Abstract
Although the role of magnesium in blood pressure has been well studied among hypertensive patients, no study has explored the role of magnesium in hypertensive crises. The primary objective of this study is to evaluate the differences in serum magnesium levels between hypertensive crises patients and matched controls (age‐, sex‐, race‐, and diabetes‐matched) in a 1:1 random match. This study is a single‐center, retrospective, chart review, case‐control study of patients with hypertensive crises (case group) and patients without hypertensive crises (control group). Patients were included in the case group if they were 18 years of age or older with hypertensive crises and have a documented magnesium level. The control group patients were required to be 18 years of age or older, have no diagnosis of hypertensive crises, and have a documented magnesium level. The primary outcome of the study was to compare the mean serum magnesium in patients with hypertensive crises versus patients without hypertensive crises. Three hundred and fifty‐eight patients were included in the study: 179 patients in both the case group and control group. The primary outcome results showed that serum magnesium concentration was not significantly different between the case group (1.89 ± 0.29 mg/dl) and control group (1.90 ± 0.31 mg/dl) (p = .787). This study found no significant difference in serum magnesium levels in patients with hypertensive crises compared to a random matched control group. Larger observational or experimental studies may be useful to evaluate the effect of magnesium on blood pressure in hypertensive crises.
Keywords: calcium, hypertension, hypertensive crises, magnesium, potassium
1. INTRODUCTION
Magnesium is the second most abundant intracellular cation after potassium and the fourth most common abundant cation in the body. 1 , 2 , 3 Magnesium is involved in a myriad of physiologic processes in the body namely: intracellular signaling, serving as a cofactor for DNA and protein synthesis, oxidative phosphorylation, cardiac excitability, neuromuscular transmission, vasomotor tone, blood pressure regulation, and bone formation. 1 , 2 , 3 Total body magnesium is approximately 24 grams in adult humans with 99% existing intracellularly in bone (53%), muscle (27%), and soft tissue (19%). 1 , 2 In humans, only 1% of total body magnesium exists in the extracellular space (serum and erythrocytes). 1 , 2 Normal total serum concentration is in the range of 1.7‐2.6 mg/dl (0.7‐1.1 mmol/L). 1 This range of serum magnesium represents only 0.3% of total body magnesium and may not accurately reflect the total magnesium status. 2 , 4 Ten percent of serum magnesium is complexed to serum anions, thirty percent of serum magnesium is albumin‐bound, and sixty percent of serum magnesium exists in the ionized, free physiologically active form. 1 Serum magnesium is regulated by the dynamic balance and interplay between intestinal transport, bone transport, and renal exchange. 1
Hypertension is a condition marked by elevation in the systolic blood pressure (SBP) and/or diastolic blood pressure (DBP). 5 , 6 Hypertension is defined variably in clinical practice guidelines as either SBP ≥ 130 mmHg and/or DBP ≥ 80 mmHg 7 or SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg. 8 , 9 The overall global prevalence of hypertension in adults is between 30% and 45%, with a global age‐adjusted prevalence of 24% and 20% in men and women, respectively. 9 The prevalence of hypertension among US adults depends on the clinical practice guideline cutpoints to categorize blood pressure, with an overall prevalence of hypertension among US adults between 32% and 46% and age‐sex adjusted prevalence range of 31%‐48% for men and 32%‐43% in women. 7 , 8 Hypertension is a leading risk factor for cardiovascular diseases (hemorrhagic stroke, ischemic stroke, myocardial infarction, angina, heart failure, peripheral artery disease, and aortic aneurysm), end‐stage renal disease, death, and disability. 5 , 6 , 7 , 8 , 9 , 10 Hypertensive crises are defined as SBP greater than 180 mmHg and/or DBP greater than 120 mmHg. 7 , 8 Hypertensive crises can be further classified into: hypertensive emergency (when there is evidence of target organ damage) and hypertensive urgency (where there is no evidence of target organ damage). 7 , 8 Although hypertensive urgency reflects a marked elevation in blood pressure, it can be managed with maximizing of oral antihypertensive agents; however, hypertensive emergency is characterized with organ damage and is associated with a 1‐year mortality rate of >79% thus necessitating swift blood pressure reduction with intravenous antihypertensive agents to prevent sustained deterioration of target organ damage. 7 , 8 The role of magnesium on hypertensive crises is the central focus of our study which is novel; however, much foundational supporting data for our study are drawn from studies evaluating the role of magnesium on hypertension.
Results from many clinical trials, albeit inconsistently, have shown magnesium deficiency (serum and/or tissue) to some degree in hypertensive subjects, with low magnesium levels linked to a significant undesirable effect on blood pressure. 11 , 12 , 13 , 14 , 15 , 16 Although magnesium has been postulated to modulate blood pressure regulation to some extent, the precise mechanism of altered magnesium metabolism in hypertensive individuals remains unclear. 11 The prevailing postulated mechanism of effect of magnesium on blood pressure is that magnesium acts as a natural calcium antagonist on most types of calcium channels in vascular smooth muscles, reducing arterial blood pressure via lowering of peripheral and cerebral vascular resistance. 11 More specifically, the activity of magnesium as a calcium antagonist produces endothelial‐dependent vasodilation and blood pressure reduction through increases of extracellular magnesium and reduction of calcium influx. 4 , 11 , 17 , 18 Additionally, magnesium has been shown to produce vasodilation by increasing prostaglandin E—a vasodilator and platelet inhibitor—by acting as a cofactor for delta‐6‐desaturase enzyme leading to conversion of linoleic acid to gamma‐linolenic acid, a precursor to prostaglandin E. 17 , 18 A strong interplay has been found between magnesium and other electrolytes (potassium, calcium, and sodium) in blood pressure reduction, with reduction of intracellular sodium and calcium, and increases in intracellular magnesium and potassium shown to improve blood pressure. 17 , 18
Several observational clinical studies (cohort, case‐control, and cross‐sectional) and a meta‐analysis have evaluated the relationship between serum magnesium and blood pressure in patients with and without hypertension 12 , 13 , 14 , 15 , 16 , 27 ; however, no study to our knowledge has evaluated serum magnesium and blood pressure relationship among patients with hypertensive crises. Among existing studies in patients with and without hypertension, some of the studies performed tests of association (correlation, odds ratio, risk ratios, and hazard ratios) between magnesium and blood pressures 12 , 27 while some studies looked at mean differences in magnesium among hypertensive versus normotensive patients. 12 , 13 , 14 , 15 , 22 Collectively, these studies have revealed conflicting evidence on the relationship between serum magnesium levels and blood pressure, with some studies showing negative association, 12 , 13 , 14 , 15 , 16 , 19 , 21 , 24 , 25 and others showing no association 20 , 22 , 23 , 26 , 27 or a positive association. 20 In a related fashion, effect of magnesium supplementation on blood pressure has been studied extensively. Nine out of the ten studies (clinical trials, Cochrane Review, and meta‐analyses) reviewed showed mostly positive association/effect of magnesium supplementation with lower SBP, DBP, or both 26 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ; and only one study found no significant effect of magnesium supplementation on blood pressure. 36 This prevailing positive effect of magnesium supplementation lowering blood pressure proved compelling and served as a major foundational basis for our study evaluating whether serum magnesium is a factor that contributes to the dysregulated high blood pressure seen in patients with hypertensive crises. We hypothesized that serum magnesium will be significantly lower in patients with hypertensive crises and that low serum magnesium will be significantly associated with blood pressures (SBP and DBP) in patients with hypertensive crises.
The primary objective of this study is to evaluate the differences in serum magnesium levels between patients with hypertensive crises and matched controls (age‐, sex‐, race‐, and diabetes‐matched) in a 1:1 random match. The aim of the study is to determine the relationship between serum magnesium and blood pressure in hypertensive crises and provide preliminary data for future studies exploring the use of magnesium supplementation in patients with hypertensive crises. Secondary objectives were to evaluate the association between serum magnesium (and other electrolytes) and blood pressure in patients with hypertensive crises, determine the effects of covariates (age, sex, race, body mass index [BMI], and history of diabetes mellitus) on the relationship between serum magnesium and blood pressures, and to compare the mean BMI in patients with hypertensive crises versus patients without hypertensive crises.
2. STUDY DESIGN AND METHODS
This study is a single‐center, retrospective, chart review, case‐control study conducted at University Medical Center New Orleans (UMCNO) in New Orleans, Louisiana. In this case‐control study, patients with hypertensive crises were included in the case group, while the control group consisted of patients without hypertensive crises who were admitted to the hospital during the same time period from August 2013 to August 2015. This study was approved by the Xavier University of Louisiana Institutional Review Board (IRB) and UMCNO Research Review Committee (RRC).
Patients who were 18 years of age or older with an international classification disease ninth revision (ICD‐9) code of 401.9 (hypertensive crises: emergency or urgency) and a documented magnesium level on their electronic medical record (during the hypertensive crises hospital admission) were included in the case group. Hypertensive crises were defined as systolic blood pressure (SBP) greater than 180 mmHg and/or diastolic blood pressure (DBP) greater than 120 mmHg. Patients identified as having hypertensive crises based on ICD‐9 codes were confirmed to have two occurrences of either systolic blood pressure (SBP) greater than 180 mmHg and/or diastolic blood pressures (DBP) greater than 120 mmHg within 48 hours of the hospital encounter. Hypertensive crises were further categorized as either hypertensive urgency (absence of acute or on‐going target organ damage) or hypertensive emergency (presence of acute or on‐going target organ damage). Target organ damage by system included neurologic (hypertensive encephalopathy and intracranial hemorrhage), cardiac (acute myocardial infarction, acute left ventricular failure, unstable angina, and dissecting aortic aneurysm), and renal (acute kidney injury). All diagnoses of target organ damage were confirmed with both the physician diagnosis documented on the patients' problem list and clinical findings (laboratory results, imaging, signs, and symptoms) made on the patients. Hypertensive encephalopathy diagnosis was verified based on physical examination findings of headache and altered level of consciousness. Diagnosis of intracranial hemorrhage was confirmed using a computed tomography scan (or magnetic resonance imaging) of the head with or without contrast, performed on patients with neurologic symptoms, which includes change in mental status or focal neurologic signs indicative of cerebrovascular accident or hemorrhage. Unstable angina diagnosis was made clinically and confirmed with documented new or sudden chest pain, while myocardial infarction diagnosis was confirmed with elevated serum troponin levels and electrocardiogram (EKG) findings. Acute left ventricular failure was diagnosed with echocardiographic findings of a decreased ejection fraction less than 40% as well as physical examination findings of elevated jugular venous pressures (distension), crackles, or edema. Diagnosis of dissecting aortic aneurysm was confirmed from imaging studies revealing wide mediastinum on chest x‐ray and/or chest CT scan with or without contrast. Acute kidney injury was defined as a serum creatinine greater than 2 mg/dl, which is new onset in absence of prior renal disease and/or increase in SCr of 0.5 mg/dl or greater. The control group patients were required to be 18 years of age or older with a documented magnesium level on their medical record during the hospital admission. Control group patients were excluded if they experienced a blood pressure fitting the criteria of hypertensive crises, as defined above.
Exclusion criteria were based on patient conditions interfering with serum magnesium levels including: chronic kidney disease (CKD) stages 3, 4, and 5, end‐stage renal disease (ESRD), hepatic cirrhosis, pheochromocytoma, alcoholism, chronic diarrhea, and hyperaldosteronism. Patients who received magnesium supplementation (IV or oral) prior to serum magnesium level collection (both at home and in the hospital) were excluded. Additionally, patients who received inotropes or vasopressors (including epinephrine, norepinephrine, dopamine, phenylephrine, vasopressin, dobutamine, or milrinone) prior to blood pressure collection were excluded from the study. Patients who had an unidentifiable glomerular filtration rate (GFR) value were excluded in the final analyses.
All patient data were obtained from UMCNO's electronic medical record. The following demographic data were collected: age, sex, race, body mass index (BMI), and history of diabetes mellitus. Outcome variables collected included serum magnesium (mg/dl), serum calcium (mg/dl), serum potassium (mEq/L), SBP (mmHg), and DBP (mmHg). Corrected calcium (mg/dl) was calculated using the formula: corrected calcium = patient's measured serum calcium in mg/dl + (0.8 × [4 gm/dl − patient's measured albumin in gm/dl]). The adjusted correct serum used the actual patient's measured serum calcium when albumin was 4 gm/dl or greater, while the unadjusted corrected calcium applied the corrected calcium formula regardless of the patient's measured albumin level. Additionally, predictor variables were collected and include: at home and hospital use of loop diuretics (furosemide, bumetanide, torsemide, and ethacrynic acid), at home and hospital use of thiazide and thiazide‐like diuretics (hydrochlorothiazide, chlorthalidone, chlorothiazide, metolazone, and indapamide), at home and hospital use of proton pump inhibitors, albumin levels (gm/dl), Modification of Diet in Renal Disease (MDRD) glomerular filtration rate (GFR) (ml/min/1.73 m2), and CKD stages 1, and 2 diagnosis.
2.1. Outcomes
The primary outcome of the study was to compare the mean serum magnesium in patients with hypertensive crises versus patients without hypertensive crises. Secondary outcomes of the study are to assess the correlation between serum magnesium (and other electrolytes) on blood pressures (SBP and DBP) in patients with hypertensive crises; determine the effects of covariates such as age, race, sex, body mass index (BMI), and history of diabetes mellitus on the relationship between serum magnesium and blood pressure; and compare the mean BMI in patients with hypertensive crises versus patients without hypertensive crises.
2.2. Statistical analyses
The control group was randomly matched to the cases in a 1:1 ratio based on the covariates of age, sex, race, and history of diabetes. The matching for race was performed using two categories of African Americans and non‐African Americans (Whites, Asians, Native Hawaiian/Pacific Islanders, and racial category of “other”) because the sample size for the non‐African American races, except Whites, was small.
Descriptive statistical analysis was performed on demographic characteristics. A chi‐square test or Fisher's exact test was used to compare between‐group differences for categorical variables in the baseline characteristics. Student's t test was used to compare the continuous variables between groups (mean serum magnesium levels, mean albumin levels, MDRD GFR, and body mass index) between the case and control group. Simple linear regression was performed to assess the correlation (r) and coefficient of determination (R 2) between serum magnesium, serum calcium, corrected calcium, and serum potassium on blood pressures (SBP and DBP) in patients with hypertensive crises. Multivariable linear regression was performed to assess effects of magnesium on blood pressures (SBP and DBP) at the time of hypertensive crises while adjusting for covariates. Logistic regression analyses were performed to assess the odds of having either abnormally low (<1.5 mg/dl) or high (>2.6 mg/dl) serum magnesium in cases compared to matched controls. Statistical analyses were performed using SAS® version 9.4. An α value <.05 was considered statistically significant.
3. RESULTS
There were 358 patients who were included in the study: 179 patients in both the case group and the randomly matched control group (see Figures 1 and 2). Baseline demographics were similar between groups for the matched variables of age, sex, race (using two categories of African Americans and non‐African Americans), and history of diabetes (see Table 1). There were 49 non‐African Americans in both the case group and control groups. Majority of the non‐African Americans were White: case group (n = 39) and control group (n = 45). Altogether, Asians, Native Hawaiian/Pacific Islanders, and racial category of “other” were small and made up less than 6% of the case and control groups. There were significant baseline differences between the case and control group in SBP, DBP, the use of hospital loop diuretics, use of home thiazide diuretics, use of hospital thiazide diuretics, CKD staging, and albumin. SBP, DBP, the use of hospital loop diuretic, home thiazide diuretics, and hospital thiazide diuretics were all significantly higher in cases compared to controls. CKD staging was significantly different between cases and controls. Additionally, albumin level was significantly lower in the control group compared to the cases. The blood pressure average and ranges are reported in Table 1 and reflect that patients may have met the hypertensive crises definition through either SBP greater than 180 mmHg, DBP greater than 120 mmHg, or both SBP greater than 180 mmHg and DBP greater than 120 mmHg. The lower values in the range account for the likelihood of patients meeting the hypertensive crises definition through one of the criteria: either SBP or DBP alone.
FIGURE 1.
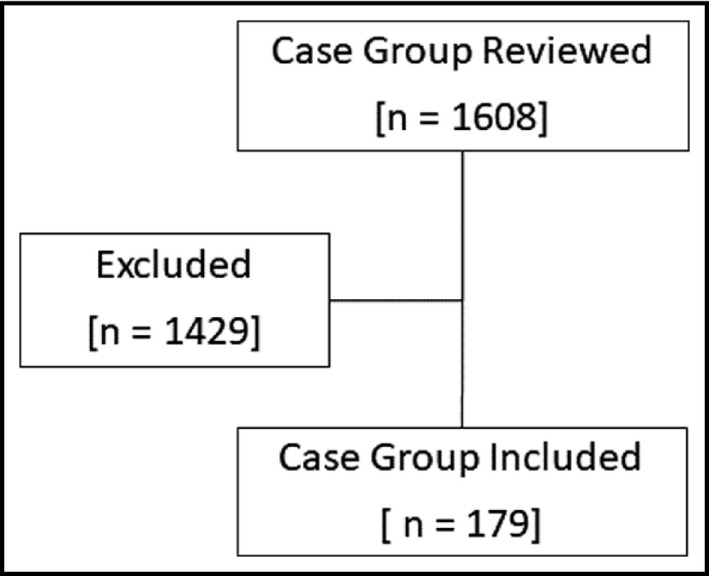
Flow chart of patient selection into the case group
FIGURE 2.
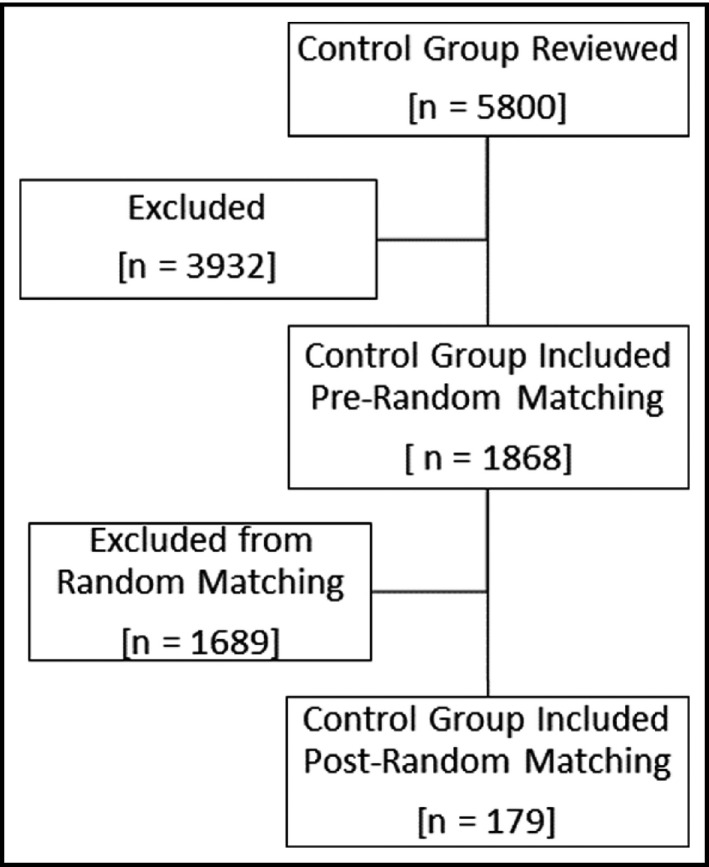
Flow chart of patient selection into the random matched control group
TABLE 1.
Baseline demographics
| Baseline demographics | |||
|---|---|---|---|
|
Cases N = 179 |
Controls N = 179 |
p‐Value | |
| Age [Mean ± SD years; (range)] | 57.23 ± 12.88 (24–91) | 56.74 ± 15.41 (18–98) | .744 |
| Sex | Male 93 (51.96%) | Male 98 (54.75%) | .5963 |
| Female 86 (48.04%) | Female 81 (45.25%) | ||
| Race | African American 130 (72.63%) | African American 130 (72.63%) | 1.000 |
| Non‐African American 49 (27.37%) | Non‐African American 49 (27.37%) | ||
| History of diabetes mellitus | Diabetic: 49 (27.37%) | Diabetic: 51 (28.49%) | .8137 |
| Non‐diabetic: 130 (72.63%) | Non‐diabetic: 128 (71.51%) | ||
| Hypertensive crises diagnosis | Urgency 151 (84.36%) | N/A | N/A |
| Emergency 28 (15.64%) | |||
| Systolic blood pressure [Mean ± SD mmHg; (range)] | 194.7 ± 24.92 (106–286) | 123.3 ± 21.85 (68–168) | <.0001 |
| Diastolic blood pressure [Mean ± SD mmHg; (range)] | 114.3 ± 19.46 (70–162) | 77.21 ± 14.22 (40–118) | <.0001 |
| Use of home loop diuretic | 22 (12.29%) | 24 (13.41%) | .7521 |
| Use of hospital loop diuretic | 72 (40.22%) | 32 (17.88%) | <.0001 |
| Use of home thiazide diuretic | 45 (25.14%) | 16 (8.94%) | <.0001 |
| Use of hospital thiazide diuretic | 66 (36.87%) | 10 (5.59%) | <.0001 |
| CKD staging | Stage 1: 6 (3.35%) | Stage 1: 0 (0%) | <.0001 |
| Stage 2: 15 (8.38%) | Stage 2: 2 (1.12%) | ||
| No CKD: 158 (88.27%) | No CKD: 176 (98.88%) | ||
| Albumin [Mean ± SD gm/dl] | 3.58 ± 0.61 | 3.43 ± 0.73 | <.038 |
| MDRD Glomerular filtration rate (GFR) [Mean ± SD ml/min/1.73 m2] | 66.09 ± 26.31 | 68.18 ± 28.04 | .468 |
Abbreviations: CKD, chronic kidney disease; MDRD, Modification of Diet in Renal Disease.
The primary outcome result is displayed on Table 2 and showed that serum magnesium concentration was not significantly different between the case group and control group. Table 2 also displays results comparing the BMI between the case group and the control group. The result revealed a significantly higher BMI in the case group compared to a lower BMI in the control group.
TABLE 2.
Differences in serum magnesium and BMI between hypertensive crises patients and controls
| Differences in serum magnesium and BMI between hypertensive crises patients and controls | |||
|---|---|---|---|
| Cases (N = 179) | Controls (N = 179) | p‐Value | |
| Mean serum magnesium levels (mg/dl) | 1.89 ± 0.29 | 1.90 ± 0.31 | .787 |
| Mean BMI (kg/m2) | 30.67 ± 8.53 | 27.54 ± 6.87 | <.001 |
Tables 3 displays the relationship between magnesium and systolic blood pressure or diastolic blood pressures in a simple linear regression analysis performed only on the case group. The results show that there is no significant association between magnesium and either SBP or DBP. Additionally, the results of the relationship between several electrolytes (calcium, corrected calcium (adjusted and unadjusted), and potassium) and SBP or DBP in a simple linear regression analysis are reported on Table 3. Of all the additional electrolytes assessed, only calcium showed a significant positive correlation (r = .147) to SBP with 2.2% of the variability in SBP attributed to calcium (R 2 = .022, p = .049).
TABLE 3.
Relationship between serum magnesium (other electrolytes) and SBP at crises or DBP at crises–cases
| Relationship between magnesium and SBP at crises or DBP at crises (N = 179) | |||
|---|---|---|---|
| Variables | r | R 2 | p‐Value |
| SBP at crises | |||
| Magnesium | .054 | .003 | .474 |
| Calcium | .147 | .022 | .049 |
| Corrected calcium (Adjusted) (N = 177) | .054 | .003 | .478 |
| Corrected calcium (Unadjusted) (N = 177) | .036 | .001 | .632 |
| Potassium | −.117 | .014 | .119 |
| DBP at crises | |||
| Magnesium | .009 | .000 | .899 |
| Calcium | .046 | .002 | .537 |
| Corrected calcium (Adjusted) (N = 177) | .004 | .000 | .953 |
| Corrected calcium (Unadjusted) (N = 177) | −.015 | .000 | .842 |
| Potassium | −.110 | .012 | .143 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
In the results from multivariable regression analysis performed only on the case group (Table 4), after adjusting for covariates (age, sex, race, history of diabetes, BMI, use of diuretics (loop and thiazides) at home and hospital, use of proton pump inhibitors at home and hospital, CKD staging, Albumin, GFR), magnesium was not significantly correlated with either SBP or DBP in patients with hypertensive crises. The results of the logistic regression analysis are reported on Table 5. The odds of having either abnormally low serum magnesium or abnormally high serum magnesium were not significantly different between the case group and the control group.
TABLE 4.
Relationship between serum magnesium and SBP at crises or DBP at crises–cases (Adjustment for covariates)
| Relationship between serum magnesium and SBP at crises or DBP at crises (N = 179) | ||
|---|---|---|
| Variables | β ± SE | p‐Value |
| SBP at crises | ||
| Magnesium | 5.12 ± 6.70 | .440 |
| DBP at crises | ||
| Magnesium | 3.67 ± 5.12 | .470 |
Adjusted for covariates—Age, sex, race, history of diabetes, BMI, use of diuretics (loop and thiazides) at home and hospital, use of proton pump inhibitors at home and hospital, CKD staging, Albumin, GFR.
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
TABLE 5.
Odds of having abnormally low serum magnesium (<1.5 mg/dl) or abnormally high serum magnesium (>2.6 mg/dl) in cases versus controls
| Estimate | Standard error | Odds ratio | 95% confidence interval | p‐Value | |
|---|---|---|---|---|---|
| Odds of abnormally low serum magnesium (<1.5 mg/dl) | −0.3604 | .4283 | 0.697 | 0.300, 1.619 | .401 |
| Odds of abnormally high serum magnesium (>2.6 mg/dl) | 0.4111 | .9191 | 1.509 | 0.248, 9.194 | .655 |
4. DISCUSSION
This study contributes much to the understanding of the role of magnesium in patients with hypertensive crises. Our study found no significant serum magnesium difference between the case group and the matched control group. This finding is consistent with the results from Kozielec et al 22 study which showed no significant difference in serum ionized magnesium levels among hypertensive patients and controls. However, our study result is divergent from majority of the studies that compare mean magnesium in hypertensive patients compared to normotensive individuals; four of these studies showed statistically significant lower serum or erythrocyte magnesium level in hypertensives compared to normotensive individuals. 12 , 13 , 14 , 15 It is worth noting that the matched control group in our study included patients who could be normotensive or hypertensive; and so our control group is not a normotensive group and should be extrapolated carefully. It is plausible to consider that our pilot/exploratory study was not adequately powered to detect significant differences in magnesium between patients with hypertensive crises and matched controls without hypertensive crises.
The major secondary outcome of our study evaluated the relationship between magnesium and blood pressures (SBP and DBP) in patients with hypertensive crises. Our study did not find a significant correlation between magnesium and either SBP or DBP in both a simple linear regression analysis (Table 3) and multivariable linear regression analysis (Table 4). This negative finding conflicts with the results from several studies which showed either a significant negative correlation 12 , 14 , 15 , 16 , 19 , 24 , 25 or a positive correlation. 20 However, this negative finding of no significant association between magnesium and blood pressure has been reported in several studies previously. 20 , 23 , 26 , 27 More specifically, a robustly designed meta‐analysis by Han et al 26 found no significant association between serum magnesium and blood pressure (risk ratio [RR] = 0.91, 95% CI: 0.80–1.02). We also performed a logistic regression and found no significant difference in the odds of having an abnormally high or low magnesium among patients with hypertensive crises compared to matched controls. Altogether, our study did not detect a significant association between magnesium and blood pressure (SBP or DBP) in patients with hypertensive crises which suggests that serum magnesium may not play a pronounced role in the dysregulated blood pressure seen in patients with hypertensive crises.
Among the additional electrolytes (calcium, corrected calcium (adjusted and unadjusted), and potassium) we assessed for a relationship with blood pressures (Table 3), we found that only serum calcium showed significant correlation to SBP but not DBP. The positive association of serum calcium to SBP has been reported in several studies previously 16 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ; however, conflicting relationship (negative association or no association) between serum calcium and blood pressure has been reported in the literature as well. 44 , 45 , 46 , 47 , 48 Calcium and potassium were measured because of strong linkages of these electrolytes with blood pressure, especially in concert with magnesium. 7 , 13 , 15 , 16 , 17 , 18 , 20 Our study also explored the role of body weight on patients with hypertensive crises compared to control group, and we found that BMI was significantly higher in the case group (30.67 ± 8.53 kg/m2) compared to the control group (27.54 ± 6.87 kg/m2). Our finding of higher BMI in the case group compared to the control group is consistent with literature that has shown obesity and overweight as a strong predisposing risk factor for hypertension and elevated blood pressure. 49 , 50 , 51 , 52 , 53 , 54 , 55
The strengths of our study include its case‐control study design, use of statistical tests to create randomly matched groups, and employment of diverse statistics that explored between‐group differences and association within group, and the pilot/exploratory nature of our study. The case‐control study design allowed us to investigate whether there is a true difference in mean serum magnesium between the hypertensive crises patients and control group. Additionally, we performed correlational analysis to examine the association between magnesium and blood pressure in hypertensive crises. The random matching process also allowed us to have a control group similar to the cases on the covariates of age, sex, race, and history of diabetes which softened the effects of these covariates on the study outcomes, improving the study's internal validity. This study is also a pilot/exploratory study and is thus a hypothesis‐generating study which can provide population estimates to help determine the appropriate power and sample size to study the effect of magnesium on hypertensive crises in future studies.
Our study has several limitations which impact the internal and external validity of our study. First, this study is a single‐center study and as such limits the generalizability of our study to patients across institutions. The findings from this single‐center study should be extrapolated cautiously to individual patients and patient populations with hypertensive crises. The matched control group in our study included patients who were either normotensive or hypertensive. This may weaken the internal validity of the study given the heterogeneity of our matched control group and the lack of result delineation between normotensive versus hypertensive patients in our study. This study was a retrospective study which introduces variability on the time when variables were collected; as variables were not collected uniformly at narrow and specific times. Our study was also a non‐interventional/non‐experimental study which impacts the internal validity and excludes our study from the ability to assess causation. Our study provides results that examine association between variables evaluated. Our study was not a randomized study and confounding variables may have impacted our study results given that some baseline characteristics were significantly different between groups. Given the retrospective nature of our study, some notable variables were not accounted for or controlled and therefore may confound our main study variables (magnesium and/or blood pressure). These potential confounding variables include, but are not limited to: BMI, use of diuretics therapy, type of treatment used to manage hypertension/hypertensive crises, underlying primary etiology/cause of hypertension/hypertensive crises, use of oral contraceptives in females, etc When accounting for BMI as a potential confounding variable, our study did not match our control group to the case group on BMI consistent with many studies 44 , 45 , 56 , 57 , 58 , 59 , 60 ; because although BMI has been associated with hypomagnesemia in studies, 61 , 62 , 63 diabetes was the major driver of hypomagnesemia in a study of obese patients. 56 Additionally, we found that BMI was not correlated to serum magnesium in our study in a linear regression analysis. Another important limitation of our study is that magnesium is predominantly an intracellular cation, and given that intracellular magnesium is not routinely measured clinically at our hospital, the serum magnesium obtained from our electronic hospital record may not be a good reflection of patients' magnesium stores. 1 , 2 Lastly, our study had a small sample size (n = 358), which increases the probability of type II errors—a weakened probability to detect significant differences that may exist in the true population of patients from which our sample population was obtained. Our inability to detect significant differences in the serum magnesium levels between the case and control groups of our study may be linked to the small sample size of our study.
5. CONCLUSION
This study found no significant difference in serum magnesium levels in patients with hypertensive crises compared to a random matched control group. There was no significant association between magnesium and either systolic blood pressure or diastolic blood pressure in patients with hypertensive crises. When adjusting for covariates, magnesium was not significantly correlated with systolic blood pressure or diastolic blood pressure in patients with hypertensive crises. Association of magnesium to blood pressure in hypertensive crises may be insignificant based on this case‐control study. Larger studies may be warranted to evaluate true effect of magnesium on blood pressure in hypertensive crises. Results from larger studies investigating the effect of magnesium on blood pressure in hypertensive crises patients will move clinical evidence closer to the true effect of magnesium on blood pressure in hypertensive crises and should serve as the basis for an experimental study testing the therapeutic utility of magnesium in hypertensive crises.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Ifeanyi Onor contributed to the conception of the study, study design, data analysis/interpretation, and first manuscript draft preparation. John Okogbaa, Christopher Gillard, Daniel Sarpong, Mihran Naljayan, Shane Sanne, and Shane Guillory participated in the study design and data analysis/interpretations. Robbie Beyl contributed primarily to the statistical analysis of the data set and data interpretation. Emily Johnston, Nicole Little, Lashira Hill, Oluwabunmi Lawal, Casey Payne, Mallory Coleman, Carolkim Huynh, Sarah Bilbe, Ahlam Ayyad, Kabrea Jones, Jasmine Kinnard, Rosanna Dastoori, Devin Rolland, and Amanda Miller contributed to the literature review, data collection, and data analysis/interpretation. Rim Hadgu, Amne Borghol, and Samuel Okpechi contributed to literature review and data analysis/interpretation. All authors critically commented and revised the manuscript and gave the final approval. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author's own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
This study was supported in part by several grants: RCMI—NIH/NIMHD5G12MD007595 and NIMHD 2U54MD007595‐11 from the National Institute of Health (NIH), National Institute on Minority Health and Health Disparities (NIMHD), and Research Centers in Minority Institutions Program (RCMI); Center for Minority Health and Health Disparities Research and Education—5 S21 MD 000100‐12 from the National Institute on Minority Health and Health Disparities (NIMHD); LaCATS—1 U54 GM104940 and 2U54GM104940‐02 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center (LaCATS); Xavier Center of Excellence—HRSA D34HP00006 from the Health Resources and Service Administration of the Department of Health and Human Services (DHHS); and HRSA D34HP00006 via US Department of Education—Title III, Part B Program.
Onor IO, Johnston EK, Little NG, et al. Evaluation of serum magnesium differences in hypertensive crises and control patients: A randomly matched case‐control study. J Clin Hypertens. 2021;23:1229–1238. 10.1111/jch.14244
REFERENCES
- 1. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 2015;10(7):1257‐1272. 10.2215/CJN.09750913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology. Br J Anaesth. 1999;83(2):302‐320. 10.1093/bja/83.2.302 [DOI] [PubMed] [Google Scholar]
- 3. Champagne CM. Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: a review. Nutr Clin Pract. 2008;23(2):142‐151. 10.1177/0884533608314533 [DOI] [PubMed] [Google Scholar]
- 4. Kupetsky‐Rincon EA, Uitto J. Magnesium: novel applications in cardiovascular disease – a review of the literature. Ann Nutr Metab. 2012;61(2):102‐110. 10.1159/000339380 [DOI] [PubMed] [Google Scholar]
- 5. Solomon CG, Taler SJ. Initial treatment of hypertension. N Engl J Med. 2018;378(7):636‐644. 10.1056/NEJMcp1613481 [DOI] [PubMed] [Google Scholar]
- 6. Oparil S, Acelajado MC, Bakris GL, et al. Hypertension. Nat Rev Dis Prim. 2018;4:18014. 10.1038/nrdp.2018.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Pr. J Am Coll Cardiol. 2018;71(19):e127‐e248. 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 8. National High Blood Pressure Education Program . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute (US); 2004. https://www.ncbi.nlm.nih.gov/books/NBK9630/. Accessed March 19, 2020. [PubMed] [Google Scholar]
- 9. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021‐3104. 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 10. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223‐237. 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geiger H, Wanner C. Magnesium in disease. CKJ Clin Kidney J. 2012;5(Suppl 1):i25‐i38. 10.1093/ndtplus/sfr165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Resnick LM, Gupta RK, Laragh JH. Intracellular free magnesium in erythrocytes of essential hypertension: relation to blood pressure and serum divalent cations. Proc Natl Acad Sci. 1984;81(20):6511‐6515. 10.1073/pnas.81.20.6511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Resnick LM, Bardicef O, Altura BT, Alderman MH, Altura BM. Serum ionized magnesium: relation to blood pressure and racial factors. Am J Hypertens. 1997;10(12 Pt 1):1420‐1424. 10.1016/s0895-7061(97)00364-6 [DOI] [PubMed] [Google Scholar]
- 14. Ma J, Folsom AR, Melnick SL, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the aric study. J Clin Epidemiol. 1995;48(7):927‐940. 10.1016/0895-4356(94)00200-A [DOI] [PubMed] [Google Scholar]
- 15. Touyz RM, Milne FJ, Seftel HC, Reinach SG. Magnesium, calcium, sodium and potassium status in normotensive and hypertensive Johannesburg residents. South African Med J. 1987;72(6):377‐381. [PubMed] [Google Scholar]
- 16. Abbasi IU, Haque SU, Kausar MW, Karira KA, Zubaris NA. Correlation of divalent Cat ions (Ca++, Mg++) and serum renin in pateints of essential hypertension. J Pak Med Assoc. 2012;62(2):134‐138. [PubMed] [Google Scholar]
- 17. Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens (Greenwich). 2008;10(7 Suppl 2):3‐11. 10.1111/j.1751-7176.2008.08575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Houston M. The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens. 2011;13(11):843‐847. 10.1111/j.1751-7176.2011.00538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen B, Schroll M, Christiansen C, Transbøl I. Serum and erythrocyte magnesium in normal elderly Danish people: relationship to blood pressure and serum lipids. Acta Med Scand. 1977;201(1–6):31‐34. 10.1111/j.0954-6820.1977.tb15650.x [DOI] [PubMed] [Google Scholar]
- 20. Rinner MD, van Spliet Laar L Kromhout D. Serum sodium, potassium, calcium and magnesium and blood pressure in a Dutch population. J Hypertens. 1989;7(12):977‐981. 10.1097/00004872-198912000-00008 [DOI] [PubMed] [Google Scholar]
- 21. Shibutani Y, Sakamoto K, Katsuno S, Yoshimoto S, Matsuura T. Relation of serum and erythrocyte magnesium levels to blood pressure and a family history of hypertension. A follow‐up study in Japanese children, 12–14 years old. Acta Paediatr Scand. 1990;79(3):316‐321. 10.1111/j.1651-2227.1990.tb11463.x [DOI] [PubMed] [Google Scholar]
- 22. Kozielec P, Kotkowiak L, Późniak J, et al. Assessment of serum ionized magnesium levels in healthy volunteers, in patients with coronary artery disease and/or hypertension and in hypertension alone. Magnes Res. 2005;18(4):241‐244. [PubMed] [Google Scholar]
- 23. Khan AM, Sullivan L, McCabe E, Levy D, Vasan RS, Wang TJ. Lack of association between serum magnesium and the risks of hypertension and cardiovascular disease. Am Heart J. 2010;160(4):715‐720. 10.1016/j.ahj.2010.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peacock JM, Folsom AR, Arnett DK, Eckfeldt JH, Szklo M. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 1999;9(3):159‐165. 10.1016/S1047-2797(98)00040-4 [DOI] [PubMed] [Google Scholar]
- 25. Guerrero‐Romero F, Rodríguez‐Morán M, Hernández‐Ronquillo G, et al. Low serum magnesium levels and its association with high blood pressure in children. J Pediatr. 2016;168:93‐98.e1. 10.1016/j.jpeds.2015.09.050 [DOI] [PubMed] [Google Scholar]
- 26. Han H, Fang X, Wei X, et al. Dose‐response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: a systematic review and meta‐analysis of prospective cohort studies. Nutr J. 2017;16(1):1‐12. 10.1186/s12937-017-0247-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joosten MM, Gansevoort RT, Mukamal KJ, et al. Urinary magnesium excretion and risk of hypertension: the prevention of renal and vascular end‐stage disease study. Hypertension. 2013;61(6):1161‐1167. 10.1161/HYPERTENSIONAHA.113.01333 [DOI] [PubMed] [Google Scholar]
- 28. Guerrero‐Romero F, Rodríguez‐Morán M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, double‐blind, placebo‐controlled clinical trial. J Hum Hypertens. 2009;23(4):245‐251. 10.1038/jhh.2008.129 [DOI] [PubMed] [Google Scholar]
- 29. Sanjuliani AF, De Abreu Fagundes VG, Francischetti EA. Effects of magnesium on blood pressure and intracellular ion levels of Brazilian hypertensive patients. Int J Cardiol. 1996;56(2):177‐183. 10.1016/0167-5273(96)02716-7 [DOI] [PubMed] [Google Scholar]
- 30. Kawano Y, Matsuoka H, Takishita S, Omae T. Effects of magnesium supplementation in hypertensive patients: assessment by office, home, and ambulatory blood pressures. Hypertension. 1998;32(2):260‐265. 10.1161/01.HYP.32.2.260 [DOI] [PubMed] [Google Scholar]
- 31. Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta‐analysis. Eur J Clin Nutr. 2012;66(4):411‐418. 10.1038/ejcn.2012.4 [DOI] [PubMed] [Google Scholar]
- 32. Zhang X, Li Y, Del Gobbo LC, et al. Effects of magnesium supplementation on blood pressure: a meta‐analysis of randomized double‐blind placebo‐controlled trials. Hypertension. 2016;68(2):324‐333. 10.1161/HYPERTENSIONAHA.116.07664 [DOI] [PubMed] [Google Scholar]
- 33. Jee SHA, Miller ER, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: a meta‐analysis of randomized clinical trials. Am J Hypertens. 2002;15(8):691‐696. 10.1016/S0895-7061(02)02964-3 [DOI] [PubMed] [Google Scholar]
- 34. Rosanoff A, Plesset MR. Oral magnesium supplements decrease high blood pressure (SBP > 155mmHg) in hypertensive subjects on anti‐hypertensive medications: A targeted meta‐analysis. Magnes Res. 2013;26(3):93‐99. 10.1684/mrh.2013.0343 [DOI] [PubMed] [Google Scholar]
- 35. Dickinson HO, Nicolson D, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;3(3):CD004641. 10.1002/14651858.cd004641.pub2 [DOI] [PubMed] [Google Scholar]
- 36. Rogiers P, Vermeier W, Kesteloot H, Stroobandt R. Effect of the infusion of magnesium sulfate during atrial pacing on ECG intervals, serum electrolytes, and blood pressure. Am Heart J. 1989;117(6):1278‐1283. 10.1016/0002-8703(89)90406-7 [DOI] [PubMed] [Google Scholar]
- 37. Jorde R, Sundsfjord J, Fitzgerald P, Bønaa KH. Serum calcium and cardiovascular risk factors and diseases: the Tromso Study. Hypertension. 1999;34(3):484‐490. 10.1161/01.HYP.34.3.484 [DOI] [PubMed] [Google Scholar]
- 38. Kesteloot H, Joossens JV. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension. 1988;12(6):589‐593. 10.1161/01.HYP.12.6.589 [DOI] [PubMed] [Google Scholar]
- 39. Lind L, Jakobsson S, Lithell H, Wengle B, Ljunghall S. Relation of serum calcium concentration to metabolic risk factors for cardiovascular disease. Br Med J. 1988;297(6654):960‐963. 10.1136/bmj.297.6654.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mateus‐Hamdan L, Beauchet O, Rolland Y, Schott AM, Annweiler C. Association of calcium concentration with pulse pressure in older women: data from a large population‐based multicentric study. J Nutr Heal Aging. 2014;18(3):323‐329. 10.1007/s12603-013-0412-1 [DOI] [PubMed] [Google Scholar]
- 41. Schutte R, Huisman HW, Schutte AE, et al. Serum calcium revisited: associations with 24‐h ambulatory blood pressure and cardiovascular reactivity in Africans. Hypertens Res. 2010;33(7):688‐694. 10.1038/hr.2010.65 [DOI] [PubMed] [Google Scholar]
- 42. Sabanayagam C, Shankar A. Serum calcium levels and hypertension among US adults. J Clin Hypertens. 2011;13(10):716‐721. 10.1111/j.1751-7176.2011.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Staessen J, Sartor F, Roels H, et al. The association between blood pressure, calcium and other divalent cations: a population study. J Hum Hypertens. 1991;5(6):485‐494. [PubMed] [Google Scholar]
- 44. Hazari MAH, Arifuddin MS, Muzzakar S, Devender RV. Serum calcium level in hypertension. N Am J Med Sci. 2012;4(11):569‐572. 10.4103/1947-2714.103316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Folsom AR, Smith CL, Prineas RJ, Grimm RH. Serum calcium fractions in essential hypertensive and matched normotensive subjects. Hypertension. 1986;8(1):11‐15. 10.1161/01.HYP.8.1.11 [DOI] [PubMed] [Google Scholar]
- 46. Kunutsor SK, Laukkanen JA. Circulating active serum calcium reduces the risk of hypertension. Eur J Prev Cardiol. 2017;24(3):239‐243. 10.1177/2047487316681174 [DOI] [PubMed] [Google Scholar]
- 47. Jorde R, Bønaa KH, Sundsfjord J. Population based study on serum ionised calcium, serum parathyroid hormone, and blood pressure. The Tromso study. Eur J Endocrinol. 1999;141(4):350‐357. 10.1530/eje.0.1410350 [DOI] [PubMed] [Google Scholar]
- 48. Cutler J, Brittain E. Calcium and blood pressure: an epidemiologic perspective. Am J Hypertens. 1990;3(8 Pt 2):137S‐146S. 10.1093/ajh/3.8.137 [DOI] [PubMed] [Google Scholar]
- 49. Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41(3):625‐633. 10.1161/01.HYP.0000052314.95497.78 [DOI] [PubMed] [Google Scholar]
- 50. Hall JE, Do Carmo JM, Da Silva AA, Wang Z, Hall ME. Obesity‐induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991‐1006. 10.1161/CIRCRESAHA.116.305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26‐year follow‐up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968‐977. 10.1161/01.CIR.67.5.968 [DOI] [PubMed] [Google Scholar]
- 52. Bray GA. Life insurance and overweight. Obes Res. 1995;3(1):97‐99. 10.1002/j.1550-8528.1995.tb00125.x [DOI] [PubMed] [Google Scholar]
- 53. Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA ‐ J Am Med Assoc. 2009;302(4):401‐411. 10.1001/jama.2009.1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Garrison RJ, Kannel WB, Stokes J, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham offspring study. Prev Med (Baltim). 1987;16(2):235‐251. 10.1016/0091-7435(87)90087-9 [DOI] [PubMed] [Google Scholar]
- 55. Huang Z, Willett WC, Manson JE, et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128(2):81‐88. 10.7326/0003-4819-128-2-199801150-00001 [DOI] [PubMed] [Google Scholar]
- 56. Lecube A, Baena‐Fustegueras JA, Fort JM, Pelegrí D, Hernández C, Simó R. Diabetes is the main factor accounting for hypomagnesemia in obese subjects. PLoS One. 2012;7(1):e30599. 10.1371/journal.pone.0030599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guerrero‐Romero F, Rodríguez‐Morán M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol. 2002;39(4):209‐213. 10.1007/s005920200036 [DOI] [PubMed] [Google Scholar]
- 58. Touyz RM, Milne FJ. Alterations in intracellular cations and cell membrane atpase activity in patients with malignant hypertension. J Hypertens. 1995;13(8):867‐874. 10.1097/00004872-199508000-00007 [DOI] [PubMed] [Google Scholar]
- 59. Tillman DM, Semple PF. Calcium and magnesium in essential hypertension. Clin Sci. 1988;75(4):395‐402. 10.1042/cs0750395 [DOI] [PubMed] [Google Scholar]
- 60. Yao Y, He L, Jin Y, et al. The relationship between serum calcium level, blood lipids, and blood pressure in hypertensive and normotensive subjects who come from a Normal University in East of China. Biol Trace Elem Res. 2013;153(1–3):35‐40. 10.1007/s12011-013-9646-3 [DOI] [PubMed] [Google Scholar]
- 61. McKay J, Ho S, Jane M, Pal S. Overweight & obese Australian adults and micronutrient deficiency. BMC Nutr. 2020;6(1):12. 10.1186/s40795-020-00336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Farhanghi MA, Mahboob S, Ostadrahimi A. Obesity induced Magnesium deficiency can be treated by vitamin D supplementation. J Pak Med Assoc. 2009;59(4):258‐261. https://pubmed.ncbi.nlm.nih.gov/19402296/. Accessed February 23, 2021. [PubMed] [Google Scholar]
- 63. Rodríguez‐Morán M, Guerrero‐Romero F. Elevated concentrations of TNF‐alpha are related to low serum magnesium levels in obese subjects. Magnes Res. 2004;17(3):189‐196. https://pubmed.ncbi.nlm.nih.gov/15724867/. Accessed February 23, 2021. [PubMed] [Google Scholar]


