Abstract
Objective:
To determine whether the Dietary Approaches to Stop Hypertension (DASH) diet or an alternative, simplified diet, emphasizing high fiber fruit and vegetables, lowers serum urate (SU).
Methods:
We conducted a secondary study of the DASH feeding study, a 3-arm, parallel-design, randomized trial of 459 adults with systolic blood pressure (SBP) <160 mmHg and diastolic blood pressure (DBP) of 80–95 mmHg, not on BP medications. Participants were randomized to 8 weeks of monitored feeding and ate one of three diets: 1) a typical American diet (control), 2) a fruit and vegetable (FV)-rich diet but otherwise similar to control, or 3) the DASH diet which was rich in fruit, vegetables, and low-fat dairy products, and reduced in fat, saturated fat and cholesterol. Body weight was kept constant throughout the study. SU was measured at baseline and after 8-weeks of feeding.
Results:
Of the 327 participants with available specimens (mean age 45.4±11.0 years, 47% women, 50% black), mean baseline SU was 5.7±1.5 mg/dL. Compared to the control diet, the FV diet reduced SU by 0.17 mg/dL (95%CI: −0.34, 00; P=0.051) and DASH reduced SU by 0.25 mg/dL (95%CI: −0.43,−0.08; P=0.004). These effects increased with increasing baseline SU levels (<5, 5–5.9, 6–6.9, 7–7.9, and ≥8 mg/dL) with DASH (0.08, 0.12, 0.42, 0.44, and 0.73 mg/dL; P-trend=0.04), but not with FV.
Conclusions:
The DASH diet reduced SU, particularly among those with hyperuricemia. These findings support the growing need for a dedicated trial to test DASH among patients with hyperuricemia and gout.
Keywords: Diet, cardiovascular disease, blood pressure, cholesterol, serum urate, trial
Dietary patterns are viewed as important determinants of elevated serum urate (SU) levels and risk for gout (1). Dietary recommendations for gout have traditionally focused on reduced consumption of purines, which are precursors to uric acid (2–4). However, low purine (i.e., low protein) diets have limited palatability and sustainability. Furthermore, the reduction in protein consumption can lead to a compensatory higher intake of carbohydrates (including fructose) and fats (including trans or saturated fats), which can in turn worsen cardiovascular (CV) risk factors, such as blood pressure and serum lipids, which are frequently elevated in adults with hyperuricemia and gout (5). Recent evidence suggests that the Dietary Approaches to Stop Hypertension (DASH) diet, a dietary strategy shown to lower both BP and lipids, may lower SU and gout risk (6,7) as well as CV risk. However, these findings have not been confirmed in an independent trial (8). Furthermore, the extent to which the SU reduction from DASH is simply attributed to greater consumption of fruits and vegetables is unknown.
The DASH trial was a parallel arm, 8-week feeding study conducted in adults with elevated blood pressure (BP) or hypertension (9). Participants were fed one of three diets: 1) a typical American diet, 2) a fruit and vegetable (FV) diet, or 3) the DASH diet, which combined greater consumption of high-fiber fruit and vegetables with low-fat dairy and reduced saturated fat and total fat. This landmark study demonstrated that the DASH diet lowered BP and low density lipoprotein-cholesterol compared to a typical American diet and beyond fruit and vegetables alone (10). However, whether the DASH diet also reduced SU has not been reported.
In the present study, we measured SU in stored specimens from the DASH trial to determine the effect of the DASH diet or the FV diet on SU compared with the typical American diet. We hypothesized that the DASH diet would lower SU beyond fruits and vegetables consistent with its effects on CV risk factors.
Methods
The DASH trial was initiated and sponsored by the National Heart, Lung, and Blood Institute. It was conducted between September 1994 and March 1996 at four clinical centers within the United States (Baltimore, Maryland; Boston, Massachusetts; Durham, North Carolina; Baton Rouge, Louisiana). The study’s primary results were published (9). In summary, DASH compared the effects of three different diets on blood pressure (BP) in 459 adults with elevated blood pressure. Participants were randomized within each site to 1) a control diet that was typical of what many Americans eat, 2) the FV diet, or 3) the DASH diet. Prior to enrollment, all participants provided written, informed consent for specimen storage. The present study utilized serum curated by the NHLBI BioLINCC repository to measure urate. Notably one of the four research centers did not provide any specimens, resulting in the reduced sample for our study. Institutional Review Boards (IRBs) at each of the 4 research sites institution approved the original study protocol. Use of the publically available data was considered by IRBs of Mass General Hospital and Beth Israel Deaconess to be exempt research.
Participants
Participants in the DASH trial were aged 22 years and older with an average SBP between 120 to 159 mm Hg and an average diastolic BP (DBP) between 80 to 95 mm Hg. Exclusion criteria were diabetes mellitus, a recent CV event (within the previous six months), a body mass index >35 kg/m2, renal insufficiency, use of antihypertensive medications, and self-reported alcoholic beverage intake of more than 14 drinks per week.
Dietary Interventions
Participants were randomized to one of three diets, following a parallel design: a control diet, the FV diet, or the DASH diet (called “the combination diet” in the original publication; Supplement Table ST1). The control diet was designed after a typical American diet with potassium, magnesium, and calcium levels matching the 25th percentile of U.S. consumption, and macronutrient profiles and fiber reflecting the average intake levels in the U.S. (9). In contrast, the FV diet included potassium and magnesium at the 75th percentile of U.S. consumption and provided higher amounts of fiber. This diet had more fruits and vegetables with fewer snacks and sweets than the control diet.
The DASH diet was similar to the FV diet, providing potassium and magnesium at levels reflecting the 75th percentile of U.S. consumption, and was higher in fiber and protein (9). In addition, the DASH diet provided calcium at the 75th percentile of U.S. consumption. It also emphasized fat-free or low-fat dairy products and included whole grains, poultry, fish, and nuts while restricting saturated fat, total fat, cholesterol, sweets, and sugar-containing beverages consumption. All three diets provided a comparable amount of sodium (~3 g/day) in this trial.
Each diet was isocaloric and administered as part of a seven-day menu cycle, which included 3 meals per day at four kilocalorie levels (1600, 2100, 2600, and 3100 kcal). Each weekday, participants ate 1 main meal (lunch or dinner) on site. The remaining weekday meals and all weekend meals were provided to participants and consumed offsite. Both feeding compliance and participants’ weights were closely monitored, and adherence was high, with participants attending over 95% of person-days at scheduled on-site meals and adhering to the study protocol offsite (all study foods and no non-study foods) over 93% of person-days. In addition, objective biomarkers also confirmed high adherence as well (9).
Primary outcomes
The primary outcome measure of the present post-hoc study was SU measured in 2018 from available stored specimens from three of the four clinical centers. Serum specimens were collected in participants at baseline (N=327) and after the conclusion of the 8-week feeding periods (N=327) for each of the three diets. All specimens (baseline and 8-week) were collected after a 12-hour fast. Serum was stored at −70◦C and underwent at least 1 freeze-thaw cycle for the present measurements. SU was measured using a standard automated uricase enzymatic assay on the Roche P Modular system (Roche Diagnostics - Indianapolis, IN).
Covariates
Additional participant characteristics were determined via questionnaire, laboratory specimens, and physical examination. Sex was self-reported, and race was examined in categories of black and non-black. Body mass index (BMI) was derived from measured height and weight with obesity defined as a BMI ≥30 kg/m2. Seated SBP and DBP were measured using random-zero sphygmomanometers. Baseline BP was calculated as the average of three pairs of measurements during screening and four pairs during the run-in phase, while BP at follow-up was calculated as the average of four or five pairs of measurements during weeks 7 and 8 of the intervention phase. Hypertension in the DASH trial was defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg. Low density lipoprotein cholesterol (LDL-cholesterol) was estimated (11) from total cholesterol, high density lipoprotein cholesterol, and triglycerides, measured using enzymatic colorimetry.
Statistical analysis
We summarized population characteristics at baseline according to diet assignment using means (SD) and proportions. The distribution of baseline change in SU was examined using kernel density plots. We determined the mean (SD) SU concentration at baseline and 8 weeks according to diet and compared the change from baseline via t-tests.
We compared SU across dietary assignments with adjustment for baseline serum concentrations, using the following contrasts: FV versus control, DASH versus control, and DASH versus FV. In addition, we performed a concurrent change analysis adjusting for change in baseline SBP, DBP, LDL-c or all three CVD risk factors. All dietary comparisons and concurrent change analyses were performed using linear regression models.
We also compared baseline change in SU with baseline change in SBP, DBP, and LDL-c using scatter plots, Lowess curves, and linear regression models. In addition, we determined the association of baseline change in SU with baseline change in SBP, DBP, and LDL-c using linear regression with adjustment for age, sex, black race, and dietary assignment.
In addition, we performed pre-specified subgroup analyses of the effects of the FV diet versus control or the DASH diet compared to control in strata of age (< 50 years, ≥ 50 years), sex (men, women), race (non-black, black), baseline hypertension (baseline SBP ≥ 140 or DBP ≥ 90 mm Hg), baseline obesity (BMI ≥ 30 kg/m2), baseline hyperuricemia (baseline SU > 6 mg/dL for women or > 7 mg/dL for men), and categories of baseline SU (< 5, 5–5.9, 6–6.9, 7–7.9, and ≥ 8 mg/dL). These subgroups are largely consistent with our prior work (6). Comparisons across categories were performed using interaction terms. For categories of baseline SU, the interaction term was determined by treating the median SU value of each category as a continuous variable. This interaction term represents the P for trend. As a sensitivity analysis we also examined the following categories of baseline SU: (< 5, 5–5.9, 6–6.9, and ≥ 7 mg/dL).
All analyses were performed with Stata version 15.1 (Stata Corporation, College Station, TX, USA). Missing data (primarily from unavailable specimens) were evenly distributed across dietary assignments.
Results
Baseline characteristics
Baseline characteristics of the 327 participants of the DASH trial with stored serum are shown in Table 1. Characteristics were similar across dietary assignments. Notably, the 132 original participants that were excluded were more likely to be female, black, and hypertensive (Supplement Table ST2).
Table 1.
Baseline characteristics according to diet assignment.
| Control, N = 107 | Fruit/Vegetable, N = 110 | Combination (DASH), N = 110 | |
|---|---|---|---|
| Age, year | 45.1 (11.7) | 46.3 (10.9) | 44.8 (10.5) |
| Female, % | 43.0 | 44.5 | 52.7 |
| Black, % | 49.5 | 48.2 | 53.6 |
| Body mass index ≥ 30 kg/m2, % | 36.4 | 36.4 | 36.4 |
| Body mass index, kg/m2 | 28.0 (3.9) | 27.9 (4.0) | 28.3 (3.9) |
| Hypertension, % | 27.1 | 27.3 | 24.5 |
| Systolic blood pressure, mm Hg | 130.7 (10.8) | 131.5 (11.3) | 130.9 (10.3) |
| Diastolic blood pressure, mm Hg | 85.0 (4.5) | 84.3 (4.9) | 84.3 (4.4) |
| Low density lipoprotein cholesterol, mg/dL | 121.8 (30.8) | 127.9 (30.6) | 117.1 (33.3) |
Note that one person assigned the control diet and one person assigned the fruit/vegetable diet is missing a low density lipoprotein cholesterol value.
For LDL-cholesterol, the N was 106 among those assigned control and 109 among those assigned fruit/vegetables due to missing values. Among those exclude, only 128 had an LDL-cholesterol measurement.
Change in Serum Urate from Baseline
Mean baseline levels of SU were similar across diet assignments (Table 2). We observed significant changes from baseline SU among those on the FV diet (−0.17 mg/dL; 95% CI:−0.28, −0.06) and the DASH diet (−0.22 mg/dL; 95% CI: −0.35, −0.08) (for complete distributions of change from baseline see Supplement Figure SF1). There was no change in mean SU from baseline among those assigned the control diet.
Table 2.
Mean baseline, 8-week, and difference in serum urate, mg/dL
| Diet | Baseline* | 8-weeks* | Difference | P |
|---|---|---|---|---|
| Control, N=107 | 5.65 (1.56) | 5.68 (1.55) | 0.03 (−0.10, 0.16) | 0.67 |
| Fruit & Vegetable, N=110 | 5.85 (1.27) | 5.68 (1.27) | −0.17 (−0.28, −0.06) | 0.004 |
| DASH, N=110 | 5.59 (1.55) | 5.37 (1.42) | −0.22 (−0.35, −0.08) | 0.002 |
Between-Diet Comparisons & Concurrent Change Analysis
Compared to the control diet, the FV diet reduced SU by 0.17 mg/dL (95% CI: −0.34, 0.00) and the DASH diet reduced SU by 0.25 mg/dL (95% CI: −0.43, −0.08) (Figure 1). There was no difference in SU changes between the DASH and FV diets.
Figure 1.
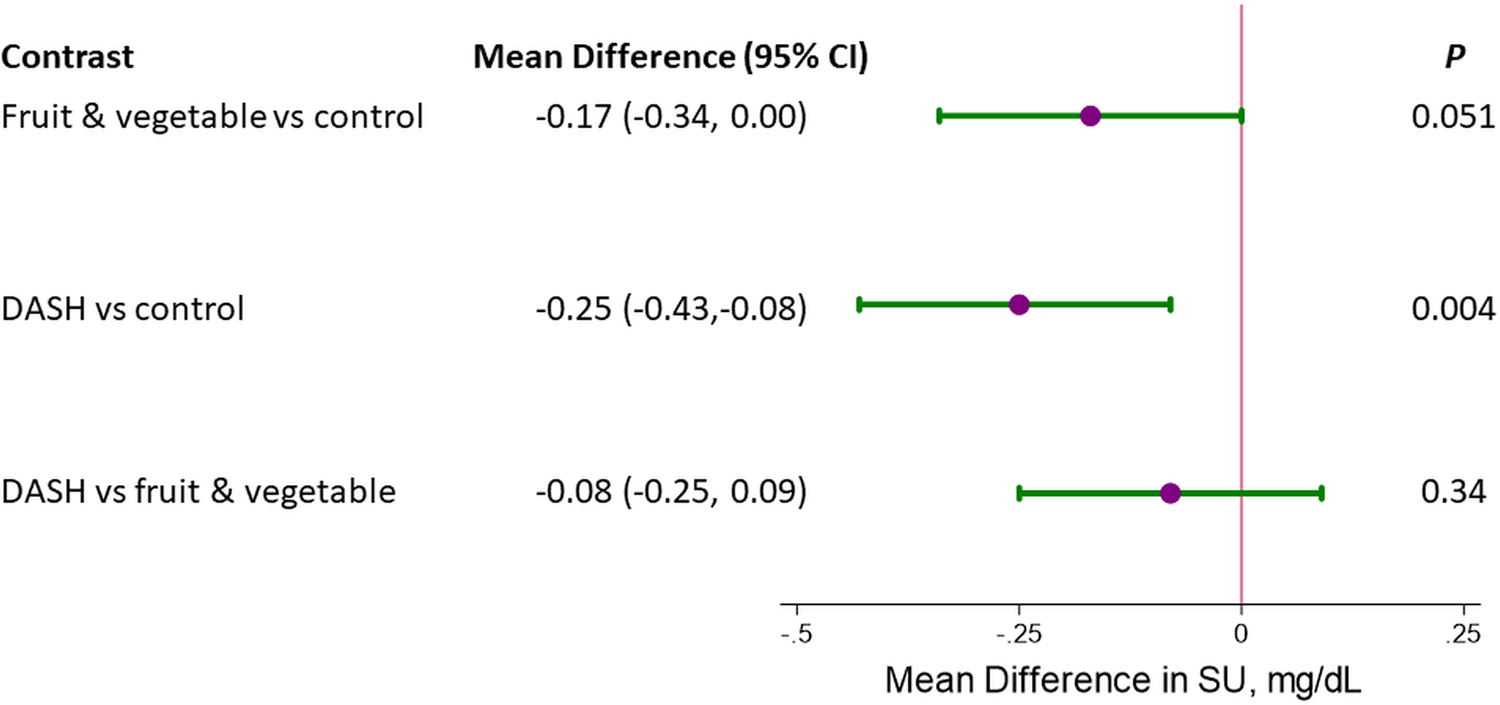
Mean difference in serum urate (mg/dL) between diets, comparing the fruit and vegetables diet versus control diet, the DASH (Combination) diet versus the control diet, and the DASH (Combination) diet versus the fruit and vegetables diet. All comparisons represent the difference in week 8 measurements adjusted for baseline measurements.
Adjustment for changes in blood pressure or LDL-cholesterol had little impact on the effects observed from the FV diet compared with the control diet (Table 3). Similarly, adjustment for blood pressure did not alter the effects of the DASH diet on SU compared with the control diet. However, adjustment for LDL-cholesterol did attenuate the effects of DASH on SU. Despite our reduced sample (327 vs 459), the effects of the diets on SBP, DBP, and LDL-cholesterol were consistent with the main study (Supplement Table ST3).
Table 3.
Between diet change in serum urate adjusted for concurrent changes in systolic blood pressure, diastolic blood pressure, and LDL-cholesterol, N=327*
| Fruit/Vegetable vs Control |
DASH vs Control |
DASH vs Fruit/Vegetable |
||||
|---|---|---|---|---|---|---|
| Mean Difference (95% CI) | P | Mean Difference (95% CI) | P | Mean Difference (95% CI) | P | |
| Unadjusted | −0.17 (−0.34, 0.00) | 0.051 | −0.25 (−0.43, −0.08) | 0.004 | −0.08 (−0.25, 0.09) | 0.34 |
| Adjusted for change in SBP | −0.17 (−0.34, 0.01) | 0.062 | −0.24 (−0.43, −0.06) | 0.009 | −0.08 (−0.25, 0.10) | 0.38 |
| Adjusted for change in DBP | −0.17 (−0.34, 0.01) | 0.058 | −0.24 (−0.41, −0.06) | 0.008 | −0.07 (−0.25, 0.10) | 0.41 |
| Adjusted for change in LDL-c* | −0.17 (−0.34, 0.00) | 0.057 | −0.21 (−0.38, −0.04) | 0.018 | −0.04 (−0.21, 0.13) | 0.63 |
| Adjusted for SBP, DBP, and LDL-c* | −0.16 (−0.34, 0.01) | 0.063 | −0.20 (−0.39, −0.02) | 0.028 | −0.04 (−0.21, 0.13) | 0.66 |
Abbreviations: SBP represents systolic blood pressure; DBP represents diastolic blood pressure; LDL-c represents low density lipoprotein-cholesterol
In models adjusted for LDL-c, N=324.
We also compared the association between baseline change in SU and change in baseline SBP, DBP, or LDL-cholesterol (Table 4; Supplement Figure SF2). Changes in SBP or DBP from baseline were not significantly associated with changes in SU from baseline; however, for SBP there was evidence of an interaction across diets. In contrast, change in LDL-cholesterol from baseline was strongly associated with change in SU from baseline (0.49 mg/dl per 1 mg/dL change in LDL-cholesterol; 95% CI: 0.16, 0.81) independent of dietary assignment.
Table 4.
Association of baseline changes with change in serum urate from baseline
| Serum Urate, mg/dL |
|||
|---|---|---|---|
| Change from baseline in CVD risk factor | N | Mean Difference (95% CI) | P |
| Systolic blood pressure, mm Hg | 327 | 0.07 (−0.97,1.12) | 0.90 |
| Diastolic blood pressure, mm Hg | 327 | 0.34 (−1.15,1.86) | 0.65 |
| LDL cholesterol, mg/dL* | 324 | 0.48 (0.15,0.80) | 0.004 |
Adjusted for age, sex, black race, diet assignment
Note some LDL cholesterol values were missing
Subgroup Analysis
The effects of the FV diet on SU compared to the control diet were not modified by strata of age, sex, race, baseline hypertension, baseline obesity, or baseline SU levels (Figure 2A). In contrast, the DASH diet had greater effects on SU in adults without baseline hypertension (P-interaction = 0.02) (Figure 2B). Furthermore, there was an incrementally greater reduction in SU among adults with higher SU at baseline (P-trend = 0.04), such that the DASH diet reduced SU by 0.08, 0.12, 0.42, 0.44, and 0.73 mg/dL with increasing baseline SU levels (<5, 5–5.9, 6–6.9, 7–7.9, and ≥8 mg/dL). Findings were unaltered when we used alternative categories of baseline SU levels (Supplement Table ST4).
Figure 2.
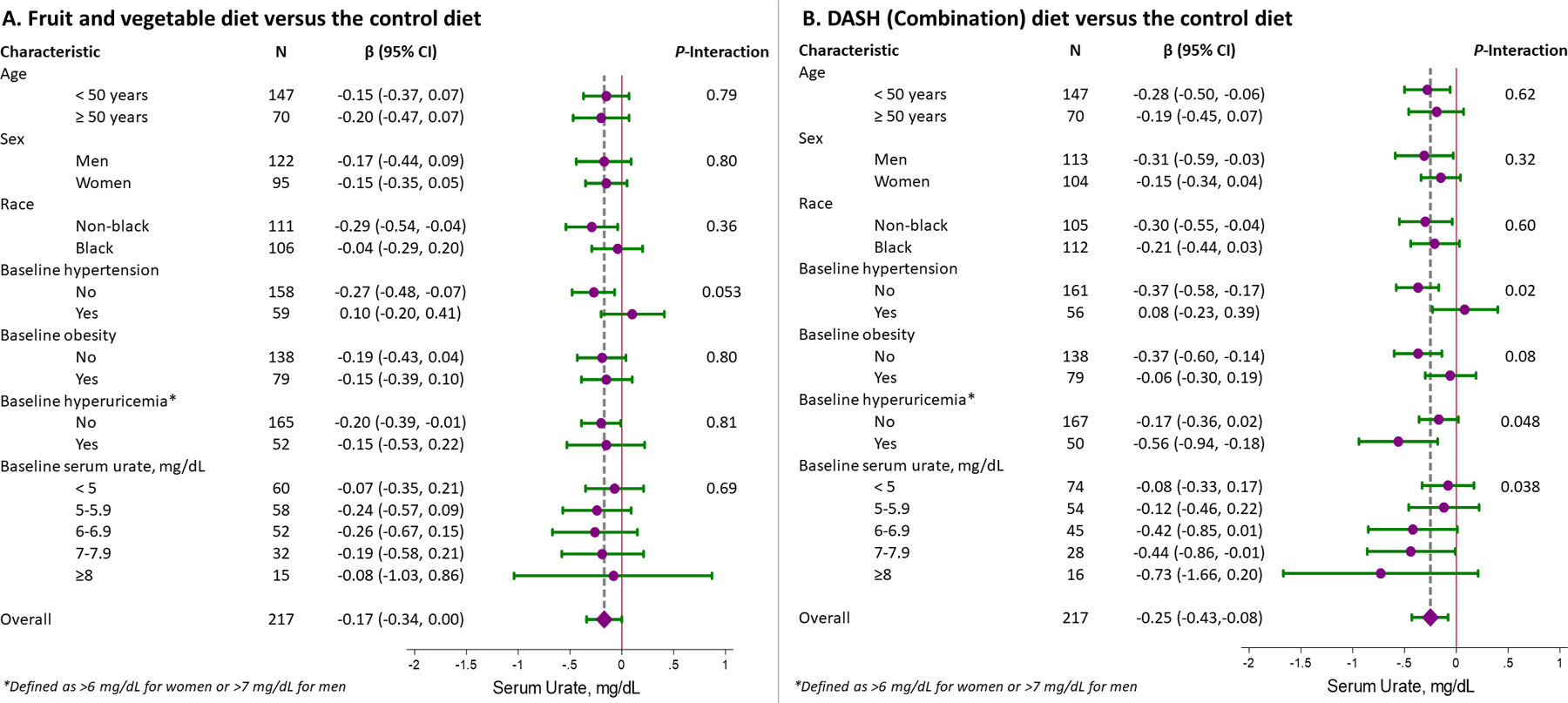
Mean difference in serum urate (mg/dL) between diets, comparing (A) the fruit and vegetables diet versus control diet and (B) the DASH (Combination) diet versus the control diet in strata of demographic groups and relevant comorbidities. All comparisons represent the difference in week 8 measurements adjusted for baseline measurements. Note that baseline hyperuricemia is defined as a baseline serum urate > 6 mg/dL for women or > 7 mg/dL for men.
Discussion
In this secondary analysis of the DASH trial, compared to a typical American diet, the DASH diet significantly reduced SU after an 8-week intervention period. These effects were significantly greater in those with higher SU concentrations at baseline. Furthermore, the observed effects were strongly associated with change in LDL-cholesterol, but not change in BP. In contrast, while the FV diet lowered SU, the urate-lowering effects were of borderline significance. Overall, our findings suggest that merely increasing fruit and vegetable consumption is less efficacious for SU reduction than adopting the complete DASH diet, particularly its features that optimize reduction in LDL-cholesterol.
Diet has been implicated as a determinant of hyperuricemia for thousands of years largely anecdotally or in observational studies (1). Nutrition recommendations for gout have predominantly focused on the purine scavenger pathway as a means of reducing urate precursors (2–4). However, the sustainability and benefits of a conventional low purine (i.e., low protein) approach has been called into question, even among those with hyperuricemia and gout. When reducing the intake of one macronutrient (e.g., protein), there must be a compensatory increase in one or both of the remaining macronutrients (e.g., carbohydrates and fats). Given the prevalence of Western-style diets and deterioration of healthy eating habits (12), there is a concern that protein-restriction may result in substitution of foods that are rich in refined carbohydrates (including fructose) and saturated or trans fats. These changes could further exacerbate insulin resistance, leading to higher plasma levels of glucose and lipids, thereby contributing to the development and worsening of metabolic syndrome and its complications in patients with hyperuricemia and gout (13,14). Meanwhile, the DASH diet has been shown to lower BP and LDL-cholesterol (9), two CV risk factors often elevated in adults with gout (5,15). Recently, we demonstrated in the DASH-Sodium trial that the DASH diet lowered SU by about −0.35 mg/dL, with a greater magnitude observed (i.e., −1.3 mg/dL) among adults with a baseline SU ≥7 mg/dL (6). In a later translational trial, Five Plus Nuts and Beans, we showed that while $30/day of high potassium fruits and vegetables modeled after DASH did not lower SU, there was a significant trend toward greater reduction among adults with a baseline SU > 8 mg/dL (8). Furthermore, longitudinal studies found that higher adherence to the DASH diet was associated with a lower risk of gout (7). The present study adds to these previous findings by showing that the DASH diet reduces SU, particularly among adults with hyperuricemia, which was not apparent with the FV diet.
Nutrition experts have questioned the necessity of the whole DASH diet for SU reduction. Indeed, an emphasis on one of its subcomponents, for example increased fruit and vegetables, represents a simplified public health prevention strategy that could promote greater adherence. However, our study shows that a diet which only increases fruit and vegetable servings may not achieve the same magnitude of urate reduction as the DASH diet, particularly among those with hyperuricemia. Given that the DASH diet differed from the FV diet in several ways, it is difficult to isolate the exact feature that was responsible for the greater effects of DASH. Nevertheless, we suspect that the DASH emphasis on low fat and fat free dairy may be contributory, as dairy protein has been shown to lower SU in other clinical trials (16). Although it is important to note that the effects of the FV diet on SU did not differ significantly from DASH, suggesting a need for further research on dietary pattern for SU reduction. In addition, these diets were isocaloric such that weight was maintained during the duration of the trial. Studies have shown that weight loss diets, even those that are high in protein, may lead to reduction in SU (14,17) by lowering adiposity and insulin resistance, thereby enhancing uric acid excretion (13,18,19). Thus, further research on the total effect of diet, including both dietary composition and weight loss, on SU is warranted.
Multiple studies demonstrate a strong relationship between hyperlipidemia and hyperuricemia (5,20). Our study further showed that changes from baseline in LDL-cholesterol were associated with changes in SU. Moreover, adjustment for change in LDL-cholesterol attenuated the effects of the DASH diet on SU. These observations imply that dietary interventions that target LDL-cholesterol reduction may also reduce SU. This may be an important consideration in future dietary studies to optimize SU reduction. Notably, change in SBP and SU were less consistently associated across dietary interventions. We have observed similar differences previously with dietary sodium intake (21). This may suggest that dietary strategies targeting BP reduction may be less reliable for SU reduction.
Our study has limitations. First, specimens from all randomized participants were not available. Of the 132 individuals with missing data, 114 (i.e., 86%) were from one site. However, given that participants were randomized by site, these missing samples were not related to trial compliance or dietary assignment. Further, despite the reduced sample in our study, we were adequately powered to observe differences in SU between the dietary assignments. Second, the trial did not focus on enrolling persons with gout. Further, the presence of gout at baseline and the incidence of gout attacks during the trial was not assessed. Third, since this trial tested dietary patterns, it is challenging to isolate specific food groups or micronutrients that may account for the distinct SU effects. Likewise, we are not able to differentiate whether the diet-associated change in SU was due to decreased endogenous production or increased renal clearance of urate. Nevertheless, the differential effects of DASH on SU suggest that micronutrients or food groups unique to DASH (versus the FV or control diets) are likely causally involved. Fourth, the original DASH trial intentionally adjusted kilocalories to minimize weight loss. It is possible that this might underestimate the full impact of diet on SU in the setting of weight loss. Fifth, our subgroup analyses should be viewed cautiously in light of the multiple comparisons performed. Sixth, the magnitude of SU reduction was small even among the hyperuricemic subgroup relative to pharmacologic urate lowering therapy. While small SU reductions are important for population-wide, prevention strategies, our findings should not be viewed as a replacement for urate lowering therapy especially among patients with gout. Seventh, the study population did not focus on adults with gout and excluded a number of conditions (e.g., diabetes, heavy alcohol drinkers) that are prototypical of patients with gout. Further research is needed to confirm these findings in a gout population. Finally, our association analyses between change in LDL-cholesterol and SU are observational.
Our study also has several strengths. First, dietary interventions were tightly controlled and administered in a randomized fashion, allowing for inferences in a diverse population of adults with CVD risk factors often encountered among adults with hyperuricemia or gout. Second, we measured SU, the therapeutic target for preventing gout flares. As a result, the observed effects on SU are generalizable to the primary prevention of gout; although, these findings should be confirmed in a gout population. Third, our isocaloric design isolated the effects of diet from weight change and its effects on SU. Finally, having three dietary patterns with some overlap, allows for greater insights as to which components of diet might be important for reducing SU. In our study, the complete DASH diet seems to be important for urate reduction, which is informative for subsequent dietary intervention studies.
In conclusion, the DASH diet lowered SU particularly among adults with hyperuricemia. This reduction in SU was highly correlated with reductions in LDL-cholesterol. DASH may represent the optimal dietary approach to lower SU as an adjunct to urate lowering therapy in adults with hyperuricemia and gout; however, a definitive trial in adults with hyperuricemia and gout is needed.
Supplementary Material
Acknowledgements
We are indebted to the study participants for their sustained commitment to the DASH Trial.
This trial is registered at clinicaltrials.gov, number: NCT00000544
The full trial protocol is available via the NHLBI BioLincc repository.
Sources of Funding
This study was supported by NIH/NIAMS P50 AR060772 and R01 AR065944.
SPJ supported by a NIH/NHLBI K23HL135273 and NIH/NHLBI R21HL144876.
CY supported by National Institutes of Health Ruth L. Kirschstein Institutional National Research Service Award [T32 AR007258] and Rheumatology Research Foundation Scientist Development Award.
HKC was supported by a Rheumatology Research Foundation grant.
The original DASH trial was supported by grants (HL50981, HL50968, HL50972, HL50977, HL50982, HL02642, RR02635, and RR00722) from the National Heart, Lung, and Blood Institute, the Office of Research on Minority Health, and the National Center for Research Resources of the National Institutes of Health.
Abbreviations used:
- BMI
body mass index
- CI
confidence interval
- CV
cardiovascular
- DASH
Dietary Approaches to Stop Hypertension
- DBP
diastolic blood pressure
- FV
fruits and vegetables
- LDL-c
low density lipoprotein cholesterol
- SBP
systolic blood pressure
Footnotes
The authors report no disclosures.
Conflicts of interest
The authors have no conflicts of interest to report.
References
- 1.Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res Ther 2006;8 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2005;52:283–289. [DOI] [PubMed] [Google Scholar]
- 3.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350:1093–1103. [DOI] [PubMed] [Google Scholar]
- 4.Frank O Nutritional state and purine metabolism. Adv Exp Med Biol 1977;76B:266–268. [DOI] [PubMed] [Google Scholar]
- 5.Juraschek SP, Kovell LC, Miller ER, Gelber AC. Dose-response association of uncontrolled blood pressure and cardiovascular disease risk factors with hyperuricemia and gout. PLoS ONE 2013;8:e56546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juraschek SP, Gelber AC, Choi HK, Appel LJ, Miller ER. Effects of the Dietary Approaches to Stop Hypertension (DASH) Diet and Sodium Intake on Serum Uric Acid. Arthritis & Rheumatology (Hoboken, NJ) 2016;68:3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai SK, Fung TT, Lu N, Keller SF, Curhan GC, Choi HK. The Dietary Approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ 2017;357:j1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juraschek SP, White K, Tang O, Yeh H-C, Cooper LA, Miller ER. Effects of a Dietary Approach to Stop Hypertension (DASH) Diet Intervention on Serum Uric Acid in African Americans With Hypertension. Arthritis Care Res (Hoboken) 2018;70:1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 10.Anon. DASH Diet: What To Know | US News Best Diets. Available at: https://health.usnews.com/best-diet/dash-diet. Accessed January 25, 2018.
- 11.Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER, Lin P-H, et al. Effects on blood lipids of a blood pressure–lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr 2001;74:80–89. [DOI] [PubMed] [Google Scholar]
- 12.Mellen PB, Gao SK, Vitolins MZ, Goff DC. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med 2008;168:308–314. [DOI] [PubMed] [Google Scholar]
- 13.Fam AG. Gout, diet, and the insulin resistance syndrome. J Rheumatol 2002;29:1350–1355. [PubMed] [Google Scholar]
- 14.Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med 2012;125:679–687.e1. [DOI] [PubMed] [Google Scholar]
- 16.Dalbeth N, Ames R, Gamble GD, Horne A, Wong S, Kuhn-Sherlock B, et al. Effects of skim milk powder enriched with glycomacropeptide and G600 milk fat extract on frequency of gout flares: a proof-of-concept randomised controlled trial. Ann Rheum Dis 2012;71:929–934. [DOI] [PubMed] [Google Scholar]
- 17.Yokose C, McCormick N, Rai SK, Lu N, Curhan G, Schwarzfuchs D, et al. Effects of Low-Fat, Mediterranean, or Low-Carbohydrate Weight Loss Diets on Serum Urate and Cardiometabolic Risk Factors: A Secondary Analysis of the Dietary Intervention Randomized Controlled Trial (DIRECT). Diabetes Care 2020. Available at: https://care.diabetesjournals.org/content/early/2020/08/31/dc20-1002. Accessed September 28, 2020. [DOI] [PMC free article] [PubMed]
- 18.Quiñones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol 1995;268:E1–5. [DOI] [PubMed] [Google Scholar]
- 19.Ter Maaten JC, Voorburg A, Heine RJ, Ter Wee PM, Donker AJ, Gans RO. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci 1997;92:51–58. [DOI] [PubMed] [Google Scholar]
- 20.Chu NF, Wang DJ, Liou SH, Shieh SM. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur J Epidemiol 2000;16:13–17. [DOI] [PubMed] [Google Scholar]
- 21.Juraschek SP, Choi HK, Tang O, Appel LJ, Miller ER. Opposing effects of sodium intake on uric acid and blood pressure and their causal implication. J Am Soc Hypertens 2016;10:939–946.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


