Abstract
We report a case of COVID-19 in a 29-week preterm infant. This child is the youngest reported case of SARS-CoV-2 infection in Malaysia, and to the best of our knowledge, one of the youngest documented cases of established vertical transmission of SARS-CoV-2 reported in literature. Our report highlights the clinical course, timelines of viral shedding by real-time reverse transcription-PCR and antibody seroconversion in a premature infant infected with SARS-CoV-2. In addition, we discuss the challenges faced in managing a preterm infant infected with SARS-CoV-2 and the knowledge gaps that need to be explored.
Keywords: COVID-19, neonatal health, congenital disorders
Background
COVID-19 has spread rapidly across the globe since it was first declared a pandemic by the WHO on 11 March 2020.1 Despite the total number of COVID-19 cases now exceeding 110 million2 globally, limited data exist on the transmission of COVID-19 to newborn infants by infected pregnant women. The clinical course and impact on infected neonates, especially preterm infants have not been well characterised. COVID-19 infection in a premature infant represents an exclusive subset of the total COVID-19 infections worldwide with its own unique challenges.
Case presentation
A 39-year-old primigravida with underlying advanced adenocarcinoma of the colon was admitted to our state hospital for a planned premature Caesarean section and tumour resection at 29 weeks gestation due to impending rupture of the tumour. Antenatal steroids were administered for fetal pulmonary maturation. The mother unexpectedly developed fever and cough on the fourth day of hospitalisation with progressive lymphopaenia on serial blood counts. A chest radiograph revealed signs of pneumonia. Her nasopharyngeal swab was positive for SARS-CoV-2 gene targets via multiplex real-time reverse transcription PCR (RT-PCR) testing (Allplex 2019-nCoV assay, Seegene).
The elective operation was postponed on the diagnosis of COVID-19 infection. However, the mother developed premature contractions and preterm labour rapidly ensued. The mother wore a surgical mask during delivery and was attended by healthcare staff who wore personal protective equipment in line with airborne and contact precautions. A male infant was born via spontaneous vaginal delivery at 29 weeks plus 5 days gestation with a birth weight of 1100 g (50th centile) and length 41 cm (50th centile). Apgar scores were 9 at 1 min and 9 at 5 min. Delayed cord clamping was not performed. The infant was directly handed over to the neonatal team without skin-to-skin contact with the mother. Stabilisation of the neonate was performed in a separate negative pressure room and was uneventful.
The infant was transferred to the neonatal intensive care unit (NICU) and placed in a closed incubator in a negative pressure isolation room with strict airborne and contact precautions. There were no other cases of COVID-19 in the unit, no visitors allowed and all healthcare workers who attended to the child remained asymptomatic in the 2 weeks following delivery. The infant required non-invasive continuous positive airway pressure ventilation. His fractional concentrations of oxygen in inspired air requirements remained at 21% until 12 hours of life when he developed worsening respiratory distress. He required intubation and a dose of surfactant therapy.
Investigations
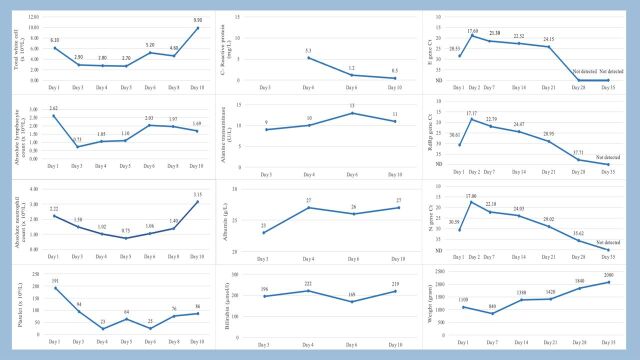
Chest radiograph findings were consistent with hyaline membrane disease. A nasopharyngeal swab for RT-PCR obtained at 2 hours of life was positive for all 3 SARS-CoV-2 target genes. The repeated respiratory sample with a tracheal aspirate remained positive 24 hours later. Neonatal serology obtained at birth, analysed by lateral flow immunoassay (Healgen Scientific, USA) was negative for SARS-CoV-2 specific IgM and IgG. The infant demonstrated seroconversion of IgM and IgG on day 14 of life. His white cell count was normal at birth but he developed neutropaenia, lymphopaenia and thrombocytopaenia by the third day of life. The infant’s main laboratory findings are shown in figure 1, including the cycle threshold (Ct) values for specific PCR targets (envelope, nucleocapsid and RNA-dependent RNA polymerase genes).
Figure 1.
Serial changes in complete blood counts, C reactive protein, serum alanine transaminase, albumin, bilirubin, weight and PCR Ct values. Ct, cycle threshold; E gene, Envelope gene; Rdrp gene, RNA-dependent RNA polymerase gene; N gene, Nucleocapsid gene; ND, not detected.
Outcome and follow-up
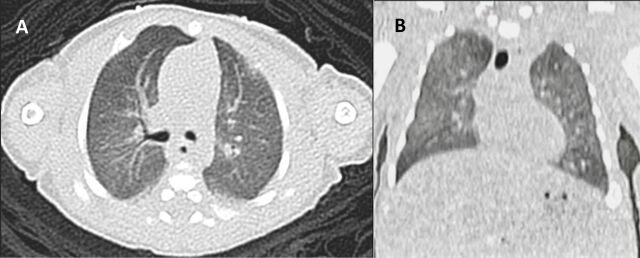
The infant was ventilated for 14 days and was successfully weaned to room air on day 50 of life. A high-resolution CT thorax performed on day 49 of life showed bilateral diffuse ground glass opacities suggestive of respiratory distress syndrome with no evidence of interstitial lung disease (figure 2). His cranial ultrasound showed bilateral grade 2 intraventricular haemorrhage with no subsequent progression. The infant was started on total parenteral nutrition (TPN) due to the extreme prematurity while gradually introducing enteral formula feeds. He experienced transient TPN cholestasis in the second week of life which resolved spontaneously. Blood cultures were negative. No antivirals or corticosteroids were given to the infant. He was discharged home on day 54 of life. Ophthalmology screening on discharge was normal.
Figure 2.
High-resolution RCT of the chest. (A) Transverse view, (B) coronal view showing ground glass opacities suggestive of respiratory distress syndrome with no evidence of interstitial lung disease.
Discussion
COVID-19 infection in pregnancy leads to an increased risk of complications to both mother and baby. Pregnant women with COVID-19 are more vulnerable to severe disease and are more likely to experience premature delivery.3 In addition to that, the possibility of vertical transmission of SARS-CoV-2 is of particular concern. Vertical transmission of the virus is thought to occur through several mechanisms: (1) in utero via haematogenous transfer across the placenta, aspirated amniotic fluid or (2) intrapartum via exposure to infected maternal blood or vaginal secretions.4
Vertical transmission of COVID-19, however, is difficult to ascertain due to the possibility of viral contamination during the labour (due to aerosols generated by infected women during active labour or maternal faecal contamination, particularly during vaginal birth), debatable reliability of serological testing, and a lack of standardised international consensus on the definition of ‘vertical’ transmission. There have been numerous reports on SARS-CoV-2 being detected in newborn samples, but the transmission route was unclear as amniotic fluid, placental tissue, vaginal swabs, maternal and newborn blood samples were not consistently analysed in every infected mother and newborn infant.5–7 Piersigilli et al8 described COVID-19 infection in a 26-week preterm neonate, but likely acquired the infection horizontally as COVID-19 was not suspected or diagnosed in the mother until 7 days post partum.
However, there are now growing evidence that vertical transmission of COVID-19 is possible, based on detection of SARS-CoV-2 specific IgM in neonatal serum,9 detection of SARS-CoV-2 nucleic acids in amniotic fluid,10 placental tissue,10–12 umbilical cord blood,12 evidence of placental invasion through immunohistochemistry10 13 and neonatal viraemia following placental infection.10
Despite vertical transmission of SARS-CoV-2 being a rare occurrence, it is a plausible route of infection for our case. The infant’s mother was acutely infected and went into premature labour. The premature delivery was likely related to COVID-19 as there were no other identifiable causes. Postnatal transmission was ruled out by the strict infection control practices described above. The baby did not receive any breast milk either, ruling out this potential route of infection. An early nasopharyngeal swab for SARS-CoV-2 RT-PCR was positive at 2 hours of life. The risk of contamination from maternal vaginal secretions at birth was reduced by performing sampling after the infant was cleaned and transported back to the neonatal unit. Contamination from maternal vaginal secretions would probably result in a positive swab on the first day, which would not be persistently detectable.4 This could have been the case with a study based on the Spanish Society of Neonatal Registry14 where 14 infants were tested positive for SARS-CoV-2 at birth, taken at median age 3 hours after delivery. However, repeated tests for 13 out of the 14 neonates were negative, suggesting the earlier positive results could have been just transient colonisation.
In contrast, our infant had a repeated sample from a tracheal aspirate on day 2 of life which showed increasing viral shedding, inferred by the Ct values. This suggests an actual neonatal infection with ongoing viral replication in the first 2 days of life. The sensitivity of SARS-CoV-2 IgG is optimal three to 4 weeks after the onset of symptoms.15 However, the mother was acutely symptomatic before delivery and had insufficient time for seroconversion and transplacental IgG transfer. Unsurprisingly, the infant’s SARS-CoV-2 specific IgG at birth was negative. He demonstrated seroconversion of IgM and IgG on day 14 of life. Given these circumstances, vertical transmission was considered the most likely route of infection. This was compatible with the recently proposed definition for vertical transmission of SARS-CoV-2 by the WHO; with confirmed infection during the pregnancy, and positive RT-PCR from a non-sterile sample (nasopharyngeal swab) at <24 hours, and a sterile sample (tracheal aspirate) at 24–48 hours of age.16
Notably, our patient did not experience a severe clinical course despite being delivered prematurely, consistent with the outcomes of most other infants reported in literature that were perinatally exposed or with possible vertical transmission of SARS-CoV-2.5–10 The infant’s respiratory distress which required intubation and ventilation was likely a result of prematurity rather than COVID-19 pneumonia. The laboratory abnormalities were transient with a nadir of absolute lymphocyte counts (ALC), absolute neutrophil counts (ANC) and platelet counts seen on day 3 (ALC 0.73×109/L), day 5 (ANC 0.75×109/L) and day 4 of life (platelet count 23×109/L), respectively. The hypoalbuminaemia resolved by day 11 of life and was likely a negative acute phase reactant towards the infection. Our report suggests that COVID-19 did not result in a worse clinical course in newborns, even in a premature infant.
Immune hyperactivation is thought to contribute to the severity of adult COVID-19.17 On the other hand, the immaturity of the neonatal immune system could be protective for newborns. This is one of the knowledge gaps that need to be explored. Antivirals such as remdesivir have been suggested in children with critical disease.18 However, given the typically mild course of COVID-19 in children and the risk of hepatotoxicity with remdesivir, supportive care alone would suffice for most cases.
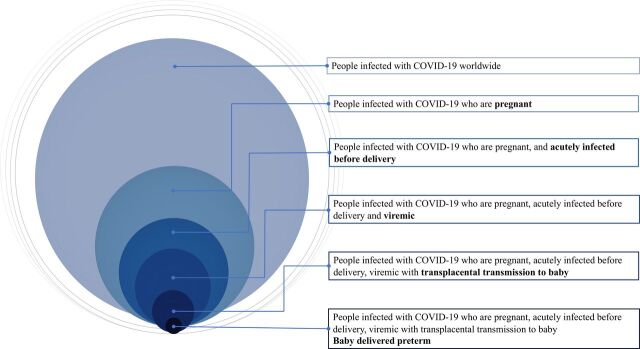
We hypothesised the infant was infected in utero from an immunocompromised mother who was acutely infected and viraemic. The bloodborne virus crossed the maternal-placental interface to reach the fetus, resulting in infection and premature delivery. The unique circumstances on how our case was infected are summarised in figure 3. However, we were unable to perform molecular testing for SARS-CoV-2 on placental tissue, cord blood, vaginal swabs and amniotic fluid in our centre. This would have provided useful information to verify the pathogenesis and would be the limitations of our report. We were also unable to perform viral cultures on the nasopharyngeal aspirate specimens. Of note, the RT-PCR results for our case remained positive until the fifth week of life. Although the infant continued to have detectable viral shedding by PCR, the exact duration of replication-competent viral shedding is unknown. The duration of live-virus shedding is well characterised in immunocompetent patients with COVID-19, but immunocompromised hosts may shed cultivable virus for months.19–21 At this point, there is still paucity of data on the extent newborns infected with SARS-CoV-2 remain contagious.
Figure 3.
Venn diagram depicting the unique circumstances of COVID-19 infection in our patient based on our hypothesis.
Management of COVID-19 in a premature infant came with unique challenges apart from the ventilation, fluid balance and feeding difficulties associated with prematurity. The struggles were compounded by the need for stringent airborne and contact precautions for an extended duration, considering the expected prolonged stay in the NICU. Therein lies the dilemma of when to release the infant from isolation. Although a positive RT-PCR did not necessarily indicate infectiousness, we used a test-based strategy as a surrogate marker of infectivity to reduce the risk of nosocomial spread. Apart from that, a premature infant’s birth is usually an unexpected event for parents that can be psychologically disruptive. The diagnosis of COVID-19 in our case amplified the stress, especially when the parents were not permitted to visit their newborn child.
In conclusion, our report highlights the clinical course, timelines of viral shedding by RT-PCR and antibody seroconversion in a premature infant infected with SARS-CoV-2. Understanding of SARS-CoV-2 infection in the neonatal population would continue to evolve over time. There is need for further studies to determine the duration of infectivity in neonates and the long-term outcomes. Lastly, we need to be cognizant of the complex psychosocial aspects of neonatal care especially when dealing with COVID-19 infection in a premature infant.
Learning points.
Vertical transmission of SARS-CoV-2 did not appear to result in a more severe clinical course despite prematurity.
Viral shedding in the respiratory tract of a newborn infected with SARS-CoV-2 can persist until the fifth week of life, but the duration the infant remains infectious is unknown.
There is a need for emotional support for parents who have a premature infant with COVID-19 warded in the neonatal unit, who are unable to visit their baby due to the pandemic restrictions.
Acknowledgments
We would like to acknowledge Dr Suhaila bt Baharuddin, MD, MPath for her contributions in the molecular work and thank Dr Lee Ming Lee, MBBS, MRCP for providing editorial assistance. We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Footnotes
Contributors: DCEN participated in the clinical care of the patient, conceptualised the manuscript, drafted the initial manuscript, reviewed and revised the manuscript. LC participated in the clinical care of the patient, coordinated data collection and drafted the initial manuscript. PPLC participated in the clinical care of the patient, supported literature review, revised the manuscript and added intellectual content. UP participated in the clinical care of the patient, supported literature review, revised the manuscript and added intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.World Health Organization . WHO Director-General’s opening remarks at the media briefing on COVID-19, 2020. Available: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 [Accessed 1 Feb 2021].
- 2.Johns Hopkins University Coronavirus Resource Center . COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University, 2020. Available: https://coronavirus.jhu.edu/ [Accessed 20 Feb 2021].
- 3.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumberg DA, Underwood MA, Hedriana HL, et al. Vertical transmission of SARS-CoV-2: what is the optimal definition? Am J Perinatol 2020;37:769–72. 10.1055/s-0040-1712457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr 2020;174:722–5. 10.1001/jamapediatrics.2020.0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 2020;369:m2107. 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzamora MC, Paredes T, Caceres D, et al. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol 2020;37:861–5. 10.1055/s-0040-1710050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piersigilli F, Carkeek K, Hocq C, et al. COVID-19 in a 26-week preterm neonate. Lancet Child Adolesc Health 2020;4:476–8. 10.1016/S2352-4642(20)30140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020;323:1846–8. 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020;11:3572. 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020;192:E647–50. 10.1503/cmaj.200821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenizia C, Biasin M, Cetin I, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun 2020;11:5128. 10.1038/s41467-020-18933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosier H, Farhadian SF, Morotti RA, et al. SARS–CoV-2 infection of the placenta. J Clin Invest 2020;130:4947–53. 10.1172/JCI139569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez-Luna M, Fernández Colomer B, de Alba Romero C, et al. Neonates born to mothers with COVID-19: data from the Spanish Society of neonatology registry. Pediatrics 2021;147:e2020015065. 10.1542/peds.2020-015065 [DOI] [PubMed] [Google Scholar]
- 15.Hanson KE, Caliendo AM, Arias CA. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19:Serologic Testing. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO . Definition and categorization of the timing of mother-to-child transmission of SARS-CoV-2. Scientific brief, 2021. Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1 [Accessed 15 Feb 2021].
- 17.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020;383:2255–73. 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter interim guidance on use of antivirals for children with coronavirus disease 2019/Severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc 2021;10:34–48. 10.1093/jpids/piaa115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 2020;383:2586–8. 10.1056/NEJMc2031670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020;383:2291–3. 10.1056/NEJMc2031364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakajima Y, Ogai A, Furukawa K, et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother 2021;27:387–9. 10.1016/j.jiac.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]