Abstract
Objective
Glucocorticoid levels rise rapidly following status epilepticus and remain elevated for weeks after the injury. To determine whether glucocorticoid receptor activation contributes to the pathological sequelae of status epilepticus, mice were treated with a novel glucocorticoid receptor modulator, C108297.
Methods
Mice were treated with either C108297 or vehicle for 10 days beginning one day after pilocarpine-induced status epilepticus. Baseline and stress-induced glucocorticoid secretion were assessed to determine whether hypothalamic-pituitary-adrenal axis hyperreactivity could be controlled. Status epilepticus-induced pathology was assessed by quantifying ectopic hippocampal granule cell density, microglial density, astrocyte density and mossy cell loss. Neuronal network function was examined indirectly by determining the density of Fos immunoreactive neurons following restraint stress.
Results
Treatment with C108297 attenuated corticosterone hypersecretion after status epilepticus. Treatment also decreased the density of hilar ectopic granule cells and reduced microglial proliferation. Mossy cell loss, on the other hand, was not prevented in treated mice. C108297 altered the cellular distribution of Fos protein but did not restore the normal pattern of expression.
Interpretation
Results demonstrate that baseline corticosterone levels can be normalized with C108297, and implicate glucocorticoid signaling in the development of structural changes following status epilepticus. These findings support the further development of glucocorticoid receptor modulators as novel therapeutics for the prevention of brain pathology following status epilepticus.
INTRODUCTION
Status epilepticus (SE) is a medical emergency defined by seizures lasting more than thirty minutes. SE is associated with high morbidity and mortality, causing acute neuronal death and the subsequent development of behavioral changes, cognitive dysfunction and epilepsy (Trinka et al., 2015; Leitinger et al., 2019; Seinfeld et al., 2016). The mainstay of current treatments – anti-seizure medications – are often ineffective at stopping SE or preventing long-term negative outcomes.
Glucocorticoids represent an intriguing target for treatment of SE. Glucocorticoids are used to treat SE in some pediatric populations (Vossler et al., 2020). These treatments may take advantage of glucocorticoids’ immune modulatory and anti-inflammatory properties, as some cases of SE are thought to have a strong immunological component. Glucocorticoids, however, may also have harmful effects. Glucocorticoids can disrupt limbic circuits (McEwen, 2006; McEwen et al., 2015), induce hippocampal hyperexcitability (Joëls and de Kloet, 1991) and increase overall epileptiform activity (Castro et al., 2012; Joëls, 2009; Kumar et al., 2011, 2007; Roberts and Keith, 1995). Moreover, glucocorticoids are elevated for weeks following SE in animal models (Mazarati et al., 2009; O’Toole et al., 2014; Wulsin et al., 2016a), and therefore could exert lasting negative consequences. Indeed, pharmacological blockade of glucocorticoid receptors (GRs) with mifepristone (RU486) in rodents ameliorates hippocampal pathologies following SE (Wulsin et al., 2016a). The complex biology of glucocorticoids, however, limits the use of simple antagonists. Glucocorticoids act on tissues throughout the body, regulate widely diverging processes, and self-regulate their own secretion by negative feedback control. Antagonists, therefore, can produce paradoxical effects, such as increasing endogenous glucocorticoid levels (Peeters et al., 2008; Spitz and Bardin, 1993).
Newly derived compounds designed to modulate, rather than completely antagonize, GR signaling hold promise for overcoming the limitations of existing antagonists. C108297 [(R)-4a-ethoxy-1-(4-fluorophenyl)-6-(4-trifluoromethylbenzenesulfonyl)-4,4a,5,6,7,8-hexahydro-1H-1,2,6-triazacylopenta[b]naphthalene; Corcept Therapeutics, Menlo Park, CA] is a non-steroidal modulator with high and selective affinity towards GRs (Ki 0.9nM). It has an almost 1000-fold lower affinity for the progesterone, estrogen, androgen and mineralocorticoid receptors (Clark et al., 2008). Animal studies suggest a wide range of therapeutic potential, particularly in conditions that affect hippocampal function (Asagami et al., 2011; Belanoff et al., 2010; Meyer et al., 2014; Pineau et al., 2016; Solomon et al., 2014; Zalachoras et al., 2013). C108297 exhibits dual agonistic and antagonistic properties that may be beneficial in the treatment of brain injury. For instance, C108297 has been shown to have anti-inflammatory effects in the hippocampus (Meyer et al., 2014) while blocking glucocorticoid-induced inhibition of hippocampal neurogenesis (Zalachoras et al., 2013) and attenuating memory loss following electroconvulsive shock therapy (Andrade et al., 2012). Moreover, treatment with C108297 reduces hypothalamic-pituitary-adrenal (HPA) axis activity but does not lead to complete disinhibition of the axis, thus maintaining negative feedback control of glucocorticoid levels (Solomon et al., 2014; Zalachoras et al., 2013). To explore the utility of this new compound, mice were treated with C108297 beginning one day after SE to assess its impact on SE-induced pathology.
METHODS
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Children’s Hospital Research Foundation and conform to NIH guidelines for the care and use of animals. All animals in the study were adult male (6–7-week-old) mice on an FVB background (Charles River International Strain Code 207). Data collection and analysis was conducted by investigators blind to animal treatment groups.
C10827 dose-response testing
Thirty adult male mice were used to determine the optimal dose of C108297. Mice were housed individually in standard shoebox cages and were acclimated to laboratory conditions for one week prior to all experimental procedures. For dosing, C108297 was dissolved at a concentration of 6mg/ml in polyethylene glycol. Mice were randomly distributed into the following four groups: 1) vehicle (polyethylene glycol) (n=6); 2) 15 mg/kg C108297 (n=8); 3) 30 mg/kg C108297 (n=8) and 4) 80 mg/kg C108297 (n=8). Drug was given once per day by subcutaneous injection for 10 consecutive days. Two hours following C108297 dosing on the fifth day, mice were exposed to restraint stress for 30 min. Tail blood samples were taken at 0, 30, 60 and 120 min following the onset of the stressor. Two hours following C108297 dosing on the tenth day mice completed the tail suspension test.
Pilocarpine-induced status epilepticus
To determine whether C108297 could mitigate brain pathology following status epilepticus (SE), a second group of 45 adult male mice was housed and acclimated as described for animals used for dose-response testing. To induce SE, mice received a single subcutaneous injection of methyl scopolamine nitrate (1 mg/kg), followed 15 min later by the cholinergic agonist pilocarpine (330 mg/kg, s.c.). Mice were monitored behaviorally for seizure activity and the onset of SE was noted as previously described (Danzer et al., 2010; Gross et al., 2016). Three hours after the onset of SE, all mice received two injections of diazepam spaced 10 min apart (10 mg/kg, s.c.) to control seizure activity. For the next 48hrs, mice were given sterile Ringer’s (saline) solution twice-daily for hydration support. Overall mortality among pilocarpine-treated mice was 32% (8 of 25). No saline-treated mice died (0 of 20). Body weight was assessed once daily. Pilocarpine and saline-treated mice were randomly assigned to treatment with either 30 mg/kg C108297 or polyethylene glycol vehicle. Drug treatment started 24hrs after SE. Mice received one subcutaneous drug or vehicle injection per day for 10 days (post-SE days 1–10), yielding the following four groups: 1) Pilocarpine status epilepticus + C108297 (SE+drug, n=9); 2) Control (non-SE) mice + C108297 (Control+drug, n=10); 3) Pilocarpine status epilepticus + polyethylene glycol vehicle (SE+vehicle, n=8) and 4) Control (non-SE) mice + polyethylene glycol vehicle (Control+vehicle, n=10).
Morning baseline corticosterone levels were collected by tail-nick 24hrs after SE, prior to treatment with C108297 or vehicle. A second blood sample was collected on day five after SE. Blood samples were collected in the morning 2 hours after lights-on and always prior to injection with Vehicle or C108297. Mice were weighed daily prior to administration of C108297 or vehicle. On the tenth day after SE, mice were exposed to restraint stress for 30 min. Restraint stress was initiated two hours following the last administration of C108297 or vehicle. Blood samples were collected by tail-nick at 0, 30 and 120 min from the beginning of the stressor. A single SE+vehicle mouse exhibited a seizure during restraint stress and was excluded from this analysis. Immediately after the 120 min blood collection time-point, mice were given pentobarbital (100 mg/kg, i.p.) and transcardially perfused with heparinized 0.1M PBS (1 U/ml), followed by 2.5% paraformaldehyde + 4% sucrose in 0.1M PBS. Adrenal glands were dissected, dried and weighed.
Brain histology and immunostaining after status epilepticus
Brains were cryoprotected in 30% sucrose in PBS for a minimum of 24hrs at 4°C. Cryoprotected brains were sectioned on a freezing microtome (35 μm thickness). Serial sections were collected into wells containing cryoprotectant solution (30% sucrose, 1% polyvinylpyrrolidone, 30% ethylene glycol in 0.1M PBS) and stored at −20°C.
Free floating brain sections were placed in blocking solution (4% normal goat serum, 0.1% bovine serum albumin and 0.2%Triton-X in 0.1M PBS) for 1hr and subsequently immunostained for 24hrs at 4°C with the following antibodies: rabbit anti-Fos (1:500 Santa Cruz Biotechnology, RRID: AB_2106783; (Wulsin et al., 2010)) mouse anti-NeuN (1:500 Millipore, RRID:AB_2298772; (Figueiredo et al., 2003)), rabbit anti-Prox1 (Sigma-Aldrich, 1:100, RRID: AB_1079691; (Murphy et al., 2012)), chicken anti-GFAP (Abcam, 1:1000, RRID: AB_304558), rabbit anti-GluR2/3 (Millipore, 1:100, RRID: AB_310741; (Hester and Danzer, 2013)) and rabbit anti-Iba1 (Synaptic Systems, 1:1000; (Hester et al., 2016; Smith et al., 2016)). Donkey anti-rabbit AlexaFluor594 (1:500 Jackson ImmunoResearch Labs, RRID:AB_2340621), goat anti-mouse AF488 (1:500 Jackson ImmunoResearch Labs, RRID:AB_2307324), donkey anti-rabbit AF647, goat anti-rabbit AF488, goat anti-chicken AF488 and goat anti-rabbit AF594 (Life Technologies, 1:750) secondary antibodies were used to visualize the respective primary antibodies. Stained tissue sections were mounted onto ultra-stick slides (Gold Seal™ Rite-On™; ThermoFisher Scientific) and coverslipped with Fluoromount-G (ThermoFisher Scientific) mounting media.
Mossy cell and ectopic granule cell quantification
Confocal image stacks through the z-depth of the hippocampal dentate hilus were collected using a Nikon A1Rsi inverted microscope with a 40× water objective (NA 1.15, resolution 0.62 μm/pixel). Image stacks were used to assess the number of granule cells (Prox1) and mossy cells (GluR2/3) in the dentate hilus (XY dimensions = 1818 μm × 512 μm, z-depth = 20 μm with a 0.4μm step). For each mouse a total of 3–4 hilar regions were imaged at bregma level −2.0 to −2.3. Hilar ectopic granule cells were defined as being Prox1 immunopositive cells located within the hilus and at least 20 μm from the granule cell layer-hilar border. Mossy cells were defined as GluR2/3 immunopositive cells located within the hilus with a soma diameter >20 μm (Jiao and Nadler, 2007). Cell counts were performed using a modified optical dissector method (Howell et al., 2002; Hofacer et al., 2013). Results are reported as hilar Prox1 or GluR2/3 cell densities (Prox1 or GluR2/3 cells/ x1000 mm3 of hilus).
Quantification of astrocytic and microglial changes; Hilar area measurements
Glial fibrillary acidic protein (GFAP) and ionized calcium-binding adaptor molecule 1 (Iba1) were imaged in 2–3 hippocampal sections per mouse at bregma level −2.3 mm. Confocal image stacks through the z-depth of the tissue were collected using a Nikon A1Rsi inverted microscope with a 40× water objective (NA 1.15, resolution 0.41 μm/pixel). The region of interest was set to include the outside border of the upper and lower blades of the dentate gyrus, enclosing both the hilus and the granule cell body layer (XY dimensions = 948 μm × 948 μm, z-depth = 20 μm with a 0.4 μm step). Image stacks were imported into Imaris Software to estimate the total hilar volume occupied by astrocytes (GFAP+) and microglia (Iba1+). Surfaces with a staining intensity of at least 4× local background were automatically identified, and the total volume occupied by the surfaces was determined. Results are represented as percentages [(total volume GFAP or Iba1 immunostained regions / total volume of hilus) × 100].
For quantification of hilar area, image stacks from tissue immunostained with GFAP and Iba1 were examined using Nikon Elements software (AR 5.21.01). DAPI labeling in each image stack was used to define a region of interest around the hilus using a boundary line drawn just below the granule cell body layer (the granule cell – hilar border), which was terminated laterally by a straight line connecting the tips of the upper and lower blades of the dentate gyrus. Areas for two dentate gyri per animal were quantified and used to generate animal averages for statistical analysis.
For quantification of microglia number and soma size, image stacks of Iba1-immunoreactive cells were obtained using a Nikon A1Rsi inverted microscope with a 60x water objective (NA 1.27, resolution 0.41 μm/pixel). The image field was centered on the dentate hilus (XY dimensions = 512 μm × 512 μm, z-depth = 15 μm with a 0.3 μm step). Microglia counts were performed in Imaris software using a modified optical dissector method (Hofacer et al., 2013). Microglia soma measurements were obtained using Nikon Elements software. Only microglia with their cell bodies fully contained within the tissue section were scored. Five to eight microglia per hemisphere were selected randomly for analysis (for a total of 10–20 cells per animal), as described previously (Hester et al., 2016). Microglial soma area data from each mouse was averaged, and these individual animal averages were used for statistical analysis.
Quantification of Fos expression
Confocal images of NeuN and Fos immunoreactivity were obtained using a Nikon C2+ microscope (NIS-Elements, RRID:SCR_014329). The following brain regions were analyzed: 1) Infralimbic portion of medial prefrontal cortex, 2) prelimbic portion of medial prefrontal cortex, 3) the hippocampal dentate gyrus, 4) central amygdala, 5) basal amygdala, 6) ventral subiculum and 7) the hypothalamic paraventricular nucleus (Paxinos and Watson, 1998). 2–3 sections per brain region were analyzed. See supplemental table 1 for imaging and localization details on each region examined. Fos and NeuN cell counts were obtained with Imaris Software (RRID: SCR_007370). Fluorescent spots automated detection method (Hosford et al., 2016) was used to determine the number of immunopositive cells. Minimum fluorescent intensity and size criteria (>4.14 μm) were used for the automated screening. Cells cropped at the surface of the image stack and at two predetermined adjacent sides in X-Y plane were excluded. Automated Fos+ and NeuN+ counts were reviewed to remove false-positives and to identify false-negatives. Results are reported as percentages [(total Fos+ cells / total NeuN+ cells)*100].
Corticosterone radioimmunoassay
Tail blood samples (~50 μl) from drug dosing and status epilepticus experiments were collected into EDTA-treated microvette collection tubes and immediately placed on ice. Sample tubes were placed in a centrifuge (6000 g, 15min, 4°C) to isolate plasma, which was stored at −20°C. Plasma corticosterone levels were measured using 125I RIA kit (MP Biomedicals Inc., Orangeburg, NY) as previously described (Wulsin et al., 2016a).
Statistical analyses
Statistical analyses were performed using SigmaPlot v.13 (RRID:SCR_003210). Behavioral data in drug-dosing experiments was analyzed with one-way ANOVA, while CORT levels after restraint stress were analyzed using a two way-repeated measures ANOVA (between-subject factor: treatment dose; within-subject factor: time-point). Two-way ANOVA was used to compare treatments (vehicle vs C108297) and conditions (control vs SE) for SE experiments. GraphPad Prism software (RRID:SCR_002798) was used to perform three-way repeated measures ANOVA for the analysis of corticosterone in response to restraint for SE experiments. Mixed-effects model (REML) was used to analyze body weight data as an alternative to three-way repeated measures ANOVA, given missing values using GraphPad Prism software. All interactions were tested by Student Newman–Keuls post-hoc analysis. Planned comparisons were tested by Fisher LSD. Statistical significance was set at p<0.05. Data that failed tests of normality or equal variance were normalized using either square root or rank transformations. Values are presented as means of raw (non-transformed) data ± standard error of the mean (SEM). All graphs were prepared using GraphPad Prism software.
RESULTS
C108297 dose-response testing
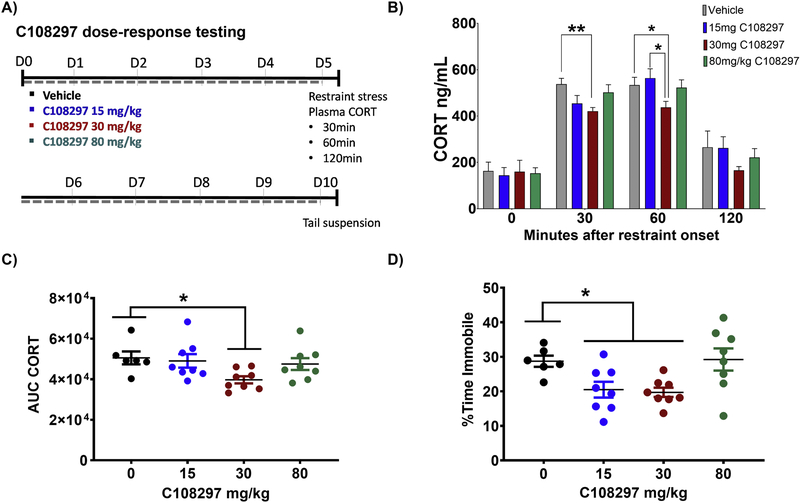
To determine the drug dose required to suppress hypothalamic-pituitary-adrenal (HPA) axis function and modulate depression-like behaviors, groups of naïve control mice were treated with 0, 15, 30 and 80 mg/kg of C108297 once daily for ten days (Fig.1A). When challenged with 30 minutes of restraint stress on day five of drug dosing, mice treated with the 30 mg/kg dose exhibited a reduction in corticosterone secretion at 30 and 60 minutes compared to 0 mg/kg (Fig.1B; Main effect time-point [F(3,78)=121.3, p<0.001], planned comparisons 30 mg/kg vs 0 mg/kg at 30min, p=0.004; 30 mg/kg vs 0 mg/kg at 60min, p=0.046; 30 mg/kg vs 15 mg/kg at 60min, p=0.021}). The 30 mg/kg dose had no effect at the 0 (p=0.96) or 120min (p= 0.22) timepoints when compared to 0 mg/kg group. Analysis of integrated corticosterone secretion (area under the curve) confirmed the efficacy of the 30 mg/kg dose (Fig.1C; [F(3,26)=3.513, p=0.029], post hoc 30 mg/kg vs 0 mg/kg, p=0.031). Neither the 15 mg/kg nor the 80 mg/kg doses were effective at reducing corticosterone secretion (Figs.1B & 1C), suggesting a bimodal response to the drug. Both the 30 mg/kg and 15 mg/kg doses were effective at reducing immobility time in the tail suspension test relative to the 0 mg/kg group (Fig.1D; [F(3,26)=4.930, p=0.008] post hoc 30 mg/kg vs 0 mg/kg, p = 0.037; 15 mg/kg vs vehicle, p=0.024). Based on these results, the 30 mg/kg dose was used for the remainder of the study.
Figure 1. Treatment with 30 mg/kg of C108297 reduces stress-induced corticosterone secretion and depressive-like behavior.
A) Timeline outlining the experimental design. Mice were treated for 10 days with vehicle or one of three different doses of C108297: 15, 30 and 80 mg/kg. On day 5 mice underwent restraint stress for timed corticosterone plasma collection. On day 10 mice underwent the tail suspension test. B) After five days of treatment, 30 mg/kg C108297 reduced corticosterone secretion at 30 and 60 minutes following the onset of restraint stress relative to vehicle treated mice. C) The overall corticosterone response to restraint stress, as measured by the area under the curve (AUC), was lower in mice treated with 30 mg/kg C108297 for five days prior to stress exposure. D) After ten days, C108297 treatment reduced immobility in the tail suspension test. Data presented as mean ± SEM, n = 8–9 mice per group. *p<0.05; **p<0.01.
C108297 treatment normalizes corticosterone levels after status epilepticus (SE)
To determine whether modulating GR signaling can mitigate the negative consequences of SE, mice were treated with 30 mg/kg C108297 once daily for 10 days beginning 24hrs after pilocarpine-induced status epilepticus (Fig.2A). Just before the animals received the first drug dose on day one, a blood sample was collected to establish morning baseline corticosterone levels. Corticosterone was elevated in the SE groups relative to no SE controls (Fig.2B; Main effect condition [F(1,36)= 105.798, p<0.001]; post hoc SE vs Control within Vehicle, p<0.001), confirming prior work showing that SE leads to HPA axis hyperreactivity (O’Toole et al., 2014; Wulsin et al., 2016a; Wulsin et al., 2018). As expected, vehicle and C108297 groups were statistically identical at this time point.
Figure 2. C108297 normalizes status epilepticus (SE)-induced baseline corticosterone hypersecretion.
A) Experimental timeline. Mice received pilocarpine to induce SE. Treatment with vehicle or 30 mg/kg of C108297 was administered 24hrs after SE and given daily thereafter for a total of 10 days. Baseline plasma corticosterone levels were collected prior to initial treatment administration and again on day 5. On day 10 mice were exposed to 30 minutes of restraint stress and plasma levels of corticosterone were collected 0, 30 and 120min after initiation of restraint. B) Prior to receiving the first C108297 dose, SE-vehicle and SE-drug mice both showed increased baseline corticosterone secretion relative to the controls. C) Five days of C108297 treatment normalized baseline corticosterone secretion relative to vehicle treated SE mice. D) Vehicle-treated SE mice showed greater corticosterone secretion in response to restraint stress at the 30min time-point relative to vehicle-treated control mice. C108297-treated SE mice were statistically identical to controls at 30min. E) The overall corticosterone response to restraint stress at 30min as measured by the area under the curve (AUC). Vehicle-treated SE mice showed a non-significant trend towards greater corticosterone levels relative to vehicle-treated controls. F) Adrenal weights are increased in SE mice independent of drug treatment. Data presented as mean ± SEM, n = 6–10 mice per group. *p<0.05; ***p<0.001.
We next set out to determine whether C108297 could mitigate the SE-induced increase in corticosterone. Thus, morning baseline corticosterone measures were assessed again five days after SE. Baseline corticosterone levels remained elevated in SE+vehicle mice relative to controls (Fig.2C; Main effect condition [F(1,29)=75.97, p<0.0001; post hoc Control+vehicle vs SE+vehicle, p<0.0001). However, corticosterone levels in SE+drug mice were significantly lower than the SE+vehicle group (Fig.2C; Interaction condition × treatment [F(1,29)= 36.778, p<0.001]; post hoc C18297 vs Vehicle within SE, p<0.001). These data demonstrate that C108297 treatment is effective at blocking the SE-induced increase in baseline corticosterone levels.
In addition to measuring baseline corticosterone levels, we also queried whether C108297 would prevent enhanced stress-induced corticosterone release evident in animals following SE (Wulsin et al., 2018). To address this question, animals were exposed to restraint stress after 10 days of drug treatment, and CORT levels were measured at 0 (pre-restraint baseline), 30 and 120 min.
Baseline CORT levels at 10 days (Fig.2D, time=0) were statistically identical among Control+vehicle, SE+vehicle and SE+drug groups (Control-vehicle vs. SE-vehicle, p=0.596; Drug treatment within SE, p=0.630). The Control+drug group, on the other hand, showed reduced CORT levels relative to Control+vehicle (p=0.019) and SE+drug groups (p=0.016). These findings support two conclusions. Firstly, elevated CORT evident at 5 days in SE groups (Fig.1C) had returned to baseline values by 10 days (Fig.1D). Secondly, C108297 treatment was only effective at reducing CORT in control (no SE) animals.
After 30 minutes of restraint, SE+vehicle mice exhibited significantly higher corticosterone levels relative to Control+vehicle mice (Fig.2D; main effect of condition [F(1,29)=6.552, p=0.016] with a strong trend towards an interaction of condition × treatment × timepoint [F(2,58)=3.018, p=0.056]; planned comparisons SE+vehicle vs Control+vehicle at 30min timepoint, p=0.016). Although slightly elevated, the SE+drug group was statistically identical to controls (p=0.44), suggesting that drug treatment prevented the increase. However, the SE+drug group was also statistically identical to the SE+vehicle group (p=0.067). Analysis of integrated CORT secretion (AUC) at 30 min produced similar findings, although the increase in the SE+vehicle group did not reach significance (Fig.2E; main effect condition [F(1,29)=4.005, p=0.055]). The findings are consistent with C108297 mitigating the stress response, however, given the mixed result, we interpret the finding cautiously.
CORT levels at the 120 time-point trended towards baseline values; however, the vehicle-treated groups were still higher than the Control+drug group (Fig.2D; Control+Vehicle, p=0.029; SE+Vehicle, p=0.028). Interestingly, the SE+drug group was now statistically identical to the Control+drug group (a shift from the 0 min timepoint), suggestive of a faster decline in CORT levels following stress.
We next queried whether treatment could prevent adrenal gland hypertrophy, a common finding in both chronically stressed animals and animals following SE (Ulrich-Lai et al., 2006; Wulsin et al., 2016b). Increased adrenal gland weight was evident in SE+vehicle mice relative to controls. This increase was not prevented by C108297 treatment (Fig.2F; Main effect of condition [F(1,29)=35.798, p<0.001]; SE vs Control within C108297 and Vehicle, p<0.001). Within control and SE groups, respectively, vehicle and drug-treated groups were statistically identical.
Expansion of the dentate hilus is not blocked by C108297 treatment
The hippocampal dentate hilus exhibits significant changes in cellular composition during epileptogenesis, including loss of interneurons and mossy cells, and accumulation of glial cells and ectopic granule cells. Changes in hilar area could contribute to observed differences in cell density. We queried, therefore, whether the gross structure of the hilus was altered in our animals by quantifying hilar area (Fig.3). Hilar area was significantly increased in pilocarpine-SE mice relative to controls (Fig.3B; main effect of condition [F(1,24)=28.806, p<0.001]; post hoc SE vs Control, p<0.001). No effect of drug was found (p=0.678). These findings indicate the drug treatment does not prevent SE-induced expansion of the dentate hilus.
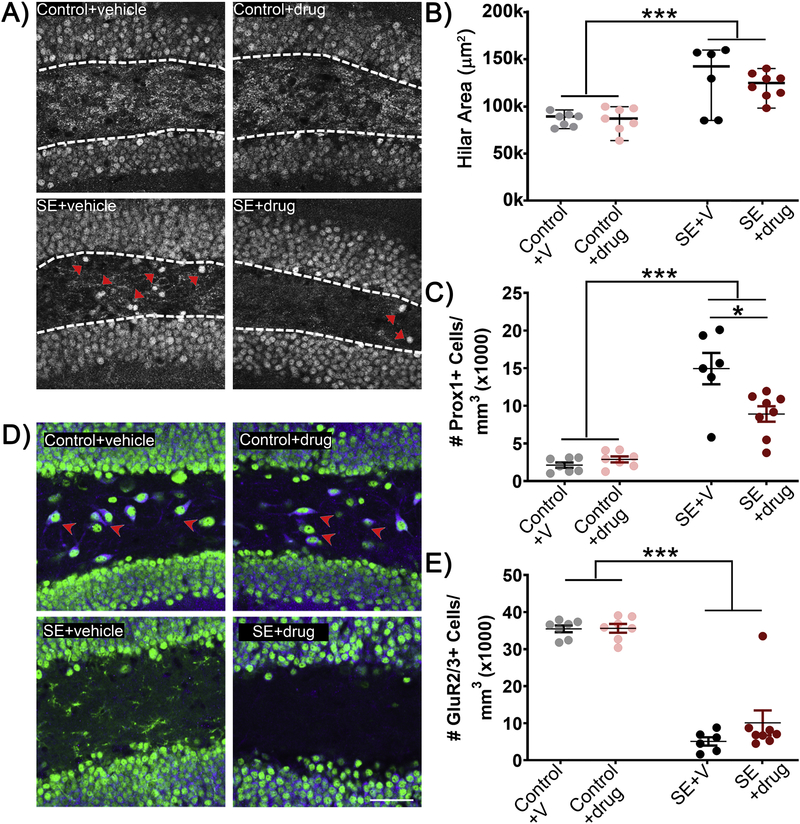
Figure 3: C108297 reduces the number of ectopic dentate granule cells following SE.
A) Representative micrographs of dentate hilus. Arrowheads show Prox 1+ ectopic granule cells. B) The area of the dentate hilus was increased following SE, however, C108297 treatment had no effect in either control or SE groups. C) Treatment with C108297 following SE reduces the number of Prox1+ ectopic granule cells relative to vehicle treated SE mice. D) Representative micrographs of dentate hilus. Arrows show GluR2/3+ mossy cells. E) Treatment with C108297 did not rescue SE-induced mossy cell loss. Data presented as mean ± SEM, n = 6–8 mice per group. *p<0.05; ***p<0.001.
C108297 reduces SE-induced dentate granule cell hippocampal pathology
SE is associated with the ectopic accumulation of granule cells in the dentate hilus which have been implicated in the subsequent development of epilepsy (Cho et al., 2015; Hosford et al., 2016, 2017; Parent et al., 1997; Scharfman et al., 2003; Singh et al., 2015; Walter et al., 2007). In the present study, pilocarpine induced-SE led to an increase in the density of hilar ectopic granule cells, evident as somatic profiles in the dentate hilus immunoreactive for the granule cell specific marker Prox-1 (Fig.3A & C; main effect of condition [F(1,24)=89.690, p<0.001]; post hoc SE vs Control, p<0.001). Increases were observed in both SE+vehicle and SE+drug groups, however, further analysis revealed that C108297 treatment significantly reduced the density of ectopic cells in the SE group (interaction condition × treatment F(1,24)=8.697, p=0.007] post hoc C108297 vs vehicle within SE, p=0.016).
Mossy cell loss is not prevented by C108297 treatment
Hilar mossy cells mediate feedback inhibitory control of the dentate and are vulnerable to seizure-induced cell death (Jiao and Nadler, 2007; Scharfman and Myers, 2012; Zhang et al., 2014). Consistent with this work, SE resulted in the overall reduction in GluR2/3-immunoreactive mossy cells (Fig.3D & E; main effect condition [F(1,24)= 59.990, p<0.001]; post hoc SE vs Control, p<0.001). Treatment with C108297, however, did not prevent mossy cell loss
C108297 reduces reactive microgliosis in SE mice
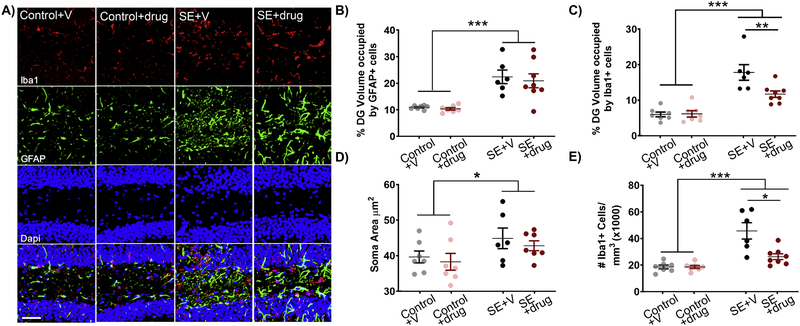
SE increased astrogliosis in the hippocampal dentate gyrus as measured by the volume of the dentate occupied by GFAP-immunoreactive cells (Fig.4A & B; main effect condition [F(1,24)=35.677, p<0.001]; post hoc SE vs Control, p<0.001). Similarly, SE resulted in increased microgliosis in the same region as determined using the microglia marker Iba1 (Fig.4A & C; main effect condition [F(1,24)= 67.892, p<0.001]; post hoc SE vs Control, p<0.001). Treatment with C108297 had no effect on the volume of the dentate occupied by GFAP-immunoreactive cells within control or SE mice (Fig.4B). C108297 treatment did, however, reduce the volume of the dentate occupied by Iba1-immunoreactive cells in SE+drug mice relative to SE+vehicle mice (Fig.4C; interaction condition × treatment [F(1,24) = 4.497, p=0.044]; post hoc C108297 vs vehicle within SE, p=0.008). To further elucidate exactly how Iba1+ cells change, we determined the average soma area and density of immunoreactive Iba1+ cells in the dentate. Measurements of Iba1+ cell soma area did not reveal a significant difference with drug treatment, although soma area was increased in SE mice vs. controls (Fig.4D; main effect condition F(1,24)=7.070, p=0.014]; post hoc SE vs Control, p=0.014). Iba1+ cell counts, on the other hand, revealed that microglial density was increased among SE groups (Fig.4E; main effect condition [F(1,24)=44.134, p<0.001]). C108297 treatment mitigated this increase in Iba1+ cell density in SE mice (post hoc C108297 vs vehicle within SE, p=0.021). Drug treatment had no effect on density in controls. Together, these findings suggest that C108297 limits SE-induced microglial proliferation, but not hypertrophy.
Figure 4. C108297 treatment decreased SE-induced microgliosis.
A) Representative micrographs of the hippocampal dentate gyrus showing Iba1+ microglia (red) and GFAP+ astrocytes (green). B) SE increases the percentage of the hilus occupied by GFAP immunoreactivity relative to controls, but drug treatment is without effect. C) SE increases the percentage of the hilus occupied by Iba1 immunoreactivity relative to controls. Treatment with C108297 reduced hilar Iba1 immunoreactivity in SE mice relative to their vehicle-treated counterparts. D) The soma area of Iba1+ microglia was increased following SE. C108297 treatment does not block this effect. E) The density of Iba1+ microglia was increased after SE, and this increase in density was significantly reduced by C108297 treatment. Data presented as mean ± SEM, n = 6–8 mice per group. *p<0.05; **p<0.01; ***p<0.001.
C108297-induced changes in Fos expression
To gain insight into patterns of brain activity following restraint stress, we investigated expression of the immediate early gene Fos (Fig.5). Patterns of Fos activation can reveal whether the brain’s stress regulatory circuitry is responding in a normal or atypical pattern. For the present study, the number of Fos immunoreactive neurons was normalized to total neuron number, accommodating for any SE-induced cell loss or changes in tissue volume.
Figure 5: Percentage of Fos-expressing neurons following SE and C108297 treatment.
In the paraventricular nucleus (A, PVN) there was a non-significant trend (p=0.05) towards a greater percentage of Fos-expressing neurons in the SE-vehicle group relative to the Control-vehicle group, and a significant reduction in the SE-drug group vs the SE-vehicle group – suggesting that treatment blocked the apparent increase (B). In prelimbic and infralimbic prefrontal cortex (C, PL, IL), SE reduced the percentage of Fos+ neurons regardless of C108297 treatment (D, E), although treatment significantly increased Fos expression in infralimbic cortex in drug-treated controls (D). In dentate gyrus and ventral subiculum (F; DG, VS), SE reduced the percentage of Fos+ neurons regardless of C108297 treatment (G, H). In basal and central amygdala (I, BLA, CeA), SE also reduced the percentage of Fos+ neurons regardless of C108297 treatment (J, K). Data presented as mean ± SEM, n = 6–8 mice per group. *p<0.05; **p<0.01; ***p<0.001.
SE animals exhibited significantly lower densities of Fos immunoreactive cells in infralimbic (Fig.5D; main effect condition [F(1,21)=62.619, p<0.001]; post hoc SE vs Control, p<0.001) and prelimbic (Fig.5E; main effect condition [F(1,21)=40.895 p<0.001]; post hoc SE vs Control, p<0.001) regions of the prefrontal cortex, the dentate gyrus (Fig.5G; main effect condition [F(1,21)=69.878, p<0.001]; post hoc SE vs Control, p<0.001), the ventral subiculum (Fig.5H; main effect condition [F(1,21)= 55.291, p<0.001]; post hoc SE vs Control, p<0.001), the basal lateral amygdala (Fig.5J; main effect of condition [F(1,21)=108.885, p<0.001]; post hoc SE vs Control, p<0.001) and the central nucleus of the amygdala (Fig.5K; main effect condition [F(1,21)=10.326, p=0.004]; post hoc SE vs Control within C108297, p=0.004). Treatment with C108297 did not prevent any of these changes. Drug treatment did, however, produce several significant effects. Within SE animals, the percentage of Fos+ cells was significantly reduced in the paraventricular nucleus in SE+drug mice vs. SE+vehicle mice (Fig.5B; Interaction condition × treatment [F(1,21)=5.541, p=0.028]; post hoc C108297 vs Vehicle within SE, p=0.011). Although not statistically significant, there was a strong trend showing increased Fos+ cells in SE+vehicle mice compared to Control+vehicle mice (post hoc SE vs Control, p=0.050). Within control animals, the percentage of Fos+ cells was significantly increased in the infralimbic cortex with drug treatment (Fig.5D; Interaction condition × treatment [F(1,21)=7.298, p=0.013]; post hoc C18297 vs Vehicle within Control, p<0.001). Although these findings do not provide strong evidence that C108297 normalizes activity patterns in the HPA axis, they do demonstrate that the drug can modulate activity.
C108297 treatment does not mitigate weight loss after status
SE can alter animal growth, with weight loss evident in acute recovery periods – likely due to the physical stress of status – followed by chronic weight gain (Hester et al., 2016). Daily weight measurements revealed a significant reduction in SE groups relative to controls during the 10 day period after SE (Fig.6; interaction condition × days [F(10,315)=5.582, p<0.0001]; post hoc SE vs Control within vehicle showed statistical significance on D1-D10, p<0.001, SE vs Control within C108297 showed statistical significance on D1-D9, p<0.05). C108297 treatment had no effect on animal weight.
Figure 6:
SE and C108297 effects on weight. Body weight data for the 10-day drug treatment period shows significant weight loss following SE relative to controls that did not undergo SE. No effect of C108297 was found. The shaded line represents the time of SE and initiation of treatment.
DISCUSSION
The present study was designed to determine whether modulating glucocorticoid receptor (GR) signaling with C108297 following pilocarpine-induced SE could prevent chronic elevations in corticosteroid levels, ameliorate hippocampal pathologies and restore the normal pattern of stress-induced Fos activation in forebrain stress-regulatory circuits known to be disrupted in temporal lobe epilepsy (Wulsin et al., 2018). Our results demonstrate that treatment with C108297 normalizes baseline corticosterone secretion, reduces the number of ectopically located dentate granule cells and decreases microglial proliferation following status epilepticus. While treatment did not normalize stress-induced Fos activation, it did alter the pattern of expression in several brain regions. These studies implicate glucocorticoid receptor signaling in the development of SE-induced hippocampal remodeling, highlighting the potential of GR modulators as novel drug class to mitigate the lasting negative consequences of SE.
Pharmacological manipulation of GR signaling
GRs are widely expressed throughout the body, and stress hormone levels are regulated by complex interactions within the HPA axis. Systemically-applied C108297, therefore, has the potential to act on a wide range of tissues. GRs are nuclear receptors that can modify gene transcription by either directly binding to DNA or via protein interactions with transcription factors or nuclear receptor coregulators (De Bosscher et al., 2003). Selective GR modulators like C108297 were developed in an attempt to separate beneficial from unwanted effects (e.g., disrupted negative feedback) by changing the specificity of ligand-induced interactions between GR and its downstream effectors or coregulators (Coghlan et al., 2003). While C108297 can block corticosterone-GR binding, it appears to have the capacity to translocate and bind nuclear co-activator proteins, resulting in tissue-specific partial agonist activity. For example, C108297-GR complexes do not recruit the same co-regulators as the GR antagonist mifepristone, but instead show partial agonistic effects by recruitment of some (but not all) of the co-regulators induced by the agonist dexamethasone (Zachaloras et al., 2013).
One such coregulator is steroid coactivator (SRC)-1. SRC1 regulates GR specific genes, and splice variants have differential effects on stress adaptation and tissue specific corticotropin releasing hormone (CRH) expression (van der Laan et al., 2008). C108297 can act as a GR antagonist – blocking effects of corticosterone in the amygdala and on hippocampal neurogenesis – but can also act as an agonist in suppressing CRH mRNA expression in the paraventricular nucleus and mimicking the effects of corticosterone on avoidance learning (Zalachoras et al., 2013). Effects in the amygdala appear to be related to selective expression of the C108297-sensitive SRC-1E as opposed to SCR-1A, suggesting that mechanisms of action may depend on molecular interactions of C108297-bound GR (Zalachoras et al., 2016). Notably, work by Baram and colleagues suggests that excess CRH has robust epileptogenic effects in the infant brain (Baram 1992, Baram 1995; Gunn and Baram; 2017). While the effects observed in the present study could be mediated by direct interaction between C108297 and GRs on hippocampal neurons, the possibility that drug effects are driven by other brain regions or even by hormonal changes initiated outside the CNS cannot be excluded.
C108297 partially normalizes corticosterone secretion after SE
Pilocarpine-induced SE results in the persistent elevation of baseline and stress-induced glucocorticoid secretion (Mazarati et al., 2009; O’Toole et al., 2014; Wulsin et al., 2016a; Wulsin et al., 2018). Five days of treatment with C108297 effectively normalized baseline corticosterone secretion in SE mice. Drug treatment did not, however, fully normalize corticosterone levels following restraint stress, with the levels for SE+drug-treated mice landing in a statistically ambiguous position between controls and vehicle-treated SE mice. In addition, drug treatment failed to prevent adrenal hypertrophy. The mixed effect is consistent with C108297’s GR agonist and antagonist properties (Zalachoras et al., 2013), and provides an opportunity to explore whether partial normalization of glucocorticoid secretion after SE has positive effects.
C108297 reduces the number of hilar ectopic granule cells
Ectopic granule cells are newly-generated neurons that are aberrantly localized to the dentate hilus following SE (Kron et al., 2010). The ectopic neurons are hyperexcitable and are linked to the development of epilepsy (McCloskey et al., 2006; Myers et al., 2013; Hester and Danzer, 2014; Cho et al., 2015). Anti-neurogenic and cell ablation approaches that reduce the density of ectopic cells have been found to reduce seizure frequency in many studies (Danzer, 2019). The reduction in the density of ectopic granule cells with C108297 is consistent with prior work showing that RU486 – a less selective GR antagonist that also blocks progesterone receptors – reduced the accumulation of ectopic neurons (Wulsin et al., 2016a). The present results provide converging lines of evidence supporting a role for GR activation in ectopic granule cell accumulation. Although not explored here, the reduction in ectopic cells suggests that C108297 could have anti-epileptogenic properties if given shortly after SE.
Mossy cells are not rescued by C108297 treatment
Hilar mossy cells are glutamatergic neurons that mediate negative feedback control of the dentate gyrus via inhibitory basket cells. They consistently die during SE (Scharfman, 2016). C108297 treatment did not prevent the loss of mossy cells. This result was not unexpected, as C108297 treatment was begun 24 hours after SE, a time point at which most mossy cells are already dead. By contrast, we have previously shown that initiation of treatment 3hrs after the onset of SE with RU486 rescues mossy cells (Wulsin et al., 2016a). While earlier treatment with C108297 might also prevent this loss, later treatment – as used here – is more clinically relevant with better translational potential.
C108297 reduces reactive microgliosis in SE mice
SE results in an expansion in hippocampal area occupied by microglia and astrocytes (Borges et al., 2003; Garzillo and Mello, 2002). Treatment with C108297 following SE reduced microglia proliferation in the hippocampal dentate gyrus without affecting the increase in area occupied by astrocytes. Although glucocorticoids are widely known to have anti-inflammatory effects in the central nervous system (Schweingruber et al., 2012), recent studies suggest that in the context of excess activation of the HPA axis, glucocorticoids may be pro-inflammatory (Carrillo-de Sauvage et al., 2013; Frank et al., 2015; Munhoz et al., 2010; Vyas et al., 2016). C108297, for example, has been shown to have anti-inflammatory effects in the hippocampus of Wobbler mice, a model of ALS. Like SE mice, Wobbler mice show HPA axis hyperactivity, and treatment with C108297 decreases astrocyte hypertrophy and reverts microglia to their resting state (Meyer et al., 2014).
Mechanisms of C108297 action on brain pathology
The mechanisms by which C108297 reduces microgliosis and ectopic granule cell accumulation remain to be ascertained. Interestingly, C108297 treatment can prevent stress-induced reductions in granule cell neurogenesis (Zalachoras et al., 2013). Hilar ectopic granule cells in the epileptic brain are newly generated (Walter et al., 2007; Kron et al., 2010; Santos et al., 2011). By preventing stress-induced reductions in neurogenesis, C108297 might be predicted to increase ectopic cell accumulation after SE. Whether and how the drug alters neurogenesis in SE, however, remains to be determined. Moreover, it is also conceivable that C108297 could promote neurogenesis and the proper integration of these new cells. Follow-up studies using BrdU labeling and neuronal fate-mapping approaches are needed to elucidate whether C108297 alters neuronal proliferation and how it acts to reduce ectopic cell accumulation following SE.
It is possible that C108297’s effects on ectopic granule cells and microglia are related. Microglia express glucocorticoid receptors (Sierra et al., 2008) and therefore may be targeted by C108297. Microglia are also implicated in regulating the accumulation of ectopic granule cells. Inhibiting microglial proliferation after status epilepticus increases ectopic granule cell accumulation (Yang et al., 2010; Luo et al., 2016). Moreover, Matsuda and colleagues (2015) describe how microglia act to reduce the accumulation of ectopic granule cells by secreting tumor necrosis factor-α. Based on these findings, one might predict that reduced microglial proliferation would exacerbate epilepsy-associated pathology. However, the role of microglia in epilepsy is complex, with evidence for both protective and harmful effects (Kinoshita and Koyama, 2021; Vezzani et al., 2015; Vezzani et al., 2016; Jassam et al., 2017; Liu et al., 2020; Wu et al., 2020; Wyatt-Johnson and Brewster, 2020). This complexity is compounded by remaining uncertainty about the normal role played by corticosteroids in regulating microglial function, and exactly how C108297, with its partial agonist and antagonist properties, might alter this regulation. Future studies using pharmacological and genetic approaches to manipulate GR function among microglia would shed light on this interesting area.
C108297 alters, but does not restore, stress activated patterns of Fos
Following SE, stress-induced Fos activation was reduced in inferior and prelimbic cortex, dentate gyrus, ventral subiculum, central and basal lateral amygdala. C108297 treatment failed to normalize the pattern of Fos immunoreactivity in these regions. Interestingly, drug treatment did reduce Fos activation in the paraventricular nucleus of the hypothalamus, a major hub regulator of the HPA axis response, corroborating known GR-agonist-like effects at the level of the hypothalamus (Solomon et al., 2014; Zalachoras et al., 2013). Overall, C108297 was capable of altering patterns of Fos activation, but since a restoration of control patterns of activation was not observed, whether the altered patterns reflect improved, exacerbated, or unchanged behavioral responses is difficult to predict.
The widespread reduction of Fos activation observed in the present study is consistent with prior work in epileptic animals. Although spontaneous seizures lead to widespread Fos activation in the minutes and hours after occurrence in the mouse pilocarpine model, Peng and Houser (2005) found reduced hippocampal Fos activation in animals examined 8 hours or more after a seizure. Moreover, chronic seizure induction has been found to suppress Fos in a variety of animal models (Winston et al., 1990; Mello et al., 1996; Simler et al., 1999; Calais et al., 2013). For the present study, mice were collected 10 days after status epilepticus. Seizures are more frequent at longer time periods after status but can occur in the first week (Cavalheiro et al., 1996; Mazzuferi et al., 2012). It is possible, therefore, that widespread reductions in Fos levels reflect the lasting suppressive effects of spontaneous seizures. It is also possible that status epilepticus produces a lasting reduction in Fos activity, still evident at 10 days. The present results, therefore, should be interpreted in light of the early time point examined. The impact of chronic epilepsy, recurrent seizures and GR modulation on stress-induced patterns of Fos activation – as well as other measures of epilepsy-associated pathology – remains an intriguing area for further study.
Limitations of the current study
For the present study, we found that C108297 reduced the density of hilar ectopic (Prox1+) dentate granule cells and Iba1+ microglial cells relative to SE mice that received vehicle. Both vehicle and drug-treated SE mice exhibited significant expansion of the dentate hilus (Fig.3B). Given equivalent total cell number, a larger hilus would lead to a reduction in observed cell density. SE-induced increases in the density of Prox1+ and Iba1+ cells observed here, therefore, likely underestimate the true change relative to controls, while mossy cell loss is likely overestimated. Importantly, however, the lack of a difference in hilar area between the two SE groups favors the interpretation that the density changes (or absence of changes) presented here are real – although stereological counts through the rostral-caudal extent of the hippocampus could still produce different results. Finally, we note that measures of Fos+ cells – expressed as a percentage of NeuN+ cells – are volume independent and thus not affected by this limitation.
In conclusion, the present study demonstrates that treatment with the GR modulator C108297 beginning 24hrs after SE prevents baseline corticosteroid hypersecretion and mitigates structural brain changes evident 10 days later. Although the drug did not prevent all sequelae of SE, partial efficacy holds out promise that GR modulation could be a novel approach to prevent brain damage following SE.
Supplementary Material
Highlights.
C108297 reduces glucocorticoid hypersecretion after status epilepticus
Glucocorticoid receptor modulation reduces brain injury after status epilepticus
Glucocorticoids may promote epileptogenic brain injury
ACKNOWLEDGEMENTS
This work was supported by National Institute of Neurological Disorders and Stroke grants R01NS065020 (SCD), R01NS062806 (SCD) and F30NS095578 (ACW); National Institute of Mental Health grants MH049698 and MH101729 (JPH) and National Institute of General Medical Sciences grant T32-GM-063483 (ACW). NIH and other funding agencies had no further role in the design, collection, analysis, interpretation and presentation of the data. We thank the CCHMC Confocal Core for providing access and support with confocal microscopy. We also thank Drs. Joseph Belanoff, Hazel Hunt and Robin Clark of Corcept Therapeutics for the generous donation of the CORT 108297.
Footnotes
CONFLICTS OF INTERESTS
The authors received CORT108297 as a donation from Corcept Therapeutics. No additional funding or support was provided by Corcept Therapeutics to complete any of the experiments presented. The authors do not have any financial interest in the company.
Supplementary data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrade C, Shaikh SA, Narayan L, Blasey C, Belanoff J, 2012. Administration of a selective glucocorticoid antagonist attenuates electroconvulsive shock-induced retrograde amnesia. J. Neural Transm 119, 337–344. doi: 10.1007/s00702-011-0712-8 [DOI] [PubMed] [Google Scholar]
- Asagami T, Belanoff JK, Azuma J, Blasey CM, Clark RD, Tsao PS, 2011. Selective Glucocorticoid Receptor (GR-II) Antagonist Reduces Body Weight Gain in Mice. J. Nutr. Metab 2011, 235389. doi: 10.1155/2011/235389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanoff JK, Blasey CM, Clark RD, Roe RL, 2010. Selective glucocorticoid receptor (type II) antagonist prevents and reverses olanzapine-induced weight gain. Diabetes. Obes. Metab 12, 545–7. doi: 10.1111/j.1463-1326.2009.01185.x [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R, 2003. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp. Neurol. 182, 21–34. doi: 10.1016/S0014-4886(03)00086-4 [DOI] [PubMed] [Google Scholar]
- Calais JB, Valvassori SS, Resende WR, Feier G, Athié MC, Ribeiro S, Gattaz WF, Quevedo J, Ojopi EB (2013) Long-term decrease in immediate early gene expression after electroconvulsive seizures. J Neural Transm (Vienna). 120(2):259–66. [DOI] [PubMed] [Google Scholar]
- Carrillo-de Sauvage MÁ, Maatouk L, Arnoux I, Pasco M, Sanz Diez A, Delahaye M, Herrero MT, Newman TA, Calvo CF, Audinat E, Tronche F, Vyas S, 2013. Potent and multiple regulatory actions of microglial glucocorticoid receptors during CNS inflammation. Cell Death Differ. 20, 1546–57. doi: 10.1038/cdd.2013.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro OW, Santos VR, Pun RYK, McKlveen JM, Batie M, Holland KD, Gardner M, Garcia-Cairasco N, Herman JP, Danzer SC, 2012. Impact of corticosterone treatment on spontaneous seizure frequency and epileptiform activity in mice with chronic epilepsy. PLoS One 7, e46044. doi: 10.1371/journal.pone.0046044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Santos NF, Priel MR (1996) The pilocarpine model of epilepsy in mice. Epilepsia 37(10):1015–9. [DOI] [PubMed] [Google Scholar]
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, Scharfman HE, Eisch AJ, Hsieh J, 2015. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun 6:6606. doi: 10.1038/ncomms7606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RD, Ray NC, Williams K, Blaney P, Ward S, Crackett PH, Hurley C, Dyke HJ, Clark DE, Lockey P, Devos R, Wong M, Porres SS, Bright CP, Jenkins RE, Belanoff J, 2008. 1H-Pyrazolo[3,4-g]hexahydro-isoquinolines as selective glucocorticoid receptor antagonists with high functional activity. Bioorg Med Chem Lett. 18(4):1312–7. doi: 10.1016/j.bmcl.2008.01.027 [DOI] [PubMed] [Google Scholar]
- Coghlan MJ, Jacobson PB, Lane B, Nakane M, Lin CW, Elmore SW, Kym PR, Luly JR, Carter GW, Turner R, Tyree CM, Hu J, Elgort M, Rosen J, Miner JN., 2003. A novel antiinflammatory maintains glucocorticoid efficacy with reduced side effects. Mol Endocrinol. 17(5):860–9. [DOI] [PubMed] [Google Scholar]
- Danzer SC, He XP, Loepke AW and McNamara JO, 2010. Structural plasticity of dentate granule cell presynaptic terminals during the development of limbic epilepsy. Hippocampus 20:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, 2019. Adult Neurogenesis in the Development of Epilepsy. Epilepsy Curr 19(5):316–320. doi: 10.1177/1535759719868186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G, 2003. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 24(4):488–522. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP, 2003. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur. J. Neurosci 18(8):2357–64. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF, 2015. The permissive role of glucocorticoids in neuroinflammatory priming: mechanisms and insights. Curr. Opin. Endocrinol. Diabetes. Obes 22, 300–5. doi: 10.1097/MED.0000000000000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzillo CL, Mello LEAM, 2002. Characterization of Reactive Astrocytes in the Chronic Phase of the Pilocarpine Model of Epilepsy. Epilepsia 43, 107–109. doi: 10.1046/j.1528-1157.43.s.5.40.x [DOI] [PubMed] [Google Scholar]
- Gross C, Yao X, Engel T, Tiwari D, Xing L, Rowley S, Danielson SW, Thomas KT, Jimenez-Mateos EM, Schroeder LM, Pun RYK, Danzer SC, Henshall DC, Bassell GJ., 2016. MicroRNA-Mediated Downregulation of the Potassium Channel Kv4.2 Contributes to Seizure Onset. Cell Rep. 17(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Baram TZ, 2017.Stress and Seizures: Space, Time and Hippocampal Circuits. Trends Neurosci. 40(11):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC, 2013. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J. Neurosci 33, 8926–36. doi: 10.1523/JNEUROSCI.5161-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC, 2014. Hippocampal granule cell pathology in epilepsy - a possible structural basis for comorbidities of epilepsy? Epilepsy Behav. 38:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Hosford BE, Santos VR, Singh SP, Rolle IJ, LaSarge CL, Liska JP, Garcia-Cairasco N, Danzer SC, 2016. Impact of rapamycin on status epilepticus induced hippocampal pathology and weight gain. Exp. Neurol. 280, 1–12. doi: 10.1016/j.expneurol.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacer RD, Deng M, Ward CG, Joseph B, Hughes EA, Jiang C, Danzer SC, Loepke AW., 2013. Cell age-specific vulnerability of neurons to anesthetic toxicity. Ann Neurol. 73(6):695–704. doi: 10.1002/ana.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford B, Liska J, Danzer S, 2016. Ablation of Newly-generated Hippocampal Granule Cells has Disease-Modifying Effects in Epilepsy. J. Neurosci 36(43):11013–11023. doi: 10.1523/JNEUROSCI.1371-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford BE, Rowley S, Liska JP, Danzer SC, 2017. Ablation of peri-insult generated granule cells after epilepsy onset halts disease progression. Sci. Rep 7, 18015. doi: 10.1038/s41598-017-18237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K, Hopkins N, Mcloughlin P, 2002. Combined confocal microscopy and stereology: a highly efficient and unbiased approach to quantitative structural measurement in tissues. Exp. Physiol 87(6):747–56. doi: 10.1113/eph8702477. [DOI] [PubMed] [Google Scholar]
- Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J, 2017. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron. 95(6):1246–1265. doi: 10.1016/j.neuron.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Nadler JV, 2007. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp. Neurol 205, 569–82. doi: 10.1016/j.expneurol.2007.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, 2009. Stress, the hippocampus, and epilepsy. Epilepsia 50, 586–97. doi: 10.1111/j.1528-1167.2008.01902.x [DOI] [PubMed] [Google Scholar]
- Joëls M, de Kloet ER, 1991. Effect of corticosteroid hormones on electrical activity in rat hippocampus. J. Steroid Biochem. Mol. Biol. 40, 83–86. doi: 10.1016/0960-0760(91)90170-A [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Koyama R, 2021. Pro- and anti-epileptic roles of microglia. Neural Regen Res. 16(7):1369–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron MM, Zhang H, Parent JM, 2010. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J Neurosci. 30(6):2051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Couper A, O’Brien TJ, Salzberg MR, Jones NC, Rees SM, Morris MJ, 2007. The acceleration of amygdala kindling epileptogenesis by chronic low-dose corticosterone involves both mineralocorticoid and glucocorticoid receptors. Psychoneuroendocrinology 32, 834–42. doi: 10.1016/j.psyneuen.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Kumar G, Jones NC, Morris MJ, Rees S, Brien TJO, Michael R, 2011. Early Life Stress Enhancement of Limbic Epileptogenesis in Adult Rats: Mechanistic Insights 6(9):e24033. doi: 10.1371/journal.pone.0024033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger M, Trinka E, Giovannini G, Zimmermann G, Florea C, Rohracher A, Kalss G, Neuray C, Kreidenhuber R, Höfler J, Kuchukhidze G, Granbichler C, Dobesberger J, Novak HF, Pilz G, Meletti S, Siebert U, 2019. Epidemiology of status epilepticus in adults: A population-based study on incidence, causes, and outcomes. Epilepsia 60, 53–62. doi: 10.1111/epi.14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Jiang L, Wen M, Ke Y, Tong X, Huang W, Chen R, 2020. Microglia depletion exacerbates acute seizures and hippocampal neuronal degeneration in mouse models of epilepsy. Am J Physiol Cell Physiol. 319(3):C605–C610. doi: 10.1152/ajpcell.00205.2020. [DOI] [PubMed] [Google Scholar]
- Luo C, Koyama R, Ikegaya Y, 2016. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia 64(9):1508–17. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Murao N, Katano Y, Juliandi B, Kohyama J, Akira S, Kawai T, Nakashima K, 2015. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat Commun. 6:6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Shin D, Kwon YS, Bragin A, Pineda E, Tio D, Taylor AN, Sankar R, 2009. Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol. Dis 34, 457–61. doi: 10.1016/j.nbd.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuferi M, Kumar G, Rospo C, Kaminski RM, 2012. Rapid epileptogenesis in the mouse pilocarpine model: video-EEG, pharmacokinetic and histopathological characterization. Exp Neurol. 238(2):156–67. [DOI] [PubMed] [Google Scholar]
- McCloskey DP, Hintz TM, Pierce JP, Scharfman HE, 2006.Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 24(8):2203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 2006. Corticosteroids and Hippocampal Plasticity. Ann. N. Y. Acad. Sci 746, 134–142. doi: 10.1111/j.1749-6632.1994.tb39223.x [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD, 2015. Stress Effects on Neuronal Structure: Hippocampus, Amygdala and Prefrontal Cortex. Neuropsychopharmacology 41(1):3–23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello LE, Kohman CM, Tan AM, Cavalheiro EA, Finch DM, 1996. Lack of Fos-like immunoreactivity after spontaneous seizures or reinduction of status epilepticus by pilocarpine in rats. Neurosci Lett. 208(2):133–7. [DOI] [PubMed] [Google Scholar]
- Meyer M, Gonzalez Deniselle MC, Hunt H, de Kloet ER, De Nicola AF, 2014. The selective glucocorticoid receptor modulator CORT108297 restores faulty hippocampal parameters in Wobbler and corticosterone-treated mice. J. Steroid Biochem. Mol. Biol. 143, 40–8. doi: 10.1016/j.jsbmb.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM, 2010. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J. Neurosci 30, 13690–8. doi: 10.1523/JNEUROSCI.0303-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Hofacer RD, Faulkner CN, Loepke AW, Danzer SC, 2012. Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced postinjury neurogenesis. Epilepsia 53, 908–21. doi: 10.1111/j.1528-1167.2012.03463.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole KK, Hooper A, Wakefield S, Maguire J, 2014. Seizure-induced disinhibition of the HPA axis increases seizure susceptibility. Epilepsy Res. 108, 29–43. doi: 10.1016/j.eplepsyres.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH, 1997. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 17, 3727–38. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The rat brain in stereotaxic coordinates. Academic Press, New York. [DOI] [PubMed] [Google Scholar]
- Peng Z, Houser CR (2005) Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci. 25(31):7210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau F, Canet G, Desrumaux C, Hunt H, Chevallier N, Ollivier M, Belanoff JK, Givalois L, 2016. New selective glucocorticoid receptor modulators reverse amyloid-β peptide–induced hippocampus toxicity. Neurobiol. Aging 45, 109–122. doi: 10.1016/j.neurobiolaging.2016.05.018 [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Keith DL, 1995. Corticosteroids enhance convulsion susceptibility via central mineralocorticoid receptors. Psychoneuroendocrinology 20, 891–902. doi: 10.1016/0306-4530(95)00016-X [DOI] [PubMed] [Google Scholar]
- Santos VR, de Castro OW, Pun RY, Hester MS, Murphy BL, Loepke AW, Garcia-Cairasco N, Danzer SC, 2011. Contributions of mature granule cells to structural plasticity in temporal lobe epilepsy. Neuroscience 197:348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, 2016. The enigmatic mossy cell of the dentate gyrus. Nat. Rev. Neurosci 17(9):562–75. doi: 10.1038/nrn.2016.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Myers CE, 2012. Hilar mossy cells of the dentate gyrus: a historical perspective. Front. Neural Circuits 6, 106. doi: 10.3389/fncir.2012.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AE, Berger RE, Goodman JH, Pierce JP, 2003. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience 121, 1017–1029. doi: 10.1016/S0306-4522(03)00481-0 [DOI] [PubMed] [Google Scholar]
- Myers CE, Bermudez-Hernandez K, Scharfman HE, 2013. The influence of ectopic migration of granule cells into the hilus on dentate gyrus-CA3 function. PLoS One 8(6):e68208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweingruber N, Reichardt SD, Lühder F, Reichardt HM, 2012. Mechanisms of Glucocorticoids in the Control of Neuroinflammation. J. Neuroendocrinol 24, 174–182. doi: 10.1111/j.1365-2826.2011.02161.x [DOI] [PubMed] [Google Scholar]
- Seinfeld S, Goodkin HP, Shinnar S, 2016. Status epilepticus. Cold Spring Harb. Perspect. Med. 6. doi: 10.1101/cshperspect.a022830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K, 2008. Steroid hormone receptor expression and function in microglia. Glia. 56(6):659–74. [DOI] [PubMed] [Google Scholar]
- Simler S, Vergnes M, Marescaux C (1999) Spatial and temporal relationships between C-Fos expression and kindling of audiogenic seizures in Wistar rats. Exp Neurol. 157(1):106–19. [DOI] [PubMed] [Google Scholar]
- Singh SP, LaSarge CL, An A, McAuliffe JJ, Danzer SC, 2015. Clonal Analysis of Newborn Hippocampal Dentate Granule Cell Proliferation and Development in Temporal Lobe Epilepsy. eNeuro 2(6):ENEURO.0087–15.2015. doi: 10.1523/ENEURO.0087-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BL, Schmeltzer SN, Packard BA, Sah R, Herman JP, 2016. Divergent effects of repeated restraint versus chronic variable stress on prefrontal cortical immune status after LPS injection. Brain. Behav. Immun 57, 263–270. doi: 10.1016/j.bbi.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J, Flak JN, Ulrich-Lai Y, Herman JP, 2014. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Horm. Behav 65, 363–71. doi: 10.1016/j.yhbeh.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz IM, Bardin CW, 1993. Clinical pharmacology of RU486 - an antiprogestin and antiglucocorticoid. Contraception 48, 403–444. doi: 10.1016/0010-7824(93)90133-R [DOI] [PubMed] [Google Scholar]
- Trinka E, Höfler J, Leitinger M, Brigo F, 2015. Pharmacotherapy for Status Epilepticus. Drugs. 75(13):1499–521. doi: 10.1007/s40265-015-0454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP, 2006. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am. J. Physiol. - Endocrinol. Metab 291(5):E965–73. doi: 10.1152/ajpendo.00070.2006 [DOI] [PubMed] [Google Scholar]
- van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC., 2008. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology 149(2):725–32. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Dingledine R, Rossetti AO, 2015. Immunity and inflammation in status epilepticus and its sequelae: Possibilities for therapeutic application. Expert Rev. Neurother. 15(9):1081–92. doi: 10.1586/14737175.2015.1079130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Lang B, Aronica E, 2016. Immunity and Inflammation in Epilepsy. Cold Spring Harb. Perspect. Med. 6, a022699. doi: 10.1101/cshperspect.a022699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossler DG, Bainbridge JL, Boggs JG, Novotny EJ, Loddenkemper T, Faught E, Amengual-Gual M, Fischer SN, Gloss DS, Olson DM, Towne AR, Naritoku D, Welty TE, 2020. Treatment of Refractory Convulsive Status Epilepticus: A Comprehensive Review by the American Epilepsy Society Treatments Committee. Epilepsy Curr. 20(5):245–264. doi: 10.1177/1535759720928269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas S, Rodrigues AJ, Silva JM, Tronche F, Almeida OFX, Sousa N, Sotiropoulos I, 2016. Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural Plast 2016, 6391686. doi: 10.1155/2016/6391686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter C, Murphy BL, Pun RYK, Spieles-Engemann AL, Danzer SC, 2007. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J. Neurosci. 27, 7541–52. doi: 10.1523/JNEUROSCI.0431-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston SM, Hayward MD, Nestler EJ, Duman RS (1990) Chronic electroconvulsive seizures down-regulate expression of the immediate-early genes c-fos and c-jun in rat cerebral cortex. J Neurochem. 54(6):1920–5. [DOI] [PubMed] [Google Scholar]
- Wu W, Li Y, Wei Y, Bosco DB, Xie M, Zhao MG, Richardson JR, Wu LJ, 2020. Microglial depletion aggravates the severity of acute and chronic seizures in mice. Brain Behav Immun. 89:245–255. doi: 10.1016/j.bbi.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Franco-Villanueva A, Romancheck C, Morano RL, Smith BL, Packard BA, Danzer SC, Herman JP, 2018. Functional disruption of stress modulatory circuits in a model of temporal lobe epilepsy. PLoS One 13(5):e0197955. doi: 10.1371/journal.pone.0197955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Herman JP, Danzer SC, 2016a. RU486 mitigates hippocampal pathology following status epilepticus. Front. Neurol 7:214. doi: 10.3389/fneur.2016.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Herman JP, Solomon MB, 2010. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology 35(7):1100–12. doi: 10.1016/j.psyneuen.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Solomon MB, Privitera MD, Danzer SC, Herman JP, 2016b. Hypothalamic-pituitary-adrenocortical axis dysfunction in epilepsy. Physiol. Behav 166:22–31. doi: 10.1016/j.physbeh.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt-Johnson SK, Brewster AL, 2020. Emerging Roles for Microglial Phagocytic Signaling in Epilepsy. Epilepsy Curr. 20(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Liu Z-RR, Chen J, Zhang S-JJ, Quan Q-YY, Huang Y-GG, Jiang W, 2010. Roles of astrocytes and microglia in seizure-induced aberrant neurogenesis in the hippocampus of adult rats. J. Neurosci. Res 88, 519–529. doi: 10.1002/jnr.22224 [DOI] [PubMed] [Google Scholar]
- Zalachoras I, Houtman R, Atucha E, Devos R, Tijssen AMI, Hu P, Lockey PM, Datson NA, Belanoff JK, Lucassen PJ, Joëls M, de Kloet ER, Roozendaal B, Hunt H, Meijer OC, 2013. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc. Natl. Acad. Sci. U. S. A 110, 7910–5. doi: 10.1073/pnas.1219411110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Thamattoor AK, LeRoy C, Buckmaster PS, 2015. Surviving mossy cells enlarge and receive more excitatory synaptic input in a mouse model of temporal lobe epilepsy. Hippocampus 25(5):594–604. doi: 10.1002/hipo.22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.