Abstract
Background:
Poor social connection is a central feature of posttraumatic stress disorder (PTSD), but little is known about the neurocognitive processes associated with social difficulties in this population. We examined recruitment of the default network and behavioral responses during social working memory (i.e., maintaining and manipulating social information on a moment-to-moment basis) in relation to PTSD and social connection.
Methods:
Participants with PTSD (n = 31) and a trauma-exposed control group (n = 21) underwent functional magnetic resonance imaging while completing a task in which they reasoned about two or four people’s relationships in working memory (social condition) and alphabetized two or four people’s names in working memory (non-social condition). Participants also completed measures of social connection (e.g., loneliness, social network size).
Results:
Compared to trauma-exposed controls, individuals with PTSD reported smaller social networks (p = .032) and greater loneliness (p = .038). Individuals with PTSD showed a selective deficit in social working memory accuracy (p = .029) and hyperactivation in the default network, particularly in the dorsomedial subsystem, on trials with four relationships to consider. Moreover, default network hyperactivation in the PTSD group (vs. trauma-exposed group) differentially related to social network size and loneliness (p’s < .05). Participants with PTSD also showed less resting state functional connectivity within the dorsomedial subsystem than controls (p = .002), suggesting differences in the functional integrity of a subsystem key to social working memory.
Conclusions:
Social working memory abnormalities in the default network may be a basic mechanism underlying poorer social connection in PTSD.
Keywords: PTSD, default network, social cognition, mental states, social reasoning, social support
Introduction
Impoverished social relationships and feelings of social isolation are risk factors, consequences, and maintenance factors in posttraumatic stress disorder (PTSD; Brewin et al., 2000; King et al., 2006; Ozer et al., 2003; Schnurr et al., 2004). Yet, the neurocognitive mechanisms underlying poor social connection in PTSD are not well understood. Poor social connection in PTSD may be due, in part, to impairments in neurocognitive mechanisms that support social cognition, defined as the perception and interpretation of information conveyed by others (Stevens & Jovanovic, 2019). Individuals with PTSD exhibit deficits in a range of social cognitive processes such as emotion recognition (Fonzo et al., 2010; Knežević & Jovančević, 2004; Mazza et al., 2012; Poljac et al., 2011; Schmidt & Zachariae, 2009; Shin et al., 2005), mental state inference (Allen & Fonagy, 2006; Mazza et al., 2012; Nazarov et al., 2014; Nietlisbach et al., 2010; Parlar et al., 2014), and empathic concern and perspective-taking (Mazza et al., 2012; Moore et al., 2017). PTSD is also associated with altered responses in brain systems associated with social cognition, particularly the brain’s default network, which includes the dorsomedial prefrontal cortex (DMPFC), medial prefrontal cortex (MPFC), posterior cingulate/precuneus (PC/PCC), tempoparietal junction (TPJ), lateral temporal cortex (LTC), and temporal poles (TPs) (Andrews-Hanna et al., 2010; Yeo et al., 2011). For example, individuals with PTSD show aberrant default network responses during tasks that require social cognition (Frewen et al., 2010, 2012; Tan et al., 2019) as well as during resting state scans (DiGangi et al., 2016; Sripada et al., 2012; Tursich et al., 2015; Zhang et al., 2015).
Yet, to date, existing studies have not addressed two issues. First, the consequences of abnormalities in the default network on social cognition in PTSD are not well characterized. Previous studies have assessed social cognition in a very unconstrained manner, for example by prompting participants to imagine a social interaction (Frewen et al., 2010, 2012) or imagine why someone might feel a certain emotion (Tan et al., 2019). What is missing is consideration of the specific social cognitive operations performed by the default network that may be altered in individuals with PTSD. Second, previous studies have not assessed whether aberrant social cognitive processing in the default network links to the difficulties connecting with others that are often experienced by individuals with PTSD.
To fill these gaps, we tested whether ‘social working memory’ (SWM) may be a specific social cognitive mechanism underpinned by the default network that is altered among individuals with PTSD and contributes to their compromised social connection. SWM refers to the maintenance and manipulation of information about people’s mental states, personalities, and relationships on a moment-to-moment basis (Meyer et al., 2012; Meyer, Taylor, et al., 2015; Meyer & Lieberman, 2012). SWM preferentially engages the brain’s default network: brain regions in this network increase activity as a function of the amount of social information managed in working memory (i.e., SWM ‘load’; Meyer, Taylor, et al., 2015; Meyer & Collier, 2020; Meyer & Lieberman, 2012). Moreover, in neurotypical samples, greater default network activity in response to SWM load correlates with social skills that are compromised in PTSD, including empathy and perspective-taking (Mazza et al., 2012; Meyer et al., 2012; Meyer, Taylor, et al., 2015; Moore et al., 2017). Additionally, individual differences in SWM have been linked to social network size (Krol et al., 2018; Meyer & Collier, 2020). Collectively, this work suggests individuals with PTSD may show altered responses in the default network while managing demands to SWM, which may help explain their poor social connection.
The objective of this study was to examine whether SWM is impaired, and associated with compromised social connection, in individuals with PTSD. We predicted that, compared to trauma-exposed control participants, individuals with PTSD would (1) exhibit poorer SWM performance and (2) altered recruitment of the default network while completing a SWM task. We also examined whether these outcomes linked to greater loneliness, smaller social networks, and/or less social support. Lastly, we explored group differences in default network resting state functional connectivity to assess potential parallels between functional integrity of the default network and neural patterns observed while engaging SWM.
Methods and Materials
Participants and Procedures
Participants aged 18–55 who experienced trauma defined by the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5; American Psychiatric Association, 2013) were recruited from the community and local Veterans Health Administration medical center. PTSD diagnosis was assessed with the Clinician-Administered PTSD Scale for DSM-5 (Weathers et al., 2017). Exclusion criteria included past-month moderate or severe substance use disorder, bipolar or psychotic disorder, imminent risk of suicide, and functional magnetic resonance imaging (fMRI) contraindications. Exclusionary diagnoses were assessed with the MINI for DSM-5 (Sheehan, 2016). All participants provided informed consent. This study was approved by the institutional review board at Dartmouth College and the Veteran’s IRB of Northern New England.
Fifty-eight qualified participants completed the fMRI scan (n = 34 PTSD, 24 controls). Three participants from each group were excluded from analyses because of outlying values on task accuracy (i.e., >2 SD outside of their group’s mean). The final sample included 31 participants with PTSD and 21 trauma-exposed controls (see Table 1 for demographic information). The most frequently endorsed index traumas were childhood physical or sexual assault (26.9%), physical or sexual assault in adulthood (25%), and military combat (15.4%). See Supplemental Table 1 for a full break-down of index traumas.
Table 1:
Demographic and Clinical Characteristics by Group
| Variable | Trauma-exposed (n = 21) | PTSD (n = 31) | t or χ2 | p |
|---|---|---|---|---|
| M (SD) or n (%) | ||||
| Age (Years) | 39.10 (11.63) | 39.06 (9.89) | .01 | .99 |
| Sex (Women) | 12 (57.1%) | 19 (61.3%) | .89 | .64 |
| Race/ethnicity (White) | 21 (100%) | 27 (87.1%) | 2.94 | .09 |
| Marital status (Married/cohabitating) | 10 (47.6%) | 14 (45.2%) | .03 | .86 |
| Education (BA/BS degree or higher) | 12 (60.0%) | 19 (63.3%) | .06 | .81 |
| Veteran status | 8 (38.1%) | 9 (29.0%) | .47 | .49 |
| PTSD symptom severity (CAPS-5) | 12.33 (7.64) | 35.42 (8.36) | 10.11 | <.001* |
| Loneliness (UCLA LS) | 48.71 (14.85) | 57.93 (13.13) | 2.34 | .03* |
| Social network size (SNI) | 11.95 (5.95) | 8.58 (4.99) | 2.21 | .03* |
| Social support-Overall (MSPSS) | 56.86 (16.03) | 52.16 (17.80) | .97 | .34 |
| Support from friends | 19.24 (5.51) | 17.68 (6.60) | .89 | .38 |
| Support from significant other | 20.16 (7.79) | 19.52 (7.84) | .29 | .77 |
| Support from family | 18.10 (7.06) | 14.32 (7.56) | 1.81 | .08 |
Note.
p < 0.05 level.
PTSD = Posttraumatic stress disorder. CAPS-5 = Clinician-Administered PTSD Scale for DSM-5 (Weathers et al., 2017). MSPSS = Multidimensional Scale of Perceived Social Support (Zimet et al., 1988). UCLA LS = UCLA Loneliness Scale (Russell et al., 1980). SNI = Berkman-Syme Social Network Index (Berkman & Syme, 1979).
Social Working Memory Task
Prior to fMRI scanning, participants watched a montage introducing a social network of characters from the program ‘Orange is the New Black’. The relationships among the characters include being friends, enemies, lovers and competitors. Groups did not differ in familiarity with the program (observed show previously: 48% PTSD, 42% controls, χ2 = .18, p = .67). Next, participants watched an instructional video describing the SWM task and completed six, unique practice trials (two per condition).
While undergoing fMRI, participants completed the SWM task (Figure 1), which uses a 2 (working memory type: SWM vs. non-SWM) × 2 (working memory load: two vs. four) experimental design. The task consists of an equal distribution of trials/condition, with a total of 36 randomly presented trials. For two-load SWM trials, participants observe two characters on the screen (4 s). The mental state of one character is provided, indicated by a thumbs-up (positive) or thumbs-down (negative). The mental state of the other, ‘target’ character is not provided. Next, there is a delay period (4 s) in which participants assess how the target character would feel on a likert scale of 1–4 (1 = very negative, 4 = very positive) based on the other character’s mental state and relationship with the target character. For example, if the two characters are friends and the character with the shown mental state is feeling positive, then the participant would reason that the target character, who likes their friend, would also feel positive. Next, participants make their response and the trial advances to jittered fixation, with a randomly generated duration between 1.55–4.47 s (M = 2.94 s).
Figure 1. Social working memory paradigm.
Note. Social working memory (SWM) paradigm. For SWM trials (Panels A and B), participants determine how the target character (indicated by pink box) would feel, based on the other characters’ feelings. The red thumbs-down sign indicates that a character is feeling negatively, whereas the green thumbs-up sign indicates that a character is feeling positively. For non-SWM trials (Panels C-D), participants alphabetize the characters’ names, based on which name anchors the alphabet line (indicated by the anchor sign). Panels A and C show two-load working memory trials and Panels B and D show four-load working memory trials. Participants encode the initial stimuli for 4 seconds, followed by a 4 second delay period. Participants next have up to 3 seconds to make their response. In the example shown in Figure 1B, respondents would reason serially about Piper’s mental state in the following sequence: she would be unhappy if Larry, her fiancé, is unhappy (rating = 1); she would be happier if she learned that Tiffany, her enemy, is unhappy (rating increases to 2); and she would be happier still if she learns that Claire, her girlfriend, is happy (rating increases to 3, the correct answer).
On four-load SWM trials, participants see three characters with mental states and one target character with no mental state and again assess how the target character would feel. Participants were instructed to reason about the mental states serially and ‘from left to right’ and with each additional character’s mental state considered independently. The final rating of the target character’s mental state is again on a scale of 1–4 (1 = very negative, 4 = very positive).
In the non-social WM trials, participants alphabetize the characters’ names during the delay period. Many working memory studies parameterize the amount of alphabetizing over a delay period (D’Esposito et al., 1999; Fougnie & Marois, 2007; Maniscalco & Lau, 2015; Postle, 2006; Postle et al., 1999). this condition therefore provides consistency with prior research, while also having participants reason based on (alphabetical) relationships. Participants see a set of characters and an anchor sign under one character (4 s), which indicates the alphabet line should start with the first letter of the character’s name with the anchor. For example, if the anchor appears under the character named Piper, ‘P’ should be treated as the first letter of the alphabet. To ensure that the answer could vary for two-load trials, participants are shown the character with the anchor for half of the probe-responses and the target character for the other half of the probe-responses. Participants then indicate the alphabetical position of the shown character. Task performance is based on accuracy (percent of correctly answered trials in each condition) and reaction time (of the correctly answered trials, average response speed).
fMRI Data Collection
Brain imaging was completed with a Siemens Trio 3T Prisma. Participants first completed an 8-minute, 24-second resting state scan, during which they observed a black screen and were instructed to rest but not sleep. Then they completed a T1-weighted structural scan acquired co-planar with the functional images (TR = 2300 ms, TE = 2.32 ms, 0.9 mm slice thickness, FOV = 24 cm, matrix = 256 × 256, flip angle = 8°), during which they watched the Orange is the New Black montage again.. The task was completed during two functional runs using an EPI gradient-echo sequence with the following parameters: TR = 1000 ms, TE = 30 ms, 2.5 mm slice thickness, FOV = 24 cm, matrix = 96 × 96 and flip angle = 59°.
Individual Differences in Social Connection
Participants completed self-report measures of social connection after the fMRI scan. Loneliness was measured with the UCLA Loneliness Scale (Russell et al., 1980). The Berkman-Syme Social Network Index (SNI; Berkman & Syme, 1979) was used to measure social network size. Perceived emotional support from family, friends, and significant others was measured with the Multidimensional Scale of Perceived Social Support (Zimet et al., 1988). Each of these scales are well-validated by prior research (Berkman & Syme, 1979; Knight et al., 1988; Zimet et al., 1990) and showed good internal consistency in our sample (ɑ’s > .87).
fMRI Data Analysis
Preprocessing.
Brain imaging data were reoriented in SPM8 (Wellcome Department of Cognitive Neurology, Institute for Neurology, London, United Kingdom) and skullstripped with Brain Extraction Tool (Smith, 2002) in FSL. Preprocessing steps using SPM8 included spatial realignment (i.e., to the mean), co-registration, normalization into a standard stereotactic space (defined by the Montreal Neurological Institute), and spatial smoothing (5-mm Gaussian kernel, full width at half-maximum). Few scans exceeded 0.5-mm translation or 0.5° rotation from the previous position (PTSD group: 3%, SD = 2.14%; controls: 2.33%, SD = 1.73%; t = 1.28, p = .21).
We created a general linear model for each subject that included a regressor for each experimental condition (convolved with the hemodynamic response function [HRF]), as well as six motion regressors of no-interest for each of the motion parameters from image realignment. This step allowed us to assess neural activity in response to each experimental condition, controlling for motion. High-pass filtering was applied using a cutoff period of 128 seconds.
Whole-brain analyses.
An interaction t-contrast assessed neural activity that varied as a function of working memory content (social vs. nonsocial) and load-level (two-load vs. four-load) for each participant. These interaction contrasts were submitted to a group-level independent samples t-test to search for clusters of neural activity in this contrast that varied between groups. We also assessed differences in neural activity in PTSD vs. trauma-exposed controls for working memory content (social vs. nonsocial, collapsed across load level) and load (four-load vs. two-load, collapsed across content). Whole-brain analyses were thresholded at p < .001, with a family-wise-error (FWE) corrected, cluster-extent defining threshold of p < .001 (Eklund et al., 2016).
Region-of-interest analyses (ROIs).
ROI analyses allowed us to compare task-related neural activity in individuals with PTSD (vs. trauma-exposed controls) in default network regions defined independently of our data. Yeo and colleagues’ (Yeo et al., 2011) 17 network parcellation generated ROIs for the three default network subsystems (Andrews-Hanna et al., 2010): the dorsomedial subsystem, which includes DMPFC, TPJ, middle temporal gyrus (MTG) extending into TP, and inferior frontal gyrus (IFG); the core subsystem, which includes MPFC and PC/PCC; and the medial temporal lobe (MTL) subsystem, which includes the hippocampal formation, retrosplenial cortex, and dorsal posterior inferior parietal lobule (dPIPL).
For each subject, we extracted the parameter estimates from these ROIs in response to each experimental condition separately. Next, for each set of ROIs comprising a default network subsystem, we computed each participant’s average parameter estimates from these ROIs to create a single measure of neural activity in each subsystem, for each experimental condition. Each subnetwork was submitted to a 2 (working memory content: social vs. nonsocial) x 2 (working memory load: two-load vs. four-load) x 2 (group: PTSD vs. trauma-exposed controls) analysis-of-variance (ANOVA).
Resting state data functional connectivity analysis.
After preprocessing steps conducted with fMRIprep [version 1.1.2; see Supplemental Information (Burgess et al., 2016; Jo et al., 2013; Power et al., 2015)], connectivity within each of the default mode network subsystems was calculated by taking the mean Fisher’s Z-transformed connectivity between each non-adjacent parcel within each of the systems for each participant.
Results
Behavioral Results
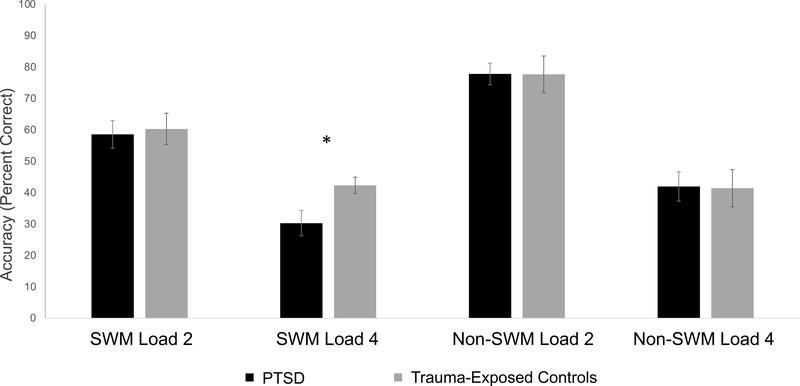
Three-way interactions from the 2 (working memory content: social vs. nonsocial) x 2 (working memory load level: two-load vs. four-load) x 2 (group: PTSD vs. trauma-exposed controls) ANOVAs were not significant for task accuracy or RT (F(1,50)accuracy = 1.44, p = .236, ηp2 = .028 and F(1, 42)RT = .68, p = .419, ηp2 = .016). However, individuals with PTSD showed a deficit in SWM four-load trial accuracy (t = 2.24, p = .029, Cohen’s d = .50; Figure 2). Task reaction times did not vary between groups for any load level (see Supplementary Figure 1).
Figure 2. Behavioral performance on the social working memory task.
Note. *p < .05. Task accuracy (percent correct) for each participant group by task condition.
Individuals with PTSD had smaller social networks (p = .032) and were lonelier (p = .038) than trauma-exposed controls but did not differ on social support (see Table 1). Relationships among social connection variables and SWM task performance are reported in Supplemental Information Table 2.
Brain Results
Whole-brain results.
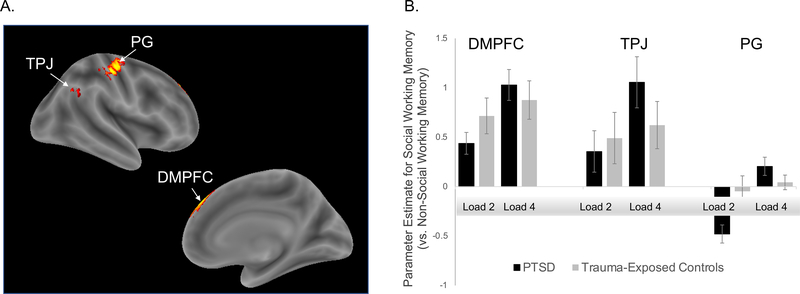
As shown in Figure 3, we observed hyperactivation in the dorsomedial subsystem of the default network in individuals with PTSD in response to the four-load SWM trials. The DMPFC and TPJ differentiated the social (vs. nonsocial) x load-level (two-load vs. four-load) interaction in the PTSD and control groups. We observed an additional cluster outside of the default network in precentral gyrus. Hyperactivation in individuals with PTSD (vs. trauma-exposed controls) was also found for the comparison of social (vs. nonsocial) working memory (collapsed across load level) throughout the default network (see Supplementary Figure 2), as well as in the lateral frontoparietal network associated with general working memory processing for the comparison of four vs. two-load working memory trials (collapsed across working memory content; see Supplementary Figure 3).
Figure 3. Whole-brain results.
Note. DMPFC = dorsomedial prefrontal cortex; TPJ = tempoparietal junction; PG = precentral gyrus. fMRI results for the interaction contrast testing for activity associated with four-load (vs. two-load) social working memory trials relative to four-load (vs. two-load) non-social working memory trials. Panel A depicts clusters of default network hyperactivation in response to four-load social working memory trials in the PTSD group. Panel B presents parameter estimates from each of these clusters for each condition within each group (PTSD vs. trauma-exposed controls).
Predefined default network ROI results.
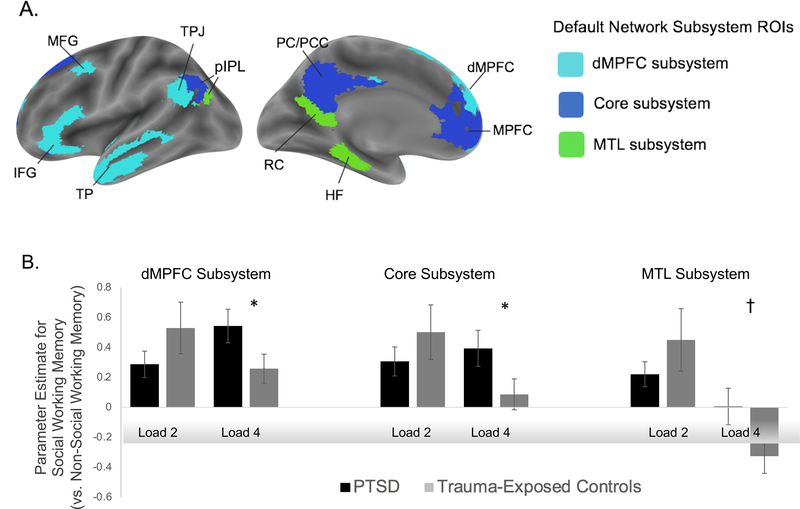
Consistent with our whole-brain results, the 2 (working memory content: social vs. nonsocial) x 2 (working memory load level: two-load vs. four-load) x 2 (group: PTSD vs. trauma-exposed controls) ANOVA was significant for the predefined, dorsomedial default network subsystem, F(1, 50) = 6.354, p = .015, ηp2 = .11. This three-way interaction was also significant for the predefined core subsystem, F(1, 50) = 5.81, p = .02, ηp2 = .10 and MTL subsystem, F(1, 50) = 4.53, p = .038, ηp2 = .08 (Figure 4). Post-hoc t-tests showed that each subsystem’s interactions were driven by hyperactivation to the four-load SWM trials. Specifically, we observed greater activation in the default network subsystems in response to SWM (vs. nonsocial working memory) four-load trials in individuals with PTSD (vs. trauma-exposed controls; DMPFCt = 1.78, p = .040, Cohen’s d = .52; Coret = 1.72, p = .045, Cohen’s d = .50; MTLt = 1.67, p = .05, Cohen’s d = .48; Figure 4). In contrast, there was no evidence of hyperactivation in response to the two-load SWM trials. That is, neural activity in response to SWM (vs. nonsocial working memory) two-load trials was not significantly different for individuals with PTSD (vs. trauma-exposed controls) in the core and MTL subsystems and were marginally less active in the dorsomedial subsystem in individuals with PTSD (vs. trauma-exposed controls; t’s < 1.28, p’s > .10, Cohen’s d’s < .38; Figure 4).
Figure 4. Regions of interest results.
Notes: Panel A shows clusters in the dorsomedial subsystem of the default network (Andrews-Hanna et al., 2010; Yeo et al., 2011). dMPFC = dorsomedial subsystem, including dorsomedial prefrontal cortex (DMPFC), temporoparietal junction (TPJ), middle temporal gyrus (MTG) extending into temporal pole (TP), and inferior frontal gyrus (IFG); core subsystem includes medial prefrontal cortex (MPFC), posterior cingulate/precuneus (PC/PCC); medial temporal lobe (MTL) subsystem includes hippocampal formation, retrosplenial cortex, and dorsal posterior inferior parietal lobule (dPIPL). Panel B depicts parameter estimates from each of the default network subsystems for each condition within each group of participants (PTSD and trauma-exposed controls). Statistical significance of posthoc t-tests are denoted as follows: *p < .05. †p < .10.
Brain-social connection relationships.
Participants’ neural responses in each ROI-defined default subsystem in response to social (vs. nonsocial) four-load trials identified as significant (i.e., the dorsomedial subsystem and core subsystem) were correlated with the variables that differed between the PTSD and control groups (i.e., loneliness and social network size), separately for each group. To assess whether hyperactivation differentially relates to less connection in the PTSD group, we directly compared these correlations between groups. The same analysis approach for perceived social support is reported in Supplementary Table 3.
Comparing the correlations between groups demonstrated that the PTSD and trauma-exposed control groups varied in how dorsomedial subsystem activity related to loneliness (Fisher’s z = 2.70, p = .007) as well as social network size (Fisher’s z = 1.98, p = .048). The core subsystem also differentially related to loneliness (Fisher’s z core subsystem = 2.58, p = .013). For each of these effects, default network activity was associated with greater social connection in the trauma-exposed control group whereas in the PTSD group, greater default network activity was associated with less social connection (Supplementary Figure 4).
Resting state functional connectivity.
Among participants that passed RSFC quality control for movement (n = 36 of the 51 participants included in task analyses), the PTSD group (n = 22) exhibited less connectivity within the dorsomedial subsystem than trauma-exposed controls (n = 14), t = −3.30, p < .001. Functional connectivity within the core and MTL subsystems did not differ across groups (p’s > .43). These results dovetail with the task-based findings, again pointing to altered responding in the dorsomedial subsystem of the default network in PTSD.
Discussion
Extant research indicates that PTSD is characterized by abnormalities in social cognitive processes and the default network (Plana et al., 2014; Stevens & Jovanovic, 2019). Yet, two questions have remained unanswered: 1) what are the consequences of abnormalities in the default network on precise social cognitive operations in PTSD, and 2) do these aberrations in the default network link to the poor social connection characteristic of individuals with PTSD? To answer these questions, we conducted the first study of social working memory (SWM) in trauma-exposed individuals with and without PTSD. We found that individuals with PTSD showed impaired SWM and associated neural abnormalities (e.g., hyperactivation in the default network), which corresponded to difficulties with social connection.
The PTSD group was less accurate than trauma-exposed individuals without PTSD on the more challenging four-load, but not two-load, SWM trials, suggesting that PTSD is associated with difficulty managing multiple people’s mental states in working memory. Whole-brain and ROI analyses revealed that the PTSD group exhibited hyperactivation within the dorsomedial subsystem of the default network during four-load SWM trials, which differentially related to their compromised social connection. At rest, the PTSD group also showed less functional connectivity within the dorsomedial subsystem than controls, suggesting PTSD-related differences in the functional integrity of the neural subsystem that supports SWM. These findings suggest that PTSD may be associated with compromised SWM skills when information processing demands are high and that hyperactivation in the default network in effort to meet these demands may be a cause and/or consequence of difficulty connecting with others.
Greater recruitment of the dorsomedial subsystem supports better SWM, which is in turn associated with better social skills and more social integration (Meyer et al., 2012; Meyer, Taylor, et al., 2015; Meyer & Collier, 2020). We found that individuals with PTSD exhibited hyperactivation within this subsystem, but also poorer accuracy on four-load trials compared to trauma-exposed controls--which we speculate may indicate that those with PTSD exert more cognitive effort when inferring others’ mental states. Critically, the compensatory cognitive effort needed to appraise complex social situations could backfire by eliciting anxiety, fatigue, and/or frustration, which may contribute to the social disengagement commonly observed in PTSD (Monson et al., 2010). This interpretation aligns with our observation that default network hyperactivation in the PTSD group was negatively associated with social connection. Therefore, poor SWM may be one neurocognitive mechanism by which PTSD degrades social connection.
In addition to abnormalities within the dorsomedial subsystem, our ROI analyses revealed PTSD-related hyperactivation in the core system (i.e., MPFC and PC/PCC) during four-load SWM trials, which was associated with greater loneliness. Components of the core subsystem support SWM when working memory trials require considering one’s own social network members (Krol et al., 2018; Meyer et al., 2012; Meyer, Taylor, et al., 2015). Interestingly, loneliness is also associated with altered mapping between neural representations of social network members in the core system (Courtney & Meyer, 2020). Given that our SWM task required managing mental state contingencies between social network members based on their relationships, it is possible that altered representations of social network relationships contributes to and/or is a consequence of loneliness in individuals with PTSD and compromises SWM computations in the core subsystem.
Our findings complement those of a recent study (Tan et al., 2019) in which participants with PTSD showed DMN hyperactivation in response to interpreting others’ emotions. This finding is compatible with our observation that individuals with PTSD exhibited hyperactivation in the default network when reasoning about multiple people’s emotional states. However, in contrast to the PTSD-related selective deficit in four-load SWM trials that we observed, this study did not reveal group differences in task performance, perhaps due to a ceiling effect in task performance. Because our SWM paradigm allows researchers to parameterize task difficulty, it is well-suited to identify individual differences in social cognitive ability. Moreover, there is evidence supporting the efficacy of SWM training for social skills (Meyer & Lieberman, 2016) pointing to the possible utility of SWM training translating to improvements in social connection among individuals with PTSD.
The ability to understand other minds is learned through interacting with others and, for many individuals with PTSD, relationships have served as sources of stress and trauma. Socially painful memories are preferentially retrieved by the default network, particularly the DMPFC (Meyer, Williams, et al., 2015), which is a key node of the dorsomedial subsystem shown here to be hyperactive in individuals with PTSD during SWM four-load trials. As such, future research can examine how harmful social experiences may impact SWM, perhaps by altering DMPFC function.
Limitations of this study include the relatively small sample and the cross-sectional design. Our sample was heterogenous in terms of index trauma type, which increases generalizability but does not allow for close examination of whether trauma type, such as interpersonal versus non-interpersonal trauma, has particularly strong associations with social working memory and social connection. As previously described (Meyer & Collier, 2020), while our calculation of task accuracy assumes that all relationship partners’ mental states impact each other equally, relationship partners likely impact each other’s mental states to different degrees, for example as a function of the importance of the relationship or the frequency of contact.
In conclusion, we provide the first evidence of abnormalities in SWM and their relationship to difficulties in social connection in PTSD. Given interpersonal factors in risk for, and maintenance of, PTSD (Nietlisbach & Maercker, 2009; Sharp et al., 2012), identifying abnormalities in specific social cognitive mechanisms in PTSD is critical. Our results highlight that individuals with PTSD may find managing multiple pieces of social information in mind particularly challenging, which may be due to atypical responding in the brain’s default network.
Supplementary Material
Acknowledgements
We gratefully acknowledge Anthony Annunziata, PhD, Michelle Bovin, PhD, Alex DaSilva, Kaitlyn Wellcome, MA, and Laurie Waterman, RRT, MS, CCRC for their assistance with data collection. Research reported in this manuscript was supported by the Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and the National Center for PTSD, U.S. Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Department of Veterans Affairs. These findings were presented at the 2021 annual meeting of the Anxiety and Depression Association of America.
Footnotes
Conflict of Interest
None of the authors have conflicts of interest for this article. Dr. Paul Holtzheimer receives consulting fees from Abbott and royalties from UpToDate and Oxford University Press. All other authors have no disclosures.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Allen JG, & Fonagy P (2006). The Handbook of Mentalization-Based Treatment. John Wiley & Sons. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65(4), 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, & Syme SL (1979). Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. Social Networks, 19. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, & Valentine JD (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology, 68(5), 748–766. 10.1037//0022-006X.68.5.748 [DOI] [PubMed] [Google Scholar]
- Burgess GC, Kandala S, Nolan D, Laumann TO, Power JD, Adeyemo B, Harms MP, Petersen SE, & Barch DM (2016). Evaluation of denoising strategies to address motion-correlated artifacts in resting-state functional magnetic resonance imaging data from the human connectome project. Brain Connectivity, 6(9), 669–680. 10.1089/brain.2016.0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney AL, & Meyer ML (2020). Self-other representation in the social brain reflects social connection. Journal of Neuroscience. 10.1523/JNEUROSCI.2826-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, & Lease J (1999). Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition, 41(1), 66–86. 10.1006/brcg.1999.1096 [DOI] [PubMed] [Google Scholar]
- DiGangi JA, Tadayyon A, Fitzgerald DA, Rabinak CA, Kennedy A, Klumpp H, Rauch SAM, & Phan KL (2016). Reduced default mode network connectivity following combat trauma. Neuroscience Letters, 615, 37–43. 10.1016/j.neulet.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, & Stein MB (2010). Exaggerated and disconnected insular-amygdalar BOLD response to threat-related emotional faces in women with intimate-partner violence PTSD. Biological Psychiatry, 68(5), 433–441. 10.1016/j.biopsych.2010.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougnie D, & Marois R (2007). Executive working memory load induces inattentional blindness. Psychonomic Bulletin & Review, 14(1), 142–147. 10.3758/BF03194041 [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Densmore M, Stevens TK, & Lanius RA (2010). Social emotions and emotional valence during imagery in women with PTSD: Affective and neural correlates. Psychological Trauma: Theory, Research, Practice, and Policy, 2(2), 145–157. 10.1037/a0019154 [DOI] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Lane RD, Densmore M, Stevens TK, & Lanius RA (2012). Emotional numbing in posttraumatic stress disorder: A functional magnetic resonance imaging study. The Journal of Clinical Psychiatry, 73(4), 431–436. 10.4088/JCP.10m06477 [DOI] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, & Saad ZS (2013, June 10). Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI [Research Article]. Journal of Applied Mathematics; Hindawi. 10.1155/2013/935154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DW, Taft C, King LA, Hammond C, & Stone ER (2006). Directionality of the association between social support and posttraumatic stress disorder: A longitudinal investigation1. Journal of Applied Social Psychology, 36(12), 2980–2992. [Google Scholar]
- Knežević M, & Jovančević M (2004). The IFEEL pictures: Psychological trauma and perception, and interpretation of child’s emotions. Nordic Journal of Psychiatry, 58(2), 139–145. 10.1080/08039480410005521 [DOI] [PubMed] [Google Scholar]
- Knight RG, Chisholm BJ, Marsh NV, & Godfrey HPD (1988). Some normative, reliability, and factor analytic data for the revised UCLA Loneliness scale. Journal of Clinical Psychology, 44(2), 203–206. [DOI] [PubMed] [Google Scholar]
- Krol SA, Meyer ML, Lieberman MD, & Bartz JA (2018). Social working memory predicts social network size in humans. Adaptive Human Behavior and Physiology, 4(4), 387–399. 10.1007/s40750-018-0100-9 [DOI] [Google Scholar]
- Maniscalco B, & Lau H (2015). Manipulation of working memory contents selectively impairs metacognitive sensitivity in a concurrent visual discrimination task. Neuroscience of Consciousness, 2015(1). 10.1093/nc/niv002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Giusti L, Albanese A, Mariano M, Pino MC, & Roncone R (2012). Social cognition disorders in military police officers affected by posttraumatic stress disorder after the attack of An-Nasiriyah in Iraq 2006. Psychiatry Research, 198(2), 248–252. 10.1016/j.psychres.2011.11.027 [DOI] [PubMed] [Google Scholar]
- Meyer ML, & Collier E (2020). Theory of minds: Managing mental state inferences in working memory is associated with the dorsomedial subsystem of the default network and social integration. Social Cognitive and Affective Neuroscience, 15(1), 63–73. 10.1093/scan/nsaa022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ML, & Lieberman MD (2012). Social working memory: Neurocognitive networks and directions for future research. Frontiers in Psychology, 3. 10.3389/fpsyg.2012.00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ML, & Lieberman MD (2016). Social working memory training improves perspective-taking accuracy. Social Psychological and Personality Science, 7(4), 381–389. 10.1177/1948550615624143 [DOI] [Google Scholar]
- Meyer ML, Spunt RP, Berkman ET, Taylor SE, & Lieberman MD (2012). Evidence for social working memory from a parametric functional MRI study. Proceedings of the National Academy of Sciences, 109(6), 1883–1888. 10.1073/pnas.1121077109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ML, Taylor SE, & Lieberman MD (2015). Social working memory and its distinctive link to social cognitive ability: An fMRI study. Social Cognitive and Affective Neuroscience, 10(10), 1338–1347. 10.1093/scan/nsv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ML, Williams KD, & Eisenberger NI (2015). Why social pain can live on: Different neural mechanisms are associated with reliving social and physical pain. PLOS ONE, 10(6), e0128294. 10.1371/journal.pone.0128294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson CM, Fredman S, & Dekel R (2010). Posttraumatic stress disorder in an interpersonal context. American Psychological Association. [Google Scholar]
- Moore TM, Risbrough VB, Baker DG, Larson GE, Glenn DE, Nievergelt CM, Maihofer A, Port AM, Jackson CT, Ruparel K, & Gur RC (2017). Effects of military service and deployment on clinical symptomatology: The role of trauma exposure and social support. Journal of Psychiatric Research, 95, 121–128. 10.1016/j.jpsychires.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov A, Frewen P, Parlar M, Oremus C, MacQueen G, McKinnon M, & Lanius R (2014). Theory of mind performance in women with posttraumatic stress disorder related to childhood abuse. Acta Psychiatrica Scandinavica, 129(3), 193–201. 10.1111/acps.12142 [DOI] [PubMed] [Google Scholar]
- Nietlisbach G, & Maercker A (2009). Social cognition and interpersonal impairments in trauma survivors with PTSD. Journal of Aggression, Maltreatment & Trauma, 18(4), 382–402. 10.1080/10926770902881489 [DOI] [Google Scholar]
- Nietlisbach G, Maercker A, Rösler W, & Haker H (2010). Are empathic abilities impaired in posttraumatic stress disorder? Psychological Reports, 106(3), 832–844. 10.2466/pr0.106.3.832-844 [DOI] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, & Weiss DS (2003). Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychological Bulletin, 129(1), 52–73. [DOI] [PubMed] [Google Scholar]
- Parlar M, Frewen P, Nazarov A, Oremus C, MacQueen G, Lanius R, & McKinnon MC (2014). Alterations in empathic responding among women with posttraumatic stress disorder associated with childhood trauma. Brain and Behavior, 4(3), 381–389. 10.1002/brb3.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana I, Lavoie M-A, Battaglia M, & Achim AM (2014). A meta-analysis and scoping review of social cognition performance in social phobia, posttraumatic stress disorder and other anxiety disorders. Journal of Anxiety Disorders, 28(2), 169–177. 10.1016/j.janxdis.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Poljac E, Montagne B, & de Haan EHF (2011). Reduced recognition of fear and sadness in post-traumatic stress disorder. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 47(8), 974–980. 10.1016/j.cortex.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Postle BR (2006). Working memory as an emergent property of the mind and brain. Neuroscience, 139(1), 23–38. 10.1016/j.neuroscience.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, & D’Esposito M (1999). Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proceedings of the National Academy of Sciences, 96(22), 12959–12964. 10.1073/pnas.96.22.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, & Petersen SE (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. 10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D, Peplau LA, & Cutrona CE (1980). The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. Journal of Personality and Social Psychology, 39(3), 472–480. 10.1037/0022-3514.39.3.472 [DOI] [PubMed] [Google Scholar]
- Schmidt JZ, & Zachariae R (2009). PTSD and impaired eye expression recognition: A preliminary study. Journal of Loss and Trauma, 14(1), 46–56. 10.1080/15325020802537096 [DOI] [Google Scholar]
- Schnurr PP, Lunney CA, & Sengupta A (2004). Risk factors for the development versus maintenance of posttraumatic stress disorder. Journal of Traumatic Stress, 17(2), 85–95. [DOI] [PubMed] [Google Scholar]
- Sharp C, Fonagy P, & Allen JG (2012). Posttraumatic stress disorder: A social-cognitive perspective. Clinical Psychology: Science and Practice, 19(3), 229–240. [Google Scholar]
- Sheehan DV (2016). The MINI International Neuropsychiatric Interview Version 7.0.2 for DSM-5.
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, & Rauch SL (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry, 62(3), 273–281. 10.1001/archpsyc.62.3.273 [DOI] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, & Liberzon I (2012). Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry and Neuroscience, 37(4), 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, & Jovanovic T (2019). Role of social cognition in post-traumatic stress disorder: A review and meta-analysis. Genes, Brain and Behavior, 18(1), e12518. 10.1111/gbb.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KM, Burklund LJ, Craske MG, & Lieberman MD (2019). Posttraumatic stress disorder and the social brain: Affect-related disruption of the default and mirror networks. Depression and Anxiety, 36(11), 1058–1071. 10.1002/da.22953 [DOI] [PubMed] [Google Scholar]
- Tursich M, Ros T, Frewen PA, Kluetsch RC, Calhoun VD, & Lanius RA (2015). Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatrica Scandinavica, 132(1), 29–38. 10.1111/acps.12387 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, & Marx BP (2017). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment. 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, & Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu F, Chen H, Li M, Duan X, Xie B, & Chen H (2015). Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. Journal of Affective Disorders, 187, 114–121. 10.1016/j.jad.2015.08.043 [DOI] [PubMed] [Google Scholar]
- Zimet GD, Dahlem NW, Zimet SG, & Farley GK (1988). The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment, 52(1), 30–41. 10.1207/s15327752jpa5201_2 [DOI] [PubMed] [Google Scholar]
- Zimet GD, Powell SS, Farley GK, Werkman S, & Berkoff KA (1990). Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment, 55(3–4), 610–617. 10.1080/00223891.1990.9674095 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.