Abstract
Background:
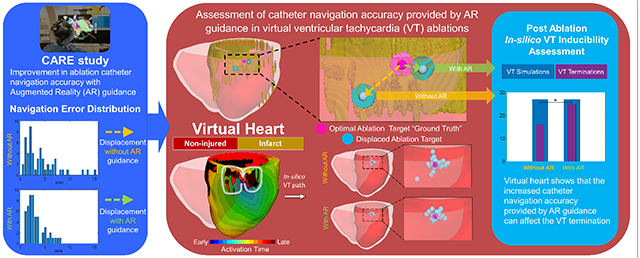
Recently, an augmented reality (AR) solution allows the physician to place the ablation catheter at the designated lesion site more accurately during cardiac electrophysiology studies. The improvement in navigation accuracy may positively affect ventricular tachycardia (VT) ablation termination, however assessment of this in the clinic would be difficult. Novel personalized virtual heart technology enables non-invasive identification of optimal lesion targets for infarct-related VT. This study aims to evaluate the potential impact of such catheter navigation accuracy improvement in virtual VT ablations.
Methods:
2 MRI-based virtual hearts with 2 in silico induced VTs (VT 1, VT 2) were included. VTs were terminated with virtual “ground truth” endocardial ablation lesions. 106 navigation error values that were previously assessed in a clinical study evaluating the improvement of ablation catheter navigation accuracy guided with AR (53 with, 53 without) were used to displace the “ground truth” ablation targets. The corresponding ablations were simulated based on these errors and VT termination for each simulation was assessed.
Results:
In 54 VT 1 ablation simulations, smaller error with AR significantly resulted in more VT termination (25) compared to the error without AR (16) (P<0.01). In 52 VT 2 ablation simulations, no significant difference was observed from error with (11) and without AR (13) (P=0.58). The substrate characteristic may impact the effect of improved accuracy to an improved VT termination.
Conclusion:
Virtual heart shows that the increased catheter navigation accuracy provided by AR guidance can affect the VT termination.
Keywords: ablation, ventricular tachycardia, computer simulation, augmented reality
Graphical Abstract
Introduction
Recent hardware advances in extended reality technology have had a direct impact on the practice of cardiology, including real-time intraprocedural use during cardiac electrophysiology studies. The Enhanced Electrophysiology Visualization and Interaction System (ĒLVIS) is an augmented reality (AR) solution that displays electroanatomic mapping data, acquired through an electroanatomic mapping system (EAMS), and real-time catheter location in true 3 dimensions (3D), as a patient specific cardiac hologram1 In addition, the system provides the electrophysiologist (EP) with the ability to maintain the sterile field and control of their digital data, so that the angle of display can be optimized while maintaining low system latency (<100msec) and display at a high frame rate (between 30-60 frames/second)2, all that allowing for a comfortable visual user experience. The downstream implication of this system for improving clinical care, and specifically patient outcomes, would be improved physician accuracy during catheter navigation when the EP is provided with real-time intraprocedural 3D digital images of the patient’s EAMS maps along with real-time catheter locations in an AR solution. In the Cardiac Augmented Reality (CARE) study,3, physicians were tasked with navigating to 5 designated points within the electroanatomic map using current standard of care (EAMS) versus ĒLVIS, for a total of 10 points per patient. EPs had improved accuracy during point navigation tasks while using ĒLVIS, resulting in an average error of 2.99±1.91mm versus 4.50±3.74mm using a standard EAMS display, for an accuracy improvement of 50%. This improvement is attributed to the combination of true 3D visualization of the cardiac geometry and catheter location, coupled with the physician’s ability to control, manipulate and move the hologram to optimize visual angle4.
We hypothesize that improvement in navigation accuracy will positively affect VT termination. Recently, a novel personalized virtual-heart modeling technology (virtual-heart arrhythmia ablation targeting, or VAAT) has enabled non-invasive identification of optimal lesion targets for infarct-related ventricular tachycardia (VT)5 The in-silico technology provides an excellent platform to explore computationally, at low cost, whether the greater point navigation accuracy achieved using AR guidance is likely to improve clinical VT termination. The goal of this study was to conduct such an assessment using the point navigation error parameters obtained during ĒLVIS clinical testing in the CARE study3.
Methods
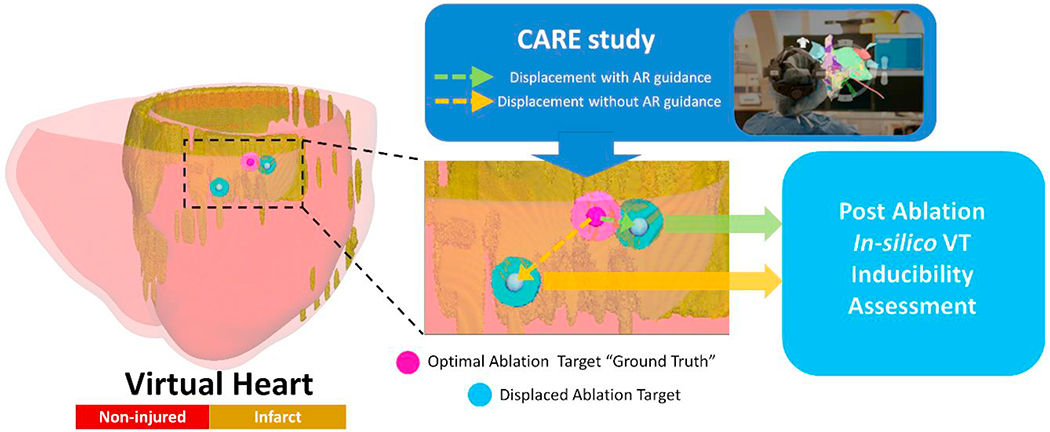
We employed personalized virtual hearts of patients with VTs, presented in our previous work6, with previously calculated optimal ablation targets that terminated the VTs. These optimal ablation targets were considered the “ground truth”. We then conducted simulations of post-ablation VT inducibility but with ablation lesion(s) placed at a distance from the “ground truth”. These distances (“errors in navigation”) corresponded to the distances measured in the CARE study3 for ablation targeting with and without AR guidance. An overview of the approach is presented in Figure 1.
Figure 1.
Study design
The personalized virtual hearts were reconstructed from Late Gadolinium Enhanced Magnetic Resonance images (LGE-MRI) of 2 ischemic cardiomyopathy patients (Figure 2). The virtual hearts incorporated patient-specific distributions of tissue remodeling in the ventricles, including core scar, gray zone (regions of intermediate intensity on LGE-MRI), and non-injured tissue. The Johns Hopkins Institutional Review Board (IRB) approved sharing of de-identified patient LGE-MRI with the modelling team5 The IRB did not require patient informed consent given the retrospective nature of the study.
Figure 2.
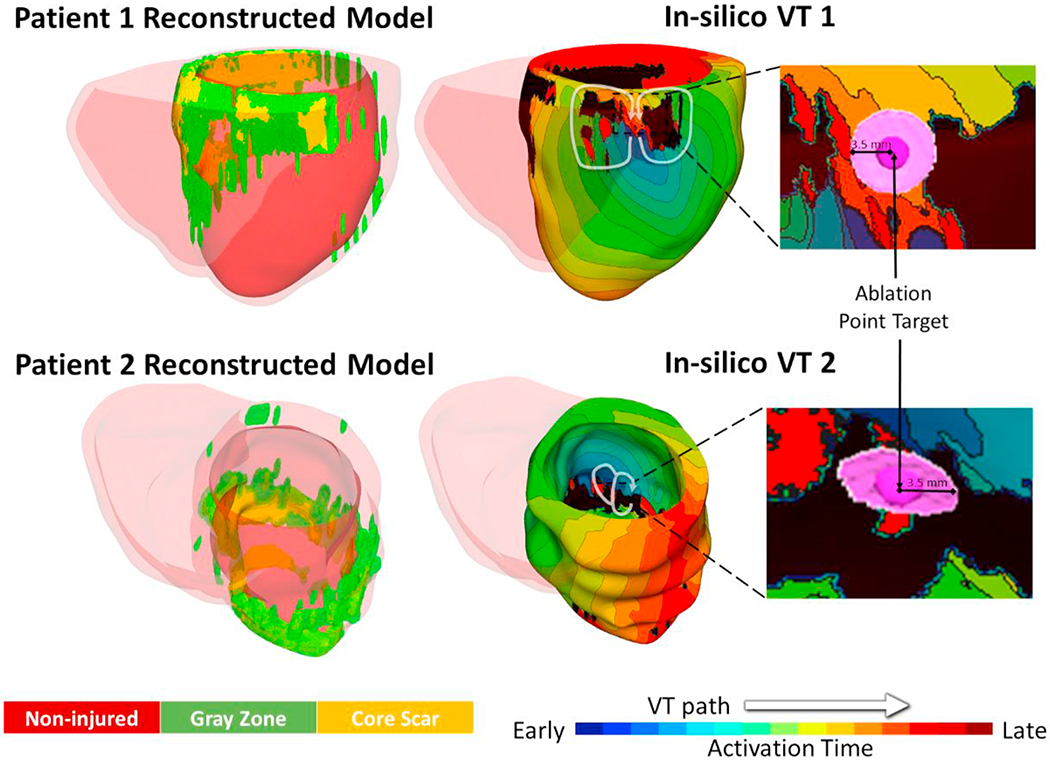
Personalized virtual hearts with induced ventricular tachycardias (VTs). An “I” type critical conduction channel (CCC) sustained the VT 1 for patient 1, and a “T” type CCC sustained the VT 2 for patient 2. Endocardial ablation targets were determined from simulations (regions with 3.5 mm radius).
To establish clearly delineated “ground truth” ablation lesions, we chose VTs sustained by conduction through a single endocardial critical channel. The “ground truth” ablations were 3.5 mm radius lesions placed in the narrowest part of the endocardial conduction channel, terminating the VT (Figure 2). The single endocardial location of the “ground truth” targets ensured easy assessment of the role of navigational error in ablation.
For virtual heart 1, the VT morphology (VT 1) was sustained by a reentrant circuit through an “I” type critical conduction channel (CCC)6 and was terminated with an endocardial right ventricular basal septal “ground truth” lesion (Figure 2 top). A “T” type CCC sustained the VT morphology in virtual heart 2 (VT 2); VT 2 was terminated with an endocardial left ventricular apical “ground truth” ablation lesion (Figure 2 bottom).
As each 3D virtual-heart simulation is computationally intensive, to ensure computational tractability, 106 different “navigation error” displacement values (Figure 3A) were randomly sampled from the 150 values reported in the CARE study3; 53 values of the 106 were acquired in procedures using AR, while the other 53 were acquired without AR. The location of the center of each “ground truth” endocardial ablation was displaced with each of these values to represent the location of the alternative lesion. First, the finite element model (FEM) nodes of the virtual heart endocardial surface were identified. The center of the “ground truth” endocardial ablation was one of these nodes and the “ground truth” endocardial ablation lesion was all FEM volume elements within 3.5 mm radius of this center node. For each one of the 106 “navigation error” values, a random FEM endocardial node at a Euclidean distance, from the “ground truth” center node, equal to this “navigation error” value was selected, and this node represented the alternative lesion center. All FEM volume elements within 3.5 mm radius of this alternative lesion center represented the alternative endocardial ablation lesion. 27 “navigation error” value from procedure with AR (2.96 +/− 1.63 mm) and 27 “navigation error” value from procedure without AR (4.39 +/− 3.20 mm) were used to displace the “ground truth” VT 1 endocardial ablation center (Fig.3B). The remaining 26 “navigation error” value from procedure with AR (3.17 +/− 2.06 mm) and 26 “navigation error” value from procedure without AR (3.93 +/− 3.45 mm) were used to displace the “ground truth” VT 2 endocardial ablation center (Fig.3B). Post-ablation VT inducibility was tested for each displaced ablation lesion; should VT occur with the new ablation location, that would indicate negative VT termination in the virtual heart. The differences of VT inducibility resulting from displacements with and without AR for each VT 1 and VT 2 were assessed using logistic regression analysis.
Figure 3.
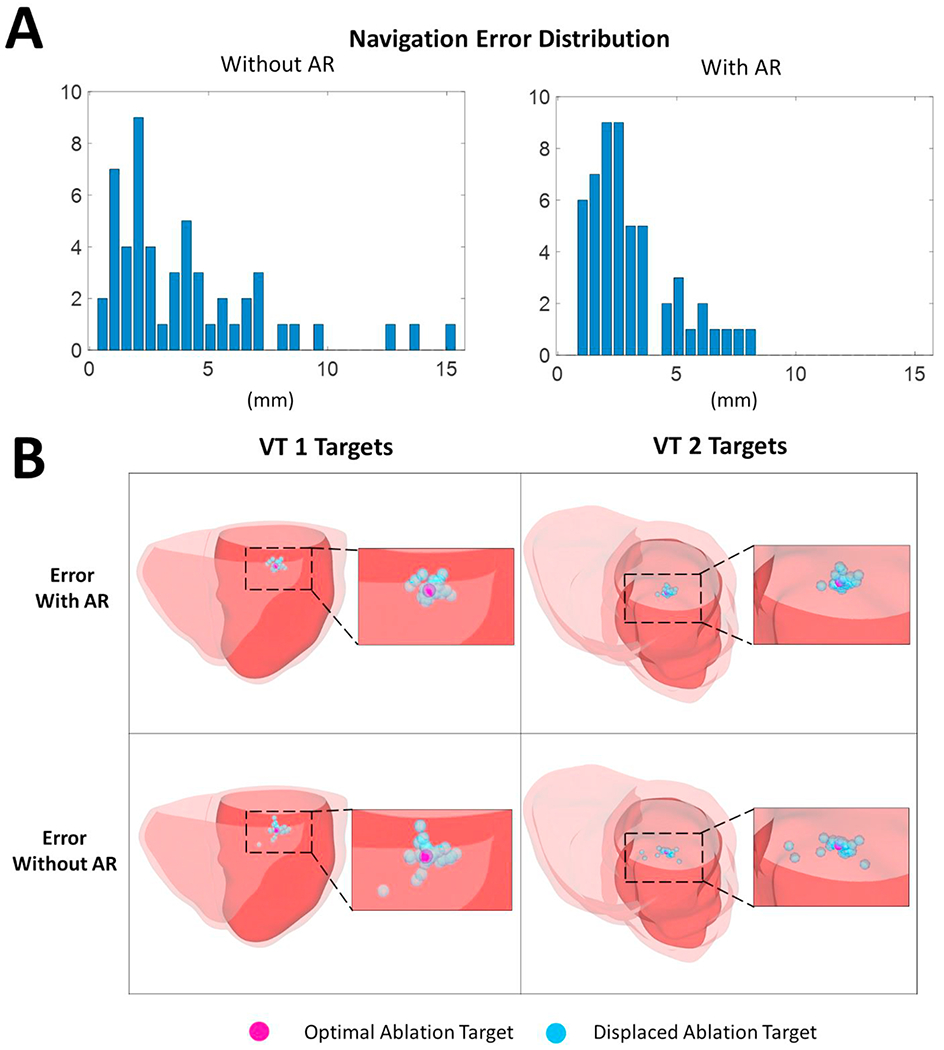
A. 106 navigation error displacement values previously obtained in a clinical study of augmented reality (AR)3. B. Displacement of the “ground truth” locations (pink) on the endocardial surface implemented using the navigation error values (blue).
Results
From the 106 3D virtual-heart simulations, consisting of 54 VT 1 ablation simulations (27 from displacement with AR, and 27 without AR) and 52 VT 2 ablation simulations (26 from displacement with AR, and 26 without AR), more VT terminations (36) were achieved using AR displaced ablation locations compared to those without AR (29). Analysis on each individual dataset showed that for VT 1, smaller displacement in the case of AR significantly resulted in more VT terminations (25) compared to displacement without AR (16) (P < 0.01) (Figure 4). For VT 2, there was no significant difference in the number of VT terminations resulting from ablation location displacement in the case of AR (11) and without AR (13) (P=0.58). The substrate characteristics, in this case the “T”-type CCC (rather than “I”) might prevent the AR-improved navigation accuracy from translating into improved VT termination.
Figure 4.
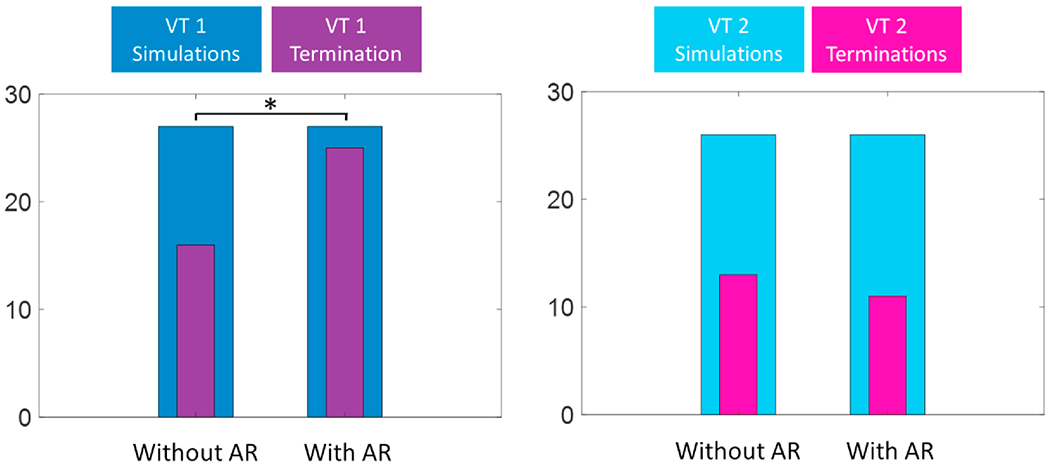
AR-displaced ablation locations resulted in significantly more VT 1 terminations while no significant differences were observed in VT 2 terminations resulted with AR and without.
Discussion
The goal of this study was to assess, using personalized virtual heart technology, whether AR guidance may improve VT termination. Use of the ĒLVIS system has been shown to improve physician ability to navigate to a given target3. This improvement in point navigation would be expected, in turn, to result in improved VT termination, however assessment of this in the clinic is difficult. Virtual heart is a technology that allows to determine ablation targets pre-procedurally and to test whether the ventricles remain inducible after executing ablation at the predicted target locations. It has been successfully tested in human prospective studies5; here, it provided a novel platform to address the issue of ablation accuracy using AR. The current study evaluated the effect of improved ablation catheter navigation accuracy on VT termination in virtual hearts using clinical measurement of catheter navigation accuracy error with and without AR guidance. The study found that the target accuracy error can affect the termination of VT. Improved accuracy due to AR guidance was found to particularly improve the VT termination on the VT dataset with a simpler (“I” type) CCC, while no improved VT termination was observed using the VT dataset with a more complex (“T” type) CCC. These findings may indicate that high ablation catheter navigation accuracy is required to terminate VT in very complex substrates. In the clinical practice, this may translate to more extensive ablation lesion required to be delivered to compensate for the ablation catheter navigational error.
In this study, we used the virtual heart technology as an assay in the evaluation of ablation accuracy, as performing such test on patients in the clinic is not possible. In the future, we also envision potentially an integrated approach combining virtual heart targets prediction and AR in clinical ablation. AR could be instrumental in improving catheter navigation accuracy to targets determined by the virtual heart and imported in EAMS.
The current study has several limitations. First, there were only two VTs evaluated with one of “I” type CCC and another of “T” type CCC, and thereby conclusions cannot be extrapolated to all “I” and “T” type VTs. We did not explore functional reentries as this made the selection of a single endocardial “ground truth” ablation target difficult. While our findings showed the lack of improved VT termination with “T” type CCC from the improved navigation accuracy with AR, this might be also influenced by less differences in displacement errors for AR and without AR evaluated on this VT compared to the ones evaluated on the VT with “I” type CCC. Furthermore, the navigation errors evaluated from the CARE study3 were obtained in Ensite navigators and evaluation of AR navigation accuracy would also need to be performed in other navigators.
Highlights.
Assessment of catheter navigation accuracy provided by AR guidance by virtual heart
Performing such VT ablation test on patients in the clinic would be difficult
Target accuracy error can affect VT termination by ablation
Improved VT termination with AR guidance in I-type VT
No improvement in VT termination with AR guidance in T-type VT
Funding:
The study was supported by NIH SBIR grant to SentiAR, including a subcontract to Johns Hopkins University (AP), and a grant from the Leducq Foundation (NT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
JNAS and JRS are co-founders, officers and directors of SentiAR Inc., JNAS, MKS, and JRS hold equity in SentiAR, Inc. MKS is an employee of SentiAR, Inc. The other authors report no conflicts.
References
- 1.Silva JNA, Southworth M, Raptis C, Silva J: Emerging Applications of Virtual Reality in Cardiovascular Medicine. JACC Basic to Transl Sci 2018; 3:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Southworth MK, Silva JNA, Blume WM, Van Hare GF, Dalal AS, Silva JR: Performance Evaluation of Mixed Reality Display for Guidance during Transcatheter Cardiac Mapping and Ablation. IEEE J Transl Eng Heal Med 2020; 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva JNA, Southworth MK, Blume WM, et al. First-In-Human Use of a Mixed Reality Display for Guidance during Transcatheter Mapping and Ablation. JACC Clin Electrophysiol 2020; 6:1023–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva JNA, Privitera MB, Southworth MK, Silva JR: Development and Human Factors Considerations for Extended Reality Applications in Medicine: The Enhanced ELectrophysiology Visualization and Interaction System ĒLVIS. In Chen JYC, Fragomeni G, eds: Virtual, Augment Mix Reality Ind Everyday Life Appl Cham: Springer International Publishing, 2020, pp. 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakosa A, Arevalo HJ, Deng D, et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng Springer US, 2018; 2:732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng D, Prakosa A, Shade J, Nikolov P, Trayanova NA: Characterizing Conduction Channels in Postinfarction Patients Using a Personalized Virtual Heart. Biophys J 2019; 117:2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]


