Abstract
Gout is a common crystal-induced arthropathy affecting mainly the joints of the appendicular skeleton; however, rarely this condition affects the axial skeleton as well. Spinal gout can cause radiculopathy, cord compression, canal stenosis and discitis. We describe a case of a 71-year-old woman where the initial presentation of destructive arthropathy and spinal masses secondary to axial gout was mistaken for a metastatic malignancy. Despite chronic polyarthropathy and bilateral subcutaneous gouty tophi, spinal gout was not considered a differential diagnosis during initial assessment.
The patient was managed conservatively with pharmacological treatment resulting in improvement of her upper limb radiculopathy and systemic joint pain, although little improvement in mobility. Such extensive involvement is rare and the masses can mimic an underlying metastatic disease. Careful history and clinical examination recognising polyarthropathy and subcutaneous tophi can aid the clinician to make the right diagnosis and institute correct treatment. Delay in recognising gout as a differential diagnosis can lead to marked morbidity as illustrated in our case.
Keywords: musculoskeletal and joint disorders, metabolic disorders, orthopaedics, degenerative joint disease, drugs: musculoskeletal and joint diseases
Background
Gout is a common metabolic disease associated with the deposition of monosodium urate crystals leading to inflammatory arthritis and eventual tissue damage.1 The prevalence of gout within the UK is estimated at 1.4%, with an annual incidence of gout ranging from 1.19 to 1.80 cases per 1000 persons per year.2 Tophaceous gout is defined as the deposition of urate crystals within joints and periarticular tissues. Typically, gouty arthritis affects the joints of the feet, ankles, knees, fingers, wrist and shoulder.3 Rarely, the condition affects the axial skeleton as well. Tophaceous gout affecting the spine was first described in 1950 by Kersley et al.4
We describe a case of a 71-year-old woman acutely diagnosed with tophaceous gout polyarthritis involving the cervical and lumbar spine.
Case presentation
A 71-year-old woman was referred to the orthopaedic team with concern of a left neck of femur pathological fracture (figure 1). The patient had a 2-week history of worsening severe left hip pain and difficulty in walking. There was a history of low-energy trauma 3 days prior to admission, falling from a chair onto her buttocks; however, she was able to mobilise independently following the fall. The patient had a history of multiple joint pains for >1o years; however, it was managed using analgesics but never had any formal diagnosis. At presentation, the patient had chronic tophaceous deposits involving bilateral hands, although not identified by the admitting team.
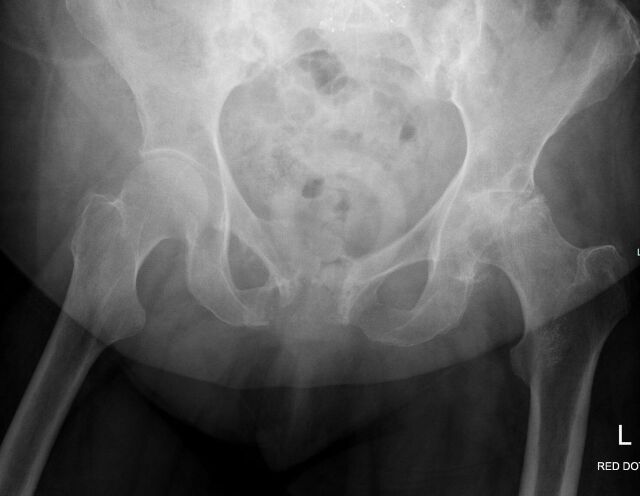
Figure 1.
AP pelvis radiograph demonstrating severe destructive changes of the left femoral head, left acetabulum and pubic symphysis. AP, anteroposterior.
Approximately 4 years prior to this presentation, the patient was admitted with an episode of lumbar discitis confirmed on the MRI. The MRI findings described appearances of osteomyelitis at L4 and L5 vertebrae with discitis at L4/5 and L5/S1, and was associated with an epidural abscess involving the anterior canal from L2 to L5 and posteriorly from T9 to L5. There was no evidence of any organisms in the blood cultures, but she received 6 weeks of intravenous antibiotics under the spinal team resulting in clinical improvement. She was started on diuretics (furosemide) 12 months ago for congestive cardiac failure. Her renal function tests were normal through this period. The patient also had a background of bilateral carpel tunnel decompression for idiopathic carpal tunnel syndrome (unrelated to gout), hypertension, heart valve defect and moderate chronic obstructive pulmonary disease.
On examination, the patient was apyrexial and haemodynamically stable. The patient was able to mobilise independently up to 10 feet with an antalgic gait affecting her left leg and in significant pain. There was evident limb length discrepancy with the left leg approximately 1 cm shorter. Left hip range of movement was significantly reduced within all planes. She demonstrated on neurological examination of her lower limbs mild weakness on left ankle dorsiflexion (Medical Research Council (MRC) grade 4) and gross sensation was intact. Normal knee and ankle reflexes were present. Upper limb examination revealed extensive wasting of the intrinsic muscles of both hands, bilateral volar wrist scars from carpal tunnel decompression and multiple gouty tophi in her hands bilaterally (figures 2 and 3). Grip strength was markedly reduced (MRC grades 2 and 3). Biceps, triceps and wrist extension were all grade 5. Her upper limb reflexes were normal although triceps were brisk bilaterally.
Figure 2.
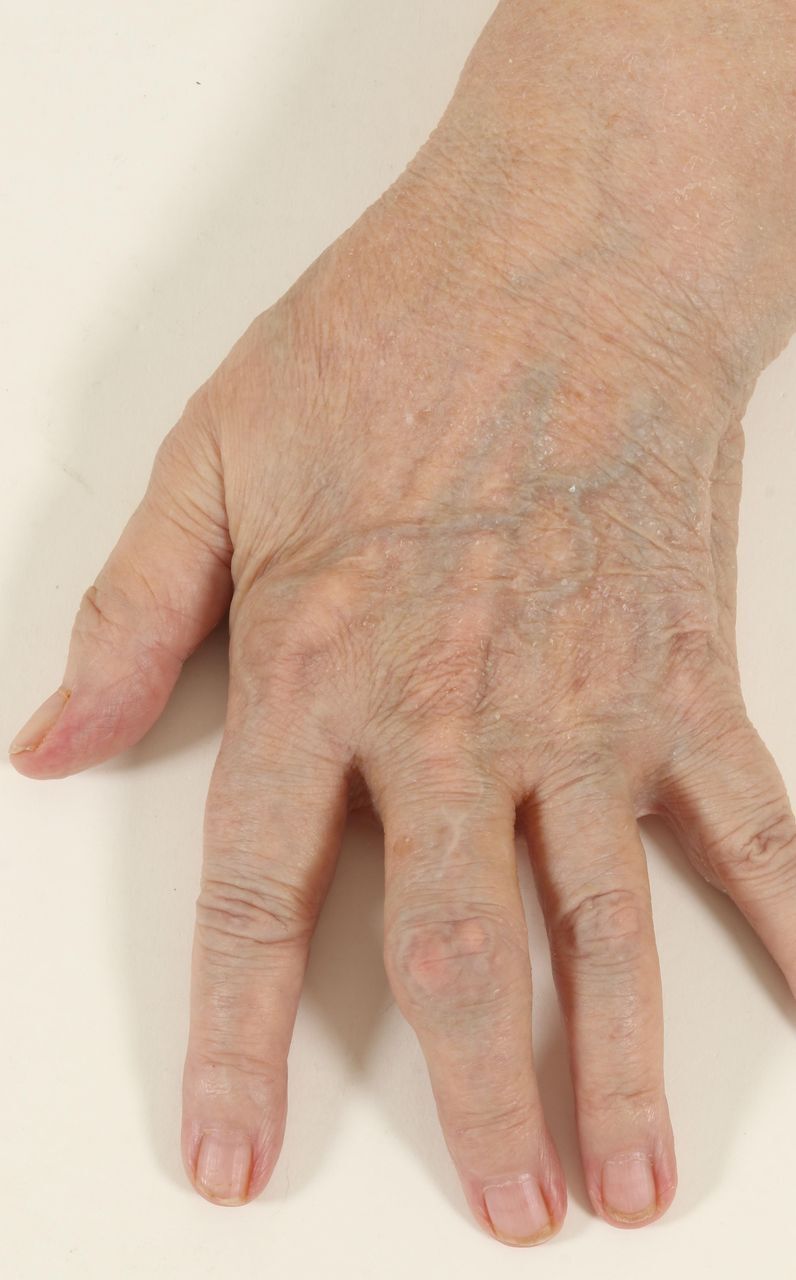
Photographic image of the left hand demonstrating arthritic deformities.
Figure 3.
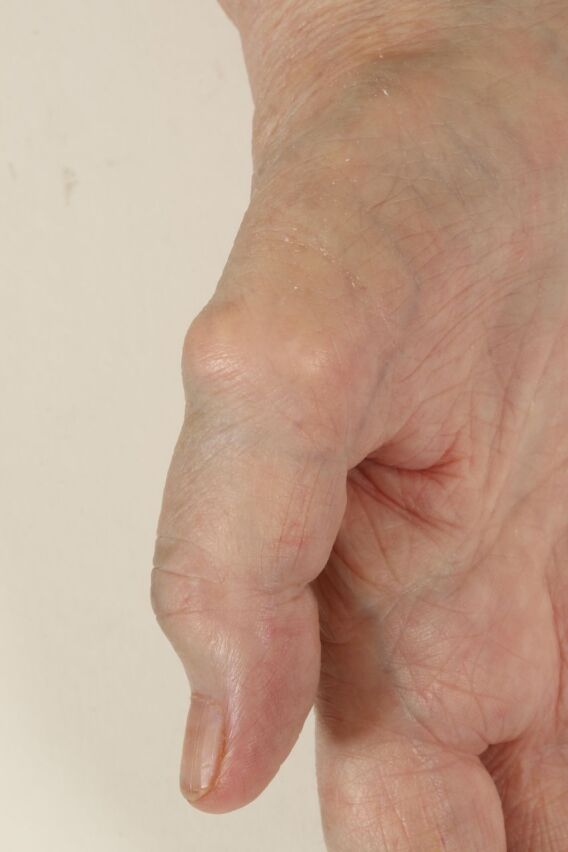
Photographic image of the right thumb demonstrating subcutaneous gouty tophi.
Investigations
Imaging
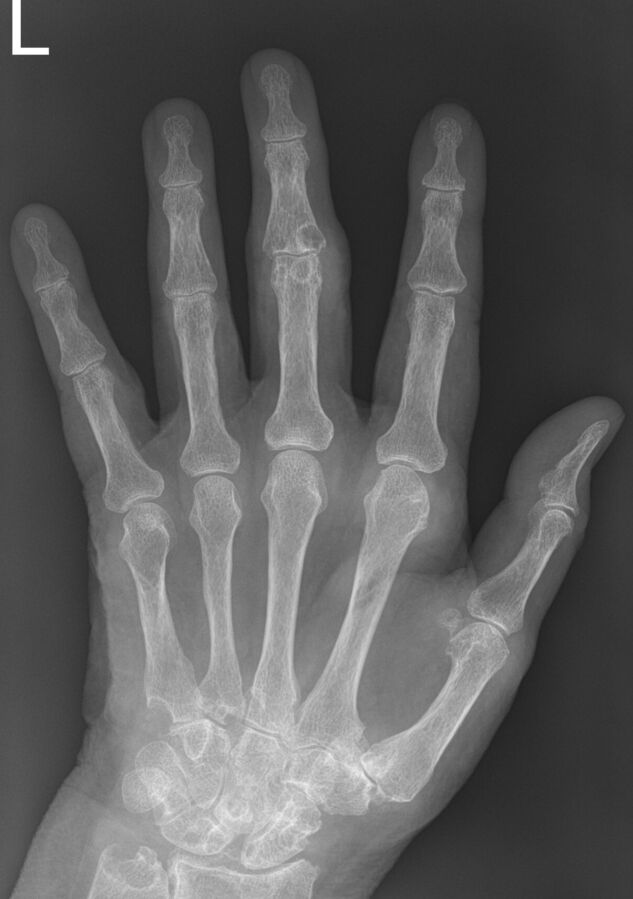
On admission, pelvic X-ray showed severe destructive changes of the left femoral head, left acetabulum and pubic symphysis suggesting a metastatic bone disease and a possible pathological fracture (figure 1). X-rays of bilateral hands demonstrated numerous large erosions of the hands and wrists, with subluxation of the metacarpal–phalangeal joint and loss of joint space within the carpal–metacarpal joints (figures 4–6).
Figure 4.
AP radiograph of the left hand demonstrating left third PIPJ severe destructive changes. AP, anteroposterior; PIPJ, proximal interphalangeal joint.
Figure 5.
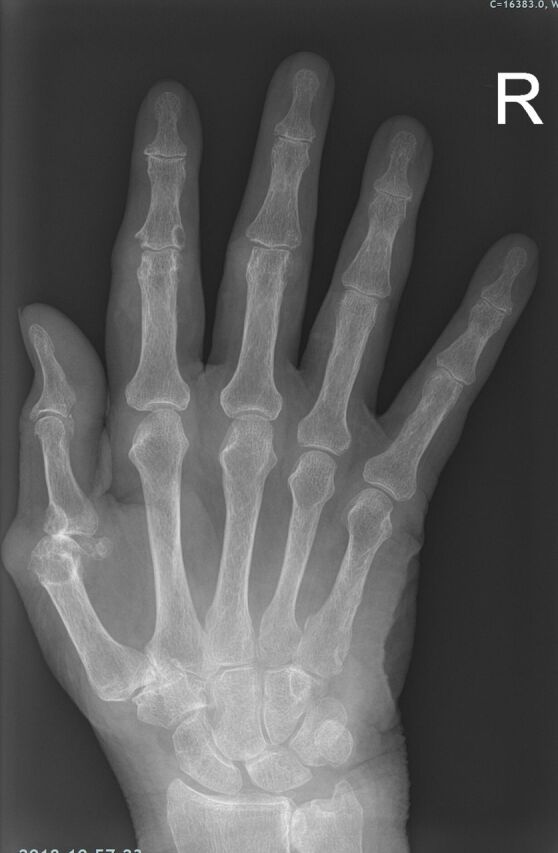
AP radiograph of the right hand demonstrating right first MCPJ destructive changes and subluxation. AP, anteroposterior; MCPJ, metacarpal phalangeal joint.
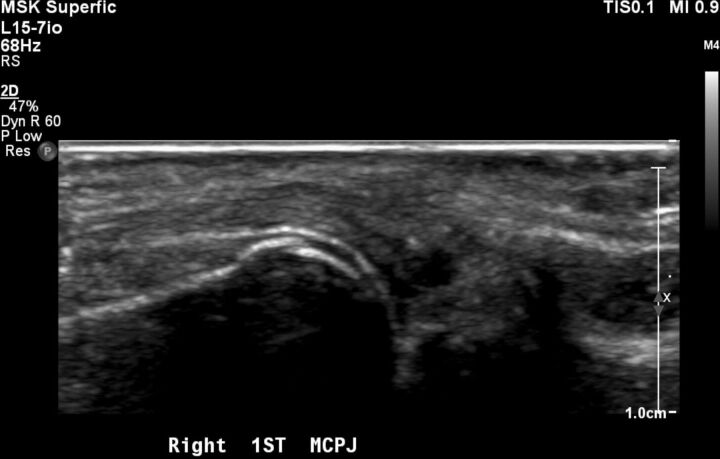
Figure 6.
USS imaging of the right first MCPJ demonstrating subluxation and associated echogenic material. MCPJ, metacarpal phalangeal joint; USS, ultrasound sonography.
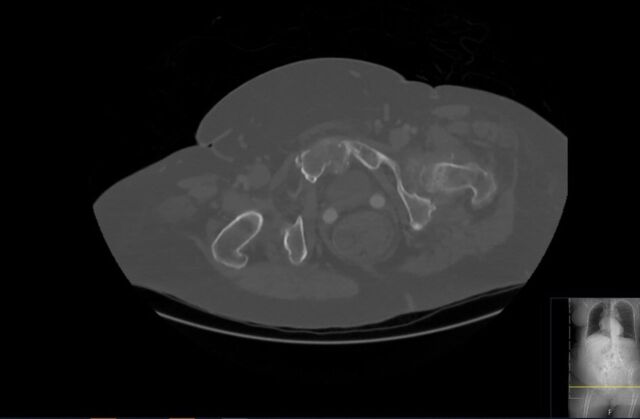
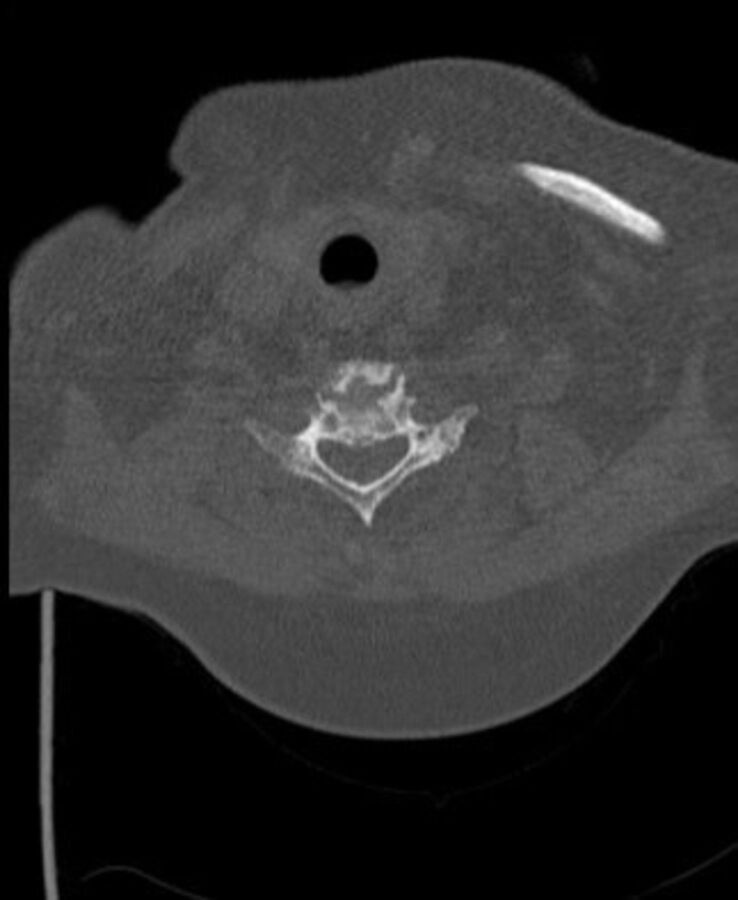
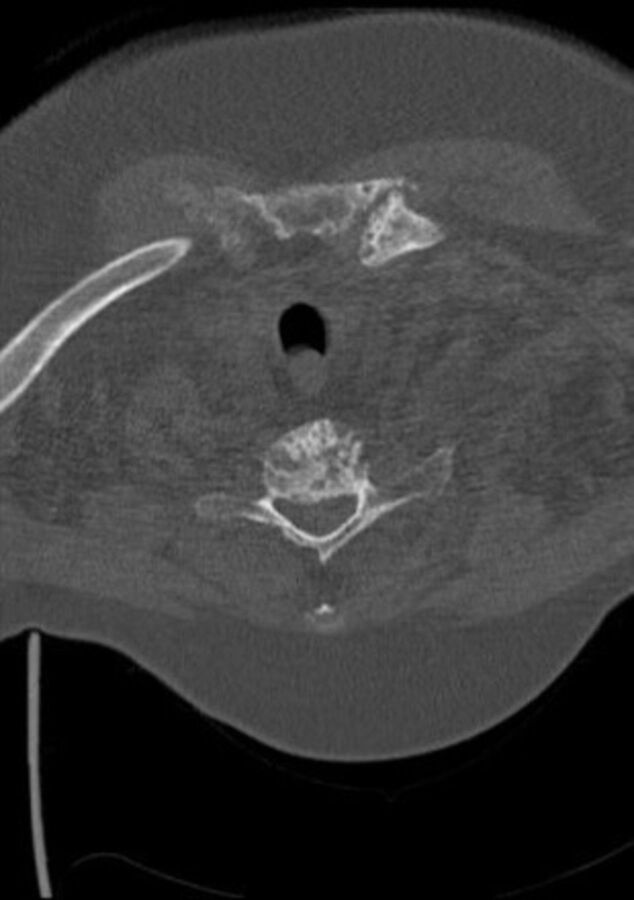
Contrast-enhanced CT of the thorax, abdomen and pelvis performed on admission to rule out malignancy revealed severe erosive arthritis with nodular enhancing synovial thickening of the pubic symphysis (figure 7), left hip, bilateral sacroiliac joints, sternoclavicular joints, shoulder and nodular lesions in the spinal facet joints at multiple levels in the lumbar vertebrae. Enhancing synovial thickening was seen encroaching into the central spinal canal at multiple levels with moderate stenosis at L3/4 level. Erosive changes were also seen in the endplates, mainly at L4/L5 level. Further investigation with cervical spine CT was requested due to clinical signs of cervical myelopathy and identified large gouty tophi affecting C4, C5 and C6 with extensive C4/5 facet joint involvement and narrowing of the thecal sac and suggestive compressive myelopathy (figures 8–10).
Figure 7.
Pelvic CT axial view demonstrating severe erosive arthritis with nodular enhancing synovial thickening of the pubic symphysis.
Figure 8.
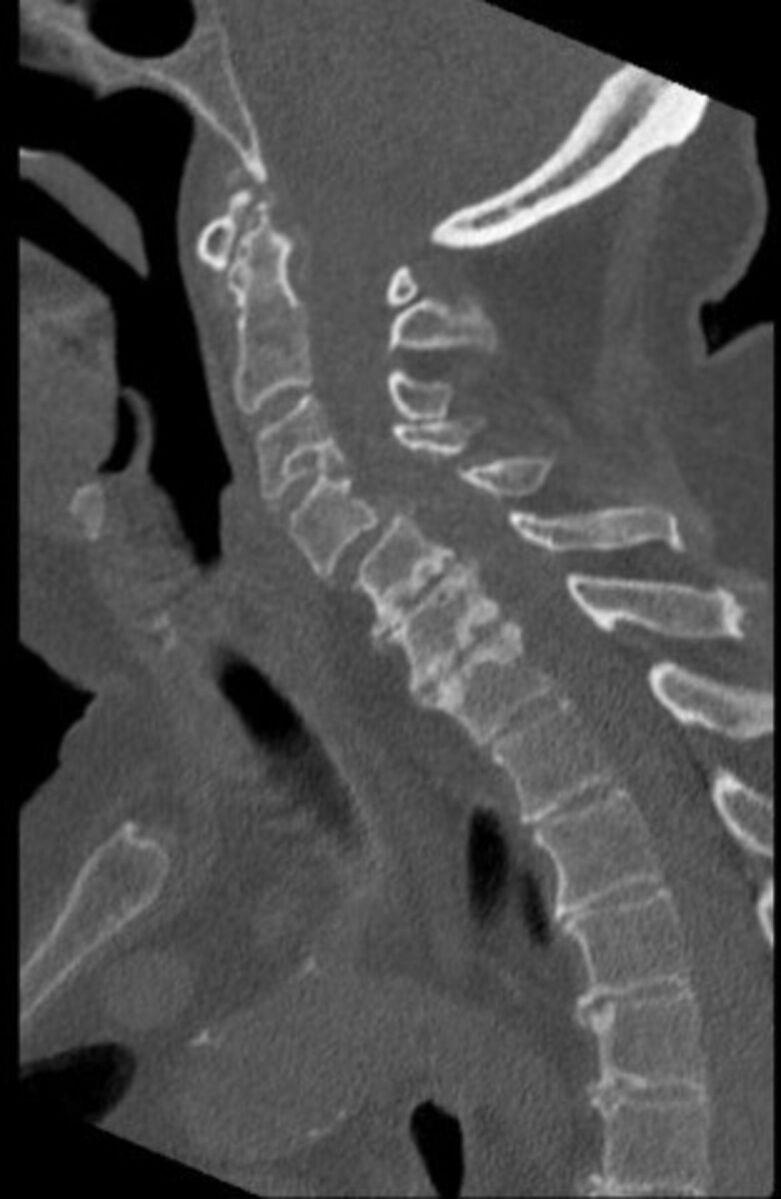
C-spine CT sagital view—demonstrating large gouty tophi affecting C4, C5 and C6 with extensive C4/5 facet joint involvement and narrowing of the thecal sac and suggestive compressive myelopathy.
Figure 9.
C-spine CT axial view at C5 vertebral body superior endplate—demonstrating large gouty tophi affecting C4, C5 and C6 with extensive C4/5 facet joint involvement and narrowing of the thecal sac and suggestive compressive myelopathy.
Figure 10.
C-spine CT axial view at C5 vertebral body inferior endplate—demonstrating large gouty tophi affecting C4, C5 and C6 with extensive C4/5 facet joint involvement and narrowing of the thecal sac and suggestive compressive myelopathy.
Ultrasound sonography (USS) examination of the right shoulder, pubic symphysis, left hip and bilateral hands confirmed similar radiographic changes suggestive of gouty polyarthritis as follows. Echogenic material with punctuated hyperechoic areas were seen within the right acromioclavicular joint and sternoclavicular joint. USS confirmed a destructive heterogenous process involving the pubic symphysis and an effusion with echogenic material within the left hip joint. Coarse hyperechoic material was seen on the superficial aspect of the cartilage of the right first metacarpal with an associated echogenic material involving the metacarpal phalangeal joint on USS (figure 6).
Blood and histological results
Notable blood results included elevated C reactive protein 114 mg/L, alkaline phosphatase level 152 IU/L and white cell count 9.8×109/L. The serum urate level was >600 mmol/dL. The overall clinical appearance was consistent with chronic tophaceous gout and USS-guided biopsy of the right acromioclavicular joint and sternoclavicular joint were performed to confirm the diagnosis. These biopsy sites were preferred to the left hip by the consultant radiologist due to ease of accessibility. Histological analysis confirmed deposition of monosodium urate crystals.
Treatment
The patient was treated conservatively with colchicine 500 μg two times per day and prednisolone 40 mg once a day for 5 days, resulting in improvement of her upper limb paresthesia and joint pain. Urate lowering drug therapy with allopurinol 100 mg once a day was commenced at 3 weeks post presentation and after the resolution of the acute attack, and the patient was transferred to a rehabilitation facility.
Outcome and follow-up
Results from follow-up under the rheumatology team showed that the patient had noticed an improvement in her hand function following treatment, although little improvement in her mobility. Repeat serum urate level was 479 mmol/dL, therefore the patient was started on an increasing regime of allopurinol and kept under close surveillance using a treat-to-target approach aiming for serum uric acid level <300 µmol/L.5
The patient was also seen as an outpatient by multiple spinal surgeons and discussed at the Tertiary Spinal Radiology Multidisciplinary Team Meeting who were in agreement that this patient was unlikely to benefit from cervical surgery due to the chronicity of symptoms and given the patient comorbidities the risks of surgical intervention greatly outweighed the benefits, thus to continue conservative management. Surgical intervention would require extensive laminectomy at C4/5 and resection of the facet joints with multilevel instrumented fusion from the cervical to the thoracic spine. The patient following 12 months of conservative management for her left hip destructive arthritis is currently on the orthopaedic waiting list for a left hip replacement.
Discussion
Although gout frequently affects the peripheral joints and kidneys, spinal involvement is rarely reported. The prevalence of spinal gout is likely greatly underestimated, as to date, available information is limited to case reports and a few cross-sectional studies by Konatalpalli et al in 20096 and 20127 and Jin et al.8 Konatalpalli et al retrospectively analysed 630 patients records (single centre) with a diagnosis of peripheral gout or gouty arthritis, of which 92 patients had a clinical or crystal-proven gout. Of the 92 patients, 64 patients had CT images of the spine performed for various medical indications. These CT images were retrospectively analysed for evidence of axial involvement of tophaceous gout, 9 of the 64 patients (14%) demonstrated radiographic changes suggestive of axial gout, although axial gout was only diagnosed clinically in one of these patients.6 In 2012, Konatalpalli et al analysed 48 patients with a diagnosis of gouty arthritis for at least 3 years, including CT imaging of the spine during the study. 35% of their patients had CT evidence of spinal erosions and/or tophi.7 More recently, Jin et al8 reported the frequency of axial deposition in patients with gout and spinal symptoms was 15.8% in their retrospective review of 95 patients presenting to their spinal centre with the diagnosis of gout and CT imaging of the vertebral column.
As demonstrated in this case report, tophaceous gout can involve multiple anatomical sites within the spine. Specifically, vertebral erosions have been most commonly reported at the discovertebral junction, facet joints, pedicles, and lamina, and a combination of tophi deposits and erosions at the vertebral body, epidural space, ligamentum flavum and pars interarticularis.6 9–13 The highest incidence of spinal involvement of tophaceous gout is within the lumbar and cervical vertebrae, 44% and 39% respectively, in comparison with only 17% of cases within the thoracic vertebrae.14
The clinical manifestation of spinal involvement of gout varies considerably, from asymptomatic to presenting symptoms ranging from isolated back pain, to myelopathy and radiculopathy, and urinary retention.15 Konatalpalli et al7 reported extremity radiographs of peripheral gouty arthropathy were strongly correlated in those patients with CT evidence of axial gout (n=17, p<0.001) and all patients with CT-identified spinal tophi had gout arthropathy radiographic changes in the hand and or feet.
When spinal gout presents with back pain, fever and elevated C reactive protein levels, the clinician is presented with difficulty of differentiating between acute spinal infection and spina gout.16–18 The hypermetabolic nature of presentation of spinal gout mimics infective, inflammatory and neoplastic aetiologies such as osteomyelitis, spondylodiscitis, abscesses and metastases, further complicating an accurate diagnosis.9 19 Similar to presentations of peripheral acute gout arthropathy, normouricemia does not exclude spinal gout, as multiple cases of spinal gout have been recorded with normal uric acid levels.6 To avoid inappropriate treatment with extended intravenous antibiotic therapy, spinal gout should be considered as a differential diagnosis. In particular, this applies to those with negative blood cultures and characteristic features suspicious of spinal gout on MRI. Ultimately in patients with suspected spinal infection or spinal gout, biopsy and inclusion of cultures is the gold standard for definitive diagnosis.
On MRI, tophaceous gout is demonstrated on T1-weighted images as a homogenous image of intermediate-to-low signal intensity and conversely with T2-weighted images tophaceous gout can vary from homogenous with low and high intensities.20 MRI with gadalolinium-based contrast material causes the tophi to demonstrate marginal enhancement, either homogeneous or heterogenous, thought to be the result of reactive vascularisation of inflammatory fibrous tissue stimulated by urate crystal deposition.20
Similar to peripheral gout arthropathy, medical therapeutic treatment during the acute attack including non-steroidal anti-inflammatory drugs (NSAIDs), colchicine and steroids, followed by urate lowering therapy (ULT) using allopurinol alone are effective in treating spinal tophaceous gout.11 14 15 21 22 The aim of ULT like allopurinol is to reduce and maintain the serum uric acid at or below a target level of 300 µmol/L to prevent gouty attacks. The lower the serum urate level, the greater the velocity of crystal elimination.5 Once the attack has settled and tophi has reduced in size, the dose of allopurinol can be titrated down. These patients with stable gout require annual follow-up to make sure that they remain in clinical remission.5
Adding colchicine 500 μg two times a day as a prophylaxis to reduce the flare up while on allopurinol is an accepted treatment strategy.23 If the response is poor, other options like febuxostat or uricosuric drugs can be tried. In the UK, the National Institute for Health and Care Excellence(NICE) have recommended the use of febuxostat if the patient cannot tolerate allopurinol, while the Scottish Medicines Consortium accepts febuxostat as a suitable second-line ULT when treatment with allopurinol is inadequate, not tolerated, or contraindicated.24 25
Serial MRI scans premedical and postmedical therapeutic treatment for spinal tophaceous gout have significant regression of the disease.26 27 Surgical decompression such as laminectomy±stabilisation, particularly those patients with neurological compromise, shows good prognosis.22 However, pharmacological treatment must be used in combination as the definitive treatment of the disease opposed to immediate symptomatic relief.
Conclusion
Gout can affect any joints in the body, however, the axial skeleton involvement is often underreported and unrecognised. Spinal gout can cause radiculopathy, cord compression, canal stenosis and discitis. Such extensive involvement is rare and the masses can mimic an underlying metastatic disease. Patients can also present with symptoms suggestive of discitis. Careful history and clinical examination recognising polyarthropathy and subcutaneous tophi can aid the clinician to make the right diagnosis and institute correct treatment. Diagnostic biopsy sample should be sent to the lab urgently using absolute alcohol as the fixative. Delay in recognising gout as a differential diagnosis can lead to marked morbidity as illustrated in our case.
Learning points.
Although gout commonly affects joints throughout the body, axial skeleton involvement is often underreported and unrecognised.
Spinal gout can cause radiculopathy, cord compression, canal stenosis and discitis. Such extensive involvement is rare and the masses can mimic an underlying metastatic disease.
Careful history and clinical examination recognising polyarthropathy and subcutaneous tophi can aid the clinician to make the right diagnosis and institute correct treatment.
Delay in recognising gout as a differential diagnosis can lead to marked morbidity as illustrated in our case.
Footnotes
Contributors: All authors discussed, reviewed and contributed to the final manuscript. JWT: completed the literature review and wrote the manuscript with support from SS and DM.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Finch A. Kubler Paul. the management of gout. Aust Prescr 2016;39:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.No listed authors . Gout | Health topics A to Z | CKS | NICE [Internet], 2018. Available: https://cks.nice.org.uk/topics/gout/ [Accessed cited 2020 Feb 20].
- 3.Thissen CACB, Frank J, Lucker GPH. Tophi as first clinical sign of gout. Int J Dermatol 2008;47 Suppl 1:49–51. 10.1111/j.1365-4632.2008.03961.x [DOI] [PubMed] [Google Scholar]
- 4.Kersley GD, Mandel L, Jeffrey MR. Gout: an unusual case with softening and subluxation of the first cervical vertebra and splenomegaly result of ACTH administration and eventual post-mortem findings. Ann Rheum Dis 1950;9:282–304. 10.1136/ard.9.4.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui M, Carr A, Cameron S, et al. The British Society for rheumatology guideline for the management of gout. Rheumatology 2017;56:1056–9. 10.1093/rheumatology/kex150 [DOI] [PubMed] [Google Scholar]
- 6.Konatalapalli RM, Demarco PJ, Jelinek JS, et al. Gout in the axial skeleton. J Rheumatol 2009;36:609–13. 10.3899/jrheum.080374 [DOI] [PubMed] [Google Scholar]
- 7.Konatalapalli RM, Lumezanu E, Jelinek JS, et al. Correlates of axial gout: a cross-sectional study. J Rheumatol 2012;39:1445–9. 10.3899/jrheum.111517 [DOI] [PubMed] [Google Scholar]
- 8.Jin H-J, Son E-S, Kim DH. The frequency of axial deposition in Korean patients with gout at a tertiary spine center. Front Med 2020;7:339. 10.3389/fmed.2020.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasturk AE, Basmaci M, Canbay S, et al. Spinal gout tophus: a very rare cause of radiculopathy. Eur Spine J 2012;21:400–3. 10.1007/s00586-011-1847-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dharmadhikari R, Dildey P, Hide IG. A rare cause of spinal cord compression: imaging appearances of gout of the cervical spine. Skeletal Radiol 2006;35:942–5. 10.1007/s00256-006-0088-2 [DOI] [PubMed] [Google Scholar]
- 11.Chan ATS, Leung JLY, Sy ANL, et al. Thoracic spinal gout mimicking metastasis. Hong Kong Med J 2009;15:143–5. [PubMed] [Google Scholar]
- 12.Cabot J, Mosel L, Kong A, et al. Tophaceous gout in the cervical spine. Skeletal Radiol 2005;34:803–6. 10.1007/s00256-005-0920-0 [DOI] [PubMed] [Google Scholar]
- 13.Sanmillan Blasco JL, Vidal Sarro N, Marnov A, et al. Cervical cord compression due to intradiscal gouty tophus: brief report. Spine 2012;37:E1534–6. 10.1097/BRS.0b013e31826f2886 [DOI] [PubMed] [Google Scholar]
- 14.King JC, Nicholas C. Gouty arthropathy of the lumbar spine: a case report and review of the literature. Spine 1997;22:2309–12. 10.1097/00007632-199710010-00023 [DOI] [PubMed] [Google Scholar]
- 15.Zheng Z-F, Shi H-L, Xing Y, et al. Thoracic cord compression due to ligamentum flavum gouty tophus: a case report and literature review. Spinal Cord 2015;53:881–6. 10.1038/sc.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doherty M. New insights into the epidemiology of gout. Rheumatology 2009;48:ii2–8. 10.1093/rheumatology/kep086 [DOI] [PubMed] [Google Scholar]
- 17.Hall AP, Barry PE, Dawber TR, et al. Epidemiology of gout and hyperuricemia. Am J Med 1967;42:27–37. 10.1016/0002-9343(67)90004-6 [DOI] [PubMed] [Google Scholar]
- 18.Wallace KL, Riedel AA, Joseph-Ridge N, et al. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 2004;31:1582–7. [PubMed] [Google Scholar]
- 19.Saketkoo LA, Robertson HJ, Dyer HR, et al. Axial gouty arthropathy. Am J Med Sci 2009;338:140–6. 10.1097/MAJ.0b013e3181a3dc14 [DOI] [PubMed] [Google Scholar]
- 20.Hsu C-Y, Shih TT-F, Huang K-M, et al. Tophaceous gout of the spine: MR imaging features. Clin Radiol 2002;57:919–25. 10.1053/crad.2001.1001 [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa EM, de Mello FM, Goldenstein-Schainberg C, et al. Gota axial. Rev Bras Reumatol 2013;53:296–302. 10.1590/S0482-50042013000300008 [DOI] [PubMed] [Google Scholar]
- 22.Wendling D, Prati C, Hoen B, et al. When gout involves the spine: five patients including two inaugural cases. Joint Bone Spine 2013;80:656–9. 10.1016/j.jbspin.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 23.Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004;31:2429–32. [PubMed] [Google Scholar]
- 24.No listed authors . febuxostat (Adenuric) [Internet]. Scottish Medicines Consortium. Available: https://www.scottishmedicines.org.uk/medicines-advice/febuxostat-adenuric-fullsubmission-63710/ [Accessed cited 2021 Jan 1].
- 25.No listed authors . Overview | Febuxostat for the management of hyperuricaemia in people with gout | Guidance | NICE [Internet], 2021. Available: https://www.nice.org.uk/Guidance/TA164
- 26.Dhôte R, Roux FX, Bachmeyer C, et al. Extradural spinal tophaceous gout: evolution with medical treatment. Clin Exp Rheumatol 1997;15:421–3. [PubMed] [Google Scholar]
- 27.Draganescu M, Leventhal LJ. Spinal gout: case report and review of the literature. J Clin Rheumatol 2004;10:74–9. 10.1097/01.rhu.0000120898.82192.f4 [DOI] [PubMed] [Google Scholar]