Abstract
The outbreak of mysterious pneumonia at the end of 2019 is associated with widespread research interest worldwide. The coronavirus disease-19 (COVID-19) targets multiple organs through inflammatory, immune, and redox mechanisms, and no effective drug for its prophylaxis or treatment has been identified until now. The use of dietary bioactive compounds, such as phenolic compounds (PC), has emerged as a putative nutritional or therapeutic adjunct approach for COVID-19. In the present study, scientific data on the mechanisms underlying the bioactivity of PC and their usefulness in COVID-19 mitigation are reviewed. In addition, antioxidant, antiviral, anti-inflammatory, and immunomodulatory effects of dietary PC are studied. Moreover, the implications of digestion on the putative benefits of dietary PC against COVID-19 are presented by addressing the bioavailability and biotransformation of PC by the gut microbiota. Lastly, safety issues and possible drug interactions of PC and their implications in COVID-19 therapeutics are discussed.
Keywords: Coronavirus, SARS-CoV-2, Curcumin, Resveratrol, Quercetin, Oxidative stress, Inflammation, Immune system
Abbreviations: AAPH, 2,2′-azobis(2-amidinopropane) dihydrochloride; ACE2, angiotensin-converting enzyme 2; ADAM17, A Disintegrin and metalloproteinase 17; ANG II, angiotensin II; ARDS, acute respiratory distress syndrome; ARE, antioxidant responsive element; AT1R, Ang II-receptor type 1; CAT, catalase; 3CLPro, chymotrypsin-like protease; CA, coumaric acid; COVID-19, coronavirus disease 19; CoVs, coronaviruses; CYP, cytochrome; DNA, deoxyribonucleic acid; EA, ellagic acid; EC, (-)-epicatechin; EC50, Half maximal effective concentration; ECG, (-)-epicatechin gallate; EFSA, European food safety authority; EGC, (-)-epigallocatechin; EGCG, epigallocatechin gallate; EGFR, epidermal growth factor receptor; FA, ferulic acid; FPP, fermented papaya preparation; FRAP, ferric reducing antioxidant power; GA, gallic acid; GAE, gallic acid equivalents; GCLC, glutamate–cysteine ligase catalytic subunit; G-CSF, granulocyte-colony stimulating factor; GSH, glutathione; HBE, human bronchial epithelial cells; HMGB1, high-mobility group box 1; HepG2, hepatocellular carcinoma; IBV, infectious bronchitis virus; ICAM-1, intercellular adhesion molecule 1; IC50, half maximal inhibitory concentration; IFN, Interferon; IKK, IκB kinase; IL, interleukin; JECFA, Joint FAO/WHO Expert Committee on Food Additives; LDL, low-density lipoprotein; MCP1, monocyte chemoattractant protein 1; MERS-CoV, Middle East respiratory syndrome coronavirus; MIP1α, macrophage inflammatory protein; MLVEC, mouse lung vascular endothelial cells; mRNA, messenger RNA; MyD88, myeloid differentiation primary response 88; NK, natural killer; NQO1, NAD(P)H quinone dehydrogenase 1; Nrf2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor kappa B; NOX, NADPH oxidase; Nsp13, non-structural protein 13 helicase; OATP, organic anion transporting protein; 8-OHdG, 8-hydroxy-deoxyguanosine; OS, oxidative stress; PC, phenolic compounds; PCA, protocatechuic acid; PLPro, papain-like protease; PRR, pattern recognition receptor; PSPL, purple sweet potato leaves; QE, quercetin equivalents; RBC, red blood cells; RNA, ribonucleic acid; ROS, reactive oxygen species; S, spike; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCFA, short-chain fatty acids; sIL-6Rα, soluble form of IL-6 receptor; SOD, superoxide dismutase; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances; TEAC-CUPRAC, trolox equivalent antioxidant capacity - cupric ion reducing antioxidant capacity; TMPRSS2, transmembrane protease serine 2; TNF-α, tumor necrosis factor; TRAP, total radical-trapping antioxidant potential; Vero E6, kidney epithelial cells; WHO, World Health Organization
1. Introduction
The outbreak of severe acute respiratory syndrome at the end of 2019 has resulted in a huge health concern worldwide. The disease caused by coronavirus (COVID-19) was initiated in Wuhan (China) and has spread around the world. Therefore, the World Health Organization (WHO) declared the disease as a pandemic. Until April 28, 2021, WHO registered more than 145 million infected cases, and the number of deaths exceeded 3 million [172]. The pathogen, a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), belongs to a large family of viruses that can infect animals and humans, causing respiratory, gastrointestinal, hepatic, and neurologic diseases [168]. The SARS-CoV-2 has higher transmissibility and infectivity but a lower mortality rate, when compared with other coronaviruses (CoVs), such as those causing severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV) [93].
The majority of SARS-CoV-2-infected individuals are asymptomatic or have mild symptoms, most likely due to the activation of the immune system. However, the disease evolves into acute respiratory distress syndrome (ARDS), acute cardiac complications, multiple organ dysfunction syndromes, septic shock, and death in about 20% of infected (usually people with some comorbidity) [52]. These complications are believed to be associated with severe inflammatory and oxidative stress responses induced by viral replication [175].
Despite the severity of the disease, no effective therapy is available to improve the outcomes in patients with either suspected or confirmed COVID-19. In this context, nutritional strategies for reducing the risk or mitigating the symptoms of COVID-19 have gained considerable attention. As a non-pharmacological complementary approach, dietary supplementation of nutraceuticals and probiotics is easily available and displays no or few side effects [66,67]. In this regard, phenolic compounds (PC) have emerged as putative nutritional or adjunct therapeutics for COVID-19 because these compounds are associated with health benefits against several pathologies [47]. Moreover, PC exhibit prebiotic effects, influencing the gut microbiota and attenuating gastrointestinal complications reported in COVID-19. PC are metabolized by colonic microbiota and the resulting products may be absorbed in the gut and exert beneficial effects on several organs [149].
Despite the existing literature on the effects of PC against several viruses, only a few studies have demonstrated their action against CoVs [8,98]. A recent study reviewed the potential ability of PC in the prevention and therapeutics of COVID-19 by addressing molecular pathways modulated by PC [89]. However, this review did not discuss the impact of digestion and metabolism on the bioavailability of PC or the effects of gut microbiota-derived PC metabolites on the putative role of PC in COVID-19. Moreover, safety issues and possible drug interactions were not addressed.
This review summarizes the current evidence regarding the bioactive mechanisms of dietary PC against COVID-19 manifestations, as well as the influence of bioavailability and gut microbiota transformations on the putative effects of PC. Moreover, safety issues and the interaction of dietary PC with drugs used to mitigate certain COVID-19 manifestations have been addressed.
2. Methods
The PubMed (https://pubmed.ncbi.nlm.nih.gov) and ScienceDirect (https://www.sciencedirect.com) databases were used to search articles by a combination of terms: coronavirus, COVID-19, SARS, MERS, influenza, NF-kB, cytokine storm, immunomodulation AND phenolic compounds, anthocyanins, flavonoids, isoflavones, nutrition, phytochemicals, bioactive compounds, and oxidative stress. As this was not a systematic review, exclusion and inclusion criteria were not defined. All articles up to and including August 20, 2020, were considered, and those providing relevant data for the discussion were included in the review.
3. Overview on SARS-CoV-2 infection
CoVs are enveloped and single-stranded RNA viruses that infect a wide variety of host species. Structurally, CoVs have four structural proteins: spike (S), membrane, envelop, and nucleocapsid [181]. S protein mediates the entering of SARS-CoV-2 into the host cell through binding to the angiotensin-converting enzyme 2 (ACE2) receptor in host cells [145]. The CoV entry activates the transmembrane protease serine 2 (TMPRSS2); this, along with ACE2, is the main determinant of the entry of this virus [145].
CoV replication is mediated by RNA polymerase to produce polyproteins. These polyproteins are processed by virus proteases, papain-like protease (PLPro), and serine main protease (chymotrypsin-like protease-3CLPro). Next, viral messenger RNA (mRNA) is used to construct viral proteins (maturation) that are subsequently released [185]. Helicase (Nsp13) is a highly conserved enzyme in all CoVs and is crucial for viral replication, making it a promising target for antiviral therapies [137].
After the SARS-CoV-2 infection, the increase in viral load causes an inflammatory cytokine storm, an out-of-control cytokine release, leading to a hyperinflammatory condition in the host [96]. The nuclear factor kappa B (NF-κB) plays a significant role in regulating the expression of a multitude of genes involved with immune and inflammatory responses [176]. Once activated, the NF-κB pathway also promotes T and B cell differentiation [92,117].
One of the major pathways for NF-ĸβ activation after CoV infection is the myeloid differentiation primary response 88 (MyD88) pathway through pattern recognition receptors (PRRs). This pathway induces a variety of pro-inflammatory cytokines, including interleukin (IL)-6 and TNF-α [60,153]. ACE2 is endocytosed along with SARS-CoV-2, resulting in the reduction of ACE2 on cells, followed by an increase in serum angiotensin II (Ang II) [61]. Ang II acts both as a vasoconstrictor and pro-inflammatory cytokine via the Ang II-receptor type 1 (AT1R). The Ang II-AT1R axis activates NF-ĸβ and induces tumor necrosis factor-α (TNF-α), epidermal growth factor receptor (EGFR), and soluble form of IL-6 receptor (sIL-6Rα) via disintegrin and metalloprotease 17 (ADAM17) [60,61,153]. Thus, the higher the viral load, the lower the concentration of ACE-2 due to virus binding, which causes increased levels of Ang II in the serum, thus activating the NF-ĸβ pathway. Certain glucocorticoids, such as methylprednisolone, prednisone, and dexamethasone, have been reported to inhibit NF-κβ activation and are used in the management of COVID-19 in several countries [150]. Thus, substances with this same mechanism of action would be important putative agents for containing this disease.
The overproduction of reactive oxygen species (ROS) and deprivation of antioxidant mechanisms are crucial events for viral replication and the subsequent virus-associated disease [21,33]. In addition, variations in cellular pH, decrease in reduced glutathione (GSH) levels, and the activity of NADPH oxidase (NOX) family are important events. The NOX4-derived ROS production is modulated by ACE2 [21,33]. Furthermore, free radicals, such as superoxide anion radical (O2 •–), chlorine oxide (ClO–), nitric oxide (NO), and peroxynitrite (ONOO–) could be the cause of virus-induced pneumonia death [173]. In addition, oxidative stress occurs not only due to ROS released but also due to pro-oxidant cytokines, such as TNF-α and IL-1, released by phagocyte activation [141].
Oxidative stress plays a crucial role in the pathogenesis of COVID-19. It perpetuates the cytokine storm as well as exacerbates hypoxia, including mitochondrial dysfunction [18]. The interplay between ROS and cytokine storm generates a self-sustaining cycle between the cytokine storm and oxidative stress, leading to multiorgan failure in severe COVID-19 patients whose condition progresses to sepsis and shock [18,173].
The Nrf2-mediated antioxidant system is an essential mechanism to protect cells from oxidative injury. Under oxidative stress, the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) is translocated to the nucleus and coordinately activates cytoprotective genes against oxidative stress (OS) by binding to antioxidant responsive element (ARE) in the promoter region of DNA. In addition, Nrf2 regulates the genes involved in immunity and inflammation, as well as in the mechanisms affecting viral susceptibility and replication of respiratory and non-respiratory infections [73,79,121,152,39,86].
Once COVID-19 has been shown to target multiple organs through inflammatory, immune, and redox mechanisms, dietary bioactive compounds that modulate these mechanisms could be a nutritional alternative to control the disease severity.
4. Potential role of PC on SARS-CoV-2 manifestations
PC have at least one aromatic ring with one or more hydroxyl groups attached. According to their chemical structure, they can be divided into several classes: phenolic acids, tannins, lignans, flavonoids, stilbenes, coumarins, and curcuminoids (Supplementary material, Fig. S1). They are products of the secondary metabolism of plants, providing essential functions, including protecting plants against herbivores and microbial infection, attraction for pollinators and seed-dispersing animals, allelopathic effects, UV protection, and signal molecules during the formation of nitrogen-fixing root nodules [56,32]. In the human diet, PC are responsible for the health-promoting effects due to their antioxidant, anti-inflammatory, immune, and prebiotic properties [151]. Increasing evidence suggests that modest long-term intakes of PC can have favorable effects on the incidence of chronic diseases ([114]; Paquette, 2017; [130]). Despite a few human intervention studies on the effect of PC to prevent and possibly treat COVID-19, these compounds have already been reported to present antiviral activity against CoV infection as well as strong antioxidant and anti-inflammatory properties, suggesting their potential role in mitigating this infectious disease.
4.1. Antiviral effect of PC against COV infections
A good antiviral agent should prevent the growth of viruses in infected cells by inhibiting their attachment, penetration, uncoating, genome replication, and gene expression. Table 1 summarizes the studies on antiviral effects of PC against CoVs.
Table 1.
Antiviral effects of dietary PC against CoVs
| PC | Concentration | Model | Virus type | Mechanism of action | Reference |
|---|---|---|---|---|---|
| Curcumin | EC50 = 10–40 µM | Vero E6 cells | SARS-CoV |
|
[169] |
| Theaflavin-3,3’-digallate | IC50 < 10 µM | Peptide cleavage assay | SARS-CoV |
|
[22] |
| EGCG | IC50 = 73 µM | FRET assay | SARS-CoV |
|
Nguyen et al., 2012 |
| Papyriflavonol A and quercetin | IC50 = 3.7 and 8.6 µM |
Purified protease | SARS-CoV |
|
[124] |
| Resveratrol | 62.5–250 µM | Vero E6 cells | MERS-CoV |
|
[91] |
| Luteolin | 10.6 µM | Vero E6 cells | SARS-CoV |
|
[182] |
| Myricetin and scutellarein | 1–10 µM | Colorimetry-based ATP hydrolysis assa | SARS-CoV |
|
[183] |
| Forsythoside A | 160–640 µM | Chicken embryo kidney cells | IBV |
|
[90] |
3CLPro, chymotrypsin-like cysteine protease; CoVs, coronavirus; EC50, Half maximal effective concentration; EGCG, epigallocatechin gallate; FRET, fluorescence ressonance energy transfer; IBV, avian infectious bronchitis; IC50, half maximal inhibitory concentration; MERS-CoV, Middle East respiratory syndrome coronavirus; PLPro = papain-like cysteine protease; SARS-CoV, SARS-CoV, severe acute respiratory syndrome coronavirus.
4.1.1. Tea PC
PC are the main bioactive components of Camellia sinensis L., whose leaves are used for green and black tea preparation [36]. The antiviral activity of green tea and black tea PC in the prophylaxis and treatment of COVID-19 has been recently reviewed [112].
Molecular docking studies (computational procedures for searching ligands that fit into the protein's binding site) have revealed 3-isotheaflavin-3-gallate, theaflavin-3,3-digallate, and tannic acid as effective 3CLPro inhibitors (IC50 < 10 µM) [22], which would putatively affect CoV replication. Researchers reported that the gallate group attached to the 3’ position is important for interaction with 3CLPro. Another recent in silico study revealed the strong interaction of epigallocatechin gallate (EGCG), epicatechin gallate (ECG), and gallocatechin-3-gallate (GCG) with one or both catalytic residues of 3CLPro [54]. Moreover, the complexes between protease and these PC were predicted to be highly stable. Theaflavin, the compound responsible for the orange/black color of black tea, is a potent inhibitor of the RNA polymerase of SARS-CoV-2 [94]. Catechin gallate (CG) and gallocatechin gallate (GCG) showed high inhibitory activity against SARS-CoV-2 N protein in a concentration-dependent manner and affected virus replication. These PC at a concentration of 0.05 µg/mL showed more than 40% inhibitory activity on a quantum dots-conjugated RNA oligonucleotide-designed chip [136].
4.1.2. Curcumin
Curcumin has been suggested as a potential treatment option for patients with COVID-19 [187] because it inhibits ACE2 and suppresses the entry of SARS-CoV-2 into the cells [158]. In another molecular docking study, curcumin exhibited an inhibitory effect on SARS-CoV-2 S protein and its cellular receptor ACE2, with a higher affinity than drugs such as nafamostat and hydroxychloroquine [105]. At an EC50 of higher than 10 µM, curcumin inhibited virus replication by reducing the number of S proteins present in the culture of Vero E6 cells infected with SARS-CoV [169].
4.1.3. Resveratrol
The protective effect of resveratrol against multiple viruses has been recently reviewed [1]. Resveratrol stably binds to the viral protein/ACE2 receptor complex of SARS-CoV-2, indicating it to be a promising agent against COVID-19 by disrupting the virus S protein [162]. In addition, the stilbene diminished the expression of N protein in SARS-CoV-2 and reduced the apoptosis of Vero E6 cells. Moreover, resveratrol alleviated the Vero E6 cell death induced by MERS-CoV, most likely due to an antiviral effect because the MERS-CoV RNA and virus titer levels were lower in resveratrol-treated cells (150–250 µM) [91].
4.1.4. Quercetin and related PC
A recent review presented evidence for the use of quercetin along with vitamin C in the therapeutics and prophylaxis of COVID-19 (Colunga [15]). Quercetin was identified by the supercomputer SUMMIT drug-docking screen and Gene Set Enrichment Analyses of expression profiling experiments as a good therapeutic candidate against SARS-CoV-2 infection [55]. According to this system, quercetin inhibited the expression of several potential COV infection-promoting genes [55]. In addition, docking studies demonstrated that myricetin and the myricetin-containing phytomedicine Equivir bind to ACE2 receptor and prevented SARS-CoV-2-induced COVID-19 [119]. Quercetin inhibited 3CLPro from MERS-CoV (IC50 = 34.8 µM), whereas no inhibitory activity was detected against MERS-CoV PLPro [124]. Other PC related to quercetin, such as myricetin and scutellarin, exhibited inhibitory action against SARS-CoV helicase [183].
Luteolin, a PC structurally related to quercetin, effectively inhibited the entry of wild-type SARS-CoV into Vero E6 cells [182]. In a recent study, the Chinese medicine Lianhuaqingwen, containing quercetin, luteolin, and kaempferol, inhibited the replication of SARS-CoV-2 with an IC50 value of 411.2 µg.mL–1 in Vero E6 cells [138].
4.1.5. PC from miscellaneous sources
Sambucus nigra extract is a source of several anthocyanins (cyanidin 3-sambubioside accounting for almost half of them) and quercetin 3-rutinoside [161]. S. nigra extract (0.004 g/mL) reduced the titers of infectious bronchitis virus (IBV). This virus is a pathogenic chicken coronavirus, and the impairment of the viral membrane is the most likely mechanism reported by workers, compromising the envelope structure and vesicle formation [23]. Forsythia suspensa Vahl. is widely used in traditional Chinese medicine and is rich in Forsythoside A. This PC inhibited CEK infection by IBV in a dose-dependent manner (0.16–0.64 mM). A direct virucidal effect was observed when the PC was administered before IBV but not when cells were previously infected [90]. Papyriflavonol A, present in Broussonetia papyrifera, is the most potent inhibitor of PLPro, with an IC50 value of 3.7 µM [124]. Other PC from the same plant (broussochalcone B, broussochalcone A, 4-hydroxyisolonchocarpin, papyriflavonol A, 3′-(3-methylbut-2-enyl)-3′,4,7-trihydroxyflavane, kazinol A, kazinol B, broussoflavan A, kazinol F, and kazinol J) were more potent against PLPro than against 3CLPro. A molecular docking study revealed that hesperidin, tangeretin, and naringenin from Citrus sp. presented high affinity for the receptor-binding domain from S protein and the protease domain from ACE2 of the host cell [158].
4.2. Antioxidant properties
The antioxidant capacity of PC has been widely investigated in the past years. It often forms the basis for several of their protective effects on living cells. The mechanisms underlying PC antioxidant capacity involve metal ion-chelating ability, scavenging of ROS, and protecting antioxidant defenses [103].
4.2.1. Direct antioxidant properties
The direct scavenging ability of PC is exerted either by participating in reactions involving the donation of one electron (i.e., as an H) or by reducing hydroperoxide to alcohol. This prevents the formation of the hydroxyl or alkoxyl radical [45]. The antioxidant activity of PC is directly related to their chemical structures [5]. The presence of -CH2COOH and -CH = CHCOOH groups on the benzene ring in phenolic acids enhances their antioxidant activities as compared with the -COOH group (Supplementary material, Fig. S1). In addition, methoxyl (-OCH3) and phenolic hydroxyl (-OH) groups promote the antioxidant activities of this class of PC [25]. For flavonoids, the most important structural characteristic contributing to a high scavenging capacity is the B ring hydroxyl structure [139] (Supplementary material, Fig. S1). The hydroxyl groups on this ring donate hydrogen and electrons to stabilize ROS, including hydroxyl and peroxyl radicals, generating a radical form of the antioxidant with greater chemical stability than the initial radical. The formation of these relatively long-lived radicals can modify radical-mediated oxidations [127] implicated in several diseases, including SARS-CoV-2 infection. In addition, the metal-chelating ability could contribute to the antioxidant properties of PC. Flavonoids present strong nucleophilic centers with a high affinity for metal ions; they are primary catalysts responsible for ROS production by cells [48].
4.2.1.1. Cell-based studies
The excessive levels of ROS along with a decrease in antioxidant defense generated by SARS-CoV-2 infection induce deleterious effects on the functions of pulmonary cells (lung epithelial and endothelium cells) and red blood cells (RBCs) (affecting cell membrane and the functionality of heme group), causing hypoxic respiratory failure observed in most severe cases of COVID-19 ([83]; [115]). Therefore, free radical scavengers, such as PC, could be beneficial co-adjuvant therapeutics for most vulnerable patients.
Table S1 (Supplementary material) presents some PC with antioxidant properties observed in several cell lines, including lung epithelial and endothelium cells, and RBCs. In particular, the stilbene resveratrol plays a potential therapeutic role in lung epithelial cells by attenuating oxidative stress generated after infection with Pseudomonas aeruginosa [19] and Streptococcus pneumoniae [188]. The antioxidant effect of resveratrol has also been demonstrated in i) lung vascular endothelial cells, where 0.1 to 10 µM of the compound attenuated HMGB1-induced mitochondrial oxidative damage and protected the lung endothelial barrier [35] and in ii) RBCs, where 100 µM of the compound prevented cell oxidation generated by H2O2 [135]. The antioxidant potential of resveratrol against H2O2-induced oxidative stress in RBCs is potentiated by the interaction of other PC present in red wine extract [154].
As shown in Table S1 (Supplementary material), PC from olive oil, green tea, and citrus fruits showed a protective antioxidant effect in lung epithelial cells and RBCs. Among certain olive oil PC, 3,4-dihydroxyphenylethanol-elenolic acid and hydroxytyrosol exerted the highest protective activity at 3 µM in AAPH-induced oxidative stress in RBCs [123]. Oleuropein (462.5 µM) reduced the oxidative stress status of lung epithelial cells A549, whereas this effect was more pronounced when the compound was encapsulated in nanostructured lipid carriers [63]. Among green tea PC, EGCG (30 µM) most effectively suppressed the AAPH-induced hemolysis in RBCs [85] and the flavonoid fraction of orange and bergamot juices (that contained vicenin-2, neohesperidin, narirutin, hesperidin, naringenin, tangeritin, and nobiletin) reduced ROS generation in lung epithelial cells [43].
4.2.1.2. Human studies
The antioxidant activity of PC has been mainly investigated either in vitro or in vivo using animal models [41], [103], whereas studies on humans, i.e., clinical trials are still limited. Table S2 (Supplementary material) summarizes studies on the antioxidant effects of some of the selected PC in humans. The possibility of a direct in vivo antioxidant action has always been questioned because it requires the presence of PC at the exact location of the formation of ROS. This presence can be limited by the low bioavailability of PC, which is largely attributed to their poor absorption in the intestine, rapid metabolization, and quick elimination [24]. The metabolism and bioavailability of PC [30,103] are crucial aspects that should be considered for a more comprehensive appraisal of the health-promoting effect of these compounds as further discussed in Section 6. Nevertheless, certain studies have been conducted using antioxidant-rich foods and beverages that showed that PC from teas (black and green), wine, grapes, olive oil, berries, and fruits and vegetables improved the antioxidant status (plasma antioxidant activity) in healthy subjects (Supplementary material, Table S2).
4.2.2. Genetic modulation of enzymatic antioxidant defenses
Recently, it has been reported that the mechanisms of action of PC include processes more than direct scavenging of ROS. For example, these compounds i) activate transcription factors involved in the Nrf2-ARE pathway and induce antioxidant enzymes, ii) exhibit xenohormetic effect, and iii) improve cell homeostasis due to their binding activity to peptides and proteins [155].
Although recent studies have reported the potential use of certain PC in the treatment of COVID-19, they were mostly focused on the antiviral activity mechanisms [101]. Next, the effects of PC on the endogenous antioxidant system by modulating the Nrf2 pathway [77] and its implication for COVID-19 therapy have been scarcely addressed. PB125, a phytochemical dietary supplement containing a mixture of extracts with carnosol (6%) and carnosic acid (15%) from Rosmarinus officinalis, withaferin A (2%) from Withania somnifera, and luteolin (98%) from Sophora japonica at a ratio of 15:5:2 (m/m/m) and extracted at 50 mg of the mixed powder per mL in ethanol, was a potent Nrf2 activator at concentrations ranging from 4 to 22 µg/mL in the HepG2 cell line [65]. Furthermore, PB125 downregulated the mRNA expression of ACE2 and TMPRSS2 at a concentration of 16 µg/mL in human liver-derived HepG2 cells [107]. In addition, PB125 markedly downregulated 36 genes encoding cytokines in endotoxin-stimulated primary human pulmonary artery endothelial cells. Considering that several of these cytokines were identified in the “cytokine storm” observed in fatal cases of COVID-19, the study group suggested that Nrf2 activation significantly decreased the intensity of the storm in patients affected by COVID-19 [107].
PC modulate the endogenous antioxidant system during certain viral infections [80]. Oral supplementation with quercetin (1 mg/day for 5 consecutive days) parallel to influenza virus instillation increased the activities of catalase (CAT) and superoxide dismutase (SOD) and the concentration of GSH. Therefore, quercetin could protect the lungs from ROS produced during influenza virus infection by restoring endogenous antioxidants. Quercetin (20 µg/L) simultaneously induced the translocation of Nrf2 from the cytosol to the nucleus and the expression of heme oxygenase (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1) (other enzymes regulated by the Nrf2 pathway) in alveolar macrophages, suggesting that the supplementation with quercetin was beneficial in treating respiratory viral infections [179]. Accordingly, increased antioxidant defenses by activating Nrf2 by flavonoids have been discussed [143] and likely contribute to their anti-inflammatory property. Furthermore, several other studies indicated that flavonoids modulate the inflammatory response by activating pathways that induce the transcription of antioxidant and detoxification defense systems [131]. This interplay between antioxidant and anti-inflammatory effects of PC reinforce their putative beneficial role against manifestations of SARS-CoV-2 infection.
4.3. Immunomodulatory and anti-inflammatory effects
The immunomodulatory ability of PC is evidenced by their ability to modulate the NF-kβ pathway by suppressing the activation of IKK or by preventing the binding of NF-κB to DNA. In addition, PC modulate the expression of pro-inflammatory genes and cytokine production, besides influencing several populations of immune cells [165,174].
Natural killer (NK), T, and B cells are particularly important for combating COVID-19 infection because they are crucial players in the immune response against bacteria and viruses. Lymphopenia (i.e., low count of T, B, and NK cells) is among the signs of COVID-19 infection. Thus, therapeutic or dietary agents that increase immune cell count are relevant [95].
The administration of Cassia auriculata-derived PC (25–100 mg/kg b.w.) increased the T and B cell counts, as well as the proliferation and sensitivity of T cells in aged rats [71]. Resveratrol (2.5 µg/mL) not only increased the percentage of CD4+ and CD8+ T cells but also stimulated CD8+ T lymphocyte and NK cell activity [42]. Honokiol, a PC extracted from the bark of the magnolia tree, at 120 mg/kg b.w., increased the frequency of dendritic cells and the count and activation of CD4+ T cells in an in vivo sepsis model [74]. In vitro and in vivo studies indicated that EGCG inhibited the migration of monocytes and increased regulatory T-cell populations [110,166].
Multiple PC, such as narirutin [58], butein [69], trans-cinnamaldehyde and 2-methoxycinnamaldehyde [134], hydroxytyrosol [9], kamebacetal A [64], kamebakaurin [64], excisanin A [64], kamebanin [64], piceatannol [12], naringin [2] (Ahmad et al., 2014), sinapic acid [186], and malvidin [31] have been described to inhibit the activation of the NF-kβ pathway. In addition to isolated PC, plant extracts containing multiple PC, namely phenolic acids, flavonoids, and even PC precursors such as quinic and shikimic acids, inhibit the NF-kβ pathway in vitro at concentrations ranging from 10 to 300 µg/mL [126,189].
Cytokine storm, mass secretion of pro-inflammatory cytokines, is one of the worst signs of the COVID-19 pathology, often leading to major complications [27,96,111]. Accordingly, studies have shown that PC can inhibit the secretion of pro-inflammatory cytokines in several conditions. For instance, kaempferol (28.62 µg/mL) significantly reduced the concentration of IFN-γ in human whole blood cultures, whereas oleuropein (54.05 µg/mL) reduced the concentration of IL-1β [113]. Resveratrol reduced the levels of TNF-α and IL-6 in vivo (100 mg/kg b.w./day) [146] and in HTLV-1-infected CD4+ T lymphocytes (20–40 µg/mL) [49]. Moreover, the secretion of TNF-α and IL-6 was reduced in human primary monocytes by oligonol (25 µg/mL), a lychee fruit-derived mixture of low-molecular-weight PC [88]. At concentrations ranging from 10.8 to 61 µg/mL, quercetin, fisetin, apigenin, resveratrol, and rutin inhibited the production of IL-6, whereas curcumin and partially fisetin (7.4 and 11.4 µg/mL, respectively) suppressed the production of TNF-α in macrophages infected with dengue virus (DENV-2) [70]. In addition, fisetin, apigenin, and resveratrol downregulated the production of IL-10, whereas rutin and fisetin inhibited the production of IFN-γ [70]. Altogether, these data showed that the immunomodulatory and anti-inflammatory properties of dietary PC support a possible role for PC-based adjuvant nutritional strategies to combat the inflammatory storm characteristic of COVID-19, apart from mitigating the complications associated with this inflammation.
5. Human studies on PC use in COVID-19
Although scarce, certain ongoing studies are investigating the therapeutic potential of PC for COVID-19 patients. In a randomized, double-blind, placebo-controlled study, COVID-19 patients receiving a daily dose of 160 mg of a nano-micellar form of curcumin for 14 days reported decreased IL-6 and IL-1β expression and secretion in serum when compared with the placebo group [159]. Currently, three clinical studies are registered at ClinicalTrials.gov using PC to target the inflammation caused by COVID-19. One of these trials will evaluate the use of a dietary supplement containing a molecular complex of quebracho, chestnut tannin extract, and vitamin B12 [128]. The second study aims to assess the use of Caesalpinia spinosa extract rich in PC, with a high antioxidant and anti-inflammatory activity, in decreasing the production of pro-inflammatory cytokines (e.g., IL-6) [99]. The third clinical trial aims to evaluate the safety and effectiveness of colchicine and herbal phenolic monoterpene fractions when added to the standard treatment in patients with COVID-19 [109]. No results about these trials have been published yet.
6. Bioavailability of dietary PC
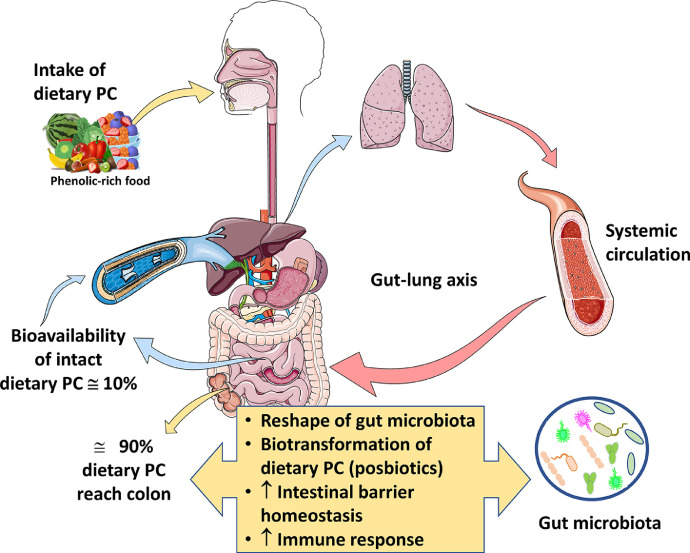
The bioavailability of dietary PC should be considered for a more comprehensive appraisal of the health-promoting effect of PC [30,103]. Despite being the most abundant bioactive phytochemical in the human diet, the bioavailability of dietary PC is usually extremely low, ranging from 1 to 10% of the initial amount. The bioavailability of PC depends on several factors, such as food processing (cooking), food-related factors (food matrix) and interactions with other compounds (fat and alcohol), and host-related factors, including intestinal factors [30].
Dietary PC are absorbed in the small intestine (Fig. 1 ), resulting in plasma concentrations rarely exceeding 1–10 µM [155]. Among all PC classes, flavones, such as quercetin and rutin, present a low absorption rate (0.3–1.5%), whereas flavonols (catechins), flavanones (naringenin), genistein, and anthocyanins show a high bioavailability (3–30%) [155]. High molecular weight tannins are poorly absorbed due to their relatively large molecular size. Sugar-bound PC exhibit limited bioavailability in their native form. Some of them are hydrolyzed in the intestine, contributing to the high variability of PC bioavailability [72].
Figure 1.
The fate of dietary PC during human digestion. Note that the low bioavailability of parent PC along with the interplay between dietary PC and gut microbiota plays a key role in human health. The gut–lung axis, which links the changes in the gastrointestinal tract to the changes in the respiratory system, would probably play a key role in the dietary approaches for attenuating COVID-19-associated ARDS.
In addition to their low absorption, dietary PC are extensively metabolized by intestinal and hepatic cells. Therefore, they are present in human plasma and tissues not only in their native form but also as phenolic metabolites. These metabolites have become the subject of several research studies showing the beneficial effects (powerful antioxidant agents) of their different forms (glucuronidated, sulfated or methylated) [144].
After oral administration, resveratrol is absorbed by passive diffusion or by forming complexes with membrane transporters followed by release into the bloodstream. In the bloodstream, they are mainly present as a glucuronide, sulfate, or in the free form [50]. The concentration of resveratrol in human plasma depends on the dose ingested; it is higher when administered in the morning [4]. In addition, its administration with ribose or piperine improves its bioavailability, whereas no changes were reported when it is ingested with or without alcohol or in combination with other PC such as quercetin [132]. In contrast, its consumption with a high-fat meal compromises its bioavailability [132]. The human gut microbiota plays an important role in the interindividual variation concerning resveratrol bioavailability, and strains such as Slackia equolifaciens sp. and Adlercreutzia equolifaciens sp. have been identified as producers of dihydroresveratrol [14].
The bioavailability of curcumin is substantially low—about 50 ng/mL is found in human plasma after oral administration (10–12 g of curcumin) [6]. The major reasons contributing to the low plasma and tissue levels of curcumin appear to be its low solubility in water, poor absorption, rapid metabolism, and rapid systemic elimination [6]. To improve its bioavailability, different approaches have been used such as the use of an adjuvant, e.g., piperine that interferes with glucuronidation, use of liposomal curcumin, use of curcumin nanoparticles, use of curcumin phospholipid complexes, and use of structural analogs of curcumin [6].
The bioavailability of quercetin is highly dependent on the type of food matrix. In particular, quercetin aglycone derived from onion skin extract powder is significantly more bioavailable than that obtained from apple skin extract [87] or even quercetin dihydrate powder-filled hard capsules [16]. The oral bioavailability of quercetin is well understood. Despite the administration of a high oral dose of quercetin, the maximum concentration of the free aglycone in plasma is only in the low nM range owing to its biotransformation during digestion, absorption, and metabolism [3]. Therefore, it is suggested that quercetin can be administered directly by alternative routes, such as a nasal or throat spray, to treat COVID-19 patients in clinical trials [171].
It is estimated that only approximately 1.68% of ingested tea catechins are present in human plasma (0.16%), urine (1.1%), and feces (0.42%) 6 h after the ingestion of tea [167]. In particular, Yang et al. reported that the maximum plasma concentrations for EGCG, EGC, and EC were 0.57, 1.60, and 0.6 µM, respectively, after the consumption of 3 g of decaffeinated green tea [177]. To improve the bioavailability of tea catechins, several approaches have been explored. For instance, the encapsulation of tea catechins in protein-based, carbohydrate-based, and lipid-based nanoparticles improved their stability, sustainable release, and cell membrane permeation, resulting in increased bioavailability [17]. In addition, molecular modification of compounds, such as synthesizing peracetylated EGCG, increased the bioavailability of this compound because it protected hydroxyl groups on EGCG from oxidative degradation until it is deacetylated into its parent EGCG by esterases in cells, decreasing biotransformation and efflux of EGCG [84]. The co-administration of catechins with other bioactive compounds produced a synergistic effect, resulting in improved absorption and inhibition of efflux transporters [17].
Most antiviral and direct antioxidant effects of dietary PC in vitro have been observed at concentrations ranging from 0.1 and 640 µM (Table 1 and Supplementary material, Table S1). As discussed above, systemic levels of PC are usually within nM or low µM range due to their low bioavailability and extensive biotransformation during digestion and after intestinal absorption [41]. Thus, concentration issues could limit the in vivo relevance of direct systemic antiviral and antioxidant effects of PC. Nevertheless, PC compounds reach concentrations within the mM and high µM range inside the gastrointestinal tract [41], where they are likely to exert antiviral and antioxidant effects.
7. Interplay between PC and gut microbiota: implications for the protection against COVID-19
About 90% of dietary PC is not absorbed in the small intestine and therefore reaches the colon [72], where it is extensively metabolized by the gut microbiota into small molecular weight compounds that usually have a higher absorption rate than their parent compounds (Fig. 1). Many of these PC metabolites have bioactive effects and are majorly responsible for the systemic biological effects of dietary PC [28]. Therefore, they meet the requirements for being considered postbiotics, i.e., microbial-derived metabolites that have beneficial effects on the host [28]. In addition, the interplay between PC and gut microbiota modulates the microbiome composition and function [28,72] (Fig. 1). This section will address how this interplay could modify the bioactive properties of PC that are relevant to their potential benefits against SARS-CoV-2 infection.
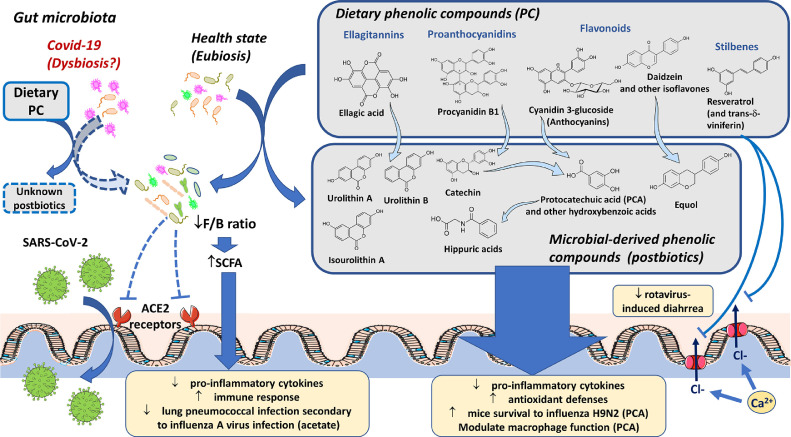
Colonic microbiota deconjugates glycoside, glucuronide, and organic acid moieties releasing phenolic-derived aglycones that are subsequently cleaved by the fission of heterocyclic and aromatic rings, and undergo dihydroxylation, decarboxylation, demethylation, reduction, and isomerization of alkene moieties [28]. Certain catabolic pathways have been elucidated (Fig. 2 ) revealing that protocatechuic and other hydroxybenzoic acids are the major metabolites of anthocyanins and other flavonoids [28], whereas urolithins are major metabolites of ellagic acid-related PC [72,129]. Proanthocyanidins are converted into catechins that are subsequently catabolized into hydroxyphenyl-γ-valerolactones and thereafter sequentially converted into the following phenolic acids: hydroxyphenylvaleric, hydroxyphenylpropionic, hydroxyphenylacetic, hydroxybenzoic, and hippuric acids [10].
Figure 2.
The interplay between dietary PC and gut microbiota, and its putative role in protection against SARS-CoV-2 infection. ACE2: angiotensin-converting enzyme 2 receptor. PC: phenolic compounds. F/B ratio: Firmicutes/Bacteroides ratio. PCA: protocatechuic acid. Dashed lines and squares indicate indirect evidence and putative effects, respectively.
Several systemic health benefits of dietary PC are dependent on phenolic metabolites generated by the gut microbiota. Certain effects demonstrated for these phenolic metabolites, such as the antioxidative, anti-inflammatory, and immunomodulatory properties, are relevant in the context of protection against COVID-19 (Fig. 2). Isoflavones, such as genistein and daidzein, are metabolized into equol that has antioxidant, anti-inflammatory, cardioprotective, neuroprotective, and estrogenic activity. In fact, equol seems to be responsible for the effects of its parent isoflavone compounds [28,106]. In addition, urolithins exhibit higher antioxidant, anti-inflammatory, and anti-proliferative activities than their parent compounds ellagitannins and ellagic acid [144], whereas 3-(3-hydroxyphenyl)propanoic acid is implicated in the protective effect of grape seed polyphenol extract against neurodegenerative diseases [164]. In contrast, the antioxidant and antiproliferative abilities of flavonoid metabolites, namely phenylpropionic, phenylacetic, and hydroxybenzoic acid derivatives was lower compared to their parent compounds [37,51].
The potential role of microbial-derived PC metabolites against SARS-CoV-2 infection comes from the studies on protocatechuic acid. After human intake of cranberry juice, plasma levels of protocatechuic acid increased and were more strongly correlated with the plasma antioxidant capacity than its parent PC [108]. In addition, the modulation of macrophage function by protocatechuic acid is majorly responsible for the antiatherogenic effects of dietary cyanidin-3-glucoside in a mice model of atherosclerosis [163]. Moreover, protocatechuic acid has been demonstrated to attenuate inflammatory response and increase viral clearance and survival rate of mice challenged with the influenza virus H9N2 [122].
The other face of the interplay between PC and gut microbiota is the reshaping of the former by dietary phenolics in a prebiotic-like effect [28]. Such effect has been implicated in several phenolic-induced benefits, including improved intestinal homeostasis [104] and immune response, among other relevant biological effects [72] (Fig. 2). These prebiotic-like effects could be particularly relevant to SARS-CoV-2 therapy because gastrointestinal problems have been reported in approximately 50% of patients in a multicenter study in Hubei, diarrhea being reported in 17% of patients [57]. The supplemental nutrition with soluble dietary fibers, which are classical prebiotics, and even with probiotics, have been recommended for nutrition therapy during the recovery of critically ill COVID-19 patients [102], [118]. Moreover, COVID-19 patients exhibited intestinal dysbiosis characterized by a decrease in the diversity and abundance of gut microbiota [57,190], which could represent a potential target for the use of PC (Fig. 2). Supporting this hypothesis, resveratrol [29] and certain resveratrol oligomers [184] have been demonstrated to alleviate diarrhea induced by rotavirus in animal models. The inhibition of epithelial Ca2+-activated Cl– channels contributes to the anti-secretory and anti-motility protective effects of these PC [184] (Fig. 2).
ACE2 receptors, which are known to mediate the entry of SARS-CoV-2 into animal cells [145], are highly expressed in the gastrointestinal epithelial cells (Harmer, Gilbert, Borman & Clark, 2002). The reconstitution of gut microbiota in gnotobiotic rats was demonstrated to decrease colonic ACE2 expression compared to that in germ-free rats [178], providing evidence that colonic expression of ACE2 is modulated by gut microbiota. Since PC increased the abundance and diversity of gut microbiota in favor of the growth of probiotic bacteria [149], reshaping of gut microbiota by PC could putatively modulate SARS-CoV-2 entry into the host (Fig. 2).
In addition, COVID-19 severity demonstrated an association with 23 bacterial taxa from fecal samples, mostly from phylum Firmicutes [190]. Clostridium ramosum and Clostridium hathewayi were positively associated with COVID-19 severity, while Erysipelotrichaceae bacterium exhibited a strong positive association with fecal SARS-CoV-2 load [190]. These Clostridium species are reportedly associated with human bacteremia [40,46]. In addition, the fecal SARS-CoV-2 load of COVID-19 patients demonstrates an inverse association with certain Bacteroides species [190], which have been reported to reduce the expression of ACE2 in murine gut [53]. These data suggest that Bacteroides species probably contribute to combating SARS-CoV-2 infection by hampering virus entry through ACE2 [190]. According to a recent review, several PC and PC-rich foods, such as curcumin, resveratrol, polymeric proanthocyanidins, de-alcoholized red wine, and green tea, reduce the fecal Firmicutes/Bacteroides ratio [72]. Considering a cause–effect relationship between gut bacterial profile and COVID-19 prognostics, PC is expected to reduce virus load and COVID-19 severity (Fig. 2).
The in vitro studies, animal models, and clinical trials provide accumulating evidence that PC, particularly hydrolyzable and condensed tannins, may exert prebiotic-like effects by promoting the growth of Lactobacilli and Bifidobacteria [28,38], which play a key role in regulating local and systemic immune responses [147]. Therefore, PC intake is expected to modulate the ecology of gut microbiota in COVID-19 patients to enable a balanced immune response against SARS-CoV-2. The mechanisms underlying the prebiotic effect of PC have not been completely elucidated so far, although it is suggested to include sugar moieties as an energy source or selective antimicrobial effects against pathogenic bacteria based on iron-chelating, anti-adhesion, and membrane protein inactivation that would favor the growth of probiotic bacteria and reshape gut microbiota [28].
The reshaping of gut microbiota increases the production of short-chain fatty acids (SCFA), such as acetate, propionate, and butyrate, which have been demonstrated to downregulate pro-inflammatory cytokines while improving systemic immune response after intestinal absorption [78] (Fig. 2). This mechanism could be particularly relevant for counteracting the SARS-CoV-2-related inflammatory storm that is usually associated with ARDS [147]. It is noteworthy that the soluble PC and mostly the matrix-bound PC from fruits increased fecal SCFA production in vitro [116,129] as well as in vivo [28,104]. A fecal transfer experiment conducted recently in mice demonstrated that changes in gut microbiota were responsible for the lung pneumococcal infection secondary to influenza A virus infection [142]. Oral supplementation with acetate, which is the predominant SCFA produced by gut microbiota, reduced the impact of this bacterial infection by modulating the activity of alveolar macrophages [142]. These data indicate SCFA as relevant therapeutic agents against the complications of viral respiratory infections and reinforce the involvement of the gut-lung axis in these pathologies (Fig. 2). The gut-lung axis comprises a two-way interaction, where the function and immune homeostasis of the lung can be affected by metabolites from gut microbiota and vice-versa [26].
COVID-19-associated dysbiosis [57] has a potential impact on the profile of microbe-derived PC metabolites, and should, therefore, be carefully evaluated when considering PC as adjuncts for SARS-CoV-2 treatment (Fig. 2). Fecal Clostridium species, which are positively associated with high-severity COVID-19 cases [190], have also been implicated in the gut metabolism of PC [28]. Moreover, emerging evidence reveals that interindividual differences in the ecology of gut microbiota result in different profiles of phenolic-derived postbiotics, which could have a key role in the biological effects of PC. Different metabolic profiles, named metabotypes, were identified for ellagitannins/ellagic acid [28] and isoflavone daidzein [106], indicating the relevance of personalized nutrition and pharmacological therapy.
Despite the overall decreased abundance of gut microbiota in SARS-CoV-2 patients, there is also an increased relative abundance of opportunistic bacteria in feces, such as Rothia and Streptococcus [57]species, which are usually associated with increased susceptibility to secondary bacterial lung infection in immunocompromised patients [100] and patients suffering from other respiratory viral infections [148]. Conversely, influenza infection has been demonstrated to modify the gut microbiome by mobilizing lung-derived immune cells (T-cells) to the small intestine, where these cells stimulate the production of IFN-γ [34]. These findings corroborate the involvement of the gut–lung axis in linking the gastrointestinal and lung dysfunctions in respiratory infections, including COVID-19. Moreover, the modulation of colonic ACE2 by gut microbiota reinforces that the gut–lung axis is probably involved in COVID-19 infection [178]. Therefore, dietary modulation of the gut microbiota might be a promising approach for the treatment of COVID-19 infection, as recently suggested by a study recommending dietary fiber and probiotics [26].
As summarized in Figure 2, the evidence discussed in this section indicates that gut microbiota probably plays a key role in the putative effects of PC against SARS-CoV-2 infection. Therefore, gut microbiota may provide metabolic pathways either for the production of specific bioactive PC-derived postbiotics or to be targeted to allow the modulation of immune response resulting in the reduction of viral infection and morbidity. Various PC-derived postbiotics exhibit high antioxidant and anti-inflammatory properties, which would be potentially beneficial against SARS-CoV-2 infection. In addition, reshaping of gut microbiota by PC has been demonstrated to trigger various mechanisms that could contribute to reducing SARS-CoV-2 infection, such as the downregulation of gut ACE2 expression, upregulation of SCFA production, and control of opportunistic bacteria. The reshaping of gut microbiota by PC could even modulate the respiratory complications of SARS-CoV-2 infection via the gut–lung axis.
8. Safety issues
Besides their natural occurrence in fruits and vegetables, PC are also present in food additives for coloring and health-improving purposes. PC are also available as tablets, capsules, or powder dietary supplements. The majority of the PC do not have sufficient toxicological studies conducted on animals to define a specific acceptable daily dose (ADI) for safe consumptions by humans. However, PC and PC-rich foods are usually considered to be safe based on the empirical evidence from their regular consumption as natural food constituents and numerous animal studies revealing their beneficial effects on health. Toxicological evaluations available for a few selected PC are discussed below. In general, quercetin appears to be well tolerated in humans when consumed orally, with a considerably low incidence of adverse effects observed at doses up to 1500 mg per day [7]. In western diets, the estimated daily intake of quercetin ranges from 3 to 40 mg (aglycone equivalents), while the recommended daily doses of quercetin aglycone via dietary supplements are usually around 500 mg. In 2010, a high-purity quercetin food ingredient was considered GRAS (“Generally Recognized As Safe”) under the intended conditions of use by the Food and Drug Administration (FDA). In this appraisal, a high intake within the estimated ADI of 19–22 mg/kg b.w. was also considered safe, which is equivalent to 1330 to 1540 mg quercetin/day for a 70-kg adult [44] However, a chronic toxicity study revealed that rats receiving 40, 400, or 1900 mg of quercetin per day for two years exhibited a dose-dependent increase in chronic nephropathy and a slightly increased incidence of focal hyperplasia of the renal tubule epithelium. Moreover, a higher incidence of kidney adenomas was observed in male rats at the doses of 400 and 1900 mg quercetin/day [157].
Resveratrol, which has a low dietary intake of 6–8 mg/day [20], is present in commercial dietary supplements at 50–500 mg of trans-resveratrol [140]. In a study, resveratrol and a nutraceutical formulation containing resveratrol (Longevinex) did not exhibit any sign of toxicity in Sprague-Dawley rats receiving daily doses of 50 and 100 mg for 28 days. Another formulation containing a high-purity trans-resveratrol (resVida) exhibited low oral toxicity, although high doses (2–3 g/kg b.w./day) appeared to adversely target the kidneys and the bladder in animals. Frequent gastrointestinal discomfort/diarrhea was observed in humans receiving high doses (2.5 g or 5 g per day) of resveratrol for 29 days [160]. On the basis of NOAEL studies, a daily dose of 450 mg of resveratrol was considered safe for a 60-kg individual, using a 10-fold safety factor [170].
Curcumin is reported to be effective, safe, and tolerable against various chronic diseases in human trials [81]. Clinical trials involving healthy human subjects revealed that curcumin induced a 50% contraction of the gall bladder at the dosage of 40 mg/day [133]. Despite this, JECFA (The Joint FAO/WHO Expert Committee on Food Additives) and EFSA (European Food Safety Authority) established an ADI of up to 3 mg/kg b.w. for curcumin which is equivalent to 210 mg/day for a 70-kg adult [76].
EGCG is the major PC in green tea. The toxicological studies have demonstrated a pattern of hepatotoxicity associated with the intake amounts of 140 to 1000 mg/day of EGCG [120]. A 13-week study on rats and dogs reported a NOAEL of 500 mg/kg b.w./day for EGCG [68]. Considering the purity and safety factor calculations, this study generated an ADI of 4.6 mg/kg b.w./day for EGCG, which is equivalent to 322 mg EGCG/day for a 70-kg adult. Other studies on EGCG toxicity conducted in both animals and humans were reviewed recently, and an intake of 338 mg EGCG/day was reported to be safe [62]. Furthermore, European regulatory agencies have proposed daily EGCG limits for supplements, which range from 300 to 1600 mg/day [180].
Although existing studies indicate high doses to be safe for most dietary PC, relevant concerns are expected when using dietary PC as adjuvant therapy for pregnant COVID-19 patients. It is recommended that the consumption of PC-rich foods and supplements be restricted during the third trimester of pregnancy due to their association with ductal constriction in fetal heart [59]. This effect is probably mediated by anti-inflammatory mechanisms and is shared by non-steroidal anti-inflammatory drugs [59]. Therefore, the possible occurrence of toxicity during PC nutritional approaches for COVID-19 therapeutics should be considered prior to reporting a final statement regarding the clinical use of PC.
9. Drug interactions
The complex interactions between food nutrients/nutraceuticals and therapeutic drugs are not yet elucidated. Nonetheless, PC may alter the effectiveness of pharmacological therapies by influencing drug absorption and bioavailability, as PC compete with drug transporters and metabolizing enzymes. Drug transporters are mainly represented by the ATP-binding cassette (ABC) and the solute carrier (SLC) transporters, which play pivotal roles in drug absorption and disposition, thereby determining drug safety and efficacy (Li et al., 2016). The drug-metabolizing enzymes include the intestinal and hepatic cytochrome P (CYP) enzymes, glucuronosyltransferases (UGTs), and sulfotransferases. PC may alter the pharmacokinetics of certain drugs by inhibiting transporters or modulating the expression of transporters and drug-metabolizing enzymes. Flavonoids, which are substrates for UGTs, when consumed in combination with certain drugs, might inhibit the glucuronidation of the drugs as a result of competitive inhibition [82].
When formulating a PC-based nutritional strategy for COVID-19 therapy, the interaction of PC with numerous therapeutic drugs, such as those used for controlling COVID-19 manifestations (antivirals, antibiotics, and glucocorticoids), must be considered. Green tea extract (containing 100 µM of EGCG) has been demonstrated to inhibit the drug transporters OATP1A1 and OATP1A2 in vitro [75]. Since these transporter proteins are involved in the transport of fluoroquinolones and antiretrovirals, green tea extract should be avoided when using these drugs [11]. On the other hand, onion and garlic extracts that are rich in PC potentiated the efficacy of streptomycin and chloramphenicol in vitro [97]. In a study, rabbits receiving the antibiotic norfloxacin (100 mg/kg b.w. p.o.) after pretreatment with curcumin (60 mg/kg b.w. per day, 3 days, p.o.) exhibited increased norfloxacin levels in plasma [125]. On a practical note, continuing treatment with curcumin resulted in a 24% and 26% decrease in the maintenance dose and loading dose of norfloxacin, respectively [125]. Therefore, caution is advised during long-term administration of curcumin and norfloxacin to avoid an increase in the adverse effects of norfloxacin.
In regard to antivirals, garlic flavonoids exerted different impacts on the hepatic pharmacokinetics of saquinavir and darunavir [13]. Moreover, chronic use of St. John's wort, a source of flavonoids, could significantly decrease the absorption and bioavailability of indinavir in humans. The phenolic-rich plants, namely, St. John's wort and Glycyrrhiza uralensis, were demonstrated to reduce the bioavailability of drugs midazolam and lidocaine, respectively, which are used for the orotracheal intubation of COVID-19 patients (Barnes et al., 2001; Tang et al., 2009). As far as we know, no studies are currently available on interactions between glucocorticoids and PC.
Besides the drugs used for counteracting COVID-19 manifestations, continuous-use medication for patients bearing comorbidities (chronic diseases such as diabetes, cardiovascular disease, and respiratory diseases) should also be evaluated for interactions with PC. Indeed, single or repeated daily doses of quercetin from 0.6 up to 300 mg quercetin/kg b.w. were reported to increase the bioavailability of drugs used by cardiovascular disease patients, such as digoxin, ranolazine, valsartan, verapamil, and diltiazem. On the other hand, the bioavailability of simvastatin was decreased upon the oral intake of quercetin [7]. Regarding diabetes management, quercetin (10 mg/kg) increased the bioavailability of intravenous and oral-administered pioglitazone by 25%–75% in female rats [156]. However, current evidence on the interactions of PC with these drugs is scarce and, therefore, caution in PC intake is advised for subjects under these therapies.
10. Conclusions
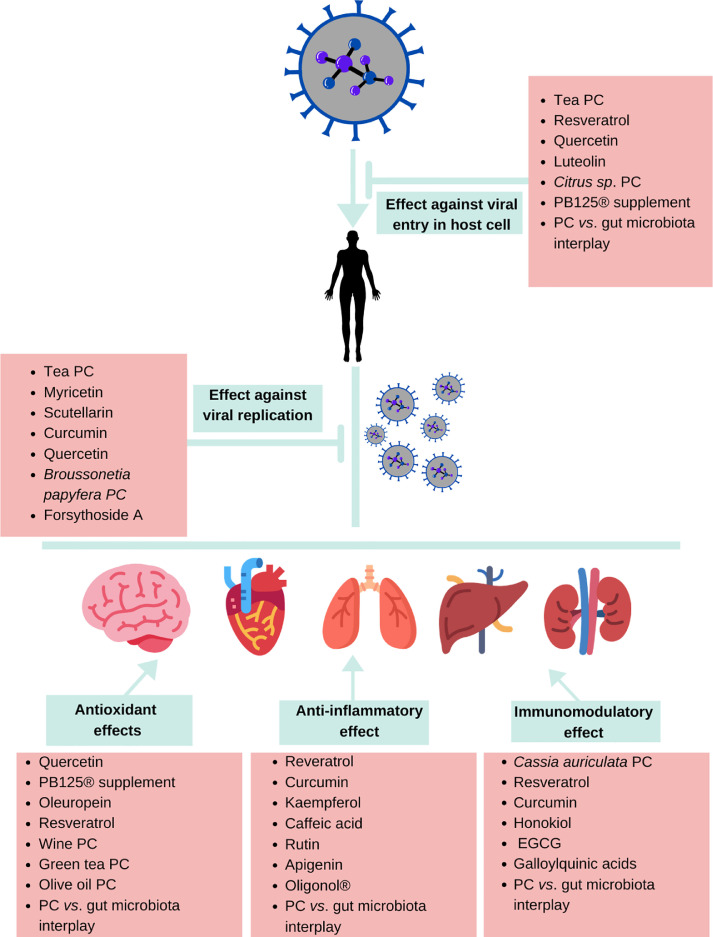
As depicted in Figure 3 , numerous PC have been demonstrated to exert multiple effects that might mitigate COVID-19 manifestations, including antiviral, antioxidant, immunomodulatory, and anti-inflammatory effects. Since the bioavailability of most dietary PC is limited, the gene-mediated antioxidant, anti-inflammatory, and immunomodulatory effects are most probably responsible for the systemic effects of PC against SARS-CoV-2 infection. Nonetheless, direct antiviral and antioxidant effects could occur in the gastrointestinal tract where PC occur in high concentrations. Moreover, the interplay between PC and gut microbiota, which includes the production of PC-derived postbiotics and the reshaping of gut microbiota, leads to the activation of different metabolic and signaling pathways that putatively reinforce host antioxidant and immune response against SARS-CoV-2 infection. It is noteworthy that several of the effects and mechanisms discussed in the present review are also relevant for a potential protective effect of PC against other viral diseases, including those caused by respiratory viruses and CoVs other than SARS-CoV-2.
Figure 3.
Representation of PCs’ effects that probably contribute to attenuating COVID-19 manifestations. EGCG, epigallocatechin gallate; PC, phenolic compounds.
Despite the promising targets identified for using PC to counteract SARS-CoV-2 infection, safety issues concerning PC and their interaction with other therapeutic drugs must be considered when strategizing the nutritional approach involving PC. In addition, the safe and rational use of dietary PC depends on further understanding of how the COVID-19 disease affects gut microbiota and its potential impact on the beneficial effects of PC. Moreover, the unique microbiome profile of different human phenolic metabotypes may yield different responses, indicating the necessity of planning personalized approaches.
11. Limitations and prospects
While the present study offers much useful information regarding the putative role of PC in COVID-19 manifestations, an important limitation of this study has to be noted, i.e., the lack of clinical trials evaluating the use of PC compounds in COVID-19 patients. So far, only one clinical trial has been concluded, revealing the positive effects of curcumin (in a nano-micellar form) in reducing the inflammatory manifestations in COVID-19 patients [159]. Although other clinical trials are currently being conducted, they concern the effects of PC-containing plant extracts and not the effects of isolated PC.
Therefore, further studies investigating the antiviral effects of PC in animal models or clinical trials are required to further corroborate the promising in silico and in vitro findings regarding the antiviral effects of certain PC. Moreover, as PC could exhibit a certain level of toxicity and may interact with drugs used in COVID-19 management, in vivo studies determining the safe dose levels of PC for therapeutic use should be conducted. Once this evaluation is completed, the next step should be to perform human clinical trials to determine the safety of using PC in humans.
Several of the potential protective mechanisms of PC against COVID-19 infection probably depend on the two-way interaction between PC and gut microbiota. Therefore, further comprehension of how COVID-19 affects gut microbiota and the impact of these changes on PC transformation during digestion would also be useful for designing the rational use of PC as adjuncts for COVID-19 therapy.
With PC becoming the protagonists in the nutraceutical scenario for COVID-19, without extensive studies on human subjects, the present review could serve as a basis for designing clinical trials in this regard.
Acknowledgments
P.R.A. acknowledges CNPq (Brazilian National Scientific and Technological Development Council) for the postdoctoral scholarship [grant number 205295/2018-5] that made this Brazil and Portugal partnership possible. T.E. is a fellowship from the National Council for Scientific and Technological Development (CNPq) [grant number 303654/2017-1]. A.T.S. acknowledges Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência for the Individual Grant CEECIND/04801/2017. iNOVA4Health – UIDB/04462/2020 and UIDP/04462/2020, a program financially supported by Fundação para a Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior, through national funds is acknowledged. Funding from INTERFACE Programme, through the Innovation, Technology and Circular Economy Fund (FITEC), is also gratefully acknowledged. Authors thank to the nutritionist Allana V. Brasil for her kind assistance in drawing figure 3 and graphical abstract.
Declaration of competing interests
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jnutbio.2021.108787.
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- 1.Abba Y., Hassim H., Hamzah H., Noordin M.M. Antiviral activity of resveratrol against human and animal viruses. Adv Virol. 2015;2015 doi: 10.1155/2015/184241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad S.F., Attia S.M., Bakheet S.A., Zoheir K.M.A., Ansari M.A., et al. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF- κb, STAT3 and pro-inflammatory mediators and enhancement of IκBα and anti-inflammatory cytokines. Inflammation. 2015;38:846–857. doi: 10.1007/s10753-014-9994-y. [DOI] [PubMed] [Google Scholar]
- 3.Almeida A.F., Borge G.I.A., Piskula M., Tudose A., Tudoreanu L., Valentová K., et al. Bioavailability of quercetin in humans with a focus on interindividual variation. Comprehensive Rev Food Sci Food Safety. 2018;17(3):714–731. doi: 10.1111/1541-4337.12342. [DOI] [PubMed] [Google Scholar]
- 4.Almeida L., Vaz-da-Silva M., Falcão A., Soares E., Costa R., Loureiro A.I., et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutrit Food Res. 2009;53(1):7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 5.Amic D., Davidovic-Amic D., Beslo D., Rastija V., Lucic B., Trinajstic N. SAR and QSAR of the antioxidant activity of flavonoids. Curr Med Chem. 2007;14:827–845. doi: 10.2174/092986707780090954. [DOI] [PubMed] [Google Scholar]
- 6.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Curr Med Chem. 2013;20(20):2572–2582. doi: 10.2174/09298673113209990120. [DOI] [PubMed] [Google Scholar]
- 7.Andres S., Pevny S., Ziegenhagen R., Bakhiya N., Schäfer B., Hirsch-Ernst K.I., et al. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutrit Food Res. 2018;62(1):1–15. doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- 8.Annunziata G., Sanduzzi Zamparelli M., Santoro C., Ciampaglia R., Stornaiuolo M., et al. May polyphenols aave a role against coronavirus infection? An overview of in vitro evidence. Front Med. 2020;7:1–7. doi: 10.3389/fmed.2020.00240. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aparicio-soto M., Redhu D., Sánchez-hidalgo M., Babina M. Olive-oil-derived polyphenols effectively attenuate inflammatory responses of human keratinocytes by interfering with the NF- κB pathway. Mol Nutrit Food Res. 2019;63(21) doi: 10.1002/mnfr.201900019. [DOI] [PubMed] [Google Scholar]
- 10.Appeldoorn M.M., Vincken J.P., Aura A.M., Hollman P.C.H., Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ- valerolactone as the major metabolites. J Agricult Food Chem. 2009;57(3):1084–1092. doi: 10.1021/jf803059z. [DOI] [PubMed] [Google Scholar]
- 11.Asher G.N., Corbett A.H., Hawke R.L. Common herbal dietary supplement-drug interactions. Am Family Phys. 2017;96(2):101–107. [PubMed] [Google Scholar]
- 12.Ashikawa K., Majumdar S., Banerjee S., Bharti A.C., Shishodia S., Aggarwal B.B. Piceatannol inhibits TNF-induced NF-κB activation and NF-κB-mediated gene expression through suppression of IκBα kinase and p65 hosphorylation. J Immunol. 2002;169(11):6490–6497. doi: 10.4049/jimmunol.169.11.6490. [DOI] [PubMed] [Google Scholar]
- 13.Berginc K., Milisav I., Kristl A. Garlic Flavonoids and organosulfur compounds:iImpact on the hepatic pharmacokinetics of saquinavir and darunavir. Drug Metab Pharmacokinetics. 2010;25(6):521–530. doi: 10.2133/dmpk.DMPK-10-RG-053. [DOI] [PubMed] [Google Scholar]
- 14.Bode L.M., Bunzel D., Huch M., Cho G.S., Ruhland D., Bunzel M., et al. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutrit. 2013;97(2):295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- 15.Biancatelli R.M.L.C., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020;11:1–11. doi: 10.3389/fimmu.2020.01451. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burak C., Brüll V., Langguth P., Zimmermann B.F., Stoffel-Wagner B., Sausen U., et al. Higher plasma quercetin levels following oral administration of an onion skin extract compared with pure quercetin dihydrate in humans. Eur J Nutrit. 2017;56(1):343–353. doi: 10.1007/s00394-015-1084-x. [DOI] [PubMed] [Google Scholar]
- 17.Cai Z.Y., Li X.M., Liang J.P., Xiang L.P., Wang K.R., Shi Y.L., et al. Bioavailability of tea catechins and its improvement. Molecules. 2018;23(9):10–13. doi: 10.3390/molecules23092346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020 doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerqueira A.M., Khaper N., Lees S.J., Ulanova M. Model of Pseudomonas aeruginosa infection of lung epithelial cells 1. Can J Physiol Pharmacol. 2013;255:248–255. doi: 10.1139/cjpp-2012-0268. January. [DOI] [PubMed] [Google Scholar]
- 20.Chachay V.S., Kirkpatrick C.M.J., Hickman I.J., Ferguson M., Prins J.B., Martin J.H. Resveratrol - pills to replace a healthy diet? Br J Clin Pharmacol. 2011;72(1):27–38. doi: 10.1111/j.1365-2125.2011.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Checconi P., De Angelis M., Marcocci M.E., Fraternale A., Magnani M., Palamara A.T., et al. Redox-modulating agents in the treatment of viral infections. Int J Mol Sci. 2020;21(11):1–21. doi: 10.3390/ijms21114084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C.N., Lin C.P.C., Huang K.K., Chen W.C., Hsieh H.P., Liang P.H., et al. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3,3′- digallate (TF3) Evidence-Based Complementary Alternat Med. 2005;2(2):209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C., Zuckerman D.M., Brantley S., Sharpe M., Childress K., Hoiczyk E., Pendleton A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet Res. 2014;10:24. doi: 10.1186/1746-6148-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C., Jiang X., Lai Y., Liu Y., Zhang Z. Resveratrol protects against arsenic trioxide-induced oxidative damage through maintenance of glutathione homeostasis and inhibition of apoptotic progression. Physiol Behav. 2016;176(12):139–148. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J., Yang J., Ma L., Li J., Shahzad N., Kim C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Scient Rep. 2020;10:2611. doi: 10.1038/s41598-020-59451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conte L., Toraldo D.M. Targeting the gut – lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Therapeut Adv Respir Dis. 2020;14:1–5. doi: 10.1177/1753466620937170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortés-Martín A., Selma M.V., Tomás-Barberán F.A., González-Sarrías A., Espín J.C. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol Nutrit Food Res. 2020;64(9):1–17. doi: 10.1002/mnfr.201900952. Tsilingiri. [DOI] [PubMed] [Google Scholar]
- 29.Cui Q., Fu Q., Zhao X., Song X., Yu J., Yang Y., et al. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PLoS One. 2018;13(2):1–11. doi: 10.1371/journal.pone.0192692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Archivio M., Filesi C., Varì R., Scazzocchio B., Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11(4):1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai T., Shi K., Chen G., Shen Y., Pan T. Malvidin attenuates pain and inflammation in rats with osteoarthritis by suppressing NF-κB signaling pathway. Inflamm Res. 2017;66(12):1075–1084. doi: 10.1007/s00011-017-1087-1096. [DOI] [PubMed] [Google Scholar]
- 32.Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (Poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants & Redox Signaling. 2013;18(14):1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgado-Roche L., Mesta F. Oxidative stress as key Player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deriu E., Boxx G.M., He X., Pan C., Benavidez S.D., Cen L., et al. Influenza virus affects intestinal microbiota and secondary Salmonella infection in the gut through Type I Interferons. PLoS Pathogens. 2016;12(5):1–26. doi: 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong W.W., Liu Y.J., Lv Z., Mao Y.F., Wang Y.W., Zhu X.Y., et al. Lung endothelial barrier protection by resveratrol involves inhibition of HMGB1 release and HMGB1-induced mitochondrial oxidative damage via an Nrf2-dependent mechanism. Free Rad Biol Med. 2015;88(Part B):404–416. doi: 10.1016/j.freeradbiomed.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Du G.J., Zhang Z., Wen X.D., Yu C., Calway T., Yuan C.S., et al. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4(11):1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dueñas M., Surco-Laos F., González-Manzano S., González-Paramás A.M., Santos-Buelga C. Antioxidant properties of major metabolites of quercetin. Eur Food Res Technol. 2011;232:103–111. doi: 10.1007/s00217-010-1363-y. [DOI] [Google Scholar]
- 38.Dueñas M., Muñoz-González I., Cueva C., Jiménez-Girón A., Sánchez-Patán F., Santos-Buelga C., et al. A Survey of modulation of gut microbiota by dietary polyphenols. BioMed Res Int. 2015 doi: 10.1155/2015/850902. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Kalamouni C., Frumence E., Bos S., Turpin J., Nativel B., Harrabi W., et al. Subversion of the heme oxygenase-1 antiviral activity by zika virus. Viruses. 2019;11(1):1–13. doi: 10.3390/v11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elsayed S., Zhang K. Human infection caused by Clostridium hatheawayi. Emerg Infect Dis. 2004;10(11):1950–1952. doi: 10.3201/eid1011.040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espín J.C., González-Sarrías A., Tomás-Barberán F.A. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017;139:82–93. doi: 10.1016/j.bcp.2017.04.033. sep. [DOI] [PubMed] [Google Scholar]
- 42.Falchetti R., Fuggetta M.P., Lanzilli G., Tricarico M., Ravagnan G. Effects of resveratrol on human immune cell function. Life Sci. 2001;70(1):81–96. doi: 10.1016/S0024-3205(01)01367-4. [DOI] [PubMed] [Google Scholar]
- 43.Ferlazzo N., Visalli G., Smeriglio A., Cirmi S., Lombardo G.E., Campiglia P., et al. Flavonoid fraction of orange and bergamot juices protect human lung epithelial cells from hydrogen peroxide-induced oxidative stress. Evidence-Based Complementary Alt Med. 2015 doi: 10.1155/2015/957031. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Food and drug administration . 2010. GRAS notice for high-purity quercetin; pp. 1–41. [Google Scholar]
- 45.Forman H.J., Davies K.J.A., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavengingin vivo. Free Rad Biol Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forrester J.D., Spain D.A. Clostridium ramosum bacteremia: case report and literature review. Surg Infect. 2014;15(3):343–346. doi: 10.1089/sur.2012.240. [DOI] [PubMed] [Google Scholar]
- 47.Fraga C.G., Croft K.D., Kennedy D.O., Tomás-Barberán F.A. The effects of polyphenols and other bioactives on human health. Food Function. 2019;10(2):514–528. doi: 10.1039/c8fo01997e. [DOI] [PubMed] [Google Scholar]
- 48.Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med. 2010;31(6):435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Fuggetta M.P., Bordignon V., Cottarelli A., Macchi B., Frezza C., Cordiali-fei P., et al. Downregulation of proinflammatory cytokines in HTLV-1-infected T cells by Resveratrol. J Exp Clin Cancer Res. 2016;35:118. doi: 10.1186/s13046-016-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gambini J., Inglés M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., et al. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med Cellular Longevity. 2015 doi: 10.1155/2015/837042. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao K., Xu A., Krul C., Venema K., Liu Y., Niu Y., et al. Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J Nutrit. 2006;136(1):52–57. doi: 10.1093/jn/136.1.52. [DOI] [PubMed] [Google Scholar]
- 52.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geva-Zatorsky N., Sefik E., Kua L., Pasman L., Tan T.G., Ortiz-Lopez A., et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168(5):928–943. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosh R., Chakraborty A., Biswas A., Chowdhuri S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors - an in silico docking and molecular dynamics simulation study. J Biomol Struct Dyn. 2020;0(0):1–13. doi: 10.1080/07391102.2020.1779818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glinsky G.V. Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines. 2020;8:129. doi: 10.3390/biomedicines8050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gould K.S., Lister C., Andersen O.M., Markham K.R. Flavonoids, chemistry, biochemistry and applications. CRC Press; Boca Raton: 2006. Flavonoid functions in plants; pp. 397–442. (Eds) [Google Scholar]
- 57.Gu S., Chen Y., Wu Z., Chen Y., Gao H., Lv L., et al. Alterations of the gut microbiota in patients with COVID-19 or H1N1 influenza. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa709. ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ha S.K., Park H.Y., Eom H., Kim Y., Choi I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food Chem Toxicol. 2012;50(10):3498–3504. doi: 10.1016/j.fct.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Hahn M., Baierle M., Charão M.F., Bubols G.B., Gravina F.S., Zielinsky P., et al. Polyphenol-rich food general and on pregnancy effects: a review. Drug Chem Toxicol. 2017;40(3):368–374. doi: 10.1080/01480545.2016.1212365. [DOI] [PubMed] [Google Scholar]
- 60.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020 doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu J., Webster D., Cao J., Shao A. The safety of green tea and green tea extract consumption in adults – results of a systematic review. Regulatory Toxicol Pharmacol. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. March. [DOI] [PubMed] [Google Scholar]
- 63.Huguet-Casquero A., Moreno-Sastre M., López-Méndez T.B., Gainza E., Pedraz J.L. Encapsulation of oleuropein in nanostructured lipid carriers: Biocompatibility and antioxidant efficacy in lung epithelial cells. Pharmaceutics. 2020;12(5):429. doi: 10.3390/pharmaceutics12050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang B.Y., Lee J.H., Koo T.H., Kim H.S., Hong Y.S., Ro J.S., et al. Kaurane diterpenes from Isodon japonicus inhibit nitric oxide and prostaglandin E2 production and NF-κB activation in LPS-stimulated macrophage RAW264.7 cells. Planta Medica. 2001;67(5):406–410. doi: 10.1055/s-2001-15808. [DOI] [PubMed] [Google Scholar]
- 65.Hybertson B.M., Gao B., Bose S., McCord J.M. Phytochemical combination PB125 activates the Nrf2 pathway and induces cellular protection against oxidative injury. Antioxidants. 2019;8(5):1–21. doi: 10.3390/antiox8050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iddir M., Brito A., Dingeo G., Del Campo S.S.F., Samouda H., La Frano M.R., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the covid-19 crisis. Nutrients. 2020;12(6):1–39. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Infusino F., Marazzato M., Mancone M., Fedele F., Mastroianni C.M., Severino P., et al. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection: a scoping review. Nutrients. 2020;12(6):1–21. doi: 10.3390/nu12061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isbrucker R.A., Edwards J.A., Wolz E., Davidovich A., Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44(5):636–650. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Ishikawa C., Senba M., Mori N. Butein inhibits NF-κB, AP-1 and Akt activation in adult T-cell Leukemia/Lymphoma. Int J Oncol. 2017;51(2):633–643. doi: 10.3892/ijo.2017.4026. [DOI] [PubMed] [Google Scholar]
- 70.Jasso-Miranda C., Herrera-Camacho I., Flores-Mendoza L.K., Dominguez F., Vallejo-Ruiz V., Sanchez-Burgos G.G., et al. Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infect Drug Resist. 2019;12:1833–1852. doi: 10.2147/IDR.S210890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.John C.M., Sandrasaigaran P., Kong C., Adam A., Ramasamy R. Immunomodulatory activity of polyphenols derived from Cassia auriculata flowers in aged rats. Cellular Immunol. 2011;271(2):474–479. doi: 10.1016/j.cellimm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Kawabata K., Yoshioka Y., Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24(2):370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kesic M.J., Simmons S.O., Bauer R., Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radical Biol Med. 2011;51(2):444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klingensmith N.J., Chen C., Liang Z., Burd E.M., Farris A.B., Arbiser J.L., et al. Honokiol increases CD4+ T cell activation and decreases TNF but fails to improve survival following sepsis. Physiology & Behavior. 2019;176(3):139–148. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knop J., Misaka S., Singer K., Hoier E., Muller F., Glaeser H., et al. Inhibitory effects of green tea and (-)-epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-glycoprotein. PLoS One. 2015;10(10):1–13. doi: 10.1371/journal.pone.0139370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kocaadam B., Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Critical Rev Food Sci Nutrit. 2017;57(13):2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- 77.Kode A., Rajendrasozhan S., Caito S., Yang S.R., Megson I.L., Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol - Lung Cellular Mol Physiol. 2008;294(3):478–488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 78.Koh A., de Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 79.Komaravelli N., Ansar M., Garofalo R.P., Casola A. Respiratory syncytial virus induces NRF2 degradation through a promyelocytic leukemia protein - ring finger protein 4 dependent pathway. Free Radical Biol Med. 2017;113:494–504. doi: 10.1016/j.freeradbiomed.2017.10.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar P., Khanna M., Srivastava V., Tyagi Y.K., Raj H.G., Ravi K. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp Lung Res. 2005;31(5):449–459. doi: 10.1080/019021490927088. [DOI] [PubMed] [Google Scholar]
- 81.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017;174(11):1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kyselova Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdisciplinary Toxicol. 2011;4(4):173–183. doi: 10.2478/v10102-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lam W.H., Kazi A., Kuhn D.J., Chow L.M.C., Chan A.S.C., Ping Dou Q., et al. A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (-)-epigallocatechin gallate [(-)-EGCG] Bioorganic Med Chem. 2004;12(21):5587–5593. doi: 10.1016/j.bmc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Lanping M.A., Zaiqun L.I.U., Bo Z., Li Y., Zhongli L.I.U. Inhibition of free radical induced oxidative hemolysis of red blood cells by green tea polyphenols. Chin Sci Bull. 2000;45(22):2052–2056. [Google Scholar]
- 86.Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxidative Med Cellular Longevity. 2018 doi: 10.1155/2018/6208067. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J., Mitchell A.E. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J Agricult Food Chem. 2012;60(15):3874–3881. doi: 10.1021/jf3001857. [DOI] [PubMed] [Google Scholar]
- 88.Lee N., Shin M.S., Kang Y., Park K., Maeda T., Nishioka H., et al. Oligonol, a lychee fruit-derived low-molecular form of polyphenol mixture, suppresses inflammatory cytokine production from human monocytes. Hum Immunol. 2016;77(6):512–515. doi: 10.1016/j.humimm.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levy E., Delvin E., Marcil V., Spahis S. May phytotherapy with polyphenols serve as a powerful approach for the prevention and therapy tool of novel coronavirus disease 2019 (Covid-19)? Am J Physiol Endocrinol Metab. 2020;319(4):E689–E708. doi: 10.1152/ajpendo.00298.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li H., Wu J., Zhang Z., Ma Y., Liao F., Zhang Y., Wu G. Forsythoside A inhibits the avian infectious bronchitis virus in cell culture. Phytother Res. 2011;25(3):338–342. doi: 10.1002/ptr.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduction Targeted Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):1–4. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lung J., Lin Y.S., Yang Y.H., Chou Y.L., Shu L.H., Cheng Y.C., et al. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J Med Virol. 2020;92(6):693–697. doi: 10.1002/jmv.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magro G. COVID-19: review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198070. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahmudpour M., Roozbeh J., Keshavarz M., Farrokhi S., Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahomoodally F., Ramcharun S., Zenginhomoodally G. Onion and garlic extracts potentiate the efficacy of conventional antibiotics against standard and clinical bacterial isolates. Curr Top Med Chem. 2018;18(9):787–796. doi: 10.2174/1568026618666180604083313. [DOI] [PubMed] [Google Scholar]
- 98.Mani J.S., Johnson J.B., Steel J.C., Broszczak D.A., Neilsen P.M., Walsh K.B., et al. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res. 2020;284 doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manrrique Margarita, Garcia A. P2Et extract in the symptomatic treatment of subjects with COVID-19. 2020. https://clinicaltrials.gov/ct2/show/record/NCT04410510 Clinical trialAvailable in. [Google Scholar]
- 100.Maraki S., Papadakis I.S. Rothia mucilaginosa pneumonia: a literature review. Infect Dis. 2015;47(3):125–129. doi: 10.3109/00365548.2014.980843. [DOI] [PubMed] [Google Scholar]
- 101.Marinella M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19. Int J Clin Pract. 2020;74(9):e13535. doi: 10.1111/ijcp.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martindale R., Patel J.J., Taylor B., Arabi Y.M., Warren M., McClave S.A. Nutrition therapy in critically Ill patients with coronavirus disease (COVID-19) J Parenteral Enteral Nutrit. 2020;44(7):174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martins N., Barros L., Ferreira I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci Technol. 2016;48:1–12. doi: 10.1016/j.tifs.2015.11.008. [DOI] [Google Scholar]
- 104.Maurer L.H., Cazarin C.B.B., Quatrin A., Minuzzi N.M., Costa E.L., Morari J., et al. Grape peel powder promotes intestinal barrier homeostasis in acute TNBS-colitis: a major role for dietary fiber and fiber-bound polyphenols. Food Res Int. 2019;123:425–439. doi: 10.1016/j.foodres.2019.04.068. April. [DOI] [PubMed] [Google Scholar]
- 105.Maurya V.K., Kumar S., Prasad A.K., Bhatt M.L.B., Saxena S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virus Dis. 2020;31(2):179–193. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mayo B., Vázquez L., Flórez A.B. Equol: a bacterial metabolite from the Daidzein isoflavone and its presumed beneficial health effects. Nutrients. 2019;11(9):2231. doi: 10.3390/nu11092231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McCord J.M., Hybertson B.M., Cota-Gomez A., Geraci K.P., Gao B. Nrf2 activator pb125® as a potential therapeutic agent against covid-19. Antioxidants. 2020 doi: 10.3390/antiox9060518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McKay D.L., Chen C-Y.O., Zampariello C.A., Blumberg J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015;168:233–240. doi: 10.1016/j.foodchem.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 109.Mostafai A. Colchicine plus phenolic monoterpenes to treat COVID-19. 2020. https://www.clinicaltrials.gov/ct2/show/NCT04392141 Clinical trialAvailable in. [Google Scholar]
- 110.Melgarejo E., Medina M.Á., Sánchez-Jiménez F., Urdiales J.L. Epigallocatechin gallate reduces human monocyte mobility and adhesion in vitro. Br J Pharmacol. 2009;158(7):1705–1712. doi: 10.1111/j.1476-5381.2009.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mhatre S., Srivastava T., Naik S., Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153286. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miles E.A., Zoubouli P., Calder P.C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition. 2005;21(3):389–394. doi: 10.1016/j.nut.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 114.Mirabelli M., Chiefari E., Arcidiacono B., Corigliano D.M., Brunetti F.S., Maggisano V., et al. Mediterranean diet nutrients to turn the tide against insulin resistance and related diseases. Nutrients. 2020;12(4):1066. doi: 10.3390/nu12041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miripour Z.S., Sarrami-Forooshani R., Sanati H., Makarem J., Taheri M.S., Shojaeian D., et al. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosensors Bioelectr. 2020;165 doi: 10.1016/j.bios.2020.112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Molino S., Fernández-Miyakawa M., Giovando S., Rufián-Henaresa J.A. Study of antioxidant capacity and metabolization of quebracho and chestnut tannins through in vitro gastrointestinal digestion-fermentation. J Funct Foods. 2018;49:188–195. doi: 10.1016/j.jff.2018.07.056. [DOI] [Google Scholar]
- 117.Moynagh P.N. The NF-κB pathway. J Cell Sci. 2005;118(20):4589–4592. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- 118.National Health Committee of the People's Republic of China. National Administration of Traditional Chinese Medicine Diagnostic and therapeutic guidance for 2019 novel coronavirus disease (version 5). (n.d.). Retrieved July 26, 2020, from http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf
- 119.Ngwa W., Kumar R., Thompson D., Lyerly W., Moore R., Reid T., et al. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules. 2020;25:2707. doi: 10.3390/molecules25112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oketch-Rabah H.A., Roe A.L., Rider C.V., Bonkovsky H.L., Giancaspro G.I., Navarro V., et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep. 2020;7:386–402. doi: 10.1016/j.toxrep.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olagnier D., Peri S., Steel C., van Montfoort N., Chiang C., Beljanski V., et al. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathogens. 2014;10(12):1–18. doi: 10.1371/journal.ppat.1004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ou C., Shi N., Yang Q., Zhang Y., Wu Z., Wang B., et al. Protocatechuic acid, a novel active substance against avian influenza virus H9N2 infection. PLoS One. 2014;9(10):3–10. doi: 10.1371/journal.pone.0111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paiva-Martins F., Fernandes J., Rocha S., Nascimento H., Vitorino R., Amado F., et al. Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol Nutrit Food Res. 2009;53(5):609–616. doi: 10.1002/mnfr.200800276. [DOI] [PubMed] [Google Scholar]
- 124.Park J.Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., et al. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhibit Med Chem. 2017;32(1):504–512. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pavithra1 B.H., Prakash N., Jayakumar K. Modification of pharmacokinetics of norfloxacin following oral administration of curcumin in rabbits. J Vet Sci. 2009;10(4):293–297. doi: 10.4142/jvs.2009.10.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pepe G., Sommella E., Cianciarulo D., Ostacolo C., Manfra M., Di Sarno V., et al. Polyphenolic extract from tarocco (Citrus sinensis l. osbeck) clone “lempso” exerts anti-inflammatory and antioxidant effects via NF-κB and Nrf-2 activation in murine macrophages. Nutrients. 2018;10(12):1961. doi: 10.3390/nu10121961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pereira D.M., Valentão P., Pereira J.A., Andrade P.B. Phenolics: from chemistry to biology. Molecules. 2009;14(6):2202–2211. doi: 10.3390/molecules14062202. [DOI] [Google Scholar]
- 128.Piskorz M.M. Tannin Specific Natural Extract for COVID-19 Infection (TaCOVID) 2020. https://clinicaltrials.gov/ct2/show/NCT04403646 Clinical Trials, available in. [Google Scholar]
- 129.Quatrin A., Rampelotto C., Pauletto R., Maurer L.H., Nichelle S.M., Klein B., et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J Funct Foods. 2020;65 doi: 10.1016/j.jff.2019.103714. [DOI] [Google Scholar]
- 130.Raimundo A.F., Felix F., Andrade R., Garcia-Conesa M.T., Gonzalez-Sarrias A., Gilsa-Lopes J., et al. Combined effect of interventions with pure or enriched mixtures of (poly)phenols and anti-diabetic medication in type diabetes management: a meta-analysis of randomized controlled human trials. Eur J Nutrit. 2020;59(4):1329–1343. doi: 10.1007/s00394-020-02189-1. [DOI] [PubMed] [Google Scholar]
- 131.Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72(11):1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Ramírez-Garza S.L., Laveriano-Santos E.P., Marhuenda-Muñoz M., Storniolo C.E., Tresserra-Rimbau A., Vallverdú-Queralt A., et al. Health effects of resveratrol: Results from human intervention trials. Nutrients. 2018;10(12):1–18. doi: 10.3390/nu10121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rasyid A., Rahman A.R.A., Jaalam K., Lelo A. Effect of different curcumin dosages on human gall bladder. Asia Pacific J Clin Nutrit. 2002;11(4):314–318. doi: 10.1046/j.1440-6047.2002.00296.x. [DOI] [PubMed] [Google Scholar]
- 134.Reddy A.M., Seo J.H., Ryu S.Y., Kim Y.S., Kim Y.S., Min K.R., et al. Cinnamaldehyde and 2-methoxycinnamaldehyde as NF-κB inhibitors from Cinnamomum cassia. Planta Medica. 2004;70(9):823–827. doi: 10.1055/s-2004-827230. [DOI] [PubMed] [Google Scholar]
- 135.Revin V.V., Gromova N.V., Revina E.S., Samonova A.Y., Tychkov A.Y., Bochkareva S.S., et al. The influence of oxidative stress and natural antioxidants on morphometric parameters of red blood cells, the hemoglobin oxygen binding capacity, and the activity of antioxidant enzymes. BioMed Res Int. 2019 doi: 10.1155/2019/2109269. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roh C. A facile inhibitor screening of SARS coronavirus N protein using nanoparticle-based RNA oligonucleotide. Int J Nanomed. 2012;7:2173–2179. doi: 10.2147/IJN.S31379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A Structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9(5):1267. doi: 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salehi B., Azzini E., Zucca P., Varoni E.M., Kumar N.V.A., Dini L., et al. Plant-derived bioactives and oxidative stress-related disorders: a key trend towards healthy aging and longevity promotion. Appl Sci (Switzerland) 2020;10(3):947. doi: 10.3390/app10030947. [DOI] [Google Scholar]
- 140.Sangeetha M.K., Eazhisai Vallabi D., Sali V.K., Thanka J., Vasanthi H.R. Sub-acute toxicity profile of a modified resveratrol supplement. Food Chem Toxicol. 2013;59:492–500. doi: 10.1016/j.fct.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 141.Schwarz K.B. Oxidative stress during viral infection: a review. Free Rad Biol Med. 1996 doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 142.Sencio V., Barthelemy A., Tavares L.P., Machado M.G., Soulard D., Cuinat C., et al. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 2020;30(9):2934–2947.e6. doi: 10.1016/j.celrep.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 143.Serafini M., Peluso I., Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutrit Soc. 2010;69(3):273–278. doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 144.Serreli G., Deiana M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019;10(11):6999–7021. doi: 10.1039/c9fo01733j. [DOI] [PubMed] [Google Scholar]
- 145.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shi D.D., Dong C.M., Ho L.C., Lam C.T.W., Zhou X.D., Wu E.X., et al. Resveratrol, a natural polyphenol, prevents chemotherapy-induced cognitive impairment: involvement of cytokine modulation and neuroprotection. Neurobiol Dis. 2018;114:164–173. doi: 10.1016/j.nbd.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 147.Shinde T., Hansbro P.M., Sohal S.S., Dingle P., Eri R., Stanley R. Microbiota modulating nutritional approaches to countering the effects of viral respiratory infections including SARS-CoV-2 through promoting metabolic and immune fitness with probiotics and plant bioactives. Microorganisms. 2020;8(6):921. doi: 10.3390/microorganisms8060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Siemens N., Oehmcke-Hecht S., Mettenleiter T.C., Kreikemeyer B., Valentin-Weigand P., Hammerschmidt S. Port d'Entrée for respiratory infections - does the influenza a virus pave the way for bacteria? Front Microbiol. 2017;8:2602. doi: 10.3389/fmicb.2017.02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singh A.K., Cabral C., Kumar R., Ganguly R., Rana H.K., Gupta A., et al. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients. 2019;11(9):2216. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Solinas C., Perra L., Aiello M., Migliori E., Petrosillo N. A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine Growth Factor Rev. 2020;54:8–23. doi: 10.1016/j.cytogfr.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Spencer J.P.E., Crozier A. CRC Press; Boca Raton, FL: 2012. Flavonoids and related compounds: bioavailability and function (oxidative stress and disease) [Google Scholar]
- 152.Staitieh B.S., Ding L., Neveu W.A., Spearman P., Guidot D.M., Fan X. HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages. J Leukocyte Biol. 2017;102(2):517–525. doi: 10.1189/jlb.4a0616-282rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Talukdar J., Bhadra B., Dattaroy T., Nagle V., Dasgupta S. Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19. Biomed Pharmacother. 2020 doi: 10.1016/j.biopha.2020.110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tedesco I., Russo M., Russo P., Iacomino G., Russo G.L., Carraturo A., et al. Antioxidant effect of red wine polyphenols on red blood cells. J Nutrit Biochem. 2000;11(2):114–119. doi: 10.1016/S0955-2863(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 155.Tresserra-Rimbau A., Lamuela-Raventos R.M., Moreno J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem Pharmacol. 2018;156:186–195. doi: 10.1016/j.bcp.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 156.Umathe S.N., Dixit P.V., Kumar V., Bansod K.U., Wanjari M.M. Quercetin pretreatment increases the bioavailability of pioglitazone in rats: involvement of CYP3A inhibition. Biochem Pharmacol. 2008;75(8):1670–1676. doi: 10.1016/j.bcp.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 157.US Department of Health and Human Services Toxicology and Carcinogenesis Studies of in F344 /N Rats. Natl Toxicol Program Tech Rep Ser. 1992;409(275):1–171. [PubMed] [Google Scholar]
- 158.Utomo R.Y., Ikawati M., Meiyanto E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. 2020. Preprints, 2020030214. [DOI] [Google Scholar]
- 159.Valizadeh H., Abdolmohammadi-vahid S., Danshina S., Gencerf M.Z., Ammarig A., Sadeghia A., et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vang O., Ahmad N., Baile C.A., Baur J.A., Brown K., Csiszar A., et al. What is new for an old molecule? systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6(6):e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Veberic R., Jakopic J., Stampar F., Schmitzer V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009;114(2):511–515. doi: 10.1016/j.foodchem.2008.09.080. [DOI] [Google Scholar]
- 162.Wahedi H.M., Ahmad S., Abbasi S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J Biomol Struct Dyn. 2021;39(9) doi: 10.1080/07391102.2020.1762743. [DOI] [PubMed] [Google Scholar]
- 163.Wang D., Xia M., Yan X., Li D., Wang L., Xu Y., et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111(8):967–981. doi: 10.1161/CIRCRESAHA.112.266502. [DOI] [PubMed] [Google Scholar]
- 164.Wang D., Ho L., Faith J., Ono K., Janle E.M., Lachcik P.J., et al. Role of intestinal microbiota in the generation of polyphenol derived phenolic acid mediated attenuation of Alzheimer's disease β-amyloid oligomerization. Mol Nutrit Food Res. 2015;59(6):1025–1040. doi: 10.1002/mnfr.201400544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J, Liu Y.T., Xiao L., Zhu L., Wang Q., Yan T. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation. 2014;37(6):2085–2090. doi: 10.1007/s10753-014-9942-x. [DOI] [PubMed] [Google Scholar]
- 166.Wang J, Ren Z., Xu Y., Xiao S., Meydani S.N., Wu D. Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4 + T-cell subsets. Am J Pathol. 2012;180(1):221–234. doi: 10.1016/j.ajpath.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Warden B.A., Smith L.S., Beecher G.R., Balentine D.A., Clevidence B.A. Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutrit. 2001;131(6):1731–1737. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- 168.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50(17):4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 170.Williams L.D., Burdock G.A., Edwards J.A., Beck M., Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47(9):2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 171.Williamson G., Kerimi A. Testing of natural products in clinical trials targeting the SARS-CoV-2 (Covid-19) viral spike protein-angiotensin converting enzyme-2 (ACE2) interaction. Biochem Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.World Health Organization . WHO Coronavirus Disease (COVID-19) Dashboard. 2021. https://covid19.who.int/ Retrieved February 21, 2021, from. [PubMed] [Google Scholar]
- 173.Wu J. Nitric Oxide - Biology and Chemistry. 2020. Tackle the free radicals damage in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu D., Wang J., Pae M., Meydani S.N. Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Mol Aspects Med. 2012;33(1):107–118. doi: 10.1016/j.mam.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 175.Wu R., Wang L., Kuo H.C.D., Shannar A., Peter R., Chou P.J., et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020 doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu L., Botchway B.O.A., Zhang S., Zhou J., Liu X. Inhibition of NF-κB signaling pathway by resveratrol improves spinal cord injury. Front Neurosci. 2018;12:1–10. doi: 10.3389/fnins.2018.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang C.S., Chen L., Lee M.-J., Balentine D., Kuo M.C., Schantz S.P. Blood and urine levels of tea catechins after ingestions of different amounts of green tea by human volunteers. Prevention. 1998;7:351–354. [PubMed] [Google Scholar]
- 178.Yang T., Chakraborty S., Saha P., Mell B., Cheng X., Yeo J.Y., et al. Gnotobiotic rats reveal that gut microbiota regulates colonic mRNA of ACE2, the receptor for SARS-CoV-2 infectivity. Hypertension. 2020;76(1):8–10. doi: 10.1161/HYPERTENSIONAHA.120.15360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yasui M., Matsushima M., Omura A., Mori K., Ogasawara N., Kodera Y., et al. The Suppressive effect of quercetin on toll-like receptor 7-mediated activation in alveolar macrophages. Pharmacology. 2015;96(5–6):201–209. doi: 10.1159/000438993. [DOI] [PubMed] [Google Scholar]
- 180.Yates A.A., Erdman J.W., Shao A., Dolan L.C., Griffiths J.C. Bioactive nutrients - time for tolerable upper intake levels to address safety. Regulatory Toxicol Pharmacol. 2017;84:94–101. doi: 10.1016/j.yrtph.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 181.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’’ in COVID-19.’. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78(20):11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu M., Lee J., Lee J.M., Kim Y., Chin Y., Jee J., et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorganic Med Chem Lett. 2012;22:4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yu B., Xie R., Jin L., Tian X., Niu Y., Ma T., et al. Trans-δ-Viniferin inhibits Ca(2+)-activated Cl(-) channels and improves diarrhea symptoms. Fitoterapia. 2019;139 doi: 10.1016/j.fitote.2019.104367. [DOI] [PubMed] [Google Scholar]
- 185.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yun K.J., Koh D.J., Kim S.H., Park S.J., Ryu J.H., Kim D.G., et al. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-κB inactivation. J Agricult Food Chem. 2008;56(21):10265–10272. doi: 10.1021/jf802095g. [DOI] [PubMed] [Google Scholar]
- 187.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-Rasadi K., et al. Potential effects of curcumin in the treatment of COVID -19 infection. Phytother Res. 2020 doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zahlten J., Kim Y.J., Doehn J.M., Pribyl T., Hocke A.C., García P., et al. Streptococcus pneumoniae-induced oxidative stress in lung epithelial cells depends on pneumococcal autolysis and is reversible by resveratrol. J Infect Dis. 2015;211(11):1822–1830. doi: 10.1093/infdis/jiu806. [DOI] [PubMed] [Google Scholar]
- 189.Zhang T.T., Hu T., Jiang J.G., Zhao J.W., Zhu W. Antioxidant and anti-inflammatory effects of polyphenols extracted from Ilex latifolia Thunb. RSC Adv. 2018;8(13):7134–7141. doi: 10.1039/c7ra13569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/