Abstract
Bacterial microcompartments (BMCs) with selectively permeable shells and encapsulated enzyme cores are well-suited candidates for nano-bioreactors because of their advantages of enhancing pathway flux and protection against toxic products. To better study and engineer protein-based BMCs, a series of protein chemistry approaches are adopted. As one of the most advanced techniques, genetic code expansion can introduce various noncanonical amino acids (ncAAs) with diverse functional groups into target proteins, thus providing powerful tools for protein studies and engineering. This review summarizes and proposes useful tools based on current development of the genetic code expansion technique towards challenges in BMC studies and engineering.
Keywords: Genetic code expansion, noncanonical amino acid, bacterial microcompartment, protein labeling, photocrosslinking, protein engineering
Introduction
Functioning analogously to eukaryotic organelles, bacterial microcompartments (BMCs) have been found in more than 20% of bacteria taxa [1–3]. BMCs consist of a thin layer of protein shell with sizes between 100 and 200 nm and an encapsulated enzyme core related to different metabolic pathways [4]. Carboxysomes (anabolic) and metabolosomes (catabolic) are the two basic types of BMCs [1]. The anabolic carboxysomes are the most well-studied BMCs due to wide distribution in cyanobacteria and importance in carbon fixation [5]. Differently, metabolosomes are more diverse and have been found to participate in the catabolism of various compounds such as 1,2-propandiol [6], ethanolamine [7], choline [8], and fucose [9]. Evolution has designed BMCs to isolate biochemical reactions with the purposes of increasing local concentrations of enzymes and substrates, enhancing pathway flux, confining volatile intermediates, and protecting cells from toxic products, thus making BMCs well-suited candidates for nano-bioreactor engineering [10]. Additionally, the short N-terminal targeting sequences of encapsulated enzymes are able to introduce heterologous proteins into BMCs [11,12], offering the possibility to encapsulate non-native proteins into BMCs for diverse purposes [13,14]. Furthermore, more efforts have been made to optimize BMC functions, such as modifying the selective permeability of BMCs by engineering shell proteins [15–17]. Indeed, as BMCs are exclusively composed of proteins, approaches for protein engineering will be key options for BMC studies and engineering.
One classic approach in protein engineering is to substitute amino acid residues at designed positions, thus modulating protein structures, functions, and properties for desired purposes. However, traditional methods only have a limited candidate pool of 20 canonical amino acids. To overcome this limitation and introduce more diversified non-canonical amino acids (ncAAs) into proteins, the genetic code expansion technique has been developed [18,19]. In this approach, an orthogonal pair of aminoacyl-tRNA synthetase (AARS) and tRNA (no crosstalk with endogenous AARSs and tRNAs in host cells) is used to incorporate an ncAA in response to an assigned codon (usually a stop codon) at the desired position in the target protein [20] (Figure 1). As the key part of genetic code expansion, a series of orthogonal AARS/tRNA pairs from archaeal or eukaryotic cells have been developed for bacterial expression systems [21]. For using Escherichia coli as host cells, the tyrosyl-tRNA synthetase (TyrRS) system from Methanocaldococcus jannaschii and the pyrrolysyl-tRNA synthetase (PylRS) system from Methanosarcinaceae are widely used [20,22]. In the last decade, a series of orthogonal translation systems (OTSs) have been evolved to introduce more than 300 ncAAs into proteins in living cells for different purposes, such as labeling proteins [23,24], studying protein posttranslational modifications [25,26], mapping protein-protein interactions [27,28], and modulating enzyme functions [29,30]. Such numbers of ncAAs harboring different functional groups with variable sizes, charges, and properties provide a much larger pool of tunable candidates than traditional approaches. Here we summarize and propose applications of genetic code expansion in BMC studies and engineering.
Figure 1.

The scheme of the genetic code expansion technique. The engineered aminoacyl-tRNA synthetase (AARS) charges its cognate tRNA with a specific noncanonical amino acid (ncAA). The ncAA-charged tRNA contains a specific anti-codon (CUA for example) to read the corresponding codon (usually a stop codon) in mRNA and, the ncAA is incorporated into protein during translation at a desired site.
Applications of genetic code expansion in BMC studies and engineering
In our recent studies, we were able to genetically incorporate ncAAs into Pdu BMCs in living Salmonella cells [31]. Developing specific OTSs is the prerequisite for applying the genetic code expansion technique in Salmonella cells. By changing promoters and optimizing gene sequences of introduced orthogonal pairs of AARSs and tRNAs as well as modifying Salmonella genes for controllable protein expression, three OTSs originally developed in E. coli (the TyrRS system, the PylRS system, and the phosphoseryl-tRNA synthetase system from methanogenic archaea) were successfully introduced into Salmonella [31]. With these customized OTSs specific for Salmonella cells, different tools based on ncAAs were developed for BMC studies. We further propose several potential applications of genetic code expansion in BMC engineering (Figure 2).
Figure 2.

Applications of genetic code expansion in BMC studies and engineering. P-azido-phenylalanine (pAzF) is used for site-specific labeling for imaging. Benzoyl-phenylalanine (Bpa) is a photocrosslinking amino acid to map protein-protein interactions. Naphthylalanine (Npa) provides more hydrophobicity, possibly increasing interactions between shell proteins and stabilizing BMCs. 2,3-diaminopropanoic acid (DAP) has positive charges in physiology conditions, thus modulating pore properties to facilitate uptake of specific molecules.
1. Labeling BMCs in living cells
Incorporation of ncAAs with specific click chemistry functionality into proteins enables unique biorthogonal chemistries which are inert to natural biological reactions, allowing protein labeling for different purposes such as imaging and proteomic studies [32]. Azide-alkyne cycloaddition is one of the most popular click chemistries with advantages of fast kinetics, low toxicity, and easy operation. The azido group of p-azido-phenylalanine (pAzF) can react with an alkyne-fluorescent dye by the click reaction and produce fluorescence signals for detections [33]. Moreover, due to the small size of pAzF, similar to canonical amino acids, it can be site-specifically incorporated into target proteins to replace selected residues, thus minimally affecting protein structures [34]. In our recent study, PduA, one of the major shell proteins of Salmonella Pdu BMCs [35,36], has been selected for the pAzF-derived labeling. Based on the crystal structure of PduA [37], N67 was chosen as the target position. After site-specifically mutating the original codon for N67 in pduA gene to an amber stop codon (UAG), the pAzF-containing PduA protein was expressed together with other Pdu proteins [31]. By the click reaction with a fluorescent dye harboring an alkyne group, Pdu BMCs were labeled for fluorescence imaging in living Salmonella cells.
2. Mapping BMC protein interactions by photocrosslinking
Protein-protein interactions between shell proteins or between shell proteins and encased enzymes are crucial for BMC assembly [38,39]. However, some of the interactions could be weak or transient due to different factors such as pH and ionic conditions. Thus, it could be challenging to map the protein-protein interaction network of BMC proteins completely. NcAAs with photocrosslinkable moieties can be easily irradiated with UV light to form covalent bonds between proximal molecules to detect transient and weak interactions, which have been widely used to study protein-protein interactions both in vitro and in vivo [40]. Benzoyl-phenylalanine (Bpa) is one of the most favorable photocrosslinking amino acids. It can be genetically incorporated into proteins in Salmonella cells by the optimized TyrRS systems [31]. Because such incorporation is site-specific by the genetic code expansion technique, this approach has the advantage over the classic cysteine-derived crosslinking which has concerns of multiple cysteine residues in a single protein. On the other hand, it is necessary to select proper Bpa incorporation sites before applying Bpa-derived photocrosslinking. Ideal positions should be at the outer surface of the target protein and avoid interface between its own subunits. It is achievable because the structures of most architectural subtypes (i.e. hexamers, trimers, and pentamers) have been solved, enabling homology modeling of most BMC shell proteins [41].
3. Stabilizing BMC shells
Studies have shown that environmental factors such as pH and ionic conditions could affect the stability and variability of BMC shell protein self-assembly [42]. The approach based on canonical amino acid substitutions (K28A and R78A mutations in BMC-H proteins) has been used to feasibly manipulate the self-assembly and dynamics of BMC shell proteins [43]. As ideal nano-bioreactors, it is important to stabilize BMC shells against extreme pH, high temperature, or high ionic strength for different applications. Due to diversified side chains, ncAAs could provide more intermolecular or intramolecular interactions between shell proteins for higher stabilities. Based on properties of key residues affecting shell stabilization, genetic code expansion could introduce corresponding ncAAs to increase BMC shells. For key residues with hydrophobic side chains such as valine, isoleucine and phenylalanine, ncAA analogs with more hydrophobicity could be introduced to provide more hydrophobic interactions. For key residues with longer side chains like glutamine and lysine which could form hydrogen bonds with protein backbones, ncAAs with additional amino groups or hydroxyl groups could offer extra hydrogen bonds. According to these criteria, several potential ncAA analogs are listed (Figure 3). Specific ncAA incorporation systems need to be evolved for those ncAAs, individually. The PylRS system will be a good choice as it has a high flexibility of substrate specificities from lysine derivatives to phenylalanine analogs [44].
Figure 3.
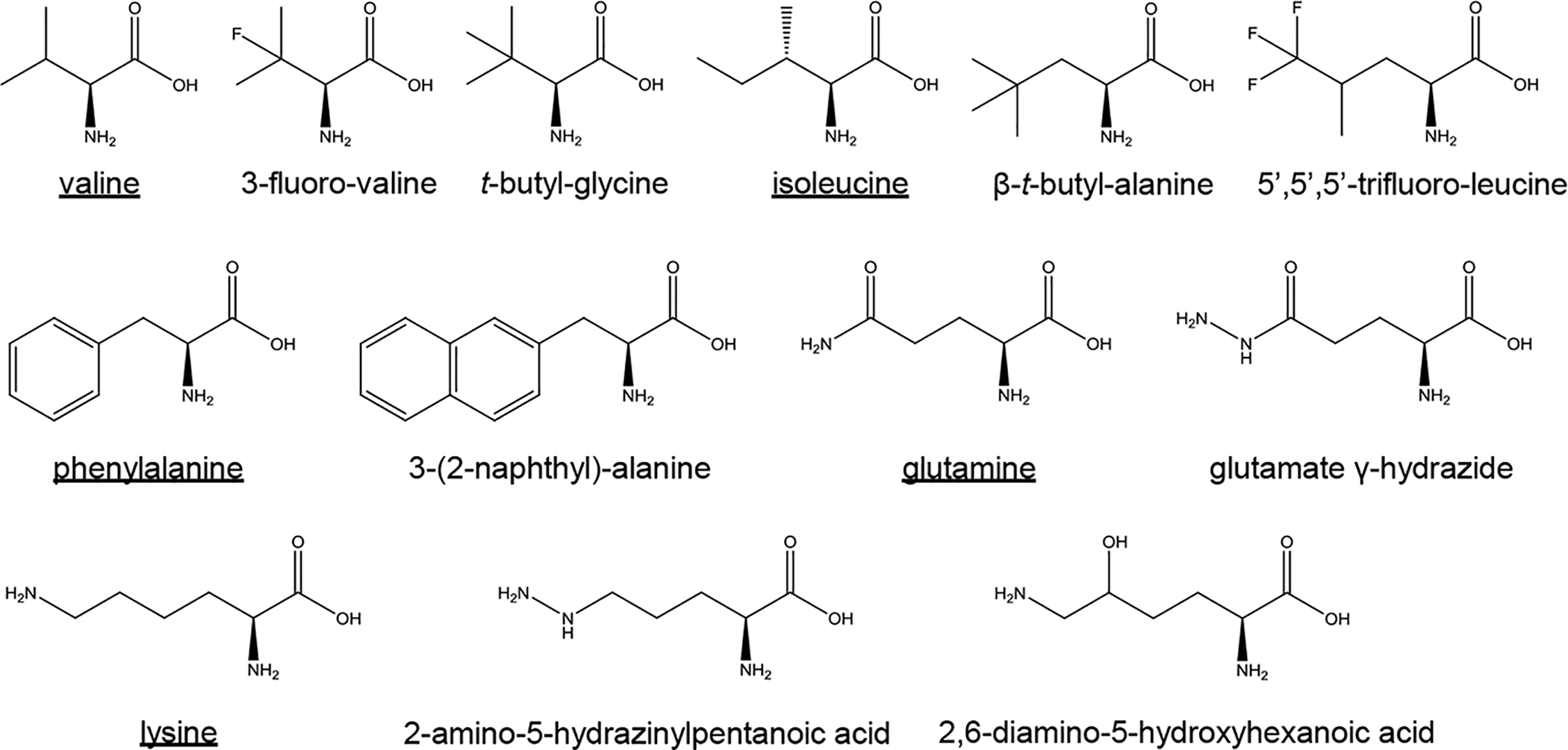
The structures of canonical amino acids and their corresponding ncAA analogs. Canonical amino acids are underlined.
4. Modulating Pdu BMC pore properties for metabolite selectivity
One unique property of BMCs as ideal nano-bioreactors is their selective pores for specific transport of substrates, products, and cofactors. Crystal structures of BMC shell proteins reveal that pores formed by different shell proteins diverge in steric/electrostatic properties, and BMCs could control metabolite movement based on various pore properties [45]. Thus, shell permeability can be tuned by modifying residues that surround the pores [15–17]. Recently, approaches of canonical amino acid substitutions have been used to modify key residues that affect the properties of shell pores. For instance, site-specific replacement of S40 of PduA could change the size and polarity of the opening pore. The substitution with histidine enables Pdu BMCs to have better uptake of glycerol than the native substrate 1,2-propandiol [17]. For another Pdu protein, PduT, different amino acid substitutions at its C38 were demonstrated with different influences on the shell permeability. PduT-C38S and PduT-C38A variants could increase the shell permeability for 1,2-propanediol, propionaldehyde, NAD+ and NADH, while PduT-C38I and PduT-C38W could not alter the permeability of Pdu BMCs [15]. Shell proteins have also been engineered for electron transport. By mutating S55 to cysteine, BMC-T1 was shown to bind a [4Fe-4S] cluster [46]. Addition of three histidine residues around the pore region of BMC-T1 generated a new binding site for copper [47]. Again, the larger pool of ncAAs could provide more candidates than the limited set of 20 canonical amino acids to modulate pore properties for desired reactions encapsulated in BMCs. Previously, pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) have been encapsulated into an ethanol reactor based on Pdu BMCs, however, the ethanol production was not significantly improved compared with non-compartmentalized reactions [48]. One possible reason could be that BMC pores have no optimal permeability to the substrate pyruvate. To increase the uptake of negatively charged pyruvate, 2,3-diaminopropanoic acid (DAP, structure in Figure 2) with one positive charge could be a potential substitution for Ser40 of PduA, and the genetic incorporation system for DAP has been established recently [49].
Conclusions and perspectives
The genetic code expansion strategy can offer various potential tools to study and engineer BMCs by incorporating a variety of ncAAs with expanded functional groups into BMC proteins. Our previous study has successfully labeled the PduA protein in Pdu BMCs with pAzF [31], and we also proposed several potential applications. Particularly, stabilizing and modifying pore selective permeability of BMCs are important for BMC engineering. Therefore, more future studies need to be conducted to develop these ncAA-based systems for stabilization and selective permeability of BMCs in experimental conditions. Additionally, more tools based on ncAA incorporations will be explored to facilitate BMC engineering due to the rapid development of genetic code expansion and the gradual enlargement of ncAAs library.
Highlights.
Genetic incorporation of noncanonical amino acids (ncAAs) can facilitate BMC studies and engineering.
NcAAs with specific click chemistry functionality can be used to label BMC proteins by bioorthogonal reactions.
NcAAs with photocrosslinking properties can help study the protein-protein interactions between BMC proteins.
NcAAs with varied functional groups can be used to stabilize BMCs and modulate selective permeability of BMC shells.
Acknowledgement:
This work was supported by NIH (R15GM140433), Robert C. and Sandra Connor Endowed Faculty Fellowship, and CEMB RA fund from University of Arkansas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: None
References
- 1.Kerfeld CA, Aussignargues C, Zarzycki J, Cai F, Sutter M: Bacterial microcompartments. Nature Review Microbiology 2018, 16:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MJ, Palmer DJ, Warren MJ: Biotechnological advances in bacterial microcompartment technology. Trends in Biotechnology 2019, 37:325–336. [DOI] [PubMed] [Google Scholar]
- 3.Stewart KL, Stewart AM, Bobik TA: Prokaryotic Organelles: Bacterial Microcompartments in E. coli and Salmonella. Ecosal Plus 2020, 9:ESP-0025–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy NW, Hershewe JM, Nichols TM, Roth EW, Wilke CD, Mills CE, Jewett MC, Tullman-Ercek D: Apparent size and morphology of bacterial microcompartments varies with technique. PLoS One 2020, 15:e0226395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill NC, Tay JW, Altus S, Bortz DM, Cameron JC: Life cycle of a cyanobacterial carboxysome. Science Advances 2020, 6:eaba1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundin AP, Stewart KL, Stewart AM, Herring TI, Chowdhury C, Bobik TA: Genetic characterization of a glycyl radical microcompartment used for 1, 2-propanediol fermentation by uropathogenic Escherichia coli CFT073. Journal of Bacteriology 2020, 202:e00017–00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadswell K, Creagh S, McCullagh E, Liang M, Brown IR, Warren MJ, McNally A, MacSharry J, Prentice MB: Bacterial Microcompartment-Mediated Ethanolamine Metabolism in Escherichia coli Urinary Tract Infection. Infection and Immunity 2019, 87:e00211–00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herring TI, Harris TN, Chowdhury C, Mohanty SK, Bobik TA: A bacterial microcompartment is used for choline fermentation by Escherichia coli 536. Journal of Bacteriology 2018, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petit E, LaTouf WG, Coppi MV, Warnick TA, Currie D, Romashko I, Deshpande S, Haas K, Alvelo-Maurosa JG, Wardman C: Involvement of a bacterial microcompartment in the metabolism of fucose and rhamnose by Clostridium phytofermentans. PLoS One 2013, 8:e54337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerfeld CA, Sutter M: Engineered bacterial microcompartments: apps for programming metabolism. Current Opinion in Biotechnology 2020, 65:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan C, Cheng S, Liu Y, Escobar CM, Crowley CS, Jefferson RE, Yeates TO, Bobik TA: Short N-terminal sequences package proteins into bacterial microcompartments. Proceedings of the National Academy of Sciences 2010, 107:7509–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juodeikis R, Lee MJ, Mayer M, Mantell J, Brown IR, Verkade P, Woolfson DN, Prentice MB, Frank S, Warren MJ: Effect of metabolosome encapsulation peptides on enzyme activity, coaggregation, incorporation, and bacterial microcompartment formation. Microbiologyopen 2020, 9:e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber I, Palmer DJ, Ludwig KN, Brown IR, Warren MJ, Frunzke J: Construction of Recombinant Pdu Metabolosome Shells for Small Molecule Production in Corynebacterium glutamicum. ACS Synthetic Biology 2017, 6:2145–2156. [DOI] [PubMed] [Google Scholar]
- 14.Nichols TM, Kennedy NW, Tullman-Ercek D: A genomic integration platform for heterologous cargo encapsulation in 1,2-propanediol utilization bacterial microcompartments. Biochemical Engineering Journal 2020, 156:107496. [Google Scholar]
- 15.Chowdhury C, Bobik TA: Engineering the PduT shell protein to modify the permeability of the 1, 2-propanediol microcompartment of Salmonella. Microbiology 2019, 165:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slininger Lee MF, Jakobson CM, Tullman-Ercek D: Evidence for improved encapsulated pathway behavior in a bacterial microcompartment through shell protein engineering. ACS Synthetic Biology 2017, 6:1880–1891. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury C, Chun S, Pang A, Sawaya MR, Sinha S, Yeates TO, Bobik TA: Selective molecular transport through the protein shell of a bacterial microcompartment organelle. Proceedings of the National Academy of Sciences 2015, 112:2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung CZ, Amikura K, Soll D: Using Genetic Code Expansion for Protein Biochemical Studies. Frontiers in Bioengineering and Biotechnology 2020, 8:598577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin JW: Expanding and reprogramming the genetic code. Nature 2017, 550:53. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Brock A, Herberich B, Schultz PG: Expanding the genetic code of Escherichia coli. Science 2001, 292:498–500. [DOI] [PubMed] [Google Scholar]
- 21.Arranz-Gibert P, Patel JR, Isaacs FJ: The role of orthogonality in genetic code expansion. Life 2019, 9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan W, Tharp JM, Liu WR: Pyrrolysyl-tRNA synthetase: an ordinary enzyme but an outstanding genetic code expansion tool. Biochimica et Biophysica Acta (BBA)Proteins and Proteomics 2014, 1844:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KJ, Kang D, Park H-S: Site-specific labeling of proteins using unnatural amino acids. Molecules and Cells 2019, 42:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meineke B, Heimgartner J, Eirich J, Landreh M, Elsasser SJ: Site-Specific Incorporation of Two ncAAs for Two-Color Bioorthogonal Labeling and Crosslinking of Proteins on Live Mammalian Cells. Cell Reports 2020, 31:107811. [DOI] [PubMed] [Google Scholar]
- 25.Venkat S, Sturges J, Stahman A, Gregory C, Gan Q, Fan C: Genetically Incorporating Two Distinct Post-translational Modifications into One Protein Simultaneously. ACS Synthetic Biology 2018, 7:689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park HS, Hohn MJ, Umehara T, Guo LT, Osborne EM, Benner J, Noren CJ, Rinehart J, Soll D: Expanding the genetic code of Escherichia coli with phosphoserine. Science 2011, 333:1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin JW, Martin AB, King DS, Wang L, Schultz PG: Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proceedings of the National Academy of Sciences 2002, 99:11020–11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Y, Jacinto MP, Zeng Y, Yu Z, Qu J, Liu WR, Lin Q: Genetically Encoded 2-Aryl-5-carboxytetrazoles for Site-Selective Protein Photo-Cross-Linking. Journal of the American Chemical Society 2017, 139:6078–6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson JC, Duffy SP, Hess KR, Mehl RA: Improving nature’s enzyme active site with genetically encoded unnatural amino acids. Journal of the American Chemical Society 2006, 128:11124–11127. [DOI] [PubMed] [Google Scholar]
- 30.Kolev JN, Zaengle JM, Ravikumar R, Fasan R: Enhancing the efficiency and regioselectivity of P450 oxidation catalysts by unnatural amino acid mutagenesis. ChemBioChem 2014, 15:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan Q, Lehman BP, Bobik TA, Fan C: Expanding the genetic code of Salmonella with non-canonical amino acids. Scientific Reports 2016, 6:39920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saleh AM, Wilding KM, Calve S, Bundy BC, Kinzer-Ursem TL: Non-canonical amino acid labeling in proteomics and biotechnology. Journal of Biological Engineering 2019, 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolb HC, Finn MG, Sharpless KB: Click chemistry: diverse chemical function from a few good reactions. Angewandte Chemie International Edition 2001, 40:2004–2021. [DOI] [PubMed] [Google Scholar]
- 34.Amiram M, Haimovich AD, Fan C, Wang Y-S, Aerni H-R, Ntai I, Moonan DW, Ma NJ, Rovner AJ, Hong SH: Evolution of translation machinery in recoded bacteria enables multi-site incorporation of nonstandard amino acids. Nature Biotechnology 2015, 33:1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, Simpson DM, Wenner N, Brownridge P, Harman VM, Hinton JCD, Beynon RJ, Liu LN: Decoding the stoichiometric composition and organisation of bacterial metabolosomes. Nature Communications 2020, 11:1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy NW, Ikonomova SP, Slininger Lee M, Raeder HW, Tullman-Ercek D: Self-assembling Shell Proteins PduA and PduJ have Essential and Redundant Roles in Bacterial Microcompartment Assembly. Journal of Molecular Biology 2021, 433:166721.33227310 [Google Scholar]
- 37.Crowley CS, Cascio D, Sawaya MR, Kopstein JS, Bobik TA, Yeates TO: Structural insight into the mechanisms of transport across the Salmonella enterica Pdu microcompartment shell. Journal of Biological Chemistry 2010, 285:37838–37846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutter M, Greber B, Aussignargues C, Kerfeld CA: Assembly principles and structure of a 6.5-MDa bacterial microcompartment shell. Science 2017, 356:1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorda J, Liu Y, Bobik TA, Yeates TO: Exploring bacterial organelle interactomes: a model of the protein-protein interaction network in the Pdu microcompartment. PLoS Computational Biology 2015, 11:e1004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coin I: Application of non-canonical crosslinking amino acids to study protein-protein interactions in live cells. Current Opinion in Chemical Biology 2018, 46:156–163. [DOI] [PubMed] [Google Scholar]
- 41.Yeates TO, Jorda J, Bobik TA: The shells of BMC-type microcompartment organelles in bacteria. Journal of Molecluar Microbiology and Biotechnology 2013, 23:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faulkner M, Zhao L-S, Barrett S, Liu L-N: Self-assembly stability and variability of bacterial microcompartment shell proteins in response to the environmental change. Nanoscale Research Letters 2019, 14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutter M, Faulkner M, Aussignargues Cm, Paasch BC, Barrett S, Kerfeld CA, Liu L-N: Visualization of bacterial microcompartment facet assembly using high-speed atomic force microscopy. Nano Letters 2016, 16:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tharp JM, Ehnbom A, Liu WR: tRNA(Pyl): Structure, function, and applications. RNA Biology 2018, 15:441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bari NK, Kumar G, Hazra JP, Kaur S, Sinha S: Functional protein shells fabricated from the self-assembling protein sheets of prokaryotic organelles. Journal of Materials Chemistry B 2020, 8:523–533. [DOI] [PubMed] [Google Scholar]
- 46.Aussignargues C, Pandelia ME, Sutter M, Plegaria JS, Zarzycki J, Turmo A, Huang J, Ducat DC, Hegg EL, Gibney BR, et al. : Structure and Function of a Bacterial Microcompartment Shell Protein Engineered to Bind a [4Fe-4S] Cluster. Journal of the American Chemical Society 2016, 138:5262–5270. [DOI] [PubMed] [Google Scholar]
- 47.Plegaria JS, Yates MD, Glaven SM, Kerfeld CA: Redox Characterization of Electrode-Immobilized Bacterial Microcompartment Shell Proteins Engineered To Bind Metal Centers. ACS Applied Bio Materials 2019, 3:685–692. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence AD, Frank S, Newnham S, Lee MJ, Brown IR, Xue W-F, Rowe ML, Mulvihill DP, Prentice MB, Howard MJ: Solution structure of a bacterial microcompartment targeting peptide and its application in the construction of an ethanol bioreactor. ACS Synthetic Biology 2014, 3:454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huguenin-Dezot N, Alonzo DA, Heberlig GW, Mahesh M, Nguyen DP, Dornan MH, Boddy CN, Schmeing TM, Chin JW: Trapping biosynthetic acyl-enzyme intermediates with encoded 2, 3-diaminopropionic acid. Nature 2019, 565:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]


