Abstract
Substance use disorders (SUDs) are common among people with HIV and can prevent achievement of optimal health outcomes. Using data from a longitudinal HIV cohort study in the District of Columbia (2011–2018), we calculated the prevalence and correlates of SUD (alcohol, stimulant, and/or opioid use disorders) and determined the association of SUD with engagement in HIV care, ART prescription, viral suppression, and mortality. Of 8420 adults, 3168 (37.6%) had a history of any SUD, most commonly history of alcohol use disorder (29.6%). SUDs disproportionately affected Black individuals (aOR 1.33) and heterosexuals (aOR 1.18), and women had a lower risk of SUD (aOR 0.65). SUD was not associated with engagement in care, ART prescription, or viral suppression. SUD was associated with mortality (aHR 1.31). Addressing alcohol use disorder and preventable causes of death among people with HIV and substance use disorders should be priorities for clinical care and public health.
Keywords: HIV, Substance use disorders, Engagement in care, Mortality
Resumen
Los trastornos por uso de sustancias (TUS) son comunes entre las personas con VIH y pueden impedir el logro de resultados óptimos de salud. Utilizando datos de un estudio sobre VIH de cohorte longitudinal en el Distrito de Columbia (2011–2018), calculamos la prevalencia y los correlatos de TUS (trastornos por consumo de alcohol, estimulantes y/o opioides) y determinamos la asociación de los TUS con la vinculación a cuidado de VIH, prescripción de terapia antirretroviral, supresión viral y mortalidad. De 8420 adultos, 3168 (37.6%) tenían historial de algún TUS, más comúnmente historial de trastorno por consumo de alcohol (29.6%). Los TUS afectaron de manera desproporcionada a las personas negras (aOR 1.33) y a los heterosexuales (aOR 1.18) y las mujeres tenían un riesgo menor de TUS (aOR 0.65). TUS no tuvo asociación estadísticamente significativa con la vinculación a cuidado de VIH, la prescripción de terapia antirretroviral o la supresión viral. TUS se asoció con mortalidad (aHR 1.31). Abordar el trastorno por consumo de alcohol y las causas prevenibles de muerte entre personas con VIH y trastornos por consumo de sustancias debe ser una prioridad para el cuidado clínico y la salud pública.
Introduction
Substance use disorders (SUDs), including alcohol, opioid, and stimulant use disorders, are common among people with HIV in the United States. Understanding the extent of SUDs among PWH is important for public health planning to ensure the availability of appropriate SUD treatment and services to support HIV care. Among people with HIV in either clinic or research cohort samples, previously published literature has reported alcohol use disorders ranging from 9 to 42% [1–7] and other substance use disorders ranging from 13 to 66% [1–3, 5, 6, 8], and early studies from Baltimore reported 30–66% of lifetime history of either alcohol and/or substance use disorder [9, 10]. Analysis from the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS), a longitudinal cohort of approximately 10,000 people with HIV in care at 8 academic clinical care sites, demonstrated the following specific SUD prevalence: 48% overall SUD, 19% alcohol use disorder, 13% methamphetamine, 11% cocaine and 4% opioid use disorder [6]. The District of Columbia (DC) has high a HIV prevalence (1.8% in 2018) [11]. Of the 12,322 people with HIV (PWH) estimated to be living in DC in 2018, 9.7% had a mode of transmission attributable to injection drug use (IDU) and 3.2% were men who have sex with men (MSM) who also had a history of IDU [11]. While these data are useful for understanding modes of HIV transmission, the extent to which PWH who receive care in DC may have alcohol, opioid, and/or stimulant use disorders has not been well-described.
Retention in care, ART initiation and viral suppression are key health outcomes for PWH. Retention in care has been associated with a reduced risk of mortality [12, 13], and viral suppression is associated with a lower risk of adverse individual health events [14] and a reduction in HIV transmission to sexual partners [15]. SUDs are frequently lifelong relapsing conditions [16], and PWH who have comorbid SUDs have demonstrated lower retention in care, ART adherence, and viral suppression [17–26].
Despite these suboptimal HIV outcomes, there are effective treatment options (opioid substitution therapy) for opioid use disorders [27, 28] and, to a lesser extent, alcohol use disorders [29]. In addition, opioid substitution therapy can help improve HIV care outcomes [28, 30]. The objectives of this analysis were to quantify and describe the pattern of SUDs among PWH participants in a longitudinal HIV clinical cohort study, and to evaluate the association of SUDs with engagement in care, ART prescription, viral suppression, and mortality, with the goal of informing future public health planning and service delivery.
Methods
Source of Data and Human Subjects Protections
The data source for this analysis was the DC Cohort, a prospective, longitudinal, clinical cohort study of PWH receiving HIV in DC. The methodology of the DC Cohort has been previously described and includes linkage between data from the clinical medical record and HIV surveillance data from DC Department of Health (including death information) [31, 32]. Participants in the DC Cohort consent to have their demographic and clinical data electronically and manually abstracted from medical records at the participating sites and entered into a centralized database. The Institutional Review Boards of George Washington University, DC Department of Health, and individual participating sites with their own IRBs have approved the DC Cohort study.
In this analysis, we included DC Cohort participants ages 18 years or older, with enrollment dates between January 1, 2011 and December 31, 2017. Follow-up data for participants through January 1, 2018 were included. At the time of analysis, there were 15 participating outpatient sites in the DC Cohort: 9 hospital-based clinics, 5 community-based clinics, and one non-public managed care organization; participants from 14 of 15 sites met inclusion criteria and had data available at the time the analysis was performed.
Outcomes
The main outcome of interest was prevalence of any past or current SUD. Having any past or current history of SUD was based upon EMR review at enrollment and/or ICD-9/10 codes throughout follow-up period that confirmed a SUD. We included ICD-9/10 codes for alcohol, stimulant, and/or opioid abuse or dependence (Supplemental Table I). We did not assess prevalence of marijuana use due to the Legalization of Minimal Amounts of Marijuana for Personal Use Initiative (D.C. Act 20–565, effective in 2015) and the potential changes on reporting of marijuana use in the EMR throughout the analysis time period.
Engagement in HIV care and ART prescription were assessed during the most recent 12 months of active DC Cohort enrollment through January 1, 2018; this was termed the “analysis year.” If a participant did not have active DC Cohort enrollment through January 1, 2018, then the last date of active enrollment was either the most recent lab or visit date. Engagement in HIV care was defined as having at least 1 HIV medical visit, CD4, or viral load (VL) test in the analysis year. All DC Cohort participating sites use electronic ordering, which means that prescriptions are entered into the EMR. ART prescription was defined as EMR evidence of combination ART having been prescribed within the analysis year. The viral load analysis year was the most recent 12 months with active DC Cohort enrollment during which a viral load test was available. Viral suppression was defined as VL < 200 copies/ml at the most recent measurement within the viral load analysis year.
All-cause mortality was based on documentation in the DC Cohort database, which gathers death data from participating clinical sites and was confirmed through linkage with the DC Department of Health vital statistics registry [32].
Covariates
We assessed the distribution of single and various combinations of multiple substance use disorders by the following variables: age at enrollment, follow-up time, gender, race/ethnicity, HIV transmission risk factor, sexual risk factor for HIV transmission, CD4 cell count at enrollment (cells/μl), and insurance status. Gender was categorized into men, women, and transgender. The transgender category included transgender men (female sex at birth with current gender man) and transgender women (male sex at birth with current gender woman); the composite group was used due to small sample size if analyzed separately. Race/ethnicity were mutually exclusive and was categorized into Non-Hispanic Black, Non-Hispanic White, Hispanic or Latino, Other (includes American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, Multiracial), or unknown. HIV transmission risk factor was categorized into men who have sex with men (MSM), PWID (including MSM/PWID), heterosexual, blood products or coagulation disorder, perinatal, other, or unknown. Sexual risk factor for HIV transmission was categorized into MSM, heterosexual, or a composite group consisting of other, none, or unknown). For the variable “sexual risk factor for HIV transmission,” MSM/PWID participants were classified as MSM. Insurance status was the most recent insurance status reported (thus reflective of insurance status after entering HIV care) and was categorized into public (includes government programs such as Medicaid, Medicare, and Ryan White), private, and a composite group consisting of other, none, or unknown.
People who had IDU listed as a mode of HIV transmission, including people who inject drugs (PWID) (n = 218) and MSM/PWID (n = 37), but did not have an ICD-9/10 code documented in the electronic medical record, were categorized into the SUD group. We believe there could be underreporting of SUDs in the EMRs supplying data to the DC Cohort for the following reasons. One, the DC Cohort collects data from clinics providing HIV care, and while IDU may be noted as part of someone’s medical history, the primary reason for the visit likely non-drug use related medical treatment (i.e., HIV care), so an ICD-9/10 code for SUD would be less likely listed as the diagnoses addressed during the visit. Two, if injection drug use had stopped prior to enrollment in the DC Cohort, an ICD-9/10 code may not be included in the patient’s current, active problem list. Individuals characterized as PWID and MSM/PWID were further categorized into the opioid use disorder (OUD) group based on local drug use patterns. Data collected through the National HIV Behavioral Surveillance study conducted in the District of Columbia that demonstrated among the 2015 cycle that focused on injection drug users, 96% injected heroin and 8% injected OxyContin [33].
In the mortality model, we included the percentage of time having a VL ≥ 200 copies/ml (unsuppressed VL) because higher cumulative viremia has been associated with higher risk of mortality [34, 35]. Percentage of time having an unsuppressed VL ≥ 200 copies/ml was calculated using all available consecutive VL pairs during active follow-up. We estimated the total number of days with VL ≥ 200 copies/ml, divided by the total number of days of observation in the denominator [36, 37]. This was distinct from the use of viral suppression as an outcome.
Statistical Methods
We determined the prevalence of any SUD, any alcohol use disorder, any opioid use disorder, and separately, disorders for alcohol use, opioid use, stimulant use, and polysubstance (all 3) use at enrollment or during follow-up, among the SUD groups using frequencies (%) and medians (interquartile range), using Kruskal–Wallis for continuous variables and Pearson’s chi-square for categorical variables. In the multivariable models, we used any SUD (vs. none) as the variable of interest, rather than individual SUD categories, due to small sample sizes of some of the SUD categories. We used logistic regression to calculate adjusted odds ratios (aOR) to assess the associations of age at enrollment, race/ethnicity, gender, sexual risk factor for HIV, and insurance status with the outcome of any SUD. We also used logistic regression (in separate models) to assess the associations of any SUD (as an exposure variable) with the outcome variables engagement in HIV care, ART prescription, and viral suppression, controlling for age at enrollment, race/ethnicity, gender, sexual risk factor for HIV transmission, and insurance status. Thus, SUD was used as an outcome and an exposure variable.
We used multivariable Cox proportional hazard models for time-to-death analysis to determine hazard from participant enrollment until date of death or censoring at end of follow-up. The variables included in the Cox model included age at enrollment, race/ethnicity, gender, sexual risk factor for HIV, insurance status, CD4 cell count at enrollment, percent of time with VL above 200 copies/ml, and any SUD. Time to endpoints were measured from the start of participant enrollment. The proportional hazards assumption was confirmed by inspecting Kaplan Meier plots for SUD and each covariate.
In the models for engagement in HIV care, ART prescription, viral suppression, and mortality, SUD was the main covariate of interest, controlling for other covariates. We used sexual risk factor for HIV transmission (instead of HIV transmission risk factor) because all PWID were classified into one of the SUD groups. We used SAS software, version 9.4 (copyright SAS Institute, Inc., Cary, NC, 2002–2012) to conduct all statistical data analyses.
Results
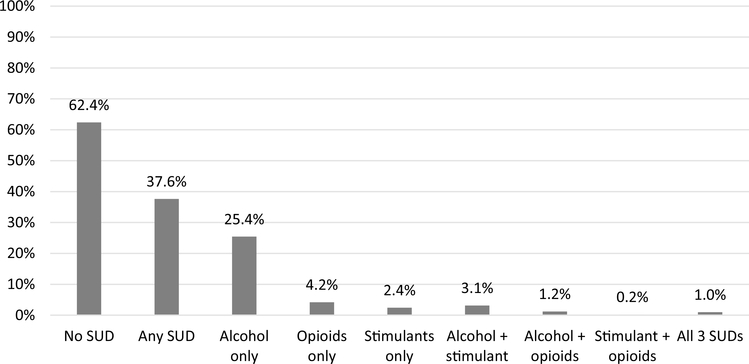
Of 8420 adult DC Cohort participants (have received HIV care in DC), 3168 (37.6%) had a history of any SUD, 2590 (30.7%) with any alcohol use disorder and 538 (6.3%) with any opioid use disorder. Among those with any SUD (n = 3168), the most prevalent SUD category was alcohol only (n = 2141, 67.6%). Black individuals and men accounted for the majority of individuals with all SUD categories (which was also true for the overall study population), and over half of those with and without SUD had CD4 > 500 cells/μl at enrollment. The full distribution of SUD categories and covariates are shown in Tables 1 and 2 and visually represented in Fig. 1.
Table 1.
Demographic characteristics and CD4 cell count at enrollment of DC Cohort participants with and without lifetime history of substance use disorders, 2011–2017 (n = 8420)
| Overall | No SUD | Any SUD | P-values | |
|---|---|---|---|---|
| n (col. %) | n (col. %) | n (col. %) | ||
| Total number of persons | 8420 | 5252 | 3168 | |
| Follow-up time, years (median, IQR) | 3.7 (2.1, 5.6) | 3.6 (2.0, 5.5) | 3.8 (2.2, 5.7) | < .0001a |
| Age at enrollment, years (median, IQR) | 47.3 (37.0, 54.8) | 45.3 (35.2, 53.1) | 50.4 (41.1, 56.9) | < .0001a |
| Race/ethnicity | < .0001b | |||
| Non-Hispanic Black | 6503 (77.2) | 3848 (73.3) | 2655 (83.8) | |
| Non-Hispanic White | 1080 (12.8) | 781 (14.9) | 299 (9.4) | |
| Hispanic or Latino | 463 (5.5) | 311 (5.9) | 152 (4.8) | |
| Other or unknown | 374 (4.4) | 312 (5.9) | 62 (2.0) | |
| Gender | 0.0014b | |||
| Men | 5981 (71.0) | 3660 (69.7) | 2321 (73.3) | |
| Women | 2282 (27.1) | 1484 (28.3) | 798 (25.2) | |
| Transgender | 157 (1.9) | 108 (2.1) | 49 (1.6) | |
| Mode of HIV transmission | < .0001b | |||
| MSM | 3220 (38.2) | 2316 (44.1) | 904 (28.5) | |
| PWID/MSM | 649 (7.7) | 0 (0.0) | 649 (20.5) | |
| Heterosexual | 2771 (32.9) | 1751 (33.3) | 1020 (32.2) | |
| Blood products or coagulation disorder | 84 (1.0) | 61 (1.2) | 23 (0.7) | |
| Perinatal | 115 (1.4) | 102 (1.9) | 13 (0.4) | |
| Other | 77 (0.9) | 59 (1.1) | 18 (0.6) | |
| Unknown | 1504 (17.9) | 963 (18.3) | 541 (17.1) | |
| Sexual risk factor for HIV transmission | < .0001b | |||
| MSM | 3298 (39.2) | 2316 (44.1) | 982 (31.0) | |
| Heterosexual | 2771 (32.9) | 1751 (33.3) | 1020 (32.2) | |
| Other, none, or unknown | 2351 (27.9) | 1185 (22.6) | 1166 (36.8) | |
| Insurance status | < .0001b | |||
| Private | 2154 (25.6) | 1764 (33.6) | 390 (12.3) | |
| Public | 5815 (69.1) | 3191 (60.8) | 2624 (82.8) | |
| Other, none, or unknown | 451 (5.4) | 297 (5.7) | 154 (4.9) | |
| CD4 at enrollment (cells/μl) | 0.2579b | |||
| < 200 | 838 (10.6) | 506 (10.3) | 332 (11.0) | |
| 200–499 | 2761 (34.8) | 1689 (34.3) | 1072 (35.5) | |
| ≥ 500 | 4347 (54.7) | 2727 (55.4) | 1620 (53.6) | |
The Kruskal–Wallis test was used to determine statistical significance for continuous variables
Pearson’s Chi-square test was used to determine statistical significance for categorical variables
Table 2.
Demographic characteristics and CD4 cell count at enrollment of DC Cohort participants with lifetime history of single and combination substance use disorders, 2011–2017 (n = 8420)
| Overall | Alcohol only | Opioids only | Stimulants only | Alcohol + stimulants | Alcohol + opioids | Stimulants + opioids | All 3 SUDs | |
|---|---|---|---|---|---|---|---|---|
| n (col. %) | n (col. %) | n (col. %) | n (col. %) | n (col. %) | n (col. %) | n (col. %) | n (col. %) | |
| Total number of persons | 8420 | 2141 | 354 | 205 | 265 | 100 | 19 | 84 |
| Follow-up time, years (median, IQR) | 3.7 (2.1, 5.6) | 3.5 (2.1, 5.4) | 3.9 (2.1, 5.9) | 4.2 (2.4, 5.9) | 4.5 (2.9, 6.1) | 4.6 (2.9, 6.4) | 4.2 (3.1, 6.5) | 5.6 (3.8, 6.5) |
| Age at enrollment, years (median, IQR) | 47.3 (37.0, 54.8) | 49.3 (38.7, 56.3) | 55.7 (50.0, 59.4) | 47.7 (38.9, 52.5) | 49.1 (42.5, 53.4) | 56.3 (51.1, 60.8) | 50.8 (45.4, 55.2) | 55.8 (50.8, 59.1) |
| Race/ethnicity | ||||||||
| Non-Hispanic Black | 6503 (77.2) | 1767 (82.5) | 319 (90.1) | 163 (79.5) | 218 (82.3) | 92 (92.0) | 16 (84.2) | 80 (95.2) |
| Non-Hispanic White | 1080 (12.8) | 199 (9.3) | 23 (6.5) | 34 (16.6) | 30 (11.3) | 8 (8.0) | 2 (10.5) | 3 (3.6) |
| Hispanic or Latino | 463 (5.5) | 129 (6.0) | 4 (1.1) | 5 (2.4) | 13 (4.9) | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Other or unknown | 374 (4.4) | 46 (2.2) | 8 (2.3) | 3 (1.5) | 4 (1.5) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Gender | ||||||||
| Men | 5981 (71.0) | 1600 (74.7) | 234 (66.1) | 140 (68.3) | 190 (71.7) | 72 (72.0) | 10 (52.6) | 75 (89.3) |
| Women | 2282 (27.1) | 510 (23.8) | 117 (33.1) | 56 (27.3) | 70 (26.4) | 27 (27.0) | 9 (47.4) | 9 (10.7) |
| Transgender | 157 (1.9) | 31 (1.5) | 3 (0.9) | 9 (4.4) | 5 (1.9) | 1 (1.0) | 0 (0.0) | 0 (0.0 |
| Mode of HIV transmission | ||||||||
| MSM | 3220 (38.2) | 705 (32.9) | 11 (3.1) | 88 (42.9) | 86 (32.5) | 6 (6.0) | 2 (10.5) | 6 (7.1) |
| PWID/MSM | 649 (7.7) | 209 (9.8) | 290 (81.9) | 12 (5.9) | 24 (9.1) | 58 (58.0) | 7 (36.8) | 49 (58.3) |
| Heterosexual | 2771 (32.9) | 774 (36.2) | 31 (8.8) | 69 (33.7) | 98 (37.0) | 19 (19.0) | 5 (26.3) | 24 (28.6) |
| Blood products or coagulation disorder | 84 (1.0) | 19 (0.9) | (0.0) | 1 (0.5) | 1 (0.4) | 2 (2.0) | (0.0) | (0.0) |
| Perinatal | 115 (1.4) | 10 (0.5) | 2 (0.6) | (0.0) | 1 (0.4) | (0.0) | (0.0) | (0.0) |
| Other | 77 (0.9) | 15 (0.7) | (0.0) | 1 (0.5) | 2 (0.8) | (0.0) | (0.0) | (0.0) |
| Unknown | 1504 (17.9) | 409 (19.1) | 20 (5.7) | 34 (16.6) | 53 (20.0) | 15 (15.0) | 5 (26.3) | 5 (6.0) |
| Sexual risk factor for HIV transmission | ||||||||
| MSM | 3298 (39.2) | 729 (34.1) | 50 (14.1) | 95 (46.3) | 91 (34.3) | 6 (6.0) | 3 (15.8) | 8 (9.5) |
| Heterosexual | 2771 (32.9) | 774 (36.2) | 31 (8.8) | 69 (33.7) | 98 (37.0) | 19 (19.0) | 5 (26.3) | 24 (28.6) |
| Other, none, or unknown | 2351 (27.9) | 638 (29.8) | 273 (77.1) | 41 (20.0) | 76 (28.7) | 75 (75.0) | 11 (57.9) | 52 (61.9) |
| Insurance status | ||||||||
| Private | 2154 (25.6) | 306 (14.3) | 46 (13.0) | 20 (9.8) | 15 (5.7) | 1 (1.0) | 0 (0.0) | 2 (2.4) |
| Public | 5815 (69.1) | 1717 (80.2) | 294 (83.1) | 175 (85.4) | 241 (90.9) | 99 (99.0) | 17 (89.5) | 81 (96.4) |
| Other, none, or unknown | 451 (5.4) | 118 (5.5) | 14 (4.0) | 10 (4.9) | 9 (3.4) | 0 (0.0) | 2 (10.5) | 1 (1.2) |
| CD4 at enrollment (cells/μl) | ||||||||
| < 200 | 838 (10.6) | 223 (11.0) | 35 (10.5) | 19 (9.6) | 25 (9.7) | 15 (15.3) | 3 (15.8) | 12 (14.3) |
| 200–499 | 2761 (34.8) | 710 (34.9) | 131 (39.2) | 65 (33.0) | 98 (37.8) | 29 (29.6) | 5 (26.3) | 34 (40.5) |
| ≥ 500 | 4347 (54.7) | 1100 (54.1) | 168 (50.3) | 113 (57.4) | 136 (52.5) | 54 (55.1) | 11 (57.9) | 38 (45.2) |
Fig. 1.
Distribution of single and combination substance use disorders among DC Cohort participants, 2011–2017 (n = 8420)
In the multivariable analysis (Table 3) in which SUD was the outcome of interest, we found that non-Hispanic Black individuals (aOR 1.33, 95% CI 1.14, 1.56, p = 0.0004), as compared to non-Hispanic White individuals, were more likely to have SUD; individuals with Other or unknown race/ethnicity were less likely to have SUD (aOR 0.52, 95% CI 0.38, 0.72, p < 0.0001). Women (versus men) were less likely to have SUD (aOR 0.65, 95% CI 0.57, 0.73, p < 0.0001). As compared to MSM, heterosexuals (aOR 1.18, 95% CI 1.04, 1.36, p = 0.0141) and people with other, none, or unknown sexual risk factor for HIV (aOR 1.84 95% CI 1.62, 2.09, p < 0.0001) were more likely to have SUD. People with public insurance (aOR 3.11, 95% CI 2.74, 3.54, p < 0.0001) or other, none, or unknown insurance (aOR 2.38, 95% CI 1.90, 3.00, p < 0.0001), versus those with private insurance, were more likely to have SUD.
Table 3.
Association of demographic factors and mode of HIV transmission with any history of substance use disorder (n = 3168) among DC Cohort participants (n = 8420), 2011–2017
| Any history of substance use disorder |
||||
|---|---|---|---|---|
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Age at enrollment (10-year increments) | 1.33 (1.28, 1.38) | < .0001 | 1.22 (1.17, 1.27) | < .0001 |
| Race/ethnicity | ||||
| Non-Hispanic White | Ref | Ref | ||
| Non-Hispanic Black | 1.80 (1.56, 2.08) | < .0001 | 1.33 (1.14, 1.56) | 0.0004 |
| Hispanic or Latino | 1.28 (1.01, 1.62) | 0.0420 | 1.26 (0.98, 1.62) | 0.0682 |
| Other or unknown | 0.52 (0.38, 0.70) | < .0001 | 0.52 (0.38, 0.72) | < .0001 |
| Gender | ||||
| Men | Ref | Ref | ||
| Women | 0.85 (0.77, 0.94) | 0.0013 | 0.65 (0.57, 0.73) | < .0001 |
| Transgender | 0.72 (0.51, 1.01) | 0.0547 | 0.70 (0.49, 0.99) | 0.0458 |
| Sexual risk factor for HIV transmission | ||||
| MSM | Ref | Ref | ||
| Heterosexual | 1.37 (1.23, 1.53) | < .0001 | 1.18 (1.04, 1.36) | 0.0141 |
| Other, none, or unknown | 2.32 (2.08, 2.59) | < .0001 | 1.84 (1.62, 2.09) | < .0001 |
| Insurance status | ||||
| Private | Ref | Ref | ||
| Public | 3.72 (3.30, 4.20) | < .0001 | 3.11 (2.74, 3.54) | < .0001 |
| Other, none, or unknown | 2.35 (1.88, 2.93) | < .0001 | 2.38 (1.90, 3.00) | < .0001 |
The proportion of DC Cohort participants engaged in HIV care was 71.5% (6022/8420), and 96.8% were prescribed ART (8148/8420). A smaller number of study participants had viral load results available for analysis; thus, 86.3% (4468/5177) were virally suppressed. In the multivariable analyses (Table 4), SUD was not associated with these three outcomes. Increasing age at enrollment was associated with all three care continuum outcomes. Hispanic or Latino individuals were more likely to be engaged in HIV care (versus non-Hispanic White individuals) (aOR 2.22, 95% CI 1.69, 2.91, p < 0.0001). Compared to those with private insurance, individuals with public insurance were more likely to be engaged in HIV care (aOR 1.57, 95% CI 1.40, 1.77, p < 0.0001), while those with other, none, or unknown insurance were less likely to be engaged in HIV care (aOR 0.68, 95% CI 0.55, 0.84, p = 0.0004). Insurance status was not statistically associated with ART prescription. For viral suppression, non-Hispanic Black individuals (aOR 0.43, 95% CI 0.29, 0.64, p < 0.0001), as well as those of other or unknown race/ethnicity (aOR 0.48, 95% CI 0.27, 0.84, p = 0.0096) were less likely to be virally suppressed (versus non-Hispanic White individuals). Those with public insurance (aOR 0.55, 95% CI 0.43, 0.72, p < 0.0001) or other, none, or unknown insurance (aOR 0.29, 95% CI 0.20, 0.43, p < 0.0001) (versus those with private insurance) were less likely to be virally suppressed.
Table 4.
Associations of demographic factors, sexual risk factor for HIV transmission, and history of any substance use disorder with engagement in HIV care, ART prescription, and viral suppression, among DC Cohort participants, 2011–2017
| Engagement in HIV care |
ART prescription |
Viral suppression |
||||
|---|---|---|---|---|---|---|
| aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Age at enrollment (10-year increments) | 1.16 (1.11, 1.21) | < .0001 | 1.07 (0.97, 1.19) | 0.1982 | 1.46 (1.36, 1.57) | < .0001 |
| Race/ethnicity | ||||||
| Non-Hispanic White | Ref | Ref | Ref | |||
| Non-Hispanic Black | 1.10 (0.95, 1.28) | 0.2048 | 1.01 (0.65, 1.55) | 0.9831 | 0.43 (0.29, 0.64) | < .0001 |
| Hispanic or Latino | 2.22 (1.69, 2.91) | < .0001 | 1.59 (0.74, 3.42) | 0.2373 | 0.75 (0.44, 1.27) | 0.2808 |
| Other or unknown | 1.05 (0.82, 1.36) | 0.6919 | 1.08 (0.51, 2.27) | 0.8408 | 0.48 (0.27, 0.84) | 0.0096 |
| Gender | ||||||
| Men | Ref | Ref | Ref | |||
| Women | 0.95 (0.84, 1.09) | 0.4834 | 0.76 (0.56, 1.04) | 0.0838 | 0.94 (0.77, 1.16) | 0.5874 |
| Transgender | 1.34 (0.91, 1.97) | 0.1336 | 0.48 (0.24, 0.98) | 0.0426 | 1.12 (0.66, 1.91) | 0.6640 |
| Sexual risk factor for HIV transmission | ||||||
| MSM | Ref | Ref | Ref | |||
| Heterosexual | 0.94 (0.82, 1.09) | 0.4181 | 0.87 (0.60, 1.25) | 0.4524 | 0.69 (0.54, 0.88) | 0.0025 |
| Other, none, or unknown | 1.00 (0.88, 1.15) | 0.9794 | 1.05 (0.73, 1.49) | 0.8080 | 0.69 (0.55, 0.86) | 0.0013 |
| Insurance status | ||||||
| Private | Ref | Ref | Ref | |||
| Public | 1.57 (1.40, 1.77) | < .0001 | 0.72 (0.52, 1.01) | 0.0554 | 0.55 (0.43, 0.72) | < .0001 |
| Other, none, or unknown | 0.68 (0.55, 0.84) | 0.0004 | 1.67 (0.75, 3.72) | 0.2088 | 0.29 (0.20, 0.43) | < .0001 |
| History of substance use disorder | ||||||
| No SUD | Ref | Ref | Ref | |||
| Any SUD | 1.07 (0.97, 1.19) | 0.1873 | 0.78 (0.60, 1.00) | 0.0525 | 0.89 (0.75, 1.06) | 0.1995 |
Factors associated with hazard of death are reported in Table 5. Any SUD was associated with a 31% increased hazard of death (aHR 1.31 95% CI 1.05, 1.65, p = 0.0194), when adjusting for age at enrollment, race/ethnicity, gender, sexual risk factor for HIV transmission, insurance status, CD4 at enrollment, and percentage of time with VL above 200 copies/ml. Race/ethnicity and gender were not associated with mortality. Lower CD4 at enrollment was associated with an increased hazard of death (CD4 < 200 cells/μl: aHR 3.42, 95% CI 2.52, 4.64, p < 0.0001; CD4 200–500 cells/μl: aHR 1.76, 95% CI 1.38, 2.25, p < 0.0001). Heterosexuals aHR 1.50, 95% CI 1.08, 2.10, p = 0.0169) and those with other, none, or unknown sexual risk factor for HIV transmission aHR 1.96, 95% CI 1.43, 2.67, p < 0.0001) had a higher likelihood of death (versus MSM), as did people with public insurance (aHR 1.73, 95% CI 1.20, 2.48, p = 0.0032) or other, none, or unknown insurance (aHR 2.14, 95% CI 1.13, 4.05, p = 0.0197) (versus those with private insurance).
Table 5.
Association of demographic characteristics and history of any substance use disorder among DC cohort participants with all-cause mortality (n = 387 deaths) after enrollment in the DC Cohort, 2011–2017
| Hazard of death due to any cause |
||||
|---|---|---|---|---|
| HR (95% CI) | p-value | aHR (95% CI) | p-value | |
| Age at enrollment s | 1.66 (1.52, 1.82) | < .0001 | 1.54 (1.38, 1.71) | < .0001 |
| Race/Ethnicity | ||||
| Non-Hispanic White | Ref | Ref | ||
| Non-Hispanic Black | 1.71 (1.21, 2.43) | 0.0025 | 1.12 (0.75, 1.67) | 0.5874 |
| Hispanic or Latino | 0.87 (0.43, 1.75) | 0.6869 | 1.12 (0.54, 2.30) | 0.7556 |
| Other or unknown | 1.19 (0.60, 2.34) | 0.6170 | 1.07 (0.51, 2.28) | 0.8572 |
| Gender | ||||
| Men | Ref | Ref | ||
| Women | 1.08 (0.86, 1.35) | 0.5175 | 1.01 (0.77, 1.32) | 0.9412 |
| Transgender | 0.54 (0.20, 1.45) | 0.2195 | 0.86 (0.27, 2.72) | 0.7985 |
| Sexual risk factor for HIV transmission | ||||
| MSM | Ref | Ref | ||
| Heterosexual | 1.94 (1.47, 2.56) | < .0001 | 1.50 (1.08, 2.10) | 0.0169 |
| Other, none, or unknown | 3.10 (2.38, 4.03) | < .0001 | 1.96 (1.43, 2.67) | < .0001 |
| Insurance Status | ||||
| Private | Ref | Ref | ||
| Public | 2.71 (1.98, 3.72) | < .0001 | 1.73 (1.20, 2.48) | 0.0032 |
| Other, none, or unknown | 2.47 (1.39, 4.38) | 0.0020 | 2.14 (1.13, 4.05) | 0.0197 |
| CD4 at enrollment (cells/μl) | ||||
| < 200 | 3.89 (2.94, 5.15) | < .0001 | 3.42 (2.52, 4.64) | < .0001 |
| 200–499 | 1.99 (1.57, 2.52) | < .0001 | 1.76 (1.38, 2.25) | < .0001 |
| ≥ 500 | Ref | Ref | ||
| Percent of time with viral load above 200 copies/ml | 0.73 (0.59, 0.90) | 0.0039 | 1.14 (0.91, 1.44) | 0.2551 |
| History of substance use disorder | ||||
| None | Ref | Ref | ||
| Any | 1.86 (1.52, 2.27) | < .0001 | 1.31 (1.05, 1.65) | 0.0194 |
The number of participants used in multivariate analyses was 7690, and there were 387 deaths. Follow-up time for hazard ratio was calculated from the effective date of participant enrollment through last active date available or until inactive date
Discussion
In this analysis using data from the DC Cohort, a longitudinal clinical cohort of people receiving HIV care in Washington, DC, substance use disorders were documented in approximately one-third of the study population. Alcohol use disorder was the most common single SUD found in this DC Cohort analysis. Non-Hispanic Black individuals, men, and heterosexuals had higher likelihood of SUDs compared to non-Hispanic White individuals, women, and MSM. Substance use disorders were associated with higher likelihood of engagement in care, viral suppression, and mortality.
Alcohol use disorder among people receiving HIV care in DC was higher than expected, given that previous studies included urban populations. The prevalence of any alcohol use disorder in this analysis was lower than what has been reported in 1995 by a Midwestern US cohort [38], similar to results from a Southeastern US clinical cohort that reported in 2006 [5] as well as a systematic review from 2019 [7], and higher than what has been reported by several other studies reported between 2001 and 2017 [2–4, 6]. In comparison to two studies that analyzed alcohol use disorder by patient report and as documented in the medical record [2, 5], our findings for alcohol use disorder were higher than both patient report and provider documentation. Further research into reducing alcohol use disorders among PWH in DC should be a priority.
In comparison to findings reported by the Center for AIDS Research Network of Integrated Clinical Systems (CNICS), our analysis similarly found that men, Black individuals, and those with heterosexual risk factor for HIV transmission were more likely to have any SUD; whereas in the CNICS analysis, younger age was correlated with SUD [6]. These differences suggest that examination of populations at risk of alcohol and drug use disorders on a local level is important for developing tailored interventions and services.
In this analysis, we observed that SUD was not associated with engagement in HIV care, ART prescription, or viral suppression. Our findings differed from previously published literature, which has found that alcohol and substance use disorders are associated with worse retention in care, lower likelihood of ART prescription, and lower likelihood of viral suppression [17–21, 23, 24, 39–41]. By definition, the study population has accessed outpatient HIV care and consented to enroll in the DC Cohort. It is plausible that people with HIV and SUD who were not enrolled in the DC Cohort may have had lower levels of engagement in HIV care, but those individuals were not represented in this analysis. The overall high proportion of participants with viral suppression (86.3%) suggests that once in care, DC Cohort participants with and without SUD were largely able to successfully achieve viral suppression.
The association of SUD with death was notable and given that we adjusted for percent of time with viral load above 200 copies/ml, this finding suggests non-HIV-related causes of death. HIV providers who care for people with a history of or current SUD should be alert to the potential risk of substance-related deaths, including overdose, or other comorbidities found among substance users, and should consider provision of preventative measures, such as naloxone prescriptions for those with opioid dependence, and regular screening for SUD relapse with appropriate referral to SUD treatment resources. Future analyses could examine causes of death and comorbidities among people with and without SUD diagnoses in the DC Cohort.
In this analysis, despite the racial distribution of DC residents being more heavily weighted towards Black individuals (46%) than the overall US population (13.4%) [42], in this analysis, non-Hispanic Black individuals had a higher likelihood of SUD and lower likelihood of viral suppression, as compared to non-Hispanic White individuals. These findings point towards persistent racial inequities in SUD and viral suppression in a large sample of people with HIV in care. Unmeasured racial discrimination could certainly be a factor affecting development of SUD and lack of viral suppression [43]; discrimination experiences should be explored in future research.
There were some limitations to this analysis. One, we combined past and current SUD because we were unsure of the extent to which historical SUDs would be coded in the EMR at the time of study enrollment. We believe that history of SUD could be revealed to the provider after initial medical visits. Thus, we felt it would be more inclusive to use any evidence of SUD throughout study participation, not just SUD documented at study enrollment. However, this definition makes the timing of active SUD in relation to the study outcomes difficult to assess. Two, the study sample was PWH enrolled in the DC Cohort study and receiving care with DC HIV providers, which may limit generalizability to other geographic areas. Three, substance use disorders may be under-documented by HIV care providers, particularly if they are not aware of someone’s previous history or they are not screening for SUDs systematically. In two previously published studies, patient-reported alcohol and drug abuse was higher than provider reports, including among veterans with HIV [2] and an academic medical center clinic sample [5]. Four, we assumed that individuals who had IDU listed as the mode of HIV transmission, without a documented SUD diagnosis in the EMR, had at some point experienced opioid use disorder. While it is true that some people can inject drugs without meeting diagnostic criteria for SUD, we believed that since IDU was listed as a mode of HIV transmission, it was a significant behavior in a person’s life and likely to represent an un-documented SUD. Finally, the follow-up period for assessing all-cause mortality was at most 6 years, which may not be enough time to fully see the association of SUDs with mortality.
The major strengths of the study are the large sample size of an urban population with HIV in the United States (nearly 10,000 enrolled, with over 75% of HIV patients seen at DC Cohort sites have been enrolled), standardized data extraction from EMRs, and match with surveillance data, which is a unique feature of the DC Cohort and enhances the completeness of laboratory and mortality data.
The high prevalence of SUD and the significant association of SUD with death in this clinical cohort are strong signals that there continues to be a need to address SUD among people receiving care for HIV. Co-located and integrated models of care for HIV and SUD have been tested to some degree, mostly for opioid dependence (in which opioid substitution therapy is provided), but these should be explored further and should address alcohol and stimulant use disorders as well [44, 45]. Adaptation of health care policies, such as inclusion of medications to treat substance use disorders on the formularies for AIDS Drug Assistance Programs, could also facilitate improved health outcomes for people with HIV and SUD [46]. The commendable national efforts around ending the HIV epidemic should include awareness of and attention towards the substantial effects of SUD among people with HIV.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the DC Cohort Study participants for their contribution to the analysis. Data in this manuscript were collected by the DC Cohort Study Group with investigators and research staff located at: Children’s National Medical Center Adolescent (Lawrence D’Angelo) and Pediatric (Natella Rakhmanina) clinics; the Senior Deputy Director of the DC Department of Health HAHSTA (Michael Kharfen); Family and Medical Counseling Service (Michael Serlin); Georgetown University (Princy Kumar); The George Washington University Biostatistics Center (Tsedenia Bezabeh, Susan Reamer, Alla Sapozhnikova, Marinella Temprosa, Nisha Grover, Greg Strylewicz, Kevin (Jiayang) Xiao); The George Washington University Department of Epidemiology (Morgan Byrne, Alan Greenberg, Maria Jaurretche, Paige Kulie, James Peterson) and Department of Biostatistics and Bioinformatics (Yan Ma); The George Washington University Medical Faculty Associates (Hana Akselrod); Howard University Adult Infectious Disease Clinic (Ronald Wilcox, Jhansi Gajjala) and Pediatric Clinic (Sohail Rana); Kaiser Permanente Mid-Atlantic States (Michael Horberg); La Clinica Del Pueblo (Ricardo Fernandez); MetroHealth (Annick Hebou); National Institutes of Health ( Henry Masur); Washington Health Institute (Jose Bordon); Unity Health Care (Gebeyehu Teferi); Veterans Affairs Medical Center (Debra Benator); Washington Hospital Center (Maria Elena Ruiz); and Whitman-Walker Health (Stephen Abbott). The authors would also like to acknowledge Carlos E. Rodriguez-Diaz, MPH, PhD (George Washington University) for his assistance with the Spanish translation of the abstract.
Funding The DC Cohort is funded by the National Institute of Allergy and Infectious Diseases, 1R24AI152598–01.
Footnotes
Conflict of interest The authors have no relevant financial or non-financial interests to disclose.
Data Availability The data and code used in this study are not publicly available. For additional information about the DC Cohort Study and contact information for inquiries, please visit the website: https://publichealth.gwu.edu/projects/dc-cohort-longitudinal-hiv-study#home-content.
Compliance with Ethical Standards
Ethics Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Boards of George Washington University, DC Department of Health, and individual participating sites with their own IRBs have approved the DC Cohort study.
Consent to Participate and Consent for Publication Participants in the DC Cohort consent to have their demographic and clinical data electronically and manually abstracted from medical records at the participating sites and entered into a centralized database, for the purposes of research and publication of findings.
Supplementary information The online version of this article (https://doi.org/10.1007/s10461-021-03157-4) contains supplementary material, which is available to authorized users.
References
- 1.Atkinson JHJ, Grant I, Kennedy CJ, Richman DD, Spector SA, McCutchan JA. Prevalence of psychiatric disorders among men infected with human immunodeficiency virus. A controlled study. Arch Gen Psychiatry. 1988;45(9):859–64. [DOI] [PubMed] [Google Scholar]
- 2.Kilbourne AM, Justice AC, Rabeneck L, Rodriguez-Barradas M, Weissman S. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. J Clin Epidemiol. 2001;54(Suppl 1):S22–8. [DOI] [PubMed] [Google Scholar]
- 3.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–8. [DOI] [PubMed] [Google Scholar]
- 4.Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retrovir. 2004;20(2):151–5. [DOI] [PubMed] [Google Scholar]
- 5.Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the Southeastern United States. J Acquir Immune Defic Syndr. 2006;42(3):298–306. [DOI] [PubMed] [Google Scholar]
- 6.Hartzler B, Dombrowski J, Crane H, Eron J, Geng E, Christopher Mathews W, et al. 2017. Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS Behav. 21(4):1138–48. Available from https://search.proquest.com/docview/1875108965. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duko B, Ayalew M, Ayano G. The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy. 2019;14(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whetten-Goldstein K, Nguyen TQ, Heald AE. Characteristics of individuals infected with the human immunodeficiency virus and provider interaction in the predominantly rural Southeast. South Med J. 2001;94(2):212–22. [PubMed] [Google Scholar]
- 9.Lyketsos CG, Hanson A, Fishman M, McHugh PR, Treisman GJ. Screening for psychiatric morbidity in a medical outpatient clinic for HIV infection: the need for a psychiatric presence. Int J Psychiatry Med. 1994;24(2):103–13. [DOI] [PubMed] [Google Scholar]
- 10.Lyketsos CG, Hutton H, Fishman M, Schwartz J, Treisman GJ. Psychiatric morbidity on entry to an HIV primary care clinic. AIDS. 1996;10(9):1033–9. [DOI] [PubMed] [Google Scholar]
- 11.District of Columbia Department of Health 2019. Annual epidemiology and surveillance report: data through December 2018 Available from https://dchealth.dc.gov/page/hivaids-hepatitis-std-and-tb-administration-hahsta. Accessed 24 Dec 2020.
- 12.Giordano TP, Gifford AL, White AC, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. 2007. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 44(11):1493–9. Available from https://www.jstor.org/stable/4485427. Accessed 24 Dec 2020. [DOI] [PubMed] [Google Scholar]
- 13.Mugavero MJ, Westfall AO, Cole SR, Geng EH, Crane HM, Kitahata MM, et al. 2014. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis. 59(10):1471–9. Available from http://www.ncbi.nlm.nih.gov/pubmed/25091306. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. 2015. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 373(9):795. Available from http://www.ncbi.nlm.nih.gov/pubmed/26192873. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. 2016. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 375(9):830. Available from http://www.ncbi.nlm.nih.gov/pubmed/27424812. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute on Drug Abuse 2019. The national institute on drug abuse media guide: how to find what you need to know about drug use and addiction. Available from www.drugabuse.gov. Accessed 24 Dec 2020.
- 17.Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43(4):411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chander G, Himelhoch S, Fleishman JA, Hellinger J, Gaist P, Moore RD, et al. HAART receipt and viral suppression among HIV-infected patients with co-occurring mental illness and illicit drug use. AIDS Care. 2009;21(5):655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe CJ, Cole SR, Napravnik S, Kaufman JS, Adimora AA, Elston B, et al. The role of at-risk alcohol/drug use and treatment in appointment attendance and virologic suppression among HIV (+) African Americans. AIDS Res Hum Retrovir. 2014;30(3):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacamano PL, Farley JE 2016. Behavioral and other characteristics associated with HIV viral load in an outpatient clinic. PLoS One 11(11):e0166016. Available from https://search.proquest.com/docview/1835682010. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monroe AK, Lau B, Mugavero MJ, Mathews WC, Mayer KH, Napravnik S, et al. Heavy alcohol use is associated with worse retention in HIV care. J Acquir Immune Defic Syndr. 2016;73(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colasanti J, Stahl N, Farber E, del Rio C, Armstrong W 2017. An exploratory study to assess individual and structural level barriers associated with poor retention and re-engagement in care among persons living with HIV/AIDS. JAIDS J Acquir Immune Defic Syndr. 74 Suppl 2:S120. Available from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=fulltext&D=ovft&AN=00126334-201702011-00006. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grau LE, Griffiths-Kundishora A, Heimer R, Hutcheson M, Nunn A, Towey C, et al. 2017. Barriers and facilitators of the HIV care continuum in Southern New England for people with drug or alcohol use and living with HIV/AIDS: perspectives of HIV surveillance experts and service providers. Addict Sci Clin Pract.12. Available from https://search.proquest.com/docview/1946219505. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook RL, Zhou Z, Kelso-Chichetto NE, Janelle J, Morano JP, Somboonwit C, et al. Alcohol consumption patterns and HIV viral suppression among persons receiving HIV care in Florida: an observational study. Addict Sci Clin Pract. 2017;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolan S, Walley AY, Heeren TC, Patts GJ, Ventura AS, Sullivan MM, et al. HIV-infected individuals who use alcohol and other drugs, and virologic suppression. AIDS Care. 2017;29(9):1129. 10.1080/09540121.2017.1327646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesko CR, Keil AP, Fojo AT, Chander G, Lau B, Moore RD. Recent substance use and probability of unsuppressed HIV viral load among persons on antiretroviral therapy in continuity care. Am J Epidemiol. 2019;188(10):1830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, et al. 2016. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Available from http://researchonline.lshtm.ac.uk/2572430/. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed]
- 29.Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS ONE. 2012;7(5):e38335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fanucchi L, Springer SA, Korthuis PT. Medications for treatment of opioid use disorder among persons living with HIV. Curr HIV/AIDS Rep. 2019;16(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg AE, Hays H, Castel AD, Subramanian T, Happ LP, Jaurretche M, et al. Development of a large urban longitudinal HIV clinical cohort using a web-based platform to merge electronically and manually abstracted data from disparate medical record systems: technical challenges and innovative solutions. J Am Med Inform Assoc. 2016;23(3):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castel AD, Terzian A, Opoku J, Happ LP, Younes N, Kharfen M, et al. Defining care patterns and outcomes among persons living with HIV in Washington, DC: linkage of clinical cohort and surveillance data. JMIR Public Heal Surveill. 2018;4(1):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo I, Olsen H, Patrick R, Phillips G 2nd, Magnus M, Opoku J, et al. Willingness to use HIV pre-exposure prophylaxis among community-recruited, older people who inject drugs in Washington. DC Drug Alcohol Depend. 2016;164:8–13. [DOI] [PubMed] [Google Scholar]
- 34.Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, et al. 2011. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 53(9):927–35. Available from http://www.jstor.org/stable/23052438. Accessed 24 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson AD, Walker AS, Suthar AB, Sabin C, Bucher HC, Jarrin I, et al. Limiting cumulative HIV Viremia copy-years by early treatment reduces risk of aids and death. J Acquir Immune Defic Syndr. 2016;73(1):100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012–2013. Clin Infect Dis. 2016;63(7):976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks G, Gardner LI, Rose CE, Zinski A, Moore RD, Holman S, et al. Time above 1500 copies: a viral load measure for assessing transmission risk of HIV-positive patients in care. AIDS. 2015;29(8):947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefevre F, O’Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, et al. Alcohol consumption among HIV-infected patients. J Gen Intern Med. 1995;10(8):458–60. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham WE, Sohler NL, Tobias C, Drainoni M, Bradford J, Davis C, et al. Health services utilization for people with HIV infection: comparison of a population targeted for outreach with the U.S. population in care. Med Care. 2006;44(11):1038–47. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez A, Barinas J, O’Cleirigh C. Substance use: impact on adherence and HIV medical treatment. Curr HIV/AIDS Rep. 2011;8(4):223–34. [DOI] [PubMed] [Google Scholar]
- 41.Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The impact of alcohol use and related disorders on the HIV continuum of care: a systematic review: alcohol and the HIV continuum of care. Curr HIV/AIDS Rep. 2015;12(4):421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Census 2020. Explore Census Data. Available from https://data.census.gov/cedsci/. Accessed 24 Dec 2020.
- 43.Krieger N The science and epidemiology of racism and health: racial/ethnic categories biological expressions of racism, and embodiment of inequality–an ecosocial perspective. In: Whitmarsh I, Jones DS, editors. What’s the use of race? Modern governance and the biology of difference. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- 44.Guise A, Seguin M, Mburu G, McLean S, Grenfell P, Islam Z, et al. Integrated opioid substitution therapy and HIV care: a qualitative systematic review and synthesis of client and provider experiences. AIDS Care. 2017;29(9):1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vold JH, Aas C, Leiva RA, Vickerman P, Chalabianloo F, Løberg E-M, et al. Integrated care of severe infectious diseases to people with substance use disorders: a systematic review. BMC Infect Dis. 2019;19(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin EG, Wang KH. Integrating substance abuse treatment into HIV care: missed opportunities in the AIDS drug assistance program. J Acquir Immune Defic Syndr. 2013;62(4):421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.