Abstract
Aims/Introduction
Psychological therapies have showed benefits for both glycemic control and psychological outcomes in people with diabetes. However, the effects of mindfulness‐based intervention (MBI) on glycemic control and psychological outcomes are inconsistent across studies, and the evidence for MBI has not been summarized. We aimed to identify the effects of MBI on glycemic control and psychological outcomes in people with diabetes by carrying out a systematic review and meta‐analysis.
Materials and Methods
Six databases (Pubmed, Embase, CINAHL, Cochrane, Web of science and PsycINFO) were searched from inception to October 2019. Randomized controlled trials of MBI for people with type 1 and type 2 diabetes were included. Two authors independently extracted relevant data and assessed the risk of bias, with a third reviewer as arbitrator. Subgroup analyses and sensitivity analyses were also carried out.
Results
Eight studies with 841 participants met the eligibility criteria. Meta‐analysis showed that MBI can slightly improve glycosylated hemoglobin (HbA1c; −0.25%, 95% confidence interval [CI] −0.43 to −0.07) and diabetes‐related distress (−5.81, 95% CI −10.10 to −1.52) contribute to a moderate effect size in reducing depression (standardized mean difference −0.56, 95% CI −0.82 to −0.30) and stress (standardized mean difference −0.53, CI −0.75 to −0.31). Subgroup analyses showed greater HbA1c reductions in subgroups with baseline HbA1c levels <8% and follow‐up duration >6 months. Mixed effects were observed for anxiety.
Conclusions
MBI appears to have benefits on HbA1c, depression, stress and diabetes‐related distress in people with diabetes. More rigorous studies with longer follow‐up duration are warranted to establish the full potential of MBI.
Keywords: Diabetes, Meta‐analysis, Mindfulness‐based intervention
This is the first meta‐analysis of RCTs to quantify the magnitude of improvement in HbA1c, depression, stress, and diabetes‐related distress from mindfulness‐based intervention (MBI) in people with dabetes. Our review found that MBI can slightly improve glycosylated hemoglobin (HbA1c) with a reduction of 0.25%. MBI can also improve depression, stress, and diabetes‐related distress. Effects of MBI may differ by baseline psychological disorder and follow‐up duration.

INTRODUCTION
People with diabetes are more likely to suffer from clinically significant psychological disorders than those without the disease 1 , 2 , 3 , 4 . Results from the literature showed that psychological disorders contributed to poor self‐care, worsened blood glucose levels, diminished quality of life and increased healthcare costs 5 , 6 . The American Diabetes Association “Standards of Medical Care in Diabetes‐2020” included psychosocial care as a part of recommended therapy in diabetes management 7 . Hence, psychosocial care has been considered an essential component of successful diabetes management.
One potential effective psychological treatment consists of mindfulness‐based intervention (MBI). In recent years, MBI has been successfully implemented to improve psychological health and coping skills 8 in a range of clinical populations, including chronic disease 9 , pain disorders 10 and cancer 11 . Encouragingly, the benefits of MBI for psychological disorders have been found in people with diabetes 12 , 13 , 14 . In addition, a few studies suggested that MBI might have a favorable effect on glycemic control in people with diabetes 15 . Previous studies supposed that psychological interventions, such as cognitive behavioral therapy (CBT) and problem solving, improved glycemic control in people with diabetes through improving adherence to medical care and the ability to manage negative emotions 16 , 17 . However, the mechanisms responsible for the effects of psychological interventions, including MBI on glycemic control, were uncertain. Mindfulness is defined as “the awareness that emerges through paying attention on purpose, in the present moment and non‐judgmentally to things as they are” 18 . MBI refers to those interventions in which mindfulness practices are explicitly taught as a key ingredient in the treatment 19 , 20 . It is known that diabetes as a disease affects both body and mind, requiring considerable physical, emotional, and psychological accommodation and coping 21 . MBI increases levels of mindfulness and non‐judgmental acceptance, and decreases negative reactivity and repetitive negative thinking, which in turn lead to positive outcomes 22 . Commonly used MBIs include mindfulness‐based stress reduction (MBSR), mindfulness‐based cognitive therapy (MBCT) and mindfulness‐based eating awareness training (MB‐EAT). MBSR usually includes a body scan, meditation, and informal daily mindfulness practice to overcome pain, stress and illness. 23 MBCT is quite similar to MBCT, but MBCT does not include the loving kindness meditation instructions 23 . MB‐EAT includes reducing episodes of overeating and improving disordered eating behaviors through mindful meditation 23 , 24 . Usually, MBI entails participating in group‐based weekly 1–2 ‐h long sessions for a period of 8 weeks 23 , 25 . Several studies modified MBI slightly to make it more feasible and acceptable, including changing the period of intervention 26 , delivering MBI through audio compact disc 27 or providing intervention for individuals instead of a group 13 .
Although MBI is considered as a promising therapy for psychosocial problems 28 , 29 , and has become increasingly popular and available in recent years, the effects on both psychological outcomes and glycemic control among people with diabetes are mixed 30 , especially for glycemic control 12 , 14 , 15 . In addition, existing reviews on MBI for people with diabetes are limited, and do not provide firm conclusions on its effectiveness on glycosylated hemoglobin (HbA1c), depression, stress, anxiety and diabetes‐related distress. One systematic review showed that MBI appeared to have psychological benefits reducing depression, anxiety and distress symptoms, but no glycemic control benefits were observed 31 . Only one meta‐analysis tested the effects of MBI on HbA1c and diabetes‐related distress, which included non‐randomized controlled trials 30 . It is worth noting that the mixed interventions were not excluded in that meta‐analysis, making it difficult to draw reliable conclusions on the specific effects of MBI due to the contamination. In addition, diabetes‐related distress was the primary outcome in that meta‐analysis, other common psychological outcomes (i.e., depression, stress and anxiety) were not tested.
Given the mixed results on glycemic control and psychological outcomes of MBI and limited meta‐analysis of MBI among people with diabetes, there is a need to quantitatively analyze evidence based on randomized controlled clinical trials (RCTs) to determine its pooled effectiveness among people with diabetes. As such, this systematic review aimed to identify the effects of MBI on HbA1c, depression, stress, diabetes‐related distress and anxiety in adults with diabetes.
METHODS
The protocol of this review was registered in PROSPERO (CRD42020159088). This study followed the Preferred Reporting Items for Systematic Review and Meta‐analyses (PRISMA) statement and checklist.
Data sources and search strategy
Six databases were searched (Pubmed, Embase, CINAHL, Cochrane, Web of science and PsycINFO) for studies from inception and to 16 October 2019. We designed strategies that included Medical Subject Headings; keywords were searched, such as “diabetes,” “T2DM,” “T1DM,” “IDDM,” “NIDDM,” “mindfulness*,” “MBI,” “meditation,” “MBSR,” “MBCT,” “MB‐EAT,” “mind body therapies” and comprehensive combinations of these search terms. The detailed search strategy can be found in Table S1. A manual search of reference lists of shortlisted articles for relevant reviews was also carried out to identify additional studies.
Study selection
Studies meeting the following inclusion criteria were included: (i) individuals aged >18 years with type 1 or type 2 diabetes; (ii) interventions included any interventions that MBI was a major component of, such as MBSR, MBCT and MB‐EAT; (iii) studies that compared MBI with usual care, wait‐list control, no intervention or health education without any mindful component; (iv) studies reported on at least one of the outcomes of depression, stress, anxiety, distress and glycemic control (HbA1c); and (v) studies that were RCTs published in English. Exclusion criteria were as follows: (i) individuals with gestational diabetes or prediabetes; (ii) exercise‐focused intervention programs with mindfulness as a component, mixed interventions (i.e., Tai Chi and meditation), because these interventions might result in contamination for the specific effects of MBI; and (iii) dialectical behavior therapy, and acceptance and commitment therapy, because these two therapies stemmed from different theoretical roots compared with other commonly used MBIs 20 . Two authors independently searched the literature and assessed the studies. Any disagreements were resolved through discussion with a third reviewer.
Data extraction
Data from the included studies were independently extracted by two authors using a standardized data extraction form guided by the Cochrane Handbook 32 . These two authors reviewed the collected data twice to ensure extraction accuracy. Extracted data included study setting, design, duration, sample size, participant demographics (i.e., age, sex), intervention characteristics (i.e., frequency, duration of each mindfulness session, intervention type), control characteristics, dropout rate and outcomes measures. When a study included multiple control arms, we utilized the control arm with the best matched comparison (i.e., waiting‐list control, patient education group). Study authors were contacted by email to provide additional data when the data was incomplete. Because three studies reported the standard error or 95% confidence interval for depression, stress, anxiety and HbA1c 14 , 26 , 33 , missing standard deviations in those studies were imputed by calculation based on the Cochrane Handbook 32 .
Quality assessment
Two authors independently assessed quality using the Cochrane risk of bias tool, which included the items of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias. Any discrepancies in data extraction or quality assessments were discussed and resolved by a third reviewer.
Statistical analysis
Pooled analyses were carried out on Review Manager (version 5.3, The Cochrane Collaboration, Copenhagen, Denmark), as recommended by the Cochrane Handbook. Meta‐analyses were based on a random effects model to obtain more conservative estimates. Changes in outcomes, or outcomes at follow up, were compared between groups. The pooled intervention effect estimates for HbA1c and distress were calculated by mean difference (MD), and the pooled intervention effect estimates for other outcomes, including depression, stress and anxiety, were calculated by standardized MD (SMD), as those outcomes were measured by different scales. The SMD, also known as the Cohen’s d, was used to evaluate the magnitude of effect size (0.2 represented a small effect, 0.5 a moderate effect and 0.8 a large effect) 34 .
Heterogeneity was identified by Cochran’s Q‐test and I 2 statistics, where I 2> 50% and a P‐value of Q‐test <0.10 showed heterogeneity between studies. Possible sources of heterogeneity were explored by subgroup analyses and sensitivity analyses. In this review, the subgroups formed were based on previous meta‐analysis results 35 , 36 , 37 , including baseline HbA1c level, baseline psychological status and duration of follow up. Initially, the diabetes type was considered as a factor to carry out subgroup analysis, as previous meta‐analysis of diabetes psychological interventions showed different pooled results among type 1 and type 2 diabetes patients 16 , 38 , 39 . However, no study included in the present review reported outcomes on people only with type 1 diabetes, making it impossible to identify diabetes type‐specific effects of MBI. Sensitivity analyses were also carried out based on the study characteristics (i.e., duration of intervention time). The funnel plots for assessing publication bias were not carried out, as available trials were <10. When quantitative synthesis was not appropriate, a narrative synthesis was carried out instead.
RESULTS
Study selection and characteristics
The literature search and selection process are shown in Figure 1. Of the 1,874 potentially relevant records identified and screened, 103 records were eligible for full text review and 92 were excluded. Finally, 11 articles describing eight different studies with 841 participants were selected for inclusion in the review 12 , 13 , 14 , 15 , 42 . The number of participants ranged from 24 to 139 in each study. Four studies had a mixture of type 1 and type 2 diabetes patients; four studies only included type 2 diabetes patients. The details of the characteristics of the included studies can be found in Table 1.
Figure 1.
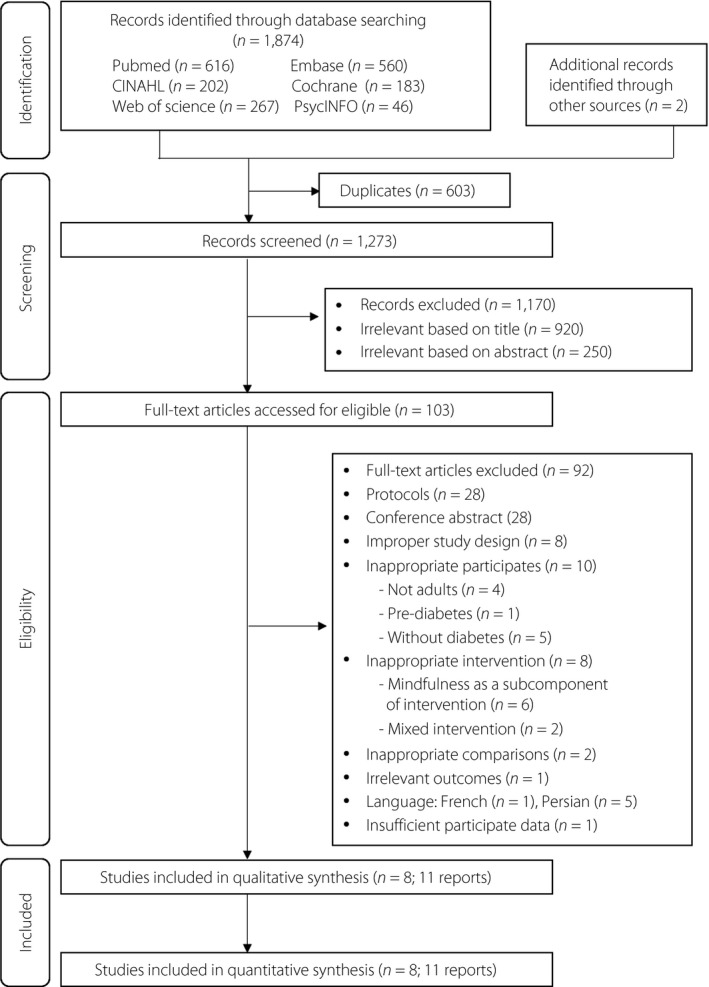
Flow diagram of included studies.
Table 1.
Summary of basic study characteristics
| Author (year) | Study design | Country, recruitment setting | Diabetes type |
No. participants |
Mean age of participants (years) | Male participants (%) | Intervention group | Comparison group | MBI protocol | Assessment time points | Outcomes | ITT analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hartmann et al., (2012); Kopf et al., (2014) |
2‐arm RCT | Germany, outpatient clinic | Type 2 |
Total: 110 IG: 53 CG: 57 |
Total: not reported IG: 68.7 CG: 59.3 |
Total: 78.2% IG: 75.5% CG: 80.7% |
MBSR | usual care | once weekly over 8 weeks, followed by one booster session after 6 months | Baseline, Postintervention, 1‐year, 2‐year, and 3‐year follow up | Depression (PHQ‐9), Stress (PHQ‐9), HbA1c | Yes |
| Jung et al., (2015) | 3‐arm RCT | South Korea, outpatient clinics | Type 2 |
Total: 84 IG: 28 CG1: 28 CG2: 28 |
Total: 66.27 IG: 67.00 CG1: 63.33 CG2: 68.47 |
Total: 48.2% IG: 47.6% CG1: 50% CG2: 47.1% |
MBSR |
CG1: walking group CG2: patient education group |
twice per week for 8 weeks and learned 8 K‐MBSR themes. Each theme was introduced and practiced twice in 1 week. Each theme lasted between 60–120 min. | Baseline, Postintervention |
Stress (Diabetes Distress Scale) |
No |
|
Miller et al., (2012); Miller et al., (2014) |
2‐arm RCT | USA, not specified | Type 2 |
Total: 68 IG:32 CG:36 |
Total: Not reported IG: 53.9 CG: 54.0 |
Total:36.6% IG:37.0% CG:36.0% |
Mindful Eating Intervention |
Smart Choices DSME–based intervention | eight weekly and 2 biweekly 2.5‐h sessions, and meditation with a compact disc 6 days/week and to practice mini‐meditations before meals | Baseline, Postintervention, 3 months follow up | Depression (BDI‐II), anxiety (Beck Anxiety Inventory), HbA1c | No |
| Nathan et al., (2017) | 2‐arm RCT | Canada, endocrine and diabetes center, community | Type 1 or type 2 |
Total: 66 IG: 33 CG: 33 |
Total: 59.7 IG: 59.7 CG: 59.8 |
Total: 44.0% IG: 50.0% CG: 37.5% |
MBSR | wait‐list control | 8 weekly, 2.5‐h sessions and 1 6‐h session on a weekend day midway through the course. | Baseline, at the time of randomization, 2 weeks, 3 months | depression (PHQ‐9), stress (PSS), HbA1c | No |
| Pearson et al., (2018) | 2‐arm RCT | Australia, outpatient clinics | Type 2 |
Total: 74 IG: 38 CG: 36 |
Total: Not reported IG: 57.5 CG: 61.1 |
Total:53.7% IG: 38.7% CG: 66.7% |
A novel approach to delivering a mindfulness‐based intervention | usual care | 30 min a day over 8 weeks to listen to the audio compact disc guided mindfulness | Baseline, 8 weeks, and 12 weeks | Depression (DASS‐21), Anxiety (DASS‐21), stress (DASS‐21), distress (PAID), HbA1c | Yes |
| Schroevers et al., (2015) | 2‐arm Pilot RCT | the Netherlands, outpatient clinic | Type 1 or type 2 |
Total:24 IG: 12 CG:12 |
Total: Not reported IG: 54.9 CG:55.9 |
Total: 58% IG: 58% CG: 58% |
individual MBCT | wait‐list control |
eight weekly individual sessions of 60 min |
Baseline, Postintervention, 3 months after the intervention | Depression (CES‐D), distress (PAID), | Yes |
| Tovote et al., (2014) | 3‐arm RCT | the Netherlands, outpatient clinics | Type 1 or type 2 |
Total:94 IG: 31 CG1: 32 CG2: 31 |
Total: 53.1 IG: 49.8 CG1: 54.6 CG2: 54.7 |
Total: 51% IG: 55% CG1: 50% CG2: 48% |
MBCT |
CG1: cognitive behavior therapy CG2: waiting‐list control |
eight weekly sessions of 45–60 min | Baseline, Postintervention (on average, 3 months after the first assessment) | Depression (BDI‐II), Anxiety (GAD‐7), distress (PAID), HbA1c | Yes |
|
van Son et al., (2013); van Son et al., (2014) |
2‐arm RCT |
the Netherlands, outpatient clinics |
Type 1 or type 2 |
Total:139 IG: 70 CG: 69 |
Total: Not reported IG: 56 CG: 57 |
Total: 50.4% IG: 47% CG: 54% |
MBCT + MBSR | wait‐list control | 8 weekly 2‐h sessions, a 2‐h booster session was added 3 months after the end of the intervention | Baseline, 4 weeks, 8 weeks and 6 months follow up | Depression (HADS), Anxiety (HADS), Stress (PSS), Distress (PAID), HbA1c | Yes |
Total n = 8; 11 reports. BDI‐II, Beck Depression Inventory‐II; CES‐D, Centre for Epidemiologic Studies Depression Scale; CG, comparison group; DASS‐21, the 21‐item Depression, Anxiety and Stress Scale; DSME, Diabetes Self‐Management Education; GAD‐7, Generalized Anxiety Disorder 7; HADS, The Hospital Anxiety and Depression Scale; HbA1c, glycosylated hemoglobin; IG, intervention group; ITT analysis, intention‐to‐treat analysis; K‐MBSR, Korean Mindfulness‐Based Stress Reduction; MBCT, Mindfulness‐Based Cognitive Therapy; MBSR, Mindfulness‐Based Stress Reduction; PAID, Problem Areas in Diabetes Survey; PHQ‐9, Patient Health Questionnaire; PSS, Perceived Stress Scale; RCT, Randomized Controlled Trial.
Studies carried out various forms of mindfulness‐based intervention, including MBSR (3 studies), MBCT (2 studies), mindful eating (1 study), combination of MBSR and MBCT (1 study), and unspecific mindfulness‐based intervention (1 study). Those three MBSR studies focused on body and meditation practices 14 , 33 , 40 ; two MBCT studies delivered intervention integrating MBSR and CBT 13 , 41 . One mindful eating intervention study applied mindful meditation to eating 26 ; one unspecific mindfulness‐based intervention study guided mindfulness practice and breath awareness 27 . The duration of intervention ranged from 8 weeks to 3 months, with sessions varying from 30 min to 150 min. All studies delivered the sessions face‐to‐face, except one study 27 through audio compact disc. Five studies provided group‐based intervention 12 , 14 , 26 , 33 , 40 , and three studies provided individual intervention 13 , 27 , 41 . Psychological outcomes were measured by different tools. A summary of the outcome data from all studies can be found in Table S2.
Risk of bias
Seven studies detailed their random sequence generation process, and four studies carried out allocation concealment 12 , 14 , 27 , 40 . One study documented participants’ blinding 13 , and another study blinded the MBI provider 27 . Three studies blinded the outcome assessors and were rated a low risk of detection bias 14 , 27 , 40 . Five studies carried out intention‐to‐treat analysis, Six studies 12 , 13 , 14 , 27 , 33 , 41 reported attrition rates of <20%, and two studies 26 , 40 reported attrition rates of >20%, but no significant difference between groups regarding attrition rates. Hence, all studies were rated as a low risk of attrition bias. Three studies published or registered a protocol and reported all prespecified outcomes (Table 2).
Table 2.
Risk of bias of individual studies according to the Cochrane guideline
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Hartmann et al., (2012) | Unclear | Unclear | High | Unclear | Low | Low | Unclear |
| Jung et al., (2015) | Low | Low | High | Low | Low | Unclear | Unclear |
| Kopf et al., (2014) | Unclear | Unclear | High | Unclear | Low | High | Unclear |
| Miller et al., (2012); | Low | Unclear | High | Unclear | Low | Unclear | Unclear |
| Miller et al., (2014) | Low | Unclear | High | Unclear | Low | Unclear | Unclear |
| Nathan et al., (2017) | Low | Low | High | Low | Low | Low | Unclear |
| Pearson et al., (2018) | Low | Low | Low | Low | Low | Low | Unclear |
| Schroevers et al., (2015) | Low | Unclear | High | Unclear | Low | Unclear | Unclear |
| Tovote et al., (2014) | Low | Unclear | Low | High | Low | High | Unclear |
| van Son et al., (2013) | Low | Low | High | Unclear | Low | Low | Unclear |
| van Son et al., (2014) | Low | Low | High | Unclear | Low | Low | Unclear |
Effects of MBI on outcomes
HbA1c
Seven studies measured HbA1c 12 , 14 , 15 , 26 , 27 , 33 , 42 , and only one study reported a reduction of HbA1c in participants accepting MBI 15 . After pooling, the mean reduction in HbA1c was 0.25% (95% CI −0.43 to −0.07; P = 0.006), with no substantial heterogeneity between studies (I 2 = 0%; P = 0.92; Figure 2a).
Figure 2.
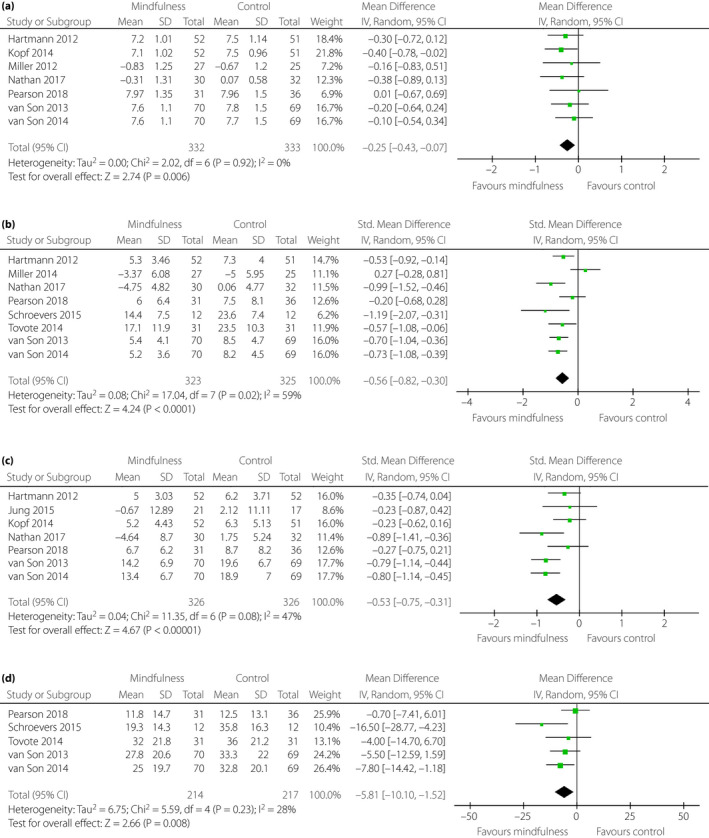
Forest plot. Effectiveness of mindfulness‐based intervention on (a) glycosylated hemoglobin, (b) depression, (c) stress and (d) diabetes‐related distress. CI, confidence interval; SD, standard deviation.
Depression
Eight studies measured depression using five tools (Patient Health Questionnaire, Beck Depression Inventory‐II, 21‐item Depression, Anxiety and Stress Scale, Center for Epidemiologic Studies Depression Scale, and the Hospital Anxiety and Depression Scale) 12 , 13 , 14 , 24 , 27 , 33 , 41 , 42 . The overall pooled effect size for depression was moderate (SMD −0.56, 95% CI −0.82 to −0.30; P < 0.0001; Figure 2b). However, substantial heterogeneity was found between the studies (I 2 = 59%; P = 0.02).
Stress
Seven studies measured stress using three tools (Patient Health Questionnaire, 21‐item Depression, Anxiety and Stress Scale and Perceived Stress Scale) 12 , 14 , 15 , 27 , 33 , 40 , 42 . The overall pooled effect size for stress was moderate (SMD −0.53, CI −0.75 to −0.31; P < 0.00001). However, the heterogeneity test was contradictory by Cochran’s Q‐test (P = 0.08) and I 2 statistics (I 2 = 47%; Figure 2c).
Diabetes‐related distress
Five studies measured distress using Problem Areas in Diabetes Survey scale 12 , 13 , 27 , 41 , 42 . The overall pooled results showed a statistical significance in reducing distress (MD −5.81, 95% CI −10.10 to −1.52; P = 0.008), with heterogeneity between studies (I 2 = 28%; P = 0.23; Figure 2d). No subgroup analyses were carried out for diabetes‐related distress, as there were fewer than six studies in the pooled meta‐analysis 43 .
Anxiety
Five studies measured anxiety using four tools (21‐item Depression, Anxiety and Stress Scale, the Hospital Anxiety and Depression Scale, Generalized Anxiety Disorder, and Beck Anxiety Inventory) 12 , 13 , 24 , 27 , 42 . Because of the varying effect measures reported across these studies, a narrative synthesis was carried out instead of meta‐analysis. Two studies reported significant reduction of anxiety in the mindfulness group compared with the control group 12 , 42 . Three studies found no significant differences in anxiety between the MBI and control group 13 , 24 , 27 .
Subgroup analyses
Three subgroup analyses were carried out to explore possible reasons for the heterogeneity (Table 3).
Table 3.
Subgroup analyses by various exclusion for glycosylated hemoglobin, depression and stress
| Outcome | No. studies | Pooled effect estimates | Heterogeneity | ||
|---|---|---|---|---|---|
| SMD (95% CI) | P‐value | I 2 | PQ | ||
| Baseline HbA1c | |||||
| HbA1c | |||||
| <8% | 4 | –0.26 † (−0.47, −0.05) | 0.01 | 0% | 0.77 |
| ≥8% | 3 | –0.22 † (−0.57, 0.13) | 0.22 | 0% | 0.66 |
| Depression | |||||
| <8% | 3 | –0.67(−0.87, −0.46) | <0.00001 | 0% | 0.73 |
| ≥8% | 5 | –0.49(−0.98, −0.01) | 0.05 | 73% | 0.006 |
| Stress | |||||
| <8% | 5 | –0.55(−0.84, −0.27) | 0.0002 | 60% | 0.06 |
| ≥8% | 2 | –0.57(−1.17, 0.04) | 0.06 | 65% | 0.09 |
| Baseline psychological status | |||||
| HbA1c | |||||
| Without psychological disorder | 5 | –0.30 † (−0.52, −0.08) | 0.007 | 0% | 0.86 |
| With psychological disorder | 2 | –0.15 † (−0.46, 0.16) | 0.34 | 0% | 0.75 |
| Depression | |||||
| Without psychological disorder | 4 | –0.37(−0.85, −0.10) | 0.13 | 74% | 0.009 |
| With psychological disorder | 4 | –0.72(−0.93, −0.51) | <0.00001 | 0% | 0.69 |
| Stress | |||||
| Without psychological disorder | 5 | –0.37(−0.60, −0.15) | 0.001 | 13% | 0.33 |
| With psychological disorder | 2 | –0.79(−1.04, −0.55) | <0.00001 | 0% | 0.97 |
| Duration of follow up | |||||
| HbA1c | |||||
| <6 months | 4 | –0.21 † (−0.48, 0.06) | 0.13 | 0% | 0.84 |
| ≥6 months | 3 | –0.28 † (−0.52, −0.04) | 0.006 | 0% | 0.60 |
| Depression | |||||
| <6 months | 6 | –0.53(−0.91, −0.15) | 0.006 | 69% | 0.007 |
| ≥6 months | 2 | –0.65(−0.90, −0.39) | <0.00001 | 0% | 0.45 |
| Stress | |||||
| <6 months | 4 | –0.58(−0.91, −0.26) | 0.0004 | 44% | 0.14 |
| ≥6 months | 3 | –0.47(−0.82, −0.12) | 0.009 | 62% | 0.07 |
Standardized mean difference was used for pooled effect estimates unless otherwise indicated.
Mean difference was used for pooled effect estimates. CI, confidence interval; HbA1c, glycosylated hemoglobin; SMD, standardized mean difference.
Baseline HbA1c
A previous meta‐analysis of diabetes psychosocial interventions showed a greater effect in participants with poor baseline HbA1c levels 35 . Therefore, we did a subgroup analysis to see if this hypothesis might also be true for MBI. As the participants in most included studies were older, we divided studies into two subgroups based on the less‐stringent glycemic goals (HbA1c <8%) for older adults, as recommended by the American Diabetes Association 44 . Combining studies with baseline HbA1c levels <8.0% showed greater reductions in HbA1c levels of 0.26% (95% CI −0.47 to −0.05; P = 0.01) compared with 0.22% (95% CI −0.57 to 0.13; P = 0.22) among those with baseline HbA1c levels ≥8.0%. In addition, the effects on HbA1c were no longer statistically significant in the subgroup of baseline HbA1c level ≥8% (Table 3).
Baseline psychological status
The baseline psychological status was divided into two groups: participants with baseline psychological disorders and participants without baseline psychological disorders. Three studies recruited participants with a certain baseline level of depression 13 , diabetes‐related distress 41 or low levels of emotional well‐being 12 , hence, participants in those studies were considered to have a psychological disorder. Combining studies with baseline psychological disorder showed a larger effect size for depression (SMD −0.72, 95% CI −0.93 to −0.51; P < 0.00001) and stress (SMD −0.79, 95% CI −1.04 to −0.55; P < 0.00001), with no substantial heterogeneity for both depression (I 2 = 0%; P = 0.69) and stress (I 2 = 0%; P = 0.97). Combining studies without baseline psychological disorder showed a smaller effect size for depression (SMD −0.37, 95% CI −0.85, −0.10; P = 0.13) and stress (SMD −0.37, 95% CI −1.04, −0.55; P = 0.001; Table 3).
Duration of follow up
The duration of the Diabetes Self‐Management Education intervention was divided into two groups (≤6 months and >6 months) guided by previous meta‐analysis 45 . Combining studies with a longer follow‐up duration (>6 months) showed a larger HbA1c reduction (MD −0.28, 95%CI −0.52 to −0.04; P = 0.006) than those with a shorter duration (≤6 months; SMD −0.21, 95% CI −0.48 to 0.06; P = 0.13). Additionally, studies with shorter follow‐up duration showed no statistically significant effects on HbA1c (Table 3).
Sensitivity analyses
Three sensitivity analyses were undertaken to explore possible reasons for the heterogeneity and test the robustness of the results. Removing one study 24 with longer duration of intervention time (>8 weeks) partially explained the substantial heterogeneity for depression (I 2 = 16%; P = 0.31), but not the significance or the direction of the effect (SMD −0.65, 95% CI −0.83 to −0.47; P < 0.00001). Removing one study delivering intervention through compact disc 27 explained all of the substantial heterogeneity for diabetes‐related distress (I 2 = 0%; P = 0.43), but did not change the significance or the direction of the effect (MD −7.44, 95% CI −11.58 to −3.29; P = 0.0004). Removing one study reporting improvement in HbA1c after intervention, the significance or the direction of the effect on HbA1c did not change (MD −0.21, 95% CI −0.41 to −0.01; P = 0.04).
DISCUSSION
To our best knowledge, this is the first meta‐analysis of RCTs to quantify the magnitude of improvement in HbA1c, depression, stress and diabetes‐related distress from MBI. The present review showed that MBI can slightly improve HbA1c and diabetes‐related distress, and lead to a moderate reduction in depression and stress. Subgroup analyses showed greater HbA1c reductions in studies with baseline HbA1c level <8% and follow‐up duration >6 months. Subgroup analyses also showed greater effects in participants with certain baseline psychological disorders than those without baseline psychological disorders in improving depression and stress. Mixed effects were observed for anxiety.
An important finding of the present review was that the effects of MBI on HbA1c were confirmed, although the effect size was small. This is in line with previous meta‐analysis 35 , 38 , which reported that psychotherapies can slightly improve HbA1c in people with diabetes. Possible mechanisms responsible for the effect on HbA1c could be that mindfulness training might assist participants to develop a healthy lifestyle or behaviors 27 . Performing optimal behavior has been confirmed to be effective in reaching targeted HbA1c 46 , 47 . Another potential pathway is through modulation of the hypothalamic–pituitary–adrenal axis and stress pathways 27 . Jung et al. 40 carried out a randomized controlled trial to test the effects of MBSR on people with type 2 diabetes, and a significant reduction in cortisol levels was observed.
Subgroup analyses showed that the effects of MBI for HbA1c were found in the longer follow‐up duration subgroup; however, the benefits for HbA1c were not observed in the shorter follow‐up duration subgroup. These results differed from the findings reported by Uchendu et al. 17 , who found short‐term (up to 4 months) and medium‐term (up to 8 months) effects on glycemic control, and no significant effect for long‐term (up to 12 months) glycemic control. This difference might be explained by the fact that the intervention approaches were different between the present review and previous systematic reviews. The present review focused on the effects of MBI, whereas previous reviews focused on CBT 17 . Some types of MBI, such as MBCT, were similar to CBT, but were not the same. MBI emphasized redirecting participants’ attention toward the present moment, but CBT focused on reappraising and modifying thought content 48 . Notably, due to the small number of studies reporting long‐term outcomes of MBI, we should be cautious to draw conclusions about the long‐term effects on HbA1c. Therefore, more studies are required to show whether MBI can improve glycemic control in the long term. Subgroup analyses suggested that the reduction in HbA1c was found among participants with lower baseline HbA1c levels (<8%), but not among participants with higher baseline HbA1c levels (≥ 8%). This implied that participants with better glycemic control at baseline benefitted more from MBI. MBI requires somewhat intense participation by patients in the exercises and modules. However, individuals with HbA1c levels ≥8% were more likely to already have diabetes complications 44 , who were less motivated with lower adherence to intervention 49 , which might explain the different effects in participants with different baseline HbA1c. Another explanation could be that participants with lower HbA1c levels might have better exercise habits 50 , contributing to contamination of the effects of MBI.
In terms of psychological outcomes, depression, stress and diabetes‐related distress were significantly improved. The present review confirmed previous findings 30 , 31 , and further found that the effects of MBI on psychological outcomes might differ by different baseline psychological status. The benefits on psychological outcomes could be attributed to the effects of MBI in improving mindfulness, contributing to facilitating insight into one’s emotional life, and enabling one to liberate oneself from negative and destructive mental states 51 . Subgroup analyses showed a larger effect size in combined studies involving participants with baseline psychological disorders than those involving participants without baseline psychological disorders, implying that participants with certain psychological disorders at baseline might benefits more from MBI. This result is consistent with a recent review on MBSR for older people, which also observed significant effects of MBSR, particularly in those with mood symptoms of distress, but limited effects on the healthy population. Similar results were also found in people with diabetes, that psychological treatment might be more efficacious for high‐severity than for low‐severity participants with depression 37 . This result might be explained by the floor effects – participants without psychological disorders at baseline achieved a lower score on the psychological screening scale, leaving little room for improvement after intervention. Nevertheless, a small number of studies included in the present review might influence the reliability and generalizability of the results. Hence, caution is warranted in interpreting these findings. Mixed effects were found for anxiety, as five of the included studies measured anxiety using five different scales, making it difficult to quantify the magnitude of effects in anxiety. It should be noted that heterogeneity was found in the pooled intervention effect estimate for depression, stress and diabetes‐related distress. This heterogeneity might be due to the use of a variety of measurement tools and different intervention components.
The present study had some strengths. A strength was that we only included RCTs, to guarantee the quality of evidence. The subgroup and sensitivity analyses further added to the strength of this study. However, there were some limitations in this review. First, most included studies failed to blind the participants and the intervention provider; this might increase the risk of performance bias. Second, besides a small number of trials, most included studies consisted of small samples, which might have resulted in exaggerated effect sizes and thus biased the results 52 , making it difficult to draw a robust conclusion. Third, due to a lack relevant trials, the present review did not draw conclusions on sex‐specific effects, diabetes type‐specific effects, and the format of MBI sessions that were most beneficial and preferred by people with diabetes. Finally, language restriction for the literature search might introduce language bias, as non‐English studies with relevant outcomes were missed.
It is not clear which population groups will benefit the most from MBI (e.g., different diabetes type), and which kind of MBI is most effective (e.g., MBSR, MBCT). Additionally, the mechanism of MBI on glycemic control and psychological outcomes has not been identified. More studies with longer follow‐up duration are required to determine the long‐term impact on glycemic control and psychological outcomes. Further research is required on evaluating the impact of MBI on other important outcomes for people with diabetes, such as cost‐effectiveness, self‐management and health‐related quality of life.
In conclusion, the present review shows that MBI can slightly improve HbA1c and diabetes‐related distress, leading to a moderate effect size in reducing depression and stress. Mixed effects are found in anxiety. The effects on depression and stress are larger in participants in the baseline psychological disorder subgroup. The impact of MBI on HbA1c might differ from the duration of follow up and baseline HbA1c level.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Table S1 | Search strategy (Pubmed).
Table S2 | Study data (n = 8; 11 reports).
Acknowledgments
We thank Dr Song Wang for assistance in methodology. This study was supported by West China Nursing Discipline Development Special Fund Project, Sichuan University (No. HXHL19009).
J Diabetes Investig 2021; 12: 1092–1103
References
- 1. de Groot M, Golden SH, Wagner J. Psychological conditions in adults with diabetes. Am Psychol 2016; 71: 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perrin NE, Davies MJ, Robertson N, et al. The prevalence of diabetes‐specific emotional distress in people with Type 2 diabetes: a systematic review and meta‐analysis. Diabetic Med 2017; 34: 1508–1520. [DOI] [PubMed] [Google Scholar]
- 3. Khaledi M, Haghighatdoost F, Feizi A, et al. The prevalence of comorbid depression in patients with type 2 diabetes: an updated systematic review and meta‐analysis on huge number of observational studies. Acta Diabetol 2019; 56: 631–650. [DOI] [PubMed] [Google Scholar]
- 4. Khalighi Z, Badfar G, Mahmoudi L, et al. The prevalence of depression and anxiety in Iranian patients with diabetes mellitus: A systematic review and meta‐analysis. Diabet Metab Synd Clin Res Rev 2019; 13: 2785–2794. [DOI] [PubMed] [Google Scholar]
- 5. Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care 2003; 26: 2822. [DOI] [PubMed] [Google Scholar]
- 6. Ali S, Stone M, Skinner TC, et al. The association between depression and health‐related quality of life in people with type 2 diabetes: A systematic literature review. Diabetes/Metab Res Rev 2010; 26: 75–89. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . 5. Facilitating behavior change and well‐being to improve health outcomes: Standards of medical care in diabetes‐2020. Diabetes Care 2020;43(Suppl 1):S48–S65. [DOI] [PubMed] [Google Scholar]
- 8. Roemer L, Williston SK, Rollins LG. Mindfulness and emotion regulation. Curr Opin Psychol 2015; 3: 52–57. [Google Scholar]
- 9. Victorson D, Kentor M, Maletich C, et al. Mindfulness meditation to promote wellness and manage chronic disease: A systematic review and meta‐analysis of mindfulness‐based randomized controlled trials relevant to lifestyle medicine. Am J Lifestyle Med 2014; 9: 185–211. [Google Scholar]
- 10. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness‐based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: A randomized clinical trial. JAMA 2016; 315: 1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johns SA, Brown LF, Beck‐Coon K, et al. Randomized controlled pilot study of mindfulness‐based stress reduction for persistently fatigued cancer survivors. Psycho‐Oncol 2015; 24: 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Son J, Nyklicek I, Pop VJ, et al. The effects of a mindfulness‐based intervention on emotional distress, quality of life, and HbA(1c) in outpatients with diabetes (DiaMind). Diabetes Care 2013; 36: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tovote KA, Fleer J, Snippe E, et al. Individual mindfulness‐based cognitive therapy and cognitive behavior therapy for treating depressive symptoms in patients with diabetes: results of a randomized controlled trial. Diabetes Care 2014; 37: 2427–2434. [DOI] [PubMed] [Google Scholar]
- 14. Nathan HJ, Poulin P, Wozny D, et al. Randomized trial of the effect of mindfulness‐based stress reduction on pain‐related disability, pain intensity, health‐related quality of life, and A1C in patients with painful diabetic peripheral neuropathy. Clin Diabet 2017; 35: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kopf S, Oikonomou D, Hartmann M, et al. Effects of stress reduction on cardiovascular risk factors in type 2 diabetes patients with early kidney disease – results of a randomized controlled trial (HEIDIS). Exp Clin Endocrinol Diabetes 2014; 122: 341–349. [DOI] [PubMed] [Google Scholar]
- 16. Viana LV, Gomes MB, Zajdenverg L, et al. Brazilian Type 1 Diabetes Study G. Interventions to improve patients’ compliance with therapies aimed at lowering glycated hemoglobin (HbA1c) in type 1 diabetes: systematic review and meta‐analyses of randomized controlled clinical trials of psychological, telecare, and educational interventions. Trials 2016; 17: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uchendu C, Blake H. Effectiveness of cognitive‐behavioural therapy on glycaemic control and psychological outcomes in adults with diabetes mellitus: A systematic review and meta‐analysis of randomized controlled trials. Diabetic Med 2017; 34: 328–339. [DOI] [PubMed] [Google Scholar]
- 18. Williams Mark, Teasdale John, Segal Zindel, Kabat‐Zinn J. The Mindful Way through Depression. New York: Guilford Press, 2007. [Google Scholar]
- 19. Kabat‐Zinn J, Hanh TN. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Dell Pub., a division of Bantam Doubleday Dell Publishing Group, 2009. [Google Scholar]
- 20. Shapiro SL, Carlson LE. The art and science of mindfulness: Integrating mindfulness into psychology and the helping professions (Second Edition). Washington, DC, US: American Psychological Association, 2017. [Google Scholar]
- 21. Whitebird RR, Kreitzer MJ, O’Connor PJ. Mindfulness‐based stress reduction and diabetes. Diabet Spect 2009; 22: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu J, Strauss C, Bond R, et al. How do mindfulness‐based cognitive therapy and mindfulness‐based stress reduction improve mental health and wellbeing? A systematic review and meta‐analysis of mediation studies. Clin Psychol Rev 2015; 37: 1–12. [DOI] [PubMed] [Google Scholar]
- 23. Shapiro SL, Carlson LE. The art and science of mindfulness: Integrating mindfulness into psychology and the helping professions. American Psychological Association, Washington, DC, US, 2009. [Google Scholar]
- 24. Miller CK, Kristeller JL, Headings A, et al. Comparison of a mindful eating intervention to a diabetes self‐management intervention among adults with type 2 diabetes: a randomized controlled trial. Health Educat Behav 2014; 41: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Segal Zindel V, Mark J, Williams G, Teasdale John D. Mindfulness‐Based Cognitive Therapy for Depression, Second Edition. New York, London: The Guilford Press; 2018. [Google Scholar]
- 26. Miller CK, Kristeller JL, Headings A, et al. Comparative effectiveness of a mindful eating intervention to a diabetes self‐management intervention among adults with type 2 diabetes: A pilot study. J Acad Nutr Diet 2012; 112: 1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pearson S, Wills K, Woods M, et al. Effects of mindfulness on psychological distress and HbA(1c) in people with diabetes. Mindfulness 2018; 9: 1615–1626. [Google Scholar]
- 28. Nagel K, Dearth‐Wesley T, Herman AN, et al. The association of dispositional mindfulness and adverse childhood experiences with glycemic control among young adults with type 1 diabetes. Diabetes 2018;67(Supplement 1):218‐OR. [Google Scholar]
- 29. Ludwig DS, Kabat‐Zinn J. Mindfulness in Medicine. JAMA 2008; 300: 1350–1352. [DOI] [PubMed] [Google Scholar]
- 30. Bogusch LM, O’Brien WH. The effects of mindfulness‐based interventions on diabetes‐related distress, quality of life, and metabolic control among persons with diabetes: A meta‐analytic review. Behav Med (Washington, DC). 2019; 45: 19–29. [DOI] [PubMed] [Google Scholar]
- 31. Noordali F, Cumming J, Thompson JL. Effectiveness of Mindfulness‐based interventions on physiological and psychological complications in adults with diabetes: A systematic review. J Health Psychol 2017; 22: 965–983. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JPH, Thomas J, Chandler J, et al.Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). 2019; www.training.cochrane.org/handbook. Accessed December 19, 2019.
- 33. Hartmann M, Kopf S, Kircher C, et al. Sustained effects of a mindfulness‐based stress‐reduction intervention in type 2 diabetic patients: design and first results of a randomized controlled trial (the Heidelberger Diabetes and Stress‐study). Diabetes Care 2012; 35: 945–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen J. Statistical power analysis for the behavioral sciences. Routledge, 2013. [Google Scholar]
- 35. Harkness E, Macdonald W, Valderas J, et al. Identifying psychosocial interventions that improve both physical and mental health in patients with diabetes: a systematic review and meta‐analysis. Diabetes Care 2010; 33: 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uchendu C, Blake H. Effectiveness of cognitive–behavioural therapy on glycaemic control and psychological outcomes in adults with diabetes mellitus: a systematic review and meta‐analysis of randomized controlled trials. Diabet Med 2017; 34: 328–339. [DOI] [PubMed] [Google Scholar]
- 37. Driessen E, Cuijpers P, Hollon SD, et al. Does pretreatment severity moderate the efficacy of psychological treatment of adult outpatient depression? A meta‐analysis. J Consult Clin Psychol 2010; 78: 668–680. [DOI] [PubMed] [Google Scholar]
- 38. Ismail K, Winkley K, Rabe‐Hesketh S. Systematic review and meta‐analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004; 363: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 39. Winkley K, Landau S, Eisler I, et al. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta‐analysis of randomised controlled trials. BMJ (Clinical research ed) 2006; 333: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jung HY, Lee H, Park J. Comparison of the effects of Korean mindfulness‐based stress reduction, walking, and patient education in diabetes mellitus. Nursing Health Sci 2015; 17: 516–525. [DOI] [PubMed] [Google Scholar]
- 41. Schroevers MJ, Tovote KA, Keers JC, et al. Individual mindfulness‐based cognitive therapy for people with diabetes: A pilot randomized controlled trial. Mindfulness 2015; 6: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Son J, Nyklicek I, Pop VJ, et al. Mindfulness‐based cognitive therapy for people with diabetes and emotional problems: long‐term follow‐up findings from the DiaMind randomized controlled trial. J Psychosom Res 2014; 77: 81–84. [DOI] [PubMed] [Google Scholar]
- 43. Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011; 64: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 44. American Diabetes A. 12. Older adults: Standards of medical care in diabetes‐2020. Diabetes Care 2020; 43(Suppl 1): S152–S162. [DOI] [PubMed] [Google Scholar]
- 45. Hildebrand JA, Billimek J, Lee J‐A, et al. Effect of diabetes self‐management education on glycemic control in Latino adults with type 2 diabetes: A systematic review and meta‐analysis. Patient Educ Couns 2020; 103: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Norris SL, Lau J, Smith SJ, et al. Self‐management education for adults with type 2 diabetes. Diabetes Care 2002; 25: 1159. [DOI] [PubMed] [Google Scholar]
- 47. Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta‐analysis. Lancet 2012; 379: 2252–2261. [DOI] [PubMed] [Google Scholar]
- 48. Arch JJ, Ayers CR, Baker A, et al. Randomized clinical trial of adapted mindfulness‐based stress reduction versus group cognitive behavioral therapy for heterogeneous anxiety disorders. Behav Res Ther 2013; 51: 185–196. [DOI] [PubMed] [Google Scholar]
- 49. Aminuddin HB, Jiao N, Jiang Y, et al. Effectiveness of smartphone‐based self‐management interventions on self‐efficacy, self‐care activities, health‐related quality of life and clinical outcomes in patients with type 2 diabetes: A systematic review and meta‐analysis. Int J Nurs Stud 2019; 103286. [DOI] [PubMed] [Google Scholar]
- 50. Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta‐analysis. JAMA 2011; 305: 1790–1799. [DOI] [PubMed] [Google Scholar]
- 51. Coffey KA, Hartman M, Fredrickson BL. Deconstructing mindfulness and constructing mental health: Understanding mindfulness and its mechanisms of action. Mindfulness 2010; 1: 235–253. [Google Scholar]
- 52. Schmidt CB, van Loon BJP, Vergouwen ACM, et al. Systematic review and meta‐analysis of psychological interventions in people with diabetes and elevated diabetes‐distress. Diabet Med 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Search strategy (Pubmed).
Table S2 | Study data (n = 8; 11 reports).


