Abstract
Objective:
Response to immunosuppression is highly variable in systemic sclerosis (SSc) related interstitial lung disease (ILD). We hypothesized that a composite serum Interferon Inducible Serum Protein Score would exhibit predictive significance for the response to immunosuppression in SSc-ILD.
Methods:
Serum samples collected in the Scleroderma Lung Study II, a randomized controlled trial of mycophenolate versus cyclophosphamide, were examined. Results were validated in an independent observational cohort on active treatment. A composite score of 6 interferon inducible proteins (IP-10, MIG, MCP-2, B2M, TNFR2, and MIP-3 beta) was calculated and its predictive significance for longitudinal forced vital capacity % predicted measurements was examined.
Results:
Higher baseline Interferon Inducible Protein Score predicted better response over 3 to 12-month visits in the mycophenolate (b=0.41, p=0.001) and cyclophosphamide (b=0.91, p=0.009) arms. In contrast, higher baseline c-reactive protein (CRP) levels predicted worse ILD course in both treatment arms. The predictive significance of Interferon Inducible Protein Score and CRP remained after adjustment for baseline demographic/clinical predictors.
During the second-year placebo treatment period of the cyclophosphamide arm, higher Interferon Inducible Protein Score at 12 months showed a trend for predicting worse ILD course (b=−0.61, p=0.068) while it continued predicting better response to active immunosuppression in the mycophenolate arm (b=0.28, p=0.029).
The predictive significance of baseline Interferon Inducible Protein Score was replicated in the independent cohort (rs=0.43; p=0.028).
Conclusions:
Higher Interferon Inducible Protein Score in SSc-ILD predicts better response to immunosuppression and could be potentially used for identifying patients who may derive the most benefit from these two treatments.
INTRODUCTION
Interstitial lung disease (ILD) is the leading cause of disease related mortality in systemic sclerosis (SSc) (1, 2). Scleroderma Lung Studies (SLS) I (3) and II (4) showed that both cyclophosphamide (CYC) and mycophenolate mofetil (MMF) were effective in the treatment of SSc-ILD as measured by serially obtained percent predicted forced vital capacity (FVC%). Moreover, a follow-up study indicated that short-term improvement in FVC% was associated with improved long-term survival (5). However, response to immunosuppression was highly variable between patients in both clinical trials. Moreover, CYC and MMF can be associated with serious side effects (3, 4, 6). Ideally, their use should be reserved for the subset of patients who are likely to respond to these medications. However, there are no widely accepted clinical or biological parameters to predict response to immunosuppression in SSc-ILD. Moreover, the extent of lung fibrosis on high resolution chest CT did not predict the change in FVC% from baseline with CYC treatment in SLS I (3). Thus, there is a substantial unmet clinical need for novel predictive biomarkers in SSc-ILD.
The interferon (IFN) signature is the most prominent and robustly replicated gene expression signature in peripheral blood cells of SSc patients. This signature was first described in whole blood samples (7, 8) but has since been replicated in peripheral blood mononuclear cells (9), as well as in lymphocytes and monocytes (10). These studies indicated that approximately half of SSc patients have a “lupus-like” IFN gene expression signature in their peripheral blood cells (7). However, serum samples are more accessible during routine clinical care and a more practical source for biomarkers development than peripheral blood cell RNA samples. Recent studies have shown that certain serum proteins correlate with the IFN gene expression signature in SSc (11, 12), enabling the utilization of these serum proteins as surrogate markers for the IFN activation status. The predictive significance of the IFN transcript or serum protein signature for response to immunosuppression has not been investigated in SSc.
Capitalizing on the valuable, prospectively collected serum samples in the SLS II study (4), we determined whether a composite serum IFN Inducible Protein Score has predictive significance for response to immunosuppression in SSc-ILD. We hypothesized that SSc patients with higher IFN inducible serum proteins levels are more responsive to immunosuppressive therapy with either MMF or CYC.
METHODS
Study participants:
All SLS II patients with an available baseline serum sample were included in the present study. The eligibility criteria of SLS II have been published previously (4). Briefly, key inclusion criteria were as follows: adults aged 18-75 years; well-defined SSc with limited or diffuse cutaneous involvement (13); active ILD as demonstrated by restrictive to borderline restrictive ventilatory impairment (FVC% <80-85 but ≥ 45) AND the presence of any ground glass opacity on high-resolution computed tomography (HRCT), exertional dyspnea (Grade 2 or worse on the Magnitude of Task component of the Mahler Baseline Dyspnea Index (14)); and disease duration less than 7 years (based on the 1st non-Raynaud’s symptom due to SSc). Key exclusion criteria included clinically significant pulmonary hypertension; clinically significant abnormalities on HRCT not attributable to SSc; smoking within the past 6 months; evidence of significant airflow obstruction; prior use of oral CYC or MMF for more than 8 weeks or use of CYC and/or MMF in the 30 days prior to randomization.
The SLS II protocol was approved by the institutional review board of participating sites, and written informed consent was obtained from all study participants.
SLS II study design:
Patients were randomized to receive either MMF for 2 years or oral CYC for one year followed by one year of placebo. Based on this design, both treatment arms were on active treatment during the first 12 months, while the participants in the MMF arm were continued on MMF therapy and those in the CYC arm were placed on placebo during the second year. The FVC% was the primary outcome and was measured every 3 months during the 24 month study period.
Serum proteins were also measured in sera collected from 39 healthy controls at the University of Texas Health Science Center at Houston (UTHSC-H)- (see Supplementary Methods)
SSc related autoantibodies were determined at the UTHSC-H divisional laboratories and the extent of disease based on involvement >20% was measured on HRCT (see Supplementary Methods for more details).
Serum protein assays and calculation of the IFN Inducible Protein Score:
Serum samples were collected at the baseline, 12-month, and 24-month visits and were immediately processed on-site on the day of collection according to a standardized protocol, and were subsequently aliquoted, stored in −80 0C freezers and shipped on dry-ice in batches to the central biorepository at the UTHSC-H. All 133 participants (63 and 71 in the MMF and CYC arms, respectively) with an available serum sample were included in the present study. Serum samples from healthy controls were processed/stored in the same manner as SLS II, except that no shipment was required. Only unthawed serum aliquots from SLS II participants and healthy controls were used.
The primary focus of the present study was the measurement of six IFN inducible proteins: Monokine Induced by Gamma Interferon (MIG), Interferon Gamma Induced Protein 10 (IP-10), Monocyte Chemotactic Protein 2 (MCP-2), Beta 2 Microglobulin (B2M), Tumor Necrosis Factor Receptor 2 (TNFR-2), and Macrophage Inflammatory Protein 3 beta (MIP-3b). The corresponding gene names of these six proteins are CXCL9, CXCL10, CCL8, B2M, TNFRSF1B, and CCL19, respectively. This protein list was selected following a two-step process. In step one, 14 serum cytokines were identified that correlated significantly (r>0.3 and PFDR <0.05) with the IFN gene expression signature in the baseline samples collected in the Scleroderma: Cyclophosphamide or Transplantation (SCOT) study (see Supplemental Material in (12)). In Step 2, six of these proteins were also confirmed as inducible by type I IFN in human peripheral blood cells based on in-vitro studies, according to the information obtained from the Interferome V 2.0 database (17).
Serum protein assays were performed by the CLIA certified Myriad Rules-Based Medicine (Austin, TX, USA) using Multi-Analyte Profiling (MAP) multiplexed immune assay. Although the primary focus of the current study was IFN inducible proteins, these serum proteins could not be measured in isolation with pre-designed multiplex panels. Therefore, 57 other serum proteins, belonging to predesigned Myriad MAPs were also measured as part of the multiplex assay. For the analysis, proteins with levels below the lower limit of quantification (LLOQ) in more than 50% of baseline SLS II participants were excluded. For the remainder of proteins, levels below the LLOQ were replaced by the LLOQ while proteins above the upper limit of quantitation (ULOQ) were replaced with ULOQ. The aforementioned six IFN inducible proteins were within the dynamic range of their respective assays for all samples and no adjustments were necessary. Thirty-four out of the other 57 proteins, including high sensitivity C-reactive protein (CRP), were detectable in more than 50% of baseline SLS II samples and were further analyzed. In addition, ultra-sensitive methods by Simoa Assays (Quanterix, Massachusetts, USA) (18) were employed to measure levels of two low-abundant cytokines, B lymphocyte chemoattractant (BLC – other name: CXCL13) and interleukin-6 (IL-6) previously implicated as biomarker in SSc-ILD (19, 20).
A composite score of MIG, IP-10, MCP-2, B2M, TNFR-2, and MIP-3b was calculated using a previously described method (7, 11, 21-23). Specifically, the protein levels were divided by the top 95th percentile level of each protein. Next, all values in the top 5% category were assigned a value of 1.0. Finally, the normalized values of the 6 proteins were summed up to obtain the IFN Inducible Protein Score.
Confirmation cohort:
For independent confirmation of study results, patients with SSc enrolled in the Prospective Registry for Early Systemic Sclerosis (PRESS) cohort were investigated. Briefly, PRESS is a multicenter, observational cohort of early diffuse SSc patients (disease duration < 3 years from onset of the first non-Raynaud’s phenomenon symptom of SSc) (24). All enrolled patients who fulfilled the following criteria were included in the present study: available serum sample at the baseline visit; no missing FVC% data at the baseline and 12-month visits; evidence consistent with SSc-ILD on HRCT, and treatment with immunosuppressive agents during the first year follow-up period. The serum samples in PRESS were processed and stored following the same procedures as in SLS II. Moreover, the IFN inducible proteins and CRP were measured using the same assays in the Myriad Rules-Based Laboratory.
Statistical analysis:
Depending on the distribution, raw or log2-transformed cytokine data were analyzed. Similar to the primary clinical outcome analysis in SLS II, (4) a joint model (25) combining a mixed effects model for the longitudinally obtained FVC%s with a survival model to handle non-ignorable missing data due to study dropouts, treatment failure, or death was used. In the primary analysis, the outcome was the course of FVC % measured at 3-month increments from 3 to 12 months, which corresponds to the time period in which patients in both treatment arms were receiving active treatment. The longitudinal model in the primary analysis included the following covariates: baseline protein level, baseline FVC%, and a linear time trend. Also an extended multivariable analysis was performed that contained baseline protein levels (ie. IFN Inducible Protein Score and CRP), in addition to baseline demographic and clinical variables that showed predictive significance in separate analyses (p<0.05), baseline FVC% and a linear time trend.
In a secondary analysis, we also investigated whether the serum protein levels at the month-12 visit had predictive significance for the course of FVC% over the 15-24 month visits. The longitudinal model in this analysis included the following covariates: protein levels at the 12-month visit; FVC% at the 12-month visit; and linear splines with a knot at 21 months to characterize the time trend.
The p-value for the analysis of individual serum protein levels was adjusted for multiple comparisons using False Discovery Rate (FDR) method by Benjamini-Hochberg (26).
In the confirmation cohort (PRESS), the majority of investigated patients had only two FVC measurements available during the first 12 months after enrolment (baseline and 12-month visit), thus a different, simplified approach for the analysis of data from these two time points was utilized. As previously described,(27) the predictive significance of IFN Inducible Protein Score for percent change in FVC% ([FVC% 12 month visit-FVC% baseline]/ FVC% baseline) was investigated by Spearman’s correlation.
All tests were 2-sided. The joint analyses were performed using the R package JMbayes, and all other analyses were conducted in SAS v9.4 (The SAS Institute, Cary, NC).
RESULTS:
Baseline characteristics
Among 142 enrolled patients, serum samples were available in 133 patients at the baseline, in 99 patients at the 12-month, and in 84 patients at the 24-month visit. The healthy controls had a similar age, gender and ethnic background to that of the SLS II participants (see Supplemental Table 1 for patient and control characteristics).
IFN Inducible Protein Score in Patients and Controls
The SLS II participants at the baseline visit had a significantly higher IFN Inducible Protein Score than healthy controls (fold difference = 2.19, p<0.001).
As shown in Supplemental Figure 1, the IFN Inducible Protein Score decreased significantly from the baseline to the 12-month visit (fold change=0.75; p <0.001 for MMF and fold change=0.76; p<0.001 for CYC). As shown in the Supplemental Figure 2, in the subgroup of patients with available serum samples at the 12- and 24-month visits (n=43 and n=41 in MMF and CYC arms, respectively), the IFN inducible protein score did not change significantly from 12-month to 24-month visits (p=0.994 and p=0.529 for MMF and CYC, respectively).
As shown in Supplemental Table 2, the baseline demographic and clinical variables did not show a significant association/correlation with the concurrent IFN Inducible Protein Score.
Predictive significance of individual serum protein levels for ILD course:
As mentioned previously, serum protein levels of six IFN inducible serum proteins, as well as 36 serum proteins involved in other immune pathways, were measured in the baseline SLS II samples as part of the multiplex assay. We subsequently investigated whether any individual baseline protein levels had predictive significance for the course of FVC% during the 3-12 follow-up period. As shown in Supplemental Table 3, only two serum proteins, MIG and IP-10 (both IFN inducible proteins) showed predictive significance for FVC% in both treatment arms in the same direction after correction for multiple comparisons. Specifically, higher baseline MIG and IP-10 levels predicted higher serial FVC% levels. The point estimates for the other four IFN inducible proteins were also towards higher serial FVC% levels, although their associations did not reach statistical significance.
Of note, two other proteins (ICAM-1 and Eotaxin-1) also reached statistical significance in both treatment arms after correction for multiple comparisons but the direction of prediction was not consistent between the two SLS II treatment arms for these two proteins.
Predictive significance of IFN Inducible Protein Score for ILD course:
Next, the predictive significance of IFN Inducible Protein Score was investigated. As shown in Table 1, higher baseline IFN Inducible Protein Score predicted better ILD course based on higher serial FVC% over 3 to 12 months in both treatment arms after adjustment for baseline FVC% (MMF/CYC: Estimate=0.41/0.91; p=0.001/0.009).
Table 1:
Predictive significance of IFN Inducible Protein Score for subsequent serial FVC%
| MMF Arm* | |||
|---|---|---|---|
| Predictive significance of baseline IFN Score for serial FVC%s 3-12 months | |||
| Point estimate | 95% CI | p-value | |
| Baseline IFN Inducible Protein Score | 0.41 | 0.23 to 0.59 | 0.001 |
| Baseline FVC% | 0.84 | 0.82 to 0.86 | <0.001 |
| Predictive significance of 12-month IFN Score for serial FVC%s 15-24 months | |||
| Point estimate | 95% CI | p-value | |
| 12-month IFN Inducible Protein Score | 0.28 | 0.11 to 0.69 | 0.029 |
| 12-month FVC% | 0.96 | 0.9 to 0.98 | <0.001 |
| CYC Arm* | |||
| Predictive significance of baseline IFN Score for serial FVC%s 3-12 months | |||
| Point estimate | 95% CI | p-value | |
| Baseline IFN Inducible Protein Score | 0.91 | 0.56 to 1.13 | 0.009 |
| Baseline FVC% | 0.87 | 0.84 to 0.9 | <0.001 |
| Predictive significance of 12-month IFN Score for serial FVC%s 15-24 months | |||
| Point estimate | 95% CI | p-value | |
| 12-month IFN Inducible Protein Score | −0.61 | −1.5 to 0.11 | 0.068 |
| 12 month FVC% | 1 | 0.96 to 1.08 | <0.001 |
All models also included time as an independent variable
In the secondary analysis pertaining to the second year of SLS II during which patients in the MMF arm continued to receive MMF and those in the CYC arm were switched to placebo (Table 1), higher IFN Inducible Protein Scores at 12 months continued to predict better response to immunosuppression in the MMF arm (estimate: 0.28, p=0.029), while higher IFN Inducible Protein Scores at 12 months showed a trend for predicting lower serial FVC% over the 15 to 24 month visits during the placebo treatment period of the CYC arm (estimate=−0.61; p=0.068).
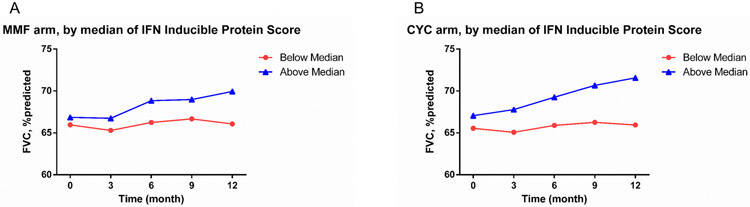
Our previous gene expression studies have shown that approximately half of patients with SSc have an IFN signature (7, 12). Building on this finding, IFN Inducible Protein Score was dichotomized based on the median value in the baseline patient samples. As shown in Figure 1, patients with a positive baseline IFN Inducible Protein Score had a more favorable ILD course over 3 to 12 months in both treatment arms compared with patients with a negative IFN Inducible Protein Score (MMF/CYC: Estimate= 1.28/2.6; p=0.003/0.004).
Figure 1:
Course of FVC% 3-12 months after randomization based on the baseline IFN Inducible Protein Score, dichotomized using the median value. Patients with a positive IFN score had higher FVC% levels in the MMF (Panel A- p=0.003) and CYC (Panel B- p=0.004) arms. This analysis is adjusted for baseline FVC% and used the same analytic approach like Tables 1-3.
We also examined whether baseline IFN Inducible Protein Score had predictive significance for the course of diffusing capacity of the lung for carbon monoxide % predicted (DLco%) over 3 to 12 months after randomization. Consistent with FVC% findings, higher IFN inducible protein score predicted higher serial DLco% in the CYC arm (estimate=0.7, 95% CI= 0.47 to 0.96, p<0.001). However, IFN inducible protein score did not significantly predict DLco% course in the MMF arm (estimate=−0.15, 95% CI= −0.42 to 0.15, p=0.146).
Predictive significance of CRP for ILD course:
Contrary to the favorable (i.e. positive) predictive value of the IFN Inducible Protein Score, higher CRP levels predicted a worse ILD course reflected in lower serial FVC% over the 3 to 12-month visits in both treatment arms after adjustment for baseline FVC% (Table 2).
Table 2:
Predictive significance of CRP for subsequent serial FVC%
| MMF Arm* | |||
|---|---|---|---|
| Predictive significance of baseline CRP for serial FVC%s 3-12 months | |||
| Point estimate | 95% CI | p-value | |
| Baseline CRP† | −0.15 | −0.31 to −0.01 | 0.038 |
| Baseline FVC% | 0.83 | 0.78 to 0.86 | <0.001 |
| Predictive significance of 12-month CRP for serial FVC%s 15-24 months | |||
| Point estimate | 95% CI | p-value | |
| 12-month CRP† | −0.61 | −0.7 to −0.51 | <0.001 |
| 12-month FVC% | 0.98 | 0.96 to 0.99 | <0.001 |
| CYC Arm* | |||
| Predictive significance of baseline CRP for serial FVC%s 3-12 months | |||
| Point estimate | 95% CI | p-value | |
| Baseline CRP† | −0.56 | −0.72 to −0.45 | <0.001 |
| Baseline FVC% | 0.90 | 0.86 to 0.92 | <0.001 |
| Predictive significance of 12-month CRP for serial FVC%s 15-24 months | |||
| Point estimate | 95% CI | p-value | |
| 12-month CRP† | −0.3 | −0.93 to −0.08 | 0.027 |
| 12 month FVC% | 1.01 | 0.97 to 1.12 | <0.001 |
All models also included time as an independent variable
Log2 transformed
In the secondary analysis, higher CRP levels at 12 months again predicted a worse ILD course reflected by lower serial FVC%s during the 15 to 24 month visits in both treatment arms (Table 2).
IFN Inducible Protein Score and CRP are independent predictors of ILD course:
As shown in Table 3, the predictive significance of baseline demographic and clinical variables for serial FVC%s over the 3 to 12 month visits were first examined in separate models after adjustment for baseline FVC% for each treatment arm.
Table 3:
Separate analyses to examine the predictive significance of baseline demographic and clinical variables for serial FVC% 3 to 12 months*
| MMF Arm | CYC Arm | |||
|---|---|---|---|---|
| Baseline variable | Point estimate (95% CI) |
p-value | Point estimate (95% CI) | p-value |
| Age in year | −0.05 (−0.18 to 0.08) | 0.462 | 0.04 (−0.06 to 0.15) | 0.411 |
| Female Sex | 0.04 (−0.53 to 0.69) | 0.891 | 1.17 (−0.09 to 2.26) | 0.058 |
| African American race | −0.68 (−1.26 to −0.13) | 0.032† | −2.4 (−3.04 to −1.9) | <0.001† |
| Diffuse disease type | 1.15 (0.43 to 2.06) | 0.005† | −1.97 (−3.34 to −0.77) | 0.008† |
| Disease duration | 0.04 (−0.06 to 0.15) | 0.314 | 0.12 (0.01 to 0.25) | 0.042† |
| mRSS | 0.07 (0.04 to 0.11) | 0.002† | −0.04 (−0.14 to 0.06) | 0.392 |
| Anti-Topoisomerase | −0.14 (−1.12 to 0.81) | 0.729 | −0.35 (−2.23 to 1.62) | 0.654 |
| Anti-RNA polymerase | 0.83 (−0.61 to 2.06) | 0.175 | 1.08 (−2.08 to 4.17) | 0.425 |
| Extensive disease on HRCT # | −2.45 (−2.85 to −2.11) | <0.001† | 0.09 (−2.18 to 2.36) | 0.79 |
Each row represents a separate model that included one baseline clinical variable, baseline FVC% and time as independent variables
Baseline demographic and clinical variables showing predictive significance in separate models that were included in the subsequent extended multivariable model (Tables 4 and 5).
QILD >20% on high resolution chest CT (HRCT)
Next, the predictive significance of IFN Inducible Protein Score and CRP (both as continuous variables) was investigated in an extended multivariable model after adjustment for baseline FVC%, in addition to variables showing predictive significance in the above separate analyses in the MMF arm (Table 4) and in the CYC arm (Table 5). Similar to above findings, higher baseline IFN Inducible Protein Scores predicted better ILD course and higher baseline CRP levels predicted worse ILD course over the 3 to 12 month visits after adjustment for baseline demographic and clinical variables in both treatment arms.
Table 4:
Predictive significance of baseline IFN Inducible Protein Score and CRP after adjustment for baseline demographic and clinical variables for serial FVC% 3 to 12 months in the MMF arm*
| Baseline variable | Point estimate | 95% CI | p-value |
|---|---|---|---|
| IFN Inducible Protein Score | 0.32 | 0.11 to 0.52 | 0.013 |
| CRP† | −0.13 | −0.24 to −0.01 | 0.041 |
| African American race | 0.95 | 0.43 to 1.41 | 0.004 |
| Diffuse disease type | 0.39 | −0.19 to 1.05 | 0.139 |
| mRSS | 0.05 | 0.03 to 0.09 | 0.008 |
| Baseline FVC% | 0.81 | 0.78 to 0.83 | <0.001 |
| Extensive disease on HRCT | −2.27 | −2.70 to −1.80 | <0.001 |
Time was also included as an independent variable
Log2 transformed
Table 5:
Predictive significance of baseline IFN Inducible Protein Score and CRP after adjustment for baseline demographic and clinical variables for serial FVC% 3 to 12 months in the CYC arm*†
| Baseline variable | Point estimate | 95% CI | p-value |
|---|---|---|---|
| IFN Inducible Protein Score | 0.92 | 0.79 to 1.04 | <0.001 |
| CRP† | −0.46 | −0.53 to −0.39 | <0.001 |
| African American race | −2.01 | −2.31 to −1.71 | <0.001 |
| Diffuse disease type | −0.60 | −0.91 to −0.33 | 0.005 |
| Disease duration | 0.19 | 0.12 to 0.26 | 0.002 |
| Baseline FVC% | 0.90 | 0.89 to 0.91 | <0.001 |
Time was also included as an independent variable
Log2 transformed
Predictive significance of IFN score was confirmed in an independent cohort:
The predictive significance of IFN Inducible Protein Score and CRP was investigated in the independent, observational PRESS cohort. In this cohort, 47 patients had a baseline serum sample and had FVC% measurements at the baseline and 12-month visits; of these, 31 (66%) had evidence of SSc-ILD on HRCT. Out of these 31 patients, 26 were treated with immunosuppressive agents (23 with MMF and 3 with methotrexate) during the first year follow-up period and were included in the present study. Supplemental Table 4 shows their demographic and clinical characteristics. Confirming our findings in SLS II, higher baseline IFN Inducible Protein Score predicted increasing FVC levels; specifically the baseline IFN Inducible Protein levels correlated positively with percent change in FVC% at 12 months (Spearman’s correlation coefficient [rs]=0.43; p=0.028). This correlation remained significant even after exclusion of the three patients treated with methotrexate (n=23; rs=0.47; p=0.023). Of note, baseline CRP was not predictive of percent change in FVC% at 12 months in the PRESS cohort (p=0.828).
DISCUSSION:
In the well-characterized SLS II clinical trial cohort, higher IFN Inducible Protein Score levels predicted better response to MMF, as well as CYC, while higher baseline CRP levels predicted a worse ILD course. Moreover, the predictive significance of the IFN Inducible Protein Score was independent of CRP and clinical/demographic predictors. In the validation analysis, the predictive significance of IFN Inducible Protein Score was confirmed in the early diffuse SSc observational PRESS cohort.
In this study, a rigorous method was employed for calculation of the serum IFN Inducible Protein Score. Specifically, serum proteins included in the IFN Inducible Score correlated with the peripheral blood cell IFN transcript signature in our previous study of untreated SSc patients using the same protein assays (12) and were induced by type I IFN in in-vitro studies of human peripheral blood cells. Moreover, the method used for calculation of the composite score weighted each protein equally (21-23), ensuring that the overall IFN Inducible Protein Score is not skewed by few outlier values of one or two proteins. Thus, the utilized IFN Inducible Protein Score provides an accurate reflection of the type I IFN activation status in circulation in SSc-ILD. Of note, there is substantial overlap between type I and type II interferon inducible genes/proteins. Based on the information in the Interferome database, the 6 utilized serum proteins can be induced by both type I and type II IFN. Therefore, we cannot exclude that the investigated IFN composite score is in part driven by type II IFN. However, in a pilot study of anifrolumab (blocking antibody against IFNAR1) in 26 SSc patients, two of the proteins (B2M and IP-10 [CXCL10]), included in the composite score, decreased significantly after blocking the type I IFN receptor (28), providing direct human evidence that the IFN Inducible Protein Score is at least in part driven by type I IFN in patients with SSc.
In the present study, SSc-ILD patients with a higher IFN Inducible Protein Score were more responsive to immunosuppression with CYC and MMF. However, the results from the second year of the CYC arm (placebo phase) indicated that patients with an IFN excess profile at the 12-month visit had a worse ILD course without concurrent immunosuppressive treatment, while higher IFN Inducible Protein Score at the same visit continued predicting better ILD course in patients assigned to the MMF arm, who stayed on active immunosuppressive treatment during the second-year study period. This finding supports the notion that a high IFN score adversely affects SSc-ILD progression unless immunosuppressive treatment is administered. Thus, the IFN Inducible Protein Score in SSc acts as a predictive biomarker identifying likely responders to treatment rather than a prognostic biomarker that predicts the natural history of disease regardless of treatment status. The deleterious effect of IFN excess in SSc is supported by previous murine model and human studies (reviewed in (29)). A previous study on the role of Interferon regulatory factor 5 (IRF5), the bleomycin induced dermal and lung fibrosis was attenuated in Irf5 deficient mice. Moreover, there was in-vitro evidence that profibrotic transcriptional activity of IRF5 in fibroblasts was enhanced by TGF-β (30). A more recent study on the role of interferon regulatory factor 7 (IRF7) in SSc pathogenesis, bleomycin induced dermal fibrosis, as well as hypodermal fibrosis in tight skin mouse were attenuated in Irf7 deficient mice. Moreover, IRF7 blockade attenuated fibrotic response to TGFβ in SSc dermal fibroblasts (31). In term of direct human data, a previous randomized controlled trial in which SSc patients were treated with recombinant IFNα or placebo had to be stopped prematurely because IFNα treated patients demonstrated a significantly worse ILD course as measured by FVC% (32). More recently, in a phase I trial of SSc patients treated with the anti- Type I IFN receptor antibody anifrolumab, skin gene expression studies showed evidence of suppressed TGF-β signalling in the anifrolumab treated group (28). Cumulatively, these data indicate that IFN excess is deleterious in SSc but also identifies patients who are more likely to benefit from immunosuppressive treatment.
In the present study, higher CRP predicted worse ILD course in SSc patients on active immunosuppressive treatment as well as during the second year placebo phase in the CYC arm, indicating that CRP, as a general inflammatory marker, is a prognostic biomarker that predicts worse FVC course regardless of treatment status. This finding is also supported by previous observational studies showing higher baseline CRP levels are predictive of reduced survival (33) and faster FVC% decline in SSc (34). More recently, in a retrospective study of 24 SSc-ILD patients treated with 6 monthly infusions of CYC, higher CRP level was significantly associated with poor response (35). Of note, higher CRP levels in the confirmation cohort did not predict the course of ILD in the present study. This might be due to the small sample size and/or the more heterogeneous patient population in PRESS, where a general inflammatory marker like CRP can be influenced by extra-pulmonary factors. Moreover, patients in the PRESS cohort had different baseline patient characteristic than SLS II participants. Specifically, all PRESS patients had diffuse cutaneous involvement and had disease duration less than three years. Moreover, 30% of investigated PRESS patients had baseline FVC% > 85% and therefore would have not met one of the inclusion criteria for SLS II.
In addition to IFN inducible proteins, 36 immune pathway related serum proteins including IL-6 were measured in the present study. In a previous observational study, higher IL-6 levels were predictive of worse ILD course (19). In the present study, IL-6 was not predictive of FVC% course neither in the MMF nor the CYC arms (Supplemental Table 3). Similarly, anti-topoisomerase I was not predictive of ILD course (Table 3). This finding is not consistent with our previous finding in an observational cohort of early SSc patients with and without ILD where anti-topoisomerase I was predictive of faster decline in FVC% (36), supporting the notion that anti-topoisomerase loses its predictive significance in a study population that includes only patients with clinically significant ILD.
This study has several strengths. To our knowledge, this is the first study examining the predictive role of serum IFN inducible proteins in a randomized controlled clinical trial of SSc-ILD. All serum protein assays were performed in the same CLIA-certified laboratory using rigorously standardized procedures. In SLS II, repeated FVC% measurements were available, allowing for a more accurate reflection of ILD progression. Patients were treated according to standardized, uniform treatment protocols, decreasing the potential confounding effect of treatment heterogeneity. Moreover, the predictive significance of the IFN Inducible Protein Score was shown in both the MMF and CYC arms separately, and confirmed in an independent observational study. Finally, SLS II was conducted in 14 centers across the U.S. and included patients from a diverse ethnic background increasing the generalizability of our findings.
The limitations of the present study include: The sample size in the confirmation cohort was relatively small. Furthermore, the SLS II did not include a placebo arm during the first year of study period, although this limitation is partially mitigated by the fact that the IFN Inducible Protein Score showed prediction in opposite directions during the second year when patients in the CYC arm were put on placebo while patients in the MMF arm continued receiving active immunosuppressive treatment. Furthermore, SLS II only included patients with disease duration less than 7 years; therefore, we could not investigate the predictive significance of the IFN Inducible Protein Score in patients with long-standing disease. Moreover, our findings should be further investigated in the recently completed study of nintedanib treatment for SSc-ILD (37) and future large clinical trials of anti-fibrotic agents in SSc-ILD with the ultimate goal of developing prediction models for identifying patients who would primarily benefit from immunosuppressive versus anti-fibrotic treatment.
In conclusion, SSc-ILD patients with higher serum IFN Inducible Protein Score are more likely to respond to MMF or CYC. The predictive significance of IFN Inducible Protein Score is independent of the general inflammatory marker CRP, which predicted worse ILD course regardless of the treatment regimen in SLS II. These serum proteins may be useful for more informed clinical decisions and clinical trial design and may ultimately lead to more personalized treatment regimens in SSc-ILD.
Supplementary Material
ACKNOWLEDGEMENT:
The authors thank Mr. Julio Charles and Ms. Hau Pham for managing the Scleroderma Lung Study II Biorepository. This manuscript is dedicated to the memory of Dr. Robert Elashoff, who passed away in October 2020. His knowledge, insights, and enthusiasm greatly contributed to this work.
Grant support: The Scleroderma Lung Study II (SLS II) was funded by NHLBI/NIH R01 HL089758 (DPT) and R01 HL089901 (RME). Roche Laboratories provided one of the study drugs (mycophenolate mofetil) in SLS II. The presented biomarker studies based on the samples collected from SLSII and PRESS cohort were funded by DoD W81XWH-16-1-0296 (SA) and NIH R01AR073284 (SA), Scleroderma Foundation SCORE Grant (SA, TF, MH, DK), NIH/NIAMS K24 AR063120 (DK) and R01 AR07047 (DK)
Reference List
- 1.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66(7):940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–15. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkmann ER, Tashkin DP, Sim M, Li N, Goldmuntz E, Keyes-Elstein L, et al. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann Rheum Dis. 2019;78(1):122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radis CD, Kahl LE, Baker GL, Wasko MC, Cash JM, Gallatin A, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum. 1995;38(8):1120–7. [DOI] [PubMed] [Google Scholar]
- 7.Assassi S, Mayes MD, Arnett FC, Gourh P, Agarwal SK, McNearney TA, et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 2010;62(2):589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan FK, Zhou X, Mayes MD, Gourh P, Guo X, Marcum C, et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford). 2006;45(6):694–702. [DOI] [PubMed] [Google Scholar]
- 9.York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56(3):1010–20. [DOI] [PubMed] [Google Scholar]
- 10.Duan R, Leo P, Bradbury L, Brown MA, Thomas GP. Gene Expression profiling reveals a down-regulation in immune-associated genes in AS patients. Ann Rheum Dis. 2009. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Mayes MD, Tan FK, Wu M, Reveille JD, Harper BE, et al. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheum. 2013;65(1):226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assassi S, Wang X, Chen G, Goldmuntz E, Keyes-Elstein L, Ying J, et al. Myeloablation followed by autologous stem cell transplantation normalises systemic sclerosis molecular signatures. Ann Rheum Dis. 2019;78(10):1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum. 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 14.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–8. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Li G, Gjertson D, Elashoff R, Shah SK, Ochs R, et al. Classification of parenchymal abnormality in scleroderma lung using a novel approach to denoise images collected via a multicenter study. Acad Radiol. 2008;15(8):1004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177(11):1248–54. [DOI] [PubMed] [Google Scholar]
- 17.Rusinova I, Forster S, Yu S, Kannan A, Masse M, Cumming H, et al. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41(Database issue):D1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivnak AJ, Rissin DM, Kan CW, Song L, Fishburn MW, Piech T, et al. A fully-automated, six-plex single molecule immunoassay for measuring cytokines in blood. J Immunol Methods. 2015;424:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De LA, Sestini P, Pantelidis P, Hoyles R, Hansell DM, Goh NS, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol. 2013;40(4):435–46. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi T, Miyagawa T, Toyama S, Yamashita T, Nakamura K, Saigusa R, et al. CXCL13 produced by macrophages due to Fli1 deficiency may contribute to the development of tissue fibrosis, vasculopathy and immune activation in systemic sclerosis. Exp Dermatol. 2018;27(9):1030–7. [DOI] [PubMed] [Google Scholar]
- 21.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 2006;3(12):e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 2009;60(10):3098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frech TM, Shanmugam VK, Shah AA, Assassi S, Gordon JK, Hant FN, et al. Treatment of early diffuse systemic sclerosis skin disease. Clin Exp Rheumatol. 2013;31(2 Suppl 76):166–71. [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, Elashoff RM, Li G. Robust joint modeling of longitudinal measurements and competing risks failure time data. Biom J. 2009;51(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Y B, Y H. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological); 1995. p. 289–300. [Google Scholar]
- 27.Salazar GA, Kuwana M, Wu M, Estrada YMRM, Ying J, Charles J, et al. KL-6 But Not CCL-18 Is a Predictor of Early Progression in Systemic Sclerosis-related Interstitial Lung Disease. J Rheumatol. 2018;45(8):1153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X, Higgs BW, Bay-Jensen AC, Karsdal MA, Yao Y, Roskos LK, et al. Suppression of T Cell Activation and Collagen Accumulation by an Anti-IFNAR1 mAb, Anifrolumab, in Adult Patients with Systemic Sclerosis. J Invest Dermatol. 2015;135(10):2402–9. [DOI] [PubMed] [Google Scholar]
- 29.Skaug B, Assassi S. Type I interferon dysregulation in Systemic Sclerosis. Cytokine. 2019. [DOI] [PubMed] [Google Scholar]
- 30.Saigusa R, Asano Y, Taniguchi T, Yamashita T, Ichimura Y, Takahashi T, et al. Multifaceted contribution of the TLR4-activated IRF5 transcription factor in systemic sclerosis. Proc Natl Acad Sci U S A. 2015;112(49):15136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu M, Skaug B, Bi X, Mills T, Salazar G, Zhou X, et al. Interferon regulatory factor 7 (IRF7) represents a link between inflammation and fibrosis in the pathogenesis of systemic sclerosis. Ann Rheum Dis. 2019;78(11):1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black CM, Silman AJ, Herrick AI, Denton CP, Wilson H, Newman J, et al. Interferon-alpha does not improve outcome at one year in patients with diffuse cutaneous scleroderma: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1999;42(2):299–305. [DOI] [PubMed] [Google Scholar]
- 33.Muangchan C, Harding S, Khimdas S, Bonner A, Group CSR, Baron M, et al. Association of C-reactive protein with high disease activity in systemic sclerosis: Results from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken ). 2012;64(9):1405–14. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Mayes MD, Pedroza C, Draeger HT, Gonzalez EB, Harper BE, et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis Care Res (Hoboken). 2013;65(8):1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumida H, Asano Y, Tamaki Z, Aozasa N, Taniguchi T, Toyama T, et al. Prediction of therapeutic response before and during i.v. cyclophosphamide pulse therapy for interstitial lung disease in systemic sclerosis: A longitudinal observational study. J Dermatol. 2018;45(12):1425–33. [DOI] [PubMed] [Google Scholar]
- 36.Assassi S, Sharif R, Lasky RE, McNearney TA, Estrada YMR, Draeger HT, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther. 2010;12(5):R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N Engl J Med. 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.