Abstract
Background
Medications with anticholinergic properties are commonly prescribed to older adults. The cumulative anticholinergic effect of all the medications a person takes is referred to as the 'anticholinergic burden' because of its potential to cause adverse effects. It is possible that high anticholinergic burden may be a risk factor for development of cognitive decline or dementia. There are various scales available to measure anticholinergic burden but agreement between them is often poor.
Objectives
To assess whether anticholinergic burden, as defined at the level of each individual scale, is a prognostic factor for future cognitive decline or dementia in cognitively unimpaired older adults.
Search methods
We searched the following databases from inception to 24 March 2021: MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), CINAHL (EBSCOhost), and ISI Web of Science Core Collection (ISI Web of Science).
Selection criteria
We included prospective and retrospective longitudinal cohort and case‐control observational studies with a minimum of one year' follow‐up that examined the association between an anticholinergic burden measurement scale and future cognitive decline or dementia in cognitively unimpaired older adults.
Data collection and analysis
Two review authors independently assessed studies for inclusion, and undertook data extraction, assessment of risk of bias, and GRADE assessment. We extracted odds ratios (OR) and hazard ratios, with 95% confidence intervals (CI), and linear data on the association between anticholinergic burden and cognitive decline or dementia. We intended to pool each metric separately; however, only OR‐based data were suitable for pooling via a random‐effects meta‐analysis. We initially established adjusted and unadjusted pooled rates for each available anticholinergic scale; then, as an exploratory analysis, established pooled rates on the prespecified association across scales. We examined variability based on severity of anticholinergic burden.
Main results
We identified 25 studies that met our inclusion criteria (968,428 older adults). Twenty studies were conducted in the community care setting, two in primary care clinics, and three in secondary care settings. Eight studies (320,906 participants) provided suitable data for meta‐analysis. The Anticholinergic Cognitive Burden scale (ACB scale) was the only scale with sufficient data for 'scale‐based' meta‐analysis. Unadjusted ORs suggested an increased risk for cognitive decline or dementia in older adults with an anticholinergic burden (OR 1.47, 95% CI 1.09 to 1.96) and adjusted ORs similarly suggested an increased risk for anticholinergic burden, defined according to the ACB scale (OR 2.63, 95% CI 1.09 to 6.29). Exploratory analysis combining adjusted ORs across available scales supported these results (OR 2.16, 95% CI 1.38 to 3.38), and there was evidence of variability in risk based on severity of anticholinergic burden (ACB scale 1: OR 2.18, 95% CI 1.11 to 4.29; ACB scale 2: OR 2.71, 95% CI 2.01 to 3.56; ACB scale 3: OR 3.27, 95% CI 1.41 to 7.61); however, overall GRADE evaluation of certainty of the evidence was low.
Authors' conclusions
There is low‐certainty evidence that older adults without cognitive impairment who take medications with anticholinergic effects may be at increased risk of cognitive decline or dementia.
Plain language summary
The impact of medications with anticholinergic effects on future problems with memory and thinking
What was the aim of this review?
Medicines can be classified by their ability to block the action of a chemical signalling system in the body called the cholinergic system. Medicines that do this are said to have anticholinergic effects. There are various measurement scales to quantify the effects of anticholinergic medicines. The overall anticholinergic effect caused by all the anticholinergic medications a person is taking is referred to as 'anticholinergic burden.'
We aimed to investigate if older people who have no problems with memory or thinking are more likely to develop dementia when prescribed anticholinergic medicines than people who are not prescribed these medicines.
Anticholinergic burden ratings can vary with the scale used because different scales score medicines in different ways. Therefore, we also wanted to know if any particular anticholinergic burden measurement scale was more strongly associated with increased risk of dementia than other scales.
Key messages
There may be a link between anticholinergic medicine use and future risk of dementia. However, there are limitations in the published evidence, and we cannot say definitively if dementia is caused by the anticholinergic medicines themselves or by other factors. There were too few studies to allow us to compare the various anticholinergic measurement tools.
What was studied in the review?
There are more than 40 million older people worldwide living with dementia. These numbers are expected to rise to over 100 million by 2050 and at present there are very limited treatment options available. Therefore, it is important to identify factors that may increase the risk of dementia.
Because the cholinergic system in the brain plays an important role in learning and memory, there are theoretical reasons to believe that medications with anticholinergic effects could cause future dementia. Research has suggested that these medications may have unintended effects on memory and thinking, potentially resulting in dementia. If this is the case, one way to reduce the numbers of older people who develop dementia may be to avoid prescribing these medicines. Many commonly used medications have anticholinergic effects, for example medications for hay fever, insomnia (difficulty getting to sleep or staying asleep for long enough to feel refreshed), and depression.
In this review, we investigated the link between anticholinergic medicines, as measured by various measurement scales, and future dementia.
What were the main results of the review?
We found 25 studies, including 968,428 people aged 50 years or more. Despite the relatively large number of studies, differences in design and methods only allowed us to combine a few of them in analyses. We found that there is a consistent link between use of anticholinergic medicines and risk of future dementia. We cannot say if these medicines play a causal role; however, if they do, taking these medicines could potentially double a person's risk of dementia.
Of the anticholinergic measurement scales available, we could assess one commonly used tool – the 'Anticholinergic Cognitive Burden scale.' If this scale identified someone as having high anticholinergic burden, the risk of future dementia was more than two times higher than for someone with no anticholinergic burden.
The evidence included in this review was of a low quality overall and may have exaggerated the strength of the association between anticholinergic medicines and dementia. For example, anticholinergic medicines may be prescribed for the early symptoms of dementia. This would give a strong link but would not imply that the medicine caused the memory problems. Similarly, there is a risk that studies are only published when they show an association between anticholinergic medicines and future dementia. It may be that the only way to truly establish if anticholinergic medications are associated with future dementia would be to conduct a study where some people have their anticholinergic medications stopped or changed to an alternative and others continue their usual medications.
How up to date was this review?
We searched for studies published up to 24 March 2021.
Summary of findings
Summary of findings 1. Older adults with anticholinergic burden compared with older adults with no anticholinergic burden for dementia.
| Older adults with anticholinergic burden compared with older adults with no anticholinergic burden for dementia | ||||
|
Patient or population: older adults without cognitive impairment at baseline Settings: mixed Intervention: older adults with an anticholinergic burden (defined according to the Anticholinergic Cognitive Burden scale) Comparison: older adults with no anticholinergic burden | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Cognitive decline or dementia | OR 2.63 (95% CI 1.09 to 6.29) | 125,359 (4 studies)a | ⊕⊕⊝⊝ Lowb,c,d,e | Findings were restricted to studies included in our primary analysis evaluating the association between anticholinergic burden (as defined by the Anticholinergic Cognitive Burden scale) and risk of cognitive decline or dementia, independent of age, sex, and comorbidities. Evidence indicates anticholinergic burden may increase the odds of developing future cognitive decline or dementia. |
|
CI: confidence interval; OR: odds ratio. GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aCampbell 2016; Hafdi 2020; Hsu 2017; Richardson 2018. Differences in design and methods restricted suitability of available studies for pooling in meta‐analysis to these four studies. bTwo of four studies were at high risk of bias, with issues around reverse causation. The remaining two studies were at unclear risk of bias, one of which had unclear risk of bias due to reverse causation. Therefore, downgraded one level for serious risk of bias. cThe confidence intervals of our pooled odds ratio differed by more than five points. Downgraded one level for imprecision. dWe were unable to evaluate risk of bias via a funnel plot; however, risk of publication bias was assumed within this literature unless evidence was found to the contrary. Downgraded one level. eA trend of increasing risk based on severity of anticholinergic burden was apparent in subgroup analysis, with the odds ratios increasing linearly from 'low severity' to 'high severity.' Upgraded one level.
Background
Description of the condition
Cognition (or cognitive function) is the mental process of acquiring knowledge and understanding through experience, senses, and thought. It includes the domains of memory, language, attention, executive functioning, and visuospatial processing. Cognitive impairment is the disruption of functioning of any one of these domains. Cognitive function may be assessed in detail using a battery of neuropsychological tests covering multiple domains; although in clinical practice, brief assessment tools such as the Mini Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA) are often used (Folstein 1975; Nasreddine 2005).
Dementia is a syndrome of decline in cognitive function beyond that expected from normal ageing and to an extent that interferes with usual functioning. It may affect memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgement. There are a variety of internationally accepted diagnostic criteria for dementia, the most widely used of which are included in the World Health Organization International Classification of Diseases (ICD) and the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM). The most recent iteration of the DSM (DSM‐5) refers to 'major neurocognitive disorder' instead of dementia.
The labels of 'dementia' or 'major neurocognitive disorder' encompass a variety of pathologies, with specific diagnostic criteria also available for pathologically defined dementia subtypes, such as the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) criteria for dementia due to Alzheimer's disease (McKhann 1984; McKhann 2011); McKeith criteria for Lewy body dementia (McKeith 2005); Lund criteria for frontotemporal dementias (McKhann 2001); and the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) criteria for vascular dementia (Román 1993).
An individual may experience a decline in cognition that is not enough to merit a label of dementia but that is more than would be expected as part of ageing. An objective cognitive impairment that is not severe enough to have a significant impact on daily activities is referred to as a mild cognitive impairment (MCI). This is a risk factor for future dementia as one in five may go on to develop dementia within five years (Petersen 2001).
Dementia and cognitive decline are major public health issues. There are currently more than 40 million people worldwide with dementia due to Alzheimer's disease – the most common subtype – and this number is projected to increase to more than 100 million by 2050 (Prince 2016). Dementia costs were estimated at USD 818,000 million in 2015, equivalent to 1.1% of global gross domestic product. It is estimated that by 2030, the global cost of dementia could grow to USD 2,000,000 million, which could overwhelm health and social care systems (Wimo 2017). The total cost of dementia to the UK alone is GBP 34,700 million, of which GBP 4,900 million is paid by the National Health Service (NHS) and GBP 15,700 million is paid by social care. The remainder is paid by those living with dementia and their families, and is classified as unpaid social care or private care (Alzheimer's Society 2019).
A number of prognostic factors have been associated with the onset of dementia, including age, sex, premorbid intelligence, genetics, medical conditions (e.g. diabetes, hypertension), and lifestyle factors (e.g. physical inactivity) (Livingston 2017). Identification of prognostic factors could assist healthcare professionals in predicting outcomes for people with cognitive syndromes and help policymakers in planning for future population healthcare needs. Identification of modifiable prognostic factors are potential targets for preventing or delaying the onset of cognitive decline and dementia.
Description of the prognostic factor
A prognostic factor is any measure that is associated with a future clinical outcome. The prognostic factor of interest for this review is anticholinergic burden from medication use.
Numerous medications commonly used in older adults have anticholinergic properties. Some medications, such as oxybutynin (for overactive bladder), exert their intended action through their anticholinergic activity. For other medications, such as amitriptyline for depression, anticholinergic activity is probably incidental to their intended mechanism of action. It is common for older adults to be taking multiple medications with anticholinergic properties (Myint 2015).
Even medications that have low anticholinergic activity individually may contribute to a significant overall anticholinergic effect if a person is taking several of them. This can be quantified as the anticholinergic burden. There is a reported relationship between anticholinergic burden and various adverse health outcomes (Singh 2008). It has been suggested that exposure to high anticholinergic burden is associated with cognitive decline and dementia in older adults (Fox 2014). Anticholinergic burden measures are used in primary and secondary care as part of the medication review process. Such reviews are increasingly recommended for older adults. The quantification of anticholinergic burden is designed to assess risk of future adverse events. Based on the anticholinergic burden score, clinicians may recommend reducing or replacing certain medications. Use of measures of anticholinergic burden to guide treatment decisions is entering clinical practice. The most recent National Institute for Health and Care Excellence (NICE) dementia guideline recommends considering anticholinergic burden as a factor that may be contributing to cognitive impairment and suggests using a validated scale to measure anticholinergic burden (NICE 2018). To date, anticholinergic burden has been considered largely as a 'stand‐alone' prognostic factor (not as part of a multifactorial prediction model).
Measures of anticholinergic burden
Anticholinergic burden can be measured using a variety of approaches. There is no consensus on which anticholinergic burden measures provide the most accurate and clinically useful prognostic information. Generally, anticholinergic burden measures use a person's medication list and assign a score to certain medications. A cumulative total based on all prescribed medications is then calculated. Although these measures should be similar, overlap is limited; they include differing medications and assign differing scores to these medications.
Our literature scoping suggests that 18 tools to measure anticholinergic burden have been published. One large population cohort (UK Biobank) reported that anticholinergic burden was strongly associated with future adverse health outcomes regardless of which anticholinergic burden measure was used (Hanlon 2020). However, at the individual patient level, there was substantial variability in the anticholinergic burden score generated by each measure. Methodologies for developing respective scales vary significantly: where some incorporate expert clinical opinion in their development and are designed to measure both central and peripheral anticholinergic effects, others focus on serum radioreceptor anticholinergic activity assay or muscarinic receptor affinity measurements and may only capture peripheral anticholinergic effects. Therefore, any prognostic review should be completed at the individual scale level in addition to creating summary estimates for all anticholinergic burden measures.
Prior to adoption in clinical practice, there should be a comprehensive assessment of the available literature to describe whether anticholinergic burden is a true prognostic factor, particularly adjusting for other dementia risk factors that may also be associated with anticholinergic prescribing. The relationship may vary based on the duration of the drug exposure period or via differences in clinical and demographic characteristics in specific clinical or community‐based settings. If anticholinergic burden is a prognostic factor, the strength of the association and the quality of the supporting evidence should also be described. Looking at the prognostic properties of each anticholinergic burden measure may assist in choosing a preferred scale.
Why is it important to do this review?
Increasing clinical interest in anticholinergic burden is accompanied by a growth in the research literature on anticholinergic burden as a prognostic factor. However, results from individual studies of anticholinergic burden and cognition or dementia are conflicting. Not all published papers have followed best practice in design, conduct, or reporting; and comparative strength of associations between respective scales and dementia has not been established. Thus, there is uncertainty around the clinical utility of anticholinergic burden and, consequently, inconsistency in clinical practice and guideline recommendations. In this systematic review, we aimed to estimate the prognostic utility (adjusted and unadjusted) of different anticholinergic burden measures for predicting cognitive decline or dementia in a cognitively healthy older adult population, and to assess the quality of the supporting evidence.
Objectives
Primary objective
To assess whether anticholinergic burden, as defined at the level of each individual scale, is a prognostic factor for future cognitive decline or dementia in cognitively unimpaired older adults.
Secondary objectives
To assess whether the strength of association between anticholinergic burden and future cognitive decline or dementia differs in various settings (e.g. primary care, secondary care, or community settings).
To compare the prognostic validity of different anticholinergic burden scales.
To examine the effect of duration of exposure and duration of follow‐up on the anticholinergic burden dementia risk association.
Methods
We followed best practice in design, conduct, and reporting of our prognosis review as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). The review was supported by the Cochrane Prognostic Methods Group, partners within the Cochrane Mental Health and Neuroscience Network, and the UK National Institute for Health Research Complex Reviews Support Unit (NIHR CRSU).
We used the PICOT (Patient/Problem; Intervention; Comparison; Outcome; Timing) system to design our review question (Schardt 2007) (Table 2). As recommended by the Cochrane Prognosis Methods Group, we followed guidelines suggested by Riley 2019, to ensure that our review was designed, conducted, and reported in keeping with best practice recommendations.
1. PICOTS.
| Population | Older adults (mean age ≥ 50 years) free of cognitive impairment at baseline |
| Interventions | Anticholinergic burden as measured by any validated ordinal anticholinergic burden scale |
| Comparators (covariates of interest) | Age, sex, and comorbidity |
| Outcomes | Incident dementia or cognitive function (multidomain) |
| Type of study | Longitudinal, observational cohort/case‐control |
| Timing and setting | Recruitment from primary, secondary, or community settings Minimum of 1‐year follow‐up |
Criteria for considering studies for this review
Types of studies
We included prospective and retrospective longitudinal cohort and case‐control observational studies. We did not include cross‐sectional studies, as it is not possible to determine prognosis from this design. We did not include prospective case studies, defined here as having fewer than 20 participants. We excluded studies that were published only as abstracts or posters at conferences, as these have not undergone peer review.
Types of participants
We included any studies that recruited older adults (as defined by the authors, but with minimum median/mean age 50 years at baseline) who were free of any known cognitive diagnosis (MCI, dementia, delirium) at time of recruitment and at time of application of the anticholinergic burden measure. We did not exclude studies that did not assess cognition at baseline; however, where a mixed population was recruited, we only included the study if the prevalence of dementia was less than 7% in a community sample (similar to general population prevalence in unselected older adults). We also included studies that recruited mixed populations in a hospital setting, where the prevalence of dementia was higher, provided that it was conducted in an unselected (i.e. consecutive admissions) sample.
We included studies where initial recruitment was in primary care, secondary care, and community settings. Participants in these respective settings may differ in important demographics (e.g. mean age, clinical or lifestyle factors) that could alter the strength of the association between anticholinergic burden and cognitive decline or dementia. We defined primary care as settings in which the patient self‐presented to a non‐specialist service, such as general practice. We defined secondary care as any settings where patients were referred for expert care, including general hospitals and more specialist settings. We defined community settings as settings in which the cohort was completely unselected, that is 'population screening.'
As this review focused on prognosis in people who were cognitively well, we did not include studies conducted in care‐home settings where the prevalence of cognitive syndromes is substantial.
We made no other restriction based on comorbidity or polypharmacy but recorded these factors in our data extraction. We assessed whether comorbid conditions that are associated with dementia (depression, stroke, other neurological diseases) were measured and considered any potential impact of this in our 'Risk of bias' assessment. We included studies conducted in specific patient subgroups, such as Parkinson's disease or stroke, provided they met our other inclusion criteria.
Index prognostic factor
The prognostic factor of interest was anticholinergic burden from medications. We included any study that used an ordinal scale that purported to measure cumulative exposure to medications with anticholinergic properties. Scales did not need to be described as validated for prediction of cognitive outcomes. Previously identified scales are listed in Appendix 1.
Different approaches to quantifying anticholinergic burden have been used. Some scales sum ordinal scores for each relevant drug to give a continuous measure; others create a summary ordinal hierarchical measure that scores, for example, 0 to 3 or 0 to 4 based on the cumulative anticholinergic exposure. Most scales define thresholds of 'low' and 'high' burden. As our focus in this review was on the extent of anticholinergic burden, we did not include studies that established anticholinergic exposure via a simple dichotomised present/absent method.
Some anticholinergic burden measures were developed specifically to predict dementia, while others were developed to predict other adverse events, including death. Anticholinergic activity should be an objective drug effect, and so we included any anticholinergic burden measure, not just those developed for cognitive outcomes.
We did not choose a particular measure of primary interest as there is no consensus on the preferred measure, and there is substantial heterogeneity in clinical practice. However, if the Drug Burden Index (DBI) scale was utilised, we only included data if anticholinergic burden data were reported separately.
Comparator prognostic factors
We were interested in the value of anticholinergic burden as a prognostic factor over and above other prognostic factors that may be common in this population. Hence, while we included studies that only assessed the unadjusted anticholinergic burden prognosis, we also evaluated the prognostic effect of anticholinergic burden adjustment for core variables identified as fundamental to the putative link between anticholinergic burden and dementia. These variables were selected on the basis of a Delphi discussion between the review authors and a wider multicentre collaborative, working in the field of anticholinergic burden research (Appendix 2). The chosen core variables were age, sex, and comorbidity. We recognise that comorbidity may be described in various ways. We accepted any classification that the original study authors defined as a measure of comorbidity, including measures that offered quantitative data, for example, number of medications, number of medical conditions listed, or formal measures such as the Charlson Comorbidity Index.
We assessed use of additional adjustments in our 'Risk of bias' assessment.
Outcome measures
Primary outcome: we included any study that assessed incident dementia, MCI, or cognitive decline (i.e. change on a measure of cognitive function) as an outcome. We accepted any validated diagnostic criteria for dementia or MCI. For the outcome of cognitive decline, we accepted any multidomain cognitive assessment tool that is validated for the direct assessment of cognition. We did not include papers that only measured a single cognitive domain.
Timing: we accepted assessment for cognitive decline or dementia at one year or longer following baseline anticholinergic burden assessment to mitigate the risk of reverse causality between any observed risk associations.
Search methods for identification of studies
Electronic searches
As reporting of prognostic factor studies is variable, it can be challenging to identify all relevant studies. We adopted the procedure proposed by Geersing 2012 to maximise our ability to identify relevant prognostic studies. Specifically, as we searched for one prognostic factor, we did not adopt any specific search filter, but instead adopted a search that combines our prognostic factor (anticholinergic burden) with the disease outcome (dementia/cognitive impairment).
We searched the following databases: MEDLINE (1946 to 24 March 2021; OvidSP), Embase (1974 to 24 March 2021; OvidSP), PsycINFO (1806 to 24 March 2021; OvidSP), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1950 to 24 March 2021; EBSCOhost), and ISI Web of Science Core Collection (1928 to 24 March 2021; ISI Web of Science) (Appendix 3). We applied no language restrictions.
Searching other resources
We supplemented this with handsearches of all included studies and identified systematic reviews.
Data collection and analysis
Selection of studies
We used Covidence systematic review software to identify relevant studies (Covidence). The review group Information Scientist performed a 'first pass' screen to remove clearly irrelevant titles. Two review authors (MT and SE) independently screened studies identified via our search method. Titles and abstracts were screened in the first instance, with the full text of potentially relevant studies then accessed to determine if the study met our inclusion criteria. In case of disagreement, a third review author (TQ) acted as arbiter and made the final decision on study inclusion/exclusion.
Data extraction and management
Two review authors (MT and SE) extracted the data to a piloted proforma based on the CHARMS‐PF (CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies, adapted for prognostic factors) template (Riley 2019). We contacted authors for missing data where required. We selected two studies to trial our data extraction proforma (Gray 2015; Richardson 2018a). We extracted all data onto a standard form (Appendix 4).
Assessment of methodological quality
Two review authors (MT and SE) independently used the QUIPS (Quality in Prognosis Studies) checklist (Hayden 2012), assessing the included studies across the domains of: study participation; study attrition; prognostic factor measurement; outcome measurement; adjustment for covariates; reverse causation; statistical analyses; and reporting. We used the QUIPS anchoring statements but modified the content to suit our review topic based on consensus within the review author team. We judged each domain as low risk of bias, moderate risk of bias, or high risk of bias (Appendix 5). In cases of uncertainty we contacted original study authors for clarification, where possible.
Discussing reporting deficiencies: prognosis research is frequently confounded by poor reporting and possible publication bias. We supplemented our 'Risk of bias' assessment with a narrative discussion of reporting issues, highlighting when missing information may have affected results. Prognostic factor studies often do not register protocols, increasing the risk that not all studies (published and unpublished) will be identified, and there is a risk of small‐study effects (in which smaller studies with higher odds ratios (ORs) are more likely to be published than smaller studies with non‐significant ORs), which can bias meta‐analyses (Peat 2014; Riley 2019). We used sensitive search filters for the disease (dementia) and the prognostic factor (anticholinergic burden) without any specific filter for prognostic research to increase retrieval, and attempted to examine the likelihood of small‐study effects in our review by generating a funnel plot.
Data synthesis
We evaluated risk of future cognitive decline or dementia for anticholinergic drug users against non‐users. Where possible, we pooled summary estimates for each anticholinergic burden tool individually; then, as an exploratory analysis, pooled summary estimates across all scales. We conducted each meta‐analysis in two ways. First, as an all‐encompassing 'any anticholinergic drug use' variable; second, as an ordinal, hierarchical variable in which low, moderate, and high users were pooled separately, to investigate possible differential relationships based on anticholinergic burden severity. Low users were defined as those with a cumulative score of 1 on an anticholinergic scale; moderate users were defined as those with a cumulative score of 2 on an anticholinergic scale; high users were defined as those with a cumulative anticholinergic scale score of 3 or above.
We pooled data in two separate ways. In the first instance, we pooled data obtained from unadjusted analyses. In the second instance, we pooled data from fully adjusted analyses, provided age, sex, and comorbidity were controlled for as a minimum. We pooled ORs and hazard ratios separately. We planned to calculate standardised mean difference (SMD) for linear data and pool this separately from dichotomous outcome data. Where data were not available, we attempted to estimate data based on methods suggested by Tierney 2007. Where data were sufficiently similar to permit pooling, we used a random‐effects approach given our expectation of high heterogeneity between studies. We used Comprehensive Meta‐ Analysis software to conduct all meta‐analyses (Comprehensive Meta‐Analysis Version 3).
We conducted a sensitivity analysis, restricting to studies that had no high risk of bias domains.
For secondary (subgroup) analysis, we assessed risk by setting. We also conducted analysis based on duration of follow‐up. We created categories of one to five years, and longer than five years and considered pooled rates for each timeframe individually. These additional outcomes were decided upon through discussion among the review authors.
We planned to assess exposure, including exposure before enrolment into the study and exposure during the study.
Finally, we planned to conduct a comparative analysis of the prognostic performance of the differing anticholinergic burden measures using a network meta‐analysis.
Investigation/description of heterogeneity
We described heterogeneity narratively based on consistency of association and effect size between anticholinergic burden and future cognitive decline or dementia, measurement of prognostic factor, outcome measurement and definition, and study design. We did not employ the I2 statistic in our evaluation of heterogeneity. In prognosis research, individual studies often have large sample sizes resulting in narrow confidence intervals (CIs); this can cause high I2 values even if inconsistency between studies is moderate (Iorio 2015).
It is possible that observed associations between anticholinergic drugs and future cognitive decline or dementia are driven by uncontrolled variables. Number of medical conditions or overall polypharmacy are two possible moderators of observed effects and could differ by setting. To investigate this, we planned to conduct a meta‐regression based on study recruitment setting (primary versus secondary care versus community care), comorbidity ('number of comorbidities' controlled for as a covariate, yes/no), and polypharmacy (controlled for as a covariate, yes/no).
Grading the evidence
We used the GRADE approach to evaluate our overall confidence in the results. We adapted the GRADE approach to suit prognosis research using methods consistent with Huguet 2013. Specifically, we evaluated reported evidence in the following eight areas.
Phase of investigation: phase 3 explanatory studies derived from bespoke cohort study designs that seek to explain the mechanisms behind an underlying association between anticholinergic burden and dementia/cognition were be considered a high level of evidence. Phase 2 explanatory studies that seek to confirm an independent association between anticholinergic burden and dementia/cognition were treated as moderate evidence, and hypothesis‐generating phase 1 explanatory studies were treated as weak evidence for any association between anticholinergic burden and dementia/cognition.
Study limitations: we used the previously described QUIPS tool to evaluate the overall risk of bias of included studies. Our GRADE judgement was based upon the overall certainty of the evidence, that is, if most (more than 50%) included studies were considered at high risk of bias in their reported association between anticholinergic burden and dementia/cognition, we downgraded the evidence accordingly.
Inconsistency: we downgraded the evidence if associations between ACB and dementia/cognition were heterogeneous (i.e. the reported ORs/hazard ratios fell either side of 1.0 on a forest plot, the measure of the prognostic factor was highly variable, outcome measurement was highly variable, and methodological heterogeneity due to study design/potential biases study design); and if the P value was low for the test of the null hypothesis that all studies in a meta‐analysis have the same underlying magnitude effect.
Indirectness: we downgraded the studies where their investigation did not fully match with our broader review question. We considered two areas of indirectness when judging if evidence should be downgraded on this basis: 1. if the population in the included studies only represented a subset of the population of interest (e.g. if only very old, i.e. older than 80 years, were assessed); 2. if the outcome investigated in the included studies was overly restricted (e.g. if the included studies explored only the association between anticholinergic burden and Alzheimer's dementia), then the evidence for the association between anticholinergic burden and all‐cause dementia was downgraded for indirectness.
Imprecision: we downgraded the evidence if there were insufficient numbers in the meta‐analysis or if the CIs were wide. We did not set an absolute value, but assessed this in the context of effect size and minimally important clinical difference.
Publication bias: due to inherent issues regarding publication bias in prognostic research, we adopted the default position that publication bias was likely and downgraded the evidence unless our assessment of publication bias provided significant evidence to the contrary (i.e. a symmetrically distributed funnel plot, and evidence that the prognostic factor has been investigated in numerous cohort studies).
Effect size: we upgraded our confidence in the effect estimate when the effect size was moderate to large (e.g. a hazard ratio of 2.5 or above).
Exposure‐response gradient: we upgraded our confidence in the effect estimate if there was evidence (via subgroup analysis) that a longer duration of anticholinergic burden was associated with an increased risk of dementia/cognitive decline. Similarly, we upgraded the evidence if there was an incremental increase in effect size with increasing anticholinergic burden.
Results
Description of studies
Results of the search
Our search identified 16,391 results. We identified a further four eligible studies via handsearching references. After deduplication and assessment of abstracts, we assessed 93 studies via full‐text screening, of which 25 met our inclusion criteria. See Characteristics of excluded studies table for reasons for exclusion. Figure 1 shows the PRISMA flow chart (Moher 2009).
1.

Study flow diagram.
Included studies
Twenty‐two studies were longitudinal 'cohort' designs and three were 'case control' studies (Coupland 2019; Richardson 2018; Yarnall 2015). All but one study (Iyer 2020) were conducted in a retrospective fashion, utilising databases in which participant information was obtained for a purpose other than investigating the association between anticholinergic burden and risk of future cognitive decline or dementia. Study sample sizes ranged from 102 to 324,703 participants and were conducted in Europe, North America, Asia, and Oceania: specifically, eight in the US; six in the UK; two in Taiwan; and one each in Sweden, Canada, France, Italy, Australia, Germany, Netherlands, Ireland, and South Korea. Follow‐up times for development of cognitive decline or dementia ranged from one to 11 years. See Characteristics of included studies table for details.
Participant characteristics
The review included 968,428 older adults; about 60% were women, mean/median age range across studies was 52 to 83 years. Participants were recruited overwhelmingly from community settings (963,081; 99.4%); 3716 (0.4%) were recruited via attendance at primary care clinics and 1525 (0.1%) were recruited in a secondary care setting. Most included studies were inclusive of the general 'older adult' population. However, two studies were conducted in people with Parkinson's disease (Sheu 2019; Yarnall 2015); one study was conducted in people with an overactive bladder (Iyer 2020); and one study was restricted to a 'young old' population (Low 2009).
Prognostic factor
Anticholinergic burden was assessed via 10 distinct methods. Eight studies used more than one method (Ancelin 2006; Brombo 2018; Coupland 2019; Gray 2015; Hsu 2017; Joung 2019; Kashyap 2014; Richardson 2018). Nineteen studies used an anticholinergic measurement scale: 17 used the Anticholinergic Cognitive Burden scale (ACB scale) (Brombo 2018; Campbell 2016; Chuang 2017; Coupland 2019; Fox 2011a; Grossi 2019; Hafdi 2020; Hsu 2017; Iyer 2020; Joung 2019; Kashyap 2014; Koyama 2014; Moriarty 2020; Richardson 2018; Risacher 2016; Shah 2013; Sheu 2019), four used the Anticholinergic Drug Scale (ADS) (Kashyap 2014; Low 2009; Richardson 2018; Yarnall 2015), three used the Anticholinergic Risk Scale (ARS) (Brombo 2018; Hsu 2017; Kashyap 2014), and two used the anticholinergic component of the Drug Burden Index (DBI‐ach) (Hsu 2017; Kashyap 2014). One study evaluated anticholinergic burden via a list defined by Chew 2008 (Jessen 2010); one study used a clinician‐rated anticholinergic score (Han 2008); one study used the Anatomical Therapeutic Chemical classification system (Papenberg 2017); two studies used the ACB scale in combination with Beers criteria (Coupland 2019; Joung 2019); one study used a literature review of drugs' anticholinergic activity (Whalley 2012); one study used Beers criteria combined with a literature review (Gray 2015); and one study used serum radioreceptor assay combined with a summation of mean estimated clinical effects of specific drugs, established via a pharmacologist, physician, and biologist (Ancelin 2006).
Outcome measures
Eleven studies reported assessing for dementia as an outcome (Ancelin 2006; Coupland 2019; Gray 2015; Grossi 2019; Hafdi 2020; Hsu 2017; Jessen 2010; Joung 2019; Richardson 2018; Sheu 2019; Whalley 2012); three reported MCI and dementia combined (Campbell 2016; Chuang 2017; Risacher 2016); four reported MCI alone (Ancelin 2006; Kashyap 2014; Low 2009; Yarnall 2015); and 13 reported multidomain cognitive impairment based on standardised cognitive test scores (Ancelin 2006; Brombo 2018; Fox 2011a; Han 2008; Iyer 2020; Kashyap 2014; Koyama 2014; Low 2009; Moriarty 2020; Papenberg 2017; Risacher 2016; Shah 2013; Whalley 2012; Yarnall 2015). Six studies established dementia or MCI via a consensus diagnosis based on a multidisciplinary evaluation (Campbell 2016; Chuang 2017; Gray 2015; Hafdi 2020; Jessen 2010; Whalley 2012). Three studies established dementia or MCI via a clinical examination from a single expert (e.g. neurologist, psychiatrist) (Ancelin 2006; Kashyap 2014; Low 2009). Five studies relied upon clinical codes recorded in medical records for diagnoses (Coupland 2019; Hsu 2017; Joung 2019; Richardson 2018; Sheu 2019), and one study employed a computer algorithm (Grossi 2019). Method for diagnosing dementia or MCI was unclear in two studies (Risacher 2016; Yarnall 2015). Dementia was diagnosed according to the DSM (APA 2000) criteria in seven studies (Ancelin 2006; Campbell 2016; Chuang 2017; Gray 2015; Grossi 2019; Hafdi 2020; Jessen 2010), and ICD‐10 (WHO 1992) criteria in three studies (Jessen 2010; Sheu 2019; Whalley 2012). Three studies did not explicitly state dementia diagnostic criteria (Coupland 2019; Hsu 2017; Richardson 2018). MCI was diagnosed according to DSM‐5 criteria in one study (Kashyap 2014), Peterson criteria (Petersen 2001) in one study (Chuang 2017), Jack criteria (Jack 1999) in one study (Low 2009), DSM III criteria in one study (Campbell 2016), Stockholm consensus group criteria (Winbald 2004) in one study (Ancelin 2006), and Movement Disorder Society criteria (Litvan 2012) in one study (Yarnall 2015). One study did not state criteria by which MCI was defined (Risacher 2016).
Risk of bias in included studies
Nineteen studies were at high risk of bias in at least one domain. No studies were at low risk of bias in every domain.
Most common issues were around lack of control for reverse causation (14 studies at high risk of bias), study attrition/missing data (nine studies at high risk of bias), and population generalisability (nine studies at high risk of bias).
Only two studies employed a dual approach to establishing medication use that would enhance identification of both prescription and non‐prescription drugs, along with recording dosage, duration of exposure, and adherence (Fox 2011a; Kashyap 2014). Two studies did not record anticholinergic drug usage at multiple time points, despite a follow‐up duration of more than one year (Koyama 2014; Risacher 2016).
Most studies controlled for age, sex, and comorbidities as covariates; however, only 13 studies controlled for psychiatric conditions, such as depression, that may heighten risk, or even be prodromal signs of dementia as well as increase anticholinergic burden (Ancelin 2006; Coupland 2019; Gray 2015; Grossi 2019; Hafdi 2020; Han 2008; Iyer 2020; Joung 2019; Moriarty 2020; Papenberg 2017; Richardson 2018; Sheu 2019; Yarnall 2015).
There were also general issues around good methodological practice: no protocols were registered; only three studies reported tests of statistical assumptions in analysis (Joung 2019; Moriarty 2020; Risacher 2016), and no studies reported blinding to outcome when assessing anticholinergic burden scores (Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Association between anticholinergic burden and dementia/cognitive decline
Twenty‐three of 25 studies reported a significant association between anticholinergic use and increased risk of cognitive impairment (of any type). However, specific associations between anticholinergic use and risk of dementia, MCI, and cognitive decline were more heterogeneous.
Dementia: 9/11 studies reported a significantly increased risk for dementia (Coupland 2019; Gray 2015; Grossi 2019; Hafdi 2020; Hsu 2017; Jessen 2010; Joung 2019; Richardson 2018; Sheu 2019); two studies reported no association (Ancelin 2006; Whalley 2012).
MCI and dementia: 3/3 studies reported significant increased risk for MCI and dementia combined (Campbell 2016; Chuang 2017; Risacher 2016).
MCI alone: 1/4 studies reported significantly increased risk for MCI (Ancelin 2006); 3/4 studies reported no association (Kashyap 2014; Low 2009; Yarnall 2015).
Cognitive decline: 12/14 studies reported significantly increased risk for reduced performance on cognitive tests (Ancelin 2006; Brombo 2018; Fox 2011a; Han 2008; Kashyap 2014; Koyama 2014; Low 2009; Moriarty 2020; Papenberg 2017; Risacher 2016; Shah 2013; Whalley 2012); two studies reported no association (Iyer 2020; Yarnall 2015). Investigated domains were extremely diverse and affected domains inconsistently reported. There were statistically significant declines for memory (seven studies; Ancelin 2006; Han 2008; Kashyap 2014; Koyama 2014; Moriarty 2020; Papenberg 2017; Risacher 2016), executive functioning (four studies; Han 2008; Kashyap 2014; Risacher 2016; Whalley 2012), visuospatial construction (one study; Ancelin 2006), language (two studies; Ancelin 2006; Koyama 2014), processing speed (two studies; Ancelin 2006; Low 2009), and attention (one study; Ancelin 2006). Three studies reported a global cognitive decline on cognitive testing (Brombo 2018; Fox 2011a; Shah 2013).
One study investigated the association between anticholinergic use and cognitive decline via a univariate regression only (Kashyap 2014). Choice of comorbidities varied across all studies that assessed the association via multiple regression (see Characteristics of included studies table).
A variety of factors influenced the observed association between anticholinergic burden and cognitive impairment.
Associations differed by anticholinergic burden score and dosage. Most studies suggested that a higher anticholinergic burden score/dosage is more strongly associated with cognitive impairment than lower scores/dosages. Two studies found the association was only apparent in those with a low anticholinergic burden (anticholinergic burden = 1), though both studies had low numbers of moderate/high anticholinergic burden (Ancelin 2006; Chuang 2017), and one study reported that those with an anticholinergic burden of 2 or 3 had a shorter duration of exposure and frequency of use than those with an anticholinergic burden of 1 (Chuang 2017).
Duration of use was a modifying factor in five studies. Three studies found an association between anticholinergic burden and cognitive impairment for recurrent/persistent drug users only (Grossi 2019; Hafdi 2020; Papenberg 2017), while one study found that continuous use of anticholinergic drugs was more strongly associated with cognitive impairment than intermittent use (Joung 2019). By contrast, one study found that the gradient of global cognitive decline was greater for incident users, but not for prevalent users (Shah 2013); while one study reported a larger decline in cognitive scores for new users compared to persistent users, and no significant difference in decline on cognitive scores between discontinued users and non‐users (Moriarty 2020).
Drug type was a factor explored in five studies. Three studies found drug type altered the relationship between anticholinergic burden and risk of cognitive impairment (Coupland 2019; Hafdi 2020; Richardson 2018). Specifically, antidepressants, analgesics, anti‐Parkinson's, antipsychotics, antiemetics, urological, and respiratory drugs were significantly associated with increased risk of cognitive impairment; while antihistamines, cardiovascular, and gastrointestinal drugs were not. Two studies found risk for dementia in the highest exposure category was independent of anticholinergic drug type (Gray 2015; Joung 2019).
The population investigated also appears to be an important moderator of the association. The two studies that reported no association between anticholinergic use and future cognitive impairment (of any type) were conducted in a people with Parkinson's disease (Yarnall 2015) and overactive bladder (Iyer 2020). All studies that investigated the association in a more 'general' older adult population reported a significant association of some type.
Meta‐analysis
Twelve studies provided OR or hazard ratio (or both) data for meta‐analysis. Thirteen studies provided linear data (Ancelin 2006; Brombo 2018; Fox 2011a; Han 2008; Iyer 2020; Koyama 2014; Low 2009; Moriarty 2020; Papenberg 2017; Risacher 2016; Shah 2013; Whalley 2012; Yarnall 2015). However, hazard ratio and linear data were too heterogeneous to pool; therefore, we report only on the results of pooled ORs. See Characteristics of included studies table for the specific covariates controlled for in each study included in our meta‐analyses.
Some of the included papers described associations for various cognitive outcomes. For our primary cognitive analysis, we had to prioritise a single outcome. We prespecified that we would favour data that described the association with incident clinical dementia. We chose this outcome as we anticipated it would be most commonly reported and arguably it is the most clinically relevant. Similarly, some papers included multiple scales that purport to measure anticholinergic burden. In these instances, we favoured the ACB scale as we anticipated that this would be the most commonly used.
Univariate association
Four studies (287,546 participants) provided unadjusted ORs (Coupland 2019; Hafdi 2020; Kashyap 2014; Richardson 2018). Three of four studies reported ORs for risk of dementia only; one study reported ORs for cognitive decline (Kashyap 2014). There were insufficient numbers to pool ORs subgrouped by anticholinergic measurement scale; hence, we pooled rates across all scales. Where studies provided ORs subgrouped by anticholinergic burden score only, we selected anticholinergic burden values of 3 or closest to evaluate 'definite' anticholinergic drugs while maximising statistical power and minimising heterogeneity. Richardson 2018 subgrouped cumulative anticholinergic burden score by defined daily dosage (DDD); therefore, we selected the largest group (DDD greater than 0 to 13) in the ACB scale 3 category. One study reported dosage‐based subgroups only; for this study, the ORs from the largest 'dosage subgroup' (1 to 90) had asymmetrical CIs, hence we selected the ORs from the dosage with the second highest participant numbers ('greater than 1900') (Coupland 2019). Pooled rates suggested a significant increase in risk of cognitive impairment or dementia for anticholinergic drug users compared to non‐users (OR 1.47, 95% CI 1.09 to 1.96) (Figure 3).
3.
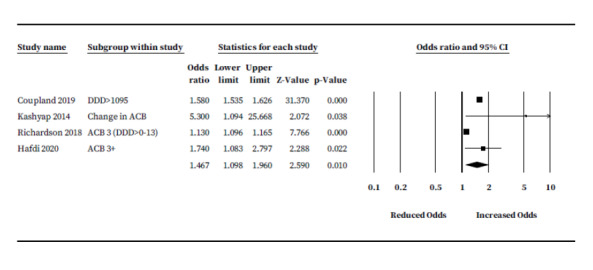
Unadjusted analysis: anticholinergic burden and odds of future cognitive decline or dementia.
Fully adjusted multivariate model
For our primary analysis on the association between anticholinergic drug use and risk of cognitive decline or dementia, three studies (121,833 participants) provided ORs based on use of the ACB scale exclusively to establish anticholinergic burden (Campbell 2016; Hsu 2017; Richardson 2018). One additional study provided ORs after contacting the author (Hafdi 2020). One other study reported ORs in a form that could be included in our ACB scale meta‐analysis (Sheu 2019). However, this study compared those with a moderate and severe anticholinergic burden against those with a low burden (instead of against non‐users) and was conducted in people with Parkinson's disease; therefore, we excluded it from analysis. As in our univariate analysis, we selected ORs with an ACB score of 3 or closest where multiple ORs were reported. Total number of ORs pooled for this analysis was six, as one study provided three suitable ORs due to subgrouping based on age (Hsu 2017). Results suggest drugs classified as having 'definite' anticholinergic properties by the ACB scale may have an increased risk of future cognitive decline or dementia (OR 2.63, 95% CI 1.09 to 6.29) (Figure 4). There were insufficient studies to establish specific associations for other anticholinergic measurement scales.
4.

Primary analysis: adjusted association between ACB scale and odds of future cognitive decline or dementia.
We ran further analyses to quantify ORs for each 'low', 'moderate', and 'high' ACB scale subgroup. From Richardson 2018, we selected the DDD greater than 1460 from each 'low', 'moderate', and 'high' ACB scale group based on utilising the highest available participant numbers while maintaining consistency of DDD across subgroups. A relationship between severity of cumulative anticholinergic burden and risk of dementia was apparent. Four ORs from two studies (145,663 participants with low ACB) were pooled for 'low' ACB (OR 2.18, 95% CI 1.11 to 4.29) (Hsu 2017; Richardson 2018). Four ORs from two studies (1719 participants with moderate ACB) were pooled for 'moderate' ACB (OR 2.71, 95% CI 2.01 to 3.56) (Hsu 2017; Richardson 2018). Five ORs from three studies (8451 participants with high ACB) were pooled for 'high' ACB (OR 3.27, 95% CI 1.41 to 7.61) (Hafdi 2020; Hsu 2017; Richardson 2018) (Figure 5).
5.
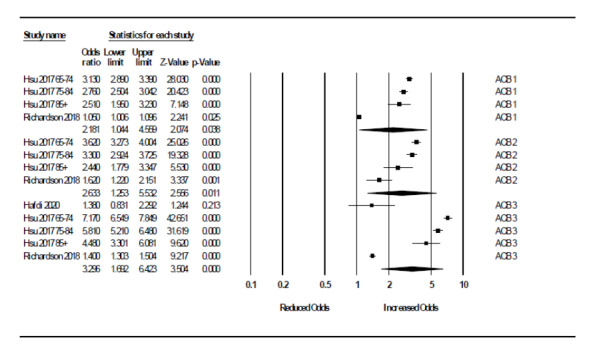
Primary analysis: relationship between severity of anticholinergic burden (defined according to ACB scale) and odds of future cognitive decline or dementia.
As an exploratory analysis, we pooled ORs across scales. Seven studies (317,216 participants) reported ORs suitable for analysis (Ancelin 2006; Campbell 2016; Coupland 2019; Hafdi 2020; Hsu 2017; Richardson 2018; Whalley 2012). Five of the seven studies reported the odds for risk of dementia only; one assessed the odds of developing MCI or dementia (combined) (Campbell 2016), and one assessed odds of developing MCI only (Ancelin 2006). We pooled ORs with an anticholinergic burden score of 3 or closest; or in the case of one study (Coupland 2019), a dosage of 'strong' anticholinergic drugs of 1 to 90. Pooled rates suggest people taking anticholinergic drugs may have a significantly increased risk of future cognitive decline or dementia (OR 2.16, 95% CI 1.38 to 3.38) (Figure 6).
6.
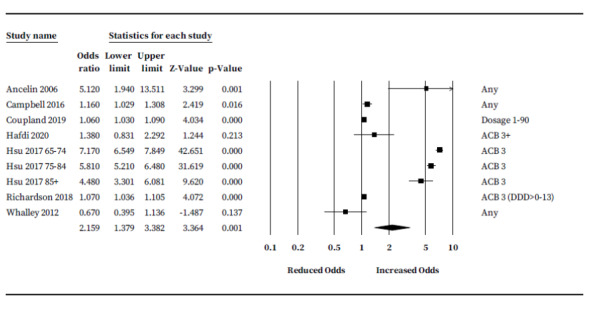
Exploratory 'cross scale' analysis: adjusted association between anticholinergic burden and odds of future cognitive decline or dementia.
We ran a post‐hoc analysis to investigate the association with dementia specifically (296,523 participants). We removed two studies that did not assess 'dementia only' as an outcome (Ancelin 2006; Campbell 2016). Results show risk of dementia may be higher for anticholinergic drug users compared to non‐users (OR 2.18, (95% CI 1.33 to 3.57) (Figure 7).
7.

Post hoc analysis: adjusted association between anticholinergic burden and odds of future dementia specifically.
We conducted a sensitivity analysis to investigate the effect of risk of bias on our pooled rates. We restricted analysis to three studies (468,830 participants) that reported ORs but had no high risk of bias categories (Coupland 2019; Hafdi 2020; Richardson 2018). Results were consistent with our exploratory analysis: people taking anticholinergic drugs may be at increased risk of developing future dementia; however, the size of the risk was lower (OR 1.07, 95% CI 1.04 to 1.09) (Figure 8).
8.
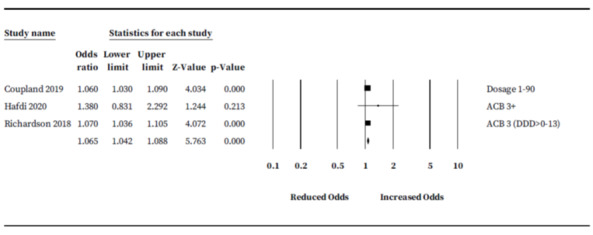
Risk of bias analysis: adjusted association between anticholinergic burden and odds of future cognitive decline or dementia restricted to low risk of bias studies only.
We were unable to formally investigate the possibility of publication/small‐study bias by generating a funnel plot due to limited study numbers. However, risk of publication bias is assumed within this field.
Certainty of evidence (GRADE)
The overall quality of evidence from our primary analysis was low. Evidence was downgraded based on extent of risk of bias within studies, imprecision, and possible publication bias. Evidence was upgraded based on the apparent incremental increase in risk based on severity (low, moderate, and high) of ACB scores. Specific judgements in each respective domain are shown in Table 3.
2. GRADE.
| Quality criteria |
Rating (circle 1 for each criterion) |
Footnotes (explain reasons for upgrading or downgrading) |
Quality of the evidence (circle 1 per outcome) |
| Prognostic factor: anticholinergic burden (defined by ACB scale) and risk of future cognitive impairment/dementia in cognitively healthy older adults | |||
| Phase of investigation | No issues | Most studies were phase 3 confirmatory investigations. | High Moderate Low Very Low |
| Study limitations | Serious (–1) | 2 studies were at high risk of bias, with issues around reverse causation. The remaining 2 studies were at unclear risk of bias, 1 of which had uncertain risk of bias due to reverse causation. | |
| Imprecision | Serious (–1) | Relatively wide confidence intervals. | |
| Inconsistency | No issues | Included studies were predominantly assessed using similar prognostic outcome measures, definitions, in similar settings with similar follow‐up durations. Direction of effect was also consistent. | |
| Indirectness | No issues | Included studies represented the review question adequately. | |
| Publication bias | Serious (–1) | Publication bias was assumed. | |
| Effect size | No | Moderate effect size in primary analysis but lower bound confidence intervals suggested a low effect size. | |
| Dose‐response gradient | Yes (+1) | Clear relationship between severity of ACB scale score and risk of dementia was apparent. | |
ACB: Anticholinergic Cognitive Burden.
Secondary analyses
We conducted a subgroup analysis based on 'setting.' Five studies (295,666 participants) were conducted in a community setting and suggested anticholinergic drug users in the community may have an increased risk of cognitive decline or dementia (OR 2.18, 95% CI 1.30 to 3.65) (Figure 9) (Coupland 2019; Hafdi 2020; Hsu 2017; Richardson 2018; Whalley 2012). There were insufficient studies conducted in primary or secondary care to establish pooled rates.
9.
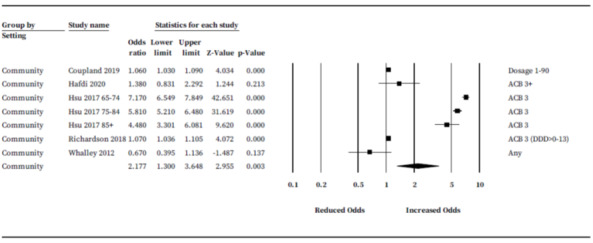
Subgroup analysis: adjusted association between anticholinergic burden and odds of future cognitive decline or dementia subgrouped by setting.
We examined the pooled ORs based on duration of follow‐up. Five studies (295,666 participants) provided suitable ORs for duration greater than five years (OR 2.18, 95% CI 1.30 to 3.65) (Figure 10) (Coupland 2019; Hafdi 2020; Hsu 2017; Richardson 2018; Whalley 2012). There were insufficient studies to investigate OR for one to five years' follow‐up.
10.

Subgroup analysis: adjusted association between anticholinergic burden and odds of future cognitive decline or dementia subgrouped by duration of follow‐up.
Duration of exposure to anticholinergic medications was too poorly reported to be investigated as a source of potential variability regarding the association between anticholinergic burden and cognitive decline or dementia.
We were unable to run a meta‐regression to investigate the influence of number of comorbidities controlled for on the relationship between anticholinergic burden and cognitive decline or dementia due to lack of study numbers. This was similarly the case for polypharmacy and setting.
Association based on scale
Low study numbers meant presenting pooled rates for all but the ACB scale was not possible; hence, we could not conduct a comparative statistical analysis, as planned. However, four studies compared scales within the same participant pools, enabling narrative comparisons to be drawn (Brombo 2018; Hsu 2017; Kashyap 2014; Richardson 2018).
Respective performance of scales was relatively heterogeneous across studies. Nevertheless, some patterns were apparent. First, while all anticholinergic measurement scales showed an association with cognitive decline or dementia, the DBI‐ach was consistently the least strongly associated scale, demonstrating lower odds for future cognitive decline or dementia than all scales it was compared against in two separate studies (Hsu 2017; Kashyap 2014). The ARS scale showed some of the strongest reported associations with cognitive decline or dementia when compared to other scales. Importantly, however, there was evidence that associations are strongest at lower and higher ARS scores, suggesting a U‐shaped relationship with cognitive decline or dementia (Hsu 2017). The ADS and ACB scales were on the whole comparable, demonstrating a similar strength of association in the two studies that compared them (Kashyap 2014; Richardson 2018). All other available scales described in this review were not evaluated in tandem with a secondary scale in the same study population and as such cannot be reliably compared.
Discussion
Summary of main results
Anticholinergic drug use is consistently associated with future cognitive decline or dementia. Our results suggest that cognitively unimpaired older adults who use drugs defined as 'definitely' anticholinergic by the ACB scale may have more than two‐times greater odds of developing future cognitive decline or dementia than non‐users, independent of age, sex, and comorbidities. Moreover, a relationship between severity of anticholinergic burden and future dementia appears to be apparent: odds of developing future cognitive decline or dementia may rise as extent of anticholinergic burden increases. People with a severe anticholinergic burden might see their odds of cognitive decline or dementia increase by as much as 227%.
While our findings are largely based on anticholinergic drugs as measured by the ACB scale, the association appears to exist regardless of which anticholinergic scale is used. All anticholinergic measurement scales evaluated in this review showed an association with cognitive decline or dementia. Interestingly, the DBI‐ach was consistently less strongly associated with future cognitive decline or dementia than other scales when making within‐study comparisons. This is contrary to previous assertions that scales incorporating dosage are more likely to be reliable risk predictors (Mayer 2015). Of all the available scales, the evidence suggests the ARS is less well suited to the role of predicting future cognitive decline or dementia; this is due to an apparent U‐shaped relationship that violates the assumption that severity of anticholinergic drug scores are additive to the overall risk (at least until a plateau is reached). This curious trend may be a by‐product of increased prescribing of drugs rated 'less severe' by the ARS scale as treatments for prodromal symptoms of dementia (Hsu 2017).
At present, the ACB scale is the most popular available anticholinergic burden measurement tool. Although no anticholinergic burden scale can be considered the 'gold standard', there is some justification for the popularity of the ACB scale in this area. Specifically, it was explicitly designed to evaluate the association between anticholinergic burden and cognition (Boustani 2009), and as such considers factors such as the anticholinergic potency of a drug when assigning an anticholinergic burden rating. Furthermore, it demonstrates a trend suggesting a steady increase in risk as severity of anticholinergic burden increases, as seen both in our meta‐analysis and in previous studies (Pasina 2013); it incorporates the second highest number of drugs (99) of all available scales (Mayer 2015), and was ranked the highest quality of 19 available scales in a comprehensive systematic review (Lisibach 2021), achieving the best score for 'rigour of development.' It is also one of only three available scales to offer guidance on use in clinical practice along with clear scoring rules that allow reproducibility. Limitations of the ACB scale relate to it not considering dosage, drug interactions, or clearance of respective drugs – all of which are likely relevant to the evident risk of future cognitive decline or dementia. Supplementation of the ACB scale with other existing anticholinergic measurement systems, such as 'Beers criteria', was an approach adopted by several studies included in this review. Augmenting the ACB scale with additional measures may seem like an attractive approach to enhance ACB scale‐based anticholinergic burden measurement; but, currently there is insufficient evidence to determine the extent to which this improves prognostic accuracy compared to when the ACB scale is used alone.
Overall completeness and applicability of evidence
Most included studies were conducted in older adult populations in various regions throughout the world. However, there was little to no representation from Middle Eastern, South American, or African populations; therefore, we cannot say our results generalise 'globally.' Similarly, included participants were overwhelmingly from community‐based settings and we could not delineate specific pooled rates for older adults in secondary care settings, or reliable pooled rates for those attending primary care. People recruited in primary or secondary care settings likely differ in important demographics, including mean age and number of health conditions, which may increase or reduce the influence of anticholinergic burden on future risk of cognitive decline or dementia. As such, our reported rates of increased risk for older adults are particularly relevant to those in the community.
Our evidence is also restricted to older adults who are 'cognitively healthy.' Anticholinergic burden may be equally important for those with an established cognitive syndrome but this was not a focus of this review.
Certainty of the evidence
Our results are tempered by lack of control for reverse causation, imprecision in reported effect size, risk of publication bias, and the general risk of bias observed throughout included studies. Consequently, our overall confidence in the evidence for our primary pooled analysis was low.
If anticholinergic drugs are prescribed as a treatment for prodromal signs of dementia, such as mood symptoms or sleep disorders, any risk association will be inflated as a consequence. Similarly, if symptoms and subsequent prescriptions increase right up to the point of the dementia diagnosis, the observed trend of increasing risk based on severity of anticholinergic burden could simply reflect this. While it is not known what lag time is sufficient to reduce protopathic bias (i.e. risk of reverse causation), when we restricted pooled rates to those studies that had at least a two‐year lag‐time and not thought to be at high risk of bias overall, the association between anticholinergic drug use and dementia remained. This runs contrary to the reverse causation hypothesis and provides some reassurance that the observed effects are related to the anticholinergic properties of the drugs themselves. Regardless, our pooled rates are likely an overestimation.
Limitations of the review process
To minimise bias in the review process, two review authors independently screened all titles, extracted data, and conducted risk of bias assessment. Despite this, we encountered several issues.
Tools that were used throughout our review were not explicitly designed for use with prognostic research. To mitigate this problem, we followed best available guidelines to adapt GRADE for evaluation of prognostic study evidence and enhanced QUIPS by incorporating specific guidance when rating each category.
We evaluated cognition and dementia as a composite variable since both are important to older adults and combining these outcomes increases power to detect smaller, but still clinically meaningful prognostic associations. Despite this, our meta‐analyses were limited by power constraints. One recurring issue was the tendency of authors to report ORs subgrouped into low, moderate, and high user categories and compare against a common reference standard (non‐users). As a consequence, we were required to select single ORs from a specific subgroup for each analysis. Although we were able to extract relevant data from many of the included studies, we received a poor response from authors who we contacted about providing additional data, further reducing the strength of our analyses. In combination, these issues challenge the veracity of our results – particularly our subgroup analyses. For instance, our quantification of the association between low, moderate, and high anticholinergic burden and future cognitive decline or dementia are derived from just three studies, thus are of questionable validity.
We were also unable to conduct a number of analyses that we prespecified in our protocol due to issues with the types of data that were presented in available studies (Quinn 2020). Specifically, we could not pool linear or hazard ratio outcome data, investigate the association between anticholinergic burden and duration of exposure, or determine if number of comorbidities or polypharmacy were sources of the high heterogeneity in observed effect sizes between studies.
Lastly, we did not include grey literature in our review. Although we were unable to formally assess for publication bias, we identified few small studies that reported an absence of association between the ACB scale and dementia. Existent studies that did report a lack of association typically reported a significant association with another form of cognitive impairment (e.g. MCI) (Whalley 2012); hence, there is a real risk of publication bias in this field.
Agreements and disagreements with other studies or reviews
Our results are broadly consistent with three previous meta‐analyses (Dmochowski 2021; Pieper 2020; Ruxton 2015). These prior reviews report a 20% to 46% increased risk for dementia (Dmochowski 2021; Pieper 2020), and a 45% increased risk for cognitive impairment (Ruxton 2015). This is lower than our primary pooled rates but in line with our analysis restricted to studies at lower risk of bias, which may be more accurate. Our findings also build upon these previous meta‐analyses by quantifying risk of ACB scale measured drugs specifically, along with risks for each ACB scale subgroup (low, moderate, high).
The increasing risk based on extent of anticholinergic burden is consistent with prior evidence: Joung 2019 found people on moderate‐to‐high dosages of anticholinergic drugs were at significantly greater risk of Alzheimer's disease than people on very low dosages, while Coupland 2019 similarly found a dose‐response effect. Low anticholinergic burden represents the most common category that those with any form of anticholinergic burden reside, and although we observed a significant association with dementia at this level, it remains a point of contention due to frequent negative findings within the literature (Hafdi 2020).
Lastly, previous studies have suggested that the cholinergic system may play a particular role in episodic memory (Bentley 2011). It has been theorised that any impact of anticholinergic drugs may be largely confined to this domain (Papenberg 2017). We found considerable variability throughout the literature regarding reported cognitive domains affected by anticholinergic drug use. As such, our results do not support this theory and the available evidence suggests that disruption of the cholinergic system leads to widespread cognitive impairment.
Authors' conclusions
Implications for practice
Clinicians should think carefully about the need to prescribe anticholinergic drugs to older adults. Estimates suggest as many as 20% to 50% of older people are prescribed at least one anticholinergic medication (Fox 2014). If the observed link between anticholinergic drug use and future dementia is causal, these exposure rates imply anticholinergics meaningfully contribute to the global dementia burden; albeit the extent of this contribution is difficult to determine due to the risk of bias present within studies. Moreover, there are associations with increased risk of mortality (Grave‐Morris 2020), impaired physical function, and reduced quality of life (Stewart 2020), emphasising the potential dangers anticholinergic drugs pose to older adults.
While we would advise caution in prescribing anticholinergic drugs, we cannot comment on the potential benefits of deprescribing for people already receiving anticholinergic drugs. It is uncertain to what extent any adverse effects of anticholinergic medicines are reversible upon cessation. One randomised controlled trial (RCT) provided no evidence that deprescribing can benefit cognition (Kersten 2013), albeit this RCT was underpowered and focused only on short‐term cognitive changes (Pieper 2020). We can also hypothesise from observational studies that circumstances may exist in which the increased risk of dementia associated with use of anticholinergic drugs is irreversible. For example, risk of future dementia for older adults with high anticholinergic burden has been found to be unaltered by recency of use: those who are historic heavy users of anticholinergic drugs might be at similar risk to recent or even long‐term continuous heavy users (Gray 2015).
Implications for research
Several outstanding issues remain to be resolved. Most prominently, clarification is needed regarding the importance of medication type on the association between anticholinergic burden and risk of future cognitive decline or dementia. Although plausible mechanisms exist by which anticholinergic drugs may impact the cognition of older adults (Shah 2013), some studies only observed significant associations for certain types of anticholinergic drugs, many of which are prescribed for prodromal symptoms of dementia. While this variability based on drug‐type was not consistent across studies, there remains a possibility that anticholinergic effects may not be driving the observed association. It is likely that the only way a causal role for anticholinergic drugs and risk of future cognitive decline or dementia can be conclusively established (or disregarded) is via an RCT in which some people have their anticholinergic medications stopped to determine if the risk of future cognitive decline or dementia is reduced.
We likewise do not know precise details regarding the interaction between severity of anticholinergic burden, duration of exposure, and the frequency of use required to cause long‐term harm. If anticholinergic drugs play a causal role, establishing the relationship between these variables could be key to our ability to alter future dementia risk. As such, this is a crucial avenue for future research.
Methodological standards also require improvement. Authors should be sure to register protocols before conducting their study. This is necessary to determine the extent of publication bias within the field as well as adequately evaluate statistical approaches. Drug use measurement is currently extremely limited. Reported study methods are typically not sufficient to reliably establish dosage or adherence, and total exposure period is often inadequately reported. Use of anticholinergic drugs can vary over the course of a study (Shah 2013), which is likely to have a bearing on results, yet not all studies take measurements at multiple time points. Researchers should also ensure that those who ascribe anticholinergic ratings are blinded to cognitive outcomes (and vice versa) to minimise bias, and ensure inclusion of psychiatric conditions when controlling for comorbidities. Addressing these limitations would go some way to improving our understanding of the relationship between anticholinergic burden and risk of cognitive decline or dementia.
History
Protocol first published: Issue 2, 2020 Review first published: Issue 4, 2021
Acknowledgements
We would like to thank our funders for supporting this work. We would like to thank the CDCIG Information Specialist for helping develop the MEDLINE search strategy.
We would also like to thank Dr Melanie Hafdi for providing OR data for our meta‐analysis.
We would like to thank peer reviewers Amanda J Cross and Christpher Etherton Beer, consumer reviewer Delia Bishara and Prognosis Methods Group reviewer Angela Aldin for their comments and feedback.
Appendices
Appendix 1. Anticholinergic burden scales
AAS: Anticholinergic Activity Scale
AAS‐r: Revised Anticholinergic Activity Scale
ABC: Anticholinergic Burden Classification
ABS: Anticholinergic Burden Scale
ACB: Anticholinergic Cognitive Burden
ADS: Anticholinergic Drug Scale
AEC: Anticholinergic Effect on Cognition
AIS: Anticholinergic Impregnation Scale
ALS: Anticholinergic Loading Scale
ARS: Anticholinergic Risk Scale
BAAS: Brazilian Anticholinergic Activity Scale
Chew's list
CrAS: Clinician‐rated Anticholinergic Scale
Ellett's list
KABS: Korean Anticholinergic Burden Scale
MARANTE: Muscarinic Acetylcholinergic Receptor Antagonist Exposure Scale
mARS: Modified Anticholinergic Risk Scale
Appendix 2. Contributions to Delphi
Contributors to Delphi for selection of adjustment variables were researchers and clinicians from a range of specialities (medicine and psychology). Specific contributors were Dr Carrie Stewart, Dr Roy Soiza, Professor Phyo Myint, Dr Terry Quinn, and Dr Yoon Loke.
Appendix 3. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE (OvidSP) from 1946 Date of search: 24 March 2021 |
1. cholinergic antag*.ti,ab. 2. anticholinergic*.ti,ab. 3. anti‐cholinergic*.ti,ab. 4. cholinergic Antagonists/tu 5. Cholinergic Antagonists/ae 6. AAS.ti,ab. 7. ACB.ti,ab. 8. ADS.ti,ab. 9. DAPs.ti,ab. 10. ARS.ti,ab. 11. DBI‐ACh.ti,ab. 12. SAMS.ti,ab. 13. ("chew* score" or "chew* list").ti,ab. 14. ("han's score" or "han score").ti,ab. 15. or/1‐14 16. Cognition/ 17. Cognition Disorders/ 18. Dementia/ 19. cognit*.ti,ab. 20. dement*.ti,ab. 21. alzheimer*.ti,ab. 22. "lewy bod*".ti,ab. 23. FTLD.ti,ab. 24. PDD.ti,ab. 25. "executive function*".ti,ab. 26. Attention/ 27. (speed adj2 processing).ti,ab. 28. memory.ti,ab. 29. Memory Disorders/ 30. "episodic memory".ti,ab. 31. Memory, Episodic/ 32. MCI.ti,ab. 33. Mild Cognitive Impairment/ 34. (nMCI or aMCI or mMCI or MCIa).ti,ab. 35. AAMI.ti,ab. 36. ACMI.ti,ab. 37. ARCD.ti,ab. 38. CIND.ti,ab. 39. VCI.ti,ab. 40. VAD.ti,ab. 41. major neurocognitive disorder*.ti,ab. 42. minor neurocognitive disorder*.ti,ab. 43. neurocognitive dysfunction.ti,ab. 44. Neurocognitive Disorders/ 45. or/16‐44 46. 15 and 45 |
Mar 2020: 2907 Mar 2021: 252 |
| EMBASE (OvidSP) from 1974 Date of search: 24 March 2021 |
1. cholinergic antag*.ti,ab. 2. anticholinergic*.ti,ab. 3. anti‐cholinergic*.ti,ab. 4. *cholinergic receptor blocking agent/ 5. AAS.ti,ab. 6. ACB.ti,ab. 7. ADS.ti,ab. 8. DAPs.ti,ab. 9. ARS.ti,ab. 10. DBI‐ACh.ti,ab. 11. SAMS.ti,ab. 12. ("chew* score" or "chew* list").ti,ab. 13. ("han’s score" or "han score").ti,ab. 14. or/1‐13 15. Cognition/ 16. Cognition Disorders/ 17. Dementia/ 18. cognit*.ti,ab. 19. dement*.ti,ab. 20. alzheimer*.ti,ab. 21. "lewy bod*".ti,ab. 22. FTLD.ti,ab. 23. PDD.ti,ab. 24. "executive function*".ti,ab. 25. Attention/ 26. (speed adj2 processing).ti,ab. 27. memory.ti,ab. 28. Memory Disorders/ 29. "episodic memory".ti,ab. 30. Memory, Episodic/ 31. MCI.ti,ab. 32. Mild Cognitive Impairment/ 33. (nMCI or aMCI or mMCI or MCIa).ti,ab. 34. AAMI.ti,ab. 35. ACMI.ti,ab. 36. ARCD.ti,ab. 37. CIND.ti,ab. 38. VCI.ti,ab. 39. VAD.ti,ab. 40. major neurocognitive disorder*.ti,ab. 41. minor neurocognitive disorder*.ti,ab. 42. neurocognitive dysfunction.ti,ab. 43. Neurocognitive Disorders/ 44. or/15‐43 45. 14 and 44 |
Mar 2020: 4544 Mar 2021: 552 |
| PsycINFO (OvidSP) from 1806 Date of search: 24 March 2021 |
1. cholinergic antag*.ti,ab. 2. anticholinergic*.ti,ab. 3. anti‐cholinergic*.ti,ab. 4. exp Cholinergic Receptors/ 5. AAS.ti,ab. 6. ACB.ti,ab. 7. ADS.ti,ab. 8. DAPs.ti,ab. 9. ARS.ti,ab. 10. DBI‐ACh.ti,ab. 11. SAMS.ti,ab. 12. ("chew* score" or "chew* list").ti,ab. 13. ("han's score" or "han score").ti,ab. 14. or/1‐13 15. exp Cognition/ 16. exp Dementia/ 17. cognit*.ti,ab. 18. dement*.ti,ab. 19. alzheimer*.ti,ab. 20. "lewy bod*".ti,ab. 21. FTLD.ti,ab. 22. PDD.ti,ab. 23. "executive function*".ti,ab. 24. exp Attention/ 25. (speed adj2 processing).ti,ab. 26. memory.ti,ab. 27. exp Memory Disorders/ 28. "episodic memory".ti,ab. 29. exp Episodic Memory/ 30. exp Cognitive Impairment/ 31. MCI.ti,ab. 32. exp Cognitive Assessment/ 33. (nMCI or aMCI or mMCI or MCIa).ti,ab. 34. AAMI.ti,ab. 35. ACMI.ti,ab. 36. ARCD.ti,ab. 37. CIND.ti,ab. 38. VCI.ti,ab. 39. VAD.ti,ab. 40. major neurocognitive disorder*.ti,ab. 41. minor neurocognitive disorder*.ti,ab. 42. neurocognitive dysfunction.ti,ab. 43. exp Neurocognitive Disorders/ 44. or/15‐43 45. 14 and 44 |
Mar 2020: 3489 Mar 2021: 164 |
| CINAHL (EBSCOhost) Date of search: 24 March 2021 |
S1 TX cholinergic antag* S2 TX anticholinergic* S3 TX anti‐cholinergic* S4 (MH "Cholinergic Antagonists+") S5 TX AAS S6 TX ACB S7 TX ADS S8 TX DAPs S9 TX ARS S10 TX DBI‐ACh S11 TX SAMS S12 TX "chew* score" or "chew* list" S13 TX "han's score" or "han score" S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15 (MH "Cognition+") S16 (MH "Cognition Disorders+") S17 (MH "Dementia+") S18 TX cognit* S19 TX dement* S20 TX alzheimer* S21 TX "lewy bod*" S22 TX FTLD S23 TX PDD S24 TX "executive function*" S25 (MH "Attention") S26 TX speed AND processing S27 TX memory S28 (MH "Memory Disorders") S29 TX "episodic memory" S30 (MH "Memory Disorders") OR (MH "Memory") S31 TX MCI S32 "Mild Cognitive Impairment" S33 TX nMCI or aMCI or mMCI or MCIa S34 TX AAMI S35 TX ACMI S36 TX ARCD S37 TX CIND S38 TX VCI S39 TX VAD S40 TX major neurocognitive disorder* S41 TX minor neurocognitive disorder* S42 TX neurocognitive dysfunction S43 "Neurocognitive Disorders" S44 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 S45 S14 AND S44 | Mar 2020: 2229 Mar 2021: 260 |
| Web of Science core collection (ISI Web of Science) Date of search: 24 March 2021 |
TOPIC: ("cholinergic antag*" OR anticholinergic* OR "anti‐cholinergic*" OR AAS OR ACB OR ADS OR DAPs OR ARS OR "DBI‐ACh" OR SAMS OR "chew* score" OR "chew* list" OR "hands score" OR "hans score" OR "han score") AND TOPIC: (cognit* OR dement* OR alzheimer* OR "lewy bod*" OR FTLD OR PDD OR "executive function*" OR attention OR memory OR MCI OR "major neurocognitive disorder*" OR "minor neurocognitive disorder*") Timespan: All years. Indexes: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC. |
Mar 2020: 1348 Mar 2021: 646 |
| TOTAL before deduplication | Mar 2020: 14,517 Mar 2021: 1874 |
|
| TOTAL after deduplication | Mar 2020: 9767 Mar 2021: 1493 |
|
| TOTAL after first assessment of abstracts by CDCIG Information Specialist | Mar 2021: 1034 Mar 2020: 168 |
|
Appendix 4. Contents of proforma
| Extracted information | Included details |
| General information | Author, title, source, publication date, language, related or duplicate publications. |
| Source of data | Cohort (retrospective or prospective data collection), case‐control, randomised trial, or secondary analysis of registry data. |
| Participant information | Participant eligibility and recruitment method (e.g. consecutive or other recruitment, number of centres, inclusion and exclusion criteria); participant demographics (e.g. age, sex); details of ongoing treatments/medications; study dates; country of recruitment; setting (using our definitions of primary, secondary, and community settings). |
| Prognostic factor | Definition and method of measurement of prognostic factor. Duration of exposure (pre‐ or post‐study commencement) was not regularly recorded; however, where possible, we recorded timing of prognostic factor measurement (number of weeks participants had been on the anticholinergic drugs prior to baseline assessment); where data were available, we also collected duration of exposure during the study. |
| Outcomes to be predicted | Definition and method of measurement of outcome; time of outcome ascertainment, or summary of duration of follow‐up. |
| Adjustment for other prognostic factors (covariates) | List of all the covariates that were adjusted for in any regression model. |
| Sample size | Number of participants and number of outcomes/events; how missing data were handled (e.g. complete‐case analysis, imputation, or other methods). |
| Reported results | We recorded incidence of dementia and cognitive decline. Where possible, we extracted estimates and corresponding confidence intervals from each included paper. We also recorded overall survival (including duration of follow‐up) and dementia‐free survival (including duration of follow‐up). |
Appendix 5. QUIPS (Quality in Prognosis Studies) anchoring statements
Specific considerations
Study participation: we considered whether the method of recruitment was at risk of selection bias (e.g. consecutive recruitment versus convenience sample) and if there was adequate reporting of comorbidities and demographics (age and sex). If either a convenience sample was used or there was inadequate reporting of comorbidities/demographics, we assigned a moderate risk of bias.
Attrition: we anticipated that most studies would utilise databases, hence attrition was less of an issue. Therefore, we focused on reporting of, and methods for dealing with, missing data. We assigned a moderate risk of bias if no analysis was carried out to evaluate if participants with missing data differed in baseline anticholinergic burden score compared to those with full data.
Prognostic factor measurement: we considered how medication data were obtained. If medication was not established via at least two methods and capable of establishing non‐prescription medications taken, along with duration of exposure and adherence, we assigned a moderate risk of bias. If repeated anticholinergic burden measurements were not made over time for studies with a follow‐up duration of more than one year, we assigned a high risk of bias. We anticipated that some studies would utilise validated anticholinergic burden scales but adjust these scales, for instance to incorporate dosage into the anticholinergic calculation. We did not consider utilisation of anticholinergic burden scales as part of the 'Risk of Bias' assessment, as it is the purpose of the review to establish which anticholinergic burden scales have the greatest prognostic accuracy.
Outcome measurement: we considered the method utilised for dealing with missing data in relation to the outcome. If 'last diagnosis carried forward' was used when final outcome data were not available, we assigned a high risk of bias. We assessed whether the outcome was established via a clinical follow‐up or was reliant upon database diagnoses. If outcome was reliant upon database diagnoses alone, we assigned a moderate risk of bias. We also assessed if the outcome was determined without knowledge of the prognostic factor. If there was no blinding to outcome and the cognitive diagnosis was conducted after the anticholinergic burden measurement was taken, we assigned a high risk of bias; if the cognitive diagnosis preceded the anticholinergic burden measurement, we assigned a moderate risk of bias.
Covariates: we assessed whether studies adjusted for age, sex, and comorbidities as a minimum. If these covariates were not adjusted for, we assigned a high risk of bias. Assessment for comorbidities required control for at least three comorbidities that covered both physical and psychiatric domains; failure to do so resulted in a rating of moderate risk of bias.
Reverse causation: we evaluated studies regarding perceived risk that anticholinergic drug use was prescribed for treatment of early signs of dementia. If studies did not explicitly report restricting anticholinergic burden measurement to at least 12 months before dementia onset, a rating of high risk of bias was applied. If studies imposed restrictions of anticholinergic burden measurement for one to two years before the dementia index date, a moderate risk of bias rating was applied.
Statistical analysis: we evaluated how the analysis was conducted. Specific issues of consideration in each area were decided upon via discussion among the review authors. We assigned a high risk of bias if: a multivariate analysis was not conducted; if the analysis was not appropriately powered based on the '10 events per covariate' rule; if the method for selecting covariates for inclusion in a multivariate model was based on P values in a univariate analysis without incorporation of prior knowledge of relevant associations into selection; if the method of analysis was inconsistent with the stated protocol; and if the reported results were inconsistent with the stated method of analysis. We assigned a moderate risk of bias if relevant assumptions were not checked.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ancelin 2006.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: primary care Country: France Duration of follow‐up: 1 year Covariates controlled for: age, sex, education, untreated depression, and treated hypertension |
|
| Participants | Participants numbers: 372 Population type: older adults Sex: not clear Age (mean): not stated |
|
| Prognostic factors | Anticholinergic burden measurement method: combined rating of drugs' serum radioreceptor assay and mean estimated clinical effects, established via literature review | |
| Outcomes | Outcomes assessed: MCI; dementia; cognitive decline Outcome ascertainment dementia/MCI: independent clinical assessment via expert Outcome ascertainment cognitive decline: computerised neuropsychometric examination Diagnostic criteria dementia: DSM‐III criteria for neurogeriatric disorder Diagnostic criteria MCI: revised criteria proposed by the Stockholm consensus group Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | French Social Security (CNAM‐TS), the Fondation de France, the Direction Générale de la Santé, and the Region Languedoc Roussillon | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Unclear | Poor reporting of comorbidities. |
| Study attrition | Unclear | Only 80% followed up. No analysis carried out to evaluate if participants with missing data differed in baseline anticholinergic burden score compared to participants with full data. |
| Prognostic factor measurement | Unclear | Repeated measurements of drug use at 1 year. Used self‐report to establish prescribed and non‐prescribed medication use. Had second method of establishing drugs (checking with GP). No mention of recording dosage, frequency, or adherence. |
| Outcome measurement | Unclear | No blinding but anticholinergic burden scores assigned after cognitive diagnosis. |
| Study confounding | Unclear | Controlled for age, sex, and comorbidities that covered both physical and psychiatric conditions, but only controlled for 2 comorbidities. |
| Reverse causation | No | No mention of restricting anticholinergic drug use to 1 year before MCI onset. |
| Statistical analysis and reporting | Unclear | No protocol or assumptions checked. |
Brombo 2018.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: secondary care Country: Italy Duration of follow‐up: 1 year Covariates controlled for: age, sex, education, smoking, hypertension, coronary heart disease, renal failure, anaemia, infectious diseases, and ARS score at first follow‐up |
|
| Participants | Participants numbers: 1123 Population type: older adults Sex: 56% women (629) Age (mean): 81 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale; ARS scale | |
| Outcomes | Outcomes assessed: cognitive decline Outcome ascertainment: MMSE Diagnostic criteria: cognitive decline: scores on cognitive testing |
|
| Source of funding | Italian Ministry of Labour, Health and Social Policy | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | Baseline dementia prevalence was 20%. |
| Study attrition | No | Cognition of follow‐up data only available for 53.6% of baseline sample. |
| Prognostic factor measurement | Unclear | Medication use established via questionnaire only. |
| Outcome measurement | Unclear | No blinding but anticholinergic burden likely assessed after cognitive score established. |
| Study confounding | Unclear | Age, sex, and comorbidities (including physical and depression) all measured; however, did not control for depression in regression analysis. |
| Reverse causation | No | No restriction to anticholinergic burden measurement (assessed at 12 months before and 9 months before). |
| Statistical analysis and reporting | Unclear | Relevant assumptions not checked. |
Campbell 2016.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: primary care Country: USA Duration of follow‐up: 1 year Covariates controlled for: age, sex, race, arthritis, cancer, CAD, CHF, COPD, diabetes, liver disease, renal disease, stroke, number of non‐anticholinergic drugs, prior inpatient admissions, prior ED visits, prior outpatient visits |
|
| Participants | Participants numbers: 3344 Population type: older adults Sex: 71% women (2374) Age (mean): 71 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: dementia + MCI Outcome ascertainment dementia/MCI: consensus diagnosis based on multidisciplinary evaluation Diagnostic criteria dementia/MCI: DSM‐III criteria |
|
| Source of funding | Not stated | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | Describes age, sex, and comorbidities of population. However, almost all had hypertension (99%, which was not reported in this study but in Cai 2013, which uses the same data source) and high prevalence of stroke and other cardiovascular conditions so population may not be very generalisable. |
| Study attrition | No | No tests of missing data. |
| Prognostic factor measurement | Unclear | Only 1 method used to determine medications. |
| Outcome measurement | Unclear | No blinding but anticholinergic burden likely assessed after cognitive assessment. |
| Study confounding | Unclear | Controls for age, sex, and comorbidities, but comorbidities did not cover psychiatric domain. |
| Reverse causation | No | Anticholinergic burden measurement was collected over the 12 months before cognitive evaluation. Does not mention restricting to anticholinergic drugs that were prescribed only 12 months before cognitive assessment. |
| Statistical analysis and reporting | Unclear | No protocol or assumptions checked. |
Chuang 2017.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: USA Duration of follow‐up: 11 years Covariates controlled for: sex, race, birth year (age), years of education, follow‐up time, smoking status (current, former, never), drinker (never, ever), number of cardiovascular comorbidities |
|
| Participants | Participants numbers: 723 Population type: older adults Sex: 31% women (226) Age (mean): 52 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: Alzheimer's disease + MCI Outcome ascertainment dementia/MCI: consensus diagnosis based on multidisciplinary evaluation Diagnostic criteria for dementia and Alzheimer's disease: DSM‐III and NINCDS‐ADRDA criteria Diagnostic criteria for MCI: Petersen's criteria |
|
| Source of funding | Intramural Research Program, National Institute on Aging, and National Institutes of Health | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Unclear | Demographics and comorbidities all reported; however, population was majority male (69%) with low mean age (52 years). |
| Study attrition | No | Less than a third of baseline sample remaining for inclusion in analysis. |
| Prognostic factor measurement | Unclear | Patients brought unused medications to visit. Cross‐checked by study nurses but no measurement of dosage or frequency of use. Repeated measurements made at each visit. |
| Outcome measurement | Unclear | No blinding but using data obtained from previously conducted longitudinal cohort study so anticholinergic burden likely measured after cognitive assessment already complete. |
| Study confounding | Unclear | Comorbidities controlled for did not include psychiatric conditions. |
| Reverse causation | No | No restriction mentioned on anticholinergic drug use time frame before cognitive assessment and information on duration of use only available for 49% of participants. |
| Statistical analysis and reporting | Unclear | No assumptions checked or protocol registered. |
Coupland 2019.
| Study characteristics | ||
| Methods | Design: retrospective case‐control Setting: community Country: UK Duration of follow‐up: 11 years Covariates controlled for: BMI, smoking status, alcohol consumption, Townsend deprivation score, ethnic group, coronary heart disease, atrial fibrillation, heart failure, hypertension, hyperlipidaemia, diabetes (type 1 and type 2), stroke, transient ischaemic attack, subarachnoid haemorrhage, renal disease, asthma, COPD, anxiety, depression, bipolar disorder, schizophrenia, severe head injury, cognitive decline/memory loss, antihypertensive drugs, aspirin, hypnotics, anxiolytic drugs, non‐steroidal anti‐inflammatory drugs. Cases and controls were matched for age (within 1 year), sex, general practice, and calendar time. |
|
| Participants | Participants numbers: 284,343 Population type: older adults Sex: 63% women (179,420) Age (mean): 82 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale and Beers criteria | |
| Outcomes | Outcomes assessed: dementia Outcome ascertainment dementia: clinical codes recorded in official records Diagnostic criteria for dementia: not stated |
|
| Source of funding | National Institute for Health Research School for Primary Care Research | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Yes | Age, sex, and comorbidities all described. |
| Study attrition | Unclear | No comparison of missing values with anticholinergic burden. |
| Prognostic factor measurement | Unclear | Relied on prescriptions for establishing medications. |
| Outcome measurement | Unclear | Relied on database diagnosis. |
| Study confounding | Yes | Cases and controls were matched for age and sex; comorbidities controlled for in regression analysis and covered physical and psychiatric domains. |
| Reverse causation | Yes | Restricted medications to 12 months, 3 and 5 years before diagnosis. |
| Statistical analysis and reporting | Unclear | Did not check assumptions or register a protocol. |
Fox 2011a.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: UK Duration of follow‐up: 2 years Covariates controlled for: age, sex, baseline MMSE, education, social class, number of non‐anticholinergic medications, and number of comorbidities |
|
| Participants | Participants numbers: 12,423 Population type: older adults Sex: 60% women (7417) Age (mean): not stated |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: cognitive decline Outcome ascertainment cognitive decline: MMSE Diagnostic criteria for cognitive decline: scores on cognitive testing |
|
| Source of funding | Medical Research Council, UK | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Unclear | Lack of detail in reporting exclusion criteria. Did not state numbers with baseline dementia. |
| Study attrition | No | No analysis of missing data. High % of attrition (only 64% remaining at 2‐year follow‐up). |
| Prognostic factor measurement | Yes | Used self‐report cross‐checked by counting remaining medications. Recorded dosage, frequency, and quantity. |
| Outcome measurement | Unclear | No blinding to outcome but anticholinergic burden likely established after cognitive assessment. |
| Study confounding | Unclear | Age, sex, and comorbidities all measured (comorbidities included both physical and psychiatric). However, controls for 'number of health conditions' rather than each condition individually. |
| Reverse causation | Unclear | Restricted analysis to anticholinergic burden baseline association with change in MMSE score at 2 years. |
| Statistical analysis and reporting | Unclear | No assumptions checked or protocol registered. |
Gray 2015.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: USA Duration of follow‐up (mean): 7.3 years Covariates controlled for: ACT cohort, age, age at ACT study entry, sex, education, BMI, current smoking, regular exercise, self‐rated health, hypertension, diabetes, stroke, coronary heart disease, Parkinson's disease, history of depressive symptoms, and current benzodiazepine use |
|
| Participants | Participants numbers: 3434 Population type: older adults Sex: 60% women (20,470) Age (mean): 74 years |
|
| Prognostic factors | Anticholinergic burden measurement method: Beers criteria combined with a literature review | |
| Outcomes | Outcomes assessed: dementia Outcome ascertainment dementia: consensus diagnosis based on multidisciplinary evaluation Diagnostic criteria dementia: DMS‐IV and NINCDS‐ADRDA criteria |
|
| Source of funding | National Institute on Aging, NIH Grants, and the Branta Foundation | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Yes | Random community sampling and reports age, sex, and comorbidities. |
| Study attrition | Unclear | Conducted series of sensitivity analyses but did not assess for differences in missing and anticholinergic burden score. |
| Prognostic factor measurement | Unclear | Relied on pharmacy dispensing data. Updated exposure as participants were followed forward in time. |
| Outcome measurement | Unclear | No blinding to outcome but anticholinergic burden likely measured after cognitive assessment. |
| Study confounding | Yes | Controlled for age, sex, and comorbidities. |
| Reverse causation | Unclear | Excluded prescriptions in the most recent 1‐year period. |
| Statistical analysis and reporting | Unclear | Analysis was appropriately conducted (including tests of assumptions) but no protocol. |
Grossi 2019.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: UK Duration of follow‐up: 10 years Covariates controlled for: sex, age, education, social class, residential accommodation, centre of recruitment, study arm, health conditions at year 0 or year 2 (stroke, Parkinson's disease, epilepsy, sleep problems, anxiety, depression), self‐reported health at year 2, disability at year 2, MMSE at year 2, MMSE orientation subscore at year 2, decrease in MMSE score between year 0 and year 2, and self‐perceived change in memory function between year 0 and year 2. |
|
| Participants | Participants numbers: 8216 Population type: older adults Sex: about 69% women (5669) Age (mean): 74 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: dementia Outcome ascertainment dementia: computer‐assisted algorithm Diagnostic criteria dementia: AGECAT scores ≥ 3 (which is equivalent to dementia as diagnosed by DSM‐III‐R) |
|
| Source of funding | UK Alzheimer's Society | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Yes | Reported age, sex, and comorbidities. |
| Study attrition | No | Major loss to follow‐up. |
| Prognostic factor measurement | Unclear | Self‐report only. No measurement of dosage, frequency of use, or adherence. Anticholinergic burden measurement repeated at each interview (year 0 and year 2). |
| Outcome measurement | Unclear | No blinding but seemed like anticholinergic burden likely assigned after dementia diagnosis ascertained. |
| Study confounding | Yes | Age, sex, and relevant comorbidities all controlled for. |
| Reverse causation | Yes | Anticholinergic burden measurement taken 8 years before dementia diagnosis and those who developed dementia at year 2 (2 years after anticholinergic burden use was first measured) were excluded. |
| Statistical analysis and reporting | Unclear | No protocol or assumptions checked. |
Hafdi 2020.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: Netherlands Duration of follow‐up (mean): 6.7 years Covariates controlled for: age, sex, history of cardiovascular disease or stroke (or both), education, MMSE at baseline, and Geriatric Depression Scale at baseline |
|
| Participants | Participants numbers: 3526 Population type: older adults Sex: 54% women (1919) Age (mean): 74 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: dementia Outcome ascertainment dementia: consensus diagnosis based on multidisciplinary evaluation Diagnostic criteria dementia: DSM‐IV |
|
| Source of funding | Dutch Ministry of Health, Welfare and Sport; the Dutch Innovation Fund of Collaborative Health Insurances; and the Netherlands Organisation for Health Research and Development | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Yes | Reported age, sex, and comorbidities. Minor issue with slightly restricted age population. |
| Study attrition | Unclear | No comparison between completers vs non‐completers/missing data. |
| Prognostic factor measurement | Unclear | 2 methods (self‐report cross‐checked with electronic records). Repeated measurement at 2 years – though only once and this study ran for 8 years; no mention of whether anticholinergic scores changed and lack of detail on the question so could not be certain if asked about non‐prescribed drugs. No measure of adherence or dosage. On basis of these issues assigned unclear risk of bias. |
| Outcome measurement | Unclear | No blinding but anticholinergic burden assignment conducted after cognitive diagnosis established. |
| Study confounding | Yes | Age, sex, and comorbidities all measured. |
| Reverse causation | Unclear | Anticholinergic burden measurement taken 2 years before dementia assessment and excluded those who developed dementia in first 2 years after taking anticholinergics. |
| Statistical analysis and reporting | Unclear | No protocol or assumptions. |
Han 2008.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: USA Duration of follow‐up: 1 year Covariates controlled for: age, education, race, living arrangement, before enrolment. history of alcohol use and smoking, and depressive symptoms, ADL function, Charlson Comorbidity Index |
|
| Participants | Participants numbers: 544 Population type: older adults Sex: 100% men Age (mean): 74 years |
|
| Prognostic factors | Anticholinergic burden measurement method: clinician‐rated | |
| Outcomes | Outcomes assessed: cognitive decline Outcome ascertainment cognitive decline: HVRT, IADL Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | Men only with hypertension. |
| Study attrition | Unclear | Attrition was high but just below the 20% high risk mark. Conducted analysis to investigate potential impact of missing values on final model and did not alter conclusions but did not explain what these missing data analyses entailed. |
| Prognostic factor measurement | Unclear | No measurement of dosage or frequency of use. No measurement of non‐VA sources of drugs. Conducted repeated measurements over the follow‐up period for the 12 months preceding each assessment at 1 and 2 years. |
| Outcome measurement | Unclear | No mention of blinding. Unclear if anticholinergic burden was applied before or after cognitive assessment score was determined. |
| Study confounding | Yes | Only men in sample so no sex. Controlled for comorbidities via Charlson Comorbidity Index along with depression. |
| Reverse causation | No | Anticholinergic burden exposure time frame defined as the 12 months preceding each follow‐up at 1 year and 2 years. (so seems to have covered medication use right up to the point of each follow‐up assessment). |
| Statistical analysis and reporting | Unclear | No assessment of assumptions. Referred to a protocol but was not a protocol specifically for this study so no mention of an analysis plan. |
Hsu 2017.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: Taiwan Duration of follow‐up: 10 years Covariates controlled for: sex and time‐varying comorbidities. ARS and ACB scales were further adjusted for defined daily dose of drugs with anticholinergic properties (age controlled for by subgrouping) |
|
| Participants | Participants numbers: 116,043 Population type: older adults Sex: 50% women (57,552) Age (mean): not stated |
|
| Prognostic factors | Anticholinergic burden measurement method: ARS scale; ACB scale; DBI‐ach scale | |
| Outcomes | Outcomes assessed: dementia Outcome ascertainment dementia: clinical codes recorded in health records Diagnostic criteria dementia: not stated |
|
| Source of funding | Ministry of Science and Technology | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Yes | Reported age, sex, and covariates. Random sampling of population contained in a national insurance database registry. |
| Study attrition | Unclear | No mention of missing data. No mention of mortality over 10‐year follow‐up or how dealt with this. |
| Prognostic factor measurement | Unclear | Only 1 method (prescription records) employed for determining medications. Anticholinergic burden assessed monthly for 120 months. |
| Outcome measurement | Unclear | Relied on medical record diagnoses. |
| Study confounding | Unclear | Controlled for most relevant variables including comorbidities (used Charlson Comorbidity Index). Age not controlled for in the model but categorised people into distinct age groups and conducted 3 analyses on that basis. No psychiatric domains assessed. |
| Reverse causation | No | No mention of restricting anticholinergic burden measurement other than removing people with dementia at baseline (1 year after enrolment). Anticholinergic burden was measured monthly over the 10‐year time frame so appeared that beyond the initial 1 year following study enrolment, anticholinergic drug use could be at risk of being prescribed for prodromal dementia symptom. |
| Statistical analysis and reporting | Unclear | No protocol described and no measure of assumptions. |
Iyer 2020.
| Study characteristics | ||
| Methods | Design: prospective cohort Setting: secondary care Country: USA Duration of follow‐up: 1 year Covariates controlled for: age, BMI, time, group, GDS, total number of medications, neurological diagnosis |
|
| Participants | Participants numbers: 106 Population type: overactive bladder population Sex: 100% women Age (mean): 77 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: cognitive decline Outcome ascertainment cognitive decline: MoCA Diagnostic criteria cognitive decline: change in scores on cognitive testing |
|
| Source of funding | None. | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | Women only. Overactive bladder population. |
| Study attrition | No | Considerable attrition. |
| Prognostic factor measurement | Unclear | Only 1 method employed (ACB scale). No mention of assessing non‐prescription anticholinergic medications. |
| Outcome measurement | No | No mention of blinding and not a retrospective design so knowledge of anticholinergic use may have biased MoCA scoring. Repeated MoCA‐based testing could also induce practice effects, masking any cognitive decline. |
| Study confounding | Yes | Age and comorbidities controlled for. |
| Reverse causation | No | No restrictions placed on ACB use before follow‐up assessments up to 12 months. |
| Statistical analysis and reporting | Unclear | No protocol or assumptions checked. |
Jessen 2010.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: Germany Duration of follow‐up (mean): 4.5 years Covariates controlled for: sex, age, level of education, depression, ApoE genotype |
|
| Participants | Participants numbers: 2605 Population type: older adults Sex: 65% women (1701) Age (mean): 79 years |
|
| Prognostic factors | Anticholinergic burden measurement method: Chew list | |
| Outcomes | Outcomes assessed: dementia Outcome ascertainment dementia: consensus diagnosis based on multidisciplinary evaluation Diagnostic criteria dementia: DSM‐IV and ICD‐10 criteria |
|
| Source of funding | German Federal Ministry for Education and Research, and the Federal Institute for Drugs and Medical Devices | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Unclear | Random sampling of GP registries. > 50% participation rate. No reporting of comorbidities (other than depression and ApoE) so scored as moderate. |
| Study attrition | Unclear | No mention of missing data or attrition (despite extensive follow‐up period). In original study that they used data from, the baseline cohort was 3327; however, may be more exclusion criteria for this study so assigned unclear risk of bias. |
| Prognostic factor measurement | Unclear | Lack of detail regarding how medication use was established. Repeated measurements of anticholinergic use at each 8‐month follow‐ups. |
| Outcome measurement | Unclear | No blinding but seemed likely that anticholinergic burden score was assigned retrospectively after cognitive diagnosis was established in earlier study. |
| Study confounding | No | Did not control for comorbidities other than depression and ApoE genotype. |
| Reverse causation | No | No restriction on anticholinergic burden time frame measurement reported. |
| Statistical analysis and reporting | Unclear | No protocol and no assessment of assumptions. |
Joung 2019.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: South Korea Duration of follow‐up: 10 years Covariates controlled for: age, sex, level of income, diabetes mellitus, hypertension, myocardial infarction, cardiovascular diseases, dizziness, genitourinary diseases, epilepsy, Parkinson's disease, neuralgia, skin disease, sleep disorder, prescribed doses of weak anticholinergics, and cumulative prescribed days of other non‐anticholinergic agents that could impair cognitive function (all psychotic diseases including depression, psychosis, and anxiety were excluded in the step of participant selection in advance). |
|
| Participants | Participants numbers: 191,805 Population type: older adults Sex: 55% women (105,493) Age (mean): 67 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale plus Beers criteria | |
| Outcomes | Outcomes assessed: Alzheimer's disease Outcome ascertainment dementia: clinical codes recorded in medical records Diagnostic criteria dementia: ICD‐10: F00 or G30) and a prescription for anti‐Alzheimer's disease agents (i.e. donepezil, rivastigmine, galantamine, or memantine) |
|
| Source of funding | Not stated | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Yes | Population‐based study with adequate reporting of demographics and comorbidities. |
| Study attrition | Unclear | No mention of missing data. |
| Prognostic factor measurement | Unclear | Relied on prescription records. Exposure measurement taken throughout follow‐up. |
| Outcome measurement | Unclear | Relied on medical record diagnoses. |
| Study confounding | Yes | All appropriate variables controlled for. Depression controlled for via exclusion of antidepressant use in sensitivity analysis only. |
| Reverse causation | Yes | Restricted to dementia onset 2 years after baseline anticholinergic use. Also various statistical analysis to control for protopathic bias (reverse causation) did not change results so scored as low risk of bias on this basis. |
| Statistical analysis and reporting | Unclear | Checked assumptions. No protocol registered, however. |
Kashyap 2014.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: Canada Duration of follow‐up: 1 year Covariates controlled for: None. |
|
| Participants | Participants numbers: 102 Population type: older adults Sex: 84% women (86) Age (mean): 72 years |
|
| Prognostic factors | Anticholinergic burden measurement method: DBI‐ach scale; ACB scale; ADS scale; ARS scale | |
| Outcomes | Outcomes assessed: MCI; cognitive decline Outcome ascertainment MCI: independent clinical assessment via expert Outcome ascertainment cognitive decline: neuropsychological tests and an MoCA Diagnostic criteria MCI: DSM‐5 criteria for a mild neurocognitive disorder. Diagnostic criteria cognitive decline: 3 different ways: worsening in the raw neuropsychological test scores for each test; a change in scores on each test that exceeded an expected cut‐off based on the test–retest reliable change index; and a change in scores on each test that persisted when regression to the mean, practice effects, and age were accounted for using the standardised regression‐based measure method |
|
| Source of funding | Canadian Institutes of Health Research | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Unclear | Report on age and sex but did not report which comorbidities they measured. |
| Study attrition | Unclear | No comparison with missing data. Did not describe the proportion or potential impact of loss to follow‐up. |
| Prognostic factor measurement | Yes | Established by patient self‐report. Validated by nurse checking medication box. Recorded frequency, adherence, and dosage. |
| Outcome measurement | Unclear | There was blinding to the mild neurocognitive disorder diagnosis but not to the change in cognition evaluation; retrospective anticholinergic burden determination. |
| Study confounding | No | Only conducted univariate analysis. No confounders adjusted for. |
| Reverse causation | No | No time restriction on anticholinergic burden measurement (anticholinergic burden measured at baseline then again at 12 months – combined measures and used 'increase in anticholinergic burden' as the independent variable. |
| Statistical analysis and reporting | No | No multivariate analysis conducted. |
Koyama 2014.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: USA Duration of follow‐up: 4.9 years Covariates controlled for: age, race, education, smoking, physical activity, modified Charlson Comorbidity Index, and baseline MMSE score |
|
| Participants | Participants numbers: 1429 Population type: older adults Sex: 100% women (1429) Age (mean): 83 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: cognitive decline Outcome ascertainment cognitive decline: battery of 7 cognitive tests Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | National Institute of Aging at the National Institutes of Health and the Alzheimer's Association | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | Women only. |
| Study attrition | No | Final sample was 1429/4606 at baseline. |
| Prognostic factor measurement | No | Participants asked to bring in all medication used in 30 days prior to baseline visit. No measurement of dosage or frequency of use. No subsequent measurement of medication use despite 5‐year follow‐up. |
| Outcome measurement | Unclear | No blinding but cognitive assessment results likely defined before anticholinergic burden calculated. |
| Study confounding | Unclear | Controlled for comorbidities via a modified Charlson Comorbidity Index; however, no depression controlled for. |
| Reverse causation | Unclear | Anticholinergic drug use at baseline only and follow‐up was at 2 years. However, no exclusion around dementia at baseline or 1 year reported. |
| Statistical analysis and reporting | Unclear | No assumption checked. |
Low 2009.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: Australia Duration of follow‐up: 4 years Covariates controlled for: age, gender, education, depression, and self‐rated health |
|
| Participants | Participants numbers: 2058 Population type: 'young old' Sex: 48% women (992) Age (mean): 62 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ADS scale | |
| Outcomes | Outcomes assessed: cognitive decline; MCI Outcome ascertainment MCI: independent clinical assessment via expert Outcome ascertainment cognitive decline: a neuropsychological assessment Diagnostic criteria MCI: Jack 1999 criteria Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | National Health and Medical Research Council grants | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Unclear | Sample was restricted to 'young old' (aged 60–64 years) and had high mean education (14 years). |
| Study attrition | Unclear | 87% of participants followed up. Comparison of lost to follow‐up vs followed up participants conducted but showed people lost to follow‐up were more likely to be on anticholinergic medicine. |
| Prognostic factor measurement | Unclear | Anticholinergic drug use reported at baseline and 4‐year follow‐up. Used self‐reported medication use but only 1 method employed. No measurement of frequency, duration, or dose. |
| Outcome measurement | No | No blinding to anticholinergic burden during clinical diagnosis, but anticholinergic burden likely measured retrospectively. MCI was defined according to dated criteria (Jack 1999 rather than Peterson). MMSE used to assess cognition but study had high education mean and displays a ceiling effect. |
| Study confounding | Unclear | Controlled for age, sex, and comorbidities that cover both physical and psychiatric domains. However, minimal comorbidities. |
| Reverse causation | No | Excluded people with dementia at baseline but did not seem to be any formal restriction in anticholinergic drug use and time of outcome assessment at 4 years. |
| Statistical analysis and reporting | No | No protocol and no assumptions checked. Also analysis for MCI outcome was underpowered as only 38 MCI cases at follow‐up. |
Moriarty 2020.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: Ireland Duration of follow‐up: 2 years Covariates controlled for: baseline wave (i.e. whether the period was wave 1–2 or 2–3), age at baseline wave, sex, education, marital status, employment, incontinence, quintiles of CES‐D score, pain, trouble falling asleep, disabilities, smoker, vision, hearing, self‐reported doctor‐diagnosed conditions, eye conditions, cardiovascular conditions (listed in paper supplementary materials), other health conditions (listed in paper supplementary materials) |
|
| Participants | Participants numbers: 7027 Population type: older adults Sex: 55.6% women (3906) Age (median): 61 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: cognitive decline Outcome ascertainment cognitive decline: 3 cognitive tests (MMSE, animal naming, assessment of recall) Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | Alzheimer's Society, Grant/Award Number: AS‐PG‐2013‐017; Atlantic Philanthropies; Irish Government; Irish Life | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Unclear | Relatively young participant pool. |
| Study attrition | Yes | Minimal attrition (7027 included out of 8504 total study sample) |
| Prognostic factor measurement | Unclear | Assessed via 2 methods but duration and dosage only recorded for a subset of participants and adherence not recorded. |
| Outcome measurement | Unclear | No blinding to cognitive assessment mentioned but ACB scores likely applied after cognition scores derived. |
| Study confounding | Yes | All essential covariates adjusted for including psychiatric conditions. |
| Reverse causation | Unclear | Most people had ≥ 2 years between medication assessment and outcome assessment; however, time frame for new users not restricted so some risk of protopathic bias. |
| Statistical analysis and reporting | Unclear | Appropriate analysis. Checked assumptions but no protocol registered. |
Papenberg 2017.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: Sweden Duration of follow‐up: 6 years Covariates controlled for: age, sex, education, CRFs, cardiovascular disease, physical inactivity, total number of drugs, and depression |
|
| Participants | Participants numbers: 1473 Population type: older adults Sex: 61% women (902) Age (mean): 70 years |
|
| Prognostic factors | Anticholinergic burden measurement method: Anatomical Therapeutic Chemical classification system | |
| Outcomes | Outcomes assessed: cognitive decline Outcome ascertainment cognitive decline: cognitive test battery Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | Ministry of Health and Social Affairs | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Yes | Age, sex, and comorbidities all reported adequately. Random selection of community. |
| Study attrition | No | High degree of attrition (only 1724/3363 underwent cognitive testing at baseline and follow‐up). |
| Prognostic factor measurement | Unclear | Patients asked to bring in medications they were taking(including non‐prescription medications); medical records checked if participant could not provide information. No mention of recording dosage, frequency, or adherence. |
| Outcome measurement | Unclear | No blinding but cognition recorded after cognitive assessment. |
| Study confounding | Yes | All relevant comorbidities controlled for. |
| Reverse causation | No | No restriction to time frame of anticholinergic drug use mentioned. |
| Statistical analysis and reporting | Unclear | No protocol or assumptions checked. |
Richardson 2018.
| Study characteristics | ||
| Methods | Design: retrospective case‐control Setting: community Country: UK Duration of follow‐up: 4 years Covariates controlled for: age, region, any falls, any fractures, number of doctor consultations in the 12 months before the drug exposure period, number of prescriptions during the drug exposure period, BMI, smoking status, harmful alcohol use, depression duration, cardiovascular or cerebrovascular and related diagnoses, mental health diagnoses, other comorbidities. Cases and controls matched on sex, year of birth (within 3 years), years of 'up to standard' data history, and area level deprivation measured by the index of multiple deprivation quintile of each practice based on its postcode. |
|
| Participants | Participants numbers: 324,703 Population type: older adults Sex: 63% women (204,563) Age (mean): 83 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale; ADS scale | |
| Outcomes | Outcomes assessed: dementia Outcome ascertainment dementia: clinical codes recorded in medical records or prescription for a cognitive enhancer Diagnostic criteria dementia: not stated |
|
| Source of funding | Alzheimer's Society | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Yes | Reported age, sex, and comorbidities. All seemed generalisable. |
| Study attrition | Unclear | Conducted several sensitivity analyses based on missing data and outcomes, but did not compare missing data with anticholinergic burden scores. |
| Prognostic factor measurement | Unclear | Only used prescribing records to establish current drug use. |
| Outcome measurement | Unclear | Relied on database diagnosis. |
| Study confounding | Yes | Controlled for a vast number of covariates including psychiatric and physical conditions. |
| Reverse causation | Yes | Restricted measurement of anticholinergic drug use to ≥ 4 years before index date for dementia diagnosis. |
| Statistical analysis and reporting | Unclear | No assessment of validity of assumptions for logistic regression analysis. |
Risacher 2016.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: secondary care Country: USA Duration of follow‐up: 32 months Covariates controlled for: age, sex, total number of medications, cardiac surgery, total number of comorbid conditions, other psychiatric conditions and amyloid‐β positivity |
|
| Participants | Participants numbers: 402 Population type: older adults Sex: 53% women (213) Age (mean): 73 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: dementia and MCI; cognitive decline Outcome ascertainment dementia/MCI/cognitive decline: comprehensive cognitive and clinical battery Diagnostic criteria dementia/MCI: not stated Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | ADNI and Department of Defense ADNI | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | High numbers of participants with subjective cognitive decline. |
| Study attrition | Unclear | No analysis carried out to evaluate if participants with missing data differed in baseline anticholinergic burden score compared to those with full data. |
| Prognostic factor measurement | No | Relied on self‐report data (according to discussion) for medication use. Not clear if there were multiple measurements but seems to only be based on baseline use and follow‐up was for several years. |
| Outcome measurement | Unclear | No blinding. Is unclear how Alzheimer's disease and MCI were diagnosed in the study. |
| Study confounding | Unclear | Age, sex, and relevant comorbidities all controlled for. |
| Reverse causation | No | There were dementia/MCI converters in first 6 and 12 months (figure 4) and no mention of any exclusions of these participants from analysis. |
| Statistical analysis and reporting | No | Checks assumptions. No protocol. Only 58 odd cases of dementia so seemed underpowered based on number of covariates they claimed to have controlled for (which seemed to differ in the methods section vs in the results table). Did not control for depression in analysis despite this differing between anticholinergic users and non‐users. |
Shah 2013.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: USA Duration of follow‐up: 10 years Covariates controlled for: age, gender, and education. For incident users only in a secondary analysis: ApoE e4 allele, MCI, number of chronic medical conditions, urinary incontinence, number of depressive symptoms, physical activity, and disability on the Katz Index |
|
| Participants | Participants numbers: 895 Population type: older adult priests and nuns Sex: 70% women (623) Age (mean): 74 years (never users); 75.9 years (incident users); 76.5 years (prevalent users) |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: cognitive decline Outcome ascertainment cognitive decline: battery of 19 cognitive function tests was administered in addition to the MMSE Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | American Philosophical Society, the National Institute on Aging grants, and by the Illinois Department of Public Health to DAB | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | Sample restricted to priests and nuns. Majority of population were women (> 70%) so not particularly generalisable. |
| Study attrition | Unclear | No evaluation of people excluded vs included. No comparison of missing values against anticholinergic burden score. |
| Prognostic factor measurement | Unclear | Repeated measurements conducted (at each follow‐up assessment). Medication assessed using only 1 method (self‐report). No measurement of dosage and frequency of use. |
| Outcome measurement | Unclear | Anticholinergic burden likely measured after cognitive assessment complete (medications recorded before each evaluation but was part of religious order study so anticholinergic burden not primary focus during assessments). |
| Study confounding | Unclear | Only controls for age, sex, and education in primary analysis. Did not control for any comorbidities, despite measuring these. In secondary analysis it then controls for covariates but is restricted to incident anticholinergic drug users only. |
| Reverse causation | No | No restrictions placed on time frame of anticholinergic drug use before cognitive assessment. |
| Statistical analysis and reporting | Unclear | No protocol or assumptions checked. |
Sheu 2019.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: Taiwan Duration of follow‐up: 4 years Covariates controlled for: age, sex, duration of Parkinson's disease before index date, conditions (hypertension, stroke, hypercholesterolaemia, diabetes mellitus, depression, anxiety, psychotic‐related disorder, alcohol‐related disorder, sleep disorder, and head injury), and medications (antihypertensives, antidiabetics, anticoagulants, antihyperlipidaemics, antidepressants, benzodiazepines, and antipsychotics) |
|
| Participants | Participants numbers: 1232 Population type: people with Parkinson's disease Sex: 44% women (542) Age (mean): not stated |
|
| Prognostic factors | Anticholinergic burden measurement method: ACB scale | |
| Outcomes | Outcomes assessed: dementia Outcome ascertainment dementia: clinical codes recorded in medical records Diagnostic criteria dementia: ICD‐9‐CM codes 290.0–290.4, 294.1, 331.0–331.2 |
|
| Source of funding | Ministry of Science and Technology | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | Parkinson's disease population. |
| Study attrition | Unclear | No mention of attrition. |
| Prognostic factor measurement | Unclear | Relied on prescription records. Measured cumulative dose over 3‐year exposure period. |
| Outcome measurement | Unclear | Relied on database diagnoses. |
| Study confounding | Yes | All relevant covariates controlled for. |
| Reverse causation | Unclear | Excluded participants taking anticholinergic drugs 1 year before diagnosis. |
| Statistical analysis and reporting | Unclear | No assumptions checked or protocol registered. |
Whalley 2012.
| Study characteristics | ||
| Methods | Design: retrospective cohort Setting: community Country: UK Duration of follow‐up: 6.25 years Covariates controlled for: age and childhood IQ for linear regression; age, sex, family history of dementia, education, heart history, blood pressure history, ApoE e4 for logistic regression |
|
| Participants | Participants numbers: 281 Population type: older adults Sex: 42% women (119) Age (mean): 77 years |
|
| Prognostic factors | Anticholinergic burden measurement method: literature review of drugs' mean anticholinergic activity | |
| Outcomes | Outcomes assessed: dementia; cognitive decline Outcome ascertainment dementia: consensus diagnosis based on multidisciplinary evaluation Outcome ascertainment cognitive decline: MMSE and cognitive test battery of 5 cognitive tests Diagnostic criteria dementia: ICD‐10 criteria Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | Wellcome Trust | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | Unclear | Adequate reporting of age, sex, and comorbidities. However, limited age range included (77–82 years). |
| Study attrition | No | 281/324 participated. Only 136/281 (48%) participants had complete cognitive data sets available after follow‐up. |
| Prognostic factor measurement | Unclear | Relied on patient self‐report. |
| Outcome measurement | Unclear | No blinding but anticholinergic burden score likely applied in retrospect. |
| Study confounding | Unclear | No control for depression. |
| Reverse causation | Unclear | Excluded people with lower MMSE at baseline from analysis. Follow‐up 15 months minimum after baseline anticholinergic measurement taken. |
| Statistical analysis and reporting | No | Model was underpowered to assess dementia association (only 45 cases of dementia included in model yet 9 covariates). |
Yarnall 2015.
| Study characteristics | ||
| Methods | Design: retrospective case‐control Setting: community and outpatient Country: UK Duration of follow‐up: 1.5 years Covariates controlled for: age, gender, years of education, disease duration, MDS‐UPDRS III, levodopa equivalent daily dose and GDS‐15 (note: unclear what was controlled for in final model) |
|
| Participants | Participants numbers: 219 Population type: people with Parkinson's disease Sex: 40% women (88) Age (mean): 69 years |
|
| Prognostic factors | Anticholinergic burden measurement method: ADS scale | |
| Outcomes | Outcomes assessed: MCI; cognitive decline Outcome ascertainment MCI: not clear Outcome ascertainment cognitive decline: MMSE, MoCA, in addition to tests of attention, visual memory, executive, language and visuospatial function Diagnostic criteria MCI: PD‐MCI was determined using the MDS criteria Diagnostic criteria cognitive decline: scores on cognitive testing |
|
| Source of funding | Parkinson's UK grant | |
| Notes | ||
| Item | Authors' judgement | Support for judgement |
| Study participation | No | Parkinson's disease population. |
| Study attrition | Unclear | No comparison of completers vs non‐completers. |
| Prognostic factor measurement | Unclear | Not particularly clear how prognostic factor was measured but seems to be self‐report. Repeated measurements taken at baseline and 18 months. |
| Outcome measurement | Unclear | No blinding but ADS ratings likely assigned in retrospect. |
| Study confounding | Yes | Appropriate variables controlled for. |
| Reverse causation | No | ADS at baseline and at 18 months pooled so no restriction on ADS use before cog assessment at 18 months. |
| Statistical analysis and reporting | No | Uses backward stepwise regression to determine model covariates for use in exploring ADS association with various measures of cognition. Not stated what variables were controlled for in the final model. |
ACB: Anticholinergic Cognitive Burden; ACT: Adult Changes in Thought; ADS: Anticholinergic Drug Scale; AGECAT: Automated Geriatric Examination for Computer Assisted Taxonomy; ANDI: Alzheimer's Disease Neuroimaging Initiative; ApoE: apolipoprotein E; ARS: Anticholinergic Risk Scale; BMI: body mass index; CAD: coronary artery disease; CES‐D: Center for Epidemiologic Studies Depression Scale; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CNAM‐TS: Caisse Nationale d'Assurance Maladie des Travailleurs Salariés; CRF: cardiovascular risk factor; DBI‐ach: anticholinergic component of the Drug Burden Index; DSM‐III: Diagnostic and Statistical Manual of Mental Disorders Third Edition; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders Third Edition Revised; DMS‐IV: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders Fifth Edition; ED: emergency department; GDS: Geriatric Depression Screen; GP: general practitioner; HVRT: Hopkins Verbal Recall Test; IADL: instrumental activity of daily living; ICD‐9‐CM: International Classification of Diseases, Ninth Revision, Clinical Modification; ICD‐10: International Classification of Diseases, Tenth Revision; MCI: mild cognitive impairment; MDS‐UPDRS III: Motor Rating Scale – Unified Parkinson's Disease Rating Scale III; MMSE: Mini‐Mental State Examination; MoCA: Montreal Cognitive Assessment; NIH: National Institutes of Health; NINCDS‐ADRDA: Association Internationale pour la Recherche et l'Enseignement en Neurosciences; PD‐MCI: Parkinson's disease mild cognitive impairment; VA: Veteran's Affairs.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aalto 2018 | Wrong study design. |
| Andrade 2019 | Wrong study design. |
| Andre 2019 | Wrong patient population. |
| Ang 2015 | Abstract only. |
| Ang 2017 | Wrong patient population. |
| Aparasu 2014 | Wrong patient population. |
| Asano 2019 | Abstract only. |
| Ben Omar 2013 | Abstract only. |
| Bostock 2013 | Wrong study design. |
| Bottiggi 2006 | Dichotomises anticholinergics. |
| Bouchard 2017 | Grey literature (dissertation). |
| Broder 2020 | Abstract only. |
| Cai 2013 | Repeat data set of already included study. |
| Campbell 2010 | Repeat data set of already included study. |
| Campbell 2018 | Repeat data set of already included study. |
| Cancelli 2008 | Wrong study design. |
| Cao 2008 | Wrong study design. |
| Cardwell 2020 | Wrong prognostic factor (DBI‐ach not reported separately). |
| Carriere 2009 | Dichotomised anticholinergic burden. |
| Cherniaeva 2019 | Abstract only. |
| Cooley 2020 | Duplicate. |
| Cooley 2021 | Wrong population. |
| Dmochowski 2021 | Wrong study design (systematic review). |
| Drag 2012 | Abstract only. |
| Ehrt 2010 | Wrong patient population (baseline dementia = 20% for community dwelling population). |
| Fox 2011b | Wrong patient population. |
| Gnjidic 2012 | Wrong prognostic factor (DBI‐ach not reported separately). |
| Hanlon 2020 | Wrong population (middle aged). |
| Hong 2019 | Dichotomised anticholinergic burden. |
| Huang 2012 | Non‐English language. |
| Jakeman 2020 | Conference abstract. |
| Kada 2019 | Non‐English language. |
| Kamkwalala 2020 | Abstract only. |
| Kar 2004 | Wrong study design (review). |
| Kashyap 2013 | Abstract only. |
| Khan 2021 | Wrong study design. |
| Lampela 2013 | Wrong study design. |
| Lattanzio 2018 | Wrong outcome. |
| Lavrador 2020 | Duplicate. |
| Lavrador 2021 | Wrong study design (duration of follow‐up too short). |
| Lawson 2015 | Abstract only. |
| Lechevallier‐Michel 2005 | Wrong study design. |
| Liu 2020 | Repeat data set of already included study. |
| Lucas 2016 | Abstract only. |
| Naharci 2017 | Wrong patient population. |
| Neelamegam 2020 | Repeat data set of already included study. |
| Nishtala Prasad 2020 | Wrong patient population. |
| Park 2017 | Repeat data set of already included study. |
| Pasina 2013 | Wrong study design (follow‐up only for Barthel index; none for cognition). |
| Pasina 2020 | Wrong study design. |
| Perez 2019 | Abstract only. |
| Pfistermeister 2017 | Wrong study design. |
| Richardson 2018b | Abstract only. |
| Rudolph 2008 | Wrong outcome. |
| Salyer 2019 | Wrong study design. |
| Shetty 2020 | Abstract only. |
| Shiota 2020 | Wrong study design. |
| Suh 2020 | Repeat data set of already included study. |
| Tanaka 2019 | Non‐English language. |
| Tristancho‐Perez 2019 | Abstract only. |
| Tsoutsoulas 2017 | Wrong study design. |
| Verdoux 2020 | Wrong population. |
| Wang 2019 | Repeat data set of already included study. |
| Weigand 2020 | Repeat data set of already included study. |
| Welk 2020 | Dichotomises anticholinergic use. |
| Wouters 2017 | Wrong prognostic factor (DBI‐ach not reported separately). |
| Wouters 2020 | Wrong prognostic factor (DBI scale; anticholinergic not reported separately). |
| Ziad 2021 | Wrong patient population (middle‐aged adults). |
DBI: Drug Burden Index; DBI‐ach: anticholinergic component of the Drug Burden Index.
Differences between protocol and review
We made some minor changes to our review process to those specified in the protocol (Quinn 2020).
Four review authors joined the review team (SE, ANS, RS, and YL).
We did not employ the I2 statistic to evaluate heterogeneity in included studies, choosing instead to evaluate heterogeneity narratively. This was due to evidence that the I2 statistic can exaggerate levels of heterogeneity in prognostic research.
We decided not to report mortality, overall survival or dementia‐free survival as secondary outcomes on the basis that we found very few data on this in included reviews and since mortality has already been the focus of other recently published systematic reviews.
We conducted a sensitivity analysis restricted to studies with no 'high risk of bias' categories, rather than restricted to studies at 'low risk of bias' overall, due to the lack of studies judged at low risk of bias.
We added additional criteria to our outcome risk of bias and reverse causation evaluation. Specifically, if the cognitive diagnosis preceded the anticholinergic burden measurement, we assigned a moderate risk of bias to the outcome. If studies imposed a restriction on anticholinergic drug use to one to two years before the dementia index date, we applied a moderate risk of bias rating.
We were unable to perform our planned meta‐regressions due to lack of study numbers. Similarly, we could not pool linear or hazard ratio outcome data, investigate the association between anticholinergic burden, and duration of exposure due to insufficient data.
Contributions of authors
TQ conceived the idea.
ANS conducted the search.
MT and SE screened the search results, selected studies for inclusion and exclusion, and performed data extraction and 'Risk of bias' assessment for each study.
MT conducted the analysis and wrote the manuscript.
PM, TQ, JM, RS, CS, and YL contributed to data interpretation and the writing of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK
This protocol was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
-
The Dunhill Medical Trust, UK
This work was supported by a Dunhill Medical Project Grant (RPGF1806/66)
Declarations of interest
MT: none
SE: none
ANS: none
JM: none
PM: none
RS: none
CS: none
YK: none
TQ: none
New
References
References to studies included in this review
Ancelin 2006 {published data only}
- Ancelin ML, Artero S, Portet F, Dupuy A-M, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ 2006;332(7539):455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brombo 2018 {published data only}
- Brombo G, Bianchi L, Maietti E, Malacarne F, Coronello A, Cherubini A, et al. Association of anticholinergic drug burden with cognitive and functional decline over time in older inpatients: results from the CRIME Project. Drugs & Aging 2018;35:917-24. [DOI] [PubMed] [Google Scholar]
Campbell 2016 {published data only}
- Campbell NL, Perkins AJ, Bradt P, Perk S, Wielage RC, Boustani MA. Association of anticholinergic burden with cognitive impairment and health care utilization among a diverse ambulatory older adult population. Pharmacotherapy 2016;36(11):1123-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuang 2017 {published data only}
- Chuang YF, Elango P, Gonzalez CE, Thambisetty M. Midlife anticholinergic drug use, risk of Alzheimer's disease, and brain atrophy in community-dwelling older adults. Alzheimer's & Dementia 2017;3(3):471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coupland 2019 {published data only}
- Coupland CA, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Internal Medicine 2019;179(8):1084-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2011a {published data only}
- Fox C, Richardson K, Maidment ID, Savva GM, Mathews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the Medical Research Council Cognitive Function and Ageing Study. Journal of the American Geriatrics Society 2011;59:1477-83. [DOI] [PubMed] [Google Scholar]
Gray 2015 {published data only}
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergic medications and incident dementia. JAMA Internal Medicine 2015;175(3):401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grossi 2019 {published data only}
- Grossi CM, Richardson K, Fox C, Maidment I, Steel N, Loke YK, et al. Anticholinergic and benzodiazepine medication use and risk of incident dementia: a UK cohort study. BMC Geriatrics 2019;19:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hafdi 2020 {published data only}
- Hafdi M, Hoevenaar-Blom MP, Beishuizen CR, Moll van Charante EP, Richard E, Gool WA. Association of benzodiazepine and anticholinergic drug usage with incident dementia: a prospective cohort study of community-dwelling older adults. Journal of the American Medical Directors Association 2020;21:188-93. [DOI] [PubMed] [Google Scholar]
Han 2008 {published data only}
- Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. Journal of the American Geriatric Society 2008;56(12):2203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2017 {published data only}
- Hsu WH, Wen YW, Chen LK, Hsiao FY. Comparative associations between measures of anticholinergic burden and adverse clinical outcomes. Annals of Family Medicine 2017;15:561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iyer 2020 {published data only}
- Iyer S, Lozo S, Botros C, Wang C, Warren A, Sand P, et al. Cognitive changes in women starting anticholinergic medications for overactive bladder: a prospective study. International Urogynecology Journal 2020;12:2653-60. [DOI] [PubMed] [Google Scholar]
Jessen 2010 {published data only}
- Jessen F, Kaduszkiewicz H, Daerr M, Bickel H, Pentzek M, Riedel-Heller S, et al. Anticholinergic drug use and risk for dementia: target for dementia prevention. European Archives of Psychiatry and Clinical Neuroscience 2010;260:S111-5. [DOI] [PubMed] [Google Scholar]
Joung 2019 {published data only}
- Joung K, Kim S, Cho Y, Cho S. Association of anticholinergic use with incidence of Alzheimer's disease: population-based cohort study. Scientific Reports 2019;9:6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kashyap 2014 {published data only}
- Kashyap M, Belleville S, Mulsant BH, Hilmer SN, Paquette A, Tu LM, et al. Methodological challenges in determining longitudinal associations between anticholinergic drug use and incident cognitive decline. Journal of the American Geriatrics Society 2014;62:336-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koyama 2014 {published data only}
- Koyama A, Steinman M, Ensrud K, Hillier TA, Yaffe K. Long-term cognitive and functional effects of potentially inappropriate medications in older women. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2014;69(4):423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Low 2009 {published data only}
- Low LF, Anstey KJ, Sachdev P. Use of medications with anticholinergic properties and cognitive function in a young-old community sample. International Journal of Geriatric Psychiatry 2009;24:578-84. [DOI] [PubMed] [Google Scholar]
Moriarty 2020 {published data only}
- Moriarty F, Savva GM, Grossi CM, Bennett K, Fox C, Maidment I, et al. Cognitive decline associated with anticholinergics, benzodiazepines and Z-drugs: findings from the Irish Longitudinal Study on Ageing (TILDA). British Journal of Clinical Pharmacology 2020 Dec 3 [Epub ahead of print]. [DOI: 10.1111/bcp.14687] [DOI] [PubMed] [Google Scholar]
Papenberg 2017 {published data only}
- Papenberg G, Backman L, Fratiglioni L, Laukka EJ, Fastbom J, Johnell K. Anticholinergic drug use is associated with episodic memory decline in older adults without dementia. Neurobiology of Aging 2017;55:27-32. [DOI] [PubMed] [Google Scholar]
Richardson 2018 {published data only}
- Richardson K, Fox C, Maidment I, Steel N, Loke YK, Arthur A, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ 2018;360:k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Risacher 2016 {published data only}
- Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurology 2016;73(6):721-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2013 {published data only}
- Shah RC, Janos AL, Kline JE, Yu L, Leurgans SE, Wilson RS, et al. Cognitive decline in older persons initiating anticholinergic medications. PloS One 2013;8(5):e64111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheu 2019 {published data only}
- Sheu JJ, Tsai MT, Ericksen SR, Wu CH. Association between anticholinergic medication use and risk of dementia among patients with Parkinson's disease. Pharmacotherapy 2019;39(8):798-808. [DOI] [PubMed] [Google Scholar]
Whalley 2012 {published data only}
- Whalley LJ, Sharma S, Fox HC, Murray AD, Staff RT, Duthie AC, et al. Anticholinergic drugs in late life: adverse effects on cognition but not on progress to dementia. Journal of Alzheimer's Disease 2012;30:253-61. [DOI] [PubMed] [Google Scholar]
Yarnall 2015 {published data only}
- Yarnall AJ, Lawson RA, Duncan GW, Breen DP, Khoo TK, Brooks D, et al. Anticholinergic load: is there a cognitive cost in early Parkinson's disease? Journal of Parkinson's Disease 2015;5:743-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aalto 2018 {published data only}
- Aalto UL, Roitto HM, Finne-Soveri H, Kautiainen H, Pitkala K. Use of anticholinergic drugs and its relationship with psychological well-being and mortality in long-term care facilities in Helsinki. Journal of the American Medical Directors Association 2018;19(6):511-5. [DOI] [PubMed] [Google Scholar]
Andrade 2019 {published data only}
- Andrade C. Anticholinergic drug exposure and the risk of dementia: there is modest evidence for an association but not for causality. Journal of Clinical Psychiatry 2019;80(4):19f13000. [DOI] [PubMed] [Google Scholar]
Andre 2019 {published data only}
- Andre L, Gallini A, Montastruc F, Coley N, Montastruc JL, Vellas B, et al. Anticholinergic exposure and cognitive decline in older adults: effect of anticholinergic exposure definitions in a 3-year analysis of the Multidomain Alzheimer Preventive Trial (MAPT) study. British Journal of Clinical Pharmacology 2019;85(1):71-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ang 2015 {published data only}
- Ang MS, Abdul Rashid NA, Lee J. Impact of anticholinergic burden on cognitive performance in patients with schizophrenia. Academy of Medicine Singapore 2015;44:S55. [Google Scholar]
Ang 2017 {published data only}
- Ang MS, Abdul Rashid NA, Lam M, Rapisarda A, Kraus M, Keefe RS, et al. The impact of medication anticholinergic burden on cognitive performance in people with schizophrenia. Journal of Clinical Psychopharmacology 2017;37(6):651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aparasu 2014 {published data only}
- Aparasu RR, Chatterjee S, Carnahan RM, Chen H, Johnson ML. Anticholinergic medication use and risk of dementia among elderly nursing home residents with depression. Value in Health 2014;17(3):A208. [DOI] [PubMed] [Google Scholar]
Asano 2019 {published data only}
- Asano R, Tanaka A, Arai Y, Hirata T, Abe Y, Oguma Y. Drug burden of polypharmacy and anticholinergic/sedative drugs and physical/cognitive/mental related outcomes of the community-dwelling elderly people: the Kawasaki well-being project. Pharmacoepidemiology and Drug Safety 2019;28:293. [Google Scholar]
Ben Omar 2013 {published data only}
- Ben Omar N, Wenzel-Seifert K, Haen E. Age-dependency of psychotropic drug-induced anticholinergic adverse events: analysis of the data bank of the pharmacovigilance system AGATE. Naunyn-Schmiedeberg's Archives of Pharmacology 2013;386:S90. [Google Scholar]
Bostock 2013 {published data only}
- Bostock CV, Soiza RL, Mangoni AA. Associations between different measures of anticholinergic drug exposure and Barthel Index in older hospitalized patients. Therapeutic Advances in Drug Safety 2013;4(6):235-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bottiggi 2006 {published data only}
- Bottiggi KA, Salazar JC, Yu L, Caban-Holt AM, Ryan M, Mendiondo MS, et al. Long-term cognitive impact of anticholinergic medications in older adults. American Journal of Geriatric Psychiatry 2006;14(11):980-4. [DOI] [PubMed] [Google Scholar]
Bouchard 2017 {published data only}
- Bouchard AB. Longitudinal relations among anticholinergic drug burden, neurocognition, and community functioning in outpatients with serious mental illness. Dissertation abstracts international 2017;78:No pagnation. [Google Scholar]
Broder 2020 {published data only}
- Broder J, Lockery J, Wolfe R, Ryan J, Woods R, Ernst M. Anticholinergic medication burden and longitudinal cognitive function in older persons. Journal of the American College of Clinical Pharmacy 2020;3(8):1625. [Google Scholar]
Cai 2013 {published data only}
- Cai X, Campbell N, Khan B, Callahan C, Boustani M. Chronic anticholinergic use and the aging brain. Alzheimers & Dementia 2013;9(4):377-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2010 {published data only}
- Campbell NL, Boustani MA, Lane KA, Gao S, Hendrie H, Khan BA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology 2010;75(2):152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2018 {published data only}
- Campbell NL, Lane KA, Gao S, Boustani MA, Unverzagt F. Anticholinergics influence transition from normal cognition to mild cognitive impairment in older adults in primary care. Pharmacotherapy 2018;38(5):511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cancelli 2008 {published data only}
- Cancelli I, Gigli GL, Piani A, Zanchettin B, Janes F, Rinaldi A, et al. Drugs with anticholinergic properties as a risk factor for cognitive impairment in elderly people: a population-based study. Journal of Clinical Psychopharmacology 2008;28(6):654-9. [DOI] [PubMed] [Google Scholar]
Cao 2008 {published data only}
- Cao YJ, Mager DE, Simonsick EM, Hilmer SN, Ling SM, Windham BG, et al. Physical and cognitive performance and burden of anticholinergics, sedatives, and ACE inhibitors in older women. Clinical Pharmacology and Therapeutics 2008;83(3):422-9. [DOI] [PubMed] [Google Scholar]
Cardwell 2020 {published data only}
- Cardwell K, Kerse N, Ryan C, Teh R, Moyes SA, Menzies O, et al. The association between Drug Burden Index (DBI) and health-related outcomes: a longitudinal study of the 'oldest old' (LiLACS NZ). Drugs & Aging 2020;37(3):205-13. [DOI] [PubMed] [Google Scholar]
Carriere 2009 {published data only}
- Carriere I, Fourrier-Reglat A, Dartigues JF, Rouaud O, Pasquier F, Ritchie K, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-City Study. Archives of Internal Medicine 2009;169(14):1317-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherniaeva 2019 {published data only}
- Cherniaeva M, Kulikova M, Ostroumova O, Golovina O, Kochetkov A, Sychev D. Impact of the anticholinergic burden on cognitive functions in very old patients. European Journal of Clinical Pharmacology 2019;75:S71. [Google Scholar]
Cooley 2020 {published data only}
- Cooley SA, Ances B. Effect of anticholinergic medications on brain integrity in older HIV-positive adults. Topics in Antiviral Medicine 2020;28(1):137. [Google Scholar]
Cooley 2021 {published data only}
- Cooley SA, Paul RH, Strain JF, Boerwinkle A, Kilgore C, Ances BM. Effects of anticholinergic medication use on brain integrity in persons living with HIV and persons without HIV. AIDS 2021;35(3):381-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dmochowski 2021 {published data only}
- Dmochowski RR, Thai S, Iglay K, Enemchukwu E, Tee S, Varano S, et al. Increased risk of incident dementia following use of anticholinergic agents: a systematic literature review and meta-analysis. Neurourology and Urodynamics 2021;40(1):28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drag 2012 {published data only}
- Drag LL, Wright SL, Bieliauskas LA. Prescribing practices of anticholinergic medications and their association with cognition in an extended care setting. Journal of Applied Gerontology 2012;31(2):239-59. [Google Scholar]
Ehrt 2010 {published data only}
- Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson's disease: a cohort study. Journal of Neurology, Neurosurgery and Psychiatry 2010;81(2):160-5. [DOI] [PubMed] [Google Scholar]
Fox 2011b {published data only}
- Fox C, Livingston G, Maidment ID, Coulton S, Smithard DG, Boustani M. The impact of anticholinergic burden in Alzheimer's dementia – the LASER-AD study. Age and Ageing 2011;40(6):730-5. [DOI] [PubMed] [Google Scholar]
Gnjidic 2012 {published data only}
- Gnjidic D, Le Couteur DG, Naganathan V, Cumming RG, Creasey H, Waite LM, et al. Effects of drug burden index on cognitive function in older men. Journal of Clinical Psychopharmacology 2012;32(2):273-7. [DOI] [PubMed] [Google Scholar]
Hanlon 2020 {published data only}
- Hanlon P, Quinn TJ, Gallacher KI, Myint PK, Jani BD, Nicholl BI, et al. Assessing risks of polypharmacy involving medications with anticholinergic properties. Annals of Family Medicine 2020;18(2):148-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hong 2019 {published data only}
- Hong C-T, Chan L, Wu D, Chen W-T, Chien L-N. Antiparkinsonism anticholinergics increase dementia risk in patients with Parkinson's disease. Parkinsonism & Related Disorders 2019;65:224-9. [DOI] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang K-H, Chan Y-F, Shih H-C, Lee C-Y. Relationship between potentially inappropriate anticholinergic drugs (PIADs) and adverse outcomes among elderly patients in Taiwan. Journal of Food and Drug Analysis 2012;20(4):930-7. [Google Scholar]
Jakeman 2020 {published data only}
- Jakeman B, Scherrer A, Darling K, Nadin I, Hasse B, Hauser C, et al. Effect of non-HIV drugs on neurocognitive domains in a well treated HIV population. Topics in Antiviral Medicine 2020;28(1):142. [Google Scholar]
Kada 2019 {published data only}
- Kada S. Long-term effects of anticholinergics and sedatives on cognitive and physical function. Pharmaceutisch Weekblad 2019;154(44):32-6. [Google Scholar]
Kamkwalala 2020 {published data only}
- Kamkwalala AR, Ma Q, Karris MY, Sundermann E, Ellis RJ, Moore DJ, et al. Anticholinergic burden is associated with depressive symptoms in persons with HIV. Topics in Antiviral Medicine 2020;28(1):144. [Google Scholar]
Kar 2004 {published data only}
- Kar S, Slowikowski SP, Westaway D, Mount HT. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer's disease. Journal of Psychiatry & Neuroscience 2004;29:427-41. [PMC free article] [PubMed] [Google Scholar]
Kashyap 2013 {published data only}
- Kashyap M, Belleville S, Mulsant B, Hilmer S, Tannenbaum C. The effect of misclassification of the outcome on the relationship between an increase in anticholinergic drug burden and memory impairment in older adults. Pharmacoepidemiology and Drug Safety 2013;22:505-6. [Google Scholar]
Khan 2021 {published data only}
- Khan WU, Ghazala Z, Brooks HJ, Subramaniam P, Mulsant BH, Kumar S, et al. The impact of anticholinergic burden on functional capacity in persons with schizophrenia across the adult life span. Schizophrenia Bulletin 2021;47(1):249-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lampela 2013 {published data only}
- Lampela P, Lavikainen P, Garcia-Horsman JA, Bell JS, Huupponen R, Hartikainen S. Anticholinergic drug use, serum anticholinergic activity, and adverse drug events among older people: a population-based study. Drugs & Aging 2013;30(5):321-30. [DOI] [PubMed] [Google Scholar]
Lattanzio 2018 {published data only}
- Lattanzio F, Corica F, Schepisi R, Amantea D, Bruno F, Cozza A, et al. Anticholinergic burden and 1-year mortality among older patients discharged from acute care hospital. Geriatrics & Gerontology International 2018;18(5):705-13. [DOI] [PubMed] [Google Scholar]
Lavrador 2020 {published data only}
- Lavrador M, Cabral AC, Figueiredo IV, Verissimo MT, Castel-Branco M, Fernandez-Llimos F. Using different anticholinergic lists for DBI calculation: effects on anticholinergic outcome prediction. International Journal of Clinical Pharmacy 2020;42(2):816. [Google Scholar]
Lavrador 2021 {published data only}
- Lavrador M, Cabral AC, Figueiredo IV, Verissimo MT, Castel-Branco MM, Fernandez-Llimos F. Size of the associations between anticholinergic burden tool scores and adverse outcomes in older patients. International Journal of Clinical Pharmacy 2021;43(1):128-36. [DOI] [PubMed] [Google Scholar]
Lawson 2015 {published data only}
- Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, Brooks D, et al. Does prolonged use of anticholinergic medication contribute to cognitive impairment in early Parkinson's disease? Movement Disorders 2015;30:S344. [Google Scholar]
Lechevallier‐Michel 2005 {published data only}
- Lechevallier-Michel N, Molimard M, Dartigues JF, Fabrigoule C, Fourrier-Reglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. British Journal of Clinical Pharmacology 2005;59(2):143-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2020 {published data only}
- Liu Y-P, Chien W-C, Chung C-H, Chang H-A, Kao Y-C, Tzeng N-S. Are anticholinergic medications associated with increased risk of dementia and behavioral and psychological symptoms of dementia? A nationwide 15-year follow-up cohort study in Taiwan. Frontiers in Pharmacology 2020;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 2016 {published data only}
- Lucas C, Greene M, Esmaeili-Firidouni P, Valcour V. Anticholinergic burden and cognition in HIV+ older adults. Journal of the American Geriatrics Society 2016;64:S132. [Google Scholar]
Naharci 2017 {published data only}
- Naharci MI, Cintosun U, Ozturk A, Oztin H, Turker T, Bozoglu E, et al. Effect of anticholinergic burden on the development of dementia in older adults with subjective cognitive decline. Psychiatry and Clinical Psychopharmacology 2017;27(3):269-76. [Google Scholar]
Neelamegam 2020 {published data only}
- Neelamegam M, Zgibor J, Chen H, O'Rourke K, Bakour C, Rajaram L, et al. The effect of cumulative anticholinergic use on the cognitive function of older adults: results from the Personality and Total Health (PATH) through life study. Journals of Gerontology Series A. Biological Sciences & Medical Sciences 2020;75(9):1706-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishtala Prasad 2020 {published data only}
- Nishtala Prasad S, Allore H, Han L, Jamieson HA, Hilmer SN, Chyou T-Y. Impact of anticholinergic burden on cognitive performance: a cohort study of community-dwelling older adults. Journal of the American Medical Directors Association 2020;21(9):1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2017 {published data only}
- Park HY, Park JW, Song HJ, Sohn HS, Kwon JW. The association between polypharmacy and dementia: a nested case control study based on a 12-year longitudinal cohort database in South Korea. PloS One 2017;12:e0169463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasina 2013 {published data only}
- Pasina L, Djade CD, Lucca U, Nobili A, Tettamanti M, Franchi C, et al. Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: comparison of the anticholinergic cognitive burden scale and anticholinergic risk scale: results from the REPOSI study. Drugs & Aging 2013;30(2):103-12. [DOI] [PubMed] [Google Scholar]
Pasina 2020 {published data only}
- Pasina L, Lucca U, Tettamanti M. Relation between anticholinergic burden and cognitive impairment: results from the Monzino 80-plus population-based study. Pharmacoepidemiology and Drug Safety 2020;29(12):1696-702. [DOI] [PubMed] [Google Scholar]
Perez 2019 {published data only}
- Perez AT, Moreno AV, Ramos BS, Alfaro Lara ER, Santos Rubio MD, Rustarazo SB, et al. Anticholinergic burden scales as predictors of cognitive impairment in elderly multimorbidity patients. European Geriatric Medicine 2019;10:S227. [Google Scholar]
Pfistermeister 2017 {published data only}
- Pfistermeister B, Tumena T, Gasmann KG, Maas R, Fromm MF. Anticholinergic burden and cognitive function in a large German cohort of hospitalized geriatric patients. PloS One 2017;12(2):e0171353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richardson 2018b {published data only}
- Richardson K, Grossi C, Fox C, Maidment ID, Loke Y, Steel N, et al. Anticholinergic medications, benzodiazepines, and long-term cognitive decline in large observational studies: findings from the Anticholinergics, Benzodiazepines, Cognition and Dementia (ABCD) study. Alzheimer's & Dementia 2018;14:P966. [Google Scholar]
Rudolph 2008 {published data only}
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Archives of Internal Medicine 2008;168(5):508-13. [DOI] [PubMed] [Google Scholar]
Salyer 2019 {published data only}
- Salyer J, Sargent L, Tirado C, Flattery MP, Shah KB. Anticholinergic burden and cognitive impairment in patients with heart failure. Journal of Heart and Lung Transplantation 2019;38(4 Suppl):S299. [Google Scholar]
Shetty 2020 {published data only}
- Shetty N, Dubaz O, Yu Y, Gao H, Simuni T. Anticholinergic use in Parkinson's disease: practice patterns in patients with cognitive impairment. Neurology 2020;94(15 Suppl):2720. [Google Scholar]
Shiota 2020 {published data only}
- Shiota T, Torimoto K, Okuda M, Iwata R, Kumamoto H, Miyake M, et al. Cognitive burden and polypharmacy in elderly Japanese patients treated with anticholinergics for an overactive bladder. Lower Urinary Tract Symptoms 2020;12(1):54-61. [DOI] [PubMed] [Google Scholar]
Suh 2020 {published data only}
- Suh Y, Ah YM, Han E, Jun K, Hwang S, Choi KH, et al. Dose response relationship of cumulative anticholinergic exposure with incident dementia: validation study of Korean anticholinergic burden scale. BMC Geriatrics 2020;20(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2019 {published data only}
- Tanaka A, Arai Y, Hirata T, Abe Y, Oguma Y, Urushihara H. Effects of polypharmacy and anticholinergic/sedative drugs on the physical/cognitive/mental related outcomes of community-dwelling elderly people: the Kawasaki Wellbeing Project. Japanese Journal of Geriatrics 2019;56(4):504-15. [DOI] [PubMed] [Google Scholar]
Tristancho‐Perez 2019 {published data only}
- Tristancho-Perez A, Belda Rustarazo S, Lopez-Malo MD, Santos-Rubio MD, Galvan-Banqueri M, Sanchez Fidalgo S. Impact of anticholinergic burden, quantified by anticholinergic risk scales, on cognitive and functional status and falls in patients with multimorbidity: a preliminary study. European Journal of Hospital Pharmacy 2019;26:A274. [Google Scholar]
Tsoutsoulas 2017 {published data only}
- Tsoutsoulas C, Mulsant BH, Kumar S, Ghazala Z, Voineskos AN, Menon M, et al. Anticholinergic burden and cognition in older patients with schizophrenia. Journal of Clinical Psychiatry 2017;78(9):e1284-90. [DOI] [PubMed] [Google Scholar]
Verdoux 2020 {published data only}
- Verdoux H, Quiles C, Bon L, Chereau-Boudet I, Dubreucq J, Fiegi L, et al. Impact of anticholinergic load on functioning and cognitive performances of persons with psychosis referred to psychosocial rehabilitation centers. Psychological Medicine 2020:1-9. [DOI] [PubMed] [Google Scholar]
Wang 2019 {published data only}
- Wang YC, Chen YL, Huang CC, Ho CH, Huang YT, Wu MP, et al. Cumulative use of therapeutic bladder anticholinergics and the risk of dementia in patients with lower urinary tract symptoms: a nationwide 12-year cohort study. BMC Geriatrics 2019;19(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weigand 2020 {published data only}
- Weigand AJ, Bondi MW, Thomas KR, Campbell NL, Galasko DR, Salmon DP, et al. Association of anticholinergic medications and AD biomarkers with incidence of MCI among cognitively normal older adults. Neurology 2020;95(16):e2295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welk 2020 {published data only}
- Welk B, McArthur E. Increased risk of dementia among patients with overactive bladder treated with an anticholinergic medication compared to a beta-3 agonist: a population-based cohort study. BJU International 2020;126(1):183-90. [DOI] [PubMed] [Google Scholar]
Wouters 2017 {published data only}
- Wouters H, Hilmer S, Gnjidic D, Campen J, Teichert M, Meer H, Schaap LA, Huisman M, Comijis HC, Denig P, Lamoth CJ, Taxis K. Long-term exposure to anticholinergic and sedative drugs and cognitive and physical function in later life. European Geriatric Medicine 2017. [DOI] [PubMed] [Google Scholar]
Wouters 2020 {published data only}
- Wouters H, Hilmer SN, Gnjidic D, Campen JP, Teichert M, Meer HG, et al. Long-term exposure to anticholinergic and sedative medications and cognitive and physical function in later life. Journals of Gerontology Series A. Biological Sciences & Medical Sciences 2020;75(2):357-65. [DOI] [PubMed] [Google Scholar]
Ziad 2021 {published data only}
- Ziad A, Berr C, Ruiz F, Begaud B, Lemogne C, Goldberg M, et al. Anticholinergic activity of psychotropic drugs and cognitive impairment among participants aged 45 and over: the CONSTANCES study. Drug Safety 2021;44(5):565-79. [DOI] [PubMed] [Google Scholar]
Additional references
Alzheimer's Society 2019
- Alzheimer's Society. Annual report overview 2018/19. www.alzheimers.org.uk/about-us/policy-and-influencing/dementia-scale-impact-numbers (accessed prior to 27 April 2021).
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Text Revision edition. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
Bentley 2011
- Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Progress in Neurobiology 2011;94:360-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boustani 2009
- Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health 2009;4(3):311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chew 2008
- Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, et al. Anticholinergic activity of 107 medications commonly used by older adults. Journal of American Geriatric Society 2008;56:1333-41. [DOI] [PubMed] [Google Scholar]
Comprehensive Meta‐Analysis Version 3 [Computer program]
- Comprehensive Meta-Analysis Version 3. Englewood (NJ): Biostat, 2013.
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189-98. [DOI] [PubMed] [Google Scholar]
Fox 2014
- Fox C, Smith T, Maidment I, Chan WY, Bua N, Myint PK, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age and Ageing 2014;43:604-15. [DOI] [PubMed] [Google Scholar]
Geersing 2012
- Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG. Search filters for finding prognostic and diagnostic prediction studies in MEDLINE to enhance systematic reviews. PloS One 2012;7:e32844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grave‐Morris 2020
- Grave-Morris K, Stewart C, Soiza RL, Taylor-Rowan M, Quinn TJ, Loke YN, et al. The prognostic value of anticholinergic burden measures in relation to mortality in older individuals: a systematic review and meta-analysis. Frontiers in Pharmacology 2020;11:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 2012
- Hayden JA, Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Annals of International Medicine 2012;158(4):280-6. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Huguet 2013
- Huguet A, Hayden JA, Stinson J, McGrath PJ, Chambers CT, Tougas ME, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Systematic Reviews 2013;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iorio 2015
- Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870. [DOI] [PubMed] [Google Scholar]
Jack 1999
- Jack CR Jr, Petersen RC, Xu Yc, Obrien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kersten 2013
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2013;68:271-8. [DOI] [PubMed] [Google Scholar]
Lisibach 2021
- Lisibach A, Benelli V, Ceppi MG, Waldner-Knogler K, Csajka C, Lutters M. Quality of anticholinergic burden scales and their impact on clinical outcomes: a systematic review. European Journal of Clinical Pharmacology 2021;77(2):147-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litvan 2012
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Movement Disorder 2012;27:349-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingston 2017
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673-734. [DOI] [PubMed] [Google Scholar]
Mayer 2015
- Mayer T, Haefeli WE, Seidling HM. Different methods, different results – how do available methods link a patient's anticholinergic load with adverse outcomes? European Journal of Clinical Pharmacology 2015;71(11):1299-314. [DOI] [PubMed] [Google Scholar]
McKeith 2005
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863-72. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939-44. [DOI] [PubMed] [Google Scholar]
McKhann 2001
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Archives of Neurology 2001;58:1803-9. [DOI] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging – Alzheimer's Association work group on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7:263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myint 2015
- Myint PK, Fox C, Kwok CS, Luben RN, Wareham NJ, Khaw KT. Total anticholinergic burden and risk of mortality and cardiovascular disease over 10 years in 21,636 middle-aged and older men and women of EPIC-Norfolk prospective population study. Age and Ageing 2015;44:219-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasreddine 2005
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society 2005;53:695-9. [DOI] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Dementia: assessment, management and support for people living with dementia and their carers, 2018. www.nice.org.uk/guidance/ng97 (accessed prior to 27 April 2021). [PubMed]
Peat 2014
- Peat G, Riley RD, Croft P, Morley KI, Kyzas PA, Moons KG, et al, PROGRESS Group. Improving the transparency of prognosis research: the role of reporting, data sharing, registration, and protocols. PLoS Medicine 2014;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2001
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133-42. [DOI] [PubMed] [Google Scholar]
Pieper 2020
- Pieper NT, Grossi CM, Chan WY, Loke YK, Savva GM, Haroulis C, et al. Anticholinergic drugs and incident dementia, mild cognitive impairment and cognitive decline: a meta-analysis. Age and Ageing 2020;00:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prince 2016
- Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. Improving healthcare for people living with dementia: coverage, quality and costs now and in the future. www.alzint.org/resource/world-alzheimer-report-2016/ (accessed prior to 27 April 2021).
Richardson 2018a
- Richardson K, Fox C, Maidment I, Steel N, Loke YK, Arthur A, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ 2018;361:k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2019
- Riley RD, Moons KG, Snell KI, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019;30:k4597. [DOI] [PubMed] [Google Scholar]
Román 1993
- Román GC, Tatemichi TK, Erkinjutti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43:250-60. [DOI] [PubMed] [Google Scholar]
Ruxton 2015
- Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: a systematic review and meta-analysis. British Journal of Clinical Pharmacology 2015;80(2):209-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schardt 2007
- Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Medical Informatics and Decision Making 2007;15:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2008
- Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008;300:1439-50. [DOI] [PubMed] [Google Scholar]
Stewart 2020
- Stewart C, Yrjana K, Kishor M, Soiza R, Taylor-Rowan M, Quinn TJ, et al. Anticholinergic burden measures predict older people's physical function and quality of life: a systematic review. Journal of the American Medical Directors Association 2020:S1525-8610. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines, 1992. apps.who.int/iris/handle/10665/37958 (accessed prior to 27 April 2021).
Wimo 2017
- Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer's & Dementia 2017;1:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winbald 2004
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine 2004;256:240-6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Quinn 2020
- Quinn TJ, Myint PK, McCleery J, Taylor-Rowan M, Stewart C. Anticholinergic burden (prognostic factor) for prediction of dementia or cognitive decline in older adults with no known cognitive syndrome. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD013540. [DOI: 10.1002/14651858.CD013540] [DOI] [PMC free article] [PubMed] [Google Scholar]


