Abstract
Background—
Global electrical heterogeneity (GEH) is associated with sudden cardiac death in the general population. Its utility in patients with systolic heart failure (HF) who are candidates for primary prevention (PP) implantable cardioverter-defibrillators (ICDs) is unclear.
Objective—
To investigate whether GEH is associated with sustained ventricular tachycardia (VT)/ventricular fibrillation (VF) leading to appropriate ICD therapies in HF patients with PP ICDs.
Methods—
We conducted a multicenter retrospective cohort study. GEH was measured by spatial ventricular gradient (SVG) direction (azimuth and elevation) and magnitude, QRS-T angle, and sum absolute QRST integral (SAIQRST) on pre-implant 12-lead ECGs. Survival analysis using cause-specific hazard functions compared the strength of associations with two competing outcomes: sustained VT/VF leading to appropriate ICD therapies and all-cause death without appropriate ICD therapies.
Results—
We analyzed 2,668 patients (age 63±12y; 23% female; 78% white; 43% nonischemic cardiomyopathy (NICM); left ventricular ejection fraction 28±11% from 6 academic medical centers). After adjustment for demographic, clinical, device, and traditional ECG characteristics, SVG elevation (Hazard Ratio (HR) per 1 standard deviation (SD) 1.14 (95% CI 1.04–1.25); P=0.004), SVG azimuth (HR per 1 SD 1.12(1.01–1.24); P=0.039); SVG magnitude (HR per 1 SD 0.75(0.66–0.85); P<0.0001), and QRS-T angle (HR per 1 SD 1.21 (95% CI 1.08–1.36); P=0.001) were associated with appropriate ICD therapies. SAIQRST had different associations in infarct-related [HR 1.29(1.04–1.60)] and NICM [HR 0.78(0.62–0.96); Pinteraction=0.022].
Conclusion—
In patients with PP ICDs, GEH is independently associated with appropriate ICD therapies. The SVG vector points in distinctly different directions in patients with two competing outcomes.
Keywords: ICD, VCG, HF, VT/VF
Introduction
Patients with cardiomyopathy (CM) and reduced left ventricular ejection fraction (LVEF) are at elevated risk of sudden cardiac death (SCD), and in this population, guidelines recommend consideration of implantable cardioverter-defibrillators (ICDs) for primary prevention (PP) of SCD1. However, current guidelines for implantation of PP ICDs rely primarily on LVEF and New York Heart Association (NYHA) functional class, which are overall poor predictors of ICD benefit, especially in patients with nonischemic CM (NICM).2 As ICD implantation is associated with significant costs for the medical system and risk to individual patients, improved methods of identifying patients most likely to benefit from PP ICDs are needed.
Global electrical heterogeneity (GEH)3, 4 is a comprehensive characterization of the spatial ventricular gradient (SVG) vector5 by 5 features: SVG vector direction (azimuth and elevation), magnitude, its scalar value, and spatial QRS-T angle.3 It integrates static and dynamic alternations in ventricular repolarization and conduction, which have been linked to ventricular arrhythmias in studies from the bench to the bedside.6 Abnormal GEH is associated with SCD in the general population3, 7 where it is explicitly associated with SCD over non-arrhythmic modes of death3, and an increased risk of drug-induced torsades-de-pointes.8, 9 The utility of assessing GEH for SCD risk stratification in CM patients with reduced LVEF who meet PP ICD indications, however, is unknown. As GEH might be a useful way of identifying patients who are most (or least) likely to benefit from PP ICD implantation, we conducted a retrospective multicenter cohort study10 to determine the associations of clinical, device, traditional ECG, and GEH metrics with sustained ventricular arrhythmias requiring appropriate ICD therapies and competing death without appropriate ICD therapies.
Methods
The multicenter study has been approved by the OHSU IRB and each participating center’s IRB. The research reported in this paper adhered to the 1964 Helsinki declaration and its later amendments.
Study design
The study design and protocol have been previously described.10 In brief, we conducted a retrospective, multicenter cohort study that included data from six US academic medical centers. We included all patients above 18 years of age with chronic systolic heart failure (HF) due to infarct-related CM and/or NICM, who underwent implantation of any ICD [including single/dual-chamber, cardiac resynchronization therapy defibrillator (CRT-D), or subcutaneous] for PP of SCD in 1996–2019, and had an available digital 12-lead ECG. Patients with inherited channelopathies/cardiomyopathies, congenital heart disease, and secondary prevention ICDs were excluded.
ECG analysis: global electrical heterogeneity measurement
A 12-lead ECG recorded around the time of ICD implantation was collected (median 0 days (interquartile range 0–29 days)). Raw, digital ECG signals were analyzed as previously described.3, 7, 11, 12 At least two physician-investigators manually labeled each cardiac beat (SH, AB, LGT). The Kors transformation matrix transformed 12-lead ECGs into orthogonal XYZ vectorcardiograms. The time-coherent global median beat was constructed using the dominant beat type, and the vectorcardiogram origin point was identified.12 We included three categories of median beats: normal (N) beats included sinus rhythm, atrial pacing, junctional rhythm, and ectopic atrial rhythm; ventricular paced (VP) beats included ventricular pacing; supraventricular (S) beats included atrial fibrillation or atrial flutter with a consistent QRS morphology. QT interval was corrected using the Hodges equation. The presence of premature ventricular complexes (PVCs) was noted.
We constructed spatial peak and area QRS and T vectors as well as their vector sum (SVG) and measured their magnitude, direction [azimuth (orientation in the XZ plane) and elevation (orientation in the XY plane)], and spatial QRS-T angles (Figure 1).7, 11, 12 Scalar approximations of the SVG were measured via the sum absolute QRST integral (SAIQRST)13–15 and the QT integral on vector magnitude (VMQTi).11 Quality control of automated ECG analysis was performed to verify appropriate beat identification and fiducial point annotation (KTH). Open-source MATLAB (MathWorks, Natick,MA) code is provided at https://physionet.org/physiotools/geh & https://github.com/Tereshchenkolab/Origin.
Figure 1.
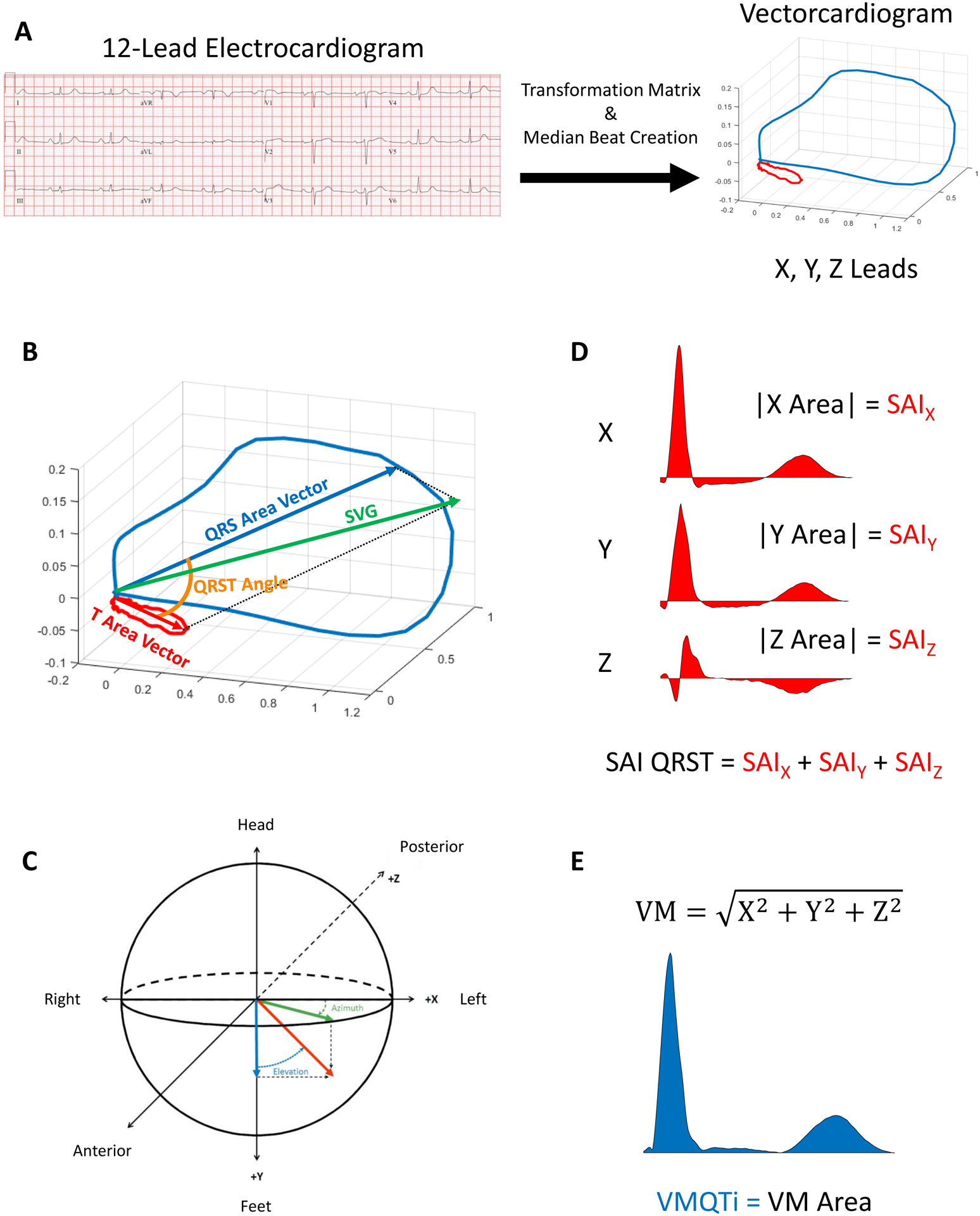
GEH measurements.
Primary Competing Outcomes
The primary outcome was defined as the first sustained ventricular tachyarrhythmia event (VT or VF) with appropriate ICD therapy (either anti-tachycardia pacing or shock). All-cause death without preceding appropriate ICD therapy served as the primary competing outcome.
Clinical characteristics and ICD programming
Patients’ demographics, clinical history, and laboratory values at the pre-implant clinical evaluation were abstracted from the medical record. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. ICD detection parameters were obtained at the time of device implant or appropriate ICD therapies if they occurred during follow-up. To harmonize VT/VF detection duration programming across different device manufacturers, we grouped patients16 in (1) long detection duration (number of intervals to detect (NID) VT ≥30/40 or VT detection duration ≥10s or VF detection duration ≥3s) or (2) short detection duration (NID VT<30/40 or VT detection duration <10s or VF detection duration <3s) categories (Supplemental Table 1).
Statistical analyses
Detailed description of the statistical methods is provided in the Supplement. Normally distributed continuous variables were reported as means and standard deviations (SD). Circular variables were reported as mean and the strength of the resultant vector (mean resultant length).
As we conducted a retrospective study, the data contained missing values for reasons unrelated to the study itself. Missingness data for individual covariates are reported in Supplemental Table 2. We employed multiple imputations using chained equations, which does not require the multivariate normal assumption. Our investigation of data missingness showed an arbitrary pattern. Observations with missing primary outcomes were excluded.
As the risk of sustained VT/VF leading to appropriate ICD therapy competes with the risk of death without appropriate ICD therapy, we employed cause-specific hazards functions, estimated using Cox proportional hazards models. We compared the strength of association of demographic, clinical, ECG, and device characteristics between the two competing outcomes.
To study the association of GEH with the primary competing outcomes, we constructed four models. All models (1–4) with GEH exposure variables were adjusted for the type of median beat, mean RR interval, presence of PVCs, and possible distortion of median beat by premature beats. Model 1 was adjusted for demographic characteristics (age, sex, race) and study center. Model 2 included model 1 variables with additional adjustment for known clinical risk factors of the primary competing outcomes [LVEF, NYHA class, CM type, hypertension, diabetes, stroke, atrial fibrillation, eGFRCKD-EPI, and medications (use of beta-blockers, ACEi/ARBs, and class 1 or III antiarrhythmic drugs (AAD)]. Model 3 contained all covariates included in model 2 and device characteristics: device type (ICD or CRT-D), manufacturer, and programming (VT and VF zone cutoffs and detection duration category). Model 4, in addition to model 3 variables, was adjusted for traditional ECG metrics: heart rate, QRS duration, and Hodges-corrected QT interval. To standardize comparisons, we expressed continuous variables per 1 SD. To check for multicollinearity, we calculated variance inflation factor (VIF) in model 4. VIF was below 3.5 for all covariates (Supplemental Table 3), confirming an absence of multicollinearity.
To investigate a previously reported discrepancy in the association of SAIQRST with ventricular tachyarrhythmias/SCD between studies that included entirely or mostly infarct-related CM (ICM) patients3, 17 and studies that included mixed ICM and NICM patients,15, 18 we tested the interaction of CM type with SAIQRST and VMQTi.
To appropriately consider nonlinear hazards, associations of continuous GEH variables with primary competing outcomes were also studied using adjusted (model 1) Cox models incorporating cubic splines with 4 knots.
To test the study findings’ robustness under the missing at random assumption, we conducted sensitivity analyses using an imputed dataset based on complete ECG data. The dataset without missing pre-implant ECG data included 2251 patients; 480 had sustained VT/VF with appropriate ICD therapy, and 401 died without appropriate ICD therapy.
Statistical analyses were performed using STATA MP 16.1 (StataCorp LP, College Station, TX). A P value < 0.05 was considered statistically significant. STATA code is available at https://github.com/Tereshchenkolab/gehco.
Results
Study Population
After excluding ineligible patientsand those with missing outcomes, the study population included 2,668 patients (Figure 2). Clinical characteristics are shown in Table 1.
Figure 2.
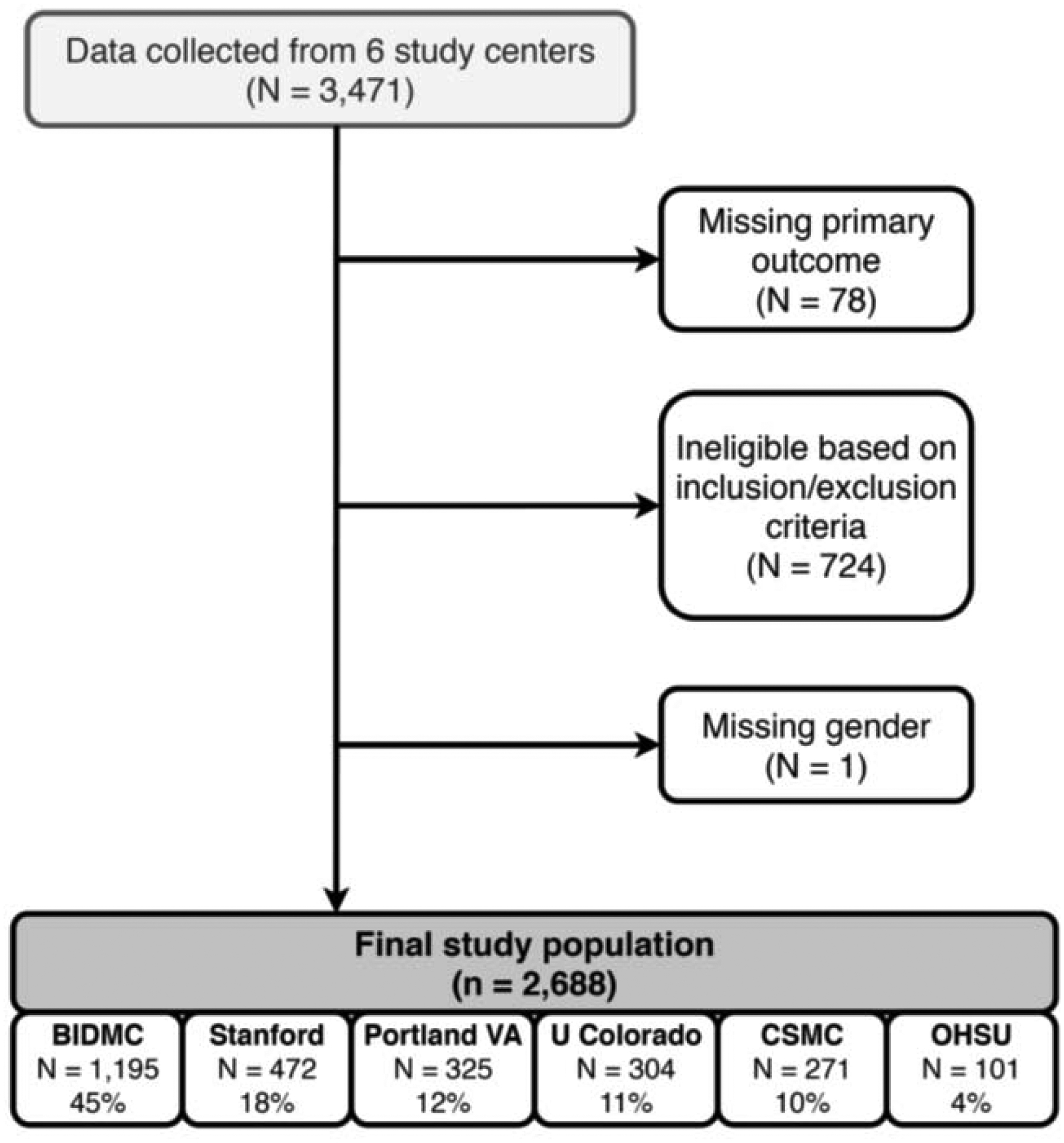
Study flowchart.
Table 1.
Clinical characteristics of the study population
| Characteristic | N=2668 |
|---|---|
| Age(SD), y | 63.1(13.0) |
| Female, % | 23.4 |
| White,% | 78.4 |
| Black,% | 9.7 |
| Hispanic,% | 7.5 |
| Ischemic CM, % | 57.0 |
| NICM, % | 43.0 |
| LVEF(SD),% | 28.2(11.1) |
| NYHA class I,% | 14.5 |
| NYHA class II,% | 39.6 |
| NYHA III,% | 43.2 |
| NYHA IV,% | 2.7 |
| Myocardial infarction,% | 49.1 |
| History of revascularization,% | 43.1 |
| Hypertension,% | 69.4 |
| Diabetes,% | 36.2 |
| Atrial fibrillation,% | 33.9 |
| History of stroke,% | 11.1 |
| Class I or III antiarrhythmic drugs,% | 11.2 |
| RAAS-modifying drugs,% | 78.7 |
| Use of beta-blockers,% | 85.1 |
| eGFRCKD-EPI(SD), mL/min/1.73 m2 | 70.4(29.0) |
| BUN(SD), mg/dL | 24.7(13.8) |
| ICD single-chamber,% | 36.2 |
| ICD dual-chamber,% | 26.9 |
| CRT-D,% | 36.3 |
| ICD replacement,% | 30.2 |
| VT detection zone programmed,% | 90.5 |
| Antitachycardia pacing ON,% | 85.8 |
| VT zone (SD),bpm | 178(15) |
| VF zone (SD),bpm | 225.4(48.3) |
| Historic ICD therapies programming,% | 67.3 |
| Delayed ICD therapies programming,% | 32.7 |
| Medtronic device,% | 60.5 |
| Boston Scientific device,% | 24.3 |
| St. Jude device,% | 13.4 |
| Heart rate(SD), bpm | 71.5(15.3) |
| QRS duration(SD), ms | 117.0(35.8) |
| QTc Hodges(SD), ms | 448.5(47.0) |
| Area QRS-T angle (mean(length),° | 144.6(0.853) |
| Area SVG elevation mean(length),° | 87.4(0.866) |
| Area SVG azimuth mean(length),° | 56.8(0.528) |
| Area SVG magnitude(SD). mVms | 41.1(24.6) |
| SAIQRST(SD), mVms | 194.9(110.4) |
Over a median follow-up of 4 years, 541 patients experienced sustained VT/VF leading to appropriate ICD therapy [incidence 50.1 (95% CI 46.0–54.5) per 1000 person-years], and 479 patients died without appropriate ICD therapy [incidence 44.0 (95%CI 40.2–48.1) per 1000 person-years]. The average rate of appropriate ICD therapy was similar to the rate of death without appropriate ICD therapy: ~ 5% per year.
Heart rate, QRS duration, and QTc were within normal range (Table 1). Unadjusted comparisons of ECG characteristics are reported in Supplemental Table 4.
Demographic and clinical characteristics associated with primary competing outcomes
In minimally adjusted model 1 (Supplemental Table 5), male sex, black race, atrial fibrillation, use of AADs, history of MI and revascularization procedures, NYHA class III, and higher BUN were associated with increased risk of appropriate ICD therapies. In contrast, greater age, eGFR, and LVEF were associated with a lower risk of appropriate ICD therapies. Adjustment for cardiac disease substrate, risk factors (model 2), and device programming (model 3) explained the association of race, atrial fibrillation, MI and revascularization, NYHA class, and BUN with VT/VF, which became non-significant. In fully adjusted analysis (model 4), only age, male sex, use of AADs, LVEF, and eGFR remained significantly associated with VT/VF and appropriate ICD therapies.
In model 1 assessing the competing outcome of death without appropriate ICD therapy (Supplemental Table 6), age, ICM, MI, revascularization history, NYHA class II-IV, diabetes, atrial fibrillation, and BUN were associated with a higher risk of all-cause death, while use of beta-blockers, ACEIs, higher LVEF, and higher eGFR were associated with a lower risk of death. Adjustment for the HF characteristics and risk factors (model 2), device characteristics (model 3), and traditional ECG metrics (model 4) only slightly attenuated the association of clinical risk factors with the competing mortality outcome.
Even after comprehensive adjustment (model 3), several clinical risk factors had significantly different cause-specific hazards associated with the two competing outcomes (Supplemental Table 7). For every 13 years (1 SD) increase in age, the hazard of appropriate ICD therapy decreased by 20% (Figure 3), whereas the hazard of death without appropriate ICD therapy increased by 32%. Diabetes increased the hazard of death without appropriate ICD therapy by 45% but was not associated with appropriate ICD therapy. As expected, NYHA class and BUN had stronger associations with death than with appropriate ICD therapy. Use of beta-blockers and ACEIs were associated with a reduced hazard of death but not with the hazard of appropriate ICD therapy.
Figure 3.
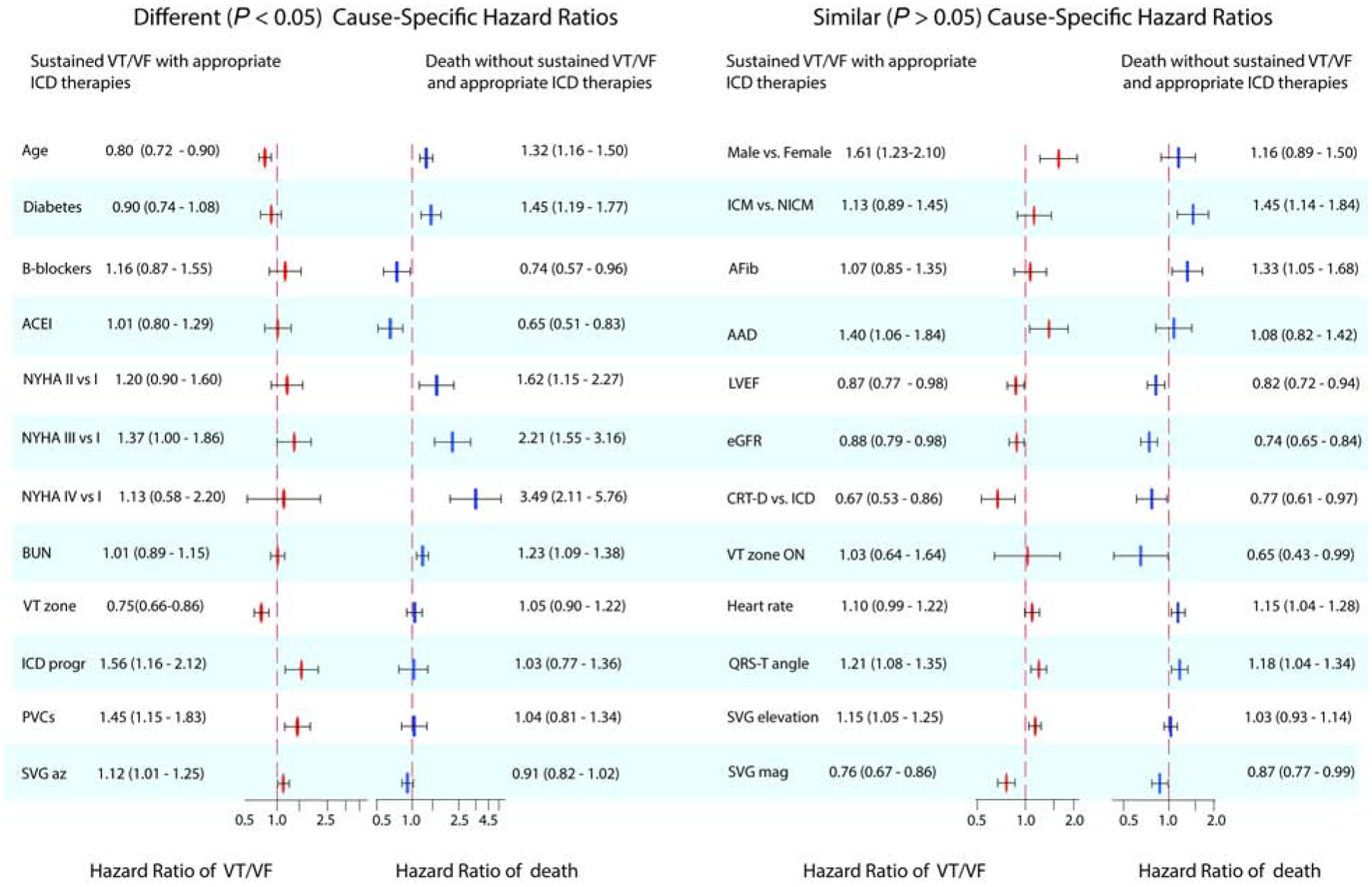
Statistically significantly different (P<0.05) or similar (P≥0.05) adjusted (model 3) cause-specific Cox HR(95% CI) of two competing outcomes: sustained VT/VF with appropriate ICD therapies (red ovals) and all-cause death without appropriate ICD therapies (blue rectangles).
In contrast, there was no statistically significant difference in adjusted cause-specific hazards of two competing outcomes for male sex, ICM, atrial fibrillation, use of AADs, LVEF, and eGFR (Figure 3).
Device characteristics associated with primary competing outcomes
In minimally adjusted model 1, ICD device type and programming were significantly associated with VT/VF and appropriate ICD therapies (Supplemental Table 5). Adjustment for HF characteristics, risk factors, comorbidities, and traditional ECG metrics (models 2–4) did not change the strength of the association. In contrast, very few device characteristics were associated with mortality without appropriate ICD therapy.
In adjusted competing risk analyses, device characteristics had significantly different cause-specific hazards associated with the two competing outcomes (Supplemental Table 7). Unsurprisingly, for each additional 15 bpm increase in VT zone threshold, the hazard of VT/VF leading to appropriate ICD therapies decreased by 25% (Figure 3), but it did not affect all-cause mortality without sustained VT/VF. Consistent with prior observations, more aggressive ICD programming was associated with a 56% higher risk of appropriate ICD therapies but was not associated with the competing death outcome.
CRT-D implantation was associated with a lower risk of both competing outcomes (Figure 3).
Association of GEH with primary competing outcomes
In minimally adjusted analysis, PVCs and all GEH metrics were associated with appropriate ICD therapies (Supplemental Table 5). However, the adjustment for known clinical risk factors and device characteristics explained that association for SAIQRST and VMQTi. Notably, after full adjustment in model 4, area and peak spatial QRS-T angle,SVG elevation, area SVG magnitude, and peak SVG azimuth were associated with appropriate ICD therapies.
In comparison, only resting heart rate, spatial QRS-T angle, and area SVG magnitude associated with the competing mortality outcome. The strength of associations diminished from model 1 to model 4, suggesting confounding by HF substrate (Supplemental Table 6).
In minimally adjusted competing risk analysis, heart rate and QTc had significantly different cause-specific hazards associated with the two competing outcomes (Supplemental Table 7). However, these differences were explained by differences in clinical risk factors. In adjusted models, QRS duration and corrected QT interval were not associated with either appropriate ICD therapies or total mortality. After adjustment, only PVCs and peak SVG azimuth had significantly different cause-specific hazards associated with two competing outcomes (Figure 3). The presence of PVCs was associated with a 42% higher risk of appropriate ICD therapies but did not associate with mortality.
Each 1 SD increase in peak SVG azimuth (+36°, backward) was associated with a 12% increase in the hazard of appropriate ICD therapies and a 10% reduction in the hazard of total mortality without appropriate ICD therapy. Accordingly, a 1 SD reduction in peak SVG azimuth (−36°, forward) was associated with a 12% decrease in the hazard of appropriate ICD therapies and a 10% increase in the risk of death without appropriate ICD therapy. Figure 4 illustrates opposing directions of hazards of two competing outcomes across the peak SVG azimuth distribution. Area SVG azimuth showed a U-shaped association with appropriate ICD therapy (Supplemental Table 5) but not competing death risk (Supplemental Table 6).
Figure 4.
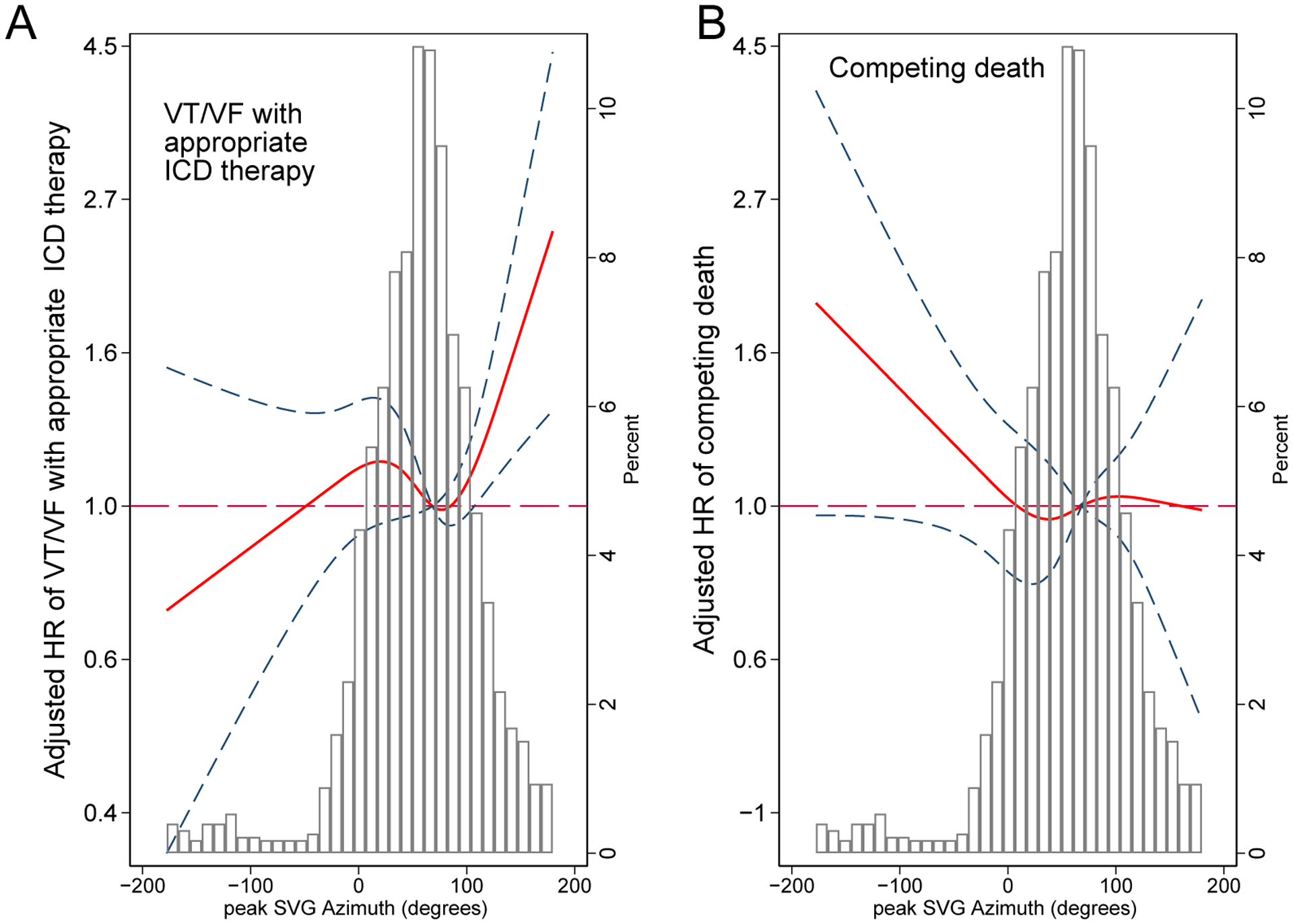
Adjusted (model 1) risk of (A) sustained VT/VF with appropriate ICD therapy and (B) competing death without appropriate ICD therapy associated with peak SVG azimuth. Restricted cubic spline with 95% CI shows a change in cause-specific Cox HR (Y-axis) in response to peak SVG azimuth change (X-axis). The 50th percentile of the SVG azimuth is a reference. Knots of the peak SVG azimuth are at (-21)-42-75-137 degrees.
Spatial QRS-T angle and SVG magnitude were associated with both appropriate ICD therapy and death without appropriate ICD therapy. For each 1 SD (33°) increase in area QRS-T angle, we observed an ~20% increase in the hazard of both outcomes (Figure 3). Each additional 1 SD increase in area SVG magnitude (25 mV*ms) was associated with a 25% reduction in the hazard of appropriate ICD therapies and a 14% reduction in the hazard of death without appropriate ICD therapy. For area SVG elevation, each 1 SD (31°) increase was associated with a 14% increase in the hazard of appropriate ICD therapies.
Sensitivity analysis (Supplemental Table 8) showed similar results.
Interaction of SAIQRST with cardiomyopathy type
Cardiomyopathy type significantly modified the association of SAIQRST (and VMQTi) with the primary arrhythmic (but not competing death) outcome (Supplemental Table 9). After adjustment for HF characteristics, risk factors, and comorbidities (model 3), for each additional 111 mVms increase in SAIQRST, the hazard of sustained VT/VF with appropriate ICD therapies increased by 29% in ICM patients, but decreased by 22% in NICM subgroup (Figure 5).
Figure 5.
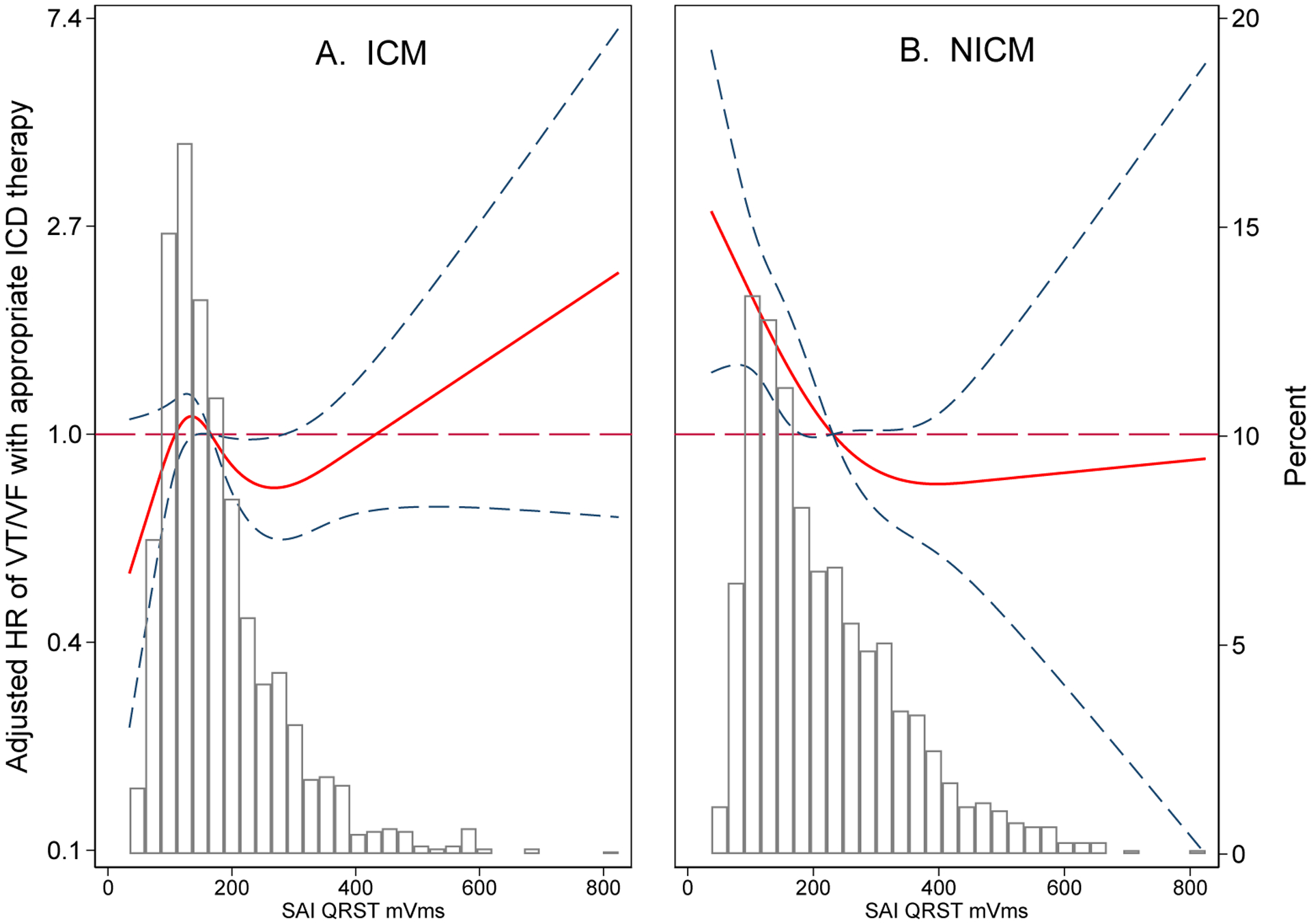
Adjusted (model 1) risk of sustained VT/VF with appropriate ICD therapy associated with SAIQRST in ICM (A) and NICM (B). Restricted cubic spline with 95% CI shows a change in cause-specific Cox HR (Y-axis) in response to SAIQRST change (X-axis). The 50th percentile of the SAIQRST is a reference. Knots of the SAIQRST in ICM are at 74-127-186-372 mVms, and in NICM are at 80-146-242-462 mVms.
Discussion
This large, multicenter, retrospective cohort study of novel risk factors for SCD had two important findings. First, in competing risk analyses, we showed an independent association of GEH with VT/VF leading to appropriate ICD therapies. After adjusting for demographic, clinical, ECG, and device characteristics, the SVG vector pointed in distinctly different directions in patients who developed sustained VT/VF treated by appropriate ICD therapies (backward-upward, counterclockwise) versus those who died without ICD therapy (forward, clockwise). SVG magnitude and spatial QRS-T angle were associated with both competing outcomes. The association of SAIQRST with sustained VT/VF was modified by CM type. Smaller SAIQRST was associated with increased VT/VF risk with appropriate ICD therapies in NICM, but decreased risk in ICM.
Second, we showed significantly different strengths of association of age, diabetes, use of beta-blockers and ACEIs, NYHA class, BUN, ICD device programming features and VT zone cutoff, and the presence of PVCs between the two competing outcomes (appropriate ICD therapies and all-cause death). There was no difference in the associations of sex, CM type, atrial fibrillation, AAD use, LVEF, eGFR, and ICD device type with both competing outcomes.
Differences in the risk factors of two competing outcomes
Considering naturally competing outcomes in ICD patients is critically important.19 However, very few studies conducted a formal comparison of the strengths of association of a risk factor with two competing outcomes.20 In our study of PP ICD recipients, unsurprisingly, VT/VF detection duration and VT zone cutoff were distinctly different attributes of the risk of ventricular tachyarrhythmia with appropriate ICD therapies.21 Our results are consistent with the well-recognized fact that appropriate ICD therapies occur more frequently than SCD,22 and ICD programming determines whether patients receive unnecessary therapies for what would have been nonsustained VT with longer ICD detection times.21
We observed that diabetes, BUN, NYHA class, and HF therapy (ACEIs, beta-blockers) were strongly associated with all-cause death without appropriate ICD therapies but did not associate with the primary arrhythmic outcome. These clinical characteristics are often perceived as risk factors of both outcomes. Observed differences in these clinical risk factors’ association with two competing outcomes should be considered in planning future clinical studies and developing SCD risk models.
Age was associated with both outcomes in distinctly different ways. After adjusting for confounders, for every 1 SD (13 years) increase in age, the hazard of death without preceding appropriate ICD therapy increased by 35%, while the hazard of sustained ventricular tachyarrhythmia leading to appropriate ICD therapy decreased by 21%. Age is a ubiquitous risk marker. It is well-recognized that many ICD recipients, despite having an ICD, often die secondary to progressive heart failure or other non-cardiac comorbidities that congregate with advanced age.
Similarities in the risk factors of two competing outcomes
Noticeably, a set of clinical risk factors (sex, CM type, atrial fibrillation, use of AADs, LVEF, eGFR), and ICD type had similar associations with both outcomes, suggesting common or overlapping mechanisms. All these risk factors are well-known. Common mechanisms of two competing outcomes highlight challenges in selecting an ideal candidate for a PP ICD.
Global electrical heterogeneity
Our study confirmed that after multivariable adjustment in competing risk analysis, there is an independent association between SVG direction and sustained ventricular tachyarrhythmias leading to appropriate ICD therapies, but not the competing risk of death. The SVG vector points towards the area of the myocardium with the shortest excited state,4, 10 and deviations from normal suggest the accumulation of a critical mass of abnormal electrical substrate, which might predispose to ventricular arrhythmias. Our results support the use of GEH, and specifically the orientation of the area SVG vector, as a marker of abnormal underlying electrophysiological substrate responsible for a propensity for sustained ventricular arrhythmias.8, 9 Further studies of mechanisms behind SVG directions are warranted.
Notably, we observed that the presence of PVCs on the 12-lead ECG was also associated with the competing risk of appropriate ICD therapy, but not all-cause mortality. Cardiac memory developing in response to PVCs may affect GEH.23 It is well known that PVCs are associated with the development or worsening of HF, and that PVCs can trigger life-threatening ventricular tachyarrhythmias. Further studies of the interaction between an underlying arrhythmogenic substrate (manifest by GEH) and PVCs (manifest by cardiac memory development) are needed to uncover underlying mechanisms, leading to novel therapies.
In contrast to SVG direction, which had significantly different cause-specific hazards associated with two competing outcomes, area SVG magnitude and spatial QRS-T angle had similar associations with both outcomes. The finding of QRS-T angle being associated with both competing outcomes is expected. Numerous previous studies have shown an association between QRS-T angle and broadly defined cardiovascular disease.24 Our results underscore the importance and added value of a comprehensive GEH concept, which includes measurement of SVG direction, in addition to the QRS-T angle.
Our results also explain a previously observed discrepancy in SAIQRST. Similar to this study, in another PP ICD study of NICM patients (PROSE-ICD),15, 18 smaller SAIQRST was associated with increased risk of appropriate ICD therapies. In contrast, in the ICM MADIT-II study,17 and general population,3 larger SAIQRST was associated with ventricular arrhythmias and SCD. The current study demonstrated an opposite association of SAIQRST with sustained VT/VF and appropriate ICD therapies in patients with ICM or NICM.
It should be noted that prior attempts to noninvasively quantify electrical heterogeneity and use it as a marker for arrhythmic risk, with measures such as QT dispersion and T-wave alternans, have failed to demonstrate a clear utility for SCD risk stratification. GEH, however, is unique in that it has a strong theoretical and mathematical foundation based on ECG dipole theory. Additionally, GEH parameters remained associated with ICD therapies despite extensive adjustment (27 variables) for demographic and clinical characteristics, device programming, and traditional ECG characteristics. Further prospective studies are planned to confirm the associations between GEH and ventricular arrhythmias/SCD.
Strengths/Limitations
Our study’s strengths include the large sample size and population drawn from a diverse group of medical centers within the US. However, because the study included only academic medical centers, the results may not apply to all ICD patients. Because of our study’s retrospective nature, ICD programming was not standardized, and differences in ICD detection/treatment parameters could have influenced the results, although we attempted to correct this by including ICD programming in our multivariable models. There was no postmortem ICD interrogation data, and we cannot precisely determine if there were ventricular arrhythmias just proximate to death. Importantly, appropriate ICD therapy is a surrogate for SCD, and all ventricular arrhythmias treated by the ICD would not necessarily have led to cardiac arrest or SCD. As in any observational study, residual confounding cannot be completely eliminated. Prospective studies evaluating the association between GEH and arrhythmic outcomes in PP ICD patients will be needed before GEH can be adopted for use in clinical practice.
Supplementary Material
Acknowledgment
The authors thank Zachary Zouyed, BS, Nichole Rogovoy, BS, Katherine Yang, BS, and Christopher Hamilton, BA, for their help with data preparation.
Funding: 17GRNT33670428 (LGT), HL118277 (LGT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration—URL:www.clinicaltrials.gov Unique identifier:NCT03210883.
Disclosures:None.
References:
- 1.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2018;15:e190–e252. [DOI] [PubMed] [Google Scholar]
- 2.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 3.Waks JW, Sitlani CM, Soliman EZ, et al. Global Electric Heterogeneity Risk Score for Prediction of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation 2016;133:2222–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waks JW, Tereshchenko LG. Global electrical heterogeneity: A review of the spatial ventricular gradient. J Electrocardiol 2016;49:824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burch GE, Abildskov JA, Cronvich JA. A Study of the Spatial Vectorcardiogram of the Ventricular Gradient. Circulation 1954;9:267–275. [DOI] [PubMed] [Google Scholar]
- 6.Goldberger JJ, Basu A, Boineau R, et al. Risk stratification for sudden cardiac death: a plan for the future. Circulation 2014;129:516–526. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Alday EA, Bender A, German D, et al. Dynamic predictive accuracy of electrocardiographic biomarkers of sudden cardiac death within a survival framework: the Atherosclerosis Risk in Communities (ARIC) study. BMC cardiovascular disorders 2019;19:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stabenau HF, Shen C, Tereshchenko LG, Waks JW. Changes in global electrical heterogeneity associated with dofetilide, quinidine, ranolazine, and verapamil. Heart Rhythm 2020;17:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stabenau HF, Shen C, Zimetbaum P, Buxton AE, Tereshchenko LG, Waks JW. Global electrical heterogeneity associated with drug-induced torsades de pointes. Heart Rhythm 2021;18:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waks JW, Hamilton C, Das S, et al. Improving sudden cardiac death risk stratification by evaluating electrocardiographic measures of global electrical heterogeneity and clinical outcomes among patients with implantable cardioverter-defibrillators: rationale and design for a retrospective, multicenter, cohort study. J Interv Card Electrophysiol 2018;52:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas JA, E AP-A, Junell A, et al. Vectorcardiogram in athletes: The Sun Valley Ski Study. Ann Noninvasive Electrocardiol 2019;24:e12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Alday EA, Li-Pershing Y, Bender A, et al. Importance of the heart vector origin point definition for an ECG analysis: The Atherosclerosis Risk in Communities (ARIC) study. Comput Biol Med 2019;104:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sur S, Han L, Tereshchenko LG. Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One 2013;8:e57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tereshchenko LG, Cheng A, Fetics BJ, et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. J Electrocardiol 2011;44:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tereshchenko LG, Cheng A, Fetics BJ, et al. Ventricular arrhythmia is predicted by sum absolute QRST integral but not by QRS width. J Electrocardiol 2010;43:548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunderson BD, Abeyratne AI, Olson WH, Swerdlow CD. Effect of Programmed Number of Intervals to Detect Ventricular Fibrillation on Implantable Cardioverter-Defibrillator Aborted and Unnecessary Shocks. Pacing and Clinical Electrophysiology 2007;30:157–165. [DOI] [PubMed] [Google Scholar]
- 17.Tereshchenko LG, McNitt S, Han L, Berger RD, Zareba W. ECG marker of adverse electrical remodeling post-myocardial infarction predicts outcomes in MADIT II study. PLoS One 2012;7:e51812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tereshchenko LG, Cheng A, Fetics BJ, et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. J Electrocardiol 2011;44:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koller MT, Schaer B, Wolbers M, Sticherling C, Bucher HC, Osswald S. Death without prior appropriate implantable cardioverter-defibrillator therapy: a competing risk study. Circulation 2008;117:1918–1926. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee NA, Tikkanen JT, Panicker GK, et al. Simple electrocardiographic measures improve sudden arrhythmic death prediction in coronary disease. Eur Heart J 2020;41:1988–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuger C, Daubert JP, Zareba W, et al. Reassessing the role of Antitachycardia Pacing in Fast Ventricular Arrhythmias in Primary Prevention Implantable Cardioverter Defibrillator Recipients: Results from MADIT-RIT. Heart Rhythm 2020. [DOI] [PubMed] [Google Scholar]
- 22.Ellenbogen KA, Levine JH, Berger RD, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation 2006;113:776–782. [DOI] [PubMed] [Google Scholar]
- 23.Haq K, Cao J, Tereshchenko LG. Characteristics of Cardiac Memory in Patients with Implanted Cardioverter Defibrillator: the CAMI study. medRxiv 2019. 10.1101/19005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oehler A, Feldman T, Henrikson CA, Tereshchenko LG. QRS-T Angle: A Review. Ann Noninvasive Electrocardiol 2014;19:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


