Abstract
Purpose:
The ability of the fracture risk assessment tool (FRAX) in discriminating fracture and non-fracture in postmenopausal women remains suboptimal. Adding a genetic profile may improve the performance of FRAX.
Methods:
Three genetic risk scores (GRSs) (GRS_fracture, GRS_BMD, GRS_eBMD) were calculated for each participant in the Women’s Health Initiative Study (n=23,981), based on the summary statistics of three comprehensive osteoporosis-related genome-wide association studies (GWAS). The primary outcomes were incident major osteoporotic fracture (MOF) and hip fracture (HF). The association between each GRS and fracture risk were evaluated in separate Cox Proportional Hazard models, with FRAX clinical risk factors adjusted for. The discrimination ability of each model was assessed using Area Under the Curve (AUC). The predictive improvement attributable to each GRSs was assessed using the net reclassification improvement (NRI) and the integrated discrimination improvement (IDI).
Results:
GRS_BMD and GRS_eBMD were significantly associated with MOF and HF risk, independent of the base FRAX risk factors. Compare to the base FRAX model, the models with GRS_fracture, GRS_BMD, and GRS_eBMD improved the reclassification of MOF by 0.5 % (95% CI, 0.2% to 0.9%, p= p<.01), 0.3% (95% CI, 0.1% to 0.6%, p=0.01), and 2.1% (95% CI, 0.3% to 2.8%, p<.01), respectively. Similar results were also observed when using HF as an outcome.
Conclusion:
Our study suggested that the addition of genetic profiles provide limited improvements in the reclassification of FRAX for MOF and HF.
Keywords: genetic risk score (GRS), bone mineral density (BMD), single nucleotide polymorphism (SNP), Fracture risk assessment tool (FRAX)
Mini abstract:
Whether genetic profiling would improve the performance of the existing FRAX tool is unclear. This study found that osteoporosis-related genetic risk scores can improve the reclassification of MOF and HF in addition to the clinical risk factors currently included in FRAX.
Introduction
Osteoporosis is an age-related bone disease characterized by low bone mineral density (BMD) and increased fracture risk [1]. Due to the high post-fracture disability and mortality rate, as the most relevant consequence of osteoporosis, fracture has become a critical public health problem worldwide [2, 3]. Each year, osteoporosis causes more than 8.9 million fractures globally [4]. In the United States, approximately 10 million people are affected by osteoporosis, and nearly 50% of postmenopausal women will experience at least one osteoporosis-related fracture in their lifetime [5–7]. With longevity increasing globally, the potentially high cumulative rate of osteoporosis and fractures will lead to an inevitable increase in social and economic burdens [8, 9]. Therefore, early identification of the high-risk individuals who may benefit from osteoporosis preventive treatment becomes essential.
In the past two decades, several fracture predictive tools have been developed, such as the Fracture Risk Assessment Tool (FRAX) [10] and the Garvan fracture risk calculator [11, 12]. These models are differentiated mainly by input variables, output, and model construct, all of which lead to marked differences in the computed risk from each calculator. FRAX was the only one that was calibrated for different countries in which it was used; thus, FRAX has been one of the most widely employed fracture prediction tools in the United States. The method uses individual clinical risk factors, either with or without femoral neck BMD information, to compute the 10-year probability of major osteoporotic fracture (MOF) and hip fracture (HF). However, the performance of FRAX in discriminating between people who will or will not sustain a subsequent fracture is unsatisfactory [13–15]. A recent meta-analysis reported that the average AUC for total fracture by FRAX was merely 0.67 [16]. Among younger postmenopausal women, the discriminating ability of FRAX is no better than chance, with AUC around 0.56 [17, 18]. Moreover, previous studies have also demonstrated the low sensitivity of FRAX in fracture prediction. Data shows that FRAX missed 90% of individuals who sustained a MOF at screening [19]. Therefore, there is room for further improvement in fracture prediction.
Besides the established clinical risk factors, mounting evidence has suggested that bone deterioration and fracture susceptibility are genetically determined [20]. Many genome-wide association studies (GWASs) and genome-wide association meta-analyses (GWAMA) have successfully identified dozens of single nucleotide polymorphism (SNPs) associated with BMD and fracture [21–26]. In the most comprehensive GWAMA published in 2018, Medina-Gomez et al. reported genetic variants associated with total body BMD (TB-BMD) at 80 loci, explaining approximately 10% of the TB-BMD variance [25]. In 2018, Trajanoska and colleagues discovered 15 genetic determinants of fracture, all related to BMD [26]. Moreover, as a fracture-related trait independent of BMD, estimated BMD (eBMD) that be derived from heel quantitative ultrasound speed of sound (SOS) and the broadband ultrasound attenuation (BUA), has received increasing attention in recent years in the study of genetic influences on osteoporosis/fracture susceptibility. The most comprehensive GWAS, conducted with 426,824 individuals from UK Biobank (UKB), have successfully discovered 518 eBMD-related loci, which accumulatively explain 20% of the eBMD variance [23]. These genetic variants are common in the general population and have only small effects, corresponding to a limited fraction of eBMD variation. To summarize the collective impact of the identified genetic variants, a genetic risk score (GRS) was developed as a single estimation of an individual’s propensity to a phenotype.
Prior studies focusing on using GRS to improve fracture prediction accuracy yielded mixed results, and most of these GRSs were constructed based on Estrada’s research findings [21]. Thao et al. reported that genetic profiling of 63 BMD-related genetic variants could improve fracture prediction performance over and above that of clinical risk factors in the Garvan fracture risk calculator [27]. In contrast, Eriksson and colleagues revealed that GRS provided limited value in improving fracture prediction [28]. Besides, Forgetta et al. derived a polygenic risk score (PRS) for heel quantitative ultrasound speed of sound using the least absolute shrinkage and selection operator (LASSO) regression and tested the performance of this PRS in a screening strategy. Their findings suggest that the use of PRS in fracture risk screening could decrease the number of individuals requiring FRAX test screening [29]. However, this study’s purpose was not to predict fractures; whether an osteoporosis-related GRS could provide added value to the current FRAX has never been assessed before. Therefore, in the present study, we aimed to determine and compare the clinical usefulness of GRSs calculations based on the three most recent and comprehensive GWAS summary statistics for fracture-related traits [23, 25, 26] in improving fracture prediction beyond the existing FRAX.
Method
Data Source
The Women’s Health Initiative [30] is a nationwide, long-term health study that was conducted to address heart disease, breast and colorectal cancer, and fragility fractures in postmenopausal women. The WHI initial study enrolled 161,808 participants from 1993 through 1998. Recruitment occurred at 40 clinical centers nationwide, and women aged 50 to 79 years were grouped into one or more randomized Clinical Trials (CT) or to an Observational Study (OS). Participants were provided by mail or telephone with questionnaires annually in the observational study and semiannually in the clinical trials. The Institutional Review Board at each participating institution approved study protocols and consent forms.
Participants
In our study, WHI genotype and phenotype data were acquired through the database of Genotype and Phenotype (dbGap) (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000200.v12.p3) with the approval of the institutional review board at the University of Nevada, Las Vegas. Participants included in the present study were combined from four WHI sub-studies: WHI Genomics and Randomized Trials Network (GARNET); National Heart Lung and Blood Institute (NHLBI); Population Architecture using Genomics and Epidemiology (PAGE); and Women’s Health Initiative Memory Study (WHIMS). We excluded the participants who reported taking any medication known to influence osteoporosis, including bisphosphonates, calcitonin, parathyroid hormone, selective estrogen receptor modulators, luteinizing hormone-releasing hormone agents, and somatostatin agents. Also, subjects who had incomplete information regarding risk factors included in FRAX were also excluded from this study. In total, 23,981 eligible women from multiple racial backgrounds were included in the data analysis.
Assessment of Incident Fractures
The primary outcomes are major osteoporotic fractures (MOF) and hip fractures (HF). The WHI participant’s follow-up time was calculated from the baseline visit to the first fracture observed or the subject’s death. People who did not experience a fracture or death were followed until the end of the initial WHI study. Self-reported fracture outcomes were identified by questionnaires, administered semiannually for CT participants and annually for OS participants. Radiology reports were used to adjudicate all fractures in the CT and HF in the OS. HF was centrally or locally adjudicated using the same criteria. The agreement between central and local adjudication for HF was 96%.
Genotype Imputation
Genotype data produced from blood samples were acquired through dbGap. The data genotyping was completed using either the Illumina (Illumina, San Diego, California) or Affymetrix 6.0 Array Set Platform (Affymetrix, Santa Clara, California). Genotype imputation was completed at the Sanger Imputation Server. The Haplotype Reference Consortium (HRC) reference panel and Positional Burrows-Wheeler Transform (PBWT) imputation algorithm were used for genotype imputation.
Genetic Risk Scores
Genetic risk for fracture was quantified using a standardized metric described previously [21]. Briefly, this metric allows the composite assessment of genetic risk in complex traits by summarizing the genetic predisposition. We defined three GRSs (GRS_BMD, GRS_fracture, and GRS_eBMD) based on three previously described comprehensive GWA studies, which reported 81 independent total body BMD-related [25], 15 fracture-related [26], and 1103 eBMD-related SNPs [23]. SNPs with low call rates (<95%) or low imputation quality (r2<0.3) were excluded. Before conducting the GRS calculation, we performed linkage disequilibrium (LD) pruning with a window size of 200 variants, sliding across the genome with a step size of 50 variants at a time using “--indep-pairwise” command in Plink to remove highly correlated SNPs (r2 > 0.25) in the WHI cohort [31]. In WHI, 75, 15, and 1055 SNPs, respectively, out of the 81, 15, and 1103 SNPs had high-quality imputed dosage information available and were used to derive GRS_BMD, GRS_fracture, and GRS_eBMD. Plink 1.9 was used to generate the GRS [31]. Three weighted GRSs were calculated for each individual in the study as GRS = sum (xi ∗ bi); where xi are individual’s genotype (0, 1, 2) for BMD-decreasing SNP i, and bi are the effect size of this SNP. Individual carries more BMD-decreasing risk allele have higher GRS.
Statistical Analysis
Demographic and baseline clinical characteristics are summarized as mean ± SD for continuous variables or frequencies (%) for categorical variables. Differences between fracture and non-fracture groups were examined using Student’s t-test for continuous variables and using chi-square tests or analysis of variance (ANOVA) for categorical variables.
To illustrate the different cumulative incidence of fracture in participants with distinct genetic profiles, we grouped them into three bins based on each GRS, taking the first and the third quantile as illustrative cut-points (bottom 25%, 25–75%, and top 25%). GRS was treated as a categorical variable in all subsequent analyses. The cumulative incidence function (CIF) was applied to derive the observed 10-year cumulative incidence of MOF and HF by GRS groups, with the competing mortality risk accounted for.
For both MOF and HF, the estimates of hazard ratios (HRs) were obtained by comparing the top percentiles (25%) and the middle percentiles (25–75%) with the bottom percentiles (25%) of each GRS using Cox proportional hazard models. The model was adjusted for the FRAX risk factors, including age, weight, height, previous fracture, parent hip fracture, current smoking, glucocorticoids, rheumatoid arthritis, secondary osteoporosis, and alcohol consumption three or more units per day, as well as genotyping platforms. The Cox proportional hazard models’ assumptions were checked beforehand using the Schoenfeld residual test [32] and the martingale residual test [33]. The WHI data satisfied both the proportional hazards and linearity assumptions. FRAX without BMD was used as the base model as BMD measurement was not available for most WHI participants. The four models were formulated as follows: Model 1--FRAX risk factors (base model); Model 2--FRAX risk factors + GRS_fracture; Model 3--FRAX risk factors + GRS_BMD; and Model 4--FRAX risk factors + GRS_eBMD. The magnitude of the association between GRS and fracture risk was assessed by the hazard ratio and its corresponding 95% confidence interval. The difference between log-odds for the separate GRS was tested for significance by using a z-test.
Model Evaluation
The Area Under the Curve (AUC) was used to evaluate the four models’ discriminatory ability in identifying individuals who might sustain a fracture from those who might not. The statistical significance of the change in the AUC between models was tested, according to DeLong et al. [34]. To investigate whether the GRSs could specifically improve the classification of fracture and non-fracture among individuals without a family history of hip fracture, we also estimated the AUCs of the four models in predicting fracture among this subgroup. We also assessed the model calibration by dividing the population into deciles based on the level of predicted fracture risk and compared the observed vs. expected event probabilities using the Greenwood-Nam-D’Agostino X2 test [35].
The net reclassification improvement (NRI) was used to assess each model’s performance in reclassification. The predicted risk fracture was estimated for each individual by the different models and then classified into three risk groups. The high risk was defined as 3% for HF, and 20% for MOF based on the National Osteoporosis Foundation recommended fixed intervention cutoffs [36]. The NRI focuses on reclassification tables constructed separately for participants with and without fractures and quantifies the correct movement in categories--upward for fractures and downward for non-fractures. Integrated discrimination improvement (IDI) was also calculated to incorporate both the direction of change in the calculated risk and the extent of change. In addition, all analyses conducted in the primary analyses were repeated in the sensitivity analysis with only Caucasian women included (N=9,203).
Results
The study included a total of 23,981 women for analysis. Participants were followed for an average of 12 years. Out of 1,637 (6.9%) women who sustained at least one MOF during the follow-up, 770 (3.33%) were hip fractures. The baseline characteristics of the participants stratified by fracture status are shown in Table 1. Women who sustained a fracture were also older, had lower body mass index (BMI), more alcohol consumption, a higher prevalence of prior fractures, more hip fractures in their family history, and more falls during the past 12 months. GRS_fracture, GRS_BMD, and GRS_eBMD were all significantly higher in women who sustained a fracture (MOF or HF) than in those who did not (p<.01). GRS_BMD and GRS_eBMD were also significantly higher among women with a positive family history of hip fractures than those who did not have (p<0.001). The distribution of GRS_BMD, GRS_fracture, and GRS_eBMD was approximately normal. The individuals who have GRS_fracture, GRS_BMD, and GRS_eBMD lower than 3.65, 3.67, and 25.92, respectively, are in the bottom 25% percentile of the GRS distribution. The participants who have GRS_fracture, GRS_BMD, and GRS_eBMD greater than 5.45, 4.36, and 27.29, respectively, are in the top 25% percentile of the GRS distribution. No significant difference on three GRSs was observed between fracture and non-fracture Caucasian women (Supplementary Table 1).
Table 1.
Baseline Characteristics of 23,981 women stratified by Major Osteoporotic fracture (MOF) and Hip fracture (HF) status.
| Subjects with MOF (n =1,637) | Subjects without MOF (n =22,281) | p Value | Subjects with HF (n=770) | Subjects without HF (n=23,148) | p Value | |
|---|---|---|---|---|---|---|
| Age (year), mean (SD) | 67.99 (±6.52) | 63.26 (±7.32) | <0.0001 | 69.48 (±5.71) | 63.39 (±7.34) | <0.0001 |
| Weight (kg), mean (SD) | 73.59 (±15.21) | 77.32 (±16.92) | <0.0001 | 72.42 (±15.28) | 77.22 (±16.86) | <0.0001 |
| Height (cm), mean (SD) | 161.25 (±6.30) | 161.06 (±6.29) | 0.28 | 161.8 (±6.27) | 161.1 (±6.29) | <0.01 |
| Body mass index (kg/m2), mean (SD) | 28.27 (±6.30) | 29.73 (±6.09) | <0.0001 | 27.61 (±5.44) | 29.69 (±6.07) | <0.0001 |
| Race/Ethnicity, n (%) | ||||||
| Whites | 1,255 (76.66) | 7,948 (35.67) | <.0001 | 600 (77.92) | 8,603 (37.17) | <.0001 |
| African American | 189 (11.55) | 9,231 (41.43) | 103 (13.38) | 9,317 (40.25) | ||
| Asian | 10 (0.61) | 467 (2.10) | 5 (0.65) | 472 (2.04) | ||
| Hispanic/Latino | 159 (9.71) | 4100 (18.40) | 51 (6.62) | 4,208 (18.18) | ||
| American Indian or Alaska Native | 24 (1.47) | 535 (2.40) | 11 (1.43) | 548 (2.37) | ||
| Smoking, n (%) | ||||||
| Never | 858 (52.42) | 11,704 (52.52) | 0.35 | 410 (53.25) | 12,152 (52.50) | 0.13 |
| Past | 639 (39.03) | 8,448 (37.92) | 303 (39.35) | 8,784 (37.95) | ||
| Current | 140 (8.55) | 2,129 (9.56) | 57 (7.40) | 2,212 (9.56) | ||
| ≥3 alcoholic drinks per day, n (%) | ||||||
| Yes | 24 (1.47) | 216 (0.97) | 0.05 | 10 (1.30) | 230 (0.99) | 0.40 |
| No | 1,613 (98.53) | 22,065 (99.03) | 760 (98.70) | 22,918 (99.01) | ||
| Rheumatoid arthritis, n (%) | ||||||
| Yes | 109 (6.66) | 1,500 (6.73) | 0.91 | 63 (8.18) | 1,546 (6.68) | 0.10 |
| No | 1,528 (93.34) | 20,781 (93.27) | 707 (91.82) | 21,602 (93.32) | ||
| Previous fragility fractures, n (%) | ||||||
| Yes | 835 (51.01) | 6,902 (30.98) | <0.0001 | 395 (51.30) | 7,342 (31.72) | <0.0001 |
| No | 802 (48.99) | 15,379 (95.04) | 375 (48.70) | 15,806 (68.28) | ||
| Familial history of hip fracture, n (%) | ||||||
| Yes | 271 (16.55) | 2,156 (9.68) | <0.0001 | 138 (17.92) | 2,289 (9.89) | <0.0001 |
| No | 1,366 (83.45) | 20,125 (93.64) | 632 (82.08) | 20,859 (90.11) | ||
| GRS_fracture, mean (SD) | 4.85 (±1.99) | 4.56 (±1.44) | <.0001 | 4.84 (±1.98) | 4.48 (±1.33) | <.0001 |
| GRS_BMD, mean (SD) | 4.30 (±0.40) | 4.00 (±0.45) | <.0001 | 4.29 (±0.39) | 4.01 (±0.45) | <.0001 |
| GRS_eBMD, mean (SD) | 27.19 (±0.77) | 26.56 (±0.87) | <0.0001 | 27.19 (±0.77) | 26.58 (±0.88) | <0.0001 |
Significant results are in boldface.
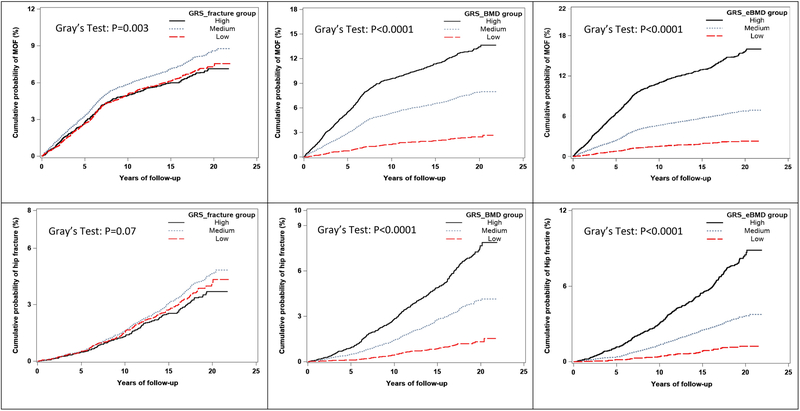
The crude 10-year cumulative incidence of MOF and HF by the three GRS groups is shown in Figure 1. With competing mortality accounted for, significant differences were observed across GRS_BMD and GRS_eBMD groups for both MOF (p<.0001) and HF (p<.0001). The incidences of MOF and hip fracture were greater in high GRS groups. GRS_fracture was also significantly associated with the 10-year cumulative incidence of MOF (p<0.01), but not with HF (p=0.07). In Caucasian women, the crude 10-year cumulative incidence of MOF differed significantly only across GRS_eBMD groups (p=0.03). No significant difference was observed across GRS groups for HF (Supplementary figure 1).
Figure 1.
Crude (unadjusted) 10-year cumulative incidence of MOF and HF stratified by GRS groups, including competing mortality risk (N=23,981).
The Cox Proportional Hazard model showed that, after adjusting for FRAX risk factors, GRS_eBMD was significantly associated with MOF. Individuals in the top 25% of GRS_eBMD distribution had 1.52-fold (95%CI, 1.17 – 1.98) increased MOF risk compared with the bottom 25% of the individuals (Table 2). Similar findings with hip fracture outcomes were also observed. Compare to the women in the low GRS groups, women in the high GRS_eBMD groups have increased, although not statistically significant, risk of HF (Table 3). GRS_fracture and GRS_BMD were not significant risk factors of either MOF (Table 2) or HF (Table 3). The z-test indicates that the effects of separate GRS on fracture risk were not statistically different from each other. Similar results were observed in sensitivity analyses (Supplementary Table 2&3)
Table 2.
HR for the hazard function for significant predictive variables for MOF in models with and without GRS
| Variable | Without GRS HR per 1 unit (95% CI) in MOF |
With GRS_fracture HR per 1 unit (95% CI) in MOF |
With GRS_BMD HR per 1 unit (95% CI) in MOF |
With GRS_eBMD HR per 1 unit (95% CI) in MOF |
|---|---|---|---|---|
| Age | 1.07 (1.06 – 1.08) | 1.07 (1.06 – 1.08) | 1.07 (1.06 – 1.08) | 1.07 (1.06 – 1.08) |
| Weight | 0.99 (0.99 – 1.00) | 0.99 (0.99 – 1.00) | 0.99 (0.99 – 1.00) | 0.99 (0.99 – 1.00) |
| Height | 1.02 (1.01 – 1.03) | 1.02 (1.01 – 1.03) | 1.02 (1.01 – 1.03) | 1.02 (1.01 – 1.03) |
| Previous osteoporotic fracture | 1.54 (1.38 – 1.71) | 1.54 (1.38 – 1.71) | 1.54 (1.38 – 1.71) | 1.53 (1.37 – 1.70) |
| Parental history of hip fracture | 1.23 (1.07 – 1.41) | 1.23 (1.07 – 1.41) | 1.23 (1.07 – 1.41) | 1.22 (1.07 – 1.41) |
| Rheumatoid arthritis | 1.18 (0.95 – 1.47) | 1.18 (0.95 – 1.46) | 1.18 (0.95 – 1.47) | 1.18 (0.95 – 1.47) |
| Current smoking | 1.29 (1.06 – 1.56) | 1.29 (1.07 – 1.57) | 1.29 (1.06 – 1.57) | 1.29 (1.06 – 1.56) |
| Daily drinking > 3 | 1.08 (0.70 – 1.66) | 1.08 (0.70 – 1.66) | 1.08 (0.70 – 1.66) | 1.08 (0.70 – 1.66) |
| Secondary osteoporosis | 1.07 (0.96 – 1.20) | 1.07 (0.96 – 1.20) | 1.07 (0.96 – 1.20) | 1.07 (0.96 – 1.20) |
| GRS | ||||
| 0–25% | NA | Ref | Ref | Ref |
| 25–75% | NA | 1.067 (0.94 – 1.21) | 1.15 (0.90 – 1.46) | 1.34 (1.05 – 1.70) |
| 75–100% | NA | 1.175 (1.00 – 1.38) | 1.12 (0.86 – 1.46) | 1.52 (1.17 – 1.98) |
Significant results are in boldface.
Table 3.
HR for the hazard function for significant predictive variables for Hip fracture (HF) in models with and without GRS
| Variable | Without GRS HR per 1 unit (95% CI) in HF |
With GRS_fracture HR per 1 unit (95% CI) in HF |
With GRS_BMD HR per 1 unit (95% CI) in HF |
With GRS_eBMD HR per 1 unit (95% CI) in HF |
|---|---|---|---|---|
| Age | 1.13 (1.11 – 1.15) | 1.13 (1.11 – 1.15) | 1.13 (1.12 – 1.15) | 1.13 (1.11 – 1.15) |
| Weight | 0.99 (0.98 – 0.99) | 0.99 (0.98 – 0.99) | 0.99 (0.98 – 0.99) | 0.99 (0.98 – 0.99) |
| Height | 1.04 (1.03 – 1.06) | 1.04 (1.03 – 1.06) | 1.04 (1.03 – 1.06) | 1.04 (1.03 – 1.06) |
| Previous osteoporotic fracture | 1.44 (1.24 – 1.68) | 1.44 (1.24 – 1.68) | 1.44 (1.24 – 1.67) | 1.43 (1.23 – 1.67) |
| Parental history of hip fracture | 1.30 (1.07 – 1.57) | 1.30 (1.07 – 1.57) | 1.29 (1.06 – 1.56) | 1.29 (1.07 – 1.57) |
| Rheumatoid arthritis | 1.62 (1.23 – 2.14) | 1.62 (1.22 – 2.14) | 1.61 (1.22 – 2.13) | 1.63 (1.23 – 2.15) |
| Current smoking | 1.29 (1.06 – 1.56) | 1.33 (0.99 – 1.79) | 1.33 (0.99 – 1.79) | 1.33 (0.99 – 1.79) |
| Daily drinking > 3 | 0.88 (0.46 – 1.70) | 0.88 (0.45 – 1.70) | 0.88 (0.45 – 1.69) | 0.88 (0.46 – 1.71) |
| Secondary osteoporosis | 1.14 (0.97 – 1.34) | 1.14 (0.97 – 1.34) | 1.14 (0.97 – 1.34) | 1.14 (0.97 – 1.34) |
| GRS | ||||
| 0–25% | NA | Ref | Ref | Ref |
| 25–75% | NA | 1.03 (0.87 – 1.24) | 1.14 (0.80 – 1.60) | 1.16 (0.82 – 1.64) |
| 75–100% | NA | 1.01 (0.80 – 1.28) | 0.97 (0.67 – 1.42) | 1.43 (0.98 – 2.10) |
Significant results are in boldface.
The fracture discrimination ability of GRSs over FRAX was assessed using the concordance index (C-index) (Table 4). The model that included only FRAX risk factors showed moderate discrimination of MOF and HF, with a C-index of 0.753 (95% CI, 0.704 to 0.800) and 0.804 (95% CI, 0.741 to 0.813), respectively. Minor to no improvement in discriminating MOF and HF was observed when adding GRSs to the base FRAX model. The GRS_eBMD improved the discrimination from 0.753 to 0.754 for MOF (P=0.19), and from 0.804 to 0.805 for HF (p=0.32), respectively. Results were similar among women without a family history of fracture; the AUC did not improve specifically (Supplementary Table 6). When restricted to Caucasian women, the discrimination ability of models with and without GRSs became lower. No significant improvement was observed when GRSs were added to the base FRAX model (Supplementary Table 4).
Table 4.
Concordance Index (and 95% confidence interval) of predicted and observed fracture risk for the model with and without GRS
| Model 1 (Base model) |
Model 2 (Base model + GRS_fracture) |
Model 3 (Base model + GRS_BMD) |
Model 4 (Base model + GRS_eBMD) |
||||
|---|---|---|---|---|---|---|---|
| C index | C index | p-value | C index | p-value | C index | p-value | |
| MOF | 0.753 (0.704– 0.800) |
0.754 (0.704 – 0.800) |
0.24 | 0.754 (0.704 – 0.800) |
0.53 | 0.754 (0.705 – 0.800) |
0.19 |
| Hip Fracture | 0.804 (0.741 – 0.813) |
0.804 (0.733 – 0.866) |
0.98 | 0.804 (0.733 – 0.866) |
0.87 | 0.805 (0.743 – 0.814) |
0.32 |
Significant results are in boldface.
Calibration was assessed by comparing observed and expected event rates for FRAX models with and without the GRSs. The results suggested that, for the MOF prediction, the model without GRS (Greenwood-Nam-D’Agostino X2, p=0.023) have better calibration than the model with GRS_fracture (p=0.011). However, better calibration was observed in the model with GRS_BMD (p=0.080), and GRS_eBMD included (p=0.098). For the HF prediction, both models showed good calibration (Greenwood-Nam-D’Agostino X2, p=0.607, p=0.641, and p=0.622, respectively, for models without and with the GRS_BMD and GRS_eBMD).
In the reclassification analysis, compared to the model without GRS, the model with GRS_fracture, GRS_BMD, and GRS_eBMD improved the reclassification of MOF by 0.5 % (95% CI, 0.2% to 0.9%, p<0.01), 0.3% (95% CI, 0.1% to 0.6%, p=0.01), and 2.1% (95%CI, 0.3% to 2.8%, p<0.01), respectively. For the model that included GRS_eBMD, 395 of them were correctly reclassified up to the high-risk group, and 325 women who did not experience a MOF were correctly reclassified from the high-risk group to the low-risk group. Overall, the three GRS models improve the reclassification of fracture events. The continuous NRI showed that improvement in MOF reclassification contributed by GRS_fracture, GRS_BMD, and GRS_eBMD overall were 6.7% (p=0.01), 10.8% (p<0.01), and 14.5% (p<0.01), respectively (Table 5). Results of sensitivity analysis showed that only GRS_eBMD significantly improved the base FRAX model in both MOF and HF prediction, with an IDI of 3.5% (95% CI, 0.00% to 4.1 %, p<0.01) and 3.5% (95%CI, 0 to 4.3%, p=0.01), respectively (Supplementary Table 5).
Table 5:
Reclassification table of 10-Year Major Osteoporotic Fracture (MOF) and Hip Fracture (HF) Stratified by Event Status. Results of Reclassification Analysis: Percent of reclassification compared with the FRAX base model (Model 1).
| Reclassification | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-fracture group | Fracture group | NRI (category) |
p Value | NRI (continuous) |
p Value | IDI | p Value | |||
| Reclassification down | Reclassification up | Reclassification up | Reclassification down | |||||||
| GRS_fracture | ||||||||||
| MOF | 0.005 | 0.003 | 0.006 | 0.006 | 0.005 (−0.005 to 0.014) |
0.28 | 0.067 (0.014 to 0.121) |
0.01 | 0.005 (0.002 to 0.009) |
<.01 |
| HF | 0.000 | 0.001 | 0.007 | 0.000 | 0.007 (−0.003 to 0.016) |
0.18 | 0.020 (−0.083 to 0.124) |
0.70 | 0.000 (0.001 to 0.030) |
0.99 |
| GRS_BMD | ||||||||||
| MOF | 0.007 | 0.006 | 0.003 | 0.009 | −0.004 (−0.011 to 0.002) |
0.62 | 0.145 (0.084to 0.207) |
<.01 | 0.003 (0.001 to 0.006) |
0.01 |
| HF | 0.005 | 0.006 | 0.018 | 0.004 | 0.011 (−0.005 to 0.027) |
0.17 | −0.146 (−0.263 –0.029) |
0.02 | 0.001 (0.000 to 0.039) |
0.90 |
| GRS_eBMD | ||||||||||
| MOF | 0.020 | 0.016 | 0.025 | 0.022 | 0.007 (−0.006 to 0.020) |
0.33 | 0.114 (−0.001 to 0.123) |
<.01 | 0.021 (0.003 to 0.028) |
<.01 |
| HF | 0.027 | 0.026 | 0.025 | 0.029 | 0.014 (−0.030 to 0.025) |
0.85 | 0.222 (0.107 to 0.336) |
<.01 | 0.028 (0.003 to 0.041) |
<.01 |
Significant results are in boldface.
NRI=net reclassification improvement; IDI=integrated discriminative improvement; 95% confidence intervals are given within brackets.
The improvement in terms of reclassification provided by GRSs was similar in HF prediction, with little to no improvement in HF prediction was observed when GRS_BMD or GRS_fracture was added into the base FRAX model. However, GRS_eBMD improved the reclassification of HF significantly by 2.8% (95%CI, 0.3% to 4.1%, p<0.01). There was a 2.5% improvement in reclassification among cases using the FRAX+ GRS_eBMD model, compared with FRAX alone. Among women with HF, 25 out of 699 participants who had hip fractures were correctly reclassified upward with the FRAX+GRS_eBMD model. Among women who did not experience HF, 492 out of 18529 were correctly reclassified downward to the low-risk group with the FRAX+GRS_eBMD model (Table 5).
Discussion
Prior studies have well demonstrated that fracture is highly heritable. However, genetic information has not been included in any existing fracture prediction tools. Considering that more than half of the variance in fracture susceptibility is due to hereditary factors [37] and the suboptimal performance of existing fracture prediction tools, genetic profiling is expected to help improve the accuracy of fracture prediction substantially. In the present study, we first assessed the association between different GRSs, which were generated based on GWAS of different osteoporosis-related traits, and the outcome of MOF and HF after an average of 12 years of follow-up. Furthermore, we quantified the predictive value of various GRSs in fracture prediction in addition to the clinical risk factors currently included in the FRAX model. Since the SNPs were discovered from GWAS conducted in Caucasian predominantly cohorts, prior studies using GWAS to assess the predictive value of PRS across a range of traits and populations have made a consistent observation: PRS predict individual risk far more accurately in Europeans [38]. Therefore, we additionally conducted sensitivity analyses among Caucasians only. We demonstrated that the genetic profile was significantly associated with fracture risk: the BMD and eBMD-based GRSs yield statistically significant improvements in the reclassification of fracture versus non-fracture.
The GRS generated based on BMD and eBMD-related GWAS were all significantly associated with MOF’s outcome, independent of the clinical risk factors included in FRAX. However, the fracture-related GRS was not a significant predictor of either MOF or HF. When assessing GRS’s usefulness for fracture discrimination and reclassification beyond the established clinical risk factors, only minor improvement in C-statistics of either MOF or HF was observed. When tested with the more sensitive IDI and NRI reclassification metrics, GRS_fracture, GRS_BMD, and GRS_eBMD slightly, but statistically significantly, improved the base FRAX model in MOF prediction. GRS_eBMD also improved the base FRAX model in HF prediction.
Our findings were consistent with prior studies. Based on the GWAMA study, 56 loci associated with femoral neck and lumbar spine BMD published in 2012 [21], Eriksson and colleagues reported minor improvement of fracture prediction when adding GRS into the base model consisting of age, height, and weight [28]. Similarly, another study reported that a genetic profile provides limited improvement to the accuracy of the GARVAN model [27]. In the current study, GRS_BMD was derived based on a more comprehensive GWAMA that identified variants in 76 independent loci associated with TB-BMD. However, with more genetic variables included, the improvement in terms of discrimination and reclassification provided by GRS_BMD was greater in the above mentioned two studies than in our study, mainly because that FRAX takes account of more risk factors in the model. Risk factors exclusively included in the FRAX model, such as the family history of fracture and race, contributing to the underlying genetic architecture of fracture, would attenuate the genetic component’s effect on fracture risk.
Most of the prior studies that assessed GRS’s potential in improving fracture prediction were mainly focused on BMD-related genetic variants. With more osteoporosis-related traits been studied in GWAS and new genetic variants been discovered, in the current study, we assessed different GRS in improving the accuracy of FRAX. BMD and eBMD are two highly correlated traits, and a recent eBMD-related GWAS has successfully identified 84% of all currently known genome-wide significant loci for DXA-derived BMD [23]. Notably, GRS_eBMD constructed based on 203 loci was outperformed GRS_BMD, which was constructed based on 76 loci in improving fracture reclassification, suggesting that more genetic variants included in GRS calculation could potentially increase the predictive value of GRS. Although prior twin and family studies have demonstrated that fracture susceptibility is highly heritable (up to 46%) [36], GRS in the current study captured only a minimal fraction of fracture risk. There is a gap between heritability estimated from GRS and that calculated from twin and family studies. Considering that fracture is a multifactorial disease, GRS related to only one trait may not sufficiently capture the genetic components of fracture. Additional studies are warranted to research this “missing heritability.” The existing GWAS studies may not have the capacity to detect all related genetic variants, especially those with small effects on fracture-related traits. GRS’s increased predictive power in fracture risk assessment can be anticipated with more genetic variants being discovered. Our study has several strengths. First, this is the first study assessing whether the addition of osteoporosis-related GRS would improve the performance of the existing FRAX. Second, we compared the predictive value of different osteoporosis-related GRSs in fracture prediction, in addition to the well-established clinical risk factors. All GRS was calculated based on the newly discovered genetic variants identified from the most recent, well-powered GWASs. The present study provides a comprehensive assessment of the genetic contribution to fracture prediction compared to other studies. Third, this study was conducted with large sample size. The original study design enabled a prospective assessment of incidence fracture for over ten years, which ensured our robustness findings.
Limitations of this study are acknowledged. First, the WHI data we used only included women 50–79 years, so our findings may not apply to men or women who are not in the study age range. Second, the GRS was constructed based solely on the genome-wide significant SNPs (typically, p<5*10−8). Genome-wide, more advanced methods of polygenic score construction will be explored in our future research. Therefore, the genetic effect on fracture may not be fully captured, which might explain why GRSs provided such limited predictive value to the existing FRAX tool. Third, BMD measurement was unavailable for most of the participants; thus, our study only focuses on FRAX without BMD. Finally, the study sample was unbalanced because Asian and American Indian subjects’ sample size was very small. The results may, therefore, not truly represent the general population and should be interpreted with caution.
Conclusions
In conclusion, our study demonstrated that genetic information of osteoporosis-related traits could improve, although on a limited basis, fracture prediction performance beyond the clinical risk factors currently included in FRAX. With more genetic variants related to osteoporosis and fracture being rapidly discovered, GRS’s predictive value can add to the existing fracture prediction tool is anticipated to increase considerably. Given the high prevalence of osteoporotic fracture, GRS’s clinical utility can provide a more accurate fracture risk classification at the individual level.
Supplementary Material
Acknowledgments:
The data/analyses presented in the current publication are based on the use of study data downloaded from the dbGaP website, under phs000200 (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000200.v12.p3). The research and analysis described in the current publication were supported by the Genome Acquisition to Analytics Research Core of the Personalized Medicine Center of Biomedical Research Excellence in the Nevada Institute of Personalized Medicine, and the National Supercomputing Institute at the University of Nevada Las Vegas provided facilities for bioinformatical analysis in this study. The research and analysis described in the current publication were supported by a grant from the National Institute of General Medical Sciences (P20GM121325), a grant from the National Institute on Minority Health and Health Disparities of the National Institutes of Health (R15MD010475). The funding sponsors were not involved in the analysis design, genotype imputation, data analysis, interpretation of the analysis results, or the preparation, review, or approval of this manuscript.
Funding: The research was funded by a grant from the National Institute of General Medical Sciences (P20GM121325), a grant from the National Institute on Minority Health and Health Disparities of the National Institutes of Health (R15MD010475). The funding sponsors were not involved in the analysis design, genotype imputation, data analysis, interpretation of the analysis results, or the preparation, review, or approval of this manuscript.
Footnotes
Conflicts of Interest: Qing Wu and Xiangxue Xiao declare that they have no conflict of interest.
Declarations:
Ethics approval: Our study was approved by the institutional review board at the University of Nevada, Las Vegas.
Consent to participant: Not applicable
Consent for publication: Not applicable
Availability of data and material: The data used in the current study is publically available through the database of Genotype and Phenotype (dbGap) (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000200.v12.p3)
Code availability: Not applicable
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Xiangxue Xiao, Nevada Institute of Personalized Medicine, College of Science, University of Nevada, Las Vegas, Las Vegas, Nevada; Department of Epidemiology and Biostatistics, School of Public Health, University of Nevada Las Vegas, Las Vegas, Nevada.
Qing Wu, Nevada Institute of Personalized Medicine, College of Science, University of Nevada, Las Vegas, Las Vegas, Nevada; Department of Epidemiology and Biostatistics, School of Public Health, University of Nevada Las Vegas, Las Vegas, Nevada.
References:
- 1.Sözen T, Özışık L & Başaran NÇ (2017) An overview and management of osteoporosis, Eur J Rheumatol. 4, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnell O & Kanis JA (2004) An estimate of the worldwide prevalence, mortality and disability associated with hip fracture, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 15, 897–902. [DOI] [PubMed] [Google Scholar]
- 3.Johnell O & Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 17, 1726–33. [DOI] [PubMed] [Google Scholar]
- 4.Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV & Jönsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA), Archives of osteoporosis. 6, 59–155. [DOI] [PubMed] [Google Scholar]
- 5.Reginster JY & Burlet N (2006) Osteoporosis: a still increasing prevalence, Bone. 38, S4–9. [DOI] [PubMed] [Google Scholar]
- 6.Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, Kleerekoper M, Luckey MM, McClung MR, Pollack RP & Petak SM (2010) American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis, Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 16 Suppl 3, 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S & Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 29, 2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sànchez-Riera L, Carnahan E, Vos T, Veerman L, Norman R, Lim SS, Hoy D, Smith E, Wilson N, Nolla JM, Chen JS, Macara M, Kamalaraj N, Li Y, Kok C, Santos-Hernández C & March L (2014) The global burden attributable to low bone mineral density, Annals of the rheumatic diseases. 73, 1635–45. [DOI] [PubMed] [Google Scholar]
- 9.Harvey N, Dennison E & Cooper C (2010) Osteoporosis: impact on health and economics, Nature reviews Rheumatology. 6, 99–105. [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA & Diseases, W. H. O. C. f. M. B. (2008) Assessment of Osteoporosis at the Primary Health Care Level, WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield Medical School. [Google Scholar]
- 11.Nguyen ND, Frost SA, Center JR, Eisman JA & Nguyen TV (2007) Development of a nomogram for individualizing hip fracture risk in men and women, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 18, 1109–1117. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen ND, Frost SA, Center JR, Eisman JA & Nguyen TV (2008) Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 19, 1431–1444. [DOI] [PubMed] [Google Scholar]
- 13.Briot K, Paternotte S, Kolta S, Eastell R, Felsenberg D, Reid DM, Glüer C-C & Roux C (2014) FRAX®: Prediction of Major Osteoporotic Fractures in Women from the General Population: The OPUS Study, PLoS One. 8, e83436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crandall CJ, Schousboe JT, Morin SN, Lix LM & Leslie W (2019) Performance of FRAX and FRAX-Based Treatment Thresholds in Women Aged 40 Years and Older: The Manitoba BMD Registry, Journal of Bone and Mineral Research. 34, 1419–1427. [DOI] [PubMed] [Google Scholar]
- 15.Sornay-Rendu E, Munoz F, Delmas PD & Chapurlat RD (2010) The FRAX tool in French women: How well does it describe the real incidence of fracture in the OFELY cohort?, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 25, 2101–7. [DOI] [PubMed] [Google Scholar]
- 16.Marques A, Ferreira RJ, Santos E, Loza E, Carmona L & da Silva JA (2015) The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis, Annals of the rheumatic diseases. 74, 1958–67. [DOI] [PubMed] [Google Scholar]
- 17.Crandall CJ, Larson JC, Watts NB, Gourlay ML, Donaldson MG, LaCroix A, Cauley JA, Wactawski-Wende J, Gass ML, Robbins JA & Ensrud KE (2014) Comparison of fracture risk prediction by the US Preventive Services Task Force strategy and two alternative strategies in women 50–64 years old in the Women’s Health Initiative, J Clin Endocrinol Metab. 99, 4514–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crandall CJ, Larson J, LaCroix A, Cauley JA, LeBoff MS, Li W, LeBlanc ES, Edwards BJ, Manson JE & Ensrud K (2019) Predicting Fracture Risk in Younger Postmenopausal Women: Comparison of the Garvan and FRAX Risk Calculators in the Women’s Health Initiative Study, Journal of general internal medicine. 34, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X, Gruner M, Trémollieres F, Pluskiewicz W, Sornay-Rendu E, Adamczyk P & Schnatz PF (2017) Diagnostic accuracy of FRAX in predicting the 10-year risk of osteoporotic fractures using the USA treatment thresholds: A systematic review and meta-analysis, Bone. 99, 20–25. [DOI] [PubMed] [Google Scholar]
- 20.Stewart TL & Ralston SH (2000) Role of genetic factors in the pathogenesis of osteoporosis, The Journal of endocrinology. 166, 235–45. [DOI] [PubMed] [Google Scholar]
- 21.Estrada, K.Styrkarsdottir, Evangelou U, Hsu E, Duncan YH, Ntzani EL, Oei EE, Albagha L, Amin OM, Kemp N, Koller JP, Li DL, Liu G, Minster CT, Moayyeri RL, Vandenput A, Willner L, Xiao D, Yerges-Armstrong SM, Zheng LM, Alonso HF, Eriksson N, Kammerer J, Kaptoge CM, Leo SK, Thorleifsson PJ, Wilson G, Wilson SG, Aalto JF, Alen V, Aragaki M, Aspelund AK, Center T, Dailiana JR, Duggan Z, Garcia DJ, Garcia-Giralt M, Giroux N, Hallmans S, Hocking G, Husted LJ, Jameson LB, Khusainova KA, Kim R, Kooperberg GS, Koromila C, Kruk T, Laaksonen M, Lacroix M, Lee AZ, Leung SH, Lewis PC, Masi JR, Mencej-Bedrac L, Nguyen S, Nogues TV, Patel X, Prezelj MS, Rose J, Scollen LM, Siggeirsdottir S, Smith K, Svensson AV, Trompet O, Trummer S, van Schoor O, Woo NM, Zhu J, Balcells K, Brandi S, Buckley ML, Cheng BM, Christiansen S, Cooper C, Dedoussis C, Ford G, Frost I, Goltzman M, Gonzalez-Macias D, Kahonen J, Karlsson M, Khusnutdinova M, Koh E, Kollia JM, Langdahl P, Leslie BL, Lips WD, Ljunggren P, Lorenc O, Marc RS, Mellstrom J, Obermayer-Pietsch D, Olmos B, Pettersson-Kymmer JM, Reid U, Riancho DM, Ridker JA, Rousseau PM, Slagboom F, Tang PE, N. L., et al. (2012) Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture, Nature genetics. 44, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SK (2018) Identification of 613 new loci associated with heel bone mineral density and a polygenic risk score for bone mineral density, osteoporosis and fracture, PLoS One. 13, e0200785–e0200785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, Vulpescu NA, Forgetta V, Kleinman A, Mohanty ST, Sergio CM, Quinn J, Nguyen-Yamamoto L, Luco A-L, Vijay J, Simon M-M, Pramatarova A, Medina-Gomez C, Trajanoska K, Ghirardello EJ, Butterfield NC, Curry KF, Leitch VD, Sparkes PC, Adoum A-T, Mannan NS, Komla-Ebri DSK, Pollard AS, Dewhurst HF, Hassall TAD, Beltejar M-JG, andMe Research T, Adams DJ, Vaillancourt SM, Kaptoge S, Baldock P, Cooper C, Reeve J, Ntzani EE, Evangelou E, Ohlsson C, Karasik D, Rivadeneira F, Kiel DP, Tobias JH, Gregson CL, Harvey NC, Grundberg E, Goltzman D, Adams DJ, Lelliott CJ, Hinds DA, Ackert-Bicknell CL, Hsu Y-H, Maurano MT, Croucher PI, Williams GR, Bassett JHD, Evans DM & Richards JB (2019) An atlas of genetic influences on osteoporosis in humans and mice, Nature genetics. 51, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp JP, Morris JA, Medina-Gomez C, Forgetta V, Warrington NM, Youlten SE, Zheng J, Gregson CL, Grundberg E, Trajanoska K, Logan JG, Pollard AS, Sparkes PC, Ghirardello EJ, Allen R, Leitch VD, Butterfield NC, Komla-Ebri D, Adoum A-T, Curry KF, White JK, Kussy F, Greenlaw KM, Xu C, Harvey NC, Cooper C, Adams DJ, Greenwood CMT, Maurano MT, Kaptoge S, Rivadeneira F, Tobias JH, Croucher PI, Ackert-Bicknell CL, Bassett JHD, Williams GR, Richards JB & Evans DM (2017) Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis, Nature genetics. 49, 1468–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina-Gomez C, Kemp JP, Trajanoska K, Luan J, Chesi A, Ahluwalia TS, Mook-Kanamori DO, Ham A, Hartwig FP, Evans DS, Joro R, Nedeljkovic I, Zheng HF, Zhu K, Atalay M, Liu CT, Nethander M, Broer L, Porleifsson G, Mullin BH, Handelman SK, Nalls MA, Jessen LE, Heppe DHM, Richards JB, Wang C, Chawes B, Schraut KE, Amin N, Wareham N, Karasik D, Van der Velde N, Ikram MA, Zemel BS, Zhou Y, Carlsson CJ, Liu Y, McGuigan FE, Boer CG, Bønnelykke K, Ralston SH, Robbins JA, Walsh JP, Zillikens MC, Langenberg C, Li-Gao R, Williams FMK, Harris TB, Akesson K, Jackson RD, Sigurdsson G, den Heijer M, van der Eerden BCJ, van de Peppel J, Spector TD, Pennell C, Horta BL, Felix JF, Zhao JH, Wilson SG, de Mutsert R, Bisgaard H, Styrkársdóttir U, Jaddoe VW, Orwoll E, Lakka TA, Scott R, Grant SFA, Lorentzon M, van Duijn CM, Wilson JF, Stefansson K, Psaty BM, Kiel DP, Ohlsson C, Ntzani E, van Wijnen AJ, Forgetta V, Ghanbari M, Logan JG, Williams GR, Bassett JHD, Croucher PI, Evangelou E, Uitterlinden AG, Ackert-Bicknell CL, Tobias JH, Evans DM & Rivadeneira F (2018) Life-Course Genome-wide Association Study Meta-analysis of Total Body BMD and Assessment of Age-Specific Effects, American journal of human genetics. 102, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trajanoska K, Morris JA, Oei L, Zheng HF, Evans DM, Kiel DP, Ohlsson C, Richards JB & Rivadeneira F (2018) Assessment of the genetic and clinical determinants of fracture risk: genome wide association and mendelian randomisation study, BMJ (Clinical research ed). 362, k3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho-Le TP, Center JR, Eisman JA, Nguyen HT & Nguyen TV (2017) Prediction of Bone Mineral Density and Fragility Fracture by Genetic Profiling, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 32, 285–293. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson J, Evans DS, Nielson CM, Shen J, Srikanth P, Hochberg M, McWeeney S, Cawthon PM, Wilmot B, Zmuda J, Tranah G, Mirel DB, Challa S, Mooney M, Crenshaw A, Karlsson M, Mellstrom D, Vandenput L, Orwoll E & Ohlsson C (2015) Limited clinical utility of a genetic risk score for the prediction of fracture risk in elderly subjects, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 30, 184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forgetta V, Keller-Baruch J, Forest M, Durand A, Bhatnagar S, Kemp JP, Nethander M, Evans D, Morris JA, Kiel DP, Rivadeneira F, Johansson H, Harvey NC, Mellström D, Karlsson M, Cooper C, Evans DM, Clarke R, Kanis JA, Orwoll E, McCloskey EV, Ohlsson C, Pineau J, Leslie WD, Greenwood CMT & Richards JB (2020) Development of a polygenic risk score to improve screening for fracture risk: A genetic risk prediction study, PLoS medicine. 17, e1003152–e1003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(1998) Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group, Controlled clinical trials. 19, 61–109. [DOI] [PubMed] [Google Scholar]
- 31.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ & Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses, American journal of human genetics. 81, 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenfeld D (1982) Partial Residuals for The Proportional Hazards Regression Model, Biometrika. 69, 239–241. [Google Scholar]
- 33.Therneau TM, Grambsch PM & Fleming TR (1990) Martingale-based residuals for survival models, Biometrika. 77, 147–160. [Google Scholar]
- 34.DeLong ER, DeLong DM & Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach, Biometrics. 44, 837–45. [PubMed] [Google Scholar]
- 35.Demler OV, Paynter NP & Cook NR (2015) Tests of calibration and goodness-of-fit in the survival setting, Statistics in medicine. 34, 1659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R & National Osteoporosis F (2014) Clinician’s Guide to Prevention and Treatment of Osteoporosis, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 25, 2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaëlsson K, Melhus H, Ferm H, Ahlbom A & Pedersen NL (2005) Genetic liability to fractures in the elderly, Archives of internal medicine. 165, 1825–30. [DOI] [PubMed] [Google Scholar]
- 38.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM & Daly MJ (2019) Clinical use of current polygenic risk scores may exacerbate health disparities, Nature genetics. 51, 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.