Abstract
Receptors to glutamate of the AMPA type (AMPARs) serve as the major gates of excitation in the human brain, where they participate in fundamental processes underlying perception, cognition and movement. Due to their central role in brain function, dysregulation of these receptors has been implicated in neuropathological states associated with a large variety of diseases that manifest with abnormal behaviors. The participation of functional abnormalities of AMPARs in brain disorders is strongly supported by genomic, transcriptomic and proteomic studies. Most of these studies have focused on the expression and function of the subunits that make up the channel and define AMPARs (GRIA1-GRIA4), as well of some accessory proteins. However, it is increasingly evident that native AMPARs are composed of a complex array of accessory proteins that regulate their trafficking, localization, kinetics and pharmacology, and a better understanding of the diversity and regional expression of these accessory proteins is largely needed. In this review we will provide an update on the state of current knowledge of AMPA receptors subunits in the context of their accessory proteins at the transcriptome level. We also summarize the regional expression in the human brain and its correlation with the channel forming subunits. Finally, we discuss some of the current limitations of transcriptomic analysis and propose potential ways to overcome them.
Keywords: Accessory proteins, Glutamate receptors, AMPA, gene expression, transcriptomics
1. Introduction
Glutamate receptors of the AMPA type (AMPARs) are the principal drivers of fast excitatory transmission in the brain, and in concert with receptors to GABA, the principal inhibitory neurotransmitter, establish the synaptic Excitation to Inhibition balance which is fundamental for proper brain function [19, 88]. Therefore, disturbances in AMPARs have been linked to numerous psychiatric, neurodevelopmental, and neurodegenerative disorders including epilepsy, schizophrenia, autism, and Alzheimer’s disease [8, 24, 26, 78, 87]. The involvement of AMPARs in disease has driven strong research efforts that have greatly advanced our understanding of these receptors; yet there is only one FDA-approved drug whose mechanism of action is through the modulation of AMPARs. Perampanel is an AMPAR antagonist indicated as an adjuvant drug for refractory epilepsy [25] and its success indicates that modulation of AMPARs is a viable and effective strategy for correcting abnormal neuronal activity. However, perampanel effects on the ubiquitously expressed AMPARs seem to be responsible for some of the adverse effects, like gait disturbance and aggressive behavior, observed in clinical practice [124]. These observations have prompted the search for modulators of AMPARs with a more regionalized effect. The discovery of LY3130481 [51] or JNJ-55511118 [68] is a step in that direction. These drugs are anticonvulsants with potent antagonist activity on AMPAR complexes containing the auxiliary subunit TARP-γ8 (transmembrane AMPAR regulatory protein gamma 8, gene name: CACNG8). Because TARP-γ8 is largely expressed in the forebrain, especially in the hippocampus, but not in the cerebellum, it could have therapeutic effects in diseases characterized by hippocampal hyperexcitability without the adverse effects associated with the cerebellum such as motor control [51, 68, 129]. Proteomic studies have identified a plethora of auxiliary subunits of AMPARs which are anatomically isolated in discrete brain regions and have modulatory roles on the physiology of AMPARs [94]. Further, functional studies have confirmed that changes in TARP stoichiometry, particularly in the hippocampus, affect AMPAR gating and kainate efficacy [75, 102]. It is here, where a large gap of knowledge in the regional expression of AMPARs in humans under even healthy conditions, is present and where transcriptomics provides a golden opportunity to start filling this gap.
2. Transcriptomic diversity of AMPA receptor complexes
AMPA receptors are heteromeric dimers-of-dimers that draw from a pool of four subunits (GluA1-GluA4) encoded by four genes (GRIA1-4) [66, 110, 126]. The exact composition of these pore-forming subunits appears to be input-specific [30, 34, 35, 41, 44, 89, 112], cell-specific [34, 35], and activity-dependent [61, 62]. A larger functional heterogeneity arises from diverse heteromeric assemblies, alternative splicing [105], RNA editing [119] and post-translational modifications [67]. For instance, the presence of the GluA2 subunit participates in the calcium ion permeability of the receptor as receptors lacking this subunit exhibit increased calcium permeability and are sensitive to voltage-dependent polyamine modulation [7, 27, 40, 114]. Recoding of arginine to glycine (R/G) or glutamine to arginine (Q/R) in three of the four AMPAR pore forming subunits provide an additional point of regulation for calcium permeability and the kinetics of the receptor responses [65].
AMPARs also rely on a host of auxiliary proteins that interact directly or indirectly with the pore-forming subunits [10, 18]. The accessory proteins can be split into those composing a defined core together with GluA1-GluA4 subunits, and those located in the periphery of the core and assemble through direct binding or temporal interactions (Table 1). Accessory proteins in the core are thought to act as a bridge for the effects of the peripheral proteins on the pore forming subunits [94]. This core can then be further subdivided into inner core proteins that occupy two pairs of distinct binding sites in the complex and outer core components that bind to other interaction sites. These accessory proteins regulate assembly, trafficking, synaptic localization, anchoring, and receptor kinetics and pharmacology [1, 6, 17, 18, 42, 49]. The importance of studying AMPARs with their accessory proteins is clearly highlighted by the work of Menuz and colleagues [74]. CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) is widely used as an antagonist of AMPARs [38]; indeed, in heterologous systems CNQX blocks the effect of agonists by competing for the same site [115]. However, when members of the TARP family are present in the AMPAR complex, CNQX acts instead as a partial agonist and will induce a fraction of the activation of the receptor [74]. Since most native AMPARs in the brain contain at least one member of the TARP family CNQX most likely acts as a partial agonist of AMPARs in vivo [74]. The direct activation of AMPAR in complex with TARP proteins by CNQX would explain why CNQX leads to an increased spiking activity of interneurons in the hippocampus [4, 32, 73], and neurons in the thalamic reticular nucleus [56] and locus coeruleus [86].
Table 1.
Accessory proteins of AMPA receptors
| TYPE | COMPLEX | REGULATES | GENE NAME | REFERENCES |
|---|---|---|---|---|
| CORE | Inner Core | Pharmacology, kinetics, trafficking | CACNG2, CACNG3, CACNG4, CACNG8 | [12, 48, 50, 52, 106, 111] |
| Pharmacology, kinetics | CACNG5, CACNG7 | |||
| Surface expression & gating properties | CNIH2, CNIH3 | [95] | ||
| Trafficking & channel conductance | GSG1L | [94, 99] | ||
| Outer Core | Complex assembly | FRRS1L, NRN1 | [9, 31, 94] | |
| Trafficking & stabilization | PRRT1, PRRT2 | [59, 94, 113] | ||
| Receptor kinetics | SHISA9 | [22] | ||
| PERIPHERY | Periphery | Uncertain | ABHD12, LRRTM4, MPP2, OLFM2, OLMF3, SACM1L, VWC2, VWC2L | [94] |
| Lateral mobility | OLFM1 | [83] | ||
| Complex assembly | CPT1C, ATAD1, PORCN | [125] | ||
| Regulates receptor activity | SHISA7, CAMK2A, CAMK2B, CAMK2D, CAMK2G | [23, 92] | ||
| Signaling | GNAS | [43] | ||
| Surface expression | ABHD6, MYO5B, MYO5A, NSF, SYT1, SYT7 | [2, 3, 15, 79, 82, 117, 118, 120, 122] | ||
| Homeostasis and Synaptic plasticity | GOPC | [16] | ||
| Synaptic plasticity | NRP2, PLXNA3, AKAP5, MAGI2, AAK1, AP2A1, AP2A2, ACTN1, STX4, NAPB, CDH2, PICK1, RAB5A, RAP2B | [3, 5, 17, 47, 53, 57, 58, 72, 90, 109, 116, 128] | ||
| Trafficking and clustering | KIF1A, KIF5B, PPFIA1, GRIPAP1, DLG1, DLG2, DLG3, DLG4, EPB41, EXOC4, PLXNA4, IQSEC1, STX12, SHISA6, GRIP1, GRIP2, AP4B1, AP4E1, AP4M1, AP4S1, SYNDIG1, SEMA3A | [3, 13, 21, 28, 39, 45, 55, 60, 69, 71, 77, 98, 101, 103, 116, 121, 123] |
Based on these complexities, studying AMPA receptors by looking at individual subunits separately or in the absence of their accessory proteins provides limited access to the potential role of AMPARs in disease.
3. Regional expression of AMPA receptors and its auxiliary subunits
The AMPAR knowledge base is larger for the hippocampus than any other brain region. For example, a PubMed search with the terms “AMPA receptors x”, where x is hippocampus, cortex, cerebellum, or thalamus, provides a total of 4594, 3071, 980 and 320 articles, respectively, published between the years 1983 and 2021, indicating that our knowledge of the particular stoichiometry of receptors in this region is more abundant than other equally important regions. The efforts of the Allen Institute have provided the field with a mine of information about the gene expression across the whole human brain [33, 108] facilitating the 30,000 ft view of gene expression and additional patterns not observed by studying only few brain regions. This data has shown that the gene expression of the pore-forming subunits GRIA1-4 in the brain is highly stereotypical across healthy individuals [100], following a pattern of brain region specificity according to their embryonic origin [54]. Indeed, gene expression separation by unsupervised clustering analysis suggests that GRIA1-4 are strong gene markers of synaptic components found in bioinformatic analysis [46, 54]. Specifically, the GRIA1 subunit is highly expressed in the hippocampus and amygdala and is highly conserved across species [29]. The cerebellum exhibits the highest levels of GRIA4 expression while the hippocampus exhibits the lowest [100].
GRIA2 is the most expressed subunit in the brain, which is a logical consequence of it forming part of the three major populations of AMPARs complexes in the brain (e.g. GluA1/2, GluA2/3 and GluA1/2/3) [127]. Using subunit specific antibodies in combination with cryo-electron microscopy (Cryo-EM), Zhao and colleagues were able to map the subunit composition and spatial arrangement of the GluA2 containing native AMPARs, as well as the relative abundance of the distinct native AMPARs compositions. They described 10 complexes, representative of native AMPARs across the whole brain, noting that the spatial arrangement of native AMPARs in specific brain regions is still missing and will require further studies [127]. Importantly, the structure of the most abundant AMPAR in the brain, GluA2/3, differs between the native complex analyzed by cryo-EM and the one analyzed in recombinant studies [36], thus highlighting once again the importance in including accessory proteins when studying the receptor complexes.
The recent development of the first positron emission tomography (PET) tracer to visualize AMPARs is a new tool to validate or reevaluate previous results from transcriptomics analysis. A derivative of 4-[2-(phenylsulfonylamino)ethylthio]-2,6-difluoro-phenoxyacetamide radiolabeled with 11C ([11C]K-2) recently showed specific binding to AMPARs [76]. This advancement has allowed for the first time the visualization of these receptors in live humans. Interestingly, [11C]K-2 binding to hippocampus and amygdala was lower than the cortex, which in turn was also lower than cerebellum [76], suggesting that the hippocampus has less AMPARs compared to other brain regions. At first glance, this contrasts with transcriptomic analysis that found the highest mRNA levels for AMPARs in the hippocampus [100]. However, [11C]K-2 has a higher affinity for GluA2 and GluA4 and only modest affinity to GluA1 and GluA3 subunits. Therefore, the pharmacological profile of [11C]K-2 do reflects the transcriptional levels of these subunits in the brain [100], with little binding of AMPARs in the hippocampus due to high GRIA1 expression and robust binding in the cerebellum due to high GRIA4 expression. Although the relationship between mRNA and proteins is gene specific and not necessarily linear [20], recent analysis of GABAARs in the human brain [97] found strong correlations between mRNA levels and benzodiazepine binding measured in PET studies of cognitively normal controls [80], indicating that transcriptomic analysis across the brain correlates with PET imaging studies in at least some instances. Further analyses also have shown that the abundance and relationships between transcripts for families of receptors can be very informative of the major receptor complexes previously unknown in specific cell types. For example, by using the fractional contribution of mRNA for GABAARs in oligodendrocyte precursor cells, which represents the available pool of GABAAR subunits mRNA, the stoichiometry of the most expressed GABAAR receptor in these cells can be deduced and functionally validated by electrophysiological and pharmacological studies [81]. Therefore, it is expected that the pharmacological and binding profile of [11C]K-2 will be an important tool in research and clinical practice involving AMPA receptors in alive people. Indeed, recent [11C]K-2 studies confirmed an increased number of AMPARs in mesial epilepsy which was initially suggested by early measurements of mRNA levels by in situ hybridization studies in surgically collected and autopsy tissue from patients with intractable temporal lobe seizures [70].
The regional expression of accessory proteins is well known in some areas like the hippocampus and cerebellum, but to the extent of our knowledge the regional expression across the whole brain has not yet been explored. Here, we analyzed the gene expression of AMPAR accessory proteins across 87 brain regions (Figure 1). It is clear that accessory proteins also follow a regional expression which overlaps to some extent with the differential expression of the GRIA1-4 subunits, although some show a divergent pattern. This regional expression pattern is further demonstrated using a covariance analysis which also shows that these divisions into major brain regions can be driven by specific genes (Figure 2). For instance, MPP3, EPB41 and LGALS12 completely separate the cerebellum from all other brain regions. Similarly, a combination of accessory proteins like CACNG4 and SHISA6 drives the separation of subcortical regions deriving from the diencephalon. Thus, the inclusion of accessory protein expression provides a more comprehensive method of defining brain regions than relying solely on the GRIA1-4 subunits.
Figure 1. Whole brain gene expression of AMPA receptor subunits and their accessory subunits.
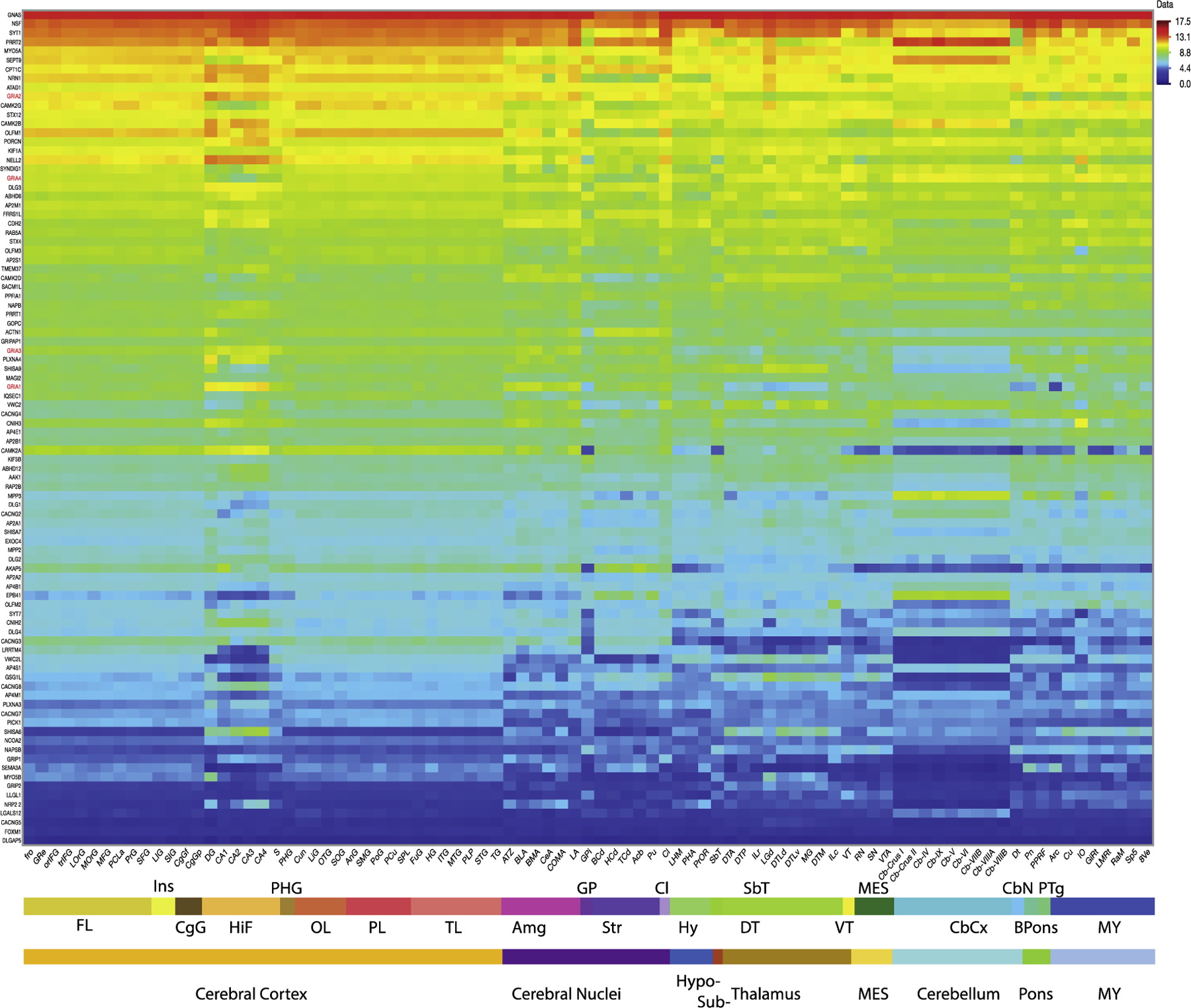
Heat plot shows the average Log2 mRNA gene expression (n= 6 subjects) downloaded from the Allen Human Brain Atlas (https://human.brain-map.org), for the most representative probes for each gene selected by factorial analysis as previously reported (see Supplementary Table 1 for additional information about the selected probes) [96, 100]. GRIA1-4 are shown in red. Labels for brain substructures are colored as per the insets. FL frontal lobe, Ins insula, CgG cingulate gyrus, HiF hippocampal formation, PHG parahippocampal gyrus, OL occipital lobe, PL parietal lobe, TL temporal lobe, Amg amygdala, GP globus pallidus, Str striatum, Cl claustrum, Hy hypothalamus, SbT subthalamus, DT dorsal thalamus, VT ventral thalamus, MES mesencephalon, CbCx cerebellar cortex, CbN cerebellar nuclei, Bpons basal part of the pons, PTg pontine tegmentum, MY myelencephalon. For substructure abbreviations, please see Supplementary Table 2
Figure 2. Region-specific gene expression of AMPA receptor subunits and their accessory subunits in the human brain.

Principal component analysis on covariances using JMP v.14 shows the separation of brain regions based on the expression of AMPARs complexes. Major brain regions are coded as per the insert. Each individual point corresponds to a single structure using data shown in Figure 1.
Past proteomics experiments in rats have established region-specific expression among the auxiliary proteins [93]. While the expression of AMPAR subunits and inner core genes aligned closely with the proteomics data, the expression of many outer core and peripheral genes did not (Figure 1). For instance, CACNG2 and CACNG4 exhibited the highest expression among the TARPs, corresponding to the higher protein expression while CACNG5 had the lowest transcript and protein expression. On the other hand, the cornichons exhibited flipped expression patterns, with CNIH2 exhibiting higher expression than CNIH3, although they retained their regional patterns of high expression in the hippocampus. Similarly, PRRT2 exhibited lower levels of expression than PRRT1, which had the highest expression of all genes analyzed, despite PRRT2 protein levels being higher. Among the peripheral genes, many exhibited altered regional patterns, particularly in the hippocampus and cerebellum. These differences may be due to variation between rats and humans, possible effects of post-transcriptional or translational modifications, or regulatory mechanisms that affect complex assembly. Therefore, integration of proteomics data with transcriptomics from the same individual would be helpful for determining the causes for this difference.
4. Conclusion
Transcriptomics offers a high-throughput method of distilling complex protein aggregation into simple metrics that can be used to compare between different brain states.
However, like any other method, this approach has its limitations. For example, although surveying for splice variants is possible, the determination of splice variants across the whole brain has not been done yet. This is important as all four pore forming subunits have two potential isoforms termed ‘flip’ and ‘flop’ that exhibit different channel kinetics and trafficking [14, 65, 84, 85, 105] and the distribution of these isoforms for the GluR1-3 subunits has been demonstrated to vary between brain regions [11]. Further, deregulation of the editing mechanisms has been suggested to underlie neuropsychiatric disorders such as schizophrenia and bipolar disorder [104]. Therefore, future analysis of data that account for different isoforms and RNA editing from tools such as REDItools [63, 64] could prove useful.
An important, although still difficult, goal in the search to understand neurotransmitter receptors in health and disease is to have a single cell transcriptome Atlas for the distinct cellular types in the whole brain. Taking in consideration that 1) there are 75 transcriptomically distinct cell types just in the middle temporal gyrus [37], 2) the human cortex can be parcellated histologically in 48 Broadman areas (BA) from which the middle temporal gyrus is BA21 (areas 13–16 are not defined in humans)[107], 3) and the abundant subcortical nuclei with increased cellular diversity [91], the task may seem daunting. However, collaborative research and systematic approaches like those promoted by Allen Institute and other groups will likely make this possible.
Supplementary Material
Highlights.
Therapeutic challenges and opportunities of modulating AMPA receptors accessory proteins
Regional-specificity of AMPA receptor auxiliary subunit gene expression across the whole human bain.
Transcriptomic and in vivo imaging studies of AMPA receptors
Funding sources:
This work was supported by NIA/NIH grant R01AG070255 to AL, and Peterson Coutin fund to AL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Anggono V, Huganir RL, Regulation of AMPA Receptor Trafficking and Synaptic Plasticity, Current opinion in neurobiology 22 (2012) 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beretta F, Sala C, Saglietti L, Hirling H, Sheng M, Passafaro M, NSF interaction is important for direct insertion of GluR2 at synaptic sites, Molecular and Cellular Neurosciences 28 (2005) 650–660. [DOI] [PubMed] [Google Scholar]
- [3].Braithwaite SP, Xia H, Malenka RC, Differential roles for NSF and GRIP/ABP in AMPA receptor cycling, Proceedings of the National Academy of Sciences of the United States of America 99 (2002) 7096–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brickley SG, Farrant M, Swanson GT, Cull-Candy SG, CNQX increases GABA-mediated synaptic transmission in the cerebellum by an AMPA/kainate receptor-independent mechanism, Neuropharmacology 41 (2001) 730–736. [DOI] [PubMed] [Google Scholar]
- [5].Brown TC, Tran IC, Backos DS, Esteban JA, NMDA Receptor-Dependent Activation of the Small GTPase Rab5 Drives the Removal of Synaptic AMPA Receptors during Hippocampal LTD, Neuron 45 (2005) 81–94. [DOI] [PubMed] [Google Scholar]
- [6].Buonarati OR, Hammes EA, Watson JF, Greger IH, Hell JW, Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation, Science signaling 12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burnashev N, Monyer H, Seeburg PH, Sakmann B, Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit, Neuron 8 (1992) 189–198. [DOI] [PubMed] [Google Scholar]
- [8].Busche MA, Konnerth A, Impairments of neural circuit function in Alzheimer’s disease, Philos Trans R Soc Lond B Biol Sci 371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cantallops I, Haas K, Cline HT, Postsynaptic CPG15 promotes synaptic maturation and presynaptic axon arbor elaboration in vivo, Nature Neuroscience 3 (2000) 1004–1011. [DOI] [PubMed] [Google Scholar]
- [10].Carbone AL, Plested AJ, Superactivation of AMPA receptors by auxiliary proteins, Nat Commun 7 (2016) 10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carlson NG, Howard J, Gahring LC, Rogers SW, RNA editing (Q/R site) and flop/flip splicing of AMPA receptor transcripts in young and old brains, Neurobiol Aging 21 (2000) 599–606. [DOI] [PubMed] [Google Scholar]
- [12].Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA, Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms, Nature 408 (2000) 936–943. [DOI] [PubMed] [Google Scholar]
- [13].Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA, Reese TS, PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density, Proceedings of the National Academy of Sciences of the United States of America 112 (2015) E6983–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coleman SK, Moykkynen T, Cai C, von Ossowski L, Kuismanen E, Korpi ER, Keinanen K, Isoform-specific early trafficking of AMPA receptor flip and flop variants, J Neurosci 26 (2006) 11220–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Correia SS, Bassani S, Brown TC, Lisé M-F, Backos DS, El-Husseini A, Passafaro M, Esteban JA, Motor protein–dependent transport of AMPA receptors into spines during long-term potentiation, Nature Neuroscience 11 (2008) 457–466. [DOI] [PubMed] [Google Scholar]
- [16].Cuadra AE, Kuo S-H, Kawasaki Y, Bredt DS, Chetkovich DM, AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST, The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 24 (2004) 7491–7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Danielson E, Zhang N, Metallo J, Kaleka K, Shin SM, Gerges N, Lee SH, S-SCAM/MAGI-2 is an essential synaptic scaffolding molecule for the GluA2-containing maintenance pool of AMPA receptors, The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 32 (2012) 6967–6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Diaz E, Regulation of AMPA receptors by transmembrane accessory proteins, Eur J Neurosci 32 (2010) 261–268. [DOI] [PubMed] [Google Scholar]
- [19].Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC, Developmental sensory experience balances cortical excitation and inhibition, Nature 465 (2010) 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Edfors F, Danielsson F, Hallstrom BM, Kall L, Lundberg E, Ponten F, Forsstrom B, Uhlen M, Gene-specific correlation of RNA and protein levels in human cells and tissues, Mol Syst Biol 12 (2016) 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA, Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins, Neuron 52 (2006) 307–320. [DOI] [PubMed] [Google Scholar]
- [22].Engelhardt J.v., Mack V, Sprengel R, Kavenstock N, Li KW, Stern-Bach Y, Smit AB, Seeburg PH, Monyer H, CKAMP44: A Brain-Specific Protein Attenuating Short-Term Synaptic Plasticity in the Dentate Gyrus, Science 327 (2010) 1518–1522. [DOI] [PubMed] [Google Scholar]
- [23].Farrow P, Khodosevich K, Sapir Y, Schulmann A, Aslam M, Stern-Bach Y, Monyer H, von Engelhardt J, Auxiliary subunits of the CKAMP family differentially modulate AMPA receptor properties, eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, Krystal JH, Murray JD, Anticevic A, Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders, Biol Psychiatry 81 (2017) 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, Kumar D, Rogawski MA, Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304, Neurology 79 (2012) 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gao R, Penzes P, Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders, Curr Mol Med 15 (2015) 146–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H, Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS, Neuron 15 (1995) 193–204. [DOI] [PubMed] [Google Scholar]
- [28].Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA, Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane, The EMBO Journal 25 (2006) 1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gold SJ, Ambros-Ingerson J, Horowitz JR, Lynch G, Gall CM, Stoichiometries of AMPA receptor subunit mRNAs in rat brain fall into discrete categories, J Comp Neurol 385 (1997) 491–502. [DOI] [PubMed] [Google Scholar]
- [30].Gryder DS, Castaneda DC, Rogawski MA, Evidence for low GluR2 AMPA receptor subunit expression at synapses in the rat basolateral amygdala, Journal of Neurochemistry 94 (2005) 1728–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Han W, Wang H, Li J, Zhang S, Lu W, Ferric Chelate Reductase 1 Like Protein (FRRS1L) Associates with Dynein Vesicles and Regulates Glutamatergic Synaptic Transmission, Frontiers in Molecular Neuroscience 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hashimoto Y, Miyakawa H, Kudo Y, Inoue M, 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) increases GABAA receptor-mediated spontaneous postsynaptic currents in the dentate granule cells of rat hippocampal slices, Neurosci Lett 358 (2004) 33–36. [DOI] [PubMed] [Google Scholar]
- [33].Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, David Daly B, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SGN, Jones AR, An anatomically comprehensive atlas of the adult human brain transcriptome, Nature 489 (2012) 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].He Y, Hof PR, Janssen WG, Vissavajjhala P, Morrison JH, AMPA GluR2 subunit is differentially distributed on GABAergic neurons and pyramidal cells in the macaque monkey visual cortex, Brain Research 921 (2001) 60–67. [DOI] [PubMed] [Google Scholar]
- [35].He Y, Janssen WG, Vissavajjhala P, Morrison JH, Synaptic distribution of GluR2 in hippocampal GABAergic interneurons and pyramidal cells: a double-label immunogold analysis, Experimental Neurology 150 (1998) 1–13. [DOI] [PubMed] [Google Scholar]
- [36].Herguedas B, Garcia-Nafria J, Cais O, Fernandez-Leiro R, Krieger J, Ho H, Greger IH, Structure and organization of heteromeric AMPA-type glutamate receptors, Science 352 (2016) aad3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Johansen N, Penn O, Yao Z, Eggermont J, Hollt T, Levi BP, Shehata SI, Aevermann B, Beller A, Bertagnolli D, Brouner K, Casper T, Cobbs C, Dalley R, Dee N, Ding SL, Ellenbogen RG, Fong O, Garren E, Goldy J, Gwinn RP, Hirschstein D, Keene CD, Keshk M, Ko AL, Lathia K, Mahfouz A, Maltzer Z, McGraw M, Nguyen TN, Nyhus J, Ojemann JG, Oldre A, Parry S, Reynolds S, Rimorin C, Shapovalova NV, Somasundaram S, Szafer A, Thomsen ER, Tieu M, Quon G, Scheuermann RH, Yuste R, Sunkin SM, Lelieveldt B, Feng D, Ng L, Bernard A, Hawrylycz M, Phillips JW, Tasic B, Zeng H, Jones AR, Koch C, Lein ES, Conserved cell types with divergent features in human versus mouse cortex, Nature 573 (2019) 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Honore T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE, Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists, Science 241 (1988) 701–703. [DOI] [PubMed] [Google Scholar]
- [39].Hoogenraad CC, van der Sluijs P, GRASP-1 regulates endocytic receptor recycling and synaptic plasticity, Communicative & Integrative Biology 3 (2010) 433–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hume RI, Dingledine R, Heinemann SF, Identification of a site in glutamate receptor subunits that controls calcium permeability, Science 253 (1991) 1028–1031. [DOI] [PubMed] [Google Scholar]
- [41].Humeau Y, Reisel D, Johnson AW, Borchardt T, Jensen V, Gebhardt C, Bosch V, Gass P, Bannerman DM, Good MA, Hvalby Ø, Sprengel R, Lüthi A, A Pathway-Specific Function for Different AMPA Receptor Subunits in Amygdala Long-Term Potentiation and Fear Conditioning, Journal of Neuroscience 27 (2007) 10947–10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jacobi E, von Engelhardt J, AMPA receptor complex constituents: Control of receptor assembly, membrane trafficking and subcellular localization, Molecular and Cellular Neuroscience 91 (2018) 67–75. [DOI] [PubMed] [Google Scholar]
- [43].Joiner M.-l.A., Lisé M-F, Yuen EY, Kam AYF, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD, Burette AC, Weinberg RJ, Law P-Y, El-Husseini A, Yan Z, Hell JW, Assembly of a β2-adrenergic receptor—GluR1 signalling complex for localized cAMP signalling, The EMBO Journal 29 (2010) 482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kakegawa W, Tsuzuki K, Yoshida Y, Kameyama K, Ozawa S, Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses, The European Journal of Neuroscience 20 (2004) 101–110. [DOI] [PubMed] [Google Scholar]
- [45].Kalashnikova E, Lorca RA, Kaur I, Barisone GA, Li B, Ishimaru T, Trimmer JS, Mohapatra DP, Díaz E, SynDIG1: An Activity-Regulated, AMPA-Receptor-Interacting Transmembrane Protein that Regulates Excitatory Synapse Development, Neuron 65 (2010) 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, Mane S, Hyde TM, Huttner A, Reimers M, Kleinman JE, Sestan N, Spatio-temporal transcriptome of the human brain, Nature 478 (2011) 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kastning K, Kukhtina V, Kittler JT, Chen G, Pechstein A, Enders S, Lee SH, Sheng M, Yan Z, Haucke V, Molecular determinants for the interaction between AMPA receptors and the clathrin adaptor complex AP-2, Proceedings of the National Academy of Sciences of the United States of America 104 (2007) 2991–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS, TARPs differentially decorate AMPA receptors to specify neuropharmacology, Trends in Neurosciences 33 (2010) 241–248. [DOI] [PubMed] [Google Scholar]
- [49].Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS, TARPs differentially decorate AMPA receptors to specify neuropharmacology, Trends Neurosci 33 (2010) 241–248. [DOI] [PubMed] [Google Scholar]
- [50].Kato AS, Siuda ER, Nisenbaum ES, Bredt DS, AMPA Receptor Subunit-Specific Regulation by a Distinct Family of Type II TARPs, Neuron 59 (2008) 986–996. [DOI] [PubMed] [Google Scholar]
- [51].Kato AS, Witkin JM, Auxiliary subunits of AMPA receptors: The discovery of a forebrain-selective antagonist, LY3130481/CERC-611, Biochem Pharmacol 147 (2018) 191–200. [DOI] [PubMed] [Google Scholar]
- [52].Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS, New Transmembrane AMPA Receptor Regulatory Protein Isoform, γ−7, Differentially Regulates AMPA Receptors, Journal of Neuroscience 27 (2007) 4969–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kennedy MJ, Davison IG, Robinson CG, Ehlers MD, Syntaxin-4 Defines a Domain for Activity-Dependent Exocytosis in Dendritic Spines, Cell 141 (2010) 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kirsch L, Chechik G, On Expression Patterns and Developmental Origin of Human Brain Regions, PLoS Comput Biol 12 (2016) e1005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Klaassen RV, Stroeder J, Coussen F, Hafner AS, Petersen JD, Renancio C, Schmitz LJ, Normand E, Lodder JC, Rotaru-Marcu DC, Rao-Ruiz P, Spijker S, Mansvelder HD, Choquet D, Smit AB, Shisa6 traps AMPA receptors at postsynaptic sites and prevents their desensitization during synaptic activity., Nature Communications 7 (2016) 10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee SH, Govindaiah G, Cox CL, Selective excitatory actions of DNQX and CNQX in rat thalamic neurons, J Neurophysiol 103 (2010) 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lee SH, Liu L, Wang YT, Sheng M, Clathrin Adaptor AP2 and NSF Interact with Overlapping Sites of GluR2 and Play Distinct Roles in AMPA Receptor Trafficking and Hippocampal LTD, Neuron 36 (2002) 661–674. [DOI] [PubMed] [Google Scholar]
- [58].Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW, SAP97 Is Associated with the α-Amino-3-hydroxy-5-methylisoxazole-4-propionic Acid Receptor GluR1 Subunit*, Journal of Biological Chemistry 273 (1998) 19518–19524. [DOI] [PubMed] [Google Scholar]
- [59].Li M, Niu F, Zhu X, Wu X, Shen N, Peng X, Liu Y, PRRT2 Mutant Leads to Dysfunction of Glutamate Signaling, International Journal of Molecular Sciences 16 (2015) 9134–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL, Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation, Nature neuroscience 12 (2009) 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liu S-QJ, Cull-Candy SG, Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype, Nature 405 (2000) 454–458. [DOI] [PubMed] [Google Scholar]
- [62].Liu SJ, Cull-Candy SG, Activity-Dependent Change in AMPA Receptor Properties in Cerebellar Stellate Cells, The Journal of Neuroscience 22 (2002) 3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lo Giudice C, Silvestris DA, Roth SH, Eisenberg E, Pesole G, Gallo A, Picardi E, Quantifying RNA Editing in Deep Transcriptome Datasets, Front Genet 11 (2020) 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lo Giudice C, Tangaro MA, Pesole G, Picardi E, Investigating RNA editing in deep transcriptome datasets with REDItools and REDIportal, Nat Protoc 15 (2020) 1098–1131. [DOI] [PubMed] [Google Scholar]
- [65].Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH, Control of kinetic properties of AMPA receptor channels by nuclear RNA editing, Science 266 (1994) 1709–1713. [DOI] [PubMed] [Google Scholar]
- [66].Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA, Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach, Neuron 62 (2009) 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lussier MP, Sanz-Clemente A, Roche KW, Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications, J Biol Chem 290 (2015) 28596–28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Maher MP, Wu N, Ravula S, Ameriks MK, Savall BM, Liu C, Lord B, Wyatt RM, Matta JA, Dugovic C, Yun S, Ver Donck L, Steckler T, Wickenden AD, Carruthers NI, Lovenberg TW, Discovery and Characterization of AMPA Receptor Modulators Selective for TARP-gamma8, J Pharmacol Exp Ther 357 (2016) 394–414. [DOI] [PubMed] [Google Scholar]
- [69].Mao L, Takamiya K, Thomas G, Lin D-T, Huganir RL, GRIP1 and 2 regulate activity-dependent AMPA receptor recycling via exocyst complex interactions, Proceedings of the National Academy of Sciences 107 (2010) 19038–19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mathern GW, Pretorius JK, Kornblum HI, Mendoza D, Lozada A, Leite JP, Chimelli LM, Fried I, Sakamoto AC, Assirati JA, Levesque MF, Adelson PD, Peacock WJ, Human hippocampal AMPA and NMDA mRNA levels in temporal lobe epilepsy patients, Brain 120 (Pt 11) (1997) 1937–1959. [DOI] [PubMed] [Google Scholar]
- [71].Matsuda S, Miura E, Matsuda K, Kakegawa W, Kohda K, Watanabe M, Yuzaki M, Accumulation of AMPA Receptors in Autophagosomes in Neuronal Axons Lacking Adaptor Protein AP-4, Neuron 57 (2008) 730–745. [DOI] [PubMed] [Google Scholar]
- [72].Matt L, Kim K, Hergarden AC, Patriarchi T, Malik ZA, Park DK, Chowdhury D, Buonarati OR, Henderson PB, Saraç ÇG, Zhang Y, Mohapatra D, Horne MC, Ames JB, Hell JW, α-Actinin Anchors PSD-95 at Postsynaptic Sites, Neuron 97 (2018) 1094–1109.e1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].McBain CJ, Eaton JV, Brown T, Dingledine R, CNQX increases spontaneous inhibitory input to CA3 pyramidal neurones in neonatal rat hippocampal slices, Brain Res 592 (1992) 255–260. [DOI] [PubMed] [Google Scholar]
- [74].Menuz K, Stroud RM, Nicoll RA, Hays FA, TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists, Science 318 (2007) 815–817. [DOI] [PubMed] [Google Scholar]
- [75].Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA, TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating, Neuron 55 (2007) 905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Miyazaki T, Nakajima W, Hatano M, Shibata Y, Kuroki Y, Arisawa T, Serizawa A, Sano A, Kogami S, Yamanoue T, Kimura K, Hirata Y, Takada Y, Ishiwata Y, Sonoda M, Tokunaga M, Seki C, Nagai Y, Minamimoto T, Kawamura K, Zhang MR, Ikegaya N, Iwasaki M, Kunii N, Kimura Y, Yamashita F, Taguri M, Tani H, Nagai N, Koizumi T, Nakajima S, Mimura M, Yuzaki M, Kato H, Higuchi M, Uchida H, Takahashi T, Visualization of AMPA receptors in living human brain with positron emission tomography, Nat Med 26 (2020) 281–288. [DOI] [PubMed] [Google Scholar]
- [77].N Y, H U, F N, S C, Y S, T H, F S, M T, K T, Y G, Plexin-A4-dependent retrograde semaphorin 3A signalling regulates the dendritic localization of GluA2-containing AMPA receptors., Nature Communications 5 (2014) 3424–3424. [DOI] [PubMed] [Google Scholar]
- [78].Nelson SB, Valakh V, Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders, Neuron 87 (2015) 684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nishimune A, Isaac JTR, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM, NSF Binding to GluR2 Regulates Synaptic Transmission, Neuron 21 (1998) 87–97. [DOI] [PubMed] [Google Scholar]
- [80].Norgaard M, Beliveau V, Ganz M, Svarer C, Pinborg LH, Keller SH, Jensen PS, Greve DN, Knudsen GM, A high-resolution in vivo atlas of the human brain’s benzodiazepine binding site of GABAA receptors, Neuroimage 232 (2021) 117878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ordaz RP, Garay E, Limon A, Perez-Samartin A, Sanchez-Gomez MV, Robles-Martinez L, Cisneros-Mejorado A, Matute C, Arellano RO, GABAA Receptors Expressed in Oligodendrocytes Cultured from the Neonatal Rat Contain alpha3 and gamma1 Subunits and Present Differential Functional and Pharmacological Properties, Mol Pharmacol 99 (2021) 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB, The AMPA Receptor GluR2 C Terminus Can Mediate a Reversible, ATP-Dependent Interaction with NSF and α- and β-SNAPs, Neuron 21 (1998) 99–110. [DOI] [PubMed] [Google Scholar]
- [83].Pandya NJ, Seeger C, Babai N, Gonzalez-Lozano MA, Mack V, Lodder JC, Gouwenberg Y, Mansvelder HD, Danielson UH, Li KW, Heine M, Spijker S, Frischknecht R, Smit AB, Noelin1 Affects Lateral Mobility of Synaptic AMPA Receptors, Cell Reports 24 (2018) 1218–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pei W, Huang Z, Wang C, Han Y, Park JS, Niu L, Flip and flop: a molecular determinant for AMPA receptor channel opening, Biochemistry 48 (2009) 3767–3777. [DOI] [PubMed] [Google Scholar]
- [85].Pei W, Ritz M, McCarthy M, Huang Z, Niu L, Receptor occupancy and channel-opening kinetics: a study of GLUR1 L497Y AMPA receptor, J Biol Chem 282 (2007) 22731–22736. [DOI] [PubMed] [Google Scholar]
- [86].Rawal B, Rancic V, Ballanyi K, TARP mediation of accelerated and more regular locus coeruleus network bursting in neonatal rat brain slices, Neuropharmacology 148 (2019) 169–177. [DOI] [PubMed] [Google Scholar]
- [87].Ren SQ, Yao W, Yan JZ, Jin C, Yin JJ, Yuan J, Yu S, Cheng Z, Amyloid beta causes excitation/inhibition imbalance through dopamine receptor 1-dependent disruption of fast-spiking GABAergic input in anterior cingulate cortex, Sci Rep 8 (2018) 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rubin R, Abbott LF, Sompolinsky H, Balanced excitation and inhibition are required for high-capacity, noise-robust neuronal selectivity, Proc Natl Acad Sci U S A 114 (2017) E9366–E9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rubio ME, Wenthold RJ, Glutamate Receptors Are Selectively Targeted to Postsynaptic Sites in Neurons, Neuron 18 (1997) 939–950. [DOI] [PubMed] [Google Scholar]
- [90].Saglietti L, Dequidt C, Kamieniarz K, Rousset M-C, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, Sheng M, Passafaro M, Extracellular Interactions between GluR2 and N-Cadherin in Spine Regulation, Neuron 54 (2007) 461–477. [DOI] [PubMed] [Google Scholar]
- [91].Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D, McCarroll SA, Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain, Cell 174 (2018) 1015–1030 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schmitz LJM, Klaassen RV, Ruiperez-Alonso M, Zamri AE, Stroeder J, Rao-Ruiz P, Lodder JC, van der Loo RJ, Mansvelder HD, Smit AB, Spijker S, The AMPA receptor-associated protein Shisa7 regulates hippocampal synaptic function and contextual memory, eLife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schwenk J, Baehrens D, Haupt A, Bildl W, Boudkkazi S, Roeper J, Fakler B, Schulte U, Regional diversity and developmental dynamics of the AMPA-receptor proteome in the mammalian brain, Neuron 84 (2014) 41–54. [DOI] [PubMed] [Google Scholar]
- [94].Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Muller CS, Bildl W, Baehrens D, Huber B, Kulik A, Klocker N, Schulte U, Fakler B, High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes, Neuron 74 (2012) 621–633. [DOI] [PubMed] [Google Scholar]
- [95].Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, Klöcker N, Functional Proteomics Identify Cornichon Proteins as Auxiliary Subunits of AMPA Receptors, Science 323 (2009) 1313–1319. [DOI] [PubMed] [Google Scholar]
- [96].Sequeira A, Shen K, Gottlieb A, Limon A, Human brain transcriptome analysis finds region- and subject-specific expression signatures of GABAAR subunits, Commun Biol 2 (2019) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sequeira ASK, Gottlieb A, Limon A, Human brain transcriptome analysis finds region- and subject-specific expression signatures of GABAAR subunits, Communications Biology 2 (2019) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Setou M, Seog D-H, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N, Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites, Nature 417 (2002) 83–87. [DOI] [PubMed] [Google Scholar]
- [99].Shanks Natalie F., Savas Jeffrey N., Maruo T, Cais O, Hirao A, Oe S, Ghosh A, Noda Y, Greger Ingo H., Yates John R., Nakagawa T, Differences in AMPA and Kainate Receptor Interactomes Facilitate Identification of AMPA Receptor Auxiliary Subunit GSG1L, Cell Reports 1 (2012) 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shen K, Zeppillo T, Limon A, Regional transcriptome analysis of AMPA and GABAA receptor subunit expression generates E/I signatures of the human brain, Sci Rep 10 (2020) 11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Shen L, Liang F, Walensky LD, Huganir RL, Regulation of AMPA Receptor GluR1 Subunit Surface Expression by a 4.1N-Linked Actin Cytoskeletal Association, The Journal of Neuroscience 20 (2000) 7932–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Shi Y, Lu W, Milstein AD, Nicoll RA, The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type, Neuron 62 (2009) 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Shin H, Wyszynski M, Huh K-H, Valtschanoff JG, Lee J-R, Ko J, Streuli M, Weinberg RJ, Sheng M, Kim E, Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha, The Journal of Biological Chemistry 278 (2003) 11393–11401. [DOI] [PubMed] [Google Scholar]
- [104].Silberberg G, Lundin D, Navon R, Ohman M, Deregulation of the A-to-I RNA editing mechanism in psychiatric disorders, Hum Mol Genet 21 (2012) 311–321. [DOI] [PubMed] [Google Scholar]
- [105].Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH, Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS, Science 249 (1990) 1580–1585. [DOI] [PubMed] [Google Scholar]
- [106].Soto D, Coombs ID, Renzi M, Zonouzi M, Farrant M, Cull-Candy SG, Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, γ−5, Nature Neuroscience 12 (2009) 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Strotzer M, One century of brain mapping using Brodmann areas, Klin Neuroradiol 19 (2009) 179–186. [DOI] [PubMed] [Google Scholar]
- [108].Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, Hawrylycz M, Dang C, Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system, Nucleic Acids Res 41 (2013) D996–D1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD, Regulation of GluR1 by the A-Kinase Anchoring Protein 79 (AKAP79) Signaling Complex Shares Properties with Long-Term Depression, Journal of Neuroscience 22 (2002) 3044–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tichelaar W, Safferling M, Keinänen K, Stark H, Madden DR, The Three-dimensional Structure of an Ionotropic Glutamate Receptor Reveals a Dimer-of-dimers Assembly, Journal of Molecular Biology 344 (2004) 435–442. [DOI] [PubMed] [Google Scholar]
- [111].Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS, Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins, Journal of Cell Biology 161 (2003) 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tóth K, McBain CJ, Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons, Nature Neuroscience 1 (1998) 572–578. [DOI] [PubMed] [Google Scholar]
- [113].Troyano-Rodriguez E, Mann S, Ullah R, Ahmad M, PRRT1 regulates basal and plasticity-induced AMPA receptor trafficking, Molecular and Cellular Neuroscience 98 (2019) 155–163. [DOI] [PubMed] [Google Scholar]
- [114].Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B, Structural determinants of ion flow through recombinant glutamate receptor channels, Science 252 (1991) 1715–1718. [DOI] [PubMed] [Google Scholar]
- [115].Verdoorn TA, Kleckner NW, Dingledine R, N-methyl-D-aspartate/glycine and quisqualate/kainate receptors expressed in Xenopus oocytes: antagonist pharmacology, Mol Pharmacol 35 (1989) 360–368. [PubMed] [Google Scholar]
- [116].Wang Q, Chiu S-L, Koropouli E, Hong I, Mitchell S, Easwaran TP, Hamilton NR, Gustina AS, Zhu Q, Ginty DD, Huganir RL, Kolodkin AL, Neuropilin-2/PlexinA3 Receptors Associate with GluA1 and Mediate Sema3F-Dependent Homeostatic Scaling in Cortical Neurons, Neuron 96 (2017) 1084–1098.e1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang Z, Edwards JG, Riley N, Provance DW, Karcher R, Li X.-d., Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD, Myosin Vb Mobilizes Recycling Endosomes and AMPA Receptors for Postsynaptic Plasticity, Cell 135 (2008) 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wei M, Zhang J, Jia M, Yang C, Pan Y, Li S, Luo Y, Zheng J, Ji J, Chen J, Hu X, Xiong J, Shi Y, Zhang C, α/β-Hydrolase domain-containing 6 (ABHD6) negatively regulates the surface delivery and synaptic function of AMPA receptors, Proceedings of the National Academy of Sciences 113 (2016) E2695–E2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wright A, Vissel B, The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain, Front Mol Neurosci 5 (2012) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wu D, Bacaj T, Morishita W, Goswami D, Arendt KL, Xu W, Chen L, Malenka RC, Südhof TC, Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP, Nature 544 (2017) 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pagès C, Streuli M, Weinberg RJ, Sheng M, Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting, Neuron 34 (2002) 39–52. [DOI] [PubMed] [Google Scholar]
- [122].Xiong H, Cassé F, Zhou M, Xiong Z-Q, Joels M, Martin S, Krugers HJ, Interactions between N-Ethylmaleimide-sensitive factor and GluA2 contribute to effects of glucocorticoid hormones on AMPA receptor function in the rodent hippocampus, Hippocampus 26 (2016) 848–856. [DOI] [PubMed] [Google Scholar]
- [123].Ye B, Liao D, Zhang X, Zhang P, Dong H, Huganir RL, GRASP-1: A Neuronal RasGEF Associated with the AMPA Receptor/GRIP Complex, Neuron 26 (2000) 603–617. [DOI] [PubMed] [Google Scholar]
- [124].Youn SE, Kim SH, Ko A, Lee SH, Lee YM, Kang HC, Lee JS, Kim HD, Adverse Events During Perampanel Adjunctive Therapy in Intractable Epilepsy, J Clin Neurol 14 (2018) 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhang J, Wang Y, Chi Z, Keuss MJ, Pai Y-ME, Kang HC, Shin J, Bugayenko A, Wang H, Xiong Y, Pletnikov MV, Mattson MP, Dawson TM, Dawson VL, The AAA+ ATPase, Thorase Regulates AMPA Receptor-Dependent Synaptic Plasticity and Behavior, Cell 145 (2011) 284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhao Y, Chen S, Swensen AC, Qian W-J, Gouaux E, Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM, Science (New York, N.Y.) 364 (2019) 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhao Y, Chen S, Swensen AC, Qian WJ, Gouaux E, Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM, Science 364 (2019) 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, Velamoor V, Auberson YP, Osten P, van Aelst L, Sheng M, Zhu JJ, Rap2-JNK Removes Synaptic AMPA Receptors during Depotentiation, Neuron 46 (2005) 905–916. [DOI] [PubMed] [Google Scholar]
- [129].Zwart R, Sher E, Ping X, Jin X, Sims JR Jr., Chappell AS, Gleason SD, Hahn PJ, Gardinier K, Gernert DL, Hobbs J, Smith JL, Valli SN, Witkin JM, Perampanel, an antagonist of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, for the treatment of epilepsy: studies in human epileptic brain and nonepileptic brain and in rodent models, J Pharmacol Exp Ther 351 (2014) 124–133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


