Abstract
Macrophages are almost everywhere in the body, where they serve pivotal functions in maintaining tissue homeostasis, remodeling, and immunoregulation. Macrophages are traditionally thought to differentiate from bone marrow-derived hematopoietic stem cells (HSCs). Emerging studies suggest that some tissue macrophages at steady state originate from embryonic precursors in the yolk sac or fetal liver and are maintained in situ by self-renewal, but bone marrow-derived monocytes can give rise to tissue macrophages in pathogenic settings, such as inflammatory injuries and cancer. Macrophages are popularly classified as Th1 cytokine (e.g. IFNγ)-activated M1 macrophages (the classical activation) or Th2 cytokine (e.g. IL-4)-activated M2 macrophages (the alternative activation). However, given the myriad arrays of stimuli macrophages may encounter from local environment, macrophages exhibit notorious heterogeneity in their phenotypes and functions. Determining the underlying metabolic pathways engaged during macrophage activation is critical for understanding macrophage phenotypic and functional adaptivity under different disease settings. Fatty acid binding proteins (FABPs) represent a family of evolutionarily conserved proteins facilitating lipid transport, metabolism and responses inside cells. More specifically, adipose-FABP (A-FABP) and epidermal-FABP (E-FABP) are highly expressed in macrophages and play a central role in integrating metabolic and inflammatory pathways. In this review we highlight how A-FABP and E-FABP are respectively upregulated in different subsets of activated macrophages and provide a unique perspective in defining macrophage phenotypic and functional heterogeneity through FABP-regulated lipid metabolic and inflammatory pathways.
1. Introduction
Fatty acid binding proteins (FABPs) comprise a family of 14–15 kDa cytoplasmic lipid chaperones that coordinate lipid distribution and responses inside cells [1–3]. Composed of 10 anti-parallel β stands and capped by a helix-turn-helix motif, FABP members are highly homologous with a similar tertiary structure. FABPs are capable of binding a variety of fatty acids (FAs) and other hydrophobic ligands in the cavity of their β barrel structure with different specificity and affinity. FABP members display tightly-regulated patterns of tissue distribution, such as liver FABP (L-FABP, also known FABP1), intestinal FABP (I-FABP, FABP2) and heart FABP (H-FABP, FABP3), which are mainly expressed in liver, intestine and heart, respectively [4]. However, accumulating studies demonstrated that some FABP members exhibit expression beyond the tissues where they were originally cloned (Table 1). For example, adipose FABP (A-FABP or FABP4) is expressed in endothelial cells and macrophages besides adipocytes [5, 6]. Epidermal FABP (E-FABP or FABP5) exhibits a more ubiquitous expression profile, including skin, adipose tissue and multiple immune populations [7–9], suggesting a critical role of E-FABP in maintaining basic cellular energy metabolism and functions. In our studies focusing on immune cell lipid metabolism and function, we found that A-FABP and E-FABP display unique expression patterns in tissue macrophages which regulate their metabolic and inflammatory signaling pathways [4, 9–11]. Given the phenotypic diversity and functional versatility of macrophages, this review provides a unique perspective by focusing on defining macrophage functions through FABP-mediated lipid responses in different disease settings.
Table 1.
The distribution, binding ligands and functions of FABP family members
| Gene | Protein | Alternative names | Predominant tissue localization | Major binding ligands | Pathological functions |
|---|---|---|---|---|---|
| FABP1 | L-FABP | Liver FABP, heme-binding FABP | Liver, duodenum, small intestine, colon, rectum, kidney, appendix | Broad hydrophobic ligands, such as heme, bile acids, acyl-CoA, vitamins, xenobiotic drugs | Hepatic steatosis, nonalcoholic fatty liver disease |
| FABP2 | I-FABP | Intestine FABP, gut FABP | Duodenum, small intestine | Long chain fatty acids (LCFA) | Metabolic syndromes, colorectal cancer |
| FABP3 | H-FABP | Heart FABP, muscle FABP | Muscles (heart, skeletal) | LCFA, eicosanoids, retinoic acids | Biomarker for acute myocardial infarction |
| FABP4 | A-FABP | Adipose FABP, aP2 | Adipose tissue (adipocytes and macrophages), endothelium | LCFA, eicosanoids, retinoic acids | Metabolic diseases (such as type 2 diabetes, atherosclerosis, insulin resistance), cardiovascular disease, asthma, cancer |
| FABP5 | E-FABP | Epidermal FABP, mal1, psoriasis-associated FABP, keratinocyte FABP | Skin, adipose tissue, lung, immune cells (macrophages, T cells,etc), esophagus, stomach, colon | LCFA, eicosanoids, retinoic acids, cannabinoids | Inflammatory skin diseases (such as psoriasis, dermatitis), atherosclerosis, autoimmune diseases, cancer |
| FABP6 | IL-FABP | IIeal FABP, gastrotropin | Small intestine (distal) | Bile acids, cholate, LCFA | Type 2 diabetes, bile acid-associated gut diseases |
| FABP7 | B-FABP | Brain FABP, brain lipid binding protein (BLBP) | Cerebellum, hippocampus | Polyunsaturated FAs, particulary DHA | Overexpression in Down’s syndrome and Schizophrenia |
| FABP8 | M-FABP | Myelin FABP | Cerebral cortex | LCFA, eicosanoids, retinoic acids, cholesterol | Guillain-Barre syndrome |
| FABP9 | T-FABP | Testis FABP, testis lipid binding protein (TLBP) | Testis, esophagus | LCFA, eicosanoids, retinoic acids | Sperm head abnormalities |
2. Macrophage phenotypic and functional heterogeneity
2.1. Origin of macrophages
Macrophages are present in all tissues in the body, in which they play a central role in maintaining tissue homeostasis, repair, and immunomodulation. Colony-stimulating factor 1 (CSF1, also known as macrophage CSF, M-CSF) is essential to the development and survival of monocytes/macrophages. Genetic depletion of the functional receptor for CSF1 (CSF1R or CD115) is lethal in mice [12, 13], suggesting that the congenital presence of the macrophage lineage is essential for murine survival.
Macrophages are generally believed to come from bone marrow-derived monocytes, which sequentially differentiate through the hematopoietic stem cells (HSCs)/macrophage and dendritic cell precursor (MDP)/common monocyte progenitor (cMoP) axis. The mononuclear phagocyte system (MPS) proposes that homeostasis of tissue macrophages rely on constant recruitment of circulating monocytes [14]. However, emerging genetic fate mapping studies reveal that steady state tissue macrophages (e.g. brain microglia, liver Kupffer cells) are mainly derived from embryonic precursors in the yolk sac or fetal liver and maintained in situ by self-renewal [15]. Of note, circulating monocytes are able to give rise to tissue macrophages in certain pathogenic settings, such as inflammatory injuries and cancer [16, 17]. Thus, depending on the local microenvironment [18], both embryonic- and bone marrow-derived macrophages may dynamically and coordinately control tissue homeostasis, infection and inflammation.
2.2. Macrophage phenotypic heterogeneity
Originally developed from bone marrow MDPs and cMoPs, blood monocytes are phenotypically heterogeneous, which are usually defined by specific surface proteins. In mice, monocytes (CD115+) are usually divided into two main subsets based on the expression of Ly6C: Ly6C+ and Ly6C− monocytes [19]. Ly6C belongs to the family of Ly6 proteins which are widely used as lineage-specific markers in leukocyte subset identification [20]. As a GPI (glycosylphosphatidylinositol)-anchor membrane protein, Ly6C has been shown to mediate the migration of different leukocyte subsets [21, 22], suggesting that Ly6C expression is critical to monocyte migration. In line with this perspective, Ly6C is highly expressed on bone marrow monocytes, which can easily migrate to the peripheral blood and to sites of infection and inflammation to engage inflammatory responses. By contrast, Ly6C− monocytes normally patrol the endothelial surface of blood vessels and are recruited to inflamed sites at a late phase for tissue repair [23]. Ly6C+ and Ly6C− macrophages also exist in lymphoid (e.g. draining lymph nodes and spleen) and nonlymphoid tissues (e.g. skin, intestine). During tissue injuries, blood Ly6C+ monocytes can rapidly migrate to the injury sites and differentiate into tissue inflammatory macrophages, TNFα/iNOS produced dendritic cells (TipDCs) or tumor associated myeloid-derived suppress cells (MDSCs), whereas Ly6C− monocytes can give rise to alveolar macrophages [24]. In humans, based on surface expression of CD14 and CD16 glycoproteins, human blood monocytes can be divided into CD14highCD16negative and CD14lowCD16positive subsets. Analysis of gene expression arrays suggests that human CD14high and CD14low monocytes functionally resemble murine Ly6C+ and Ly6C− monocytes, respectively [25]. It is of great interest to understand which factors contribute to the phenotypic heterogeneity of monocytes/macrophages in mice and humans.
2.3. Macrophage functional versatility
Besides phenotypic differences, macrophages exhibit functional versatility in vivo. Under homeostatic conditions, macrophages maintain normal physiologic functioning of organisms by shaping their architecture (e.g. brain, bone and mammary glands) and regulating diverse activities (e.g. metabolism, damage control and tissue repair). However, under pathological conditions, continuous insults from infection or chronic inflammation can subvert the trophic and regulatory roles of macrophages, thereby contributing to the progression of many diseases (e.g. cardiovascular disease and tumor) [26]. Given the myriad of possible environmental stimuli, macrophages are believed to exist in a vast array of functional states or “a functional spectrum” [27, 28]. To understand their functional complexity, macrophages are simply classified as M1/M2 dichotomy based on the widely accepted concept of Th1/Th2 polarization. During adaptive immune responses, Th1 lymphocytes mainly produce IFNγ while Th2 cells mainly produce IL-4, IL-13, etc. Accordingly, macrophages activated by Th1 or Th2 cytokines are called M1 or M2 macrophages, respectively [29, 30]. The classification of M1/M2 has been widely accepted and data generated based on this classification have provided insights into understanding macrophage functionality in different disease settings. Generally, the classical activation of M1 macrophages is characterized by pro-inflammatory and anti-tumor activities whereas the alternative activation of M2 macrophages increases angiogenesis and exhibits tumor-promoting functions [31, 32]. However, given the spectrum of functional states of macrophages in vivo [33], these two extreme classifications are apparently oversimplified, especially in the tumor microenvironment. Recent studies demonstrate that pro-tumor macrophages in either the peritoneum or in tumor stroma do not follow the M1/M2 classification [6, 34]. Thus, the concept of functional adaptivity has been proposed that macrophages can change their functional phenotype in response to an array of microenvironmental stimuli [35, 36]. Emerging evidence suggests that macrophage metabolism determines their functional outcome [37, 38]. Dissecting the underlying metabolic pathways engaged during macrophage activation is critical to understanding their phenotypic and functional adaptivity in the progression of different diseases.
2.4. Macrophage metabolic pathways
As sentinels of the immune system, macrophages sense and respond to early signs of infection and inflammation [39]. In response to microbial invaders, monocytes/macrophages activated by IFNγ and LPS exhibit an increase of aerobic glycolysis and a decrease in fatty acid oxidation (FAO). Accordingly, this classical activation of M1 macrophages is considered as pro-inflammatory macrophages with high phagocytic potential in clearance of bacteria and tumor cells [26]. By contrast, macrophages can be alternatively activated by other environmental stimuli, such as parasitic infections. It is well known that alternative activation of M2 macrophages by IL-4 or IL-13 relies on FAO to fuel macrophage functions in anti-parasitic infection as well as in angiogenesis, tissue remodeling, and tumor progression [30]. Despite these two well-characterized metabolic pathways, macrophages can be metabolically activated by many other local tissue inputs. For example, obesity is associated with elevated levels of low density of lipoprotein (LDL) [40], which can be taken up by macrophages and induce alternative activation of macrophages through lysosomal acid lipase-mediated lipolysis pathway [41]. Given the alarming rate of obesity, it is intriguing to understand how lipid metabolism regulates macrophage functions in obesity and obesity-associated maladies.
3. FABP expression profile in macrophages
FABP family members facilitate lipid trafficking, metabolism and responses inside cells, circumventing low lipid solubility. Macrophages have been shown to mainly express A-FABP and E-FABP, which provides a unique perspective to dissect how A-FABP and E-FABP regulate FA metabolism and inflammatory pathways, thus shaping macrophage functional output.
Developed from HSCs and precursors, murine bone marrow monocytes (CD115+Ly6C+) express neither A-FABP nor E-FABP. Resting human monocytes also do not express detectable FABPs. Interestingly, in vitro activation of human monocytes with PMA (phorbol 13-myristate 12-acetate) upregulates the expression of both A-FABP and E-FABP [42]. These data demonstrate that FABPs are not essential to monocyte development in the bone marrow, but can be upregulated in monocytes activated by external stimuli, suggesting an important role of FABPs in activated monocytes/macrophages. Considering the heterogeneous subsets of monocytes/macrophages in vivo, we postulated that FABP expression in these cells is not uniform. Indeed, when we analyzed FABP expression in monocytes/macrophages separated from different tissues, we found that FABP exhibited unique expression profiles in different macrophage subsets. For example, splenic macrophages (CD11b+F4/80+) can be divided into four subsets based on the surface expression of Ly6C and MHCII. While E-FABP is highly expressed in the MHCII+ Q2 and Q3 subsets, A-FABP is only expressed in the MHCII− Q4 subsets [6, 9, 11]. Neither FABPs is expressed in the Q1 subset (Figure 1). The unique expression pattern of A-FABP and E-FABP in distinct macrophage subsets has been confirmed in other peripheral lymphoid tissues. For example, bone marrow monocytes are all located in Q1 subset, thus no/low FABP expression. Peripheral blood monocytes are mainly located in the Q1 and Q4 subsets, and A-FABP is only upregulated in the Q4 monocyte subset. Macrophages in draining lymph nodes are located in the Q2 and Q3 subsets, thus expressing E-FABP, but not A-FABP. Of note, E-FABP+ macrophage subsets also highly express MHCII, a professional antigen-presenting molecule, whereas A-FABP+ Q4 subset do not express MHCII molecules. Instead, A-FABP+ macrophages highly express CD36, a membrane scavenger receptor for lipid uptake and bacterial phagocytosis [43–45]. These distinct characteristics suggest that E-FABP+ macrophages are involved in accessory functions through antigen presentation and bridging innate and adaptive immunity. By contrast, A-FABP+MHCII−CD36+ macrophages appear to be engaged in direct pathogen clearance, lipid processing and other patrolling function along the lumen of blood vessels[46–48].
Figure 1. A-FABP and E-FABP expression pattern in splenic macrophages.
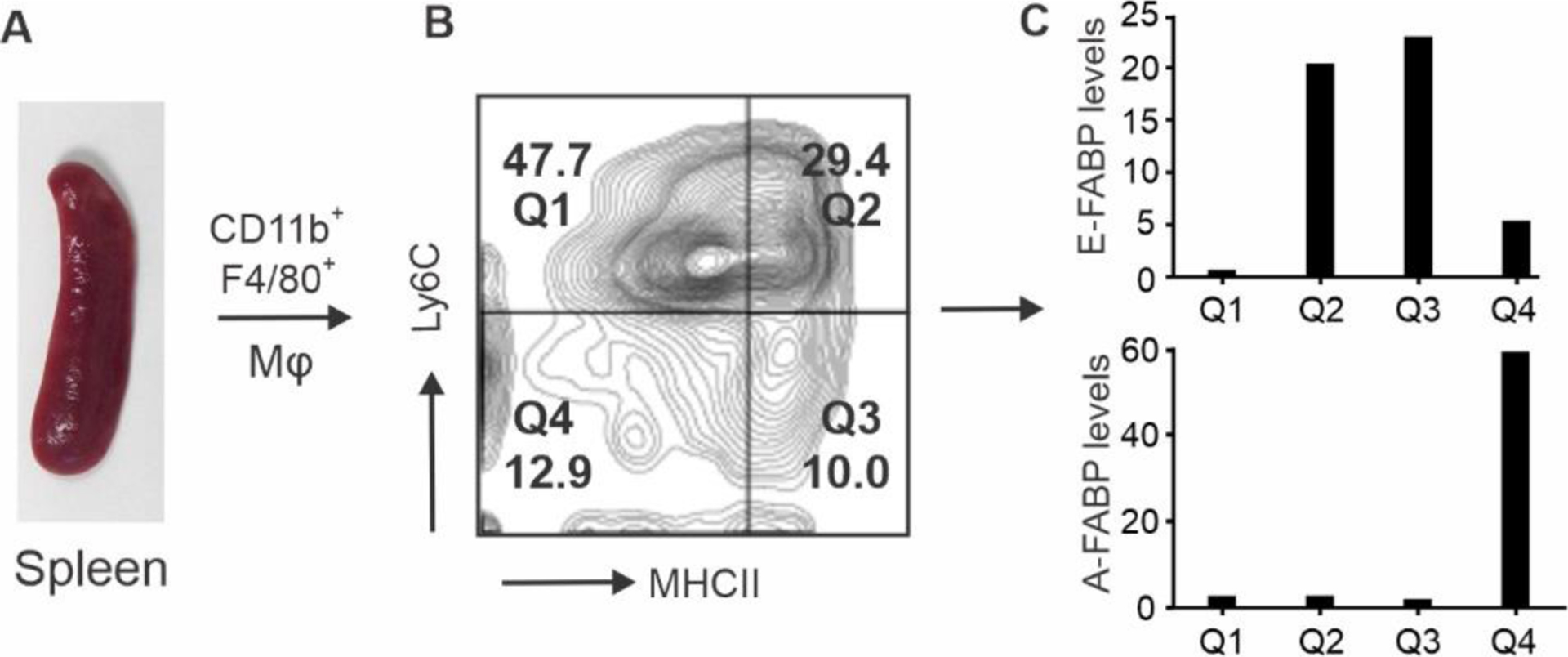
Splenic macrophages (CD11b+F4/80+) (A) are divided into four subsets (Q1-Q4) by the surface markers Ly6C and MHCII (B). Individual subsets were separated by a flow sorter and relative levels of A-FABP and E-FABP in each subset were assessed by real-time PCR (C).
4. Regulation of macrophage functions by FABPs in different diseases
As discussed above, heterogeneous murine blood monocytes contain Ly6C+ and Ly6C− two main subsets. Numerous studies have reported that Ly6C+ monocytes express chemokine receptors (e.g. CCR2) and are rapidly recruited to sites of injury, where they are activated to become either inflammatory macrophages/DCs (MHCII+) or other subsets (MHCII−) depending on local environmental cues [49–51]. By contrast, Ly6C− monocytes patrol the endothelium of blood vessel to scavenge pathogens, oxidized lipids or other debris. Ly6C− monocytes (MHCII−) can also be recruited to injury sites at a late phase to mediate tissue remodeling and immunomodulatory functions [52–54]. Due to the distinct FABP expression profile in activated macrophages, we hypothesized that E-FABP expression in MHCII+ macrophages and A-FABP expression in MHCII− CD36+ macrophages contribute to the functional versatility of macrophages in different diseases.
4.1. Tumors
As the most abundant myeloid cells in the tumor stroma, tumor associated macrophages (TAMs) are known to exhibit phenotypic and functional heterogeneity [32, 55, 56]. Although it has been proposed that IFNγ-activated M1 macrophages exert antitumor activities by producing abundant pro-inflammatory cytokines whereas IL-4-activated M2 macrophages enhance angiogenesis and exhibit pro-tumor functions [29], it is difficult to identify which macrophage subsets exert anti-tumor or pro-tumor functions due to the lack of specific phenotypic and functional markers. Studies have shown that in the tumor stroma, TAM progenitors are originally come from Ly6C+ monocytes, which gradually differentiate into either anti-tumor M1 or pro-tumor M2 TAMs depending on their locations in the tumor stroma [57, 58]. In line with these studies, we found that TAMs (CD11b+F4/80+) in syngeneic mammary tumor models exhibited a dynamic alteration in their phenotype and function (Figure 2). Right after tumor implantation (0–3 days), TAMs were mainly Ly6C+MHCII− monocytes (Q1 subset), whereas 1–2 weeks later, the major TAM population exhibited M1-like phenotype (Ly6C+MHCII+CD11c+) (Q2 subset). Three weeks later, the predominant TAM subset exhibited the M2-like phenotype (Ly6C−MHCII−) (Q4 subset)[6, 9]. Importantly, anti-tumor M1-like TAMs highly express E-FABP while pro-tumor M2-like TAMs highly express A-FABP. Using genetic knockout mouse models, we demonstrated that E-FABP expression in M1-like TAMs promotes their anti-tumor function by enhancing type I IFNβ responses through enhancing lipid droplet/viperin signaling [9]. Moreover, A-FABP expression in M2-like TAMs is critical to their pro-tumor function by promoting IL-6/STAT3 signaling through regulation of the NFκB/miR-29 pathway [6]. Of note, anti-tumor E-FABP+ macrophages differentiate early in tumor development [9]. If tumor cells are not eliminated at this stage, A-FABP+ TAMs gradually become dominant in the tumor stroma to promote tumor growth and progression [6] (Figure 2). Thus, E-FABP and A-FABP can be considered functional markers for anti-tumor and pro-tumor TAMs, respectively.
Figure 2. Expression of E-FABP and A-FABP in different subsets of TAMs.
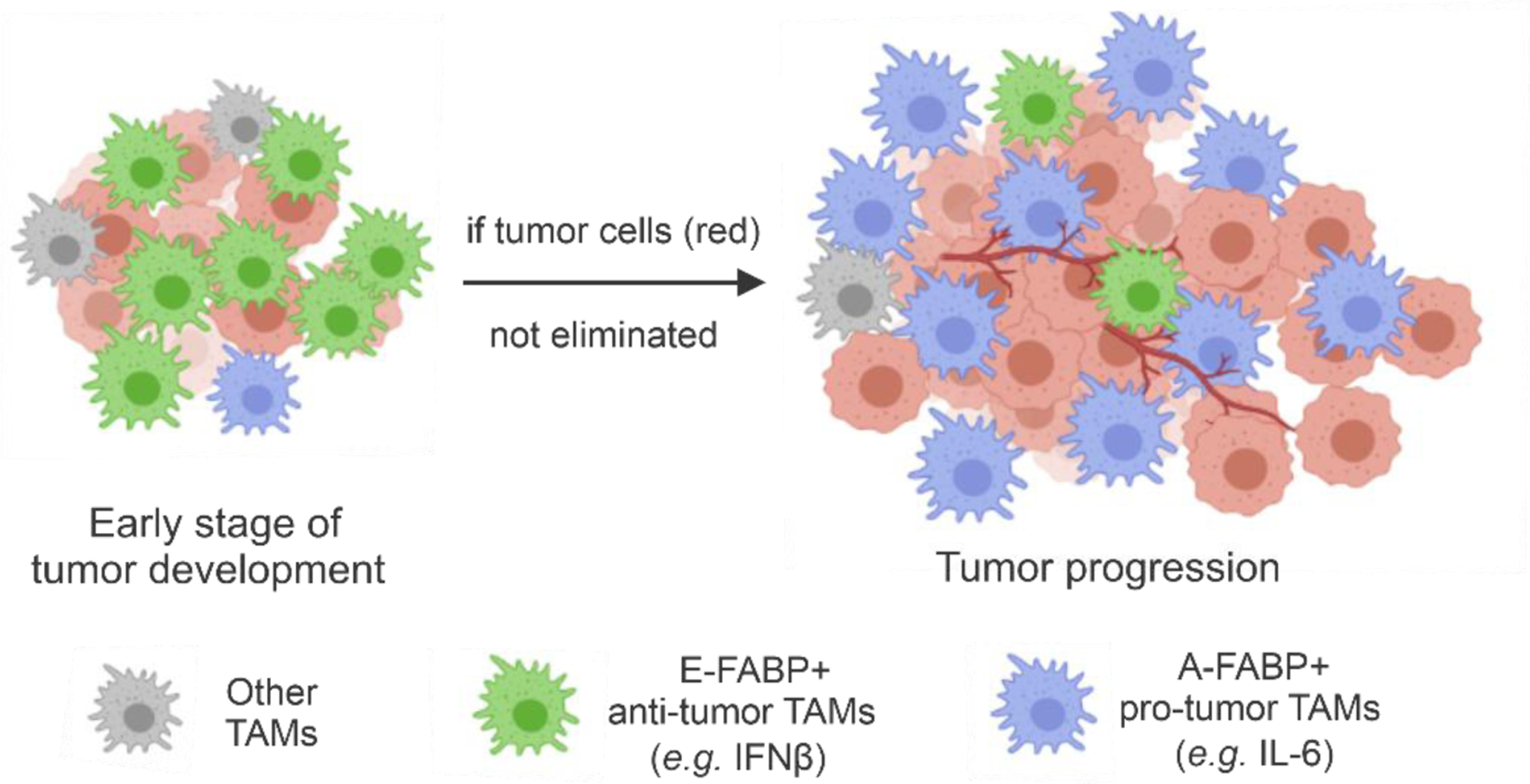
E-FABP expression in TAMs exerts anti-tumor effects by promoting type I IFNβ signaling and adaptive immune responses, while TAM expression of A-FABP promotes tumor progression by enhancing pro-tumor IL-6/STAT3 signaling.
4.2. Inflammatory diseases
Buildup of lipid-laden macrophages (foam cells) is the most characteristic feature of atherosclerosis [59]. As such, atherosclerosis represents a good model to determine the contribution of macrophages to the pathogenesis of inflammatory diseases. A-FABP expression in macrophages was first reported to contribute to foam cell accumulation and atherosclerotic lesions using the ApoE−/− A-FABP−/− mouse model in a normal chow diet [42]. The critical atherogenic role of A-FABP expression in macrophages was further demonstrated in ApoE−/− mice fed a high fat Western diet that developed advanced atherosclerosis [60, 61]. E-FABP expression in macrophages also promotes atherosclerotic lesions by enhancing CCR2-mediated recruitment [62]. The conclusion that A-FABP and E-FABP contribute to atherosclerosis is mainly based on two observations: (1) macrophages express both E-FABP and A-FABP, and 2) A-FABP and E-FABP have a similar tertiary structure and ligand binding affinity [63]. Thus, it is assumed that A-FABP and E-FABP might have similar or redundant roles in macrophage-mediated atherogenesis. However, the observations that A-FABP deficiency is not compensated by E-FABP overexpression, nor does E-FABP deficiency result in A-FABP overexpression, do not support their functional redundancy in macrophages. Given that A-FABP and E-FABP have distinct expression profiles in different subsets of bone marrow-derived monocytes/macrophages, we proposed that A-FABP+ Ly6C− CD36+ patrolling monocytes mainly contribute to oxidized lipid uptake and foam cell formation. Hyperlipidemia (e.g. oxLDL) in either ApoE−/− or LDLR−/− mice induces A-FABP-dependent lipotoxicity and macrophage death along the vascular endothelium, initiating local inflammation, and then recruits inflammatory E-FABP+ Ly6C+ CCR2+ monocytes, exacerbating atherosclerotic lesions already present (Figure 3). Thus, A-FABP and E-FABP each contribute to atherogenesis through the regulation of different subsets of macrophages.
Figure 3. A-FABP and E-FABP contribute to atherosclerosis by regulating different subsets of monocytes/macrophages.
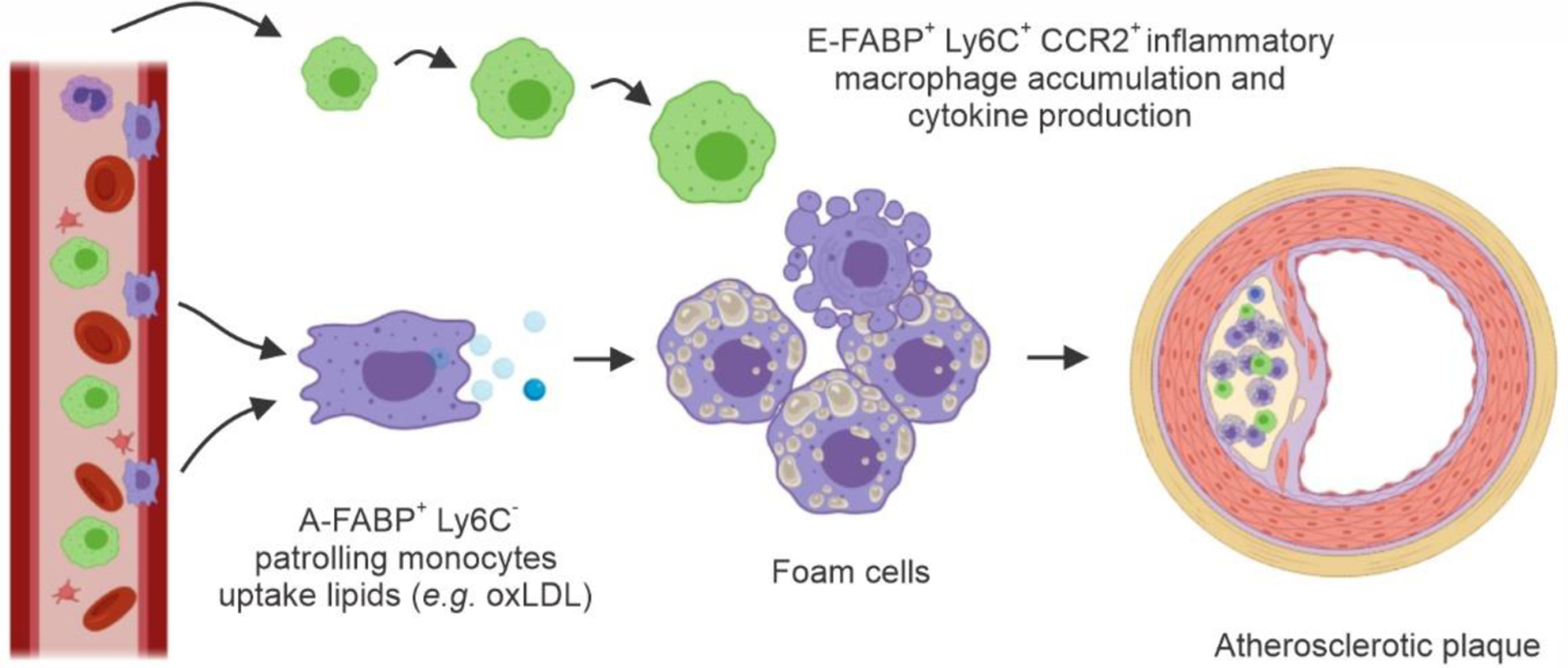
A-FABP+Ly6C−CD36+ patrolling monocytes uptake oxLDL and initiate atherosclerotic lesions while recruited Ly6C+ CCR2+ monocytes express E-FABP, contributing to local inflammation in the atherosclerotic plaque.
4.3. Obesity and other diseases
In the past three decades, the prevalence of obesity has increased at an alarming rate [64–66]. Obesity is associated with chronic inflammation and many of mankind’s most common diseases, including type II diabetes, cardiovascular disease, and at least 13 types of cancer [67, 68]. The underlying molecular mechanisms linking obesity and obesity-associated diseases are under active investigation. We used high fat diet (HFD)-induce murine obese models to demonstrate that obesity increases the risk of mammary tumor incidence and growth through at least two mechanisms: 1) Consumption of a HFD rich in saturated fat enhances differentiation of the A-FABP+ Q4 subset in obese mice, thus increasing obesity-associated tumor risk [69]; 2) An HFD rich in saturated fat increases circulating levels of A-FABP, which directly target mammary tumor cells by enhancing tumor stemness for tumor progression [70]. Unlike A-FABP, circulating levels of E-FABP are similar in obese mice compared to lean mice, suggesting that A-FABP is an important link increasing the risk of obesity-associated cancer [71].
In several murine models we observed that HFDs, particular high saturated fat diets, induce chronic skin inflammation, which is associated with increased accumulation of MHCII+ CD11c+ macrophages in the lesion skin. E-FABP is highly expressed in these macrophages and promotes inflammatory IL-1β signaling, which leads to adaptive T cell responses. Importantly, E-FABP deficiency reduces IL-1β responses and completely prevents the HFD-induced skin lesion. Thus, E-FABP expression in MHCII+CD11c+ macrophages is critical in mediating IL-1β-induced inflammatory diseases in obesity. In addition, FABPs play a central role in other disease models. For instance, LPS injection induces infiltration of E-FABP+ macrophages in the liver, which contributes to LPS-induced liver inflammation and injury [72]. IL-4 stimulation promotes macrophage polarization by upregulation of A-FABP through the IL-4/STAT6/PPARγ signaling axis [73–75]. A-FABP overexpression in liver Kupffer cells positively correlates with the poor outcomes of decompensated cirrhosis [76]. Recently studies demonstrate that A-FABP expression in alveolar macrophages is required for neutrophil recruitment and infection clearance [77, 78]. It is of great interest to determine whether severe symptoms often observed in obese COVID-19 patients are associated with dysregulation of A-FABP expression and neutrophil hyperinflammation. infection may patients. These mounting lines of evidence further suggest that E-FABP and A-FABP promote inflammatory diseases through regulating different macrophages in vivo.
5. Regulation of lipid-metabolic pathways by FABPs in macrophages
To further understand how A-FABP and E-FABP respectively regulate macrophage functions, we noticed that while both FABPs bind various dietary FAs with similar affinity [63, 79], they are unique in channeling different FAs to specific cellular organelles and in regulating their metabolic pathways in macrophages.
5.1. A-FABP regulates FA-mediated pathways in macrophages
Protein-ligand analysis demonstrated that A-FABP selectively transports specific FAs into the nucleus for transcriptional activation of nuclear receptor PPARγ [80]. Although A-FABP is able to bind multiple FAs with similar affinity, including palmitic acid, stearic acid, oleic acid, linoleic acid, arachidonic acid, etc., A-FABP only delivers certain ligands (e.g. linoleic acid, troglitazone) to the nucleus. Structural analysis indicated that linoleic acid binding to A-FABP alters its tertiary structure to form a nuclear localization signal (NLS) whereas binding of non-activating ligands (e.g. saturated FAs) masks the NLS, thus preventing their nuclear transport by A-FABP. Of note, ligand activation of PPARγ controls expression of multiple PPARγ-target genes, among which CD36 is well known to mediate oxLDL uptake and promote the formation of macrophage-derived foam cells [81] (Figure 4). A-FABP is also a direct PPARγ transcriptional activation gene, and oxLDL-induced PPARγ activation in turn promoted A-FABP expression and monocyte/macrophage differentiation [82, 83]. Thus, once egressed from bone marrow, Ly6C+ monocytes can differentiate into Ly6C− patrolling monocytes through activation of the PPARγ/CD36/A-FABP pathway in response to external stimuli in the blood (e.g. oxLDL).
Figure 4. A-FABP coordinates FA responses in macrophages.
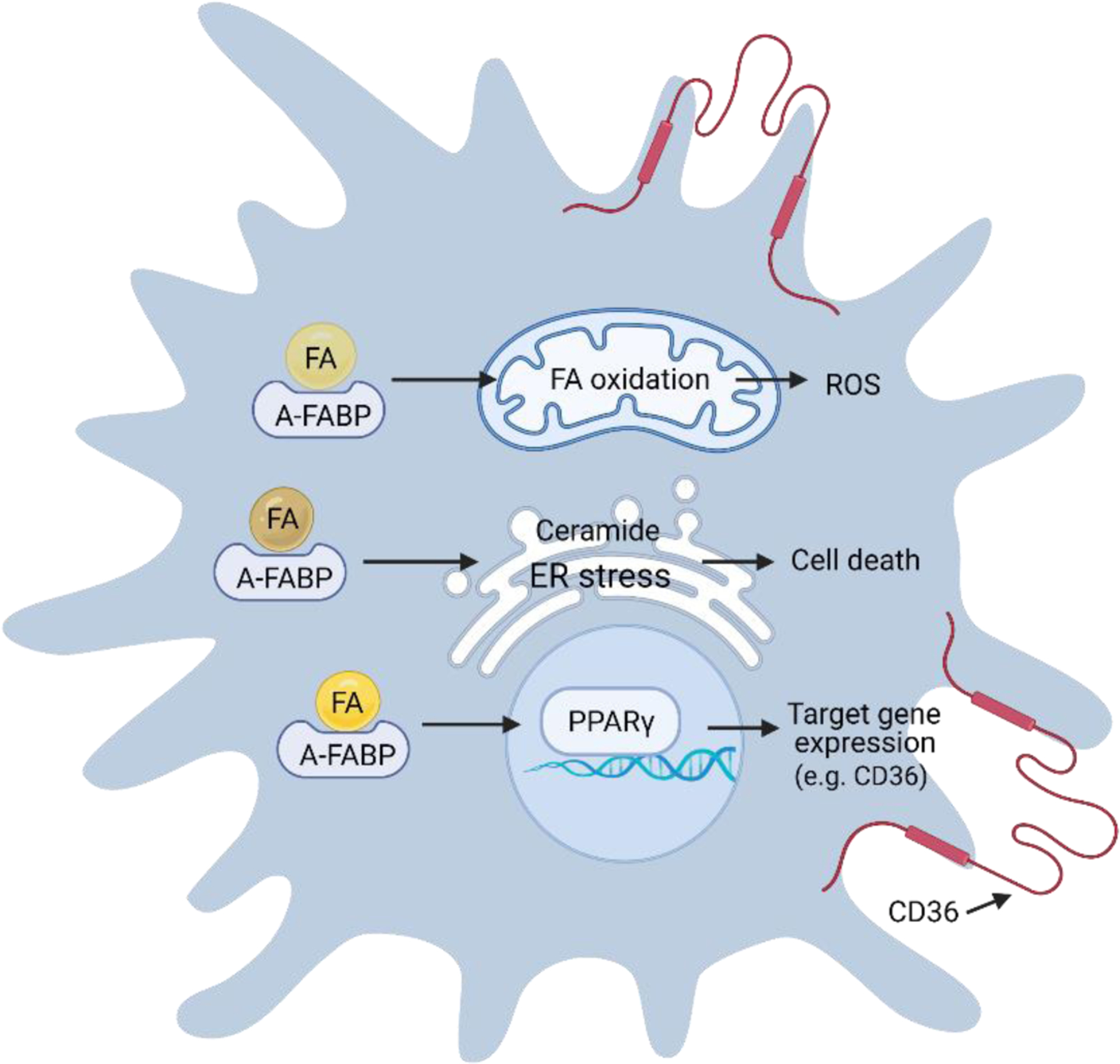
In A-FABP+ macrophages, A-FABP can facilitate multiple FA-mediated responses, which include FA oxidation and ROS production in mitochondria, ceramide production and ER stress, and activation of nuclear transcriptional factors (e.g. PPARγ) activation and regulation of gene expression (e.g. CD36).
Besides channeling linoleic acids into the nucleus for PPARγ activation, A-FABP mediates other unsaturated FA (e.g. omega-3 FAs)-induced mitochondrial oxygen consumption and production of reactive oxygen species (ROS) in macrophages [69]. Macrophages deficient in A-FABP exhibit increased intracellular levels of unsaturated FAs and upregulation of UCP2 [84], further supporting a critical role of A-FABP in mediating unsaturated FA oxidation in macrophages. A-FABP also plays a critical role in coordinating saturated FA-mediated responses in macrophages, including palmitic acid-induced endoplasmic reticulum (ER) stress [85]. Our recent studies demonstrate that A-FABP is pivotal in mediating saturated FA-induced ceramide production and macrophage cell death [11, 86]. Thus, depending on the FA ligands, A-FABP transports them to different cellular compartments to coordinate unique metabolic pathways in macrophages (Figure 4).
5.2. E-FABP-mediated FA pathways in macrophages
Although E-FABP has a high degree of homology with A-FABP, it exhibits a distinct expression profile in bone-marrow derived monocytes/macrophages, implying unique features in mediating lipid metabolic pathways. A-FABP is highly expressed in Ly6C−MHCII−CD36+ monocytes/macrophages, whereas E-FABP is highly expressed in Ly6C+MHCII+CD36− macrophages. E-FABP+ macrophages are lower in PPARγ, but higher in PPARβ/δ expression, suggesting a specific E-FABP/PPARβ/δ interaction. Mounting evidence indicates that E-FABP channels ligands from the cytoplasm to the nucleus for transcriptional activation of PPARβ/δ, and PPARβ/δ activation can directly induce E-FABP expression, thus forming a positive feedback loop [87–89]. Structural analysis indicated that E-FABP can bind a wide array of FAs and other hydrophobic ligands (e.g. trans-retinoic acid, N-acylethanolamine), but only certain ligands alter E-FABP conformation and tertiary NLS formation, leading to ligand-driven nuclear translocation [90]. It is clear now that E-FABP can transport unsaturated FAs, especially these with a U-shape conformation (e.g. linoleic acid, arachidonic acid) to the nucleus for PPARβ/δ transactivation. Besides nuclear transportation, E-FABP expression in TAMs facilitates unsaturated FA-induced lipid droplet (LD) formation in macrophages [9]. As LDs are essential in mediating IFNβ signaling [91, 92], we demonstrated that E-FABP expression in TAMs plays a critical role to promote anti-tumor type I IFNβ responses in mammary tumor models. Thus, E-FABP can also channel unsaturated FAs for LD formation, which provides a platform for unsaturated FA-mediated IFNβ responses in macrophages (Figure 5).
Figure 5. E-FABP coordinates FA responses in macrophages.
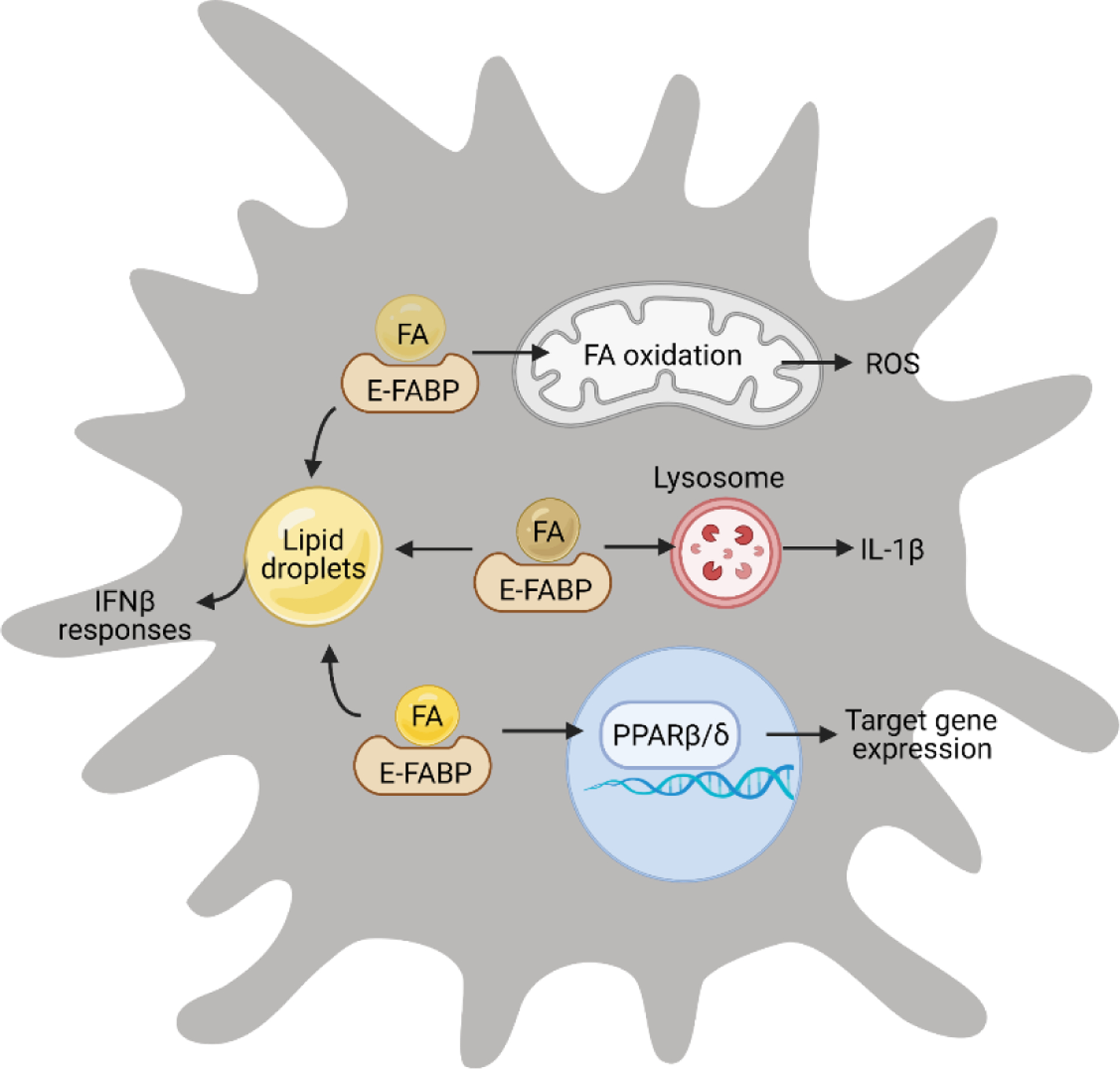
In E-FABP+ macrophages, E-FABP mediates multiple FA-induced responses, including FA oxidation and ROS production, inflammasome activation and IL-1β upregulation, lipid droplet formation and IFNβ responses, and nuclear transcriptional factor activation and gene regulation.
As E-FABP does not appear to channel saturated FAs (e.g. palmitic acid) to the nucleus for PPARβ/δ activation or formation of LDs in the cytosol, it is intriguing to know whether and how E-FABP regulates saturated FA-mediated responses inside macrophages. In an obese mouse model induced by a diet high in lard, we observed that the HFD-induced skin lesions were associated with CD11c+ macrophage accumulation. Further analysis of the skin indicated that IL-1β signaling was significantly upregulated in CD11c+ macrophages. Interestingly, saturated, but not unsaturated, FAs promote CD11c expression and induce IL-1β secretion in an E-FABP-dependent manner, suggesting that E-FABP enhances saturated FAs-mediated CD11c+ macrophage differentiation and IL-1β signaling pathways [10, 93]. Recent studies demonstrate that saturated FAs are transported to lysosomes to form crystals for inflammasome activation and IL-1β release [94], suggesting that E-FABP may deliver saturated FAs to lysosomes in macrophages (Figure 5).
Of note, E-FABP expression is more ubiquitous than A-FABP and other FABP members. Besides expressed in macrophage subsets, E-FABP is also expressed in other immune cells and tissues (e.g. T cells, mammary gland, brain, lung) [95, 96], suggesting that E-FABP serves as a ubiquitous lipid carrier. Dysregulation of lipid metabolism links ER stress[97–99], exhaustion and ferroptosis[100, 101], which have been suggested to regulate immune cell activation and survival. Thus, FABP-mediated ER stress and other lipid signaling pathways can affect immune cell fate and disease progress. Further study is warranted to determine how FABPs regulate lipid metabolism and tissue/cell specific responses in obesity, tumor and other inflammatory diseases.
6. Summary
The family of FABP members is widely expressed in different tissue/organs, facilitating lipid uptake, transport, and coordinating lipid-mediated responses. Among FABP members, A-FABP and E-FABP are highly upregulated in activated macrophages, regulating different functions of macrophages in multiple disease settings. A-FABP and E-FABP are thought to be equally expressed in macrophages at similar levels [42]. Due to the similarity of their amino-acid sequences, protein structure and binding ligands, A-FABP and E-FABP are generally believed to function redundantly in macrophages [62]. Studies using A-FABP and E-FABP double knockout mice have generated striking phenotypes in preventing insulin resistance, chronic inflammation and other metabolic diseases [102–104]. However, macrophages exhibit heterogeneous phenotypes and functions in vivo. Depending on their origin, tissue distribution and wide arrays of stimuli received from the local environment, macrophages exhibit diverse activation status when engaged with different metabolic and inflammatory pathways. As such, emerging evidence indicates that A-FABP and E-FABP can be upregulated in different subsets of activated macrophages to coordinate lipid-mediated responses. For bone marrow-derived macrophages, A-FABP is highly expressed in Ly6C− MHCII−CD36+ patrolling monocyte/macrophages to facilitate oxidative lipid uptake, foam cell formation, angiogenesis, tissue remodeling and pro-tumor functions. By contrast, E-FABP is highly expressed in MHCII+CD11c+ macrophages and promotes inflammatory responses (e.g. IL-1 signaling), antigen presentation and anti-tumor immunity (e.g. IFNβ response). A-FABP and E-FABP are also upregulated with obesity to handle dysregulated levels of FAs and channel them to specific cellular organelles inducing different metabolic and inflammatory signaling in macrophages. Thus, A-FABP and E-FABP represent a new line of functional markers defining macrophage functions to maintain homeostasis in health and engaging pathogenesis under various disease conditions.
Highlights.
Macrophages exhibit phenotypic, metabolic and functional heterogeneity.
FABPs, in particularly A-FABP and E-FABP, are expressed in different subsets of macrophages.
A-FABP and E-FABP regulate lipid metabolism by coordinating different FA-mediated pathways in macrophages.
A-FABP and E-FABP may serve as new metabolic/functional markers defining macrophage heterogeneity.
Acknowledgements
We thank the funding support from NIH grants R01AI137324 and R01CA180986. The opinions expressed in this review are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest Statement
There is no conflict of interests for all listed authors.
References
- [1].Furuhashi M, Hotamisligil GS, Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets, Nat Rev Drug Discov, 7 (2008) 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Storch J, Corsico B, The emerging functions and mechanisms of mammalian fatty acid-binding proteins, Annu Rev Nutr, 28 (2008) 73–95. [DOI] [PubMed] [Google Scholar]
- [3].Hertzel AV, Bernlohr DA, The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function, Trends Endocrinol Metab, 11 (2000) 175–180. [DOI] [PubMed] [Google Scholar]
- [4].Li B, Hao J, Zeng J, Sauter ER, SnapShot: FABP Functions, Cell, 182 (2020) 1066–1066 e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gogg S, Nerstedt A, Boren J, Smith U, Human adipose tissue microvascular endothelial cells secrete PPARgamma ligands and regulate adipose tissue lipid uptake, JCI Insight, 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hao J, Yan F, Zhang Y, Triplett A, Zhang Y, Schultz DA, Sun Y, Zeng J, Silverstein KAT, Zheng Q, Bernlohr DA, Cleary MP, Egilmez NK, Sauter E, Liu S, Suttles J, Li B, Expression of Adipocyte/Macrophage Fatty Acid-Binding Protein in Tumor-Associated Macrophages Promotes Breast Cancer Progression, Cancer Res, 78 (2018) 2343–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li B, Reynolds JM, Stout RD, Bernlohr DA, Suttles J, Regulation of Th17 differentiation by epidermal fatty acid-binding protein, J Immunol, 182 (2009) 7625–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Y, Li B, E-FABP: regulator of immune function, Oncoscience, 1 (2014) 398–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang Y, Sun Y, Rao E, Yan F, Li Q, Zhang Y, Silverstein KA, Liu S, Sauter E, Cleary MP, Li B, Fatty acid-binding protein E-FABP restricts tumor growth by promoting IFN-beta responses in tumor-associated macrophages, Cancer Res, 74 (2014) 2986–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zeng J, Zhang Y, Hao J, Sun Y, Liu S, Bernlohr DA, Sauter ER, Cleary MP, Suttles J, Li B, Stearic Acid Induces CD11c Expression in Proinflammatory Macrophages via Epidermal Fatty Acid Binding Protein, J Immunol, 200 (2018) 3407–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang Y, Rao E, Zeng J, Hao J, Sun Y, Liu S, Sauter ER, Bernlohr DA, Cleary MP, Suttles J, Li B, Adipose Fatty Acid Binding Protein Promotes Saturated Fatty Acid-Induced Macrophage Cell Death through Enhancing Ceramide Production, J Immunol, 198 (2017) 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wiktor-Jedrzejczak W, Gordon S, Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1-deficient op/op mouse, Physiol Rev, 76 (1996) 927–947. [DOI] [PubMed] [Google Scholar]
- [13].Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER, Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects, Blood, 99 (2002) 111–120. [DOI] [PubMed] [Google Scholar]
- [14].van Furth R, Cohn ZA, The origin and kinetics of mononuclear phagocytes, J Exp Med, 128 (1968) 415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR, Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors, Nature, 518 (2015) 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO, The cellular and molecular origin of tumor-associated macrophages, Science, 344 (2014) 921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL, Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation, Immunity, 40 (2014) 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ginhoux F, Jung S, Monocytes and macrophages: developmental pathways and tissue homeostasis, Nat Rev Immunol, 14 (2014) 392–404. [DOI] [PubMed] [Google Scholar]
- [19].Shi C, Pamer EG, Monocyte recruitment during infection and inflammation, Nat Rev Immunol, 11 (2011) 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA, Ly6 family proteins in neutrophil biology, J Leukoc Biol, 94 (2013) 585–594. [DOI] [PubMed] [Google Scholar]
- [21].Fumagalli L, Zhang H, Baruzzi A, Lowell CA, Berton G, The Src family kinases Hck and Fgr regulate neutrophil responses to N-formyl-methionyl-leucyl-phenylalanine, J Immunol, 178 (2007) 3874–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jaakkola I, Merinen M, Jalkanen S, Hanninen A, Ly6C induces clustering of LFA-1 (CD11a/CD18) and is involved in subtype-specific adhesion of CD8 T cells, J Immunol, 170 (2003) 1283–1290. [DOI] [PubMed] [Google Scholar]
- [23].Auffray C, Sieweke MH, Geissmann F, Blood monocytes: development, heterogeneity, and relationship with dendritic cells, Annu Rev Immunol, 27 (2009) 669–692. [DOI] [PubMed] [Google Scholar]
- [24].Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K, Development of monocytes, macrophages, and dendritic cells, Science, 327 (2010) 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB, Nomenclature of monocytes and dendritic cells in blood, Blood, 116 (2010) e74–80. [DOI] [PubMed] [Google Scholar]
- [26].Wynn TA, Chawla A, Pollard JW, Macrophage biology in development, homeostasis and disease, Nature, 496 (2013) 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL, Transcriptome-based network analysis reveals a spectrum model of human macrophage activation, Immunity, 40 (2014) 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stout RD, Suttles J, Functional plasticity of macrophages: reversible adaptation to changing microenvironments, J Leukoc Biol, 76 (2004) 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nat Rev Immunol, 8 (2008) 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martinez FO, Helming L, Gordon S, Alternative activation of macrophages: an immunologic functional perspective, Annu Rev Immunol, 27 (2009) 451–483. [DOI] [PubMed] [Google Scholar]
- [31].Sica A, Saccani A, Mantovani A, Tumor-associated macrophages: a molecular perspective, Int Immunopharmacol, 2 (2002) 1045–1054. [DOI] [PubMed] [Google Scholar]
- [32].Mantovani A, Sica A, Macrophages, innate immunity and cancer: balance, tolerance, and diversity, Curr Opin Immunol, 22 (2010) 231–237. [DOI] [PubMed] [Google Scholar]
- [33].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA, Macrophage activation and polarization: nomenclature and experimental guidelines, Immunity, 41 (2014) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Weiss JM, Davies LC, Karwan M, Ileva L, Ozaki MK, Cheng RY, Ridnour LA, Annunziata CM, Wink DA, McVicar DW, Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors, J Clin Invest, 128 (2018) 3794–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Biswas SK, Allavena P, Mantovani A, Tumor-associated macrophages: functional diversity, clinical significance, and open questions, Semin Immunopathol, 35 (2013) 585–600. [DOI] [PubMed] [Google Scholar]
- [36].Stout RD, Suttles J, Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes, Immunol Rev, 205 (2005) 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R, Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages, Science, 356 (2017) 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].O’Neill LA, Pearce EJ, Immunometabolism governs dendritic cell and macrophage function, J Exp Med, 213 (2016) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Odegaard JI, Chawla A, Alternative macrophage activation and metabolism, Annu Rev Pathol, 6 (2011) 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Klop B, Elte JW, Cabezas MC, Dyslipidemia in obesity: mechanisms and potential targets, Nutrients, 5 (2013) 1218–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O’Neill CM, Yan C, Du H, Abumrad NA, Urban JF Jr., Artyomov MN, Pearce EL, Pearce EJ, Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages, Nat Immunol, 15 (2014) 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF, Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis, Nat Med, 7 (2001) 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baranova IN, Kurlander R, Bocharov AV, Vishnyakova TG, Chen Z, Remaley AT, Csako G, Patterson AP, Eggerman TL, Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling, J Immunol, 181 (2008) 7147–7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Goldberg IJ, Eckel RH, Abumrad NA, Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways, J Lipid Res, 50 Suppl (2009) S86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu J, Wu H, An J, Ballantyne CM, Cyster JG, Critical role of integrin CD11c in splenic dendritic cell capture of missing-self CD47 cells to induce adaptive immunity, Proc Natl Acad Sci U S A, 115 (2018) 6786–6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Thomas G, Tacke R, Hedrick CC, Hanna RN, Nonclassical Patrolling Monocyte Function in the Vasculature, Arterioscl Throm Vas, 35 (2015) 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park YM, CD36, a scavenger receptor implicated in atherosclerosis, Exp Mol Med, 46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sun L, Nicholson AC, Hajjar DP, Gotto AM, Hau JH, Adipogenic differentiating agents regulate expression of fatty acid binding protein and CD36 in the J744 macrophage cell line, Journal of Lipid Research, 44 (2003) 1877–1886. [DOI] [PubMed] [Google Scholar]
- [49].Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S, Monocytes give rise to mucosal, but not splenic, conventional dendritic cells, J Exp Med, 204 (2007) 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Serbina NV, Pamer EG, Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2, Nat Immunol, 7 (2006) 311–317. [DOI] [PubMed] [Google Scholar]
- [51].Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B, Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis, J Exp Med, 204 (2007) 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ, Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response, J Immunol, 172 (2004) 4410–4417. [DOI] [PubMed] [Google Scholar]
- [53].Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ, The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions, J Exp Med, 204 (2007) 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Landsman L, Jung S, Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages, J Immunol, 179 (2007) 3488–3494. [DOI] [PubMed] [Google Scholar]
- [55].Yang M, McKay D, Pollard JW, Lewis CE, Diverse Functions of Macrophages in Different Tumor Microenvironments, Cancer Res, 78 (2018) 5492–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Condeelis J, Pollard JW, Macrophages: obligate partners for tumor cell migration, invasion, and metastasis, Cell, 124 (2006) 263–266. [DOI] [PubMed] [Google Scholar]
- [57].Van Overmeire E, Stijlemans B, Heymann F, Keirsse J, Morias Y, Elkrim Y, Brys L, Abels C, Lahmar Q, Ergen C, Vereecke L, Tacke F, De Baetselier P, Van Ginderachter JA, Laoui D, M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment, Cancer Res, 76 (2016) 35–42. [DOI] [PubMed] [Google Scholar]
- [58].Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA, Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes, Cancer Res, 70 (2010) 5728–5739. [DOI] [PubMed] [Google Scholar]
- [59].Amengual J, Barrett TJ, Monocytes and macrophages in atherogenesis, Curr Opin Lipidol, 30 (2019) 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS, Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia, Arterioscler Thromb Vasc Biol, 22 (2002) 1686–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Perrella MA, Pellacani A, Layne MD, Patel A, Zhao D, Schreiber BM, Storch J, Feinberg MW, Hsieh CM, Haber E, Lee ME, Absence of adipocyte fatty acid binding protein prevents the development of accelerated atherosclerosis in hypercholesterolemic mice, FASEB J, 15 (2001) 1774–1776. [DOI] [PubMed] [Google Scholar]
- [62].Babaev VR, Runner RP, Fan D, Ding L, Zhang Y, Tao H, Erbay E, Gorgun CZ, Fazio S, Hotamisligil GS, Linton MF, Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-gamma-regulated genes, Arterioscler Thromb Vasc Biol, 31 (2011) 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Haunerland NH, Spener F, Fatty acid-binding proteins--insights from genetic manipulations, Prog Lipid Res, 43 (2004) 328–349. [DOI] [PubMed] [Google Scholar]
- [64].Ogden CL, Fryar CD, Hales CM, Carroll MD, Aoki Y, Freedman DS, Differences in Obesity Prevalence by Demographics and Urbanization in US Children and Adolescents, 2013–2016, JAMA, 319 (2018) 2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hales CM, Carroll MD, Fryar CD, Ogden CL, Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018, NCHS Data Brief, (2020) 1–8. [PubMed] [Google Scholar]
- [66].Imes CC, Burke LE, The Obesity Epidemic: The United States as a Cautionary Tale for the Rest of the World, Curr Epidemiol Rep, 1 (2014) 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pontiroli AE, Ceriani V, Sarro G, Micheletto G, Giovanelli A, Zakaria AS, Fanchini M, Osio C, Nosari I, Veronelli AM, Folli F, L.w. group, Incidence of Diabetes Mellitus, Cardiovascular Diseases, and Cancer in Patients Undergoing Malabsorptive Surgery (Biliopancreatic Diversion and Biliointestinal Bypass) vs Medical Treatment, Obes Surg, 29 (2019) 935–942. [DOI] [PubMed] [Google Scholar]
- [68].Mathe G, Obesity not only holds cardiovascular diseases, it also hold tumors, Biomed Pharmacother, 54 (2000) 67–68. [DOI] [PubMed] [Google Scholar]
- [69].Liu L, Jin R, Hao J, Zeng J, Yin D, Yi Y, Zhu M, Mandal A, Hua Y, Ng CK, Egilmez NK, Sauter ER, Li B, Consumption of the Fish Oil High-Fat Diet Uncouples Obesity and Mammary Tumor Growth through Induction of Reactive Oxygen Species in Protumor Macrophages, Cancer Res, 80 (2020) 2564–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hao J, Zhang Y, Yan X, Yan F, Sun Y, Zeng J, Waigel S, Yin Y, Fraig MM, Egilmez NK, Suttles J, Kong M, Liu S, Cleary MP, Sauter E, Li B, Circulating Adipose Fatty Acid Binding Protein Is a New Link Underlying Obesity-Associated Breast/Mammary Tumor Development, Cell Metab, 28 (2018) 689–705 e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zeng J, Sauter ER, Li B, FABP4: A New Player in Obesity-Associated Breast Cancer, Trends Mol Med, 26 (2020) 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Moore SM, Holt VV, Malpass LR, Hines IN, Wheeler MD, Fatty acid-binding protein 5 limits the anti-inflammatory response in murine macrophages, Mol Immunol, 67 (2015) 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Szanto A, Balint BL, Nagy ZS, Barta E, Balazs D, Pap A, Szeles L, Poliska S, Oros M, Evans RM, Barak Y, Schwabe J, Nagy L, STAT6 Transcription Factor Is a Facilitator of the Nuclear Receptor PPAR gamma-Regulated Gene Expression in Macrophages and Dendritic Cells, Immunity, 33 (2010) 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Daniel B, Nagy G, Horvath A, Czimmerer Z, Cuaranta-Monroy I, Poliska S, Hays TT, Sauer S, Francois-Deleuze J, Nagy L, The IL-4/STAT6/PPAR gamma signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages, Nucleic Acids Res, 46 (2018) 4425–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Daniel B, Nagy G, Czimmerer Z, Horvath A, Hammers DW, Cuaranta-Monroy I, Poliska S, Tzerpos P, Kolostyak Z, Hays TT, Patsalos A, Houtman R, Sauer S, Francois-Deleuze J, Rastinejad F, Balint BL, Sweeney HL, Nagy L, The Nuclear Receptor PPAR gamma Controls Progressive Macrophage Polarization as a Ligand-Insensitive Epigenomic Ratchet of Transcriptional Memory, Immunity, 49 (2018) 615–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Graupera I, Coll M, Pose E, Elia C, Piano S, Sola E, Blaya D, Huelin P, Sole C, Moreira R, de Prada G, Fabrellas N, Juanola A, Morales-Ruiz M, Sancho-Bru P, Villanueva C, Gines P, Adipocyte Fatty-Acid Binding Protein is Overexpressed in Cirrhosis and Correlates with Clinical Outcomes, Sci Rep, 7 (2017) 1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z, Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19, Nat Med, 26 (2020) 842–844. [DOI] [PubMed] [Google Scholar]
- [78].Liang X, Gupta K, Quintero JR, Cernadas M, Kobzik L, Christou H, Pier GB, Owen CA, Cataltepe S, Macrophage FABP4 is required for neutrophil recruitment and bacterial clearance in Pseudomonas aeruginosa pneumonia, FASEB J, 33 (2019) 3562–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lee CW, Kim JE, Do H, Kim RO, Lee SG, Park HH, Chang JH, Yim JH, Park H, Kim IC, Lee JH, Structural basis for the ligand-binding specificity of fatty acid-binding proteins (pFABP4 and pFABP5) in gentoo penguin, Biochem Biophys Res Commun, 465 (2015) 12–18. [DOI] [PubMed] [Google Scholar]
- [80].Gillilan RE, Ayers SD, Noy N, Structural basis for activation of fatty acid-binding protein 4, J Mol Biol, 372 (2007) 1246–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM, Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma, Cell, 93 (1998) 229–240. [DOI] [PubMed] [Google Scholar]
- [82].Liu C, Tate T, Batourina E, Truschel ST, Potter S, Adam M, Xiang T, Picard M, Reiley M, Schneider K, Tamargo M, Lu C, Chen X, He J, Kim H, Mendelsohn CL, Pparg promotes differentiation and regulates mitochondrial gene expression in bladder epithelial cells, Nat Commun, 10 (2019) 4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM, PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL, Cell, 93 (1998) 241–252. [DOI] [PubMed] [Google Scholar]
- [84].Xu H, Hertzel AV, Steen KA, Wang Q, Suttles J, Bernlohr DA, Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2, Mol Cell Biol, 35 (2015) 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS, Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis, Nat Med, 15 (2009) 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhang Y, Hao J, Sun Y, Li B, Saturated Fatty Acids Induce Ceramide-associated Macrophage Cell Death, J Vis Exp, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Morgan E, Kannan-Thulasiraman P, Noy N, Involvement of Fatty Acid Binding Protein 5 and PPARbeta/delta in Prostate Cancer Cell Growth, PPAR Res, 2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kannan-Thulasiraman P, Seachrist DD, Mahabeleshwar GH, Jain MK, Noy N, Fatty acid-binding protein 5 and PPARbeta/delta are critical mediators of epidermal growth factor receptor-induced carcinoma cell growth, J Biol Chem, 285 (2010) 19106–19115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Schug TT, Berry DC, Shaw NS, Travis SN, Noy N, Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors, Cell, 129 (2007) 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Armstrong EH, Goswami D, Griffin PR, Noy N, Ortlund EA, Structural basis for ligand regulation of the fatty acid-binding protein 5, peroxisome proliferator-activated receptor beta/delta (FABP5-PPARbeta/delta) signaling pathway, J Biol Chem, 289 (2014) 14941–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Saitoh T, Satoh T, Yamamoto N, Uematsu S, Takeuchi O, Kawai T, Akira S, Antiviral protein Viperin promotes Toll-like receptor 7- and Toll-like receptor 9-mediated type I interferon production in plasmacytoid dendritic cells, Immunity, 34 (2011) 352–363. [DOI] [PubMed] [Google Scholar]
- [92].Jiang X, Chen ZJ, Viperin links lipid bodies to immune defense, Immunity, 34 (2011) 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhang Y, Li Q, Rao E, Sun Y, Grossmann ME, Morris RJ, Cleary MP, Li B, Epidermal Fatty Acid binding protein promotes skin inflammation induced by high-fat diet, Immunity, 42 (2015) 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Karasawa T, Kawashima A, Usui-Kawanishi F, Watanabe S, Kimura H, Kamata R, Shirasuna K, Koyama Y, Sato-Tomita A, Matsuzaka T, Tomoda H, Park SY, Shibayama N, Shimano H, Kasahara T, Takahashi M, Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages, Arterioscler Thromb Vasc Biol, 38 (2018) 744–756. [DOI] [PubMed] [Google Scholar]
- [95].Chmurzynska A, The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism, J Appl Genet, 47 (2006) 39–48. [DOI] [PubMed] [Google Scholar]
- [96].Smathers RL, Petersen DR, The human fatty acid-binding protein family: evolutionary divergences and functions, Hum Genomics, 5 (2011) 170–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chattopadhyay A, Kwartler CS, Kaw K, Li Y, Kaw A, Chen J, LeMaire SA, Shen YH, Milewicz DM, Cholesterol-Induced Phenotypic Modulation of Smooth Muscle Cells to Macrophage/Fibroblast-like Cells Is Driven by an Unfolded Protein Response, Arterioscler Thromb Vasc Biol, 41 (2021) 302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Szpigel A, Hainault I, Carlier A, Venteclef N, Batto AF, Hajduch E, Bernard C, Ktorza A, Gautier JF, Ferre P, Bourron O, Foufelle F, Lipid environment induces ER stress, TXNIP expression and inflammation in immune cells of individuals with type 2 diabetes, Diabetologia, 61 (2018) 399–412. [DOI] [PubMed] [Google Scholar]
- [99].Han J, Kaufman RJ, The role of ER stress in lipid metabolism and lipotoxicity, J Lipid Res, 57 (2016) 1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, Wang Q, Yang M, Qian J, Yi Q, CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability, Cell Metab, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J, Zhang A, Gupte AA, Hamilton DJ, Zheng C, Yi Q, Cholesterol Induces CD8(+) T Cell Exhaustion in the Tumor Microenvironment, Cell Metab, 30 (2019) 143–156 e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker RA, Hotamisligil GS, Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes, Cell Metab, 1 (2005) 107–119. [DOI] [PubMed] [Google Scholar]
- [103].Hotamisligil GS, Bernlohr DA, Metabolic functions of FABPs--mechanisms and therapeutic implications, Nat Rev Endocrinol, 11 (2015) 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Charles KN, Li MD, Engin F, Arruda AP, Inouye K, Hotamisligil GS, Uncoupling of Metabolic Health from Longevity through Genetic Alteration of Adipose Tissue Lipid-Binding Proteins, Cell Rep, 21 (2017) 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]


