Abstract
Purpose of review
Clostridioides difficile infection (CDI) may complicate the course of ulcerative colitis and Crohn’s disease. The clinical presentation of CDI in this population is often atypical, and patients may experience exacerbations of their underlying inflammatory bowel disease (IBD) secondary to C. difficile. In this review, we aim to review the risk factors, diagnosis, and management of CDI in the context of IBD.
Recent findings
Patients with colonic involvement of their IBD are at higher risk for CDI and colonization may be more common than in the general population. Therefore, CDI is confirmed using a two-step approach to stool testing. Oral vancomycin or fidaxomicin are the preferred agents for non-fulminant disease, and oral metronidazole is no longer recommended as first-line therapy. For all patients with CDI recurrence, fecal microbiota transplant (FMT) should be considered, as this has been shown to be safe and effective. Among those who have worsening of their underlying IBD, retrospective research suggest that outcomes are improved for those who undergo escalation of immunosuppression with appropriate antimicrobial treatment of C. difficile, however prospective data is needed.
Summary
CDI may complicate the course of IBD, however the presentation may not be typical. Therefore, all patients with worsening gastrointestinal symptoms should be evaluated for both CDI and IBD exacerbation. Providers should consider FMT for all patients with recurrent CDI as well as escalation of immunosuppression for patients who fail to improve with appropriate antimicrobial therapy.
Keywords: Clostridioides difficile, inflammatory bowel disease, ulcerative colitis, Crohn’s disease
Introduction
Clostridioides difficile is an anaerobic, spore-forming bacterium and a leading cause of nosocomial diarrhea in the United States.1 In 2011 there were an estimated 453,000 cases of initial C. difficile infection (CDI) and 29,300 CDI-associated deaths.2 Due to efforts to reduce healthcare associated infections, the incidence of CDI improved from 154.9 to 143.6 per 100,000 persons between 2011 and 2017.3 However, patients with IBD have a nearly 5-fold increase in risk of CDI compared to the general population.4 In this review, we will summarize the most recent data regarding risk factors of CDI, the clinical presentations and diagnostic criteria for CDI, management of the initial episode as well as recurrent CDI, and management of IBD exacerbation in the setting of CDI.
Risk factors for Clostridoides difficile infection
Risk factors for CDI in the general population include hospitalization, antibiotic exposure, age > 65 years, female sex, immune suppression, and multiple medical comorbidities.2, 5 However, patients with inflammatory bowel disease (IBD) appear be at greater risk for CDI. This population has increased interactions with healthcare settings, exposure to immunosuppressive medications, and altered gut microbiomes which may predispose individuals to CDI. In a prospective analysis of IBD patients in clinical remission with no recent hospitalizations or exposures to corticosteroids, immunomodulators, or antibiotics, asymptomatic carriage of toxigenic C. difficile was higher among IBD patients compared to healthy controls (8.2% vs. 1.0%).6 While no patients experienced symptoms of CDI during the 6-month follow-up period, the higher rate of colonization among IBD patients could be due to alterations in gut commensal bacteria and the mucosal immune response. Estimates of CDI from the Nationwide Inpatient Sample between 1998 and 2004 suggest that the prevalence of CDI among ulcerative colitis (UC) and Crohn’s disease (CD) patients were 37.3 per 1,000 and 10.9 per 1,000, respectively, compared to 4.5 per 1,000 non-IBD patients.7
Within the IBD population, risk factors for CDI have been reported variably in the literature. Emergency room visits, hospitalizations, and recent nonsteroidal anti-inflammatory drug (NSAID) use appear to be associated with a higher risk of CDI among IBD patients.8, 9 Data regarding the association between CDI and use of proton-pump inhibitors (PPIs) and antibiotics are conflicting.10–18 Similarly, some studies have implicated immunomodulator use as an independent risk factor for CDI while others have argued no association or even a protective effect against CDI.5, 9, 12, 14, 19–21 In a recent meta-analysis of 22 studies, colonic involvement of IBD, biologic use, and antibiotic use were found to be independent risk factors associated with CDI among patients with IBD.22
There also appears to be an increased risk of complications for IBD patients with concomitant CDI. These individuals have a higher rate of IBD exacerbation requiring escalation of therapy, emergency department visits, longer hospital stays, and higher rates of surgery.20, 23–26 In a nationwide analysis, the rate of colectomy among hospitalized UC patients increased from 4.3% to 8.8%.27 Additionally, two recent meta-analyses suggest a higher risk of colectomy among IBD patients with CDI and two suggest a higher risk of mortality.22, 28–30 Therefore, the prompt recognition and appropriate management of both CDI and IBD in this vulnerable population is critical to minimize adverse outcomes.
Clinical Features and Diagnosis of CDI in IBD
Recognizing CDI in the setting of active IBD presents a challenge, as the clinical presentations of these conditions are similar. A typical presentation of CDI in the general population includes watery diarrhea with associated abdominal cramping, nausea, and low-grade fever often in the setting of recent antibiotic therapy or healthcare exposure.31 Common laboratory findings include an elevated white blood cell count to 15,000 cells/mL and the presence of fecal leukocytes.32 Patients with IBD who develop CDI may have atypical clinical features including younger age, lack of recent antibiotic use, and community acquisition.6, 19, 33
When patients with IBD present with new or worsening diarrhea, stool testing for CDI is indicated regardless of recent antibiotic exposure.23 A two-step approach for diagnosis is recommended.34 An initial screen should be conducted with a highly sensitive test: either the enzyme immunoassays (EIAs) for clostridial glutamate dehydrogenase (GDH), which detects the presence of clostridia organisms, both toxigenic and non-toxigenic strains, or polymerase chain reaction (PCR) for toxin genes. A positive screen is then followed by higher-specificity testing using EIA for C. difficile toxins A and B. Patients who have a positive PCR for toxin genes but negative EIA for toxins A and B are colonized rather than infected. This two-step process ensures that patients with active CDI are treated and patients who are simply colonized with C. difficile undergo investigation for alternative etiologies for their symptoms. In a hospital-based study of universal screening for C. difficile, colonization via PCR testing was determined to be 4.2% of 47,048 patients screened over 3 years.35 Asymptomatic carriage of C. difficile may be as high as 8% among patients with IBD.6
Sigmoidoscopy and biopsy plays a unique role in the assessment of IBD patients with concomitant CDI. The mucosal appearance in this setting may not allow for the differentiation of CDI from IBD, particularly as the hallmark pseudomembranes of CDI seen in the general population are rarely seen among patients with underlying IBD.36 However, sigmoidoscopy can identify the extent and severity of inflammation and histologic examination of biopsies can potentially exclude other important causes of bloody diarrhea in this population, including cytomegalovirus infection, amoebiasis, and ischemic colitis.
Radiographic imaging does not provide characteristic features to differentiate CDI from IBD activation. However, patients who present with abdominal distention, fever, ileus, and/or hemodynamic instability require plain films or computed tomography (CT) of the abdomen and pelvis to exclude bowel perforation and toxic megacolon. Toxic megacolon is a potentially lethal complication of both severe CDI and IBD characterized by systemic toxicity and colonic dilatation to > 6 cm.37 Patients with toxic megacolon due to either CDI or IBD require supportive care, bowel rest, appropriate antimicrobial and/or immunosuppressive therapy, and surgical evaluation.
Medical Management of Initial Episode of CDI
Management of initial CDI in the IBD population can be challenging, as symptoms may be due in part to both infection and exacerbation of the underlying IBD. Regardless, all symptomatic IBD patients with confirmed CDI by stool testing should receive appropriate antimicrobial therapy, which is guided by data from the general population. The Infectious Diseases Society of America (IDSA) defines the initial episode of CDI as non-severe, severe, or fulminant.1 Non-severe disease includes patients with a white blood cell count of ≤15,000 cells/mL and serum creatinine <1.5 mg/dL, severe disease includes those with white blood cell count >15,000 cells/mL and/or serum creatinine ≥1.5 mg/dL, and fulminant colitis includes those with hypotension, ileus, or toxic megacolon.
IBD patients with an initial episode of either non-severe or severe CDI should be treated with oral vancomycin 125 mg 4 times daily for 10 days or fidaxomicin 200 mg twice daily for 10 days. Metronidazole is no longer recommended as first-line therapy given higher failure rates with this drug.23, 38, 39 Fidaxomicin, which is bactericidal against C. difficile, appears to have a lower rate of post-treatment recurrence, but its significantly higher cost compared to oral vancomycin currently limits its use.40, 41 A recent retrospective study has identified a lower rate of CDI recurrence among IBD patients initially treated with long-duration (21–42 days) as opposed to short duration (10–14 days) oral vancomycin, however these results require validation in prospective trials.42 IBD patients who fail to demonstrate clinical improvement with appropriate antimicrobial therapy may require concomitant escalation of immunosuppressive therapy, which will be discussed separately.
All patients with fulminant CDI should be treated with vancomycin administered orally or via nasogastric tube 500 mg 4 times daily and intravenous metronidazole 500 mg every 8 hours. Most patients with fulminant CDI should also receive intracolonic vancomycin administered rectally, as ileus is common and may be underrecognized. Those with fulminant disease also need serial abdominal examinations to detect the development of toxic megacolon or bowel perforation which could indicate emergent colectomy. The development of altered mental status, fever, cardiorespiratory failure, lactic acidosis, and worsening leukocytosis are poor prognostic indicators which may help identify individuals in whom to consider early surgical intervention.43–47 There is also retrospective data that suggests a mortality benefit for use of early fecal microbiota transplantation (FMT) in place of colectomy for fulminant CDI, however prospective trials are needed to confirm these findings.48, 49 Additional indications for FMT will be discussed separately.
Medical Management of Recurrent CDI
Recurrence of CDI requires the resolution of symptoms followed by recurrence of symptoms and a positive stool test for C. difficile within eight weeks after discontinuation of appropriate CDI therapy.1 This can be due to relapse of the original strain or infection with a new strain of C. difficile.50, 51 In the general population, CDI recurs in 10–35% of patients.52–54 However patients with IBD are 33% more likely to experience CDI recurrence.55 Risk factors for recurrent CDI in the IBD population include Crohn’s colitis and recent use of antibiotics, 5-aminosalicylic acid (5-ASA) medications, corticosteroids, or infliximab relative to index CDI.55
All patients with recurrent diarrhea but negative stool testing for C. difficile should be evaluated for other etiologies, including post-infectious irritable bowel syndrome (IBS). The prevalence of IBS at 12 months after an enteritis due to any infectious organism is estimated at 10%.56 In a retrospective cohort study of patients treated successfully for CDI, 25% of patients with no prior history of IBS met Rome III criteria for IBS with diarrhea after 6 months.57 Additional risk factors for post-CDI IBS included history of anxiety and higher BMI. In a recent prospective cohort study, pre-existing IBD was independently associated with new gastrointestinal symptoms (bloating, constipation, or loose stools) after successful cure of CDI by FMT.58
If a first recurrence of CDI is confirmed by two-step stool testing, subsequent therapy should be distinct from the first regimen. Patients initially treated with a standard regimen of oral vancomycin should receive a pulse-tapered regimen of oral vancomycin which includes 125 mg daily for 14 days followed by twice daily for 7 days, once daily for 7 days, and then once every 2–3 days for 2–8 weeks.1 Oral fidaxomicin 200 mg twice daily for 10 days is another option for these patient.59 For those that initially received oral fidaxomicin or metronidazole, a standard 10-day regimen of oral vancomycin is the appropriate next therapy. Data regarding the medication regimens effective for second or further recurrence of CDI are limited, and no distinct recommendations exist for patients with IBD. An oral vancomycin pulse-tapered regimen or oral fidaxomicin are considerations for all patients with two or more episodes of recurrent CDI.
A number of strategies for prevention of CDI and its recurrence have been investigated. In general, minimization of systemic antibiotic use and unnecessary proton pump inhibitor therapy is recommended.1Additionally, handwashing for caregivers of all patients with CDI is critical to preventing transmission, as alcohol-based hand sanitizers do not kill C. difficile spores. Data regarding oral vancomycin for secondary prophylaxis among patients requiring systemic antibiotic therapy is conflicting, but there may be a lower rate of 90-day recurrence among patients with only one prior episode of CDI.60 Studies of various probiotics have presented inconsistent results, with some demonstrating a greater risk of CDI recurrence with probiotic exposure.1, 61–65 Additionally, probiotics appear to impair reconstitution of the gut mucosal microbiome after antibiotic use.66 Therefore probiotics are not recommended for CDI prophylaxis.
Bezlotoxumab is a monoclonal antibody that targets C. difficile toxin B that was approved in 2016 for the prevention of recurrent CDI among individuals at high risk of recurrence. In the MODIFY I/II phase 3 RCTs, bezlotoxumab was associated with a significantly lower rate of recurrent CDI compared to placebo.67 In a post-hoc analysis of high-risk subpopulations, a single infusion of bezlotoxumab resulted in a 27% absolute reduction in CDI recurrence within 12 weeks among patients with IBD. However, these results do not represent a statistically significant difference due to the relatively small number of IBD patients included in this analysis (28 randomized to bezlotoxumab, 14 randomized to placebo). Therefore, larger studies of bezlotoxumab are needed in the IBD population.68
Fecal Microbiota Transplantation for Recurrent CDI in IBD
IBD patients with recurrent CDI should be referred for consideration of FMT, which has been shown to be safe and effective.23 As patients with colonic IBD are at higher risk for CDI recurrence, it is hypothesized that colonic dysbiosis due to chronic colitis predisposes these patients to CDI.55 This dysbiosis may be restored by FMT, which involves the instillation of microbial communities derived from healthy donor stool into the affected individual’s gastrointestinal tract. In a retrospective study of patients receiving FMT after at least 2 recurrences of CDI, FMT successfully cleared CDI in 74.4% of patients.69 However non-IBD patients in this cohort had a higher rate of CDI clearance at 92.1%. In another cohort study, 75% of patients with IBD had response to FMT for recurrent CDI, which was similar to the response in non-IBD patients.70 Tariq and colleagues performed a retrospective study of 145 IBD patients undergoing FMT for recurrent CDI and found that FMT induced a CDI cure rate of 80% with no further recurrence after a median of 9.3 months of follow-up.71 Our group recently performed a prospective multicenter cohort study of FMT in 49 IBD patients with recurrent CDI, which demonstrated a 10% rate of FMT failure at week 8.72 All initial non-responders achieved clinical cure after a second FMT and successful C. difficile decolonization was achieved in 45/49 (91.8%) patients.
Prospective data suggests that FMT may also have a beneficial effect on the clinical course of IBD.73–75 Moayyedi and colleagues performed a placebo-controlled trial of 70 patients with active UC without infectious diarrhea, which found that 24% of those receiving FMT achieved remission at 7 weeks compared to 5% of those receiving placebo.73 In another RCT of 85 UC patients, Paramsothy et al. demonstrated that FMT induced remission in 27% of patients compared to 8% for placebo.74 FMT was also associated with an increase in microbial diversity in this study. In a systematic review and meta-analysis of 4 RCTs, FMT was associated with higher rates of endoscopic and clinical remission of UC compared to placebo (number needed to treat = 5) with no difference in adverse events.76 In a secondary analysis of our group’s prospective cohort study, rates of clinical improvement were 73.3% for UC and 62% for CD at 12 weeks after FMT.77 Only 1 of 49 patients experienced a de novo flare.
Prior studies also suggest that FMT is safe in the IBD population. In a systematic review of 4 RCTs of FMT used for active UC, there was no significant increase in serious adverse events compared with controls.76 Our group also identified only two serious adverse events prospectively among 49 IBD patients after FMT for recurrent CDI, and neither of these was felt to be treatment-related.72
Management of immunosuppression in the setting of CDI and IBD
One aspect of management that clinicians may struggle with is the positioning of immunosuppression for IBD in the setting of active CDI. CDI may exacerbate underlying IBD, and therefore many patients should undergo escalation of immunosuppressive therapy. Guidance for these scenarios is mainly from expert opinion as well as retrospective studies (Table 1). In a European cohort of 155 hospitalized patients with CDI and IBD, 104 were treated with immunosuppressive medications (including corticosteroids, thiopurines, methotrexate, calcineurin inhibitors, or biologics) and antibiotics while 51 were treated with antibiotics alone. The primary composite outcome of death, colectomy, megacolon, bowel perforation, shock, or respiratory failure occurred in 12% of the combination therapy group compared to none in the antibiotic monotherapy group.78 On multivariate analysis, there was no significant independent association between combination therapy and the primary outcome, however there was an association between 2 or more immunosuppressive medications and the primary outcome. In a retrospective cohort of 294 patients with CDI and IBD, a low serum albumin and hemoglobin and elevated creatinine were independent predictors of shorter time colectomy or death, but use of immunosuppressive medications were not.79 Data specific to corticosteroids are conflicting, as one retrospective study of 137 IBD patients found that escalation of corticosteroids was associated with a 2-fold higher odds of colon surgery within 1 year after CDI.80 However three other retrospective studies of similar size did not identify adverse outcomes associated with corticosteroid therapy.79, 81, 82
Table 1.
Studies evaluating the association between immunosuppressive medications and clinical outcomes in the setting of C. difficile infection among patients with IBD
| Study (author, year, journal) | Design and population | Outcomes |
|---|---|---|
| Ben-Horin et al. 200977 Clinical Gastroenterology and Hepatology |
Multicenter retrospective cohort European Crohn’s and Colitis Organization 155 hospitalized IBD patients with CDI (2001–2008) |
1. No independent association between immunosuppressive medications and composite outcome (death within 3 months, megacolon, bowel perforation, shock, respiratory failure): LR 11.9, 95% CI 0.9–157 2. Higher adjusted odds of composite outcome associated with 2 or more immunosuppressive medications: OR 17, 95% 3.1–91 |
| Ananthakrishnan et al. 201278 Alimentary Pharmacology and Therapeutics |
Multi-institutional retrospective cohort Boston, MA, USA 294 hospitalized IBD patients with CDI (1998–2010) |
1. No association between immunomodulators and severe outcomes (time to colectomy or death): HR 0.57, 95% CI 0.4–1.2 2. No association between corticosteroids and severe outcomes: HR 0.9, 95% CI -0.5–1.5 3. No association between anti-TNFs and severe outcomes: HR 0.4, 95% 0.1–1.5 |
| Solanky et al. 201979 Inflammatory Bowel Diseases |
Single center retrospective cohort Rochester, MN, USA 137 hospitalized IBD patients with CDI (2008–2013) |
1. Higher adjusted odds of colon surgery within 1 year after CDI associated with corticosteroid use: OR 5.9, 95% CI 2.0–17.4 |
| Lukin et al. 201981 Inflammatory Bowel Diseases |
Multicenter retrospective cohort New York, NY, USA 207 hospitalized IBD patients with CDI (dates unknown) |
1. Lower adjusted odds of severe outcomes (death, sepsis, or colectomy within 90 days) associated with IBD therapy escalation to corticosteroids or biologics: OR 0.12, 95% CI 0.02–0.9 |
| Bar-Yoseph et al. 202080 International Journal of Colorectal Disease |
Single center retrospective cohort Haifa, Israel 113 hospitalized IBD patients with CDI (2002–2018) |
1. No significant difference in 1-year colectomy rates by exposure to early corticosteroids (within 48 hours of admission): 8.5% vs. 0.0%, p=0.19 2. No significant difference in 1-year mortality rates by exposure to early corticosteroids: 6.1% vs. 16.1%, p=0.13 |
Abbreviations: IBD = inflammatory bowel disease, CDI = C. difficile infection, LR = likelihood ratio, OR = odds ratio, CI = confidence interval
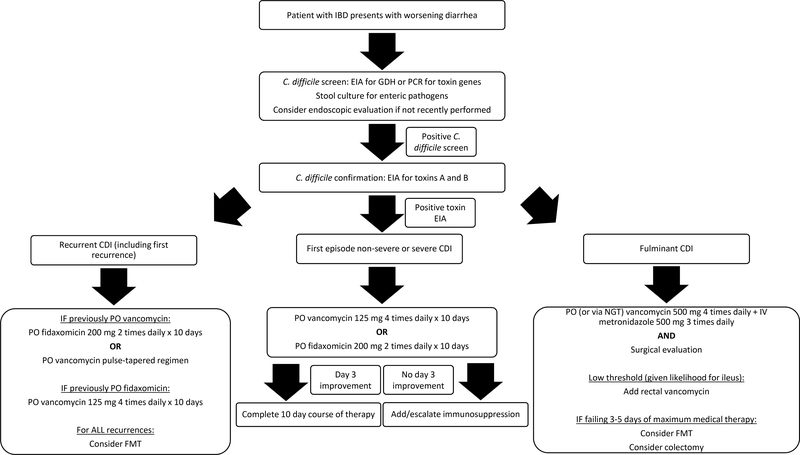
In a recent multicenter study of 207 patients with CDI and IBD, 62 patients underwent escalation to corticosteroids or biologic therapy.82 The adjusted odds of severe outcomes (death, sepsis, or colectomy) within 90 days were reduced among patients who underwent escalation of immunosuppression compared to those who were not escalated. In the absence of much-needed prospective data regarding this issue, the most recent American Gastroenterological Association clinical practice guidelines recommend initiation of corticosteroids or immunosuppressive therapy 3–4 days after persistent symptoms of colitis despite appropriate antimicrobial therapy for CDI.23 Patients should be monitored closely for progression of symptoms and other complications during this vulnerable time. A comprehensive diagnostic and management algorithm for CDI in IBD is presented in Figure 1.
Figure 1.
Comprehensive diagnostic and management algorithm of CDI in IBD
Abbreviations: IBD = inflammatory bowel disease, CDI = C. difficile infection, EIA = enzyme immunoassay, GDH = glutamate dehydrogenase, PCR = polymerase chain reaction, PO = oral, NGT = nasogastric tube, FMT = fecal microbiota transplant
Conclusion
CDI is a common but serious complication of IBD that requires simultaneous attention to both conditions. All IBD patients with worsening colitis symptoms should be evaluated for CDI and providers must acknowledge that typical risk factors such as recent antibiotic exposure and pseudomembranes may not be present. Those with positive two-step stool testing should receive oral vancomycin or fidaxomicin as initial therapy and FMT should be considered for those with recurrent disease. Escalation of immunosuppression may also be needed, especially if IBD patients fail to respond to appropriate antimicrobial therapy after 3–4 days. However, treatment decisions should always be individualized.
Key Points.
Due to relatively high rates of C. difficile colonization in the IBD population, CDI must be confirmed using a two-step approach to stool testing, including a positive EIA for toxins A and B.
Oral vancomycin or fidaxomicin are the preferred agents for non-severe or severe CDI. Metronidazole is no longer recommended as first-line therapy.
FMT should be considered for all patients with recurrent CDI. Medical therapy for recurrent CDI should always be distinct from the first regimen.
If patients do not improve with appropriate antimicrobial therapy, escalation of immunosuppression may be necessary to treat IBD flares secondary to CDI.
Acknowledgments
Financial support: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [5T32DK007533-35 to RSD].
Footnotes
Conflicts of interest: JRA serves as a consultant for Takeda, Janssen, Pfizer, Pandion, Servatus, Finch Therapeutics, Iterative Scopes and Artugen and has grant support from Merck. RSD has no financial or personal conflicts of interest to disclose.
References
- 1.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 03 2018;66(7):987–994. doi: 10.1093/cid/ciy149 [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Winston LG, McDonald LC, Team EIPCdS. Burden of Clostridium difficile infection in the United States. N Engl J Med. 06 2015;372(24):2369–70. doi: 10.1056/NEJMc1505190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guh AY, Mu Y, Winston LG, et al. Trends in U.S. Burden of. N Engl J Med. 04 2020;382(14):1320–1330. doi: 10.1056/NEJMoa1910215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh H, Nugent Z, Yu BN, Lix LM, Targownik LE, Bernstein CN. Higher Incidence of Clostridium difficile Infection Among Individuals With Inflammatory Bowel Disease. Gastroenterology. 08 2017;153(2):430–438.e2. doi: 10.1053/j.gastro.2017.04.044 [DOI] [PubMed] [Google Scholar]
- 5.Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. March 2007;5(3):339–44. doi: 10.1016/j.cgh.2006.12.027 [DOI] [PubMed] [Google Scholar]
- 6.Clayton EM, Rea MC, Shanahan F, et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. May 2009;104(5):1162–9. doi: 10.1038/ajg.2009.4 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. June 2008;103(6):1443–50. doi: 10.1111/j.1572-0241.2007.01780.x [DOI] [PubMed] [Google Scholar]
- 8.Micic D, Yarur A, Gonsalves A, et al. Risk Factors for Clostridium difficile Isolation in Inflammatory Bowel Disease: A Prospective Study. Dig Dis Sci. 04 2018;63(4):1016–1024. doi: 10.1007/s10620-018-4941-7 [DOI] [PubMed] [Google Scholar]
- 9.Regnault H, Bourrier A, Lalande V, et al. Prevalence and risk factors of Clostridium difficile infection in patients hospitalized for flare of inflammatory bowel disease: a retrospective assessment. Dig Liver Dis. Dec 2014;46(12):1086–92. doi: 10.1016/j.dld.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 10.Kariv R, Navaneethan U, Venkatesh PG, Lopez R, Shen B. Impact of Clostridium difficile infection in patients with ulcerative colitis. J Crohns Colitis. February 2011;5(1):34–40. doi: 10.1016/j.crohns.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 11.Stoica O, Trifan A, Cojocariu C, Gîrleanu I, Maxim R, Stanciu MC. Incidence and risk factors of Clostridium difficile infection in patients with inflammatory bowel disease. Rev Med Chir Soc Med Nat Iasi. 2015. Jan-Mar 2015;119(1):81–6. [PubMed] [Google Scholar]
- 12.Gu YB, Zhang MC, Sun J, Lv KZ, Zhong J. Risk factors and clinical outcome of Clostridium difficile infection in patients with IBD: A single-center retrospective study of 260 cases in China. J Dig Dis. April 2017;18(4):207–211. doi: 10.1111/1751-2980.12461 [DOI] [PubMed] [Google Scholar]
- 13.Kaneko T, Matsuda R, Taguri M, et al. Clostridium difficile infection in patients with ulcerative colitis: investigations of risk factors and efficacy of antibiotics for steroid refractory patients. Clin Res Hepatol Gastroenterol. April 2011;35(4):315–20. doi: 10.1016/j.clinre.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 14.Ramos-Martínez A, Ortiz-Balbuena J, Curto-García I, et al. Risk factors for Clostridium difficile diarrhea in patients with inflammatory bowel disease. Rev Esp Enferm Dig. January 2015;107(1):4–8. [PubMed] [Google Scholar]
- 15.Zhang T, Lin QY, Fei JX, et al. Clostridium Difficile Infection Worsen Outcome of Hospitalized Patients with Inflammatory Bowel Disease. Sci Rep. 07 2016;6:29791. doi: 10.1038/srep29791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson A, Click B, Ramos-Rivers C, et al. Lasting Impact of Clostridium difficile Infection in Inflammatory Bowel Disease: A Propensity Score Matched Analysis. Inflamm Bowel Dis. 12 2017;23(12):2180–2188. doi: 10.1097/MIB.0000000000001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossuyt P, Verhaegen J, Van Assche G, Rutgeerts P, Vermeire S. Increasing incidence of Clostridium difficile-associated diarrhea in inflammatory bowel disease. J Crohns Colitis. February 2009;3(1):4–7. doi: 10.1016/j.crohns.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 18.Maharshak N, Barzilay I, Zinger H, Hod K, Dotan I. Clostridium difficile infection in hospitalized patients with inflammatory bowel disease: Prevalence, risk factors, and prognosis. Medicine (Baltimore). February 2018;97(5):e9772. doi: 10.1097/MD.0000000000009772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. March 2007;5(3):345–51. doi: 10.1016/j.cgh.2006.12.028 [DOI] [PubMed] [Google Scholar]
- 20.D’Aoust J, Battat R, Bessissow T. Management of inflammatory bowel disease with. World J Gastroenterol. July 2017;23(27):4986–5003. doi: 10.3748/wjg.v23.i27.4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navaneethan U, Venkatesh PG, Shen B. Clostridium difficile infection and inflammatory bowel disease: understanding the evolving relationship. World J Gastroenterol. October 2010;16(39):4892–904. doi: 10.3748/wjg.v16.i39.4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balram B, Battat R, Al-Khoury A, et al. Risk Factors Associated with Clostridium difficile Infection in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J Crohns Colitis. January 2019;13(1):27–38. doi: 10.1093/ecco-jcc/jjy143.* This systematic review and meta-analysis identified key risk factors associated with C. difficile infection among patients with IBD.
- 23.Khanna S, Shin A, Kelly CP. Management of Clostridium difficile Infection in Inflammatory Bowel Disease: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 02 2017;15(2):166–174. doi: 10.1016/j.cgh.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 24.Jodorkovsky D, Young Y, Abreu MT. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Dig Dis Sci. February 2010;55(2):415–20. doi: 10.1007/s10620-009-0749-9 [DOI] [PubMed] [Google Scholar]
- 25.Murthy SK, Steinhart AH, Tinmouth J, Austin PC, Daneman N, Nguyen GC. Impact of Clostridium difficile colitis on 5-year health outcomes in patients with ulcerative colitis. Aliment Pharmacol Ther. December 2012;36(11–12):1032–9. doi: 10.1111/apt.12073 [DOI] [PubMed] [Google Scholar]
- 26.Jen MH, Saxena S, Bottle A, Aylin P, Pollok RC. Increased health burden associated with Clostridium difficile diarrhoea in patients with inflammatory bowel disease. Aliment Pharmacol Ther. June 2011;33(12):1322–31. doi: 10.1111/j.1365-2036.2011.04661.x [DOI] [PubMed] [Google Scholar]
- 27.Shrestha MP, Taleban S. Colectomy Rates Are Increasing Among Inpatients With Concomitant Ulcerative Colitis and Clostridioides difficile. J Clin Gastroenterol. August 2020;doi: 10.1097/MCG.0000000000001412 [DOI] [PubMed] [Google Scholar]
- 28.Law CC, Tariq R, Khanna S, Murthy S, McCurdy JD. Systematic review with meta-analysis: the impact of Clostridium difficile infection on the short- and long-term risks of colectomy in inflammatory bowel disease. Aliment Pharmacol Ther. 04 2017;45(8):1011–1020. doi: 10.1111/apt.13972 [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Furuya-Kanamori L, Doi SA, Ananthakrishnan AN, Kirk M. Clostridium difficile Infection and Risk of Colectomy in Patients with Inflammatory Bowel Disease: A Bias-adjusted Meta-analysis. Inflamm Bowel Dis. 02 2017;23(2):200–207. doi: 10.1097/MIB.0000000000000998 [DOI] [PubMed] [Google Scholar]
- 30.Tariq R, Law CCY, Khanna S, Murthy S, McCurdy JD. The Impact of Clostridium difficile Infection on Mortality in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 02 2019;53(2):127–133. doi: 10.1097/MCG.0000000000000968 [DOI] [PubMed] [Google Scholar]
- 31.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. January 2015;313(4):398–408. doi: 10.1001/jama.2014.17103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanahita A, Goldsmith EA, Marino BJ, Musher DM. Clostridium difficile infection in patients with unexplained leukocytosis. Am J Med. November 2003;115(7):543–6. doi: 10.1016/s0002-9343(03)00420-0 [DOI] [PubMed] [Google Scholar]
- 33.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. February 2008;57(2):205–10. doi: 10.1136/gut.2007.128231 [DOI] [PubMed] [Google Scholar]
- 34.Crobach MJ, Dekkers OM, Wilcox MH, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin Microbiol Infect. December 2009;15(12):1053–66. doi: 10.1111/j.1469-0691.2009.03098.x [DOI] [PubMed] [Google Scholar]
- 35.Collison M, Murillo C, Marrs R, et al. Universal screening for. Infect Control Hosp Epidemiol. September 2020:1–2. doi: 10.1017/ice.2020.428 [DOI] [PubMed] [Google Scholar]
- 36.Goodhand JR, Alazawi W, Rampton DS. Systematic review: Clostridium difficile and inflammatory bowel disease. Aliment Pharmacol Ther. February 2011;33(4):428–41. doi: 10.1111/j.1365-2036.2010.04548.x [DOI] [PubMed] [Google Scholar]
- 37.Autenrieth DM, Baumgart DC. Toxic megacolon. Inflamm Bowel Dis. March 2012;18(3):584–91. doi: 10.1002/ibd.21847 [DOI] [PubMed] [Google Scholar]
- 38.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. August 2014;59(3):345–54. doi: 10.1093/cid/ciu313 [DOI] [PubMed] [Google Scholar]
- 39.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. August 2007;45(3):302–7. doi: 10.1086/519265 [DOI] [PubMed] [Google Scholar]
- 40.Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. September 2011;53(5):440–7. doi: 10.1093/cid/cir404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venugopal AA, Johnson S. Fidaxomicin: a novel macrocyclic antibiotic approved for treatment of Clostridium difficile infection. Clin Infect Dis. February 2012;54(4):568–74. doi: 10.1093/cid/cir830 [DOI] [PubMed] [Google Scholar]
- 42.Lei DK, Ollech JE, Andersen M, et al. Long-Duration Oral Vancomycin to Treat Clostridioides difficile in Patients With Inflammatory Bowel Disease Is Associated With a Low Rate of Recurrence. Am J Gastroenterol. 12 2019;114(12):1904–1908. doi: 10.14309/ajg.0000000000000460.* This retrospective study identified a lower rate of C. difficile recurrence associated with long-duration oral vancomycin compared with short duration oral vancomycin among patients with IBD.
- 43.Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. May 2009;144(5):433–9; discussion 439–40. doi: 10.1001/archsurg.2009.51 [DOI] [PubMed] [Google Scholar]
- 44.van der Wilden GM, Velmahos GC, Chang Y, et al. Effects of a New Hospital-Wide Surgical Consultation Protocol in Patients with Clostridium difficile Colitis. Surg Infect (Larchmt). July 2017;18(5):563–569. doi: 10.1089/sur.2016.041 [DOI] [PubMed] [Google Scholar]
- 45.Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P, Collaborative WMR. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. November 2012;99(11):1501–13. doi: 10.1002/bjs.8868 [DOI] [PubMed] [Google Scholar]
- 46.Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. September 2008;196(3):384–8. doi: 10.1016/j.amjsurg.2007.11.017 [DOI] [PubMed] [Google Scholar]
- 47.Ferrada P, Velopulos CG, Sultan S, et al. Timing and type of surgical treatment of Clostridium difficile-associated disease: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. June 2014;76(6):1484–93. doi: 10.1097/TA.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 48.Hocquart M, Lagier JC, Cassir N, et al. Early Fecal Microbiota Transplantation Improves Survival in Severe Clostridium difficile Infections. Clin Infect Dis. 02 2018;66(5):645–650. doi: 10.1093/cid/cix762 [DOI] [PubMed] [Google Scholar]
- 49.Tixier EN, Verheyen E, Ungaro RC, Grinspan AM. Faecal microbiota transplant decreases mortality in severe and fulminant Clostridioides difficile infection in critically ill patients. Aliment Pharmacol Ther. 11 2019;50(10):1094–1099. doi: 10.1111/apt.15526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters BA, Roberts R, Stafford R, Seneviratne E. Relapse of antibiotic associated colitis: endogenous persistence of Clostridium difficile during vancomycin therapy. Gut. March 1983;24(3):206–12. doi: 10.1136/gut.24.3.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilcox MH, Fawley WN, Settle CD, Davidson A. Recurrence of symptoms in Clostridium difficile infection--relapse or reinfection? J Hosp Infect. February 1998;38(2):93–100. doi: 10.1016/s0195-6701(98)90062-7 [DOI] [PubMed] [Google Scholar]
- 52.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. December 2008;70(4):298–304. doi: 10.1016/j.jhin.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 53.Johnson S Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. June 2009;58(6):403–10. doi: 10.1016/j.jinf.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 54.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. December 2012;18 Suppl 6:21–7. doi: 10.1111/1469-0691.12046 [DOI] [PubMed] [Google Scholar]
- 55.Razik R, Rumman A, Bahreini Z, McGeer A, Nguyen GC. Recurrence of Clostridium difficile Infection in Patients with Inflammatory Bowel Disease: The RECIDIVISM Study. Am J Gastroenterol. 08 2016;111(8):1141–6. doi: 10.1038/ajg.2016.187 [DOI] [PubMed] [Google Scholar]
- 56.Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology. 04 2017;152(5):1042–1054.e1. doi: 10.1053/j.gastro.2016.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wadhwa A, Al Nahhas MF, Dierkhising RA, et al. High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol Ther. 09 2016;44(6):576–82. doi: 10.1111/apt.13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allegretti JR, Kassam Z, Fischer M, Kelly C, Chan WW. Risk Factors for Gastrointestinal Symptoms Following Successful Eradication of Clostridium difficile by Fecal Microbiota Transplantation (FMT). J Clin Gastroenterol. 10 2019;53(9):e405–e408. doi: 10.1097/MCG.0000000000001194 [DOI] [PubMed] [Google Scholar]
- 59.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. August 2012;55 Suppl 2:S154–61. doi: 10.1093/cid/cis462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caroff DA, Menchaca JT, Zhang Z, et al. Oral vancomycin prophylaxis during systemic antibiotic exposure to prevent Clostridiodes difficile infection relapses. Infect Control Hosp Epidemiol. 06 2019;40(6):662–667. doi: 10.1017/ice.2019.88 [DOI] [PubMed] [Google Scholar]
- 61.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. May 2012;307(18):1959–69. doi: 10.1001/jama.2012.3507 [DOI] [PubMed] [Google Scholar]
- 62.Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016;9:27–37. doi: 10.2147/IJGM.S98280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinclair A, Xie X, Saab L, Dendukuri N. Lactobacillus probiotics in the prevention of diarrhea associated with Clostridium difficile: a systematic review and Bayesian hierarchical meta-analysis. CMAJ Open. 2016. Oct-Dec 2016;4(4):E706–E718. doi: 10.9778/cmajo.20160087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen NT, Maw A, Tmanova LL, et al. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review With Meta-Regression Analysis. Gastroenterology. 06 2017;152(8):1889–1900.e9. doi: 10.1053/j.gastro.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 65.Allegretti JR, Kao D, Phelps E, et al. Risk of Clostridium difficile Infection with Systemic Antimicrobial Therapy Following Successful Fecal Microbiota Transplant: Should We Recommend Anti-Clostridium difficile Antibiotic Prophylaxis? Dig Dis Sci. 06 2019;64(6):1668–1671. doi: 10.1007/s10620-018-5450-4 [DOI] [PubMed] [Google Scholar]
- 66.Suez J, Zmora N, Zilberman-Schapira G, et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell. 09 2018;174(6):1406–1423.e16. doi: 10.1016/j.cell.2018.08.047 [DOI] [PubMed] [Google Scholar]
- 67.Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 01 2017;376(4):305–317. doi: 10.1056/NEJMoa1602615 [DOI] [PubMed] [Google Scholar]
- 68.Kelly CP, Wilcox MH, Glerup H, et al. Bezlotoxumab for Clostridium difficile Infection Complicating Inflammatory Bowel Disease. Gastroenterology. 10 2018;155(4):1270–1271. doi: 10.1053/j.gastro.2018.06.080 [DOI] [PubMed] [Google Scholar]
- 69.Khoruts A, Rank KM, Newman KM, et al. Inflammatory Bowel Disease Affects the Outcome of Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 10 2016;14(10):1433–8. doi: 10.1016/j.cgh.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meighani A, Hart BR, Bourgi K, Miller N, John A, Ramesh M. Outcomes of Fecal Microbiota Transplantation for Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Dig Dis Sci. 10 2017;62(10):2870–2875. doi: 10.1007/s10620-017-4580-4 [DOI] [PubMed] [Google Scholar]
- 71.Tariq R, Disbrow MB, Dibaise JK, et al. Efficacy of Fecal Microbiota Transplantation for Recurrent C. Difficile Infection in Inflammatory Bowel Disease. Inflamm Bowel Dis. August 2020;26(9):1415–1420. doi: 10.1093/ibd/izz299.* This retrospective study identified a C. difficile cure rate of 80% among IBD patients who received FMT for recurrent C. difficile infection.
- 72.Allegretti JR, Kelly CR, Grinspan A, Mullish BH, Kassam Z, Fischer M. Outcomes of Fecal Microbiota Transplantation in Patients With Inflammatory Bowel Diseases and Recurrent Clostridioides difficile Infection. Gastroenterology. July 2020;doi: 10.1053/j.gastro.2020.07.045.** his prospective, multicenter cohort study demonstrated a low rate of FMT failure for recurrent C. difficile infection among patients with IBD.
- 73.Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. July 2015;149(1):102–109.e6. doi: 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 74.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 03 2017;389(10075):1218–1228. doi: 10.1016/S0140-6736(17)30182-4 [DOI] [PubMed] [Google Scholar]
- 75.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. July 2015;149(1):110–118.e4. doi: 10.1053/j.gastro.2015.03.045 [DOI] [PubMed] [Google Scholar]
- 76.Narula N, Kassam Z, Yuan Y, et al. Systematic Review and Meta-analysis: Fecal Microbiota Transplantation for Treatment of Active Ulcerative Colitis. Inflamm Bowel Dis. 10 2017;23(10):1702–1709. doi: 10.1097/MIB.0000000000001228 [DOI] [PubMed] [Google Scholar]
- 77.Allegretti JR, Kelly CR, Grinspan A, et al. Inflammatory Bowel Disease Outcomes Following Fecal Microbiota Transplantation for Recurrent C. difficile Infection. Inflamm Bowel Dis. November 2020;doi: 10.1093/ibd/izaa283.* This secondary analysis of a prospective cohort study identified high rates of clinical improvement of IBD after FMT for recurrent C. difficile infection.
- 78.Ben-Horin S, Margalit M, Bossuyt P, et al. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and clostridium difficile infection. Clin Gastroenterol Hepatol. September 2009;7(9):981–7. doi: 10.1016/j.cgh.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 79.Ananthakrishnan AN, Guzman-Perez R, Gainer V, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. April 2012;35(7):789–95. doi: 10.1111/j.1365-2036.2012.05022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solanky D, Pardi DS, Loftus EV, Khanna S. Colon Surgery Risk With Corticosteroids Versus Immunomodulators or Biologics in Inflammatory Bowel Disease Patients With Clostridium difficile Infection. Inflamm Bowel Dis. 02 2019;25(3):610–619. doi: 10.1093/ibd/izy291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bar-Yoseph H, Daoud H, Ben Hur D, Chowers Y, Waterman M. Does early corticosteroid therapy affect prognosis in IBD patients hospitalized with Clostridioides difficile infection? Int J Colorectal Dis. March 2020;35(3):513–519. doi: 10.1007/s00384-019-03502-z [DOI] [PubMed] [Google Scholar]
- 82.Lukin DJ, Lawlor G, Hudesman DP, et al. Escalation of Immunosuppressive Therapy for Inflammatory Bowel Disease Is Not Associated With Adverse Outcomes After Infection With Clostridium difficile. Inflamm Bowel Dis. 03 2019;25(4):775–781. doi: 10.1093/ibd/izy308.* This multicenter, retrospective study identified lower adjusted odds of severe outcomes among patients with IBD and C. difficile infection who underwent escalation of immunosuppression.