diabetes 2016; 8: 45–55. [DOI] [PubMed] [Google Scholar]
- 9. Ooi S, Wong VW. Twin pregnancy with gestational diabetes mellitus: a double whammy? Diabetes Care 2018; 41: e15–e16. [DOI] [PubMed] [Google Scholar]
- 10. Hiersch L, Berger H, Okby R, et al. Gestational diabetes mellitus is associated with adverse outcomes in twin pregnancies. Am J Obstet Gynecol 2019; 220: 102 e1‐02 e8. [DOI] [PubMed] [Google Scholar]
- 11. Poulain C, Duhamel A, Garabedian C, et al. Outcome of twin pregnancies associated with glucose intolerance. Diabetes Metab 2015; 41: 387–392. [DOI] [PubMed] [Google Scholar]
- 12. Tward C, Barrett J, Berger H, et al. Does gestational diabetes affect fetal growth and pregnancy outcome in twin pregnancies? Am J Obstet Gynecol 2016; 214: 653 e1–8. [DOI] [PubMed] [Google Scholar]
- 13. Simoes T, Queiros A, Correia L, et al. Gestational diabetes mellitus complicating twin pregnancies. J Perinat Med 2011; 39: 437–440. [DOI] [PubMed] [Google Scholar]
- 14. Rauh‐Hain JA, Rana S, Tamez H, et al. Risk for developing gestational diabetes in women with twin pregnancies. J Matern Fetal Neonatal Med 2009; 22: 293–299. [DOI] [PubMed] [Google Scholar]
- 15. Cho HJ, Shin JS, Yang JH, et al. Perinatal outcome in twin pregnancies complicated by gestational diabetes mellitus: a comparative study. J Korean Med Sci 2006; 21: 457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sheehan ACM, Umstad MP, Cole S, et al. Does gestational diabetes cause additional risk in twin pregnancy? Twin Res Hum Genet 2019; 22: 62–69. [DOI] [PubMed] [Google Scholar]
- 17. Foeller ME, Zhao S, Szabo A, et al. Neonatal outcomes in twin pregnancies complicated by gestational diabetes compared with non‐diabetic twins. J Perinatol 2015; 35: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 18. Ananth CV, Chauhan SP. Epidemiology of twinning in developed countries. Semin Perinatol 2012; 36: 156–161. [DOI] [PubMed] [Google Scholar]
- 19. Hu IJ, Hsieh CJ, Jeng SF, et al. Nationwide twin birth weight percentiles by gestational age in Taiwan. Pediatr Neonatol 2015; 56: 294–300. [DOI] [PubMed] [Google Scholar]
- 20. Hiersch L, Berger H, Okby R, et al. Incidence and risk factors for gestational diabetes mellitus in twin versus singleton pregnancies. Arch Gynecol Obstet 2018; 298: 579–587. [DOI] [PubMed] [Google Scholar]
- 21. Mcgrath RT, Hocking SL, Scott ES, et al. Outcomes of twin pregnancies complicated by gestational diabetes: a meta‐analysis of observational studies. J Perinatol 2017; 37: 360–368. [DOI] [PubMed] [Google Scholar]
- 22. Klein K, Mailath‐Pokorny M, Leipold H, et al. Influence of gestational diabetes mellitus on weight discrepancy in twin pregnancies. Twin Res Hum Genet 2010; 13: 393–397. [DOI] [PubMed] [Google Scholar]
- 23. Hung TH, Chu FL, Hsieh TT. Risk factors for gestational diabetes mellitus among women screened with the two‐step and one‐step methods: a before‐and‐after study. Taiwan J Obstet Gynecol 2018; 57: 668–671. [DOI] [PubMed] [Google Scholar]
- 24. Committee on Practice Bulletins–Obstetrics and the Society for Maternal–Fetal Medicine . Practice Bulletin No. 169: multifetal gestations: twin, triplet, and higher‐order multifetal pregnancies. Obstet Gynecol 2016; 128: e131–e146. [DOI] [PubMed] [Google Scholar]
- 25. Hung TH, Chen SF, Hsu JJ, et al. Gestational weight gain and risks for adverse perinatal outcomes: a retrospective cohort study based on the 2009 Institute of Medicine guidelines. Taiwan J Obstet Gynecol 2015; 54: 421–425. [DOI] [PubMed] [Google Scholar]
- 26. Hung TH, Hsieh TT. Pregestational body mass index, gestational weight gain, and risks for adverse pregnancy outcomes among Taiwanese women: a retrospective cohort study. Taiwan J Obstet Gynecol 2016; 55: 575–581. [DOI] [PubMed] [Google Scholar]
- 27. Lee YM, Cleary‐Goldman J, Thaker HM, et al. Antenatal sonographic prediction of twin chorionicity. Am J Obstet Gynecol 2006; 195: 863–867. [DOI] [PubMed] [Google Scholar]
- 28. Committee on Obstetric Practice, American College of Obstetricians and Gynecologists . Committee opinion no. 504: Screening and diagnosis of gestational diabetes mellitus. Obstet Gynecol 2011; 118: 751–753. [DOI] [PubMed] [Google Scholar]
- 29. Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 31. Cozzolino M, Serena C, Maggio L, et al. Analysis of the main risk factors for gestational diabetes diagnosed with International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria in multiple pregnancies. J Endocrinol Invest 2017; 40: 937–943. [DOI] [PubMed] [Google Scholar]
- 32. Catalano PM, Mcintyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012; 35: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y, Wang W, Zhang D. Maternal diabetes mellitus and risk of neonatal respiratory distress syndrome: a meta‐analysis. Acta Diabetol 2019; 56: 729–740. [DOI] [PubMed] [Google Scholar]
- 34. Piper JM. Lung maturation in diabetes in pregnancy: if and when to test. Semin Perinatol 2002; 26: 206–209. [DOI] [PubMed] [Google Scholar]
- 35. Miakotina OL, Dekowski SA, Snyder JM. Insulin inhibits surfactant protein A and B gene expression in the H441 cell line. Biochim Biophys Acta 1998; 1442: 60–70. [DOI] [PubMed] [Google Scholar]
- 36. Feng B, Zhai J, Cai Y. Effect of twin pregnancy chorionic properties on maternal and fetal outcomes. Taiwan J Obstet Gynecol 2018; 57: 351–354. [DOI] [PubMed] [Google Scholar]
- 37. Hack KEA, Vereycken M, Torrance HL, et al. Perinatal outcome of monochorionic and dichorionic twins after spontaneous and assisted conception: a retrospective cohort study. Acta Obstet Gynecol Scand 2018; 97: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]


