Abstract
Introduction:
The generation of long-term durable tumor immunity and prolonged disease-free survival depends on the ability to generate and support CD8+ central memory T-cells. Microsatellite-stable colon cancer is resistant to currently available immunotherapies, thus, development of novel mechanisms to increase both lymphocyte infiltration and central memory formation are needed to improve outcomes in these patients. We have previously demonstrated that both Interleukin-2 (IL-2) and LIGHT (TNFSF14) independently enhance anti-tumor immune responses, and hypothesize that combination immunotherapy may increase the CD8+ central memory T-cell response.
Methods:
CT26 murine colorectal cancer tumors were established in syngeneic mice. Tumors were treated with control, soluble, or liposomal Interleukin-2 (IL-2) at established intervals. A subset of animal tumors overexpressed tumor necrosis superfamily factor LIGHT (TNFSF14). Peripheral blood, splenic, and tumor infiltrating lymphocytes were isolated for phenotypic studies and flow cytometry.
Results:
Tumors exposed to a combination of LIGHT and IL-2 experienced a decrease in tumor size compared to IL-2 alone that was not demonstrated in wild-type (wt) tumors or between other treatment groups. Combination exposure also increased splenic central memory CD8+ cells compared to IL-2 administration alone, while not increasing tumor-infiltrating lymphocytes. In the periphery, the combination enhanced levels of circulating CD8 T-cells and central memory T-cells, while also increasing circulating T-regulatory cells.
Conclusions:
Combination of IL-2, whether soluble or liposomal, with exposure to LIGHT results in increased CD8+ central memory cells in the spleen and periphery. New combination immunotherapy strategies that support both effector and memory T-cell functions are critical to enhancing durable anti-tumor responses and warrant further investigation.
Introduction
Cancers of the gastrointestinal (GI) tract account for the highest five-year cancer mortality of patients worldwide, and the most common GI tumor is colorectal cancer (CRC).1 As high densities of tumor infiltrating lymphocytes in primary and metastatic colon tumors have been associated with improved prognosis, immunotherapeutic approaches to GI cancer care have been pursued.2–4 Though we and others have demonstrated unprecedented responses to checkpoint blockade in other cancers,5–7 clinical trials have revealed that checkpoint blockade does not impart clinically meaningful responses in microsatellite stable (MSS, >95%) colon cancer, which is felt to have a lower neoantigen load than microsatellite instability-high (MSI-H) disease.8,9
It has been demonstrated, however, that that the degree of CD8+ T-cell immune infiltration is a better prognostic indicator of a patient’s survival than microsatellite status, and adoptive cell transfer of these cells has been associated with improved patient outcomes.10,11 We therefore developed a strategy to increase CD8 T-cell infiltration of primary and metastatic MSS colon cancer utilizing LIGHT (TNFSF14), an immunostimulatory cytokine required for activation of T-cells that can augment the antitumor immune response and overcome the stromal antigenic barrier.4,12–16 In a murine model of CRC, it was demonstrated that the immunoinhibitory threshold could be overcome by enhancing LIGHT expression in the tumor microenvironment, resulting in increased tumor infiltrating lymphocytes, lymphocyte homing signals, and CD8+ T-cell-mediated CRC tumor regressions.13
In order to further improve outcomes, generation of long-term tumor immunity is necessary. In vivo evaluation of CD8 T-cell subsets has identified central memory CD8 T-cells (TCM) as responsible for antigen recall and improved disease-free survival.17 Therefore, immunotherapy approaches that can specifically increase the CD8 TCM subset holds promise for CRC.
Interleukin-2 (IL-2) has been shown to increase the number of effector cells capable of differentiation into CD8 memory cells. The maturation and differentiation of CD8+ T-cells at all stages of the anti-tumor immune response are fine-tuned by IL-2, and exposure to exogenous IL-2 during the contraction phase of the antigen response increases the frequency of CD8+ memory T-cells.18–20 Therefore, we endeavored to increase memory CD8 T-cell populations in secondary lymphoid organs, i.e. the spleen, in a syngeneic immunocompetent model of colorectal cancer by incorporating IL-2 into the immunotherapeutic strategy. Specifically, we were interested in the impact upon central memory T-cells (Tcm), which are characterized by CD44+CD62L+, while effector memory T-cells are characterized by CD44+CD62L−.
In order to address some of the treatment limitations of soluble IL-2 administration, a multilamellar coalescent vesicle (MLCV) that packages recombinant human IL-2 (Oncolipin) was developed for clinical use.21 In this liposomal vesicle, IL-2 is entrapped between the bilayers of a multilamellar structure and attached to the outer surface so as to bind to the IL-2 receptor on T lymphocytes.22,23 The result is that IL-2 remains associated with MLCV for at least 72 hours at the site of tissue inoculation. This formulation has been utilized in a murine B16 melanoma model, where intratumoral inoculation resulted in an influx of T lymphocytes and tumor necrosis, and in human clinical trials (NCT00020462).23,24
This study seeks to enhance LIGHT-induced support of the anti-tumor immune response by combining it with intralesional administration of IL-2, soluble or in a liposomal multilamellar coalescent vesicle, with the aim of increasing central memory T-cells.
Methods:
Cell culture
Murine colorectal carcinoma cell line, CT26, was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), tested for mycoplasma, and used at low passage numbers. CT26 (ATCC CRL-2638) is an N-nitroso-N-methylurethane-(NNMU) induced, undifferentiated and microsatellite stable colon carcinoma cell line.25,26 The cells were cultured with RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate, 10 mM HEPES and 1% Penicillin/streptomycin in 37 °C incubator containing 5% CO2. All cell culture reagents were purchased from Invitrogen (Grand Island, NY, USA) and cells were passaged for 1 to 10 dilutions upon confluence. Constitutively expressing CT26LIGHT was engineered as previously described with stable expression of TNFSF14 on the cell surface with no significant difference in cell proliferation, apoptosis, or migration compared to wild-type (wt) CT26.13
Treatment of Flank Tumors
Six to12 week old female Balb/c mice weighing 18–20g were obtained from Charles River Labs (Wilmington, MA). All mouse experiments were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago (ACC 16–190) and performed in the animal facility (BRL) as per the approved protocol. Animals were utilized based on a biological difference in tumor response of at least 30% at an alpha of 0.05 and power of 80%.
Primary tumors were generated by subcutaneous injections of 2.0×10^5 CT26 or CT26LIGHT-expressing colon cancer cells in syngeneic Balb/C mice as we have previously described.13 Once the tumors were palpable and had reached a diameter of 5.0–7.5 mm, the mice were randomized blinded to tumor size into control, soluble IL-2, and liposomal IL-2 groups, n=38. In each of the groups, both wild-type (wt) and LIGHT expressing tumors were injected starting on day 7 with 25μL of empty control vesicles made of dimyristoylphosphatidyl choline (DMPC), soluble IL-2, or liposomal IL-2 using a 30g tuberculin syringe. Liposomal IL-2 (Oncolipin kindly provided by Xeme, Lombard, Illinois) was formulated at a concentration of 4×10^5 IU IL-2/mL in 6mg/mL DMPC, and was quality control tested for appearance, IL-2 content and incorporation (capture ELISA), IL-2 bioactivity (murine CTLL-2 assay), DMPC content (RP-HPCL/light scattering detector), particle size (AccuSizer™ 780), pH, sterility, and endotoxin. Bioactivity in murine T-cells was previously established.23,27 Soluble IL-2 was given at the same concentration of 4×10^5 IU IL-2/mL. The total dose of IL-2 administered in both interventions was 1×10^4 IU IL-2. The control groups received empty DMPC liposomes at a concentration of 6mg/mL with 0.6 mg of DMPC per dose, the same as in treatment groups. Mice were administered one dose of IL-2 per day for five consecutive days on days 7, 8, 9, 10, and 11.
Preparation of tissue samples
Spleens were harvested at sacrifice and placed into a cell strainer and gently homogenized using a syringe plunger. Cells were pelleted and red blood cells lysed with ACK lysis buffer. Tumors were manually dissected and minced, and incubated in 10 mL RPMI containing 5% FBS, collagenase IV (1 mg/ml, Sigma), with DNase I (50 ug/ml, Sigma) at 37 degrees for 60 minutes; and strained to obtain a single cell suspension. Peripheral blood was harvested at sacrifice directly from the vena cava. Blood specimens were loaded onto the top of a Ficoll-Hypaque centrifugation column per the manufacturer’s instructions. The lymphocyte-enriched layer was collected and red blood cells were lysed with ACK lysing buffer (Invitrogen, Grand Island, NY, USA).
Evaluation of immune cell populations
Cells were resuspended in 100μl PBS containing 0.5% fetal bovine serum albumin, incubated at 4°C for 30 minutes after adding fluorochrome conjugated antibodies, washed, and analyzed using a using a BD LSRFortessa analyzer (San Jose, CA, USA). For Foxp3 staining, the cells were permeabilized with Foxp3 fixation/permeabilization buffer prior to applying Fopx3 antibody for intracellular staining after cell surface marker staining. Antibodies utilized included anti-CD3 APC (17A2), CD4 Pacific Blue (GK1.5), CD8 PE-Cy7 (53–6.7), FoxP3 APC (3G3), CD44 PE (IM7), CD62L PE-Cy5 (MEL-14) (Biolegend, San Diego, CA). Events were collected and analyzed using Flow Jo software (Tree star Incorporated Ashland, OR).
Statistical Analysis
Statistical analysis of tumor burden and immune cell populations was performed in GraphPad software. (Graphpad Software, San Diego, CA) Differences in groups were assessed with two tailed Student’s t-test. Error bars on graphs represent SEM. Statistical significance was considered for *p<0.05, **p<0.01, and ***p<0.001.
Results:
LIGHT expression + IL-2 treatment results in decreased tumor size compared to IL-2 alone
wtCT26 tumors in all treatment groups reached humane endpoints after 21 days of tumor growth, whereas humane endpoints were not reached by the CT26LIGHT tumors over the duration of the experiment. Treatment with soluble IL-2, liposomal IL-2, or control vesicles did not impact tumor size. In the IL-2 treated tumors, when LIGHT overexpression was added to IL-2 treatment, tumors were significantly smaller in the CT26LIGHT group compared to the wtCT26 group (Figure 1).
Figure 1. LIGHT expression + IL-2 treatment results in decreased tumor volume compared to IL-2 alone or control vesicles.
On the day of sacrifice, tumor volume was determined in animals treated with soluble or liposomal IL-2. Treatment with soluble IL-2, liposomal IL-2, or control vesicles did not impact tumor volume within wt or LIGHT-expressing groups, though tumors expressing LIGHT experienced decreased tumor volumes in vivo compared to wt. *p<0.05, **p<0.01
LIGHT expression + IL-2 treatment results in increased splenic central memory CD8+ cells compared to IL-2 alone
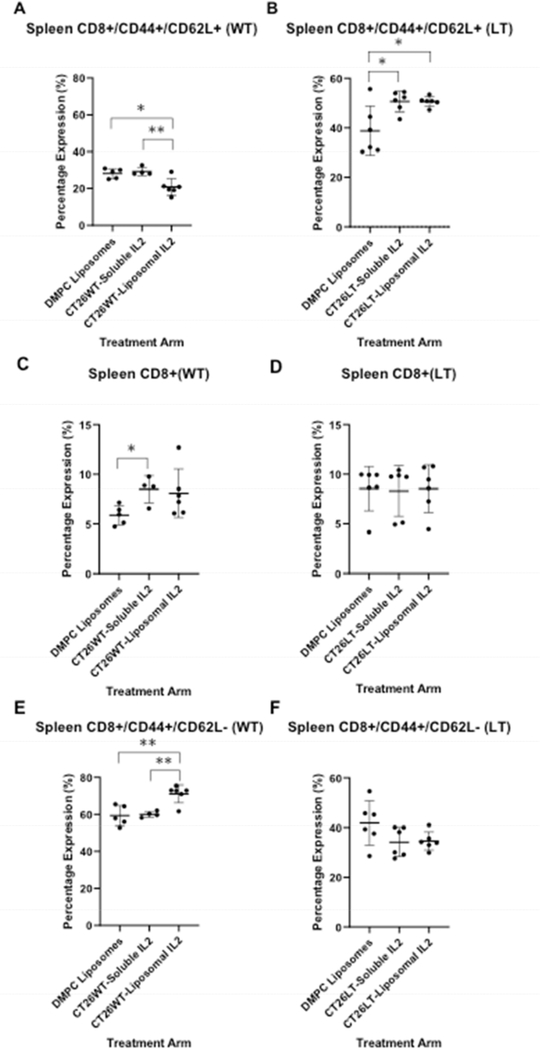
Animals with wild-type tumors experienced a significant decrease in splenic CD8+CD44+CD62L+ central memory T-cells when treated with liposomal-IL-2 compared to soluble IL-2 (p = 0.005) or control (DMPC, p = 0.01). No impact was seen on this cell population in the peripheral blood or tumor. However, in the presence of CT26LIGHT, splenic CD8+CD44+CD62L+ (Tcm) cells were increased in both soluble and liposomal IL-2 treated groups compared to DMPC alone (Figure 2, flow cytometry scatter plots in supplemental data).
Soluble, but not liposomal, IL-2 treatment led to a significant increase in CD8+ splenic T-cell presence that did not persist after LIGHT exposure. Similarly, an increase in CD8+CD44+CD62L− effector memory cells identified only upon exposure to liposomal IL-2 did not persist in animals with LIGHT expressing tumors (Figure 2). There were no differences in CD4+ cells (Supplemental figure), CD4+ effector or central memory T-cells, or CD4+FoxP3+ T-regulatory cells regardless of LIGHT exposure or IL-2 treatment.
Figure 2: The impact of LIGHT expression and IL-2 treatment on splenic CD8 and memory cells.
A decrease in percent of CD8+ cells expressing CD44+CD62L+ (central memory T-cells (TCM) was observed in liposomal IL-2 treated mice with wild-type (WT) tumors (A). In LIGHT expressing tumors (LT), both soluble and liposomal IL-2 treatment led to an increase in splenic TCM (B). Soluble IL-2 treatment led to a significant increase in splenic CD8+ T-cells in wild-type (WT) tumor animals that did not persist in animals with LIGHT (LT) expressing tumors (C, D; Y-axis represents % of splenocytes expressing CD8+). The percent of splenic CD8+ cells that are CD44+CD62L− increased after exposure only to liposomal IL-2 in WT tumor bearing animals (E). There was no difference in the percent of splenic CD8+ cells that were CD44+CD62L−, regardless of IL-2 treatment, in LT tumor bearing mice (F). *p<0.05, **p<0.01, ***p<0.001)
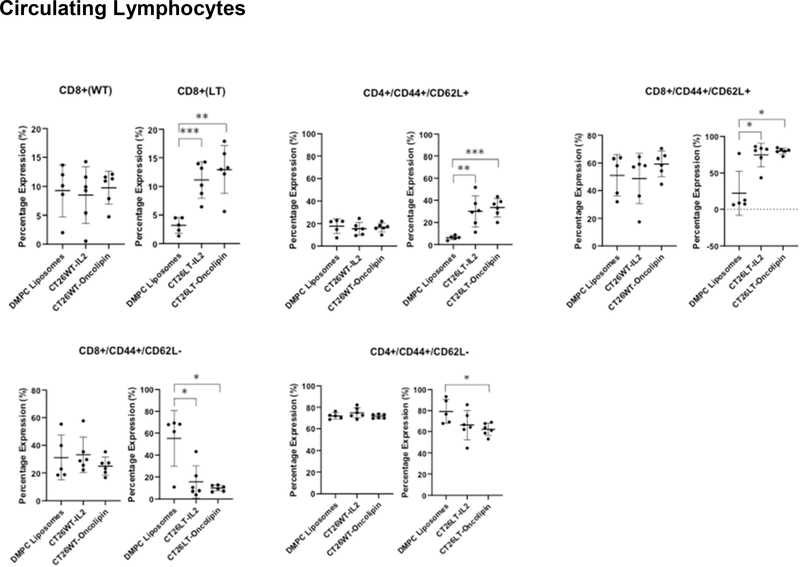
LIGHT expression + IL-2 treatment enhances circulating CD8+ T-cells and central memory T-cells
Significant increases in the percent of CD8+ cells in the peripheral blood were observed after IL-2 treatment of LIGHT expressing tumors that were not demonstrated in animals with wt tumors (Figure 3). There was also an increase in CD4+CD44+CD62L+ and CD8+CD44+CD62L+ central memory T-cells in both soluble and liposomal IL-2 treated animals with LIGHT expressing tumors that was not demonstrated in animals with wt tumors. Conversely, there was a decrease in peripheral CD4+ and CD8+ CD44+CD62L− effector memory T-cells with LIGHT + IL-2 that was not observed in animals with WT tumors (Figure 3). IL-2 treatment did not impact circulating CD4+ populations regardless of LIGHT exposure.
Figure 3. Increased presence of CD8+ and CD8+ central memory circulating lymphocytes in tumor bearing animals in the presence or absence of LIGHT and/or IL-2.
An increased percentage of CD8+ peripheral blood mononuclear cells was observed in soluble and liposomal IL-2 treated mice with LT expressing tumors. Soluble IL-2 treated mice bearing WT-tumor did not experience an increase in circulating CD8+ cells (upper left). The percent of CD4+ (upper middle) and CD8+ (upper right) T-cells expressing CD44+CD62L+ (central memory T-cells) increased with soluble and liposomal IL-2 treatment. These differences were not observed in WT-tumor bearing mice. The percent of circulating CD8+ T-cells expressing CD44+CD62L− (effector memory T-cells) significantly decreased in soluble and IL-2 treated mice with LIGHT expressing tumors (lower left). The percent of circulating CD4+ T-cells expressing CD44+CD62L− decreased in liposomal IL-2 treated mice with LIGHT expressing tumors (lower middle). Differences in circulating CD4+CD44+CD62L− cells were not observed in WT-tumor bearing mice. *p<0.05, **p<0.01, ***p<0.001. Oncolipin= liposomal IL-2. Wild-type (WT) tumor bearing animal data are labeled, and on the left in each panel to the LIGHT (LT) expressing tumor bearing animal data.
IL-2 treatment increases circulating T-regulatory cells
As IL-2 is a known stimulator of T-regulatory (Treg) cells, CD4+Foxp3+ T-cell populations were evaluated. There were no differences in Treg populations in the spleens of tumor bearing mice, however, circulating CD4+FoxP3+ cells were significantly increased in soluble IL-2 treated, and trended to increase in liposomal IL-2 treated, wt tumor bearing animals when compared to DMPC control. This pattern was consistent with increased circulating CD4+Foxp3+ cells in LIGHT+ IL-2 treated animals. (Figure 4).
Figure 4. IL-2 treatment increases circulating T-regulatory cells.
The percent of circulating CD4+ cells expressing Foxp3+ was increased in soluble IL-2 treated mice with WT tumors (p < 0.05)(A). LIGHT (LT)-tumor bearing animals also experienced increased presence of circulating CD4+Foxp3+ T-cells after treatment with both soluble and liposomal IL-2 (p<0.05)(B)
Tumor infiltrating lymphocytes
Though CD8+ T-cell infiltration was greater in CT26LIGHT tumors compared to wtCT26 tumors (p = 0.006) (Figure 5), IL-2 administration did not impact the number of tumor infiltrating lymphocytes in wild type or LIGHT expressing tumors, including intratumoral CD8+, CD4+, Treg, or memory T-cell populations (CD44CD62).
Figure 5. Tumor Infiltrating Lymphocytes.
Tumors expressing LIGHT experienced increased CD8+ T-cell infiltration compared to non-LIGHT expressing tumors. IL-2 treatment did not impact numbers of infiltrating CD4 or CD8 T-cells compared to control. *p<0.05, **p<0.01
Discussion:
Microsatellite-stable metastatic colorectal cancer is not responsive to currently available checkpoint blockade immunotherapy, thus, the current standard of care remains non-curative palliative chemotherapy that does not generate a durable antitumor immune response.6,8,9,28–30 To generate sustained anti-tumor immune responses, memory T-cells are required. Prior studies have revealed that the generation of CD8+ central memory T-cells are associated with superior antitumor immunity compared to effector memory T-cells,17 however, strategies to increase central memory T-cells remain to be identified, particularly in “cold” MSS gastrointestinal tumors.31 In this study, it was demonstrated that central memory CD8+ T-cells could be increased in secondary lymphoid organs and the periphery by utilizing combination immunotherapy of LIGHT and IL-2.
Upon recognition of tumor antigens, T-cells undergo vigorous clonal expansion and differentiate into effector cells that then traffic to the site of antigen presentation. Increasing T-cell trafficking into the tumor is thus one strategy to improve the anti-tumor immune response, and one that we have previously manipulated utilizing the immunostimulatory signal LIGHT.13,32,33 At the end of antigen-specific T-cell expansion, a contraction phase ensues where most antigen-specific effector T-cells die via apoptosis, except for a minority that survive as memory cells.34–37 Memory T-cells localize and recirculate throughout the blood and secondary lymphoid organs, which is why the current study focused on circulating and splenic T-cell populations after antitumor treatment. They have traditionally been defined as being comprised of both central memory (TCM) and effector memory subsets.38–40 Multiple adoptive T cell therapy studies in melanoma revealed that gp100 antigen-specific CD8+ TCM controlled established melanomas better than effector memory cells.17,41 The TCM cells are capable of rapid upregulation upon antigenic rechallenge and expand vigorously to aid other sets of migratory T-cells during secondary challenge.
It has been shown that IL-2 exposure during the expansion phase supports not only T-cell proliferation and activation, but also the differentiation of naïve CD8+ T-cells into memory phenotype cells.20 Furthermore, exposure to IL-2 during the contraction phase prevents elimination of anti-tumor T-cells with decreased apoptosis and increased survival, particularly of CD8+ memory cells.18,19 Therefore, it followed intuitively that combining tumor necrosis factor superfamily LIGHT, a strategy that we have demonstrated to increase T-cell activation, proliferation, and tumor infiltration in colorectal cancer and liver metastases; with IL-2 may augment the anti-tumor immune response by sustaining CD8+ T-cells in the periphery, and generating more central memory CD8+ T-cells. The Achilles heel of this stimulatory strategy, of course, is that IL-2 will also support T-regulatory cells, and in fact this was identified in the periphery of IL-2 treated animals bearing LIGHT-expressing tumors, though not in the spleen or in the tumor.42,43 This may account for why there was not a more robust response in the size of the tumors when treated with IL-2 compared to control animals. It is possible that combining IL-2 with checkpoint blockade could expand the effector T-cells while limiting suppressive T-cell influences, a strategy that we employed in patients with metastatic melanoma to improve responses over either monotherapy.5,7,44 Our preliminary studies of LIGHT with checkpoint blockade have revealed impressive tumor regressions45, therefore, it is interesting to consider the potential of LIGHT + IL-2 + checkpoint blockade based on the ability to increase TCM.46,47
There are several limitations to this study. Due to the rapid tumor growth in the absence of response to IL-2 therapy in wild type tumors, these animals reached humane endpoints. However, no differences in CD4, CD8, or Treg subsets were identified in these treated tumors, nor were they in the IL-2 treated animals bearing LIGHT-expressing tumors. In the periphery of wt tumor treated animals, there were increases in Treg with soluble but not liposomal IL-2. A reason for this observation and the differential effect may be secondary to the timing and dose exposure of IL2, where the liposomal form has a delayed release, and lower transient spikes compared to soluble bolus administration. In the spleen also there were differences in CD8+ T-cells and CD8+ memory T-cell subpopulations associated with the specific mode of IL-2 administration, which could again be due to delayed release, and/or lower transient spikes in IL-2 levels. These observations may be clinically relevant since exposure to exogenous IL-2 during the contraction phase of the antigen response increase the frequency of CD8+ memory T-cells, and when during the expansion phase, support not only T-cell proliferation and activation, but also differentiation of naïve CD8+ T-cells into memory cells. It has been shown in biopharmaceutical studies that, as opposed to soluble IL2, the liposomal formulation is able to provide receptor targeting to activated T-cells.22 Further, liposomal IL2, as opposed to soluble IL2, can deposit in the lymphoid organs, e.g. spleen, where continued delayed release occurs to impact these cell populations.22 These findings were consistent with increased peripheral Treg and CD8+ T-cells seen in the LIGHT+IL-2 exposed tumors, whether soluble or liposomal, where animals experienced not only immunostimulation from LIGHT, but also increased time after exposure to IL-2 before evaluation. Clearly, there are complex biologic interactions occurring based on the timing and dose exposure of IL2, the mechanism of which was not intended to be studied in this report, but that must be impacting the observed results. Whether blocking IL2 exposure would reverse these observations remains to be determined, however, it has been established that when the IL2 receptor is engaged with soluble IL2 or an anti-B-subunit antibody, that liposomal IL2 cell binding diminishes precipitously, as does internalization by IL2 receptor bearing cells.22
Additional evaluation of Th1 and Th2 secreted cytokines in the tumor microenvironment could potentially aid in this phenotypic evaluation. Furthermore, isolation of splenic and peripheral CD8+ T-cells for in vitro cytotoxic functional assays and in vivo adoptive transfer may help elucidate potential differences in the treatment strategies. As to why LIGHT exposure + IL-2 treatment decreased effector memory cells in the periphery, it is possible that with profound independent TNFSF14 stimulation that the decreased response to IL-2 was more pronounced in the circulation and/or that peripheral effectors destined to die during the contraction phase had already begun to decrease in number, potentially as a result of the early proliferative impact that LIGHT and IL-2 impart.48,49
Additional studies in a greater number of animals, both animal sexes, additional tumor cell lines, and/or orthotopic models are necessary to validate the current observations. Further, IL2 administration at a later time point, and other experimental designs that would reflect clinical treatment in larger established patient tumors, are warranted. That Tcm were increased is a very useful piece of information that can be utilized when developing strategies to improve recurrence-free survival and recall responses, particularly in MSS tumors where checkpoint blockade has been ineffective. That said, since a greater impact upon TIL and tumor size (pictures of tumor-bearing animals were not included in this analysis) was not encountered in this model points to the fact that IL-2 + LIGHT alone is unlikely to be a curative-intent intervention, and will likely need to be augmented with additional biologic agents to increase anti-tumor immune responses. In addition, LIGHT was presented in the tumor microenvironment on tumor cells to insure increased and stable levels of expression only in the tumor, and serves as proof-of-principal for the experimental strategy. We are investigating other more clinically relevant modalities to deliver LIGHT into the tumors including an oncovirus to selectively deliver LIGHT into the tumor microenvironment. Nonetheless, additional translational modalities to evaluate the effect of LIGHT overexpression in the tumor microenvironment, other than constant expression, combined with IL2 are necessary.
As aforementioned, it is not clear why responses would be different between soluble and liposomal IL-2, particularly with respect to decreased central memory and increased effector memory cells in wt treated tumors with liposomal IL-2, however, in the presence of LIGHT, both formulations had similar positive impact on the T-cell repertoires. IL-2 has long been utilized as an immunostimulatory cytokine to support T-cell growth and expansion. It is has been FDA-approved for decades in the treatment for patients with metastatic melanoma, but use has been limited by a short half-life of only a few minutes, and repeated bolus injections leading to severe systemic side-effects and short-acting therapeutic windows.50 Thus, the potential benefit of liposomal IL-2 may be that bioavailability is prolonged and that systemic side effects with the liposomal formulation are potentially decreased in vivo.21–24 As to why a small but significant decrease in splenic CD8 central memory T-cells was observed only with liposomal IL-2 is possibly due to the relatively prolonged exposure to a higher concentration of IL-2 in those tumors. Kaartinen et al. have elegantly demonstrated that central memory T cell populations are dependent upon the dose of IL-2 and length of T-cell expansion, with higher concentrations resulting in decreased central memory T-cells.51 In addition, in these experiments, intralesional delivery of IL-2 was evaluated as opposed to systemic delivery. Though intralesional administration of cytokines is feasible with endoscopic and interventional radiology approaches, local tumor delivery of cytokines can be achieved with an IL-2 immunocytokine or oncovirus.52 It is possible that some of the impact of intratumoral IL-2 in the current model is secondary to systemic absorption, and this could be further examined with subcutaneous, non-tumor IL-2 administration, or in a bilateral flank tumor model.
In summary, we and others have previously shown that CD+8 T cells can mediate the regression of large bulky tumors, resulting in durable long-term disease remissions. However, the most effective in vivo anti-tumor T cells may be those that target secondary lymphoid tissue, rather than tumor sites, and thus T cell populations with the phenotypic attributes of TCM may be superior to effector T cells for adoptive immunotherapies.17 Therefore, as the field of cancer immunotherapy advances, it is important to elucidate how the dissemination and maintenance of these T cells can be optimally achieved. IL-2 treatment alone of colorectal cancer tumors in vivo did not result in tumor regressions or an increase in central memory T-cells in this model, however, combination immunotherapy with LIGHT-expressing tumors and intralesional IL-2 administration, both in soluble and liposomal form, resulted in increased splenic and circulating central memory CD8+ T-cells. Further experiments evaluating the potential of this approach for generating tumor-specific anti-tumor responses and optimizing the mode of IL-2 administration are warranted.
Supplementary Material
Supplemental Figure 1
Flow cytometry scatter plots of T-cell populations included in the study
Supplemental Figure 2 Splenic CD4+ and central memory CD4+ T-cell populations Percent of splenocytes expressing CD4 in response to various interventions (upper panel). Percent of CD4+ splenic cells expressing CD44+CD62L- (lower panel).
Oncolipin = liposomal IL-2.
Supplemental Table 1
Impact of intervention on splenic, circulating, and tumor infiltrating lymphocytes
Acknowledgments
Supported by: NIH/NCI K08CA190855 and NIH/NCI R37CA238435 (AVM). Materials supported in part by Xeme Biopharma. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RLMK, Jemal A. Cancer statistics, 2015 CA: a cancer journal for clinicians 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Qin J, Kunda NM, Qiao G, Tulla K, Prabhakar BS, Maker AV. Vaccination With Mitoxantrone-Treated Primary Colon Cancer Cells Enhances Tumor-Infiltrating Lymphocytes and Clinical Responses in Colorectal Liver Metastases. J Surg Res 2019;233:57–64. [DOI] [PubMed] [Google Scholar]
- 3.Qin J, Kunda N, Qiao G, et al. Colon cancer cell treatment with rose bengal generates a protective immune response via immunogenic cell death. Cell Death Dis 2017;8:e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maker AV, Ito H, Mo Q, et al. Genetic evidence that intratumoral T-cell proliferation and activation are associated with recurrence and survival in patients with resected colorectal liver metastases. Cancer immunology research 2015;3:380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 2005;12:1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maker AV, Yang JC, Sherry RM, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother 2006;29:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010;28:3485–90. [DOI] [PubMed] [Google Scholar]
- 10.Mlecnik B, Bindea G, Angell HK, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 2016;44:698–711. [DOI] [PubMed] [Google Scholar]
- 11.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18. [DOI] [PubMed] [Google Scholar]
- 12.Maker AV. Precise identification of immunotherapeutic targets for solid malignancies using clues within the tumor microenvironment-Evidence to turn on the LIGHT. Oncoimmunology 2016;5:e1069937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao G, Qin J, Kunda N, et al. LIGHT Elevation Enhances Immune Eradication of Colon Cancer Metastases. Cancer research 2017;77:1880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin JZ, Upadhyay V, Prabhakar B, Maker AV. Shedding LIGHT (TNFSF14) on the tumor microenvironment of colorectal cancer liver metastases. J Transl Med 2013;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu P, Fu YX. Targeting tumors with LIGHT to generate metastasis-clearing immunity. Cytokine & growth factor reviews 2008;19:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu P, Lee Y, Liu W, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nature immunology 2004;5:141–9. [DOI] [PubMed] [Google Scholar]
- 17.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A 2005;102:9571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006;311:1924–7. [DOI] [PubMed] [Google Scholar]
- 19.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 2003;9:540–7. [DOI] [PubMed] [Google Scholar]
- 20.Boyman O, Cho JH, Sprent J. The role of interleukin-2 in memory CD8 cell differentiation. Adv Exp Med Biol 2010;684:28–41. [DOI] [PubMed] [Google Scholar]
- 21.Boni LT, Batenjany MM, Neville ME, et al. Interleukin-2-induced small unilamellar vesicle coalescence. Biochim Biophys Acta 2001;1514:127–38. [DOI] [PubMed] [Google Scholar]
- 22.Neville ME, Boni LT, Pflug LE, Popescu MC, Robb RJ. Biopharmaceutics of liposomal interleukin 2, oncolipin. Cytokine 2000;12:1691–701. [DOI] [PubMed] [Google Scholar]
- 23.Neville ME, Robb RJ, Popescu MC. In situ vaccination against a nonimmunogenic tumour using intratumoural injections of liposomal interleukin 2. Cytokine 2001;16:239–50. [DOI] [PubMed] [Google Scholar]
- 24.Kwak LW, Pennington R, Boni L, Ochoa AC, Robb RJ, Popescu MC. Liposomal formulation of a self lymphoma antigen induces potent protective antitumor immunity. J Immunol 1998;160:3637–41. [PubMed] [Google Scholar]
- 25.Castle JC, Loewer M, Boegel S, et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics 2014;15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germano G, Lamba S, Rospo G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017;552:116–20. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann TR, Yokota T, Kastelein R, Zurawski SM, Arai N, Takebe Y. Species-specificity of T cell stimulating activities of IL 2 and BSF-1 (IL 4): comparison of normal and recombinant, mouse and human IL 2 and BSF-1 (IL 4). J Immunol 1987; 138:1813–6. [PubMed] [Google Scholar]
- 28.Ejaz A, Casadaban L, Maker AV. Utilization and impact of adjuvant chemotherapy among patients with resected stage II colon cancer: a multi-institutional analysis. J Surg Res 2017;215:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadaban L, Rauscher G, Aklilu M, Villenes D, Freels S, Maker AV. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer 2016;122:3277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saltz LB. Value in Colorectal Cancer TreatmentL Where It Is Lacking, and Why. Cancer J 2016;22:232–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran TB, Maker VK, Maker AV. Impact of Immunotherapy after Resection of Pancreatic Cancer. J Am Coll Surg 2019;229:19–27 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- 33.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer research 1998;58:3491–4. [PubMed] [Google Scholar]
- 34.Sprent J, Tough DF. T cell death and memory. Science 2001;293:245–8. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science 1996;272:54–60. [DOI] [PubMed] [Google Scholar]
- 36.Sprent J, Surh CD. T cell memory. Annu Rev Immunol 2002;20:551–79. [DOI] [PubMed] [Google Scholar]
- 37.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity 2006;25:19–29. [DOI] [PubMed] [Google Scholar]
- 38.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 2004;20:551–62. [DOI] [PubMed] [Google Scholar]
- 39.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–12. [DOI] [PubMed] [Google Scholar]
- 40.Usherwood EJ, Hogan RJ, Crowther G, et al. Functionally heterogeneous CD8(+) T-cell memory is induced by Sendai virus infection of mice. J Virol 1999;73:7278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 2005;115:1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar P, Marinelarena A, Raghunathan D, et al. Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation. Cell Mol Immunol 2019;16:138–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alharshawi K, Marinelarena A, Kumar P, et al. PKC- is dispensable for OX40L-induced TCR-independent Treg proliferation but contributes by enabling IL-2 production from effector T-cells. Sci Rep 2017;7:6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol 2005;175:7746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maker A, Qiao G, Kunda N, Prabhakar B. Combining targeted immunotherapy with checkpoint blockade inhibits formation of colorectal liver metastases (abstract). Annals of Surgical Oncology 2017;24:1–202.27783164 [Google Scholar]
- 46.Casadaban L, Maker AV. Can Colon Cancer Recurrence and Metastases Be Determined After Surgical Resection Using a Gene Expression Signature? J Clin Oncol 2017;35:1372–3. [DOI] [PubMed] [Google Scholar]
- 47.Jamieson NB, Maker AV. Gene-expression profiling to predict responsiveness to immunotherapy. Cancer Gene Ther 2017;24:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol 2009;9:662–8. [DOI] [PubMed] [Google Scholar]
- 49.Kretschmer L, Flossdorf M, Mir J, et al. Differential expansion of T central memory precursor and effector subsets is regulated by division speed. Nat Commun 2020; 11:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vial T, Descotes J. Clinical toxicity of interleukin-2. Drug Saf 1992;7:417–33. [DOI] [PubMed] [Google Scholar]
- 51.Kaartinen T, Luostarinen A, Maliniemi P, et al. Low interleukin-2 concentration favors generation of early memory T cells over effector phenotypes during chimeric antigen receptor T-cell expansion. Cytotherapy 2017;19:689–702. [DOI] [PubMed] [Google Scholar]
- 52.Mortara L, Balza E, Bruno A, Poggi A, Orecchia P, Carnemolla B. Anti-cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-tumor Efficacy. Front Immunol 2018;9:2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1
Flow cytometry scatter plots of T-cell populations included in the study
Supplemental Figure 2 Splenic CD4+ and central memory CD4+ T-cell populations Percent of splenocytes expressing CD4 in response to various interventions (upper panel). Percent of CD4+ splenic cells expressing CD44+CD62L- (lower panel).
Oncolipin = liposomal IL-2.
Supplemental Table 1
Impact of intervention on splenic, circulating, and tumor infiltrating lymphocytes