Abstract
Two independent cohorts (N=155, N=126) of infants at high and low risk for autism spectrum disorder (ASD) were followed prospectively between 6 and 36 months of age, when n=46 were diagnosed with ASD. Gaze to adult faces was coded – during a developmental assessment (Cohort 1) or a play interaction (Cohort 2). Across both cohorts, most children developing ASD showed sharp declines in gaze to faces over time, relative to children without ASD. These findings suggest that declining developmental trajectories may be more common than previously recognized by retrospective methods. Trajectory-based screening methods could potentially identify children in the early stages of symptom onset and allow for early intervention before the full disorder has developed.
Keywords: gaze to faces, developmental trajectories, autism spectrum disorder
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by persistent impairments in social communication and interaction and by the presence of restricted repetitive patterns of behavior, interests, and activities (American Psychiatric Association, 2013; World Health Organization, 2018). Symptoms begin to be evident in the first two years of life, with a variable time course of onset (Ozonoff & Iosif, 2019; Pearson, Charman, Happé, Bolton, & McEwen, 2018). The most common procedure to collect ASD onset information is retrospective parent reports. Although efficient and cost-effective, this method contains several limitations concerning validity such as recall problems and other biases (Barger, Campbell, & McDonough, 2013; Boterberg, Charman, Marschik, Bölte, & Roeyers, 2019; Hus, Taylor, & Lord, 2011; Lord, Shulman, & DiLavore, 2004; Ozonoff, Li, Deprey, Hanzel, & Iosif, 2018). Another retrospective method which reduces several of the reporting biases of parent reports is the analysis of home movies of children later diagnosed with ASD (Dawson, 2011; Goldberg, Thorsen, Osann, & Spence, 2008; Palomo, Belinchón, & Ozonoff, 2006). However, this labor-intensive method is also subject to several issues such as the broad variability in the amount, content, and representativeness of the movies available. Therefore, studies of when and how behavioral signs of ASD unfold benefit from the use of prospective procedures to reduce these limitations, in which the development of infants is followed longitudinally until the age at which diagnosis can be confirmed for most children (Ozonoff & Iosif, 2019; Pearson et al., 2018). Most prospective studies compare the development of siblings with an older brother or sister with ASD (high-risk siblings [HR-sibs]), who are at increased risk of receiving an ASD diagnosis, with low-risk siblings (LR-sibs) with no known family history of ASD (Ozonoff et al., 2011). A comprehensive review of the advantages and challenges of the infant sibling approach can be found in several review papers (Bölte et al., 2013; E. J. H. Jones, Gliga, Bedford, Charman, & Johnson, 2014; Pearson et al., 2018; Szatmari et al., 2016).
Prospective analysis of social-communication behaviors, many of which develop before spoken language and are already reliably present early in infancy, may provide a more sensitive measurement of early symptom onset. One method that has been used to quantify longitudinal rates of key social-communication behaviors is manual moment-by-moment coding of prospectively-captured video segments. This method may be able to detect earlier—between 6 and 18 months—and more gradual, subtle declines in social-communication/social engagement skills that are less easily captured in real-time observations by parents or by standardized test instruments (Ozonoff, Gangi, et al., 2018; Ozonoff et al., 2010).
To test hypotheses about early declining developmental trajectories in ASD in the first year(s) of life, some of the most relevant preverbal social behaviors are gaze to faces and eyes of others, shared affect, response to name, and social interest or engagement (Inada, Kamio, & Koyama, 2010). Two recent review papers on onset patterns in ASD (Ozonoff & Iosif, 2019; Pearson et al., 2018) concluded that different studies using a variety of prospective methods reported largely intact, typical early development in infants between 2 and 6 months of age, followed by developmental declines and onset of symptoms around the first birthday and in the second year of life. Declines in language, social communication, and cognitive skills in the first years of life were first described by qualitative prospective case studies of HR-sibs who developed ASD (Bryson et al., 2007; Dawson, Osterling, Meltzoff, & Kuhl, 2000; Klin et al., 2004). More recently, several prospective studies including larger numbers of siblings have shown that children with ASD demonstrate declines in gaze to faces, social smiling, directed vocalizations and social engagement (Ozonoff, Gangi, et al., 2018; Ozonoff et al., 2010), eye fixation (W. Jones & Klin, 2013), gestures and shared positive affect (Landa, Gross, Stuart, & Faherty, 2013; Landa, Holman, & Garrett-Mayer, 2007), and response to name (Miller et al., 2017) between 6 and 36 months. These declines are not seen in other samples with elevated genetic risk or other developmental concerns (Ozonoff & Iosif, 2019).
Prior work by Ozonoff et al. (2010) examined social-communication behavior prospectively in a group of 25 children with ASD and 25 low-risk children without ASD. At the age of 6 months, the ASD group showed similar frequencies of coded gaze to faces, social smiling, and directed vocalizations, as well as examiner ratings of social engagement, compared to the LR non-ASD group. However, these behaviors dramatically decreased over time in the ASD group between 6 and 36 months (Ozonoff et al., 2010). Similar patterns of declining trajectories in children with ASD were also found using parent ratings of social engagement that were collected prospectively (Ozonoff, Gangi, et al., 2018). On an individual level, the majority of children in the ASD group (i.e., 69% to 88%) were classified as having developmental trajectories of declining social engagement. These studies suggest that this declining developmental pattern may be the norm for social communication in children with ASD when behaviors are studied prospectively from infancy.
To date, the prospective studies demonstrating declining developmental trajectories have used a variety of different methods to study a variety of specific social-communicative behaviors; none of these methods and findings have yet been replicated. Previous studies have also assessed behaviors in only one context—no study has evaluated the same social-communicative behavior across settings. The present prospective investigation is a confirmatory study that aims to replicate and expand previous results (Ozonoff et al., 2010) on the timing and onset of early social-communication skills. We chose to focus on gaze to faces because this behavior showed the largest declines in prior work (Ozonoff et al., 2010), gaze to faces has been examined in other trajectory-based studies (W. Jones & Klin, 2013), and eye contact is an early-reported core deficit in ASD symptomatology. We employed two independent samples of infants with and without an older sibling with ASD, examining gaze to faces across two interactive contexts with an examiner. We expected to find 1) declining trajectories of gaze to faces in the children with ASD in both samples and contexts and 2) group differences in gaze to faces between children with and without ASD, with the ASD group exhibiting lower levels of gaze to faces by 12 months.
Method
Participants
Participants were infant siblings of children with ASD (high-risk siblings) or without ASD (low-risk siblings), who were part of larger longitudinal studies. High-risk infants had at least one older sibling with ASD, confirmed using the ASD criteria on both the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) and the Social Communication Questionnaire (SCQ). Low-risk infants had older sibling(s) who were typically developing and no family history of ASD, confirmed by an Intake Screening Questionnaire and scores below the validated cutoffs for ASD on the SCQ, a widely-used parent report measure with satisfactory psychometric properties (e.g., 60–92% sensitivity and 52–92% specificity for distinguishing ASD vs. non-ASD across studies; Norris & Lecavalier, 2010). Inclusion and exclusion criteria were identical for both cohorts. Exclusion criteria for the high-risk group were a genetic disorder in the older sibling with ASD and birth before 32 weeks gestation. Exclusion criteria for the low-risk group were birth before 36 weeks gestation, genetic, developmental, or learning conditions in an older sibling, and ASD in a first-, second-, or third-degree relative. Exclusion criteria for gestational age differed between recruitment groups to maximize recruitment of high-risk families without increasing risk for developmental concerns in the low-risk group (only n = 10, or 5.5% of the high-risk participants, had gestational ages between 32 and 35 weeks). Race/ethnicity did not differ between Cohort 1 (non-Hispanic Caucasian 52%, Hispanic 9%, non-white or multiracial 32%) and Cohort 2 (non-Hispanic Caucasian 51%, Hispanic 8%, non-white or multiracial 39%), and maternal education did not differ between Cohort 1 (no college degree 34%, college degree or higher 63%) and Cohort 2 (no college degree 33%, college degree or higher 68%). Parents provided informed consent and the study was approved by the university’s Institutional Review Board.
Data were collected at up to seven ages (within two weeks of visit age) in two independent cohorts (hereafter, Cohort 1 and Cohort 2; see Table 1 for number of participants with data at each age). Participants were included in the current study if they had 36-month outcome data and gaze data at any age. Cohort 1 had gaze data from a maximum of 7 study visits at 6, 9, 12, 15, 18, 24, and 36 months (n = 62 with 7 visits, n = 55 with 6, n = 28 with 5, n = 7 with 4, and n = 3 with 3), and Cohort 2 had gaze data from a maximum of 5 study visits at 6, 12, 18, 24, and 36 months (n = 51 with 5 visits, n = 44 with 4, n = 20 with 3, n = 8 with 2, and n = 3 with 1). There were no significant differences in sex, cohort, risk group, outcome group, and gaze to faces between children with or without missing data or as a function of number of missing visits.
Table 1.
Group characteristics and mean levels of gaze to faces rate per minute by Cohort and outcome group.
| ASD group |
Non-TD group |
TD group |
||||
| Group characteristics | n | M (SD) | n | M (SD) | n | M (SD) |
| Cohort 1 | ||||||
| ADOS-2 Calibrated Severity Score | 20 | 6.8 (1.6) | 55 | 2.4 (0.9) | 80 | 1.1 (0.3) |
| Mullen Visual Reception t-score | 19 | 44.2 (16.6) | 55 | 55.5 (15.7) | 80 | 63.5 (10.2) |
| Mullen Fine Motor t-score | 20 | 37.9 (13.3) | 55 | 44.2 (14.0) | 80 | 51.5 (9.4) |
| Mullen Receptive Language t-score | 16 | 38.6 (9.5) | 54 | 47.3 (9.2) | 79 | 52.6 (8.7) |
| Mullen Expressive Language t-score | 18 | 37.8 (11.4) | 54 | 49.3 (8.9) | 80 | 54.5 (7.7) |
| Cohort 2 | ||||||
| ADOS-2 Calibrated Severity Score | 25 | 6.6 (2.1) | 33 | 2.5 (1.0) | 66 | 1.1 (0.2) |
| Mullen Visual Reception t-score | 24 | 37.5 (14.3) | 33 | 52.9 (11.5) | 66 | 60.7 (11.1) |
| Mullen Fine Motor t-score | 24 | 30.6 (11.2) | 33 | 40.5 (8.0) | 66 | 51.5 (11.7) |
| Mullen Receptive Language t-score | 19 | 38.1 (10.8) | 33 | 44.9 (7.9) | 66 | 52.5 (7.5) |
| Mullen Expressive Language t-score | 22 | 38.1 (13.0) | 33 | 49.5 (7.2) | 66 | 55.4 (7.2) |
| Gaze to faces rate per minute | n | M (SD) | n | M (SD) | n | M (SD) |
| Cohort 1 | ||||||
| 6 months | 15 | 2.8 (1.1) | 39 | 3.6 (1.5) | 46 | 3.7 (1.6) |
| 9 months | 20 | 3.2 (1.7) | 52 | 4.3 (1.9) | 70 | 4.6 (1.9) |
| 12 months | 19 | 2.6 (2.2) | 51 | 2.6 (1.3) | 74 | 3.4 (1.8) |
| 15 months | 17 | 2.6 (1.9) | 49 | 3.8 (2.1) | 71 | 4.0 (1.9) |
| 18 months | 20 | 1.6 (0.8) | 49 | 2.6 (1.5) | 76 | 3.2 (1.6) |
| 24 months | 19 | 1.5 (1.3) | 50 | 2.4 (1.5) | 70 | 2.6 (1.6) |
| 36 months | 19 | 1.4 (0.8) | 46 | 3.4 (2.2) | 69 | 3.7 (2.2) |
| Cohort 2 | ||||||
| 6 months | 16 | 4.9 (2.0) | 17 | 5.0 (2.1) | 45 | 5.8 (2.1) |
| 12 months | 24 | 6.1 (3.1) | 31 | 7.5 (2.7) | 64 | 7.4 (2.8) |
| 18 months | 20 | 2.8 (1.7) | 28 | 6.4 (3.0) | 60 | 5.9 (2.9) |
| 24 months | 18 | 2.2 (1.6) | 26 | 5.5 (3.1) | 47 | 6.4 (3.1) |
| 36 months | 21 | 3.4 (2.0) | 31 | 4.8 (2.3) | 62 | 6.8 (3.1) |
Note. ASD = Autism Spectrum Disorder, TD = Typically Developing, Non-TD = Non-Typically Developing, M = mean, SD = standard deviation. ADOS-2 and Mullen scores are reported at outcome (36 months)
ASD outcome was determined at the 36-month visit for all participants, who were classified as either ASD or no ASD by an expert examiner based on all clinical information collected. The ASD group met DSM-5 criteria for autism spectrum disorder and scored above the ASD cutoff on the ADOS-2 and the Autism Diagnostic Interview-Revised (ADI-R; Rutter, LeCouteur, & Lord, 2003). Participants without an ASD diagnosis at 36 months were further classified into one of two outcome groups based on ADOS-2 and Mullen Scales of Early Learning (a developmental assessment) scores, using criteria previously developed by the Baby Siblings Research Consortium (Ozonoff et al., 2014). The non-typically developing (Non-TD) group did not meet criteria for ASD and had ADOS-2 scores ≤3 points below the ASD cutoff and/or two or more Mullen subscale t-scores ≥1.5 standard deviations (SD) below mean and/or one or more Mullen subscale scores ≥2 SD below mean. The Typically Developing (TD) group had ADOS-2 scores >3 points below the ASD cutoff and no more than 1 Mullen subscale score ≥1.5 SD below mean and no Mullen subscale score ≥2 SD below mean. In Cohort 1, 20 children were in the ASD group (15 male, 19 HR-sibs), 55 in the Non-TD group (33 male, 37 HR-sibs), and 80 in the TD group (47 male, 38 HR-sibs). In Cohort 2, 26 children were in the ASD group (16 male, 26 HR-sibs), 34 in the Non-TD group (19 male, 25 HR-sibs), and 66 in the TD group (30 male, 38 HR-sibs). Group characteristics for Cohort 1 and Cohort 2 are presented in Table 1.
Measures
Mullen Scales of Early Learning (Mullen, 1995).
The Mullen Scales of Early Learning, a standardized developmental test for children birth to 68 months, was administered to all participants at each visit to measure developmental progress. Participants were seated on the parent’s lap at a table and the examiner was seated in a chair on the opposite side of the table facing the child. Items used for Mullen administration were kept out of the child’s sight and brought out individually for each task. Behavioral coding was conducted for a portion of the Mullen for Cohort 1.
Play Interaction.
In Cohort 2, children participated in a play interaction with the examiner at each study visit. Participants were seated in a high chair fitted with a tray and the examiner was seated in a chair facing the child. During this activity, the parent was seated in a chair behind and to the side of the examiner and asked to read or complete paperwork so as not to interact with the child. Dyads were provided with a standard set of age-appropriate toys in a bin, which was placed on a smaller chair next to the dyad. The toys included a doll, baby bottle, small blanket, car, toy key ring, shape sorter, small ball, rattle, and a pair of toy phones. Examiners were instructed to play naturally for 3 minutes and to follow the child’s lead if she requested a specific toy. Dyads played with the toys together for 3 minutes. Behavioral coding was conducted for the play interaction for Cohort 2.
ADOS-2.
The ADOS-2 is a semi-structured play-based interaction and observation designed to assess symptoms of ASD. The ADOS-2 was administered by research-reliable examiners and used in the determination of outcome at 36 months of age.
Coding
Gaze to Faces.
Gaze to faces was examined in two contexts: in Cohort 1 during administration of the Mullen Scales of Early Learning and in Cohort 2 during play interaction with an examiner. For both contexts, the room was set up to minimize distractions (e.g., posters removed from the walls, items not in use kept out of sight). Two cameras recorded each context, with the infant and examiner positioned to maximize the camera’s view of the faces. Infant gaze was coded during the first 6 minutes of the Mullen Visual Reception subtest (Cohort 1; M = 5.96 minutes) and during the 3-minute play interaction (Cohort 2; M = 2.95 minutes). Mean video length did not differ by outcome group for either cohort, ps > .80. Gaze to the examiner’s face was coded from recorded video using the same coding system and procedures outlined in Ozonoff et al. (2010), using Noldus: the Observer 5.0 behavioral observation software. Gaze to the examiner’s face was coded when an infant’s face was oriented to the face of the examiner, and this code was terminated when the infant’s face was no longer oriented to the examiner’s face. When the child was not gazing at the examiner’s face, coders determined whether gaze was toward objects presented or elsewhere in the room. The proportion of time children spent looking away from both the face of the partner and the objects presented was very low for both Cohort 1 (M = .05, SD = .07) and Cohort 2 (M = .04, SD = .06). Coders were unaware of children’s risk group status, and 20% of videos were double-coded for reliability (all ICCs > .80). Frequencies of gaze to the examiner’s face were determined from the number of coded occurrences, and these frequencies were subsequently divided by the total duration coded to create rates per minute in each context.
Statistical Analyses
Following the modeling approach in Ozonoff et al., 2010, we used generalized mixed-effects models for count data (McCulloch, Searle, & Neuhaus, 2008) to analyze the coded behavior data, examine developmental trajectories, and test differences across outcome groups. This flexible approach allows for the use of all available data for each child and produces valid inference under the assumption of data missing at random.
Separate analyses were conducted for the two cohorts. Preliminary analyses suggested higher order polynomial effects of age, especially in the ASD group. Thus, for each cohort, we first fitted a model with a fixed effect for outcome group (ASD vs. TD, Non-TD vs. TD), linear, quadratic, and cubic effects of age at visit (measured in years, from the 6 month visit), and interactions between the linear, quadratic, and cubic effects of age and outcome group. The models included child-specific intercepts and slopes, to account for within-person correlations. Age was centered at 6 months to facilitate interpretation; the intercept in the models can be interpreted as the average value in the reference outcome group (TD) at the baseline visit (6 months). After fitting this initial model, we examined the higher order interaction terms in the model, and those that did not add significantly to the model were removed. Linear contrasts were constructed to estimate trajectories (intercept, linear, quadratic, and cubic age slopes) for each outcome group and evaluate differences between ASD and Non-TD groups in baseline levels and linear, quadratic, and cubic slopes, since these differences were not explicitly estimated from the models (as ASD vs TD and Non-TD vs TD differences). Similarly, linear contrasts were used to evaluate between-group differences at each visit age, as well as within-group differences comparing behaviors at 12 vs 6, 24 vs 12, 36 vs 12, and 36 vs 6 months.
Similar analyses were conducted after incorporating risk status into outcome groups (i.e., Low-risk, High-risk TD, High-risk Non-TD, ASD) and are presented in supplementary materials. All analyses were implemented using PROC GLIMMIX in SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Results
Summary statistics (mean, SD) for gaze to faces rates per minute are presented for the three outcome groups in each cohort in Table 1. Table 2 presents estimates (on the log scale) of the negative binomial mixed-effects models fitted to the coded gaze to faces.
Table 2.
Parameter estimates (SE) for the negative binomial mixed-effects modelsapredicting gaze to faces.
| Cohort 1 | Cohort 2 | |
|---|---|---|
| Mullen | Play Interaction | |
| Estimated trajectory for TD group | ||
| Baseline (6 months) | 1.40 (0.06)*** | 1.79 (0.07)*** |
| Linear change with age | 0.09 (0.16) | 0.38 (0.24) |
| Quadratic change with age | −0.55 (0.16)*** | −0.43 (0.24) |
| Cubic change with age | 0.19 (0.04)*** | 0.12 (0.06) |
| Estimated trajectory for ASD group | ||
| Baseline (6 months) | 1.11 (0.09)*** | 1.64 (0.12)*** |
| Linear change with age | −0.14 (0.17) | 0.85 (0.46) |
| Quadratic change with age | −0.55 (0.16)*** | −1.81 (0.49)*** |
| Cubic change with age | 0.19 (0.04)*** | 0.56 (0.13)*** |
| Estimated trajectory for Non-TD group | ||
| Baseline (6 months) | 1.29 (0.06)*** | 1.63 (0.11)*** |
| Linear change with age | 0.08 (0.16) | 1.07 (0.39)** |
| Quadratic change with age | −0.55 (0.16)*** | −1.04 (0.39)** |
| Cubic change with age | 0.19 (0.04)*** | 0.24 (0.10)* |
| Estimated difference between ASD and TD groups | ||
| Baseline (6 months) | −0.29 (0.10)** | −0.15 (0.13) |
| Linear change with age | −0.23 (0.07)** | 0.47 (0.52) |
| Quadratic change with age | – | −1.38 (0.55)* |
| Cubic change with age | – | 0.44 (0.15)** |
| Estimated difference between ASD and Non-TD groups | ||
| Baseline (6 months) | −0.17 (0.10) | 0.01 (0.16) |
| Linear change with age | −0.22 (0.07)** | −0.22 (0.61) |
| Quadratic change with age | – | −0.77 (0.63) |
| Cubic change with age | – | 0.32 (0.17) |
| Estimated difference between Non-TD and TD groups | ||
| Baseline (6 months) | −0.12 (0.07) | −0.16 (0.13) |
| Linear change with age | −0.01 (0.05) | 0.69 (0.46) |
| Quadratic change with age | – | −0.60 (0.46) |
| Cubic change with age | – | 0.12 (0.12) |
Note.
p < .01
p < .001. ASD = Autism Spectrum Disorder, TD = Typically Developing, Non-TD = Non-Typically Developing.
From negative binomial mixed-effects regression models with fixed effects for outcome group (ASD, Non-TD, TD), linear, quadratic and cubic age (in years, centered at 6 months), and interactions between age effects up to cubic term and group and random effects for child-specific intercept and linear slope.
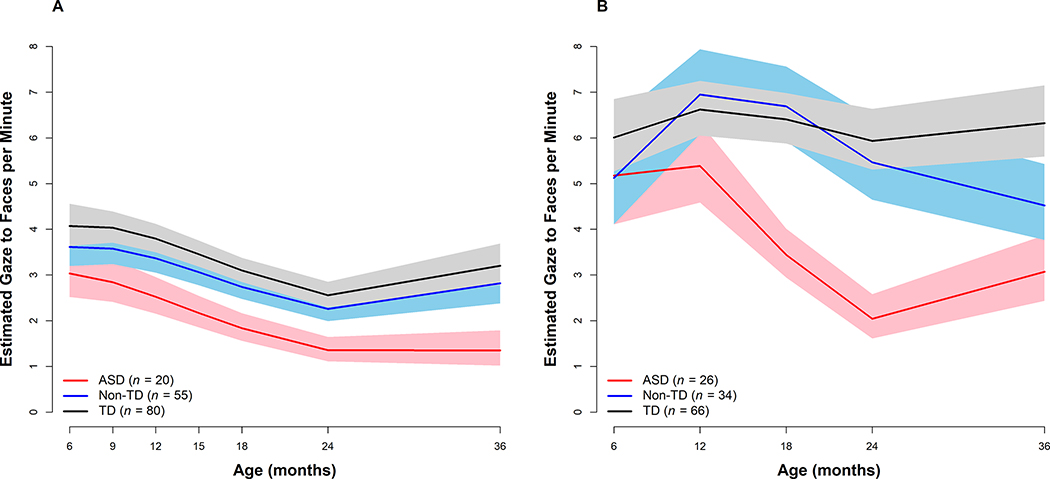
To ease interpretation, we also calculated estimates and 95% confidence intervals (CI) for coded gaze to faces on the original scale (see Figure 1 and Table 3). In Cohort 1, the ASD group exhibited significantly lower levels of gaze to faces at 6 months, as compared to the TD group. The ASD group had a sharper decline in the level of gaze to faces than both TD and Non-TD groups, which resulted in significantly lower levels in the ASD group than the other two groups from 12 through 36 months. In Cohort 2, the ASD group exhibited similar levels of gaze to faces as the other two groups at 6 months but showed a different longitudinal pattern than those groups, which resulted in significantly lower levels of gaze to faces by 12 months through 36 months compared to both TD and Non-TD groups. The Non-TD group did not differ from the TD group in gaze to faces, at baseline or in change over time, in either cohort. Examination of between-group differences in declines in gaze levels between 6 and 36 months demonstrated that in both cohorts, declines in gaze to faces in the ASD group were more pronounced than the other groups (Cohort 1: ASD vs TD p < .01, ASD vs Non-TD p < .01, Non-TD vs TD p = .94; Cohort 2: ASD vs TD p < .01, ASD vs Non-TD p = .06, Non-TD vs TD p = .29).
Figure 1.
Estimated gaze to examiner’s face for the Autism Spectrum Disorder (ASD), Non-Typically Developing (Non-TD), and Typically Developing (TD) outcome groups from 6 to 36 months of age. Shaded areas represent 95% Confidence Intervals. Panel A. Cohort 1 was coded during an administration of the Mullen Scales of Early Learning. Panel B. Cohort 2 was coded during a 3 minute play interaction task.
Table 3.
Estimated gaze to faces across age for all outcome groups from negative binomial mixed-effects modelsa
| Cohort 1 – Mullen | Cohort 2 – Play Interaction | |||||
| ASD | Non-TD | TD | ASD | Non-TD | TD | |
| M (95% CI) | M (95% CI) | M (95% CI) | M (95% CI) | M (95% CI) | M (95% CI) | |
| Gaze to Faces | ||||||
| 6 months | 3.04 (2.52–3.66)b | 3.62 (3.20–4.09) | 4.07 (3.64–4.56)d | 5.17 (4.11–6.51) | 5.12 (4.11–6.38) | 6.01 (5.27–6.85) |
| 9 months | 2.84 (2.41–3.35)b,c | 3.58 (3.24–3.95)d | 4.03 (3.71–4.39)d | – | – | – |
| 12 months | 2.53 (2.16–2.96)b,c | 3.37 (3.05–3.71)d | 3.80 (3.50–4.12)d | 5.39 (4.59–6.33)b,c | 6.95 (6.08–7.94)d | 6.62 (6.05–7.25)d |
| 15 months | 2.17 (1.85–2.54)b,c | 3.06 (2.78–3.38)b,d | 3.46 (3.18–3.75)c,d | – | – | – |
| 18 months | 1.84 (1.56–2.16)b,c | 2.74 (2.48–3.03)d | 3.10 (2.85–3.37)d | 3.44 (2.95–4.01)b,c | 6.69 (5.92–7.56)d | 6.41 (5.88–6.98)d |
| 24 months | 1.35 (1.11–1.65)b,c | 2.26 (1.99–2.56)d | 2.56 (2.30–2.85)d | 2.04 (1.62–2.58)b,c | 5.47 (4.65–6.43)d | 5.93 (5.30–6.63)d |
| 36 months | 1.35 (1.02–1.79)b,c | 2.82 (2.28–3.34)d | 3.20 (2.79–3.69)d | 3.07 (2.43–3.87)b,c | 4.52 (3.77–5.43)bd | 6.32 (5.59–7.15)c,d |
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | |
| Within-Group Differences | ||||||
| 12 vs 6 months | −0.18 (0.06)** | −0.07 (0.05) | −0.07 (0.05) | 0.04 (0.13) | 0.30 (0.12)* | 0.10 (0.07) |
| 24 vs 12 months | −0.62 (0.08)*** | −0.40 (0.06)*** | −0.39 (0.05)*** | −0.97 (0.15)*** | −0.24 (0.11)* | 1.32 (0.50)** |
| 36 vs 24 months | −0.01 (0.08) | 0.22 (0.06)*** | 0.22 (0.05)*** | 0.41 (0.14)** | −0.19 (0.10) | 0.06 (0.06) |
| 36 vs 6 months | −0.81 (0.16)*** | −0.25 (0.10)* | −0.24 (0.08)** | −0.52 (0.16)** | −0.13 (0.14) | 0.05 (0.08) |
Note. ASD = Autism Spectrum Disorder, TD = Typically Developing, Non-TD = Non-Typically Developing, M = mean, CI = confidence interval, SE = standard error.
Gaze to faces are estimated on the original scale from negative binomial mixed-effects regression models with fixed effects for outcome group (ASD, Non-TD, TD), linear, quadratic and cubic age (in years, centered at 6 months), and interactions between age effects up to cubic term and group and random effects for child-specific intercept and linear slope. Within-group differences are estimated on the log scale.
Indicates significant difference (p < .05) from TD group.
Indicates significant difference (p < .05) from Non-TD group.
Indicates significant difference (p < .05) from ASD group.
p < .05
p < .01
p < .001
The results of the supplemental analyses incorporating risk status into outcome groups (see supplemental materials, Supplemental Tables 1–2 and Figure 1) were consistent with the results of the primary analyses—the gaze levels in the ASD groups decreased over time relative to the Low-Risk group, while neither High-Risk group trajectory differed from the Low-Risk group.
Discussion
Using prospective methods and a confirmatory study design, we found in two independent samples that children who developed ASD exhibited declines in gaze to an adult’s face between 6 and 36 months, with differences evident by 6–12 months. This replicates the declines previously reported by Ozonoff et al. (2010) in two independent, larger samples, across two interactive contexts, strengthening the conclusions that 1) behavioral signs of ASD emerge over the first two years of life and 2) declining developmental trajectories are common in samples of children developing ASD when prospective methods are used. Children with TD and Non-TD outcomes did not show the same developmental pattern of early or sharp declines as demonstrated in those who developed ASD, nor did high-risk children in general (e.g., supplemental analyses).
While findings in Cohort 1 confirm findings in Ozonoff et al. (2010) using the same interactive context (structured developmental testing), findings in Cohort 2 extend this replication to an additional interactive context, a play interaction. Base rates of gaze to faces differed between the two interactive contexts, with lower rates observed during standardized developmental testing than during a play interaction. We expected this overall difference between contexts based on structural and demand differences between the tasks and prior work examining gaze to faces across these contexts (Gangi et al., 2018). However, despite these differences in overall rates of gaze to faces, children with ASD showed declines in gaze to faces over time in both cohorts relative to the TD group. This suggests that both structured settings and less structured, more naturalistic interactions can provide a window into the developmental patterns of social communication behavior in children developing ASD.
Though this study focuses on children’s gaze to faces, where children were looking when they were not looking at the social partner’s face is also relevant in evaluating whether findings merely reflect a difficulty with focused attention in general in the ASD group. The proportion of time children spent looking at neither faces nor the objects and toys presented was very low across cohorts (M ≤ .05)—the majority of time not spent looking at faces was spent looking at objects. Thus, it seems unlikely that children who spent less time gazing to the face of a social partner had difficulty with focused attention more generally, but rather that they spent more time focused on objects.
In Cohort 1, the ASD group exhibited lower levels of gaze to the face of the examiner by 6 months, while in Cohort 2 this difference was not significant until 12 months (consistent with findings from Ozonoff et al. (2010)). The children in Cohort 1 from the high-risk group with Non-TD outcomes also differed from the low-risk group at 6 months (see supplemental materials), though this difference was smaller than the difference between the ASD and low-risk groups. However, it is important to note that regardless of when group differences became significant, children with ASD consistently exhibited lower levels of gaze compared to TD and Non-TD groups through 36 months, and in both cohorts only the ASD group exhibited significant declines in gaze to the face of the examiner over time compared to the TD group.
The declining developmental trajectories seen in the ASD group may raise the question of whether this fits a particular pattern of ASD symptom onset. Four distinct patterns of ASD onset have been suggested: (i) early onset pattern defined by delays and atypicalities present in the first 12 months of life, (ii) regressive pattern described by a period of apparently typical development followed by a substantial decline in or loss of previously developed skills, (iii) mixed pattern of early delays followed by later loss, and (iv) plateau pattern in which acquired skills fail to progress to a more developmentally advanced level (Barger et al., 2013; Lord et al., 2004; Ozonoff, Heung, Byrd, Hansen, & Hertz-Picciotto, 2008; Pearson et al., 2018; Shumway et al., 2011).
Pearson and colleagues (2018) pose the question of whether declining trajectories, such as those reported in this manuscript, are phenomenologically the same as the regressions reported by parents on measures such as the ADI-R. They suggest, and we concur, that the two phenomena represent different places on a continuum of symptom emergence, from early and more gradual declines to later and more dramatic losses. Two methodological features of the current work afford the possibility of measuring losses that are more subtle and earlier appearing than typically reported by parents. First is the use of prospective, rather than retrospective, methods. The second is the focus on a behavior that is robustly present, at high frequency, in the first year of life. Early declines can only be evident when the behaviors examined are developmentally appropriate early in infancy, such as gaze to the face of a social partner. Similar patterns of developmental declines have been documented in additional areas of social development when the behaviors are early-appearing (e.g., response to name, social interest, shared affect) and are measured prospectively, regardless of whether the measurements are made by parents, expert examiners, or coded from behavioral assessments (Ozonoff, Gangi, et al., 2018).
A major strength of this work is replication of effects across two independent longitudinal cohorts, using the same coding system and different contexts. There are also limitations to this study that should be considered when interpreting our findings. While the overall sample size was large, the size of the ASD group, though approximately what would be predicted by prevalence estimates (Ozonoff et al., 2011), is relatively small. Not all participants had data at all time points and all available data were used in analyses. Our analytic approach yields valid estimates under the assumption of data missing at random and reasonably valid estimates of group effects can often be obtained even when the missing values are not completely random (Newgard & Lewis, 2015). Although we found no differences in the gaze variables as a function of amount of missing data and no group differences in amount of missing data, caution should still be taken when interpreting results. A second potential limitation is that our sample may not have had sufficient power to detect all higher order time effects and interactions (e.g., quartic age effects, interactions between outcome group and cubic or quadratic age effects). Finally, this study examined a specific social-communication behavior, gaze to the face of an examiner. It is possible that other aspects of social-communication may exhibit different developmental trajectories.
This mounting evidence of early declines in gaze to faces in ASD, now seen in two additional independent cohorts, suggests that declining developmental trajectories may occur in most children with ASD rather than just a smaller minority. Hence, the developmental pattern in which symptoms are present from birth as suggested by Kanner in his seminal paper (1943) may be less common than previously thought and declining trajectories of social-communication skills could be the norm in the development of ASD (Barger et al., 2013; Brignell et al., 2017; Ozonoff, Gangi, et al., 2018; Ozonoff et al., 2010; Thurm, Manwaring, Luckenbaugh, Lord, & Swedo, 2013).
These findings have implications for our understanding of typical and atypical developmental trajectories of social-communicative behaviors, as well as developmental screening tools and practices. Screening methods incorporating a trajectory-based approach, in which a child’s social-communication behaviors would be tracked over time in relation to normative percentiles, could be used to identify children in the early stages of a declining developmental trajectory. This could also potentially allow children who might not yet meet criteria for a full diagnosis of ASD to be identified as likely to benefit from early intervention. If infants at risk for ASD are able to be identified during the decline of skills, it might be possible to disrupt these developmental trajectories prior to the full onset of ASD symptoms. Several very early interventions targeting social and communicative skills and social engagement have been developed or adapted for infants and toddlers identified as high risk for ASD (French & Kennedy, 2018; Green et al., 2015; Kasari et al., 2014; Rogers et al., 2012; Watson et al., 2017). Evidence suggests such interventions are feasible and may be beneficial (Bradshaw, Steiner, Gengoux, & Koegel, 2015), and if children were identified in the early stages of developmental declines, such interventions could be implemented to potentially alter these trajectories of brain development and reduce symptoms (Webb, Jones, Kelly, & Dawson, 2014).
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health awards R01 MH068398, R01 MH099046, and P50 HD103526 and Autism Speaks award AS8370. We thank the children and families for their longitudinal participation in the studies.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-V. Washington, DC: American Psychiatric Association. [Google Scholar]
- Barger BD, Campbell JM, & McDonough JD (2013). Prevalence and onset of regression within autism spectrum disorders: A meta-analytic review. Journal of Autism and Developmental Disorders, 43, 817–828. doi: 10.1007/s10803-012-1621-x [DOI] [PubMed] [Google Scholar]
- Bölte S, Marschik PB, Falck-Ytter T, Charman T, Roeyers H, & Elsabbagh M (2013). Infants at risk for autism: A European perspective on current status, challenges and opportunities. European Child & Adolescent Psychiatry, 22, 341–348. doi: 10.1007/s00787-012-0368-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boterberg S, Charman T, Marschik PB, Bölte S, & Roeyers H (2019). Regression in autism spectrum disorder: A critical overview of retrospective findings and recommendations for future research. Neuroscience & Biobehavioral Reviews, 102, 24–55. doi: 10.1016/j.neubiorev.2019.03.013 [DOI] [PubMed] [Google Scholar]
- Bradshaw J, Steiner AM, Gengoux G, & Koegel LK (2015). Feasibility and effectiveness of very early intervention for infants at-risk for autism spectrum disorder: A systematic review. Journal of Autism and Developmental Disorders, 45, 778–794. doi: 10.1007/s10803-014-2235-2 [DOI] [PubMed] [Google Scholar]
- Brignell A, Williams K, Prior M, Donath S, Reilly S, Bavin EL, . . . Morgan AT (2017). Parent-reported patterns of loss and gain in communication in 1- to 2-year-old children are not unique to autism spectrum disorder. Autism, 21, 344–356. doi: 10.1177/1362361316644729 [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, & McDermott C (2007). A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders, 37, 12–24. doi: 10.1007/s10803-006-0328-2 [DOI] [PubMed] [Google Scholar]
- Dawson G (2011). Coming closer to describing the variable onset patterns in autism. Journal of the American Academy of Child & Adolescent Psychiatry, 50, 744–746. doi: 10.1016/j.jaac.2011.04.011 [DOI] [PubMed] [Google Scholar]
- Dawson G, Osterling J, Meltzoff AN, & Kuhl P (2000). Case study of the development of an infant with autism from birth to two years of age. Journal of Applied Developmental Psychology, 21, 299–313. doi: 10.1016/S0193-3973(99)00042-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French L, & Kennedy EMM (2018). Annual Research Review: Early intervention for infants and young children with, or at-risk of, autism spectrum disorder: A systematic review. Journal of Child Psychology and Psychiatry, 59, 444–456. doi: 10.1111/jcpp.12828 [DOI] [PubMed] [Google Scholar]
- Gangi DN, Schwichtenberg AJ, Iosif AM, Young GS, Baguio F, & Ozonoff S (2018). Gaze to faces across interactive contexts in infants at heightened risk for autism. Autism, 22, 763–768. doi: 10.1177/1362361317704421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg WA, Thorsen KL, Osann K, & Spence MA (2008). Use of home videotapes to confirm parental reports of regression in autism. Journal of Autism and Developmental Disorders, 38, 1136–1146. doi: 10.1007/s10803-007-0498-6 [DOI] [PubMed] [Google Scholar]
- Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, . . . Johnson MH (2015). Parent-mediated intervention versus no intervention for infants at high risk of autism: A parallel, single-blind, randomised trial. The Lancet Psychiatry, 2, 133–140. doi: 10.1016/S2215-0366(14)00091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Taylor A, & Lord C (2011). Telescoping of caregiver report on the Autism Diagnostic Interview – Revised. Journal of Child Psychology and Psychiatry, 52, 753–760. doi: doi: 10.1111/j.1469-7610.2011.02398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada N, Kamio Y, & Koyama T (2010). Developmental chronology of preverbal social behaviors in infancy using the M-CHAT: Baseline for early detection of atypical social development. Research in Autism Spectrum Disorders, 4, 605–611. doi: 10.1016/j.rasd.2009.12.003 [DOI] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, & Johnson MH (2014). Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience & Biobehavioral Reviews, 39, 1–33. doi: 10.1016/j.neubiorev.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, & Klin A (2013). Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature, 504, 427–431. doi: 10.1038/nature12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L (1943). Autistic disturbances of affective contact. Nervous child, 2, 217–250. [PubMed] [Google Scholar]
- Kasari C, Siller M, Huynh LN, Shih W, Swanson M, Hellemann GS, & Sugar CA (2014). Randomized controlled trial of parental responsiveness intervention for toddlers at high risk for autism. Infant Behavior and Development, 37, 711–721. doi: 10.1016/j.infbeh.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Chawarska K, Paul R, Rubin E, Morgan T, Wiesner L, & Volkmar F (2004). Autism in a 15-month-old child. American Journal of Psychiatry, 161, 1981–1988. doi: 10.1176/appi.ajp.161.11.1981 [DOI] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, & Faherty A (2013). Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child Development, 84, 429–442. doi: 10.1111/j.1467-8624.2012.01870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, & Garrett-Mayer E (2007). Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry, 64, 853–864. doi: 10.1001/archpsyc.64.7.853 [DOI] [PubMed] [Google Scholar]
- Lord C, Shulman C, & DiLavore P (2004). Regression and word loss in autistic spectrum disorders. Journal of Child Psychology and Psychiatry, 45, 936–955. doi: doi: 10.1111/j.1469-7610.2004.t01-1-00287.x [DOI] [PubMed] [Google Scholar]
- McCulloch C, Searle S, & Neuhaus J (2008). Generalized, Linear, and Mixed Models. Hoboken, NJ: Wiley. [Google Scholar]
- Miller M, Iosif A-M, Hill M, Young GS, Schwichtenberg AJ, & Ozonoff S (2017). Response to name in infants developing autism spectrum disorder: A prospective study. The Journal of Pediatrics, 183, 141–146.e141. doi: 10.1016/j.jpeds.2016.12.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning: AGS Circle Pines, MN. [Google Scholar]
- Newgard CD, & Lewis RJ (2015). Missing Data: How to Best Account for What Is Not Known. JAMA, 314, 940–941. doi: 10.1001/jama.2015.10516 [DOI] [PubMed] [Google Scholar]
- Norris M, & Lecavalier L (2010). Screening accuracy of level 2 autism spectrum disorder rating scales: A review of selected instruments. Autism, 14, 263–284. doi: 10.1177/1362361309348071 [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Gangi DN, Hanzel EP, Hill A, Hill MM, Miller M, . . . Iosif A-M (2018). Onset patterns in autism: Variation across informants, methods, and timing. Autism Research, 11, 788–797. doi: doi: 10.1002/aur.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Heung K, Byrd R, Hansen R, & Hertz-Picciotto I (2008). The onset of autism: Patterns of symptom emergence in the first years of life. Autism Research, 1, 320–328. doi: doi: 10.1002/aur.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, & Iosif A-M (2019). Changing conceptualizations of regression: What prospective studies reveal about the onset of autism spectrum disorder. Neuroscience & Biobehavioral Reviews, 100, 296–304. doi: 10.1016/j.neubiorev.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Li D, Deprey L, Hanzel EP, & Iosif A-M (2018). Reliability of parent recall of symptom onset and timing in autism spectrum disorder. Autism, 22, 891–896. doi: 10.1177/1362361317710798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, losif A-M, Baguio F, Cook IC, Hill MM, Hutman T, . . . Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 256–266. doi: 10.1097/00004583-201003000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, . . . Iosif A-M (2014). The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry, 53, 398–407.e392. doi: 10.1016/j.jaac.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, . . . Stone WL (2011). Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics, 128, e488–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo R, Belinchón M, & Ozonoff S (2006). Autism and family home movies: A comprehensive review. Journal of Developmental & Behavioral Pediatrics, 27, S59–S68. [DOI] [PubMed] [Google Scholar]
- Pearson N, Charman T, Happé F, Bolton PF, & McEwen FS (2018). Regression in autism spectrum disorder: Reconciling findings from retrospective and prospective research. Autism Research, 11, 1602–1620. doi: doi: 10.1002/aur.2035 [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Estes A, Lord C, Vismara L, Winter J, Fitzpatrick A, . . . Dawson G (2012). Effects of a brief Early Start Denver Model (ESDM)–based parent intervention on toddlers at risk for autism spectrum disorders: A randomized controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 1052–1065. doi: 10.1016/j.jaac.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, & Lord C (2003). Autism Diagnostic Interview Revised (ADI-R). Los Angeles: Western Psychological Services. [Google Scholar]
- Shumway S, Thurm A, Swedo SE, Deprey L, Barnett LA, Amaral DG, . . . Ozonoff S (2011). Brief report: Symptom onset patterns and functional outcomes in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 41, 1727–1732. doi: 10.1007/s10803-011-1203-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Chawarska K, Dawson G, Georgiades S, Landa R, Lord C, . . . Halladay A (2016). Prospective longitudinal studies of infant siblings of children with autism: Lessons learned and future directions. Journal of the American Academy of Child & Adolescent Psychiatry, 55, 179–187. doi: 10.1016/j.jaac.2015.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm A, Manwaring SS, Luckenbaugh DA, Lord C, & Swedo SE (2013). Patterns of skill attainment and loss in young children with autism. Development and Psychopathology, 26, 203–214. doi: 10.1017/S0954579413000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LR, Crais ER, Baranek GT, Turner-Brown L, Sideris J, Wakeford L, . . . Nowell SW (2017). Parent-mediated intervention for one-year-olds screened as at-risk for autism spectrum disorder: A randomized controlled trial. Journal of Autism and Developmental Disorders, 47, 3520–3540. doi: 10.1007/s10803-017-3268-0 [DOI] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Kelly J, & Dawson G (2014). The motivation for very early intervention for infants at high risk for autism spectrum disorders. International Journal of Speech-Language Pathology, 16, 36–42. doi: doi: 10.3109/17549507.2013.861018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2018). The 11th Revision of the International Classification of Diseases (ICD-11). Geneva. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.