Abstract
Background –
Right ventricular (RV) contractile reserve shows promise as an indicator of occult RV dysfunction in pulmonary vascular disease. We investigated which measure of RV contractile reserve during exercise best predicts occult RV dysfunction and clinical outcomes.
Methods –
We prospectively studied RV contractile reserve in 35 human subjects referred for right heart catheterization for known or suspected pulmonary hypertension. All underwent cardiac magnetic resonance imaging, echocardiography, and supine invasive cardiopulmonary exercise testing with concomitant RV pressure-volume catheterization. Event-free survival was prospectively adjudicated from time of right heart catheterization for a 4-year follow-up period.
Results –
RV contractile reserve during exercise, as measured by a positive change in end-systolic elastance (Ees) during exertion, was associated with elevation in pulmonary pressures but preservation of RV volumes. Lack of RV reserve, on the other hand, was tightly coupled with acute RV dilation during exertion (R2=0.76, P<0.001). RV Ees and dilation changes each predicted resting RV-PA dysfunction. RV ejection fraction during exercise, which captured exertional changes in both RV Ees and RV dilation, proved to be a robust surrogate for RV contractile reserve. Reduced exercise RV ejection fraction best predicted occult RV dysfunction among a variety of resting and exercise RV measures, and was also associated with clinical worsening.
Conclusions –
RV ejection fraction during exercise, as an index of RV contractile reserve, allows for excellent identification of occult RV dysfunction, more so than resting measures of RV function, and may predict clinical outcomes as well.
Keywords: pulmonary hypertension, heart ventricles, exercise
INTRODUCTION
Detecting occult right ventricular (RV) dysfunction remains an unmet need in pulmonary vascular disease.1 There exist many excellent markers of overt RV dysfunction, including reduced RV ejection fraction (RVEF), RV dilation, and elevated right-sided filling pressures.2 However, these measures all fail to identify the “at-risk” RV.1 Better detection of occult RV dysfunction would help clinicians identify at-risk patients, escalate therapies, and prognosticate outcomes. RV contractile reserve has shown promise as a novel means of detecting occult RV dysfunction,3–6 akin to how a stress test can identify coronary disease undetectable at rest. For example, exercise augmentation of RV systolic pressure was shown to predict outcomes in human pulmonary arterial hypertension (PAH).4 However, existing measures of RV reserve fall short, and none are ready for clinical use. For example, the aforementioned example employs Doppler estimates of RV pressure, which are known to be unreliable at rest and exercise.7
RV-pulmonary arterial (PA) coupling offers the most sensitive and precise way to measure RV contractile reserve.6,8–11 RV-PA coupling is defined by RV Ees/Ea, or the ratio of RV end-systolic elastance (Ees) to PA effective arterial elastance (Ea).1 RV Ees offers a load-independent method of measuring intrinsic RV contractility, while Ees/Ea measures right-sided ventriculo-arterial coupling.12–14 Using this framework, RV contractile reserve would be defined by an increase in RV Ees during stress.2 RV-PA coupling, however, falls short in its own ways. Gold-standard measurement of RV-PA coupling, for example, involves conductance catheter measurement of “multi-beat” RV Ees, a method that remains costly and impractical.15 That said, lessons learned from such measurements could yield novel ways of characterizing RV contractile reserve. Therefore, in this prospective study of humans with pulmonary vascular disease, we leveraged multi-beat measurements of Ees/Ea at rest and exercise in order to study exercise RV contractile reserve, with the ultimate goal of finding a more clinically accessible way to detect impaired RV reserve.
METHODS
Subjects.
From 2013–2016, we prospectively studied 35 human subjects referred for resting and exercise right heart catheterization (RHC) for the evaluation of known or suspected PH. Eligible and consenting subjects underwent cardiac magnetic resonance (CMR) imaging, echocardiography, resting RHC, and invasive cardiopulmonary exercise testing (iCPET) with concomitant RV PV loop catheterization. All patients provided informed consent, and the research protocol was approved by the Johns Hopkins Institutional Review Board (Protocol NA_00014540). PH and pulmonary arterial hypertension (PAH) were standardly defined.16 Data can be made available upon reasonable request.
Clinical, Imaging, and Catheterization Measurements.
Baseline clinical characteristics were obtained from PH physician assessment at time of RHC. On the study day, patients underwent transthoracic echocardiography, CMR, and RHC with iCPET with supine bicycle ergometry and concomitant resting and exercise PV measurements. The full protocol is previously outlined.6 Briefly, following clinical RHC, the 8F internal jugular introducer sheath was replaced with a dual-entry 9F sheath to facilitate simultaneous placement of a 5F PV conductance catheter (RP-CA-41103-PN, CD Leycom, Netherlands) and 4F PA wedge catheter (Teleflex, Morrisville, NC). Resting RV PV loop data were measured at end-expiration and during a Valsalva in order to calculate multi-beat RV Ees.8 For the exercise RHC portion, patients lay supine and exercised using an ergometer while both PV and PA wedge catheter remained. Continuous gas exchange was measured via metabolic cart (Innocor, Innovision, Denmark). Exercise was performed using a ramp protocol beginning at 15 Watts (W) in stage 1 and increasing in 10W increments every 2 minutes until max effort. Pressures (averaged over the respiratory cycle), gas exchange, and PV data were recorded at each stage.
PV analysis is previously described in full.6 A signal-averaged resting PV loop was calibrated using resting CMR volume. Using an iterative process, end-systolic PV points from a Valsalva series were obtained by maximizing the ratio P(t)/[V(t)-V0] of each cardiac cycle. End-systolic PV points were then fit by perpendicular regression to derive RV Ees, or the slope of the end-systolic PV relationship. This slope was applied to the end-systolic point of the resting loop in order to determine V0, or the x-intercept of the resting end-systolic PV relationship.6,8 Ea was calculated by dividing end-systolic pressure (ESP) by stroke volume (SV), both obtained from the resting PV loop. RV Ees for each exercise stage was calculated based on the slope between resting V0 and the end-systolic PV point of each exercise stage signal-averaged loop. Stroke volume, RVEF, and cardiac output were calculated using PV-derived RV EDV and ESV. Figure 1 illustrates acquisition of resting and exercise Ees and Ea for three representative subjects. Subjects were said to have RV contractile reserve if the change in RV Ees during sub-maximal workload was >0 mmHg/ml. Subjects were otherwise classified as having no RV contractile reserve.
FIGURE 1. Acquisition of resting and exercise RV pressure-volume loop measurements.
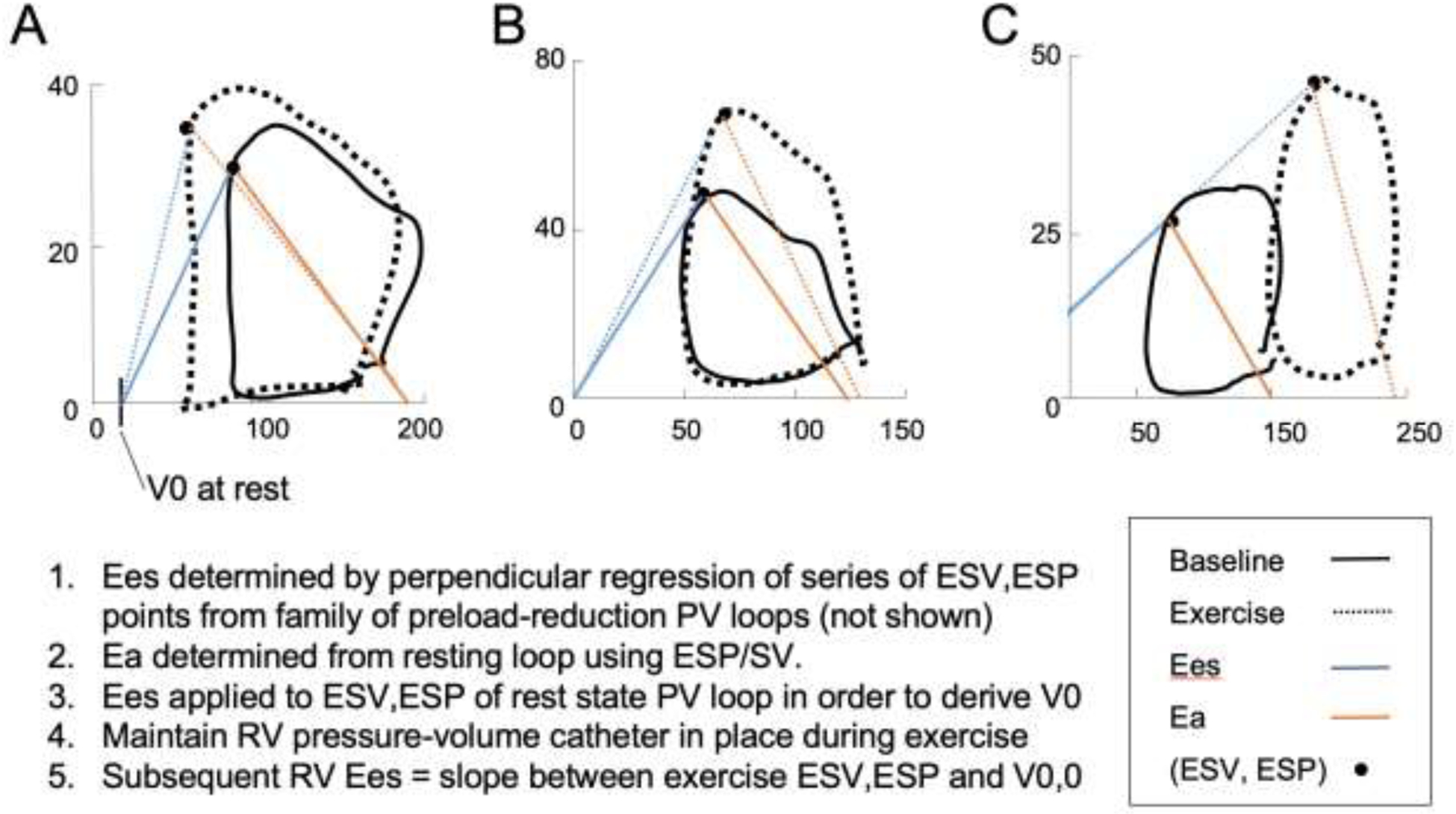
Resting exercise end-systolic elastance and effective arterial elastance were calculated, and V0 determined from the rest state. Subsequent PV loops obtained during exercise, in combination with resting V0, were used to calculate Ees and Ea at each stage. Actual pressure-volume loops shown for a subject with (A) excellent, (B) preserved, and (C) no RV contractile reserve.
From study date onward, subjects were prospectively followed to assess time to clinical worsening (TTCW), defined as the composite of right heart failure hospitalization, lung transplantation, or death. These were modified from the typical events that constitute TTCW in PAH studies,17 with some excluded because not all had PAH. All subjects were followed for four years or until clinical worsening.
Statistical Methods.
Continuous variables were reported as mean ± standard deviation; proportions used where relevant. To determine the effect of RV contractile reserve, exercise timepoint, and their interaction term on hemodynamic variables (see Figure 2), multilevel mixed-effects linear regression was employed. Non-linear regression fit to an inverse hyperbolic curve was used to compare RV Ees and RV end-diastolic volume (RVEDV). A non-parametric test for trend was used to compare exercise RVEF tertiles (see Figure 4B). Univariate linear regression was utilized to model the relationship and quantify the association between predictor variables and resting RV-PA coupling (as indexed by Ees/Ea). When applicable, receiver operator curve (ROC) analysis determined the area under the curve (AUC) for assessing Ees/Ea<1.0. Multivariate regression was used to assess the ability of exercise RVEF to predict Ees/Ea independently of other predictor variables. Cox proportional hazards modeling and Kaplan-Meier survival analysis were used to analyze survival data. A two-sided P<0.05 denoted statistical significance.
FIGURE 2. Effect of RV contractile reserve and exercise on cardiopulmonary hemodynamics.
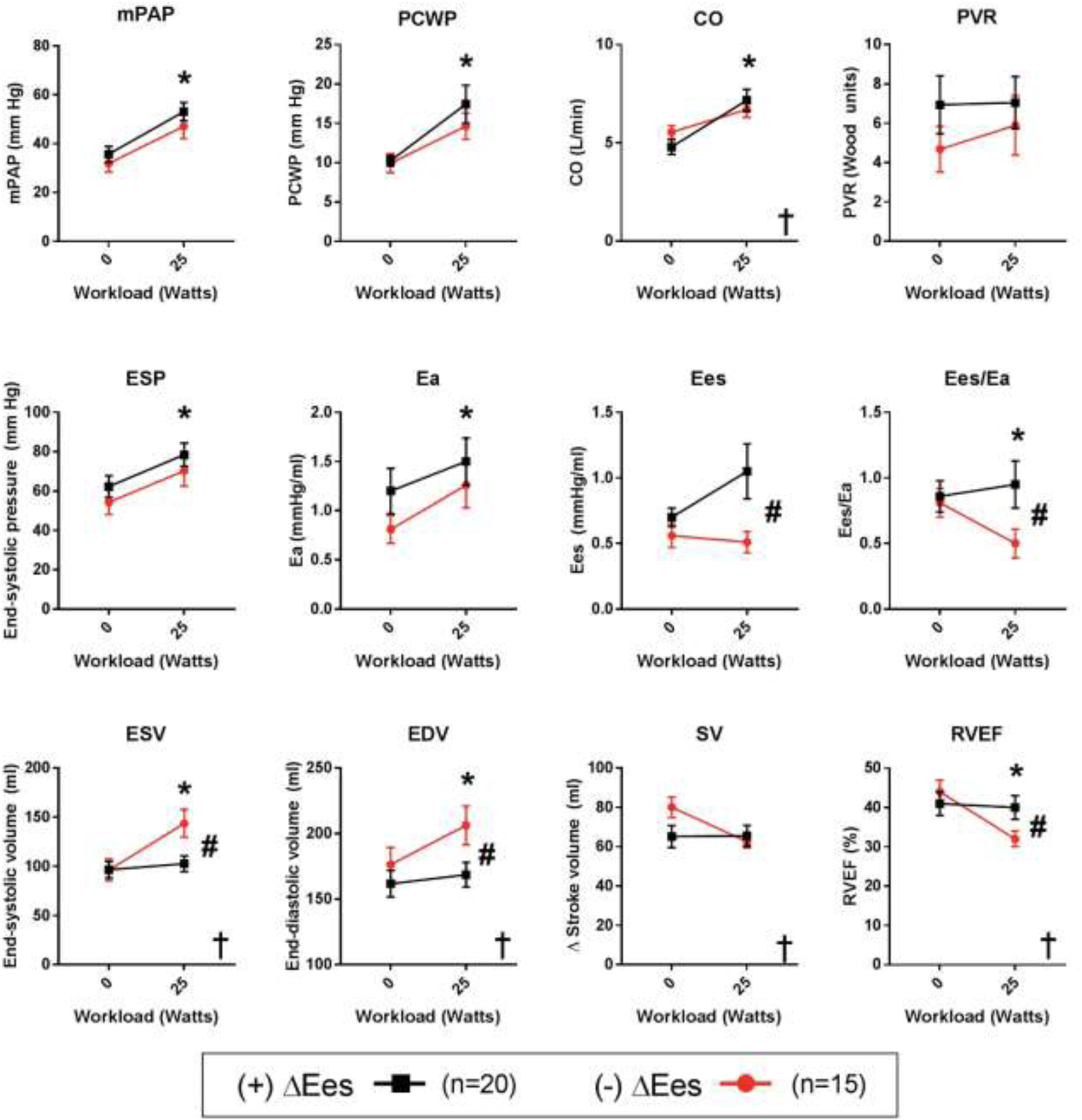
Cohort divided into those with (n=20) and without (n=15) RV contractile reserve during exercise. * P<0.05 for significant change with exercise; # P<0.05 for significant group difference; † P<0.05 for group-exercise interaction term. Overall, both groups saw similar increases in pressures and flow. However, the RV reserve group preserved RV-PA coupling, RV volumes, and RVEF, while those without RV reserve saw acute RV dilation, reduction in stroke volume, and reduced RVEF. RV reserve therefore significantly influenced the effect of exercise on RV volume changes.
FIGURE 4. Exercise RVEF Correlates with Resting RV-PA Coupling and Predicts Early RV-PA Uncoupling.
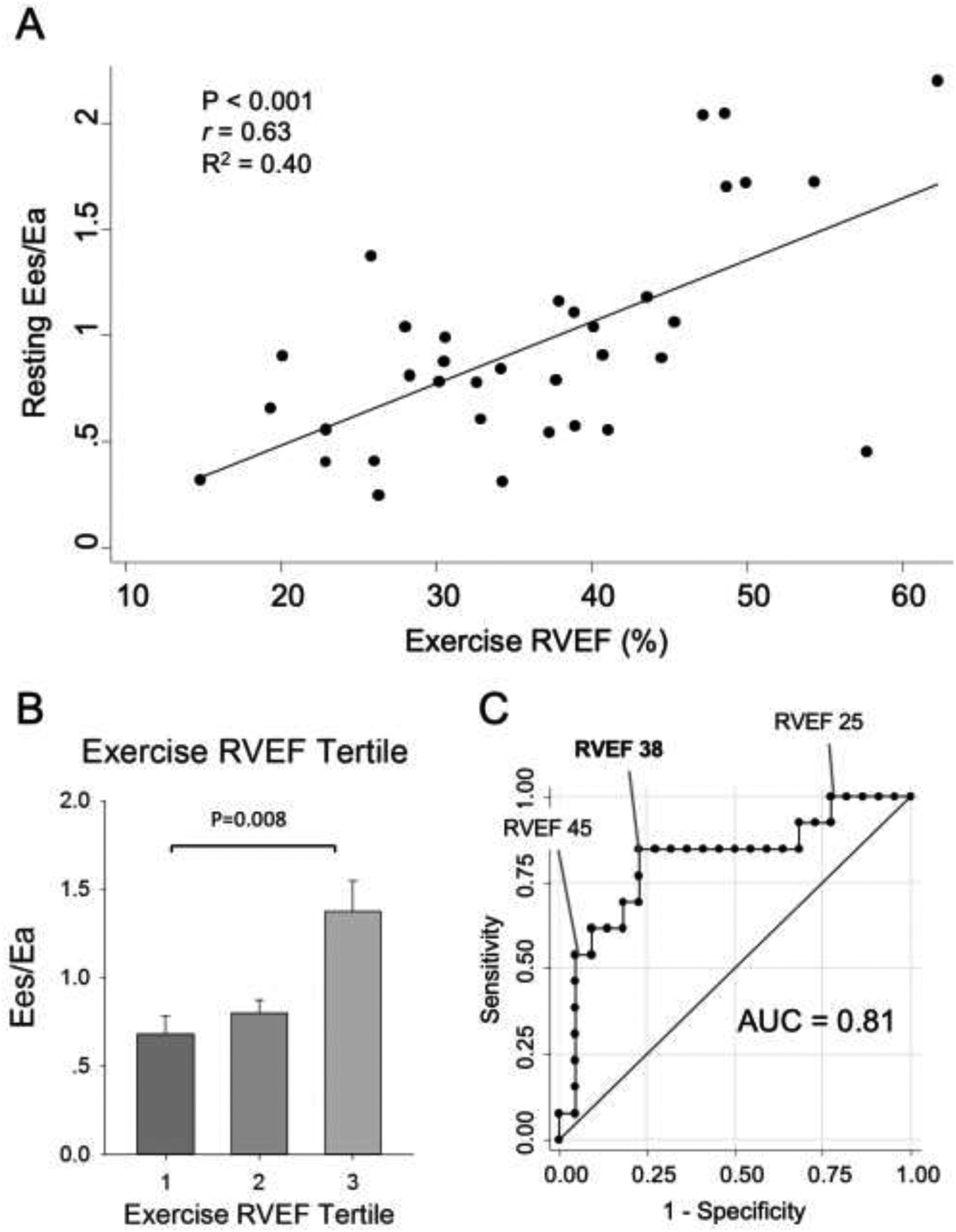
(A) Two-way scatter plot and correlation of Exercise RVEF and resting Ees/Ea (r=0.63, P<0.001). (B) Full cohort split into tertiles of exercise RVEF; Ees/Ea (mean±SEM) shown for each tertile. Only the highest (i.e., third) tertile of exercise RVEF had a preserved Ees/Ea; even a small decline in exercise RVEF signifies worsening resting Ees/Ea. (C) Exercise RVEF showed excellent ability for predicting occult RV dysfunction (Ees/Ea<1.0) with AUC=0.81. Exercise RVEF <38% had optimal sensitivity (85%) and specificity (77%).
RESULTS
Study population.
Table 1 summarizes baseline demographics, imaging, and hemodynamic characteristics. The cohort comprised subjects with known PH or exertional dyspnea and suspicion of pulmonary vascular disease referred for RHC. Subjects averaged 57±2 years of age and were predominantly female (83%). The majority (69%) had World Health Organization (WHO) group I PAH: 43% had connective tissue disease associated-PAH (CTD-PAH) while 26% had idiopathic PAH. Beyond Group I PAH, another two had Group II or III PH (6%). Nine subjects did not meet diagnostic criteria for any resting PH (26%), but of these nine, five met criteria for exercise-induced PH.18 Among all subjects, most were either WHO functional class II–III (51% and 43% respectively). By CMR, average RVEF was 51±2%, a value within normal range. Average RVEDV was 160±8 ml. During resting RHC, the average cardiac output (CO) was 5.1±0.3 L/min, mean PA pressure (mPAP) 34±2 mmHg, pulmonary vascular resistance (PVR) 6.0±1.0 WU. Resting Ees/Ea was 0.96±0.09.
TABLE 1.
Demographics, Imaging, and Hemodynamic characteristics
| Demographics | Resting Hemodynamic Data | ||
| Age (years) | 57.3 (13.9) | Resting heart rate (min−1) | 73 (12) |
| Female Sex (n, %) | 30/35, 86% | Systolic blood pressure (mm Hg) | 128 (18) |
| Race (n, %) | Diastolic blood pressure (mm Hg) | 71 (12) | |
| Caucasian | 29/35, 83% | Mean arterial pressure (mm Hg) | 93 (12) |
| African American | 4/35, 11% | Body surface area (m2) | 1.86 (0.24) |
| Other | 2/35, 6% | TD cardiac output (L/min) | 4.66 (0.99) |
| PA O2 saturation (%) | 68 (5) | ||
| Disease Characteristics | Hemoglobin (g/dl) | 13.0 (1.8) | |
| Disease subtype (n, %) | Right atrial pressure (mm Hg) | 7 (4) | |
| IPAH | 9/35, 26% | PA systolic pressure (mm Hg) | 56 (23) |
| CTD-PAH | 15/35, 43% | PA diastolic pressure (mm Hg) | 21 (10) |
| PH, Group 2/3 | 2/35, 6% | Mean PA pressure (mm Hg) | 34 (14) |
| No PH | 9/35, 26% | PA wedge pressure (mm Hg) | 10 (4) |
| WHO Functional Capacity | PVR (Wood units) | 6.0 (5.8) | |
| Class I | 2/35, 6% | RA/PAWP Ratio | 0.67 (0.33) |
| Class II | 18/35, 51% | LV Transmural Filling Pressure | 3 (3) |
| Class III | 15/35, 43% | Ees (mm Hg/ml) | 0.67 (0.39) |
| Class IV | 0/35, 0% | Ea (mm Hg/ml) | 0.84 (0.57) |
| Ees/Ea | 0.96 (0.52) | ||
| Cardiac MRI | V0 (ml) | −6 (44) | |
| LV ejection fraction (%) | 62 (6) | ||
| RV ejection fraction (%) | 51 (10) | Cardiopulmonary Exercise Data | |
| RV end-diastolic volume (ml) | 160 (49) | Exercise time (min) | 7.9 (4.1) |
| RV end-systolic volume (ml) | 80 (36) | Peak VO2 (ml/min/kg) | 10.9 (2.9) |
| RV SV/ESV | 1.13 (0.47) | VE/VCO2 | 51.7 (19.7) |
| RV mass (gm) | 27.2 (11.0) | Maximum workload (W) | 46 (22) |
IPAH, idiopathic PAH; CTD-PAH, connective tissue disease-associated PAH; WHO, World Health Organization; MRI, magnetic resonance imaging; LV, left ventricular; SV/ESV, ratio of stroke volume to end-systolic volume; TD, thermodilution; PA, pulmonary arterial; PVR, pulmonary vascular resistance; Ees, end-systolic elastance; Ea, effective arterial elastance; V0, x-intercept of Ees; peak VO2, peak oxygen consumption; VE/VCO2, ventilatory efficiency.
Effects of RV contractile reserve.
RV contractile reserve was defined by a positive change in RV Ees during exercise. In our cohort, 20 subjects demonstrated RV contractile reserve while 15 subjects exhibited no RV contractile reserve. The groups did not significantly differ with respect to demographics, PH status or disease subtype, resting hemodynamics, or imaging (Supplementary Table 1). Resting measures of RV function, including RVEF and the RV stroke volume-to-end-systolic volume (SV/ESV) ratio,19 were also similar (Supplementary Table 1).
We next assessed how RV reserve affected pressure, flow, and resistance during exercise (Figure 2). Mean PA pressures, wedge pressure, and output rose similarly with exercise, with no difference between reserve groups. No statistical differences were detected in PVR between groups or with exercise. End-systolic pressure (ESP) and effective arterial elastance (Ea) both rose significantly with exercise, but did so similarly in both groups. RV Ees augmented significantly in the group with contractile reserve, but stayed roughly unchanged in the group without (P=0.64 versus baseline), as would be expected. Driven by a differential Ees response to exercise, RV-PA coupling, as indexed by Ees/Ea, was also significantly different between groups.
RV reserve groups differed more in their volumetric response to exercise. RV end-systolic volume (ESV) and end-diastolic volume (EDV) increased significantly in the group without RV contractile reserve. Stroke volume remained similar between groups. However due to the dilation, RVEF in the cohort without RV reserve fell significantly during exercise, but remained unchanged in the group with RV reserve. These volume differences were specific to the presence or absence of RV reserve, and were not a function of a diagnosis of PH. Given the relationship between RV contractile reserve and dilation we investigated the relationship between them. Interestingly, we found that exercise changes in RV Ees and RVEDV were closely related to one another in inverse fashion (P<0.001, R2=0.76, Figure 3).
FIGURE 3. Relationship between Exercise Changes in RV End-Systolic Elastance and End-Diastolic Volume.
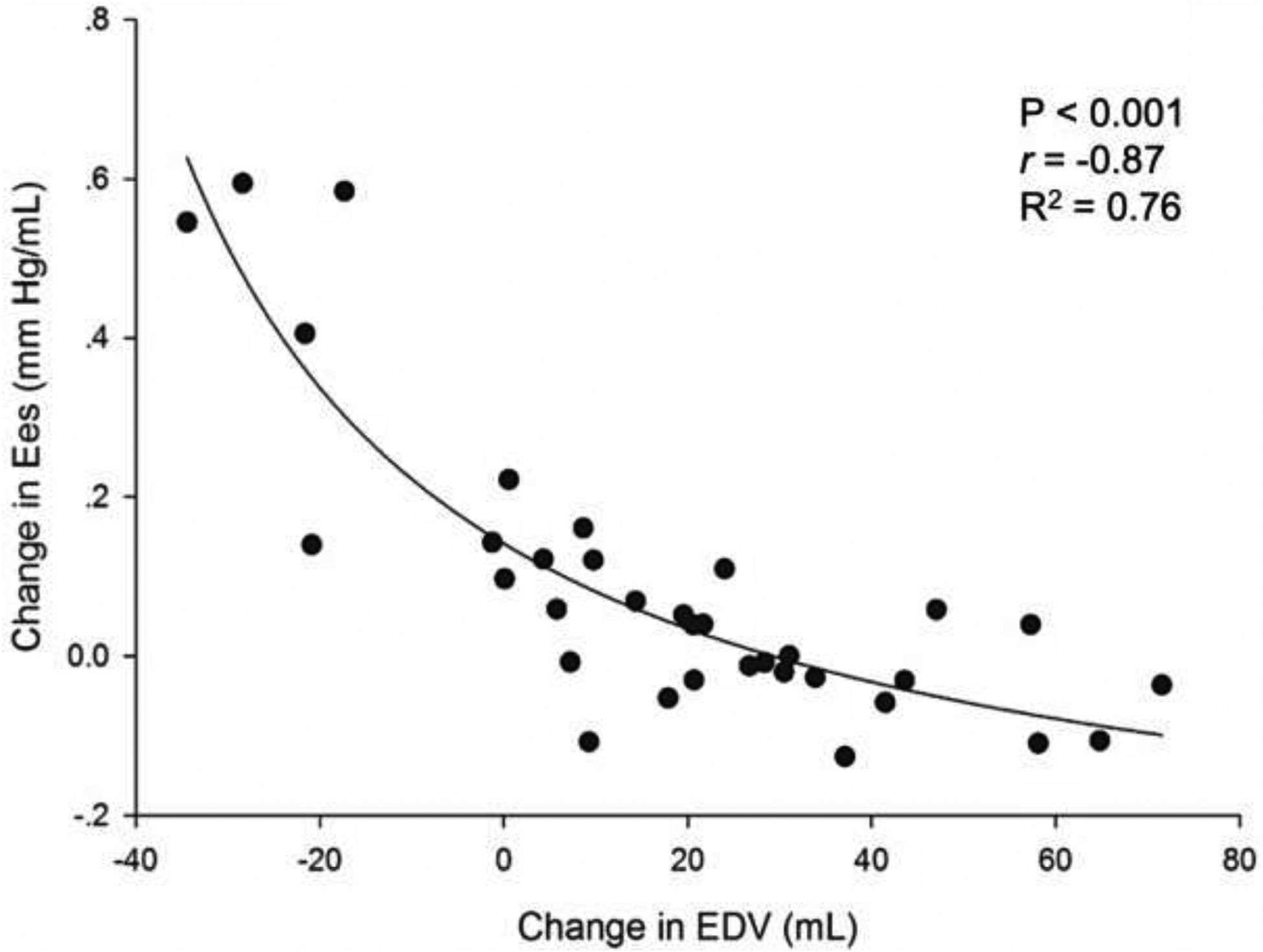
Non-linear regression shows an inverse hyperbolic relationship between RV dilation (change in EDV) and RV contractile reserve (change in Ees) during exercise.
RV contractile reserve correlates with occult RV dysfunction and clinical outcomes.
We next assessed the ability of RV contractile reserve to predict occult RV dysfunction. Both RV contractile reserve, as measured by change Ees during exercise, and RV dilation were significantly correlated to resting RV-PA coupling. Both also had reasonable ability to predict occult RV dysfunction as defined by early RV-PA uncoupling (Ees/Ea<1.0) (Table 2). Moreover, both were superior to resting measures of RV dysfunction (such as RVEF and SV/ESV) in predicting early RV-PA uncoupling (Supplementary Table 2).
TABLE 2.
RV contractile reserve measures correlate to resting RV-PA coupling
| Variable | Beta-Coefficient | r | P-value | AUC (Ees/Ea <1.0) |
|---|---|---|---|---|
| Change in RV Ees | 0.204 | 0.57 | < 0.001 | 0.84 |
| Change in RVEDV | −0.007 | 0.39 | 0.021 | 0.78 |
| Exercise RVEF | 2.914 | 0.63 | < 0.001 | 0.81 |
Univariate regression modeling the association between exercise RV reserve measures and resting RV-PA coupling (Ees/Ea). If regression significant, an area under the curve (AUC) for predicting early uncoupling (Ees/Ea <1.0) was calculated. Ees, end-systolic elastance; RV end-diastolic volume, RVEDV; RVEF, right ventricular ejection fraction.
Clinically, however, measuring a change in RV Ees would be challenging. This prompted investigation of a suitable replacement. Exercise RVEF simultaneously captures changes in both RV dilation and RV Ees by reflecting changes in RVEDV and RVESV, respectively. Exercise RVEF also does not rely on pressure measurements. Given this, we tested the capacity of exercise RVEF as an index of RV contractile reserve. Indeed, we found that sub-maximal exercise RVEF (measured via PV loop at 25W workload) correlated well with resting Ees/Ea (P<0.001, r=0.63, Table 2, Figure 4A); and remained predictive even when adjusted for other univariate predictors (P=0.004, Table 3). Preserved exercise RVEF was particularly good at identifying those with preserved resting Ees/Ea: once exercise RVEF began to fall, average Ees/Ea fell below 1.0 (Figure 4B). Consistent with these, exercise RVEF had good ability to discriminate occult RV dysfunction (Ees/Ea <1.0) with an AUC=0.81 (Table 2 and Figure 4C). Based on Youden Index analysis, a sub-maximal exercise RVEF of <38% optimally predicted RV-PA uncoupling, with 85% sensitivity and 77% specificity (Figure 4C). By contrast, a resting RVEF of <38% had 72% sensitivity and 0% specificity for detecting Ees/Ea <1.0 (data not shown).
TABLE 3.
Exercise RVEF predicts resting Ees/Ea independent of other predictors
| Variable | Beta-Coefficient | Std. Error | P-value | 95% Conf. Int. |
|---|---|---|---|---|
| Exercise RVEF (25W) | 2.460 | 0.787 | 0.004 | 0.842, 4.079 |
| Resting RVEF | 0.003 | 0.008 | 0.690 | −0.014, 0.021 |
| TDCO (L/min) | 0.142 | 0.131 | 0.288 | −0.127, 0.410 |
| VE/VCO2 | 0.001 | 0.004 | 0.988 | −0.009, 0.009 |
| PVR (Wood units) | 0.813 | 0.023 | 0.577 | −0.035, 0.615 |
| Peak TDCO (L/min) | 0.018 | 0.024 | 0.449 | −0.031, 0.068 |
| Intercept | −1.037 | 0.759 |
Multivariate analysis reveals that exercise RVEF remains predictive of Ees/Ea independent of all other predictors of Ees/Ea. Variables that shared collinearity with others (PAC, SV/ESV) were excluded. Thermodilution cardiac output (TDCO); VE/VCO2, ventilatory efficiency; PVR, pulmonary vascular resistance.
We next tested whether reduced exercise RVEF correlated with clinical worsening. Over four years, 5 subjects experienced 4 hospitalizations, 1 death, and 1 transplant. In Cox proportional hazards modeling, sub-maximal exercise RVEF predicted time to clinical worsening [Hazard Ratio 2.5 per 10% decline in exercise RVEF; 95% CI(1.0, 6.6), P=0.05]. Lastly, the cohort was divided into two groups by the median exercise RVEF of 38% (Figure 4). Subjects with exercise RVEF <38% demonstrated increased propensity for clinical worsening over 4 years (log rank P=0.014) (Figure 5).
FIGURE 5. Exercise RVEF predicts clinical worsening.
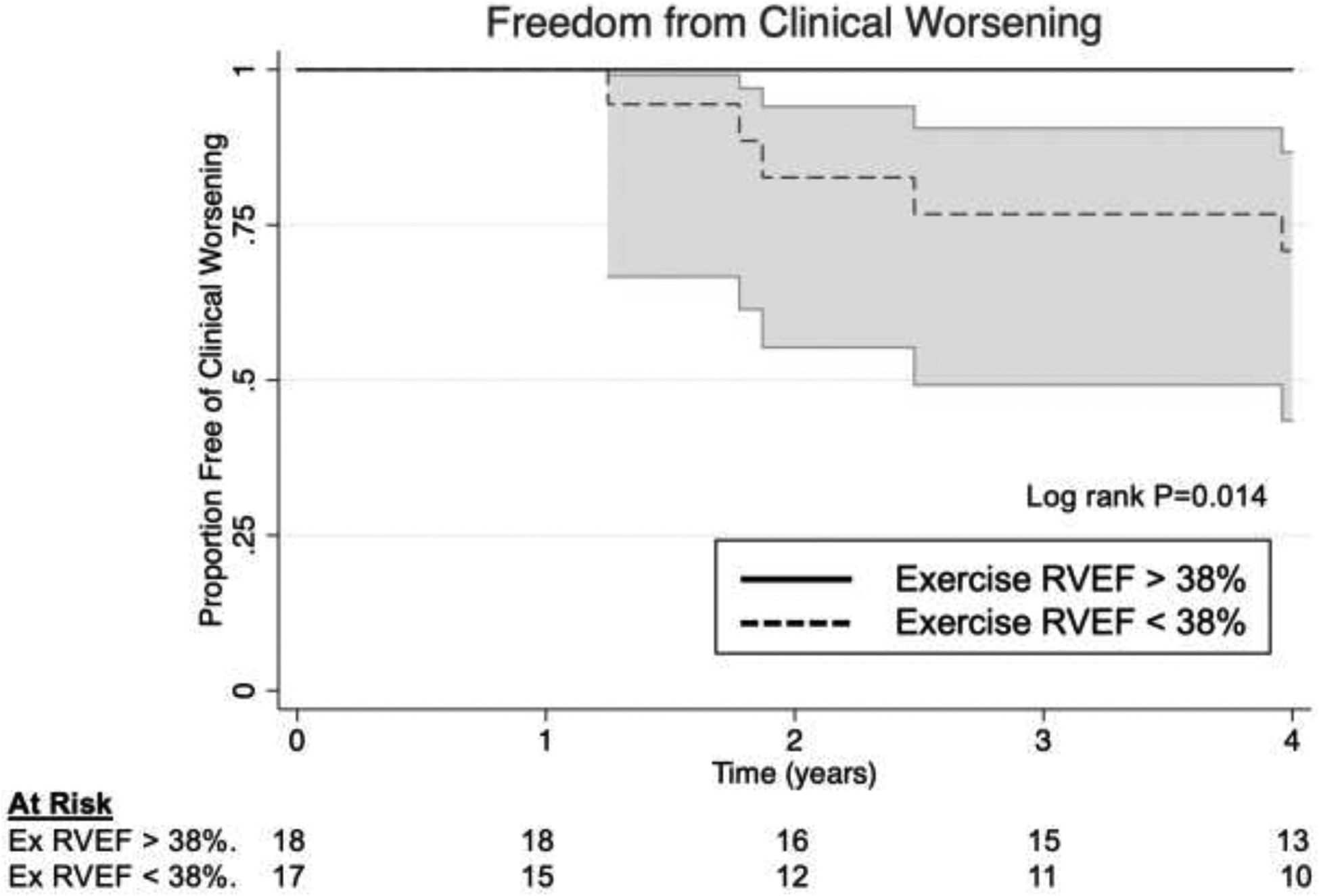
Cohort dichotomized by median exercise RVEF (38%). Subjects with exercise RVEF>38% were free of clinical worsening over the 4-year follow-up period (log rank P=0.01).
DISCUSSION
Although RV contractile reserve shows clinical promise, it remains unclear how best to measure it in the clinical setting.4,6,20 In this prospective study, we utilize RV PV loop measures during exercise to reveal poor RV contractile reserve correlates inversely with RV dilation during exercise. Based on this observation, we found that exercise RVEF, which captures exercise changes in RV ESV and RV EDV, respectively, proved to be an excellent index of RV contractile reserve. Exercise RVEF correlated well with resting RV-PA coupling, showed good ability to identify occult RV dysfunction (Ees/Ea<1.0) by ROC analysis, and predicted important clinical outcomes. Exercise RVEF therefore demonstrates potential as a clinical index of RV contractile reserve and occult RV dysfunction.
Currently available measures of RV dysfunction, such as RV dilation and systolic dysfunction, are acquired when the RV is at rest. Because of RV adaptability, these measures only become abnormal in the late disease, when the RV-PA unit is fully uncoupled (Ees/Ea <0.8).21 This was well demonstrated by Dr. Tello and colleagues, who found that low resting RVEF and decreased SV/ESV correlated well with Ees/Ea of <0.80 in human PAH.2 The present study also found similar relationships between Ees/Ea and indices of resting RV dysfunction—namely RVEF, SV/ESV, RA/PAWP, TAPSE,22 TAPSE/SPAP,23 and PAPi.24,25 However, none were able to identify early or occult RV dysfunction as measured by Ees/Ea <1.0.
Beyond resting RV measures, stressing the RV to assess its contractile reserve can better unmask early RV-PA dysfunction. The notion of a stress test to uncover occult dysfunction is common in cardiovascular physiology, and routinely employed to unmask coronary artery and valvular disease states. For the RV, exercise is a particularly suitable stressor, since it augments venous return, chronotropy, and RV wall stress.5 To accommodate these changes, the RV must tap into its reserve to augment contractility.5,26 Exercise is thus particularly suited to uncover RV contractile deficits unseen at rest. RV contractile reserve proves especially insightful in the setting of PH, where pathologic increases in pulmonary resistance impose an even greater load on the RV.27
The present study shows that poor exercise RV contractile reserve and RV dilation were both independently correlated to resting RV-PA uncoupling. Furthermore, exercise changes in RV Ees and RVEDV were inversely related to one another. This link highlights a mechanism by which subjects without RV contractile reserve are still able to maintain reasonable stroke volume, but at the price of RV dilation, increased pressure-volume area, and wasted mechanical work.12 Moreover, compensation only helped during early exercise. Reduced peak performance in the poor RV reserve group suggests that these early-stage compensatory mechanisms cannot sustain a full range of exercise tolerance.
Exercise RVEF proved to be a simpler way to capture changes in both RV Ees and RVEDV. Exercise RVEF was comparable to RV Ees in its ability to predict early uncoupling, and furthermore was associated with clinical outcomes. The mechanistic relationship between exercise RVEF, change in Ees, and RV dilation is illustrated in Figure 1. Subject A, who has excellent RV contractile reserve, augments RV Ees; RVESV thus falls while RVEDV remains stable. Therefore, subject A actually augments RVEF during exercise (SV/EDV increases). Subject B has pulmonary vascular disease but still has RV contractile reserve. There is a pathologic increase in Ea during exercise, but Ees augments as well, thereby maintaining RV volumes and preserved RVEF. Subject C, on the other hand, lacks RV contractile reserve. Thus, RV Ees fails to augment as Ea rises, leading to an acute increase in RVEDV. The net effect is an acute decrease in exercise RVEF. All three subjects have preserved RVEF at rest, but only the subject without RV contractile reserve drops RVEF during exercise. These cases help to illustrate how exercise RVEF, by capturing EDV changes and Ees-related changes in ESV during exercise, can identify occult RV dysfunction unapparent based on resting RVEF alone.
Exercise RVEF in this study was measured using invasive PV loops. The current study does not propose the clinical application of PV loop-derived exercise RVEF. Instead, our findings serve as a proof of concept for future studies. Existing studies do suggest that we can leverage non-invasive measurements of exercise RVEF. Exercise echocardiography as well as 3-dimentsional echo have both been used to assess RV contractile reserve.4,20,28 Exercise testing with RV nuclear imaging has also been utilized: DeFaria Yeh and colleagues demonstrated, using radionuclide imaging in adult congenital heart disease, that maximal RVEF and RVEF augmentation strongly predicted adverse outcomes.29 Finally, CMR is considered by many to be the best way to assess the RV, and MRI-compatible ergometers allow for CMR use during exercise.30 Exercise CMR has also shown prognostic benefit in PAH patients and other diseases.31–34 Future studies can and should prospectively test the validity of non-invasive exercise RVEF in predicting early RV decline and clinically relevant outcomes.
The present study may also impact clinical scenarios beyond pulmonary hypertension. A measure of subtle RV dysfunction may prove useful in light of newly proposed definitions of pulmonary vascular disease, which have lowered the mPAP cutoff to >20 mmHg.35,36 Also, emerging evidence suggests that RV reserve has prognostic and diagnostic value in left-sided heart disease and management post-left ventricular assist device.37–39 Exercise RVEF may thus prove useful in the assessment of occult RV dysfunction that could be unmasked if left heart disease patients undergo interventions that place undue stress upon the RV.
Study Limitations.
Our study has several limitations. One limitation is sample size, which was a function of the invasiveness of the study. While this raises the possibility of type II error, it did not impact our main conclusions. RVEF during exercise was measured using volumes derived from PV catheterization, and not by exercise CMR. However, since resting PV loop volumes rely on MRI-calibrated volume and there was no exercise shift of the PV catheter, these calculated volumes were likely close to CMR-derived values. The V0 from rest was applied to PV loops during exercise to calculate exercise changes in RV Ees. This is an assumption rooted in the fact that V0 for a given subject should not change acutely. This assumption did not affect relative changes in volume during exercise. Finally, the study cohort included subjects with a range of pulmonary vascular disease, including pulmonary arterial hypertension of various sub-types, other forms of PH, and exercise-induced PH. While this added heterogeneity to the cohort, it also helped to improve the range of RV contractile function studied and broaden the generalizability of our RV reserve findings.
Conclusions.
Poor RV contractile reserve during exercise is associated with acute RV dilation. Exercise RVEF, which captures changes in both RV end-systolic elastance and RV dilation during exercise, proved to be an excellent index of RV contractile reserve. Exercise RVEF correlated well with resting RV-PA uncoupling, predicted occult RV dysfunction, and predicted clinical worsening. Exercise RVEF may thus prove to be a useful surrogate of RV contractile reserve, and a predictor of occult RV-PA dysfunction as measured by early RV-PA uncoupling. Future studies should test the ability of non-invasively measured exercise RVEF to assess for occult RV-PA dysfunction and predict clinical outcomes.
Supplementary Material
FINANCIAL DISCLOSURE STATEMENT
Funding sources include: National Heart, Lung, and Blood Institute of the National Institutes of Health (K23-HL146889-01, S.H.; R01-HL114910-05, D.A.K., P.M.H., R.J.T.), Scleroderma Research Fund (A.A.S., F.M.W.), and Jerome Greene Scholarship (S.H.).
R.J.T. serves as a hemodynamic core lab for Actelion and Merck, a consultant and steering committee member for Medtronic, and consultant for Abiomed, Arena Pharmaeuticals, and United Therapeutics. S.C.M. serves as a consultant for Actelion, Arena Pharmaceuticals, Liquidia, and United Therapeutics. All other authors have no relevant conflicts of interest for the present work.
Non-standard abbreviations:
- RV-PA coupling
Right ventricular-pulmonary arterial coupling
- Ees
End-systolic elastance
- Ea
Effective arterial elastance
- V0
V-naught, or the x-intercept of the end-systolic elastance slope
- PV
pressure-volume
- RVEF
RV ejection fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J Am Coll Cardiol. 2017;69(2):236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 2.Tello K, Dalmer A, Axmann J, et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ Heart Fail. 2019;12(1):e005512. doi: 10.1161/CIRCHEARTFAILURE.118.005512. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 4.Grünig E, Tiede H, Enyimayew EO, et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation. 2013;128(18):2005–2015. doi: 10.1161/CIRCULATIONAHA.113.001573. [DOI] [PubMed] [Google Scholar]
- 5.Spruijt OA, de Man FS, Groepenhoff H, et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2015;191(9):1050–1057. doi: 10.1164/rccm.201412-2271OC. [DOI] [PubMed] [Google Scholar]
- 6.Hsu S, Houston BA, Tampakakis E, et al. Right Ventricular Functional Reserve in Pulmonary Arterial Hypertension. Circulation. 2016;133(24):2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6(5):953–963. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rommel K-P, Roeder von M, Oberueck C, et al. Load-Independent Systolic and Diastolic Right Ventricular Function in Heart Failure With Preserved Ejection Fraction as Assessed by Resting and Handgrip Exercise Pressure-Volume Loops. Circ Heart Fail. 2018;11(2):e004121. doi: 10.1161/CIRCHEARTFAILURE.117.004121. [DOI] [PubMed] [Google Scholar]
- 10.Hsu S, Kokkonen-Simon KM, Kirk JA, et al. Right Ventricular Myofilament Functional Differences in Humans with Systemic Sclerosis-associated versus Idiopathic Pulmonary Arterial Hypertension. Circulation. 2018;137(22):2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter MJ, Peters D, Ghofrani HA, et al. Evaluation and Prognostic Relevance of Right Ventricular-Arterial Coupling in Pulmonary Hypertension. Am J Respir Crit Care Med. September 2019:rccm.201906–1195LE. doi: 10.1164/rccm.201906-1195LE. [DOI] [PubMed] [Google Scholar]
- 12.Vonk Noordegraaf A, Chin KM, Haddad F, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53(1):1801900. doi: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fourie PR, Coetzee AR, Bolliger CT. Pulmonary artery compliance: its role in right ventricular-arterial coupling. Cardiovasc Res. 1992;26(9):839–844. doi: 10.1093/cvr/26.9.839. [DOI] [PubMed] [Google Scholar]
- 14.Burkhoff D, Sagawa K. Influence of pacing site on canine left ventricular force-interval relationship. Am J Physiol. 1986;250(3 Pt 2):H414–H418. doi: 10.1152/ajpheart.1986.250.3.H414. [DOI] [PubMed] [Google Scholar]
- 15.Hsu S Coupling Right Ventricular-Pulmonary Arterial Research to the Pulmonary Hypertension Patient Bedside. Circ Heart Fail. 2019;12(1):e005715. doi: 10.1161/CIRCHEARTFAILURE.118.005715. [DOI] [PubMed] [Google Scholar]
- 16.Hoeper MM, Bogaard H-J, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Hsu S, Simpson CE, Houston BA, et al. Multi-Beat Right Ventricular-Arterial Coupling Predicts Clinical Worsening in Pulmonary Arterial Hypertension. J Am Heart Assoc. 2020;9(10):e016031. doi: 10.1161/JAHA.119.016031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hervé P, Lau EM, Sitbon O, et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J. 2015;46(3):728–737. doi: 10.1183/09031936.00021915. [DOI] [PubMed] [Google Scholar]
- 19.Breeman KTN, Dufva M, Ploegstra MJ, et al. Right ventricular-vascular coupling ratio in pediatric pulmonary arterial hypertension: A comparison between cardiac magnetic resonance and right heart catheterization measurements. Int J Cardiol. 2019;293:211–217. doi: 10.1016/j.ijcard.2019.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma T, Lau EMT, Choudhary P, et al. Dobutamine stress for evaluation of right ventricular reserve in pulmonary arterial hypertension. European Respiratory Journal. 2015;45(3):700–708. doi: 10.1183/09031936.00089914. [DOI] [PubMed] [Google Scholar]
- 21.Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(12):1463–1482. doi: 10.1016/j.jacc.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 22.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 23.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305(9):H1373–H1381. doi: 10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 24.Korabathina R, Heffernan KS, Paruchuri V, et al. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheterization and Cardiovascular Interventions. 2012;80(4):593–600. doi: 10.1002/ccd.23309. [DOI] [PubMed] [Google Scholar]
- 25.Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK. Pulmonary Artery Pulsatility Index Is Associated With Right Ventricular Failure After Left Ventricular Assist Device Surgery. Journal of Cardiac Failure. 2016;22(2):110–116. doi: 10.1016/j.cardfail.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 26.La Gerche A, Roberts T, Claessen G. The response of the pulmonary circulation and right ventricle to exercise: exercise-induced right ventricular dysfunction and structural remodeling in endurance athletes (2013 Grover Conference series). Pulm Circ. 2014;4(3):407–416. doi: 10.1086/677355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naeije R, Vanderpool R, Dhakal BP, et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187(6):576–583. doi: 10.1164/rccm.201211-2090CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jone P-N, Schäfer M, Pan Z, Ivy DD. Right Ventricular-Arterial Coupling Ratio Derived From 3-Dimensional Echocardiography Predicts Outcomes in Pediatric Pulmonary Hypertension. Circ Cardiovasc Imaging. 2019;12(1):e008176. doi: 10.1161/CIRCIMAGING.118.008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFaria Yeh D, Stefanescu Schmidt AC, Eisman AS, et al. Impaired right ventricular reserve predicts adverse cardiac outcomes in adults with congenital right heart disease. Heart. 2018;104(24):2044–2050. doi: 10.1136/heartjnl-2017-312572. [DOI] [PubMed] [Google Scholar]
- 30.La Gerche A, Claessen G, Van de Bruaene A, et al. Cardiac MRI: a new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging. 2013;6(2):329–338. doi: 10.1161/CIRCIMAGING.112.980037. [DOI] [PubMed] [Google Scholar]
- 31.Claessen G, La Gerche A, Dymarkowski S, Claus P, Delcroix M, Heidbuchel H. Pulmonary vascular and right ventricular reserve in patients with normalized resting hemodynamics after pulmonary endarterectomy. J Am Heart Assoc. 2015;4(3):e001602–e001602. doi: 10.1161/JAHA.114.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claessen G, Schnell F, Bogaert J, et al. Exercise cardiac magnetic resonance to differentiate athlete’s heart from structural heart disease. Eur Heart J Cardiovasc Imaging. 2018;19(9):1062–1070. doi: 10.1093/ehjci/jey050. [DOI] [PubMed] [Google Scholar]
- 33.Claeys M, Claessen G, La Gerche A, et al. Impaired Cardiac Reserve and Abnormal Vascular Load Limit Exercise Capacity in Chronic Thromboembolic Disease. JACC Cardiovasc Imaging. 2019;12(8 Pt 1):1444–1456. doi: 10.1016/j.jcmg.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Howden EJ, Bigaran A, Beaudry R, et al. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol. 2019;26(3):305–315. doi: 10.1177/2047487318811181. [DOI] [PubMed] [Google Scholar]
- 35.Vachiery J-L, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1):1801897. doi: 10.1183/13993003.01897-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammed SF, Hussain I, AbouEzzeddine OF, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130(25):2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raina A, Meeran T. Right Ventricular Dysfunction and Its Contribution to Morbidity and Mortality in Left Ventricular Heart Failure. Curr Heart Fail Rep. 2018;15(2):94–105. doi: 10.1007/s11897-018-0378-8. [DOI] [PubMed] [Google Scholar]
- 39.Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015;34(9):1123–1130. doi: 10.1016/j.healun.2015.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


