Abstract
A severe complication associated with diabetes is diabetic foot ulcer (DFU). Most patients with DFU require amputation. Although treatment of non‐healing diabetic ulcers is challenging, the use of novel therapies can be effective. In this report, we present the case of a woman with type 2 diabetes with DFU‐related osteomyelitis, who was treated with a combination therapy of trichloroacetic acid, calcium alginate and foam dressings, human autologous fibroblast injection, and a fibroblast cell‐seeded collagen scaffold. The results showed the positive effects of combination therapy on DFU. In the initial treatment, the wound area was measured to be 14 × 7 cm2, with a depth of 4 cm. After 6 months, the wound was measured to be 1.5 cm2, showing a 90% reduction of the wound area. Overall, this combination therapy was highly effective in the treatment of DFU‐related osteomyelitis, and could markedly prevent amputation among DFU patients.
Keywords: Autologous fibroblast, Diabetic foot ulcer, Trichloroacetic acid
Combination therapy using trichloroacetic acid, calcium alginate and foam dressing, human autologous fibroblast injection, and fibroblast cells seeded collagen scaffold is very effective for the treatment of a diabetic foot ulcer with osteomyelitis, and can markedly prevent amputation among diabetic foot ulcer patients.
Introduction
Diabetic foot ulcer (DFU) is a common chronic complication of diabetes. DFU and diabetic nephropathy are important factors in the development of foot ulcers and lower limb amputations 1 . DFU is considered a major problem for the scientific community, as it affects the patients’ quality of life 2 . Amputation of a diabetic foot is associated with the increased risk of recurrent distal sole ulcers. It is known that ulceration can increase infection and osteomyelitis risk, and might cause progressive tissue damage.
Although various treatment strategies have been described for DFU, finding an effective prophylactic treatment is challenging 3 , 4 . Therefore, it is necessary to develop novel therapeutic approaches to improve the treatment of DFUs. Here, we describe the successful treatment of a patient with DFU, using trichloroacetic acid (TCA), calcium alginate and foam dressings, human autologous fibroblast injection, and a fibroblast‐seeded microfibrous collagen scaffold as a new effective combination therapy for DFU with osteomyelitis.
Case Presentation
The patient was a 43‐year‐old woman with a 10‐year history of type 2 diabetes and insulin dependence. The patient’s characteristics are shown in the Table 1. She had first developed a small foot ulcer (0.2 × 0.5 cm) on the lateral side of the right lower foot. After 1 month, the ulcer had progressed, and she presented to another hospital with complaints of a progressively worsening infection of the foot ulcer, swelling, exudation and pus moss on or surrounding the wound site, and tissue pain (body temperature 39.5°C). The results of magnetic resonance imaging confirmed osteomyelitis because of a non‐healing ulcer. Accordingly, she received adequate antidiabetic treatment. Therapy with intravenous antibiotics (ciprofloxacin and vancomycin), offloading and surgical debridement was carried out on the patient’s right foot, and then, wound management was initiated. However, the wound aggravated, and amputation was proposed by the orthopedist.
Table 1.
Baseline characteristics of the patient
| Characteristic | |
|---|---|
| Age (years) | 43 |
| Sex | Female |
| Height (cm) | 172 |
| Bodyweight (kg) | 92 |
| History of diabetes (years) | 10 |
| Treatment method | Insulin |
| Diabetic complications | Foot deformity |
| Healthcare utilization | |
| Hemoglobin A1C (%) | 11 |
| Fasting blood glucose (mg/dL) | 170 |
| Systolic blood pressure (mmHg) | 129.67 |
| Diastolic blood pressure (mmHg) | 81.33 |
| Total cholesterol (mg/dL) | 163 |
| LDL cholesterol (mg/dL) | 89 |
| Thyroid hormone | Normal |
| Nephropathy status | |
| Serum creatinine (mg/dL) | 1.4 |
| Estimated glomerular filtration rate | 93 |
| Albumin‐to‐creatinine ratio | 24 |
| Blood urea nitrogen (mg/dL) | 10 |
| Urine protein | Negative |
| Urine red blood cell | Negative |
| Retinopathy status | No clinical |
| Neuropathy status | No clinical |
The patient presented to our Skin and Stem Cell Research Center, Tehran, Iran, after having a non‐healing DFU (Wagner grade II) for 2 months 5 . Baseline measurements were carried out for the patient before the intervention (Table 1). However, the results were unremarkable, except for blood glucose (glycated hemoglobin 11%; fasting blood glucose 170 mg/dL). The blood glucose was controlled during the study. No evidence of diabetic nephropathy was observed in the patient laboratory tests (Table 1). Also, no clinical retinopathy and neuropathy were observed (fundus status, Achilles tendon reflex and vibration thresholds were normal). The patient did not have any drug allergies, and did not consume alcohol or smoke tobacco.
To participate in the present study, the patient signed written informed consent, and then her information was recorded.
Combination therapy was initiated for the patient in our center. The wound area was washed with a normal saline solvent, and wound debridement was carried out using a Bisturi No. 12 blade. After disinfecting the ulcer, 70% TCA solution was applied to the ulcer, and 50% TCA was applied around the ulcer area with a cotton applicator. Next, the wound area was covered with calcium alginate (Coloplast A/S, Humlebaek, Denmark) and foam (HydroTac; Hartmann, Germany) dressings. The patient received a suitable antibiotic (ciprofloxacin) to control the infection. Also, her blood glucose was controlled, pressure on the ulcer was reduced and preparation programs were applied to control the ulcer; this procedure continued for 80 sessions (three times a week). For preparation of autologous fibroblasts, a punch biopsy (6 mm2) of the skin behind the ear was taken and used to obtain skin and blood samples. The samples were transferred to the cell culture laboratory of the Skin and Stem Cell Research Center to culture autologous fibroblasts. In the cell culture laboratory, fibroblasts were cultured after preparation and culturing. After 1 month of skin biopsy explant culture, 4 × 107 fibroblasts were extracted (Figure 1).
Figure 1.
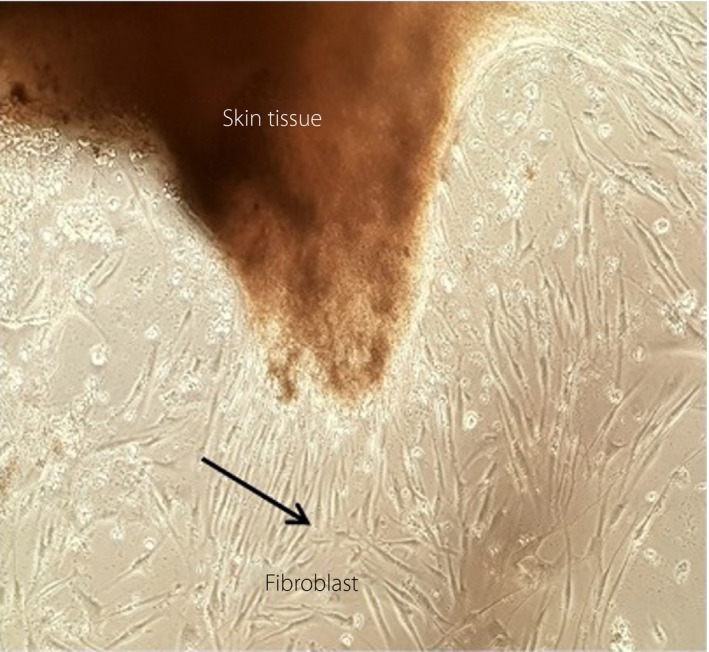
Autologous fibroblast cell culture.
After 3 weeks of treatment, the wound was prepared for fibroblast cell injection. The ulcer was washed with normal saline and debrided (TCA was not used in this session). Then, 5 × 105 cells/cm2 of wound periphery were injected into the ulcer, and a microfibrous collagen dressing, seeded with 1 × 104 cells/cm2, was administrated on the hollow parts of the ulcer. Finally, the wound area was covered by calcium alginate and foam dressings. Treatment with autologous fibroblast cells was repeated three times at 3‐week intervals. To avoid infection, ciprofloxacin was given. After fibroblast injection, treatment continued, as previously described.
In the final 10 sessions, a collagen scaffold, foam dressing and a honey alginate dressing (Medihoney, USA) were used. After treatment, the length, width and depth of the ulcer were examined monthly (Table 2). Re‐epithelialization, as well as reduced ulcer depth and surface, was detected. As shown in Figures 2 and 3, the ulcer was treated after 80 sessions of combination therapy.
Table 2.
Diabetic foot ulcer size before and after treatment
| Patient’s ulcer | Before treatment | Month(s) after treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Area (length × width) | 14 × 7 | 12 × 6 | 10 × 5 | 7 × 4 | 4 × 3 | 3 × 2 | 1.5 × 1 | 0 × 0 |
| Depth | 5.0 | 4.0 | 3.0 | 2.0 | 1.5 | 0.5 | 0.0 | 0.0 |
The values are presented as cm.
Figure 2.
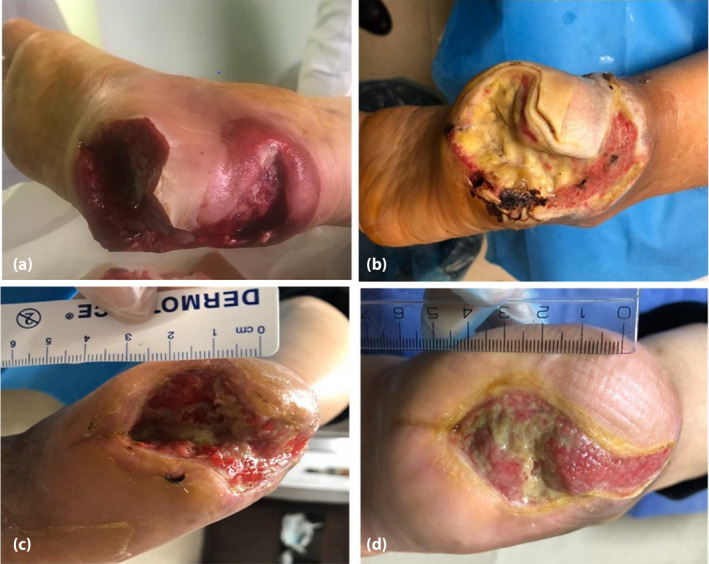
(a) The patient before treatment. The patient (b) 1 month, (c) 2 months and (d) 3 months after treatment.
Figure 3.
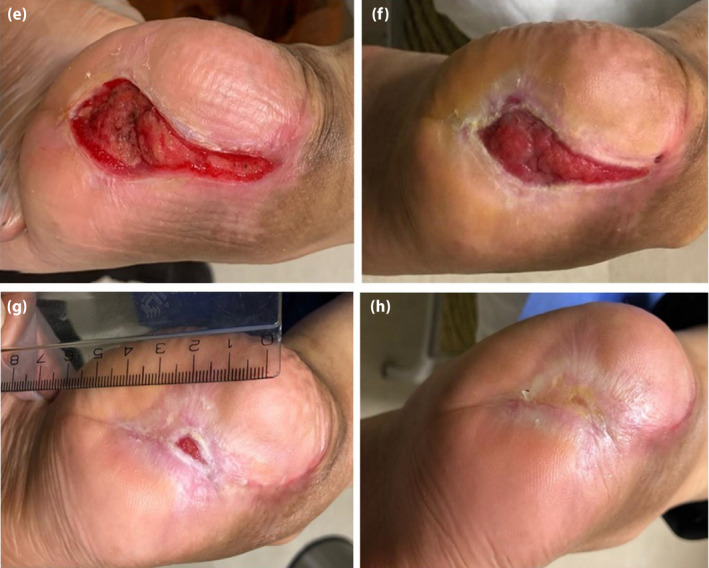
(a) The patient 4 months, (b) 5 months, (c) 6 months and (d) 7 months after treatment. As the figures show, the diabetic foot ulcer was treated.
Discussion
DFU, as an acute complication of diabetes, is the main cause of recurrent hospitalization and amputation among diabetes patients. Evidently, amputation imposes a significant burden on patients. However, available treatments for DFU are often inadequate 6 . Therefore, the current study aimed to evaluate the efficacy and safety of a combination therapy with TCA, autologous fibroblast cell injection, a collagen scaffold, and calcium alginate and foam dressings (as a new type of dressing) in DFU treatment.
TCA is a chemical agent, which has recently been used to treat recalcitrant DFU. TCA might accelerate the process of cell regeneration after 2 weeks 7 . Evidence shows that higher TCA concentrations penetrate into the mid‐reticular dermis. The skin stress response system is induced by TCA peel as a result of reconstitution of the epidermis and dermis through wound healing processes. The mechanism of action of TCA involves precipitation of proteins and coagulative necrosis of the epidermis and/or papillary dermis, resulting in the shedding of necrotic layers and reepithelization, followed by dermal collagen remodeling 8 .
In a previous study, the efficacy of TCA in the treatment of recalcitrant DFU was evaluated. All wounds significantly improved in six treated patients after 7.5 ± 0.83 weeks, and the wound areas and infection scores reduced. In that study, 68.6–100% improvements were reported for the efficacy of treatment for patients, without any complications after 3 months of follow up 3 .
In another study, TCA 80% was used in a chemical debridement technique for chronic venous leg ulcers. It was found that use of an 80% TCA solution is a simple, cost‐effective and noticeably less painful technique for venous leg ulcers 9 .
Cellular therapy is an interesting method in regenerative medicine, which uses self‐renewing cells. Researchers have used direct systemic administration, local intramuscular injection and direct application on the wound area to transport cells in cellular therapy 10 . Intramuscular injection into the wound periphery was introduced as a safer and more efficient method, compared with systemic administration in clinical studies 10 . Recently, stem cells, such as fibroblasts, either as scaffolds or suspensions, have been applied to treat ischemic DFUs. Possible mechanisms of stem cell therapy include paracrine secretion of cytokines and growth factors, their direct differentiation into vascular endothelial cells, and formation of new vessels and skin components, which ultimately result in ulcer healing 11 .
In the present study, we carried out peripheral injection of fibroblasts into the ulcer and observed a decrease in the wound size. In this regard, a randomized clinical trial also showed that improvement was significantly higher in the group with fibroblasts, compared with the control group 12 .
Studies on the efficacy of fibroblast injection for DFU have shown that the morphology of ulcers alters during the healing process, and their proliferative ability is reduced. Overall, the use of fibroblasts for the treatment of chronic and acute ulcers releases pain, and accelerates the healing process by reducing scars 4 , 7 .
The effect of autologous fibroblast suspension was evaluated on refractory DFU treatment 4 . Furthermore, in a study by Nilforoushzadeh et al. 3 , 7 , the effects of TCA and fibroblasts were investigated on recalcitrant DFU. They reported that the application of fibroblasts is an effective therapeutic approach for recalcitrant DFUs. In another study, topical TCA application, along with autologous fibroblast injection, was used for the treatment of electrical burn ulcers, and satisfactory results were reported 13 . In addition, in a study by Mansbridge et al. 14 , three‐dimensional fibroblast culture implant was used for DFU treatment. The results showed that the therapeutic properties of dermal implants are majorly dependent on the recovery of growth factor expression, protein synthesis and angiogenesis, based on metabolic activity.
In the present study, we used a fibroblast‐seeded collagen scaffold in combination with other treatments. In this regard, Cavallini et al. 15 used a mesh of in vitro expanded autologous fibroblasts as covering for 10 diabetes patients with profoundly infected leg or foot ulcers with major loss of substance to accelerate the healing of ulcers. They found that this technique is an acceptable option for the treatment of large leg ulcers and can be mainly used for patients with chronic diseases, such as diabetes 15 .
Also, in a randomized controlled trial by You et al. 16 , DFU patients were treated with grafting of an autologous fibroblast‐hyaluronic acid complex, which was protected with a polyurethane foam dressing (as a secondary dressing); a control group, treated with a non‐adherent foam dressing, was also included in that study. Their results showed that the autologous fibroblast‐hyaluronic acid complex could be a safe and effective method for DFU treatment 16 .
Furthermore, in the present study, we used calcium alginate and foam dressings. Alginate and foam dressings can be helpful in the dissolution of fibrin and necrotic tissues, preserving the temperature of the wound surface, increasing the release of various growth factors, and enabling tissue growth without scar formation or mechanical damage to the new granulation tissue. Also, dressings protect the nerve endings in the ulcer and release pain.
In a study by Saco et al., the efficacy of some new dressings, including alginate, foam, hydrofiber, hydrocolloid and hydrogel dressings, was evaluated in the treatment of DFUs. They reported that wound dressings could efficiently enhance wound healing in DFU patients and significantly reduce morbidity. Also, they found that non‐adherent dressings were more cost‐effective than hydrofiber dressings for DFUs 17 .
Recent research shows that wound dressings can accelerate the wound healing process of DFUs in patients 18 . Therefore, in the present study, we evaluated the effect of a combination therapy with TCA, calcium alginate and foam dressings, human autologous fibroblast injection, and a fibroblast‐seeded collagen scaffold on DFUs. The synergistic effects of the combination therapy can be explained as follows. TCA removes the necrotic tissue, and it can induce re‐epithelization and collagen reproduction, it can also increase cytokines, thus increasing the feeding of the wound. In the healing presses, fibroblast injection can decrease the wound surface area, and dressing can absorb the exudates. Alginate beads create an excellent immobilization material for human fibroblasts, with physiological activities and viability of the incorporated cells being preserved for times of up to 1 month.
To the best of our knowledge, this is the first study using a combination of TCA, calcium alginate and foam dressings, human autologous fibroblast injection, and a fibroblast‐seeded collagen scaffold for the treatment of DFUs. The present results showed that this combination therapy could promote the healing of DFUs and prevent amputation in patients; therefore, it can be considered as a very effective method. We are going to carry out the pilot clinical study of this method for 14 diabetes patients with 6 months’ treatment and 1‐year follow up.
The present study had some limitations. The combination therapy of TCA, calcium alginate and foam dressing, human autologous fibroblast injection, and fibroblast seeded collagen scaffold is effective on DFU, but preparing autologous fibroblasts is time‐consuming, difficult and very expensive because of clinical grade materials are used. Also, on a large scale, a very good cell bank is required. Although the present study was carried out for free, the cost of this therapy is high and the high cost can be a problem for patients. The preparation of the dressing was also difficult.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We acknowledge the colleagues and staff at Skin and Stem Cell Research Center, Tehran University of Medical Sciences, Tehran, Iran.
J Diabetes Investig 2021; 12: 1112–1117
References
- 1. Arshad M, Arshad S, Arshad S, et al. The quality of life in patients with diabetic foot ulcers. J Diab Metab 2020; 11: e101. [Google Scholar]
- 2. Chan B, Cadarette S, Wodchis W, et al. Cost‐of‐illness studies in chronic ulcers: a systematic review. J Wound Care 2017; 26(Sup4): S4–S14. [DOI] [PubMed] [Google Scholar]
- 3. Nilforoushzadeh MA, Jaffary F, Ansari N, et al. Treatment of recalcitrant diabetic ulcers using trichloroacetic acid. J Res Med Sci 2012; 17: 1.23248650 [Google Scholar]
- 4. Nilforoushzadeh MA, Jaffary F, Siavash M, et al. Autologous fibroblast suspension for the treatment of refractory diabetic foot ulcer. Indian J Dermatol Venereol Leprol 2016; 82: 105. [DOI] [PubMed] [Google Scholar]
- 5. Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981; 2: 64–122. [DOI] [PubMed] [Google Scholar]
- 6. Margolis D, Allen‐Taylor L, Hoffstad O, et al. Healing diabetic neuropathic foot ulcers: are we getting better? Diabetic Med 2005; 22: 172–176. [DOI] [PubMed] [Google Scholar]
- 7. Nilforoushzadeh MA, Jaffary F, Siavash M, et al. Treatment of recalcitrant diabetic ulcers with trichloroacetic acid and fibroblasts. J Skin Stem Cell 2014; 1: e23312 [Google Scholar]
- 8. Nilforoushzadeh M, Jaffary F, Reiszadeh M. Comparative effect of topical trichloroacetic acid and intralesional meglumine antimoniate in the treatment of acute cutaneous leishmaniasis. Int J Pharmacol 2006; 2: 633–636. [Google Scholar]
- 9. Pinheiro RR, Duarte B, Cabete J. Trichloroacetic acid (80%) as a chemical debridement method for chronic venous leg ulcers—A pilot study. Int Wound J 2018; 15: 438–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang X‐Y, Lu D‐B, Chen B. Progress in stem cell therapy for the diabetic foot. Diabetes Res Clin Pract 2012; 97: 43–50. [DOI] [PubMed] [Google Scholar]
- 11. Ulicna M, Danisovic L, Vojtassak J. Does cell therapy and tissue engineering represent a promising treatment of diabetic foot ulcers. Bratisl Lek Listy 2010; 111: 138–143. [PubMed] [Google Scholar]
- 12. Marston WA, Hanft J, Norwood P, et al. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003; 26: 1701–1705. [DOI] [PubMed] [Google Scholar]
- 13. Nilforoushzadeh MA, Esfahani MHN, Fesharaki MA, et al. Treatment of recalcitrant electrical burn ulcer with application of topical trichloroacetic acid and autologous cultured fibroblast. Cell Tissue Transplant Ther 2010. 10.4137/CTTT.S3779 [DOI] [Google Scholar]
- 14. Mansbridge J, Liu K, Patch R, et al. Three‐dimensional fibroblast culture implant for the treatment of diabetic foot ulcers: metabolic activity and therapeutic range. Tissue Eng 1998; 4: 403–414. [DOI] [PubMed] [Google Scholar]
- 15. Cavallini M. Autologous fibroblasts to treat deep and complicated leg ulcers in diabetic patients. Wound Repair Regen 2007; 15: 35–38. [DOI] [PubMed] [Google Scholar]
- 16. You H, Han S‐K, Rhie J. Randomised controlled clinical trial for autologous fibroblast‐hyaluronic acid complex in treating diabetic foot ulcers. J Wound Care 2014; 23: 521–530. [DOI] [PubMed] [Google Scholar]
- 17. Saco M, Howe N, Nathoo R, et al. Comparing the efficacies of alginate, foam, hydrocolloid, hydrofiber, and hydrogel dressings in the management of diabetic foot ulcers and venous leg ulcers: a systematic review and meta‐analysis examining how to dress for success. Dermatol Online J 2016; 22: 13030/qt7ph5v17z. [PubMed] [Google Scholar]
- 18. Rahmani S, Mooney DJ. Tissue‐Engineered Wound Dressings for Diabetic Foot Ulcers. The Diabetic Foot: Springer, 2018; p. 247–256. [Google Scholar]


