Abstract
Prenatal micronutrient supplements are cost‐effective in reducing nutritional deficiencies and adverse pregnancy and birth outcomes. However, poor adherence remains a potential barrier to the successful implementation of these supplementation programs. This systematic review assessed the effectiveness of interventions designed to increase adherence to prenatal micronutrient supplementation. Following the Cochrane Collaboration Methodology, literature searches were conducted in six electronic databases and gray literature (on July 24, 2020), and abstract screening, data extraction, and risk of bias assessment were conducted independently by two reviewers. We included 22 studies. Interventions that resulted in increased adherence were most of the education‐based strategies, consumption monitoring by volunteer health workers or family members, SMS reminders, free provision of supplements, a multicomponent intervention with community mobilization, and a participatory action research intervention. In several studies, increased adherence was accompanied by beneficial effects on pregnancy and birth outcomes. Given the heterogeneity of study designs and methods used to define and measure adherence, a meta‐analysis was not appropriate. We identified several potentially effective strategies to improve supplementation adherence, which may need to be adapted to specific contexts when considered for program implementation. However, additional high‐quality studies are critically needed to effectively guide policies and programs.
Keywords: pregnancy, micronutrients, supplementation, adherence, compliance
Prenatal micronutrient supplements are cost effective in reducing nutritional deficiencies and adverse pregnancy and birth outcomes. However, poor adherence remains a major barrier to the successful implementation of these supplementation programs. This systematic review assessed the effectiveness of interventions designed to increase adherence to prenatal micronutrient supplementation.
Introduction
The first 1000 days of life, starting at conception and continuing to 2 years of age, are a critical window for growth and development. 1 Pregnancy requires an adequate intake of key micronutrients (such as vitamins A, D, E, B6, folic acid, B12, and C, and the minerals iron, zinc, iodine, copper, and selenium) to accommodate maternal and fetal demands. Many of these micronutrients are required in increased doses, some by as much as 50%, during this critical stage of life. 2 , 3 Micronutrient deficiencies during pregnancy have immediate and long‐term detrimental effects, such as maternal mortality, pregnancy loss, congenital disorders, low birth weight, mortality in infancy, stunting, as well as increased risk of impaired cognitive development and cardiometabolic risk later in life. 2 , 4 , 5 Thus, prenatal micronutrient supplementation is recommended to address these vitamin and mineral deficiencies and to reduce the risk of adverse pregnancy and birth outcomes. Single‐nutrient interventions that have been shown to be effective in improving maternal and child outcomes include supplementation with iron to reduce iron deficiency anemia and low birth weight, preconceptional folic acid to prevent neural tube defects, iodine to prevent congenital iodine deficiency syndrome, zinc to reduce the risk of preterm birth, and calcium to reduce preeclampsia in populations with low calcium consumption. 2 , 3 Recent systematic reviews of randomized trials have also shown that multiple micronutrient supplements, containing 15 vitamins and minerals designed to address the varying micronutrient needs of pregnant women, result in decreased risks of stillbirth, low birth weight, preterm birth, and being born small‐for‐gestational age, 6 , 7 and are a cost‐effective intervention when compared with iron and folic acid (IFA) supplements. 4 , 8
Despite the availability and demonstrated efficacy of prenatal micronutrient supplements, the implementation of such interventions continues to be challenging—a special concern since micronutrient deficiencies during pregnancy remain highly prevalent, particularly in low‐ and middle‐income countries (LMICs). For instance, the 2019 Global Nutrition Report shows that anemia still affects 40% of pregnant women worldwide, and none of the 194 evaluated countries was on track to meet the 2025 Global Nutrition Target of reducing anemia by 50% in women of reproductive age. 9 Poor adherence is one of the main barriers to a successful micronutrient supplementation program, even in settings with high coverage rates (i.e., when a large proportion of pregnant women receive the supplements). An analysis of Demographic and Health Surveys from 22 LMICs showed that while 83% of pregnant women had at least one antenatal care visit and 81% received IFA supplements during that visit, only 8% adhered to the recommended dose (defined as at least 180 tablets during the entire pregnancy). 10 Another large‐scale survey conducted in China showed similar results, even though it used a lower cutoff to define adherence (i.e., 90 tablets), with adherence varying by micronutrient, from 0.6% for iron up to only 11.7% for calcium supplements. 11
Adherence has been defined as “the extent to which a patient's behavior matches the agreed recommendations from a healthcare provider.” 12 Many behavior theories exist to explain the constructs related to adopting positive healthy behavior. In particular, within the literature that has examined adherence to micronutrient supplements in pregnancy, awareness and knowledge have been considered influential constructs for behavior adoption. 13 Furthermore, other factors, such as age, education level, unplanned pregnancy, lack of time, forgetfulness, high cost, side effects, or difficulty in taking tablets, are also known to influence adherence. 14 , 15 , 16 Understanding which strategies will lead pregnant women to increased prenatal micronutrient supplement consumption will help maximize the potential benefit of this intervention. 17 Examples of such strategies could include training healthcare professionals, delivering individual counseling or group educational sessions to pregnant women, sending reminders through text messages, providing financial incentives, and providing family and peer support. This research question was, to some extent, addressed in a previous systematic review of studies designed to increase awareness, knowledge, and consumption of folic acid before and during pregnancy. 13 However, this review published in 2008 focused only on folic acid, only included studies published between 1992 and 2005, and most interventions were delivered at the population level (without a control group). By contrast, our study aimed to systematically assess and synthesize all existing evidence about targeted interventions designed to increase adherence to any micronutrient supplementation during pregnancy.
Methods
This systematic review followed the Cochrane Collaboration Methodology 18 and the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) reporting guidelines, 19 as described in a previously published protocol. 20
The protocol has also been registered on the International Prospective Register of Systematic Reviews (PROSPERO), the University of York Centre for Reviews and Dissemination (https://www.crd.york.ac.uk/prospero/; registration number CRD42019146814).
Criteria for considering studies for this review
Types of studies
The study designs included in this review were randomized controlled trials and nonrandomized studies that included a comparison group. Studies without a comparison group were excluded.
Types of participants
The target population was pregnant women who were taking any micronutrient supplements in the context of antenatal care. There was no limit on the length of gestation at the time of enrollment in the study nor on the type of setting (from low‐ to high‐income countries; urban and rural areas). Studies conducted in institutionalized pregnant women were excluded because of the influence that institutionalization can have on adherence.
Types of interventions
We included studies that used targeted interventions designed to improve adherence (i.e., intake) to the recommended prenatal micronutrient supplementation regimen, such as family support, education, or counseling. We considered studies using any micronutrient or combination of micronutrients provided as a powder, liquid (e.g., syrups and suspensions), or pill/tablet, for any duration and frequency; however, the supplementation regimen (i.e., nutrient content and duration) provided in the different groups of the study had to be the same. We included studies that compared one targeted micronutrient adherence intervention to either (1) usual care or no intervention (i.e., no intervention aimed at improving adherence to micronutrient supplements) or (2) another targeted micronutrient adherence intervention. Interventions delivered at the population level (i.e., with no comparison group, such as mass media campaigns) or using fortified or enriched foods were excluded.
Types of outcome measures
The primary outcomes were adherence to micronutrient supplements, as defined by the study authors, and adverse gastrointestinal symptoms (nausea, vomiting, and diarrhea). Secondary outcomes were other adverse effects and pregnancy and birth outcomes, such as levels of hemoglobin and rates of anemia or low birth weight.
Search methods for identification of studies
The literature searches were conducted in six electronic bibliographic databases (MEDLINE (via PubMed), Embase, Scopus, Web of Science, Scielo, and Cochrane Library), as well as in the gray literature (WHO Library), from inception to July 24, 2020. There were no language or date restrictions. An example of a search strategy used in MEDLINE (via Pubmed) is provided in the Supplementary text (online only).
Study duplicates were removed initially in the bibliographic software Zotero (version 5.0.75), where all the results were merged, followed by further deduplication in the Covidence systematic review software. 21
Data collection and analysis
Selection of studies
All the retrieved results were screened against the eligibility criteria by two reviewers (i.e., two of the authors: D.D., J.G., S.K., A.S., and F.G.) independently, using Covidence. In the case of disagreement, a third independent reviewer (M.W.B.) resolved the conflict. Similarly, the full text of the potentially eligible studies was independently assessed by two review team members to determine whether they met the inclusion criteria. When necessary, study authors were contacted to obtain additional information and the reasons for exclusion were documented.
Data extraction and management
Data extraction was conducted independently by two reviewers (i.e., two of the authors: D.D., J.G., S.K., A.S., and F.G.), using a standardized data extraction template in Covidence, and a third independent reviewer identified and resolved discrepancies. Extracted data included study design, study setting (urban, rural, or mixed), classification of country by income (as per the 2020 World Bank classification: 22 low‐income, lower‐middle‐income, upper‐middle‐income, or high‐income economies), baseline characteristics related to the study population and participant demographics, description of the provided micronutrient supplement, description of the intervention used to increase adherence and comparison group(s), and details of the relevant outcomes (including definition and method used to measure adherence). Multiple attempts to contact study authors were made to obtain missing data or clarify questions related to the methodology of the study.
Assessment of risk of bias in included studies
The risk of bias assessment was conducted by two review authors independently, and disagreements were resolved by a third independent reviewer. The study design determined the tool used to assess risk of bias.
The risk of bias for randomized controlled trials was assessed with the 2011 Cochrane Collaboration's tool for assessing risk of bias using the following criteria: random sequence generation; allocation concealment; blinding of participants, personnel, and outcomes (assessed separately for adherence and pregnancy and birth outcomes); incomplete outcome data (assessed separately for adherence and pregnancy and birth outcomes); selective outcome reporting; and other sources of bias. 18 , 23 Results were categorized as low, unclear, or high risk of bias.
The risk of bias for nonrandomized studies was assessed with the “ROBINS‐I” (Risk Of Bias in Non‐randomised Studies of Interventions) tool. 24 This tool requires the assessment of seven risk‐of‐bias domains, including bias due to confounding; bias in the selection of participants into the study; bias in the classification of interventions; bias due to deviations from intended interventions; bias due to missing data (assessed separately for adherence and pregnancy and birth outcomes); bias in the measurement of outcomes (assessed separately for adherence and pregnancy and birth outcomes); and bias in the selection of the reported results. The results of the judgment for each domain and for the final overall bias were categorized as low, moderate, serious, or critical risk of bias, or no information.
Data synthesis and analysis
Given the heterogeneity of study designs of the included studies and the variable methodologies used to report and measure adherence, it was not possible to perform a meta‐analysis. Instead, a narrative analysis of the included studies was conducted, with a synthesis of all the interventions used and a description of the effects of these interventions.
Methodological amendments to the protocol
Subgroup and sensitivity analyses were initially planned as per the protocol, 20 but the inability to conduct a meta‐analysis rendered this impossible.
Results
Results of the search
A total of 10,135 abstracts were retrieved from the literature searches (Fig. 1). After discarding duplicates, 5563 abstracts were screened in duplicate for eligibility. Of the 72 full‐text articles that were assessed for eligibility, 50 were excluded for the following reasons: duplicate articles, conference abstracts, intervention delivered at the population level (lack of the comparator group), and ineligible study design or intervention or target population. Ultimately, 22 studies were included in this review.
Figure 1.
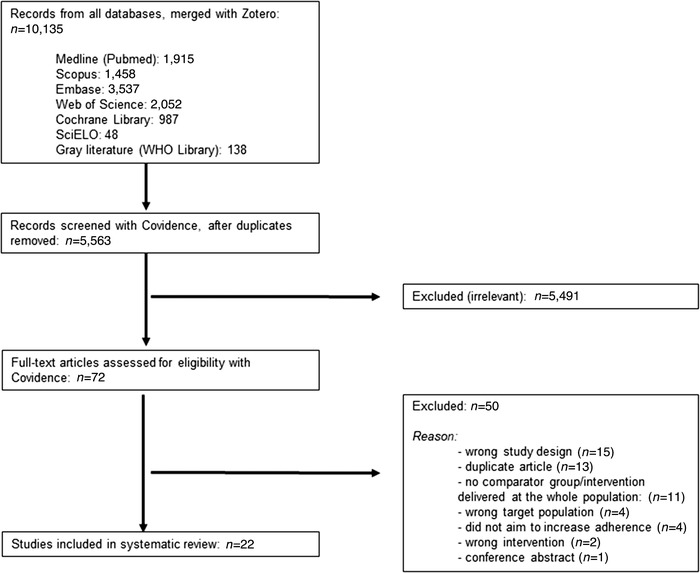
PRISMA flow diagram of literature search and selection process.
Description of included studies
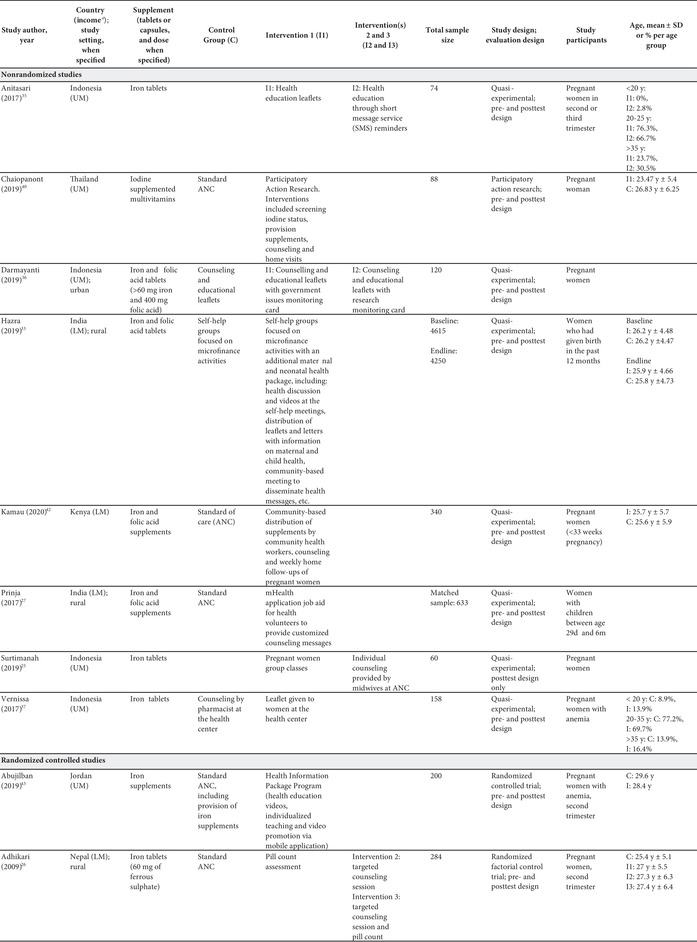
Of the 22 studies included in this systematic review, 14 were randomized controlled trials (RCTs) (including four cluster‐randomized trials) and eight were nonrandomized studies. Studies were published between 2009 and 2020, and all of them used a pre‐ and posttest design, except for one study, 25 which used a posttest design only. Table 1 provides an overview of the characteristics of the included studies.
Table 1.
Overview of the characteristics of the included studies
|
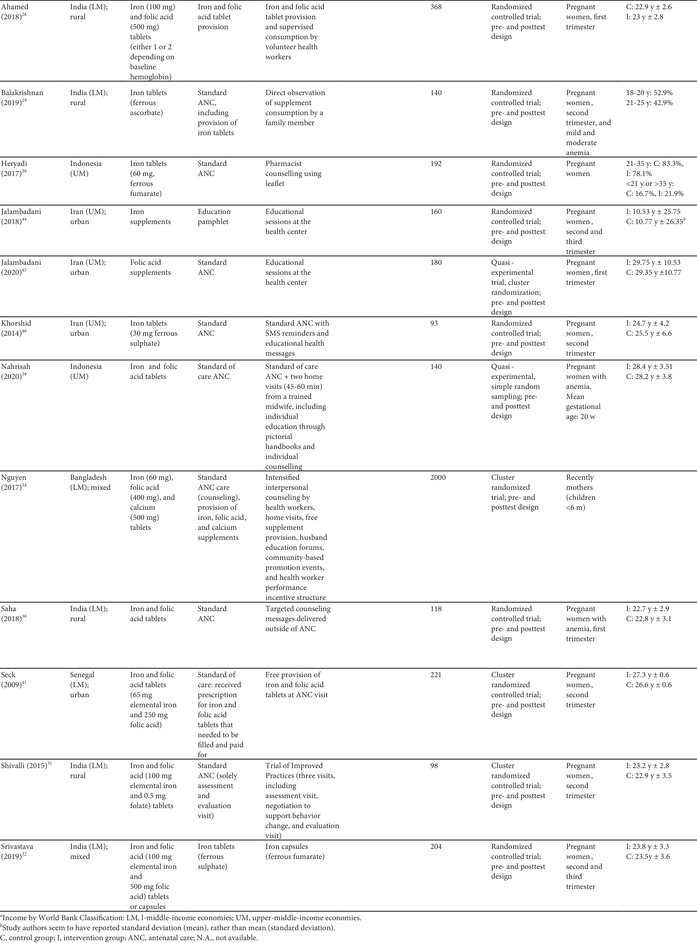
|
All studies were conducted in countries with lower‐middle or upper‐middle income economies. Over half of the studies were conducted in Asia (Nepal, 26 India, 27 , 28 , 29 , 30 , 31 , 32 , 33 Bangladesh, 34 Indonesia, 25 , 35 , 36 , 37 , 38 , 39 and Thailand 40 ), with only two studies conducted in Africa (Senegal 41 and Kenya 42 ) and four conducted in the Middle East (Jordan 43 and Iran 44 , 45 , 46 ).
Of these 22 studies, 10 used an IFA supplement (containing 60–100 mg of iron and 250–500 μg of folic acid), nine used an iron supplement (containing 30–60 mg of iron), one utilized a folic acid supplement (dose not specified), one utilized a combination of iron, folic acid, and calcium supplements (containing 60 mg of iron, 400 μg of folic acid, and 500 mg of calcium), and one used an iodine‐supplemented multivitamin (dose not specified). The majority of the studies (n = 20) had two groups, either comparing one intervention with one control group or one intervention with another intervention; however, two studies had three 36 and four groups, 26 comparing a control group with multiple intervention arms (Table 1).
Collectively, the studies covered a wide range of targeted micronutrient adherence interventions, including education‐based ones, education with consumption monitoring, consumption monitoring alone, participatory action research, SMS reminders, free provision (versus purchase) of supplements, different forms of the supplements (i.e., capsule versus tablet), and multicomponent interventions (e.g., education with community mobilization).
Study sample sizes ranged from 60 to 4615 participants; however, two‐thirds of the studies had fewer than 200 participants. While all studies were conducted on pregnant women, some specified the inclusion criteria for women within a certain trimester of pregnancy, while others did not provide information on the gestational age of the participants at the time they were enrolled or received the intervention. Five studies were conducted in anemic pregnant women. 29 , 30 , 37 , 39 , 43
As per our inclusion criteria, adherence was assessed in all studies (Tables 2 and 3). Nevertheless, the method used to measure adherence differed significantly between the studies, including prevalidated scales (e.g., Morisky Medication Adherence Scale‐8 (MMAS‐8) questionnaire), self‐reported recall, supplement compliance documentation cards, direct observation, and pill counting (Tables 2 and 3). In addition, the definition of adherence also varied significantly between studies. Definitions included a designated cutoff for supplement intake to dichotomize those who were adherent and nonadherent (e.g., at least 80% or 100 supplements taken), continuous measurements of the number of supplements consumed (e.g., a total number of tablets taken in 3 months), and Likert scale results (e.g., a behavior scale that ranged from 1 to 5). The studies utilizing a cutoff point to dichotomize results varied from consumption of 70% to 100% of the expected supplement dose, and from 75 to 180 tablets consumed (Tables 2 and 3). It should also be noted that, although almost all studies had a pre‐ and posttest design (which allows them to show the mean differences of adherence measures between baseline and endline), 12 studies reported measures of adherence for the posttest only. Because of all these methodological differences, it was not possible to directly compare the effect of the intervention on adherence between studies.
Table 2.
Outcome analysis for nonrandomized studies
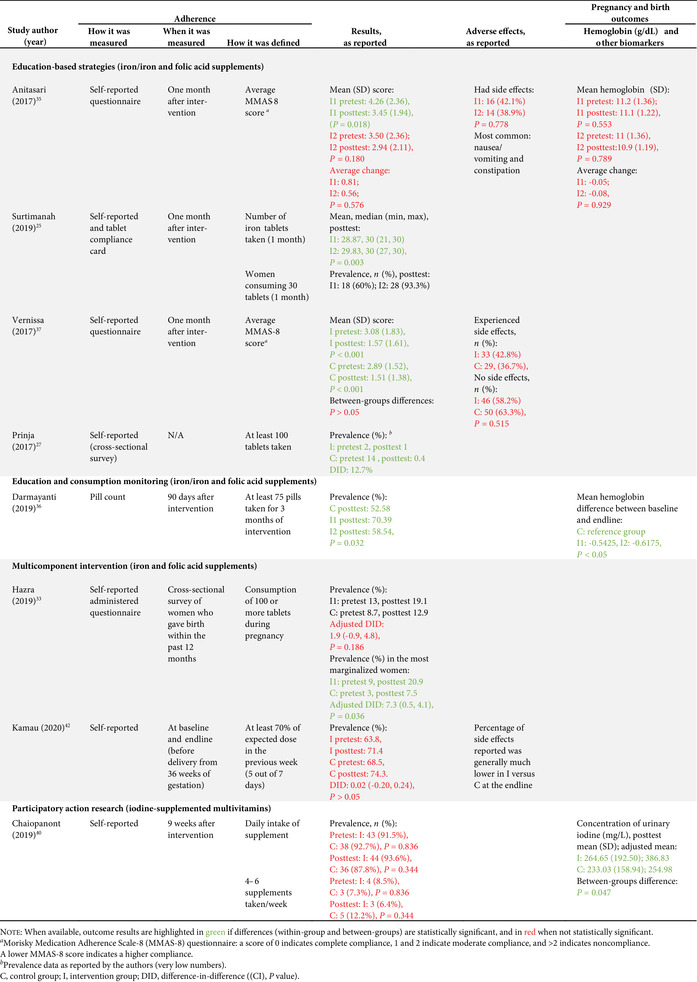
|
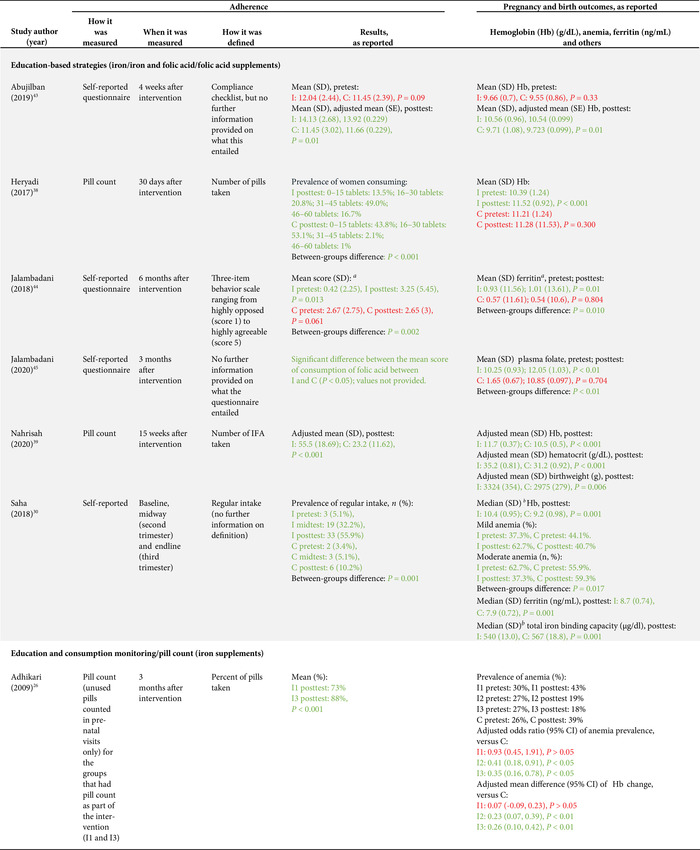
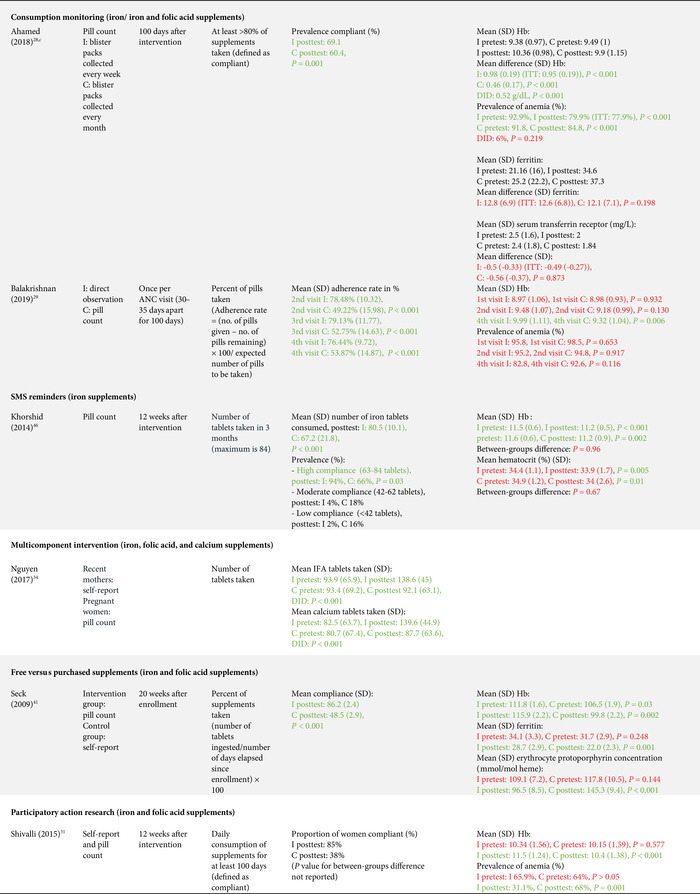
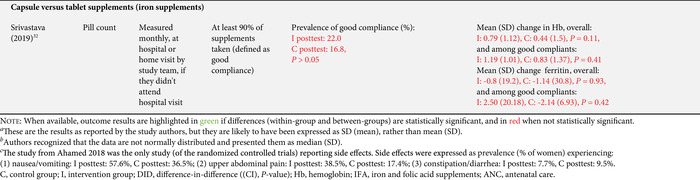
Table 3.
Outcome analysis for randomized controlled trials
|
|
|
Adverse gastrointestinal symptoms and the secondary outcomes of interest were reported by fewer studies. Adverse effects were only reported in four studies; 28 , 35 , 37 , 42 hemoglobin levels were assessed in 13 studies; 26 , 28 , 29 , 30 , 31 , 32 , 35 , 36 , 38 , 39 , 41 , 43 , 46 serum ferritin was assessed in five studies; 28 , 30 , 32 , 41 , 44 hematocrit was assessed in two studies; 39 , 46 and only one study each assessed plasma folate, 45 serum transferrin, 28 erythrocyte protoporphyrin concentration, 41 total iron binding capacity, 30 urinary iodine concentration, 40 and low birth weight 39 (Tables 2 and 3).
Effects of interventions
Sixteen of the 22 studies (73%) found a significant between‐groups difference (comparing control to intervention or multiple intervention groups) in the adherence to the prescribed supplementation regimen (Tables 2 and 3). Below is a summary of the effect of the interventions provided by type of interventions.
Education‐based interventions (10 studies)
Nine of the 10 education‐based interventions resulted in a significant increase of adherence to supplementation. Surtimanah et al. 25 found that women who received individual counseling from midwives during antenatal care had a significantly higher mean intake of iron tablets consumed in comparison with women who attended group classes for pregnant women. Vernissa et al. 37 demonstrated that both the group that received individual counseling by a pharmacist at the health center and the group that received education leaflets at the health center had a significant increase of adherence (from baseline to endline), but there was no statistically significant difference between groups. Prinja et al. 27 found that using an mHealth applicationa as a job aid for community health volunteers to provide custom counseling messages resulted in a significant difference‐in‐difference (12.7%) on adherence rates compared with the group that received standard antenatal care. Abujilban et al., 43 Heryadi et al., 38 and Saha et al. 30 used education‐based strategies (i.e., a health information package, pharmacist counseling using a leaflet, and targeted counseling outside of antenatal care, respectively) that resulted in statistically significant increases in supplementation adherence when compared with the control groups that received standard antenatal care. Jalambadani et al. 44 compared the use of education sessions (intervention group) with the provision of education pamphlets (control group) and observed a significant increase on a behavior scale in the intervention group. Two years later, the same authors (Jalambadani et al. 45 ) published another study that found significant differences in the mean scores of consumption of folic acid supplements between those who received the education sessions (intervention group) and those receiving antenatal care (control group). The intervention provided in the study of Nahrisah et al. 39 included two home visits from a trained midwife (with individual education through pictorial handbooks and counseling), which resulted in a significantly higher intake of supplements when compared with standard antenatal care (control group). In contrast to these effective interventions, Anitasari and Andrajati 35 observed that the changes in adherence were not statistically significant between the two groups.
Within these education‐based interventions, adverse side effects were measured by two studies, which observed no significant differences in this outcome between the groups. 37 , 47 The most common side effects reported by Anitasari and Andrajati 35 were nausea/vomiting and constipation. Five studies assessed hemoglobin levels, four of which observed significant increases of hemoglobin between intervention(s) and control groups, 30 , 38 , 39 , 43 whereas one study found no significant differences in hemoglobin changes between both intervention groups. 35 Ferritin was assessed in two studies and both reported significant differences between the intervention and control groups, with higher levels observed in the intervention group. 30 , 44 One study reported a significant increase in the intervention group's plasma folate levels, as opposed to the control group. 45 Only one study assessed birth weight as an outcome, which was statistically significantly higher in the intervention group (versus control). 39 This effect size was also clinically meaningful, as the adjusted mean birth weight of the intervention group was 3324 g, in comparison with the adjusted mean birth weight of control group, which was 2975 grams. The intervention of this study consisted of two home visits (45–60 min) from a trained midwife, with individual education and counseling.
Education and consumption monitoring (two studies)
The two studies that used education in conjunction with consumption monitoring strategies employed multiple intervention groups and found significant differences in adherence between study groups. The study from Darmayanti et al. 36 showed that the use of consumption monitoring cards (in addition to counseling/educational leaflets) resulted in higher adherence than the use of counseling/educational leaflets alone, and there were significant differences between the changes of hemoglobin in all three study groups. Adhikari et al. 26 showed a higher adherence in the group that received education and a pill count when compared with the group with a pill count assessment alone. Compared with the control group (standard ANC), there were significant improvements in hemoglobin changes and anemia prevalence in the group that received education with pill counting, but not in the group with pill counting alone.
Consumption monitoring (two studies)
Consumption monitoring strategies, that is, supervised consumption of supplements by volunteer health workers or family members, proved to be effective in improving adherence to supplementation in both trials that used this intervention: Ahamed et al. 28 and Balakrishnan et al. 29 While both studies found significant differences between groups in hemoglobin levels from baseline to endline, there was no difference in changes of anemia rates in the study that assessed this outcome. 28
Participatory action research interventions (two studies)
Two studies used a participatory action research approach. One of them (Chaiopanont and Taneepanichsakul) aimed at improving iodine consumption following a four‐step participatory action research design that implemented screening, provision, counseling, and home visits, and found no significant differences in adherence to the iodine‐supplemented multivitamins. 40 However, they observed significant differences in urinary iodine concentrations between the intervention and control groups at the endline (which could be explained by other variables such as the use of more iodized salt, iodine‐supplemented fish sauce, or iodine‐rich foods). The other study (Shivalli et al.) used a trial of improved practice design to increase the consumption of IFA supplements (with three visits for assessment; negotiation to support behavior change, where pregnant women were asked to select and try new recommended practices; and evaluation). The authors described large differences in the proportion of individuals defined as compliant (i.e., 85% in the intervention group and 38% in the control group); however, statistical significance was not reported. 31 This intervention resulted in significantly higher hemoglobin levels and greater reductions in the prevalence of anemia when compared with the control group (standard antenatal care).
SMS reminders (one study)
Khorshid et al. 46 assessed the effect of SMS reminders and educational health messages, which resulted in a significantly higher adherence and prevalence of “high compliance” compared with standard antenatal care. Despite these positive effects on adherence, no significant differences were observed in hemoglobin or hematocrit levels between both groups.
Free versus purchased supplements (one study)
Seck et al. 41 compared the provision of free supplements at antenatal care (intervention group) to the receipt of the prescription that required filling and paying of supplements at a pharmacy (control group), and they observed significantly higher consumption of supplements among those receiving the free supplements. Similarly, there were statistically significant differences in the mean levels of hemoglobin, erythrocyte protoporphyrin, and serum ferritin at follow‐up between the control and the intervention groups.
Capsule versus tablet supplements (one study)
Srivastava et al. 32 compared the provision of iron tablets (control group) with iron capsules (intervention group) and observed no significant differences in reported adherence, hemoglobin levels, or ferritin levels between both groups.
Multicomponent interventions (three studies)
Three studies used a combination of several interventions. Nguyen et al. 34 assessed the effect of a multicomponent intervention that included community‐based promotion events and husband education (among others), which resulted in increased adherence to both IFA and calcium supplements compared with the control group. By contrast, Kamau et al. 42 used an intervention with a community‐based distribution of IFA supplements by community health workers, counseling, and weekly follow up with pregnant women in their homes and did not observe significant differences in adherence between the intervention and the control group (receiving standard antenatal care). Nonetheless, the percentage of adverse side effects reported at follow up was generally much lower in the intervention group, which is probably a result of the counseling about managing and mitigating the common side effects of IFA supplements. A similar lack of effect on adherence was observed in the study from Hazra et al., 33 although when the analysis was confined to the subgroup of the most marginalized women (based on women's education, caste, and household wealth index), the intervention proved to be effective in increasing adherence, with a statistically significant difference‐in‐difference between intervention and control. This intervention used self‐help groups focused on microfinance activities, with an additional maternal and neonatal health package composed of health discussion and videos at the self‐help meetings, distribution of leaflets and letters with information on maternal and child health, community‐based meetings to disseminate health messages, support to attend health days, monthly celebrations, and additional meetings for pregnant women.
Risk of bias
The overall risk of bias was moderate, serious, or critical for all the included nonrandomized studies (Table 4). For the included RCTs, a few studies had low risk of bias through most of the domains (e.g., Adhikari et al., 26 Srivastava et al., 32 and Nahrisah et al. 39 ); 4 out of 14 studies had unclear risk of bias 30 , 31 , 38 , 45 and none were at high risk of bias for sequence generation; 9 out of 14 studies had unclear 29 , 30 , 31 , 38 , 44 , 45 , 46 or high 28 , 41 risk of bias for allocation concealment; and 11 out of 14 studies had unclear 30 , 34 , 38 , 39 , 41 , 43 , 44 , 45 , 46 or high 29 , 31 risk of bias for blinding of outcome assessors for adherence (Table 5).
Table 4.
Risk of bias summary for nonrandomized studies (ROBINS‐I tool)
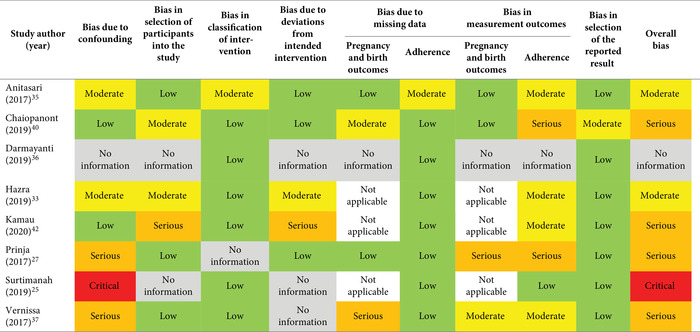
|
Table 5.
Risk of bias summary for randomized controlled trials (Cochrane risk‐of‐bias tool)
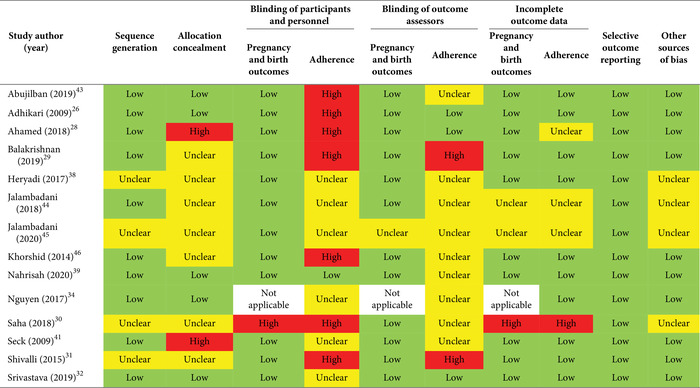
|
Discussion
Defining and quantifying adherence, as well as providing an estimate of the effect size of an intervention on adherence for a pooled body of evidence, is challenging. Previously published systematic reviews used different cutoffs to define adherence to micronutrient supplements in pregnancy, which varied from 70% 48 to 95% 7 of the recommended daily supplementation dose or was defined as the intake of micronutrient tablets for 90 days or more. 49 Given this variability in the definition of adherence and the criticism of using a specific threshold (which could not be proved to be linked to clinical outcomes 50 ), in the present work, we decided to report adherence to the micronutrient supplementation regimen as measured and defined by the study authors. Consequently, we found that the included studies used a wide range of methods to measure adherence (from prevalidated questionnaires to self‐reported recall or pill counting) and to define adherence (from a minimum number of tablets taken over a certain period of time to the use of behavior scales). In addition, adherence was assessed at different time points, from 1 to 6 months after initiation of the intervention. Moreover, the included studies had different types of study designs (RCTs versus nonrandomized studies), evaluation designs (pre‐ and posttest versus posttest only), and types of micronutrient supplements provided (with a prominent use of IFA supplements as per recommendations from global guidance 51 ). Given this heterogeneity between the studies, a statistical meta‐analysis was not feasible nor appropriate for our systematic review.
We found that a variety of interventions were effective in increasing prenatal supplement adherence. Most of these interventions were education‐based strategies (used alone or in combination with consumption monitoring), including individual counseling (from midwives at ANC, pharmacists at the health center, or community health workers at home), through education sessions, pictorial handbooks, leaflets, and videos, among others. The use of other interventions based on consumption monitoring by volunteer health workers or family members, SMS reminders, free provision of supplements, a multicomponent intervention with community mobilization, and a participatory action research intervention (with three visits for assessment, negotiation to support behavior change, and evaluation), despite being less prevalent, also effectively increased supplement adherence. Interventions that did not result in increased adherence included the provision of a capsule versus tablet, one participatory action research intervention, and two multicomponent interventions. The types of interventions identified in the present review are in line with the results of a Cochrane review of interventions for enhancing medication adherence, 52 which included complex interventions with several components, such as intense education and tailored ongoing counseling delivered by allied health professionals/pharmacists, daily treatment support, and sometimes additional support from family or peers. Nevertheless, only a few of those interventions for improving adherence with long‐term medication prescriptions improved both adherence and clinical outcome.
Our systematic review shows that most of the interventions that resulted in increased micronutrient supplement adherence also resulted in beneficial effects on pregnancy and birth outcomes (when reported by the study authors), but there were some exceptions. For example, the study from Khorshid et al. 46 showed that, when compared with standard ANC, the use of SMS reminders and educational health messages over 12 weeks resulted in higher adherence to iron supplements but no significant differences were observed in hemoglobin or hematocrit levels between both groups. It is possible that interventions need to be delivered for a longer period of time to have an effect on clinical outcomes, and there may be other factors influencing the response of the blood parameters of anemia to the iron supplements. In addition, the difference between the mean number of tablets consumed in each group (i.e., 80.5 tablets in the intervention group versus 67.2 tablets in the control group) may not be large enough to result in significant differences in hemoglobin or hematocrit levels between both groups.
While we considered studies using any type of micronutrient supplement, as mentioned above, most of included studies used iron or IFA supplements. For the studies that reported doses of these micronutrients, iron was provided in high amounts (60–100 mg of elemental iron), except for one study that used 30 mg of iron. 46 The dose of iron that is typically provided in multiple micronutrient supplements is lower, that is, 30 mg. It is unclear whether studies that use supplements containing high doses of iron present inherently lower levels of adherence (as a consequence of more side effects) than studies that use supplements with lower doses of iron, such as multiple micronutrient supplements.
While conducting the literature searches, we found some ongoing studies that could not be included at this stage but may be included in a future update of this systematic review, such as: (1) an RCT assessing whether a social norm–based intervention can increase the uptake of iron folic acid supplements and iron‐rich foods to reduce anemia in Indian pregnant women; 53 and (2) a cluster RCT assessing the effects of a video‐based health education package provided to Ethiopian pregnant and lactating women on the knowledge, attitude, and practice of recommended health, including adherence to IFA supplementation. 54
In addition to these ongoing studies, it should be noted that the 22 studies included in this systematic review were published over a span of 11 years, reflecting the urgent need to tackle this problem of suboptimal intake of micronutrient supplements during pregnancy, particularly in low‐ and middle‐income settings.
There are a number of limitations in the present work. First, most studies used self‐reported measures of adherence, which may be subject to several biases (e.g., recall bias and response bias). Second, we included a few studies that reported adherence as an outcome but did not report data on other pregnancy and birth outcomes. Thus, we do not know whether these interventions that increased adherence also resulted in more objective and clinically relevant outcomes for both the pregnant mother and her child. Nonetheless, it should be noted that a lack of clinical outcomes in implementation research is not necessarily a limitation, since such studies aim to test interventions for which the evidence of efficacy already exists and rarely can (or should) be designed to determine the assessment of clinically responsive outcomes. Third, we found that some studies had an overall low quality, as reflected in the risk of bias assessment, with serious methodological limitations (particularly among the nonrandomized studies) and relatively small sample sizes. Thus, their findings need to be interpreted with caution. Fourth, none of the included studies were conducted in low‐ or high‐income countries, which limits the extrapolation of the evidence to these settings. A previous systematic review of interventions designed to increase knowledge, awareness, and consumption of folic acid in women of reproductive age 13 found that the included studies were conducted in the United States, Australia, Europe, and Israel. The predominant use of mass media channels of communication, such as TV and internet, in the interventions of that systematic review conducted in 2008 suggests that low‐ and middle‐ and high‐income settings might need different interventions. On the other hand, advocacy capabilities and access to mobile communications in low‐ and middle‐income settings have changed dramatically in the past decade such that some of the adherence interventions tested in the past (e.g., use of education leaflets) may not be used in the future, and there is a trend for increased use of remote counseling services.
The strengths of this study include the fact that there was no language restriction (which led to the inclusion of one study written in Indonesian 37 ) and that we did multiple attempts to contact study authors, which resulted in the inclusion of important (missing) data that had not been published. In addition, we followed the rigorous Cochrane methodological requirements and conducted the literature search in a large number of databases (n = 7, including gray literature).
To our knowledge, this is the first systematic review to assess the existing literature to determine the effectiveness of interventions designed to increase adherence to micronutrient supplements in pregnancy, following the Cochrane methodology.
Current evidence suggests that a number of strategies play a role in increasing adherence to the recommended prenatal micronutrient supplementation regimen, which may need to be adapted to specific contexts (e.g., country, region, or culture). Additional high‐quality and adequately powered studies, using feasible and sustained interventions and objective adherence measures, are warranted to determine other efficacious strategies that maximize the benefit of micronutrient supplements during this critical stage of life. While it is not reasonable to recommend the use of a specific cutoff to define adherence based on the results of this systematic review, we encourage all authors of future studies assessing adherence to micronutrient supplementation to specify the recommended dosage and report the “number of supplements consumed divided by the expected number of pills to be taken.” This assessment takes into consideration the beginning of the supplementation period and allows a better comparison of adherence between different studies.
Author contributions
F.G., S.K., D.D., J.G., A.S., K.H., G.B., M.B., and S.M. were involved in the conception, design, revision of manuscript, and approval of its final version. F.G., S.K., D.D., J.G., and A.S. contributed to the acquisition of data, analysis and interpretation of data, and draft of the manuscript.
Competing interests
S.M. is an unpaid board member for and holds equity in a diagnostic start‐up focused on the measurement of nutritional biomarkers at the point‐of‐care utilizing the results from his research. All the other authors declare no competing interests.
Supporting information
Supplementary Material
Acknowledgments
We are thankful for the training that four of the study authors received during the 6th WHO/Cochrane/Cornell University Summer Institute for Systematic Reviews in Nutrition for Global Policy Making, hosted at the Division of Nutritional Sciences, Cornell University, Ithaca, NY, and for the support that the faculty members provided in the conceptualization of this systematic review. In addition, we are grateful for the support we received from the Applied Health Sciences librarians at Cornell University (Kate Ghezzi‐Kopel and Sara Rose Scinto‐Madonich) while developing our search strategies and exporting the search results to Covidence. J.G. is currently funded by the National Institutes of Health (Award T32‐DK007158). The content of this research is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Open access for this article was supported by funding from the Bill & Melinda Gates Foundation.
Footnotes
mHealth, as an abbreviation of mobile health, is a term used for the practice of medicine and public health supported by mobile devices. In this study, the mHealth application served as a job aid for community health volunteers to provide customized counseling messages for pregnant women, using economical, Java‐based mobile phones. The application had tailored content with locally relevant audio and visual prompts to equip community health volunteers with multimedia job aids to support client assessment, counseling, early identification, treatment, and/or rapid referral of pregnancy, postpartum, and newborn complications.
References
- 1. Black, R.E. , Victora C.G., Walker S.P., et al. 2013. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 382: 427–451. [DOI] [PubMed] [Google Scholar]
- 2. Gernand, A.D. , Schulze K.J., Stewart C.P., et al. 2016. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat. Rev. Endocrinol. 12: 274–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhutta, Z.A. , Das J.K., Rizvi A., et al. 2013. Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 382: 452–477. [DOI] [PubMed] [Google Scholar]
- 4. Bourassa, M.W. , Osendarp S.J.M., Adu‐Afarwuah S., et al. 2019. Review of the evidence regarding the use of antenatal multiple micronutrient supplementation in low‐ and middle‐income countries. Ann. N.Y. Acad. Sci. 1444: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black, R.E. & Dewey K.G.. 2019. Benefits of supplementation with multiple micronutrients in pregnancy. Ann. N.Y. Acad. Sci. 1444: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keats, E.C. , Haider B.A., Tam E., et al. 2019. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 3: CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith, E.R. , Shankar A.H., Wu L.S.‐F., et al. 2017. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta‐analysis of individual patient data from 17 randomised trials in low‐income and middle‐income countries. Lancet Glob. Health 5: e1090–e1100. [DOI] [PubMed] [Google Scholar]
- 8. Engle‐Stone, R. , Kumordzie S.M., Meinzen‐Dick L., et al. 2019. Replacing iron‐folic acid with multiple micronutrient supplements among pregnant women in Bangladesh and Burkina Faso: costs, impacts, and cost‐effectiveness. Ann. N.Y. Acad. Sci. 1444: 35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. 2020 Global Nutrition Report: action on equity to end malnutrition. Bristol: Development Initiatives. [Google Scholar]
- 10. Sununtnasuk, C. , D'Agostino A. & Fiedler J.L.. 2016. Iron+folic acid distribution and consumption through antenatal care: identifying barriers across countries. Public Health Nutr. 19: 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu, D. , Cheng Y., Dang S., et al. 2019. Maternal adherence to micronutrient supplementation before and during pregnancy in Northwest China: a large‐scale population‐based cross‐sectional survey. BMJ Open 9: e028843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabaté, E. 2003. Adherence to Long‐Term Therapies: Evidence for Action. Geneva: World Health Organization. [Google Scholar]
- 13. Chivu, C.M. , Tulchinsky T.H., Soares‐Weiser K., et al. 2008. A systematic review of interventions to increase awareness, knowledge, and folic acid consumption before and during pregnancy. Am. J. Health Promot. 22: 237–245. [DOI] [PubMed] [Google Scholar]
- 14. Chacko, M.R. , Anding R., Kozinetz C.A., et al. 2003. Neural tube defects: knowledge and preconceptional prevention practices in minority young women. Pediatrics 112: 536–542. [DOI] [PubMed] [Google Scholar]
- 15. van der Pal‐de Bruin, K.M. , de Walle H.E.K., de Rover C.M., et al. 2003. Influence of educational level on determinants of folic acid use. Paediatr. Perinat. Epidemiol. 17: 256–263. [DOI] [PubMed] [Google Scholar]
- 16. Robbins, J.M. , Cleves M.A., Collins H.B., et al. 2005. Randomized trial of a physician‐based intervention to increase the use of folic acid supplements among women. Am. J. Obstet. Gynecol. 192: 1126–1132. [DOI] [PubMed] [Google Scholar]
- 17. Gomes, F. , Adu‐Afarwuah S., Ajello C., et al. 2020. Setting research priorities on multiple micronutrient supplementation in pregnancy. Ann. N.Y. Acad. Sci. 1465: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins, J. & Green S.. 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration. [Google Scholar]
- 19. Hutton, B. , Salanti G., Caldwell D.M., et al. 2015. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 20. Gomes, F. , Bergeron G., Bourassa M.W., et al. 2020. Interventions to increase adherence to micronutrient supplementation during pregnancy: a protocol for a systematic review. Ann. N. Y. Acad. Sci. 1470: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Covidence systematic review software. Melbourne: Veritas Health Innovation. [Google Scholar]
- 22. World Bank . Accessed March 1, 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 23. Higgins, J.P.T. , Altman D.G., Gøtzsche P.C., et al. 2011. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterne, J.A. , Hernán M.A., Reeves B.C., et al. 2016. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Surtimanah, T. , Noviyanti E. & Meliyanti M.. 2019. Differences of family support and iron tablets consumed post pregnant women classes and midwives counseling. Indian J. Public Health Res. Dev. 10: 999. [Google Scholar]
- 26. Adhikari, K. , Liabsuetrakul T. & Pradhan N.. 2009. Effect of education and pill count on hemoglobin status during prenatal care in Nepalese women: a randomized controlled trial. J. Obstet. Gynaecol. Res. 35: 459–466. [DOI] [PubMed] [Google Scholar]
- 27. Prinja, S. , Nimesh R., Gupta A., et al. 2017. Impact of m‐health application used by community health volunteers on improving utilisation of maternal, new‐born and child health care services in a rural area of Uttar Pradesh, India. Trop. Med. Int. Health 22: 895–907. [DOI] [PubMed] [Google Scholar]
- 28. Ahamed, F. , Yadav K., Kant S., et al. 2018. Effect of directly observed oral iron supplementation during pregnancy on iron status in a rural population in Haryana: a randomized controlled trial. Indian J. Public Health 62: 287–293. [DOI] [PubMed] [Google Scholar]
- 29. Balakrishnan, D. 2019. A method to improve iron compliance in treating iron deficiency anemia in pregnancy. J. Evol. Med. Dent. Sci. 8: 298–305. [Google Scholar]
- 30. Saha, J. , Mazumder S. & Samanta A.. 2018. Does effective counseling play an important role in controlling iron deficiency anemia among pregnant women. Natl. J. Physiol. Pharm. Pharmacol. 10.5455/njppp.2018.8.0104305022018 [DOI] [Google Scholar]
- 31. Shivalli, S. , Srivastava R.K. & Singh G.P.. 2015. Trials of improved practices (TIPs) to enhance the dietary and iron‐folate intake during pregnancy—a quasi experimental study among rural pregnant women of Varanasi, India. PLoS One 10: e0137735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srivastava, R. , Kant S., Singh A., et al. 2019. Effect of iron and folic acid tablet versus capsule formulation on treatment compliance and iron status among pregnant women: a randomized controlled trial. J. Fam. Med. Prim. Care 8: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hazra, A. , Atmavilas Y., Hay K., et al. 2019. Effects of health behaviour change intervention through women's self‐help groups on maternal and newborn health practices and related inequalities in rural india: a quasi‐experimental study. EClinicalMedicine 18. 10.1016/j.eclinm.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen, P.H. , Kim S.S., Sanghvi T., et al. 2017. Integrating nutrition interventions into an existing maternal, neonatal, and child health program increased maternal dietary diversity, micronutrient intake, and exclusive breastfeeding practices in Bangladesh: results of a cluster‐randomized program evaluation. J. Nutr. 147: 2326–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anitasari, D. & Andrajati R.. 2017. Effectiveness of short message service reminders and leaflets in complying with iron supplementation in pregnant women in Depok City, Indonesia. Asian J. Pharm. Clin. Res. 10: 42–45. [Google Scholar]
- 36. Darmayanti, Mukhtar M., Rizani A. & Barkinah T.. 2019. Use of monitoring cards to increase iron substance and hemoglobin levels in pregnant women. Indian J. Public Health Res. Dev. 10: 487. [Google Scholar]
- 37. Vernissa, V. , Andrajati R. & Supardi S.. 2017. Efektivitas leaflet dan konseling terhadap kepatuhan minum tablet besi dan kadar hemoglobin ibu hamil dengan anemia di puskesmas di kabupaten bogor. Media Penelit. Dan Pengemb. Kesehat. 27: 229–236. [Google Scholar]
- 38. Heryadi, P.L. , Sauriasari R. & Andrajati R.. 2017. The influence of pharmacist counseling on changes in hemoglobin levels of pregnant women at a community health center in Indonesia. Asian J. Pharm. Clin. Res. 10: 114–117. [Google Scholar]
- 39. Nahrisah, P. , Somrongthong R., Viriyautsahakul N., et al. 2020. Effect of integrated pictorial handbook education and counseling on improving anemia status, knowledge, food intake, and iron tablet compliance among anemic pregnant women in Indonesia: a quasi‐experimental study. J. Multidiscip. Healthc. 13: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chaiopanont, S. & Taneepanichsakul S.. 2019. Effectiveness of modified iodine consumption behavior model in pregnant women by civil society integrated participation in Khon Kaen Province: a participatory action research. J. Med. Assoc. Thai. 102: 86–94. [Google Scholar]
- 41. Seck, B.C. & Jackson R.T.. 2009. Providing iron/folic acid tablets free of charge improves compliance in pregnant women in Senegal. Trans. R. Soc. Trop. Med. Hyg. 103: 485–492. [DOI] [PubMed] [Google Scholar]
- 42. Kamau, M.W. , Kimani S.T., Mirie W., et al. 2020. Effect of a community‐based approach of iron and folic acid supplementation on compliance by pregnant women in Kiambu County, Kenya: a quasi‐experimental study. PLoS One 15: e0227351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abujilban, S. , Hatamleh R. & Al‐Shuqerat S.. 2019. The impact of a planned health educational program on the compliance and knowledge of Jordanian pregnant women with anemia. Women Health 59: 748–759. [DOI] [PubMed] [Google Scholar]
- 44. Jalambadani, Z. , Borji A. & Delkhosh M.. 2018. The effect of education based on the theory of planned behavior on iron supplementation among pregnant women. Korean J. Fam. Med. 39: 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jalambadani, Z. , Delavari Heravi M. & Noori Sistani M.. 2020. Folic acid consumption based on the theory of planned behaviour in pregnant women. J. Obstet. Gynaecol. 40: 37–39. [DOI] [PubMed] [Google Scholar]
- 46. Khorshid, M.R. , Afshari P. & Abedi P.. 2014. The effect of SMS messaging on the compliance with iron supplementation among pregnant women in Iran: a randomized controlled trial. J. Telemed. Telecare 20: 201–206. [DOI] [PubMed] [Google Scholar]
- 47. Anitasari, D. & Andrajati R.. 2017. Effectiveness of short message service reminders and leaflets in complying with iron supplementation in pregnant women in Depok City, Indonesia. Asian J. Pharm. Clin. Res. 10: 42–45. [Google Scholar]
- 48. Akibu, M. , Tekelab T., Amano A., et al. 2018. Adherence to prenatal iron‐folic acid supplementation in low‐ and middle‐income countries (LMIC): a protocol for systematic review and meta‐analysis. Syst. Rev. 7: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Workineh, Y. , Semachew A., Ayalew E., et al. 2019. Compliance to Iron‐Folic Acid Supplementation and Its Association with the Number of ANC Visits in Ethiopia: Systematic Review and Meta‐Analysis. Adv. Prev. Med. 2019: 3602585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baumgartner, P.C. , Haynes R.B., Hersberger K.E., et al. 2018. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front. Pharmacol. 9. 10.3389/fphar.2018.01290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. World Health Organization . 2016. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 52. Nieuwlaat, R. , Wilczynski N., Navarro T., et al. 2014. Interventions for enhancing medication adherence. Cochrane Database Syst. Rev. 2014: CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yilma, H. , Sedlander E., Rimal R.N., et al. 2020. The reduction in anemia through normative innovations (RANI) project: study protocol for a cluster randomized controlled trial in Odisha, India. BMC Public Health 20: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. ClinicalTrials.gov. Accessed August 6, 2020. https://clinicaltrials.gov/ct2/show/NCT04414527.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


