Abstract
Background.
The equipment and methods for performing anorectal manometry and biofeedback therapy are different and not standardized. Normal values are influenced by age and sex. Our aims were to generate reference values, examine effects of gender and age, and compare anorectal pressures measured with diagnostic and biofeedback catheters and a portable manometry system.
Methods.
In this multicenter study, anorectal pressures at rest, during squeeze, and evacuation were measured with diagnostic and biofeedback catheters using Mcompass™ portable device in healthy subjects. Balloon expulsion time and rectal sensation were evaluated. The effects of age and gender were assessed.
Results.
The final dataset comprised 108 (74 women) of 124 participants with normal rectal balloon expulsion time (less than 60 seconds). During squeeze, anal resting pressure increased by approximately twofold in women and threefold in men. During evacuation, anal pressure exceeded rectal pressure in 87 participants (diagnostic catheter). The specific rectoanal pressures (eg, resting pressure) were significantly correlated and not different between diagnostic and biofeedback catheters. With the diagnostic catheter, the anal squeeze pressure and rectal pressure during evacuation were greater in men than women (P ≤ 0.02). Among women, women aged 50 years and older had lower anal resting pressure; rectal pressure and the rectoanal gradient during evacuation were greater in older than younger women (P ≤ 0.01).
Conclusions.
Anal and rectal pressures measured with diagnostic and biofeedback manometry catheters were correlated and not significantly different. Pressures were influenced by age and sex, providing reference values in men and women.
Keywords: constipation, defecatory disorders, fecal incontinence, diagnosis
Background
Fecal continence is primarily maintained by the anal sphincters in concert with rectal sensorimotor functions, intact neurological function and the pelvic floor (1, 2). Defecation requires increased rectal pressure coordinated with relaxation of the anal sphincters and pelvic floor muscles (3). Anorectal manometry is necessary to identify impaired recto-anal coordination (dyssynergia) in constipated patients who have defecatory disorders (2, 4, 5), to recognize anal sphincter weakness in FI (6), and to retrain the pelvic floor muscles with biofeedback therapy (7–9).
Anorectal pressures can be measured with non-high-resolution (ie, water perfused or solid state), high resolution, high definition, or portable manometry (4, 10–14). There are several manufacturers. The methods for data acquisition and analysis are not standardized (15) and reference values are limited. High resolution manometry catheters are widely used but expensive and relatively fragile. Taken together, these gaps reduce the utility of anorectal manometry in clinical practice and research. Indeed, until recently, no multicenter therapeutic trials (eg, of biofeedback therapy or sacral nerve stimulation) have assessed anorectal functions with manometry (16, 17).
Designed to overcome these limitations, a portable manometry system (Mcompass, Medspira Inc, Minneapolis, MN) that measures pressures and can be used to provide biofeedback therapy in the office has been developed (10). This device was used to measure anal pressures and to provide biofeedback therapy in a recent multicenter trial (17, 18). Different catheters are used to measure pressures and provide biofeedback therapy. The diagnostic catheter has been validated against high resolution manometry in 20 healthy asymptomatic women and 10 women each with fecal incontinence and constipation (10). The reference values for anorectal pressures and rectal sensation assessed with the biofeedback catheter are unknown.
This portable manometry device is being used to record anorectal pressures and to provide biofeedback therapy in an ongoing multicenter trial comparing biofeedback therapy, sacral nerve stimulation, and perianal bulking injection for fecal incontinence. The portable catheters can be used in an office based setting, are less expensive and are more user-friendly. Hence, towards the objectives of developing a database of reference values for the diagnostic and biofeedback versions of the portable catheter, the aims of this study were to evaluate rectal and anal pressures measured by portable manometry (i.e., separately with the diagnostic and the biofeedback catheters) at rest, during squeeze, evacuation, and a Valsalva maneuver as well as rectal sensation in asymptomatic healthy women and men.
Methods
Study design
This study was conducted in healthy volunteers at Colon and Rectal Surgery Associates in Minneapolis, Mn, Mayo Clinic, Rochester, Mn, Augusta University, Ga, and University of North Carolina at Chapel Hill, NC. After an initial screening visit, anorectal pressures and rectal sensation were measured with diagnostic and separately biofeedback versions of the portable manometry catheter; a rectal balloon expulsion test was also performed.
Participants
Including 20 women who were studied previously (May 2012 – October 2013) at Mayo Clinic, Rochester, MN (10) and 104 people who were evaluated in the multicenter study that was conducted between January 2018 and November 2018, there were 124 participants (83 healthy women and 41 men), who were recruited by public advertisement (Figure 1). Both studies were performed before the nternational anorectal physiology working group (IAPWG) recommendations were published {Carrington, 2020 #5858}. Participants did not have gastrointestinal symptoms as detailed below, prior pelvic radiation, anorectal procedures, including treatment for hemorrhoids, or risk factors for pelvic floor trauma, i.e. more than 4 vaginal deliveries, known birthweight greater than 4500gms (macrosomia), known 4th degree perineal tear, or known forceps use (19). Participants were not taking medications that may affect gastrointestinal motility.
Figure 1.
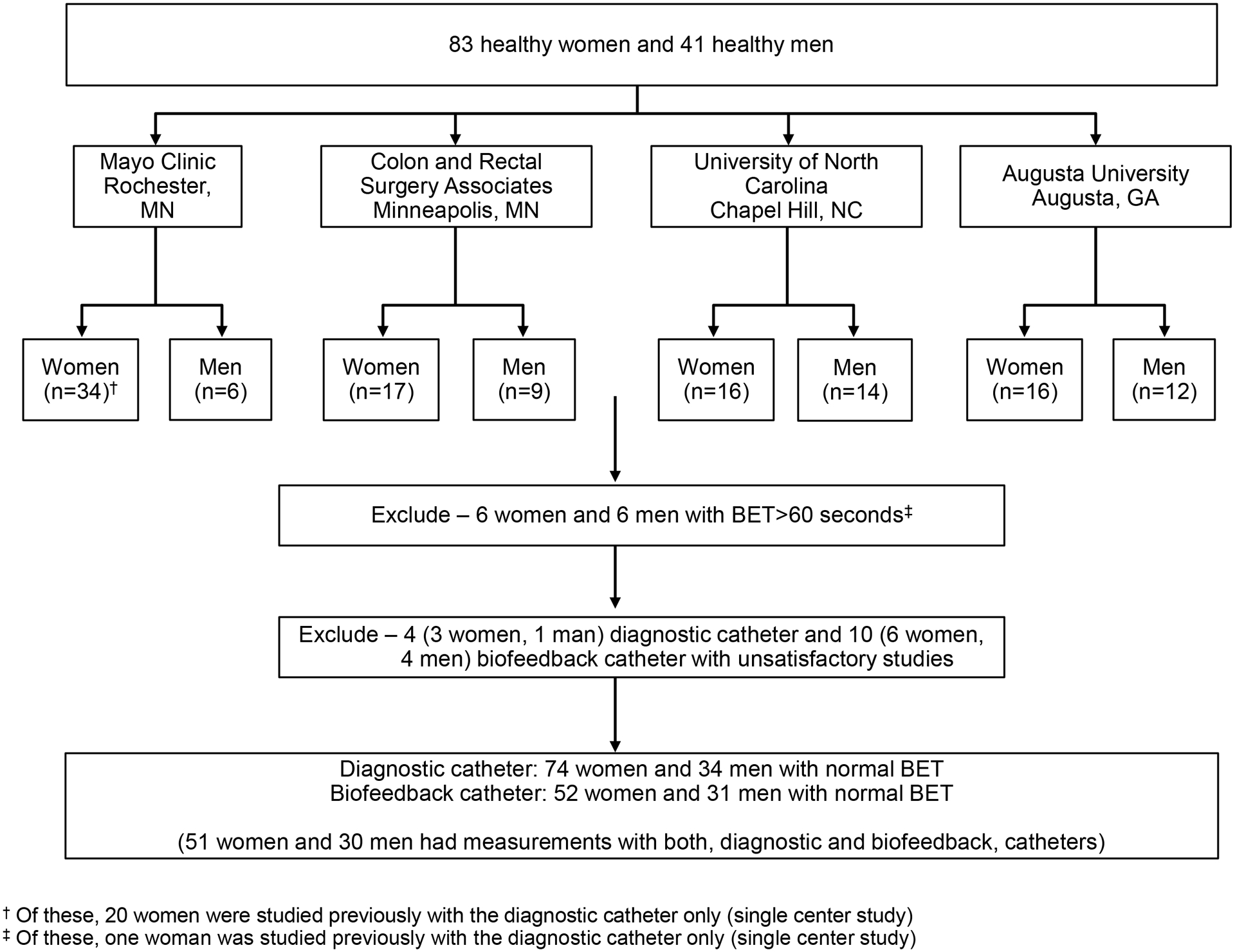
Study Flow
Bowel symptoms
Bowel symptoms were assessed by a questionnaire based on ROME II criteria (20). Participants did not have symptoms of fecal incontinence, irritable bowel syndrome, functional diarrhea or constipation, as defined by two or more of these symptoms for at least 25% of the time: excessive straining, hard or lumpy stools, incomplete evacuation, a feeling of anorectal blockage, anal digitation, or fewer than 3 bowel movements per week.
Rectal balloon expulsion time
Participants had up to 3 minutes to expel a 4-cm-long balloon filled with 50 ml water from the rectum in privacy while seated on a commode (5, 21). Consistent with studies that used a similar balloon in healthy volunteers from our and other centers, a balloon expulsion time (BET) greater than 60 seconds was considered to be abnormal (21, 22).
Portable Manometry
After up to 2 sodium phosphate enemas (Fleet saline enemas, C.B. Fleet Company, Inc), the anorectal manometry was performed in the left lateral position with the diagnostic and biofeedback catheters, in randomized order, in each participant on the same day. Both catheters have the same polyurethane rectal balloon that is used to distend the rectum and measure rectal pressures. Anal pressures are measured by four air-filled balloons (ie, one in each quadrant) in the diagnostic catheter. In order to reduce the cost of manufacturing biofeedback catheters, these catheters have only one circumferential anal balloon. These anal balloons are 2 cm long.
At the beginning of the procedure, the catheter was connected to the portable manometry device. The anal chamber was primed by inserting 3ml of air through the portable device. The catheter was then inserted into the rectum and the rectal balloon was primed by inserting 10ml of air into the rectal balloon. With both catheters, measurements were acquired with the distal end of the balloon just above the anal verge. To facilitate standardization, the user interface guides the operator in how to conduct the study. For the diagnostic manometry, rectoanal pressures were measured at rest [20s], during squeeze [3 maneuvers 20s each], Valsalva [20s] and evacuation without and with rectal distention [50 ml]). Rectal sensory thresholds for first sensation, desire, urgency, and pain were recorded by progressively distending the rectal ballon in 20 ml increments from 0–180 ml (10). Except for evacuation without rectal distension and a Valsalva maneuver, the same measurements were obtained with a biofeedback catheter. The average and maximum resting and squeeze pressures are respectively the average and highest pressures recorded during the corresponding epoch. During evacuation with rectal distention, the software subtracts the pressure (ie, 48 mmHg) in the rectal balloon inflated to 50 ml outside the body from the actual pressure recorded during the maneuver. For example, if the balloon pressure during the maneuver was 78 mmHg, the effective rectal pressure due to voluntary effort is 30 mmHg. Rectal and anal pressures during evacuation were summarized as the rectoanal gradient (ie, difference between rectal and anal pressure) and index (ie, ratio of rectal to anal pressure). These measurements were obtained during the 2s time period during evacuation with the maximum (ie, most positive or least negative) difference between rectal balloon pressure and the mean anal pressure recorded by all four balloons.
Statistical Analysis
Anal resting pressure in women and men, anal squeeze pressure in women, rectal and anal pressures and the rectoanal gradient during evacuation in women and men were normally distributed; the remaining parameters were not. For both catheters, the distribution of each anorectal parameter was summarized, separately in men and women, as the Mean [SD], 10th-90th percentile, and the 25th to 75th percentile reference values based on nonparametric distributions. The association between age and rectoanal parameters was examined with Spearman’s correlation coefficients in men and women. Wilcoxon’s Rank Sum test was used to compare measurements between men and women. Within subject differences (ie, between catheters) were assessed with Wilcoxon’s signed rank test. The concordance between anal pressures measured with the diagnostic and biofeedback catheters was assessed with Spearman’s correlation coefficient and with Lin’s concordance statistic (the concordance correlation coefficient [CCC](23). A Bland Altman assessment examined whether the magnitude of differences between 2 measurements is correlated with the magnitude of the measured responses (the average value for both studies) (24).
Results
Study flow
Because the 20 participants reported previously were only evaluated with a diagnostic catheter, this report includes 124 and 104 participants studied with the diagnostic and biofeedback catheters (Figure 1). From this cohort, the following studies were excluded from further analysis: twelve participants (ie, 6 women and 6 men) with an abnormal balloon expulsion time suggestive of pelvic floor dysfunction and four and ten participants who had technically suboptimal studies respectively with the diagnostic and biofeedback catheters. Hence, the final dataset includes 74 women (age: 40 ± 16y, [Mean ± SD]) and 34 men (age: 37 ± 15y) who were evaluated with a diagnostic catheter and 52 women (age: 40 ± 16y, [Mean ± SD]) and 31 men (age: 36 ± 14y) who were studied with a biofeedback catheter. The BET was not significantly different among participants who were evaluated with the diagnostic (women: 15 ± 12s and men 13 ± 11s; P-value = 0.72) and the biofeedback catheter (women: 17 ± 13s versus men 13 ± 11s; P-value = 0.17).
At one site, rectoanal pressures during evacuation were not measured with the biofeedback catheter in any (ie, 24) participants. Rectal pressures during evacuation with rectal distension were negative, likely inaccurate, hence excluded from analysis in 2 participants with diagnostic catheter and 7 with biofeedback catheter. Hence, the rectoanal gradient during balloon distension was measured in 106 participants with the diagnostic catheter and 52 healthy participants with the biofeedback catheter.
Rectoanal pressures in women
Among women, the anal pressure measured with diagnostic and biofeedback catheters increased by approximately twofold during squeeze (Tables 1–3). During evacuation, both without and with rectal distention, the rectal pressure was lower than anal pressure in 61 women and 26 men with the diagnostic catheter; hence the rectoanal gradient was negative and the rectoanal index was less than one.
Table 1.
Comparison of anorectal pressures measured with the diagnostic catheter in younger and older women†
| Variable | Women < 50 years with normal BET, n | Women ≥ 50 years with normal BET, n | Women < 50 years with Normal BET |
Women ≥ 50 years with Normal BET |
P-value | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD (10th, 90th percentile) |
1st, 3rd quartile | Mean ± SD (10th, 90th percentile) |
1st, 3rd quartile | ||||
| Diagnostic catheter | |||||||
| Anal resting pressure | 50 | 24 | 74 ± 21 (48, 99) | 58, 90 | 52 ± 14 (31, 70) | 44, 59 | <0.0001 |
| Anal squeeze pressure‡ | 50 | 24 | 158 ± 58 (83, 211) | 123, 192 | 134 ± 50 (75, 208) | 99, 172 | 0.09 |
| Anal squeeze increment‡,§ | 50 | 24 | 76 ± 50 (22, 149) | 42, 99 | 79 ± 47 (29, 152) | 44, 115 | 0.82 |
| BET (sec) | 50 | 24 | 16 ± 13 (4, 30) | 6, 22 | 12 ± 11 (2, 25) | 4, 16 | 0.10 |
| Evacuation without rectal distention | |||||||
| Rectal pressure | 50 | 22 | 37 ± 19 (15, 61) | 22, 49 | 54 ± 20 (37, 79) | 40, 71 | 0.001 |
| Anal pressure | 50 | 23 | 69 ± 21 (42, 94) | 56, 81 | 64 ± 18 (44, 84) | 52, 76 | 0.25 |
| Rectoanal gradient | 50 | 22 | −32 ± 25 (−65, −3) | −48, −10 | −12 ± 21 (−34, 17) | −26, 5 | 0.003 |
| Anal relaxation (%) | 50 | 23 | 2 ± 29 (−30, 27) | −9, 16 | −24 ± 35 (−74, 10) | −51, −3 | 0.0007 |
| Defecation index¶ | 50 | 23 | .54 ± .32 (.22, .93) | 0.27, 0.73 | .83 ± .43 (.32, 1.35) | 0.57, 1.11 | 0.003 |
| Evacuation with rectal distention | |||||||
| Rectal pressure | 49 | 24 | 39 ± 25 (12, 68) | 22, 49 | 60 ± 29 (33, 99) | 40, 83 | 0.002 |
| Anal pressure | 50 | 24 | 61 ± 19 (36, 83) | 47, 75 | 60 ± 20 (36, 82) | 45, 70 | 0.70 |
| Rectoanal gradient | 49 | 24 | −23 ± 26 (−59, 10) | −38, −6 | −8 ± 21 (−37, 21) | −22, 0 | 0.01 |
| Anal relaxation (%) | 50 | 24 | 3 ± 18 (−17, 28) | −12, 15 | −32 ± 47 (−104, 2) | −36, −5 | <0.0001 |
| Defecation index¶ | 50 | 24 | .60 ± .54 (.14, 1.02) | 0.31, 0.81 | .95 ± .43 (.49, 1.57) | 0.65, 1.13 | 0.001 |
| Valsalva without rectal distention | |||||||
| Anal Pressure | 50 | 23 | 109 ± 37 (65, 153) | 87, 131 | 101 ± 30 (65, 136) | 80, 126 | 0.32 |
| Rectal sensation | |||||||
| First sensation (mL) | 50 | 24 | 46 ± 21 (20, 80) | 30, 60 | 47 ± 30 (20, 74) | 30, 60 | 0.74 |
| Desire to defecate (mL) | 50 | 24 | 89 ± 34 (50, 140) | 60, 108 | 83 ± 35 (53, 107) | 60, 93 | 0.36 |
| Urgency (mL) | 50 | 24 | 127 ± 36 (80, 180) | 100, 160 | 119 ± 38 (80, 174) | 88, 153 | 0.39 |
| Pain (mL) | 40 | 15 | 138 ± 33 (99, 180) | 110, 170 | 120 ± 37 (80, 172) | 100, 155 | 0.09 |
| Biofeedback catheter | |||||||
| Anal resting pressure | 38 | 14 | 72 ± 20 (45, 95) | 62, 86 | 50 ± 14 (33, 67) | 38, 57 | 0.0002 |
| Anal squeeze pressure‡ | 38 | 14 | 159 ± 59 (76, 221) | 133, 189 | 132 ± 48 (75, 190) | 111, 162 | 0.09 |
| Anal squeeze increment‡,§ | 38 | 14 | 81 ± 47 (28, 142) | 49, 109 | 79 ± 39 (38, 117) | 55, 104 | 0.94 |
| Evacuation with rectal distention | |||||||
| Rectal pressure | 20 | 12 | 34 ± 23 (11, 67) | 14, 47 | 49 ± 35 (14, 83) | 24, 59 | 0.18 |
| Anal pressure | 25 | 12 | 67 ± 26 (40, 100) | 53, 80 | 57 ± 17 (39, 82) | 44, 65 | 0.15 |
| Rectoanal gradient | 20 | 12 | −35 ± 38 (−85, −3) | −54, −24 | −12 ± 20 (−36, 10) | −20, 0 | 0.01 |
| Anal relaxation (%) | 24 | 12 | 12 ± 70 (−27, 37) | −6, 14 | −15 ± 25 (−38, 7) | −35, −2 | 0.07 |
| Defecation index¶ | 24 | 12 | .04 ± 1.32 (−0.13, 0.69) | −0.03, 0.59 | .74 ± .39 (0.25, 1.26) | 0.46, 1.00 | 0.003 |
| Rectal sensation | |||||||
| First sensation (mL) | 38 | 14 | 36 ± 15 (20, 60) | 23, 40 | 27 ± 17 (13, 51) | 20, 30 | 0.02 |
| Desire to defecate (mL) | 38 | 14 | 87 ± 39 (40, 136) | 60, 100 | 64 ± 22 (40, 87) | 50, 78 | 0.05 |
| Urgency (mL) | 38 | 14 | 124 ± 40 (60, 180) | 100, 150 | 99 ± 32 (70, 134) | 80, 108 | 0.04 |
| Pain (mL) | 33 | 8 | 140 ± 36 (84, 180) | 110, 180 | 114 ± 38 (70, 159) | 93, 128 | 0.09 |
Values are mmHg unless stated otherwise.
These values are derived from the squeeze maneuver with the highest squeeze pressure;
Squeeze increment is (anal squeeze pressure – anal resting pressure).
No unit.
Table 3.
Comparison of anorectal pressures measured with the biofeedback catheter in women and men†
| Variable | Women, n | Men, n | Women Mean ± SD (10th, 90th percentile) |
1st, 3rd quartile | Men Mean ± SD (10th, 90th percentile) |
1st, 3rd quartile | P-value |
|---|---|---|---|---|---|---|---|
| Anal resting pressure | 52 | 31 | 66 ± 21 (37, 94) | 53, 79 | 72 ± 17 (48, 95) | 63, 83 | 0.21 |
| Anal squeeze pressure‡ | 52 | 31 | 152 ± 57 (71, 219) | 118, 188 | 227 ± 100 (140, 359) | 177, 264 | <0.0001 |
| Anal squeeze increment‡,§ | 52 | 31 | 80 ± 44 (30, 141) | 51, 110 | 154 ± 96 (67, 275) | 84, 182 | <0.0001 |
| BET (sec) | 52 | 31 | 17 ± 13 (5, 35) | 7, 23 | 13 ± 11 (5, 25) | 6, 16 | 0.17 |
| Evacuation with rectal distention | |||||||
| Rectal pressure | 32 | 20 | 39 ± 29 (12, 72) | 15, 55 | 58 ± 37 (16, 117) | 32, 69 | 0.08 |
| Anal pressure | 37 | 22 | 63 ± 24 (39, 90) | 50, 80 | 77 ± 36 (44, 105) | 59, 88 | 0.10 |
| Rectoanal gradient | 32 | 20 | −26 ± 34 (−69, 5) | −42, −11 | −26 ± 35 (−70, 15) | −52, −9 | 0.95 |
| Anal relaxation (%) | 37 | 22 | 3 ± 60 (−35, 31) | −24, 8 | −20 ± 48 (−51, 21) | −36, 1 | 0.13 |
| Defecation index¶ | 36 | 21 | 0.27 ± 1.14 (−0.11, 1.04) | 0.09, 0.71 | 0.69 ± .67 (0.00, 1.16) | 0.33, 0.84 | 0.18 |
| Valsalva without rectal distention | |||||||
| Anal Pressure | |||||||
| Rectal sensation | |||||||
| First sensation (mL) | 52 | 30 | 33 ± 16 (20, 60) | 20, 40 | 36 ± 18 (20, 61) | 20, 50 | 0.56 |
| Desire to defecate (mL) | 52 | 30 | 81 ± 37 (40, 130) | 50, 100 | 82 ± 33 (49, 121) | 60, 100 | 0.73 |
| Urgency (mL) | 52 | 30 | 117 ± 39 (61, 180) | 98, 150 | 128 ± 36 (97, 180) | 100, 158 | 0.20 |
| Pain (mL) | 41 | 22 | 135 ± 37 (80, 180) | 100, 170 | 150 ± 32 (101, 180) | 125, 180 | 0.15 |
Values are mmHg unless stated otherwise.
These values are derived from the squeeze maneuver with the highest squeeze pressure;
Squeeze increment is (anal squeeze pressure – anal resting pressure).
No unit.
For the diagnostic catheter, age was associated with lower resting pressure (ρ = −0.48, P < 0.0001), greater rectal pressure during evacuation with (ρ = 0.34, P = 0.003) and without (ρ = 0.39, P = 0.0007) rectal distention, less anal relaxation during evacuation with (ρ = −0.31, P = 0.008) and without (ρ = −0.30, P = 0.01) rectal distension, higher rectoanal gradient during evacuation with (ρ = 0.36, P = 0.002) and without (ρ = 0.42, P = 0.0002) rectal distention and a higher defecation index during evacuation with (ρ = 0.39, P = 0.0006) and without (ρ = 0.39, P = 0.0005) rectal distention (Figure 2). However, age was not significantly correlated with balloon expulsion time.
Figure 2.
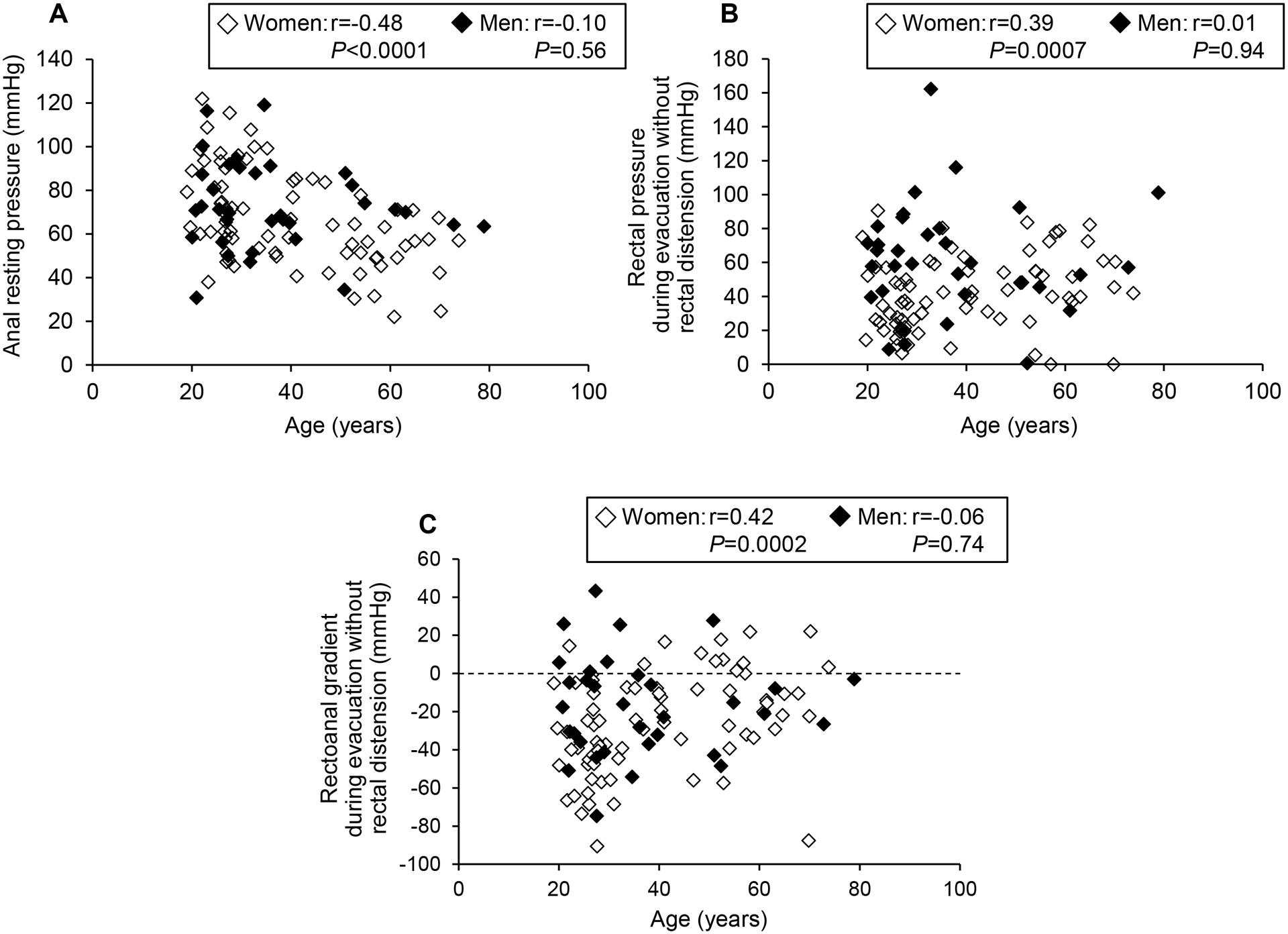
Relationship between age and resting anal pressure (A) and rectal pressure (B) and rectoanal gradient (C) during evacuation without rectal distension measured with the diagnostic catheter.
For the biofeedback catheter, age was associated with lower resting anal pressure (ρ = −0.56, P < 0.0001), lower squeeze pressure (ρ = −0.34, P =0.01) and a higher rectoanal gradient during evacuation with rectal distension (ρ = 0.39, P = 0.03).
For the diagnostic catheter, the balloon expulsion time was correlated with a lower gradient (ρ = −0.29, P = 0.01), greater anal relaxation (ρ = 0.24, P = 0.04) and a lower defecation index (ρ = −0.27, P = 0.02) during evacuation with rectal distension. For the biofeedback catheter, the balloon expulsion time was correlated with greater anal pressure during evacuation with rectal distension (ρ = 0.36, P = 0.03), Other anorectal measurements were not significantly correlated with the balloon expulsion time (data not shown).
Rectoanal pressures in men
During squeeze, the anal pressure increased by approximately threefold in men (Tables 2–4). During evacuation, both without and with rectal distention, the rectal pressure was lower than anal pressure; hence the rectoanal gradient was negative. Age had smaller effects on anal pressures in men than in women. With the diagnostic catheter, age was inversely correlated (ρ = −0.38, P = .03) with anal relaxation during evacuation with rectal distention. By contrast to women, other correlations between age and rectoanal pressures were not significant (Figure 2). With the biofeedback catheter, the correlations between age and rectoanal pressures were not significant (data not shown).
Table 2.
Comparison of anorectal pressures measured with the diagnostic catheter in women and men†
| Variable | Women, n | Men, n | Women Mean ± SD (10th, 90th percentile) |
1st, 3rd quartile | Men Mean ± SD (10th, 90th percentile) |
1st, 3rd quartile | P-value |
|---|---|---|---|---|---|---|---|
| Anal resting pressure | 74 | 34 | 67 ± 22 (42, 97) | 51, 81 | 73 ± 20 (50, 94) | 64, 88 | 0.11 |
| Anal squeeze pressure‡ | 74 | 34 | 150 ± 56 (81, 215) | 108, 181 | 223 ± 84 (149, 310) | 174, 243 | <0.0001 |
| Anal squeeze increment‡,§ | 74 | 34 | 77 ± 49 (25, 150) | 43, 105 | 143 ± 81 (71, 238) | 95, 179 | <0.0001 |
| BET (sec) | 74 | 34 | 15 ± 12 | 5, 18 | 13 ± 11 | 6, 16 | 0.72 |
| Evacuation without rectal distention | |||||||
| Rectal pressure | 72 | 32 | 42 ± 21 (18, 72) | 26, 55 | 64 ± 32 (24, 100) | 45, 80 | 0.0004 |
| Anal pressure | 73 | 33 | 67 ± 20 (42, 94) | 53, 79 | 72 ± 26 (54, 104) | 55, 86 | 0.55 |
| Rectoanal gradient | 72 | 32 | −26 ± 25 (−57, 6) | −41, −8 | −16 ± 26 (−44, 24) | −33, −2 | 0.12 |
| Anal relaxation (%) | 73 | 33 | −6 ± 33 (−51, 24) | −18, 14 | −10 ± 37 (−78, 20) | −14, 11 | 0.70 |
| Defecation index¶ | 73 | 33 | .63 ± .38 (0.22, 1.13) | 0.30, 0.84 | .80 ± .47 (0.30, 1.46) | 0.51, 0.97 | 0.07 |
| Evacuation with rectal distention | |||||||
| Rectal pressure | 73 | 33 | 46 ± 28 (13, 87) | 26, 58 | 61 ± 34 (27, 96) | 39, 75 | 0.02 |
| Anal pressure | 73 | 33 | 61 ± 19 (36, 83) | 46, 74 | 69 ± 26 (42, 91) | 51, 81 | 0.14 |
| Rectoanal gradient | 73 | 33 | −18 ± 26 (−55, 13) | −35, −2 | −15 ± 23 (−35, 18) | −30, −1 | 0.56 |
| Anal relaxation (%) | 74 | 34 | −8 ± 35 (−35, 24) | −17, 10 | −9 ± 32 (−59, 27) | −20, 13 | 0.99 |
| Defecation index¶ | 74 | 34 | .71 ± .53 (0.17, 1.28) | 0.37, 0.95 | .76 ± .42 (0.34, 1.25) | 0.56, 0.97 | 0.34 |
| Valsalva maneuver | |||||||
| Anal pressure | 73 | 33 | 106 ± 35 (65, 150) | 82, 130 | 112 ± 45 (67, 150) | 94, 128 | 0.55 |
| Rectal sensation | |||||||
| First sensation (mL) | 74 | 34 | 46 ± 25 (20, 80) | 30, 60 | 41 ± 16 (20, 60) | 30, 58 | 0.51 |
| Desire to defecate (mL) | 74 | 34 | 87 ± 34 (50, 137) | 60, 100 | 89 ± 32 (60, 127) | 60, 100 | 0.70 |
| Urgency (mL) | 74 | 34 | 124 ± 37 (80, 180) | 100, 160 | 137 ± 39 (80, 180) | 100, 170 | 0.10 |
| Pain (mL) | 55 | 34 | 133 ± 35 (84, 180) | 100, 160 | 149 ± 37 (86, 180) | 130, 180 | 0.02 |
Values are mmHg unless stated otherwise.
These values are derived from the squeeze maneuver with the highest squeeze pressure;
Squeeze increment is (anal squeeze pressure – anal resting pressure).
No unit.
Table 4.
Comparison of pressures measured with the diagnostic and biofeedback catheters in women and men†
| Variable | Women, n | Men, n | Women | Men | P-value (diagnostic vs biofeedback catheter; women) | P-value (diagnostic vs biofeedback catheter; men) | ||
|---|---|---|---|---|---|---|---|---|
| Diagnostic Mean ± SEM |
Biofeedback Mean ± SEM |
Diagnostic Mean ± SEM |
Biofeedback Mean ± SEM |
|||||
| Anal resting pressure | 51 | 30 | 70 ± 3 | 66 ± 3 | 75 ± 4 | 72 ± 4 | 0.23 | 0.49 |
| Anal squeeze pressure‡ | 51 | 30 | 154 ± 7 | 151 ± 7 | 222 ± 11 | 226 ± 11 | 0.72 | 0.71 |
| Anal squeeze increment‡,§ | 51 | 29 | 77 ± 5 | 80 ± 5 | 144 ± 10 | 152 ± 10 | 0.53 | 0.43 |
| Evacuation with rectal distention | ||||||||
| Rectal pressure | 30 | 18 | 42 ± 4 | 40 ± 4 | 59 ± 5 | 53 ± 5 | 0.57 | 0.26 |
| Anal pressure | 36 | 21 | 60 ± 5 | 62 ± 5 | 66 ± 6 | 71 ± 6 | 0.65 | 0.33 |
| Rectoanal gradient | 30 | 18 | −22 ± 7 | −24 ± 7 | −11 ± 6 | −24 ± 6 | 0.81 | 0.06 |
| Anal relaxation (%) | 36 | 21 | −2 ± 9 | 5 ± 9 | −9 ± 7 | −12 ± 7 | 0.46 | 0.71 |
| Defecation index¶ | 36 | 21 | .27 ± .19 | .53 ± .19 | .69 ± .14 | .74 ± .14 | 0.18 | 0.72 |
| Rectal sensation | ||||||||
| First sensation (mL) | 51 | 29 | 44 ± 4 | 33 ± 4 | 42 ± 4 | 36 ± 4 | 0.003 | 0.07 |
| Desire to defecate (mL) | 51 | 29 | 87 ± 4 | 81 ± 4 | 87 ± 6 | 82 ± 6 | 0.20 | 0.33 |
| Urgency (mL) | 51 | 29 | 121 ± 4 | 116 ± 4 | 140 ± 6 | 127 ± 6 | 0.23 | 0.02 |
| Pain (mL) | 41 | 22 | 134 ± 5 | 135 ± 5 | 160 ± 7 | 150 ± 7 | 0.87 | 0.06 |
Values are mmHg unless stated otherwise.
These values are derived from the squeeze maneuver with the highest squeeze pressure;
Squeeze increment is (anal squeeze pressure – anal resting pressure).
No unit.
Comparison of rectoanal pressures in men and women
Diagnostic catheter
The anal squeeze pressure (223 ± 84 versus 150 ± 56 mmHg, P < 0.0001) and the squeeze increment (143 ± 81 versus 77 ± 49 mmHg, P < 0.0001) were greater in men than in women (Table 2). The rectal pressure was greater in men than in women during evacuation with (61 ± 34 versus 46 ± 28, P = 0.02) and without (64 ± 32 versus 42 ± 21 mmHg, P = 0.0004) rectal distention. The other parameters were not significantly different between men and women.
Biofeedback catheter
Similar to the diagnostic catheter, the anal squeeze pressure (227 ± 100 versus 152 ± 57 mmHg, P<0.0001) and the squeeze increment (154 ± 96 versus 80 ± 44 mmHg, P< 0.0001) were also greater in men than in women (Table 3). However, the other parameters were not significantly different between men and women.
Rectal sensation
During rectal balloon distention with the diagnostic catheter in 108 participants, all, 106, and 95 reported thresholds respectively for first sensation, desire to defecate, and urgency. The rectal pain threshold was only assessed, subject to a maximum distention of 180 ml, in the multicenter study with 89 participants, of whom 49 reported pain.
With the biofeedback catheter, rectal distention was performed until the pain threshold or until 180 ml. At one site, the catheter was not distended after participants perceived the urge to defecate threshold (i.e., 19 participants); hence the pain threshold was not assessed. Rectal sensation was assessed in 82 of 83 participants studied with the biofeedback catheter; all reported first sensation threshold, 79 reported the desire to defecate, and 74 reported urgency. Fifty three of 63 participants perceived pain. Except for the first sensation and urgency thresholds evaluated with the biofeedback catheter, these thresholds were not significantly different between younger and older women (Table 1).
Comparison of rectoanal pressures between the diagnostic and biofeedback catheters
In both men and women, rectoanal pressures measured with the diagnostic and biofeedback catheters were correlated and not significantly different (Table 4, Figure 3). For these comparisons, Lin’s concordance correlation coefficients for resting anal pressure was 0.44 (CI: 0.19, 0.63) in women and 0.43 (CI: 0.10, 0.68) in men and for anal squeeze pressure were 0.68 (CI: 0.50, 0.80) in women and 0.81 (CI: 0.65, 0.90) in men. For rectal sensory thresholds, these values were 0.15 (CI: −0.08, 0.36) in women and 0.24 (CI: −0.10, 0.52) in men for first sensation, 0.67 (CI: 0.48, 0.79) in women and 0.40 (CI: 0.06, 0.65) in men for desire to defecate, 0.71 (CI: 0.55, 0.82) in women and 0.61 (CI: 0.34, 0.79) in men for urgency, and 0.68 (CI: 0.47, 0.81) in women and 0.59 (CI: 0.26, 0.79) in men for pain.
Figure 3.
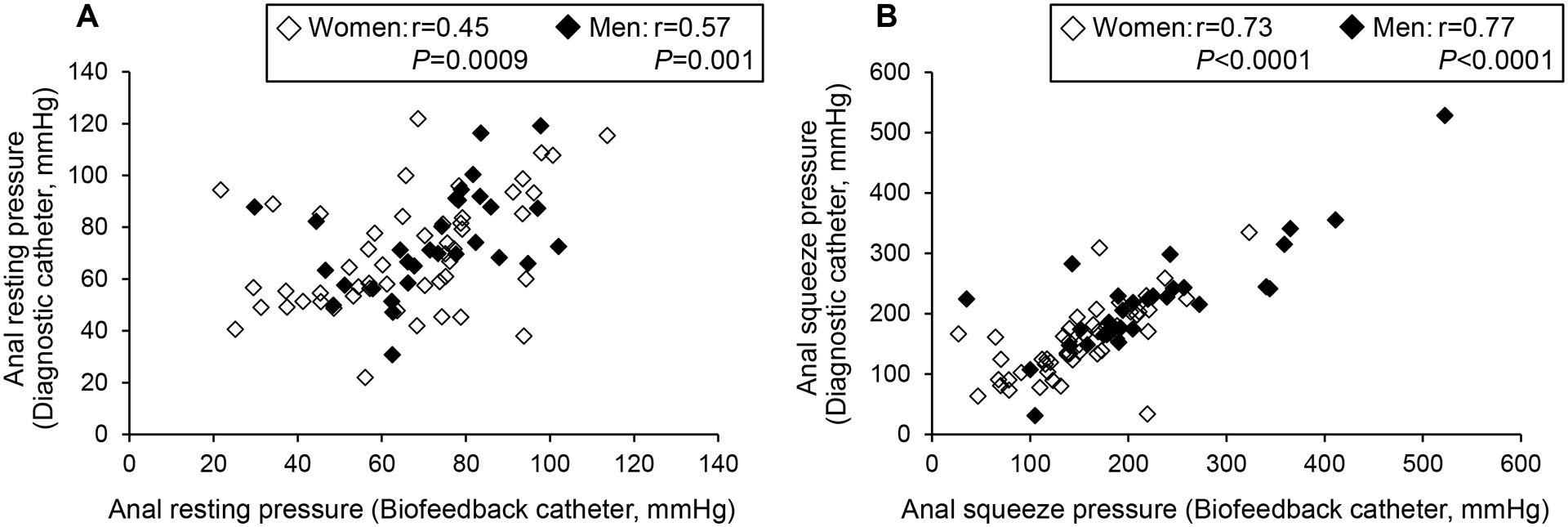
Relationship between pressures measured with the diagnostic and biofeedback catheters: resting anal pressure (A) anal anal pressure during squeeze (B).
Selected rectal sensory thresholds were greater when measured with the diagnostic than the biofeedback catheter. This includes the first sensation threshold in women (Diagnostic: 44 ± 4 ml vs Biofeedback: 33 ± 4 ml, P = 0.003) and the threshold for urgency in men (Diagnostic: 140 ± 6 ml vs Biofeedback: 127 ± 6 ml, P = 0.02). The other parameters were significantly different between diagnostic and biofeedback catheters.
The Bland-Altman plots (Figure 4) demonstrate a mean difference of 3 mmHg between anal resting pressure measured with these catheters, i.e., on average, the biofeedback catheter underestimated the average resting anal pressure by 3mmHg compared to the diagnostic catheter. For this comparison, the 95% limits of agreement were −39 mmHg and 45 mmHg. For anal squeeze pressure, the biofeedback catheter underestimated the average anal pressure during squeeze by .03 mmHg compared to the diagnostic catheter (Figure 4B); the 95% limits of agreement were −102 mmHg and 102 mmHg. For the rectoanal gradient during evacuation with rectal distension (Figure 4C), the biofeedback catheter underestimated the average rectoanal gradient during evacuation with rectal distension by 6 mmHg compared to the diagnostic catheter; the 95% limits of agreement were between −63 mmHg and 74 mmHg.
Figure 4.
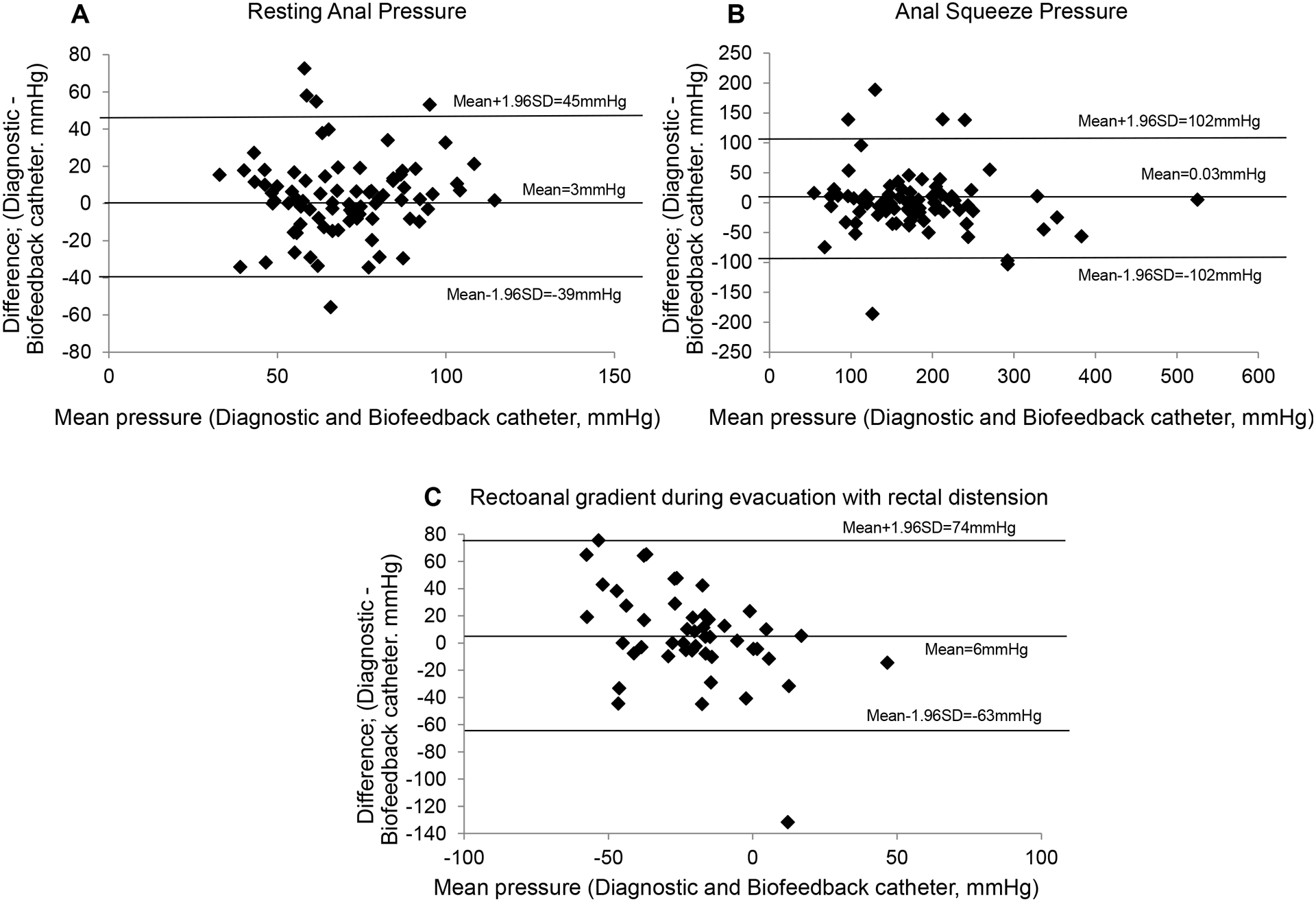
Bland Altman plots showing the agreement between pressures measured with the diagnostic and the biofeedback catheters: resting anal pressure (A) and anal pressure during squeeze (B) and rectoanal gradient during evacuation with rectal distension (C). The horizontal lines in each plot are the means and the means ± 1.96*standard deviation (SD) limits.
Discussion
This multicenter study provides reference values measured separately for the diagnostic and the biofeedback catheters using the Mcompass™ portable manometry device. The sample size (ie, 74 women and 34 men) is comparable to studies that evaluated 96 women (47 men), 42 women (36 men), and 85 women (0 men) respectively with the Medtronic high resolution manometry system (25), Medtronic high definition manometry system (11), and the Solar GI high resolution manometry system (13). Confirming previous studies, selected pressures were affected by sex and age (11, 13, 14, 25–27). In particular, the anal squeeze pressure was lower while the rectal pressure during evacuation was greater in men than women. Compared to younger women, older women had a lower anal resting pressure and a greater rectal pressure and rectoanal gradient during evacuation without rectal distension, confirming previous studies with water-perfused non-high resolution and high resolution manometry (25–27). The Spearman correlation coefficient for age versus anal resting pressure (−0.48) was similar to earlier studies with other techniques (ie, −0.34 with high resolution and −0.44 with water-perfused manometry) (25, 26). To a lesser extent, anal squeeze pressures were also affected by age and lower in older than younger women. Together, these findings underscore the need for age-appropriate reference values, especially in women. The reference values were partitioned into two categories at 50 years, which is similar to the median age at menopause (51.5 years) in a representative sample of healthy US women (28). The 10–90th percentile reference values for anal resting pressure measured with the diagnostic catheter in all women, women younger than 50 years, and women aged 50 years and older, are respectively 42–97, 48–99, and 31–70 mmHg. Because there are only 24 women aged 50 years and older, an alternative option is to consider values outside the 90th percentile values as definitely abnormal while values between the 10th and 25th percentile and between the 75th and 90th percentile values may be considered borderline abnormal.
Exemplifying differences among techniques, the 10–90th percentile values for anal resting and squeeze pressure in women were 42–97 and 81–215 mmHg, which is lower than the corresponding values (ie, 44–110 and 127–281 mmHg) with high resolution manometry. These differences are at least partly explained by differences between these techniques. The resting pressure measured by portable manometry reflects the average pressure recorded by four anal balloons, each 2 cm long, over 20 seconds while high resolution manometry provides the highest pressure averaged over 20 seconds recorded by any anal sensor. These differences are more pronounced during the squeeze maneuver probably because portable manometry expresses the greatest squeeze pressure across the entire length of the anal canal averaged across all 4 anal balloons (ie, a 2 cm span) over the 20 second squeeze period. By contrast, high resolution manometry measures the highest squeeze pressure recorded by a single sensor in the anal canal. Similar to previous studies (4, 25), the rectoanal pressure gradient during evacuation was negative in many healthy people, at least partly because manometry is performed in the left lateral position with an empty balloon. In healthy women, the correlation between the balloon expulsion time and the high resolution manometry rectoanal gradient, is comparable with an empty or distended rectal balloon (27). To reduce the risk of damaging the high resolution manometry catheter, evacuation is evaluated with an empty balloon. The 10th percentile for the rectoanal gradient in women in this study (ie, −57 mmHg without and −55 mmHg with rectal distention) is similar to the corresponding parameter measured with high resolution manometry (−66 mmHg).
By contrast to high resolution manometry, portable manometry offers a single solution for diagnostic purposes and biofeedback therapy. Hence portable manometry is less expensive and more user-friendly than using different systems for these purposes. Except for small differences in rectal sensory thresholds, the pressures measured with diagnostic and biofeedback catheters were not significantly different. This was not due to a type 2 error. Based on the observed variation in pressures for diagnostic and portable catheters, we had 80% power at an alpha level of 0.05 to detect differences of 2.5 mm Hg and 4.2 mm Hg respectively in resting and squeeze pressures between the catheters. While the correlation between pressures measured by both catheters is reassuring, the 95 percentile confidence intervals for agreement are wide (eg, −39 to 45 mmHg for anal resting pressure), which limits the extent to which pressures are interchangeable across catheters.
In this study, all 108, 106, and 95 healthy participants reported first sensation, desire to defecate, and urgency during balloon distention. Allowing for differences among techniques, the values for rectal sensory thresholds measured with the Mcompass system in this study and the ManoScan system in previous studies are comparable (25). Moreover, the in vitro dimensions and compliance of the Mcompass and the ManoScan rectal balloons are also similar (unpublished data). For example, at the highest distending volume of 180 ml, the intraballoon pressure and diameter are respectively 85 mmHg and 75 mm for the Mcompass and 74 mmHg and 69 mm for the Manoscan balloon. Patients who do not perceive the desire the defecate at this distending volume probably have reduced rectal sensation.
This multicenter study measured and analyzed anorectal pressures with standardized techniques in a relatively large cohort of healthy asymptomatic people. However, there are limitations. In some subgroups (eg, women older than 50 years), the sample size was less than 40, which is the minimum required to estimate the 2.5 and 97.5 percentiles. Ideally, at least 120 reference values are required to obtain reliable estimates (29). Future studies should evaluate the precision of these measurements by assessing the day-to-day variability.
In summary, this multicenter study provides the reference values for rectoanal pressures measured with the Mcompass™ portable anorectal manometry device and demonstrates that selected pressures are correlated with age and sex.
Funding.
This study was supported in part by USPHS NIH Grant U34 DK109191 and U01 DK 115575 to Doctors Whitehead, Bharucha, Lowry, and Rao. The authors acknowledge the contributions of Ms Shashana Fiedler, Dr Anam Herekar, Ms Kimberly Psalitis, Ms Maggie Soberay, Ms Stefanie Twist, and Dr Yun Yan to data acquisition and analysis.
Footnotes
Competing Interests. Dr. Bharucha jointly holds a patent for the Medspira portable anorectal manometry device used in this study. Dr. Bharucha and Mayo Clinic have contractual rights to receive royalties from the licensing of this technology. Mayo Clinic holds equity in the company to which the technology is licensed. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- 1.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil 2006; 18: 507–519. [DOI] [PubMed] [Google Scholar]
- 2.Carrington EV, Scott SM, Bharucha A, et al. Expert consensus document: Advances in the evaluation of anorectal function. Nature Reviews Gastroenterology & Hepatology 2018; 15: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palit S, Lunniss PJ, Scott SM. The physiology of human defecation. Dig Dis Sci 2012; 57: 1445–1464. [DOI] [PubMed] [Google Scholar]
- 4.Lee TH, Bharucha AE. How to Perform and Interpret a High-resolution Anorectal Manometry Test. J Neurogastroenterol Motil 2016; 22: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prichard DO, Lee T, Parthasarathy G, Fletcher JG, Zinsmeister AR, Bharucha AE. High-resolution Anorectal Manometry for Identifying Defecatory Disorders and Rectal Structural Abnormalities in Women. Clinical Gastroenterology & Hepatology 2017; 15: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mion F, Garros A, Brochard C, et al. 3D High-definition anorectal manometry: Values obtained in asymptomatic volunteers, fecal incontinence and chronic constipation. Results of a prospective multicenter study (NOMAD). Neurogastroenterol Motil 2017; 29. [DOI] [PubMed] [Google Scholar]
- 7.Patcharatrakul T, Valestin J, Schmeltz A, Schulze K, Rao SSC. Factors Associated With Response to Biofeedback Therapy for Dyssynergic Defecation. Clinical Gastroenterology & Hepatology 2017; 27: 27. [DOI] [PubMed] [Google Scholar]
- 8.Mazor Y, Ejova A, Andrews A, Jones M, Kellow J, Malcolm A. Long-term outcome of anorectal biofeedback for treatment of fecal incontinence. Neurogastroenterol Motil 2018: e13389. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan SP, Bharucha AE. A Practical Guide to Biofeedback Therapy for Pelvic Floor Disorders. Curr Gastroenterol Rep 2019; 21: 21. [DOI] [PubMed] [Google Scholar]
- 10.Bharucha AE, Stroetz R, Feuerhak K, Szarka LA, Zinsmeister AR. A novel technique for bedside anorectal manometry in humans. Neurogastroenterol Motil 2015: 1504–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coss-Adame E, Rao SS, Valestin J, Ali-Azamar A, Remes-Troche JM. Accuracy and Reproducibility of High-definition Anorectal Manometry and Pressure Topography Analyses in Healthy Subjects. Clinical Gastroenterology and Hepatology : the official clinical practice journal of the American Gastroenterological Association 2015; 13: 1143–1150 e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty S, Feuerhak KJ, Zinsmeister AR, Bharucha AE. Reproducibility of high-definition (3D) manometry and its agreement with high-resolution (2D) manometry in women with fecal incontinence. Neurogastroenterol Motil 2017; 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrington EV, Knowles CH, Grossi U, Scott SM. High-resolution Anorectal Manometry Measures Are More Accurate Than Conventional Measures in Detecting Anal Hypocontractility in Women With Fecal Incontinence. Clinical Gastroenterology & Hepatology 2019; 17: 477–485.e479. [DOI] [PubMed] [Google Scholar]
- 14.Gosling J, Plumb A, Taylor SA, Cohen R, Emmanuel AV. High-resolution anal manometry: Repeatability, validation, and comparison with conventional manometry. Neurogastroenterol Motil 2019; 31: e13591. [DOI] [PubMed] [Google Scholar]
- 15.Carrington EV, Heinrich H, Knowles CH, et al. Methods of anorectal manometry vary widely in clinical practice: Results from an international survey. Neurogastroenterol Motil 2017; 29: e13016. [DOI] [PubMed] [Google Scholar]
- 16.Bharucha AE, Rao SSC, Shin AS. Surgical Interventions and the Use of Device-Aided Therapy for the Treatment of Fecal Incontinence and Defecatory Disorders. Clinical Gastroenterology & Hepatology 2017; 15: 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelovsek JE, Markland AD, Whitehead WE, et al. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomised clinical trial. The Lancet Gastroenterology & Hepatology 2019; 4: 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markland AD, Jelovsek JE, Whitehead WE, et al. Improving biofeedback for the treatment of fecal incontinence in women: implementation of a standardized multi-site manometric biofeedback protocol. Neurogastroenterol Motil 2017; 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bharucha AE, Fletcher JG, Melton LJ 3rd, Zinsmeister AR. Obstetric Trauma, Pelvic Floor Injury And Fecal Incontinence: A Population-Based Case-Control Study. Am J Gastroenterol 2012; 107: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut 1999; 45 Suppl 2: II43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratuapli S, Bharucha AE, Harvey D, Zinsmeister AR. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterol Motil 2013; 25: e813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazor Y, Prott G, Jones M, Kellow J, Ejova A, Malcolm A. Anorectal physiology in health: A randomized trial to determine the optimum catheter for the balloon expulsion test. Neurogastroenterol Motil 2019; 31: e13552. [DOI] [PubMed] [Google Scholar]
- 23.Carrasco JL, Jover L. Estimating the generalized concordance correlation coefficient through variance components. Biometrics 2003; 59: 849–858. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 1995; 346: 1085–1087. [DOI] [PubMed] [Google Scholar]
- 25.Oblizajek NR, Gandhi S, Sharma M, et al. Anorectal pressures measured with high-resolution manometry in healthy people—Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterology and Motility : the official journal of the European Gastrointestinal Motility Society 2019: e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum 2006; 49: 1726–1735. [DOI] [PubMed] [Google Scholar]
- 27.Noelting J, Ratuapli SK, Bharucha AE, Harvey D, Ravi K, Zinsmeister AR. Normal Values For High-Resolution Anorectal Manometry In Healthy Women: Effects Of Age And Significance Of Rectoanal Gradient. Am J Gastroenterol 2012; 107: 1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol 1997; 145: 124–133. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz GL. Establishment and Use of Reference Values. In: Burtis CA, Bruns DE, eds. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics −. St Louis, Mo: Elsevier, 2015: 60–71. [Google Scholar]


