Abstract
Muscle spindle afferents are slowly adapting low threshold mechanoreceptors which have both dynamic and static sensitivity to muscle stretch. The exact mechanism by which these neurons translate muscle movement into action potentials is not well understood, although the PIEZO2 mechanically sensitive cation channel is essential for stretch sensitivity. PIEZO2 is rapidly adapting, suggesting the requirement for additional molecular elements to maintain firing during stretch. Spindle afferent sensory endings contain glutamate-filled synaptic-like vesicles which are released in a stretch and calcium dependent manner. Previous work has shown that glutamate can increase and a phospholipase-D coupled metabotropic glutamate antagonist can abolish firing during static stretch. Here we test the hypothesis that vesicle-released glutamate is necessary for maintaining muscle spindle afferent excitability during static but not dynamic stretch. To test this hypothesis, we used a mouse muscle-nerve ex vivo preparation to measure identified muscle spindle afferent responses to stretch and vibration. In C57BL/6 adult mice, bath applied glutamate significantly increased the firing rate during the plateau phase of stretch, but not during the dynamic phase of stretch. Blocking the packaging of glutamate into vesicles by the sole vesicular glutamate transporter, VGLUT1, either with xanthurenic acid or by using a transgenic mouse with only one copy of the VGLUT1 gene (VGLUT1+/−) decreased muscle spindle afferent firing during sustained stretch, but not during vibration. Our results suggest a model of mechanotransduction where calcium entering the PIEZO2 channel can cause the release of glutamate from synaptic-like vesicles which then helps to maintain afferent depolarization and firing.
Introduction
The muscle spindle is a complex sensory organ innervated by afferents which constantly report muscle length and movement. These afferents provide the sensory component for the myotatic reflex and the primary sensory input for proprioception (Proske & Gandevia, 2012). Muscle spindle afferents are slowly adapting low threshold mechanoreceptors with both dynamic and static stretch sensitivity and the molecular machinery necessary to confer this complex mechanotransduction is still not well understood. Loss of the PIEZO2 channel eliminates stretch sensitive activity in the majority of muscle spindle afferents (Woo et al., 2015a) and leads to a scoliosis phenotype in mice (Assaraf et al., 2020). Loss or gain of function mutations to PIEZO2 in human patients leads to deficits consistent with impaired muscle proprioceptor function, including unsteady gaits, reduced or absent tendon reflexes, and/or scoliosis (Coste et al., 2013; Chesler et al., 2016). PIEZO2 is a rapidly adapting mechanically activated cation channel (Coste et al., 2010) that has been identified as important for mechanosensation in a wide range of sensory neurons (Kefauver et al., 2020), including the Merkel cell-neurite complex (Ikeda et al., 2014; Woo et al., 2014), nociceptors (Murthy et al., 2018; Szczot et al., 2018), baroreceptors (Zeng et al., 2018), vagal and nodose ganglion airway innervating neurons (Nonomura et al., 2017), and sensory neurons that control urination (Marshall et al., 2020). Even though PIEZO2 is rapidly adapting, there is a steady-state Merkel cell current in response to mechanical stimulation that is approximately 10% of the peak response (Maksimovic et al., 2014). This residual current coupled with high membrane resistance and/or amplification by other ion channels, including voltage- or mechanically-activated channels, could produce firing that is slowly adapting (Woo et al., 2015b). This suggests that additional molecular elements work together with PIEZO2 in these different cells to produce their unique mechanically sensitive responses.
Muscle spindle afferent receptor endings have synaptic-like vesicles containing glutamate which are released in a stretch and calcium dependent manner (Bewick et al., 2005). Whole nerve firing during stretch is increased when exogenous glutamate is added to a tissue bath (Bewick et al., 2005), although individual muscle spindle afferents were not identified and it is possible that some of the increased firing was due to other sensory neurons, including nociceptors, which are known to have metabotropic glutamate receptors (Lund et al., 2010) and might therefore add to the increased firing rate. However, a 4 hour exposure to an antagonist to the phospholipase-D coupled metabotropic glutamate receptor can eliminate firing during stretch completely, which is further evidence that glutamate is necessary for maintaining muscle spindle afferent excitability during static stretch (Bewick et al., 2005). Stretch-dependent glutamate release seems ideally situated to couple mechanically activated depolarization and increased intracellular calcium levels via PIEZO2 with additional depolarizing current during prolonged stretch, but seems less likely to be able to alter dynamic responses to stretch or vibration. We hypothesized that glutamate released from synaptic-like vesicles is important for maintained firing during the static stretch but not for firing during the dynamic phase of muscle stretch. To test our hypothesis, we used complementary pharmacological and transgenic approaches. We compared identified muscle spindle afferent firing rates during ramp-and-hold stretch and vibration before and after the addition of glutamate and an inhibitor of glutamate packaging into vesicles by vesicular glutamate transporter 1 (VGLUT1), which is the transporter present in muscle spindle afferent receptor endings (Wu et al., 2004). We also compared firing rates during stretch and vibration in animals with 1 copy of the VGLUT1 gene to wild type littermate controls (Fremeau et al., 2004). As expected based on previous work (Bewick et al., 2005), we found increased muscle spindle afferent firing during static stretch following glutamate addition and decreased stretch responses after decreasing glutamate packaging into vesicles either pharmacologically or by eliminating one copy of VGLUT1. In contrast, increasing or decreasing glutamate had little effect on dynamic sensitivity other than changing general afferent excitability.
Methods
Ethical Approval
All methods and procedures in this study were in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Committee for the Update of the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Research, National Research Council (US) and were approved by the San José State University Institutional Animal Care and Use Committee (IACUC; Protocol 990 and 1047). All procedures conform to the principles and regulations laid out in Grundy (2015).
Animals
Fifty C57BL/6 adult male mice (2-4 months old; Simonsen Laboratories, Gilroy, CA, USA) were used for pharmacological experiments. Nineteen adult male and 8 female mice that were wild type (WT) or lacking one copy of VGLUT1 (VGLUT1+/−) were used for transgenic experiments (B6.129X1-Sic17a7tm1Edw/MmcD; Fremeau et al., 2004; Mutant Mouse Research and Resource Center (MMRC), Davis, CA, USA). Only two VGLUT1+/− mice did not have a WT littermate as a control. Mice were housed in cages of 4 to 10, maintained on a 12:12 light dark cycle, and given ad libitum standard laboratory chow and water. On the day of experiment, animals were anesthetized in an induction chamber with inhaled isoflurane (5% isoflurane in 100% O2; flow rate 1.5 L/min) until they were insensitive to toe pinch and their breathing had slowed to less than 20 breaths/min. Once the animals were deeply anesthetized they were decapitated using sharp scissors and the muscle and nerve tissue dissected for experimentation.
Muscle Spindle Afferent Electrophysiological Recordings
Detailed methods have been published elsewhere (Wilkinson et al., 2012; Franco et al., 2014). Briefly, the extensor digitorum longus (EDL) muscle was isolated with its innervating deep peroneal branch of the sciatic nerve in carbogenated (95% O2, 5% CO2) artificial cerebrospinal fluid (containing in mM: 128 NaCl, 1.9 KCl, 1.2 KH2PO4, 26 NaHCO3, 0.85 CaCl2, 6.5 MgSO4, and 10 glucose) with a pH of 7.4 ± 0.05. The muscle was then placed in a tissue bath perfused in 100% oxygenated synthetic interstitial fluid (containing in mM: 123 NaCl, 3.5 KCl, 0.7 MgSO4, 1.7 NaH2PO4, 2.0 CaCl2, 9.5 NaC6H11O (sodium gluconate), 5.5 glucose, 7.5 sucrose, and 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)) with a pH of 7.4 ± 0.05 and a flow rate of 15-30 ml/minute at 24°C. One tendon of the EDL muscle was attached to a stationary post and the other on the lever arm of the length and force controller by 5-0 silk sutures (300C-LR, Aurora Scientific, Inc.; Aurora, ON, Canada). Ramp-and-hold stretches or sinusoidal vibrations were applied to the muscle using the force and length controller. Platinum stimulating electrodes were mounted on either side of the muscle and connected to a stimulator (701C, Aurora Scientific, Inc.). The EDL muscle was maintained at its optimal length (Lo), which is the length at which maximum force is generated by twitch contractions after supramaximal direct stimulation. A glass suction electrode recorded sensory activity from the cut ends of the sciatic nerve. The neural data was amplified by an extracellular amplifier (Model 1800, A-M Systems Sequim, WA), then digitized and recorded (PowerLab, ADInstruments; Sydney, Australia).
Pharmacology Experiments
After finding an individual stretch-sensitive muscle spindle afferent, the EDL muscle was subjected to a series of stretches and vibrations continuously repeated with a 1-minute rest period at Lo in between each muscle stretch or vibration. A ramp-and-hold stretch of 5% Lo (ramp speed 40% Lo/second; held for 4 s) was followed by two 9 s long sinusoidal vibrations (50 μm amplitude, 50 and 100 Hz frequency). This series was repeated for 20 minutes at baseline with no drug and then for 40 minutes following the addition of one of the following drugs: glutamate (1 or 5 mM; Sigma-Aldrich), kainic acid (30, 50, or 100 μM; Tocris Bioscience), or xanthurenic acid, a potent VGLUT1 inhibitor (XA; 3 or 10 mM, Sigma-Aldrich). A subset of experiments was also run with no drug for the entire hour to serve as a control for the effect of time and repeated muscle movement.
Transgenic experiments
Muscles were subjected to a series of ramp-and-hold stretches and sinusoidal vibrations. Ramp-and-hold stretches of 3 stretch lengths were performed in triplicate: 2.5, 5.0, and 7.5% of Lo (ramp speed 40% Lo/s; 4 s hold). Afterwards, the muscle was subjected to sixteen 9 s long vibrations (5, 25, 50, 100 μm amplitude, 10, 25, 50, 100 Hz frequency). One-minute rest intervals followed each muscle length change.
Data analysis
We identified individual muscle spindle afferents in wild type mice by their characteristic slowly adapting increase in firing rate during muscle stretch (Wilkinson et al., 2012). All afferents from VGLUT1+/− mice that altered their firing frequency in response to stretch were included in our sample. Individual units were identified using the Spike Histogram feature of Lab Chart (ADInstruments). Transgenic animals were genotyped after experimentation and experimenters were blinded to the animal’s genotype during analysis. Analysis of the pharmacology experiments was not entirely blinded as the raw files did have notes about which drug was added, but due to the nature of the spike identification we think this was unlikely to bias analysis. Instantaneous firing frequency was measured 10 seconds prior to stretch (Resting Discharge or RD) and at the beginning (Initial Static Time or IST: 0.4 – 0.6 seconds into the stretch) and end of stretch (Final Static Time or FST: 3.25 – 3.75 seconds into stretch). Dynamic Index (DI) described the frequency difference between the highest frequency of the ramp up phase (Dynamic Peak or DP) and IST. In addition, average firing rate during vibration was calculated and we determined whether an afferent could entrain in a 1:1 manner with vibrations. For pharmacology studies, the firing frequencies during drug treatments were normalized to the average firing of all values measured during the pre-drug 20 min baseline period (%BL) to quantify changes in muscle spindle afferent responsiveness following drug exposure. The same normalization to average baseline firing was done for our no drug controls.
Statistics
Statistical analyses were performed using IBM Statistical Package for the Social Sciences (SPSS) Statistics, Version 25. For pharmacology experiments, there was a lot of variability in the onset of firing rate changes so we plotted group averages over time with error bars denoting standard deviation, but did not run statistical tests. We identified the maximum change in firing during the drug exposure window in FST, IST, DP, DI, 50 and 100Hz vibrations and compared by condition versus no drug control. The Shapiro Wilk test of normality was used to determine whether each group was normally distributed. Normally distributed samples were compared using two-tailed Independent Samples t-Tests, equal variances not assumed and non-normally distributed groups were compared using the Independent Samples Mann-Whitney U test. The test used is noted in text for all comparisons. For transgenic experiments, a two factor analysis of variance (ANOVA) model with genotype and stretch length as factors was used to compare the mean instantaneous firing frequencies during ramp-and-hold stretches (RD, IST, FST, DI, DP) between WT and VGLUT1+/− afferents. We compared average firing during vibration between WT and VGLUT1+/− afferents using a three factor ANOVA model, with genotype, vibration amplitude, and vibration frequency as factors. An Independent Samples Mann Whitney U test was used to compare FST at 7.5% stretch – FST at 2.5% stretch in WT mice to those of VGLUT1+/− mice . Data are reported in text as mean ± standard deviation in text. A p-value less than 0.05 was considered statistically significant.
Results
Glutamate increases muscle spindle afferent static sensitivity during ramp-and-hold stretch
We tested the effects of glutamate, the glutamate receptor agonist kainic acid, and the VGLUT1 blocker xanthurenic acid (XA) on muscle spindle afferent firing by adding them to the tissue bath and calculating firing rate changes over time as a % of pre-drug BL firing rates. Overall, glutamate increased muscle spindle afferent firing rates (Fig 1A, D, & G) and blocking glutamate release with XA caused decreases in muscle spindle afferent firing rates during stretch that were evident within minutes of drug addition (Fig 1B-D & G). Occasionally, glutamate exposure even at 1mM led to the recruitment of additional units, potentially nociceptors, that made it impossible to follow the identified muscle spindle afferent and limited our ability to use higher doses of glutamate. To control for the variability in the time of onset of drug effects, we compared the maximum change in firing rates seen following a given drug to the maximum change in firing seen in our no drug controls. Muscle spindle afferents exposed to 1 mM glutamate showed significant increases in firing frequencies during both the beginning and end of the plateau phase of stretch (IST: 120±16%, FST: 119±15% maximum increase as a % BL, n = 12 for both groups, IST: p=0.006, Independent Samples t-Test; FST p=0.005 Mann-Whitney U test, Figure 1E & H). There was significant individual variability, with about half of the afferents showing no appreciable change in firing rate. A small sample of units that we were able to follow at 5 mM glutamate showed a similar split of 2 units responding to glutamate (128% and 137% increase in FST) and 2 units that did not respond (90% and 96% change in FST). As there are many mechanisms to degrade or remove glutamate that might be limiting the effective dose at the afferent, we tried kainic acid, an agonist to the ionotropic kainate receptor and potentially some metabotropic glutamate receptors, which others have reported increases muscle spindle afferent stretch sensitivity at doses as low as 1 μM (Zanato et al., 2014). None of the 9 afferents given kainic acid showed increases in firing rates during the plateau phase of stretch that were greater than the range seen in no drug controls (n= 1 at 30 μM, 6 at 50 μM, 2 at 100 μM; Independent Samples t-Test p=0.805 for IST and p=0.700 for FST; Fig 1E & H). Our results confirm the increase in firing frequency observed in rat whole nerve recordings during stretch (Bewick et al., 2005) in mouse single unit recordings and suggest that the glutamate receptor involved in the increase in firing frequency is not affected by kainic acid.
Figure 1. Glutamate is necessary for maintained firing during stretch.

A. Representative raw neural response before and after 1 mM glutamate addition during ramp-and-hold stretch. Instantaneous frequency of muscle spindle afferent firing before (black) and after glutamate (Glu; blue) shown overlaid below. Similar representative traces following 3 mM xanthurenic acid (XA; red) that caused a reduction in firing during stretch (B; unit XA1 in Fig. 2B as well) or a complete elimination by the end of the plateau phase of stretch (C; unit XA2 in Fig. 2C as well). Time course of the response at the beginning (Initial Static Time or IST; D) or end of stretch (Final Static Time or FST; G) and the maximum firing rate during ramp phase (Dynamic Peak or DP; J) shown for no drug controls (Ctrl; black solid line; n=12), 1 mM Glu (blue dashed line; n=12) or 3 mM XA (red dotted line; n=17). Time when drug was added shown in gray bar. All values expressed as the % of the average response during the 20 min baseline (%BL). Error bars denote ± standard deviation. Maximum increases in firing during the 40 min drug exposure shown as individual points for Ctrl, Glu and kainic acid (KA) during IST (E), FST (H), and DP (K). Individual points for maximum decreases in firing for Ctrl and XA during IST (F), FST (I), and DP (L). Gray lines denote the median and * denotes IST p=0.006, Independent Samples t-Test and FST p=0.005 Mann-Whitney U test.
We next analyzed the response of muscle spindles to static stretch when glutamate packaging into vesicles is reduced by XA. Following 3 mM XA, firing rates were not significantly lower during the plateau phase of stretch on average, but many of the afferents showed large decreases in firing (IST 61±42%, FST 61±42%; XA n = 17, p=0.08 for both, Mann-Whitney U test; Figure 1F & I). Afferents treated with XA could be categorized in 3 populations where some showed little to no change in firing frequency (5 out of 17), others showed a greater than 10% decrease in FST frequency (7 out of 17, Figure 1B), and some showed complete elimination of firing during the plateau phase of stretch (5 out of 17, Figure 1C). In 4 afferents, all firing during stretch was eliminated by the end of the one hour of drug exposure. As expected, the firing during the end of stretch was eliminated first (Fig 1C). A smaller sample of 10 mM XA showed a similar split (3 no change in firing, 2 complete elimination of firing at FST, and 1 59% firing from BL at FST). Since we observed that XA could entirely block spindle afferent excitability to stretch, this confirms that basal release of glutamate is important for maintaining afferent excitability to stretch. There were no significant changes in resting muscle tension between the control and drug groups (Max Increase in RD Tension Ctrl: 104 ± 7%, Glu: 105 ± 12%; Mann-Whitney U test p=0.853; Max Decrease in RD Tension Ctrl: 94 ± 8%, XA: 95 ± 7%; Independent Samples t-Test p=0.680; n=10 for all groups), indicating that the results observed were likely due to drug effects and not changes in muscle tension.
We next tested whether dynamic sensitivity to stretch was also altered by glutamate. Peak firing changes to the dynamic phase of stretch were not significantly changed in muscle spindle afferents treated with glutamate or XA (DP; Ctrl: 116 ± 31%, Glu: 122 ± 19%; Independent Samples t-Test p=0.546; XA 38±62%, n = 17, Mann Whitney U test p=0.180; Figure 1 J-L). Dynamic Index (DP-IST) was not significantly changed for either drug (DI; Max Increase Ctrl: 128± 44%, Glu: 138 ± 46%; Mann-Whitney U test p=0.219; Max decrease Ctrl: 74± 15%, XA: 57 ± 41%; Mann-Whitney U test p=0.679). Significant increases in firing frequency were seen during 50 and 100 Hz vibrations in muscle spindle afferents treated with glutamate that were unable to entrain in a 1:1 manner to sinusoidal vibrations (Figure 2A, E-F, H-I; Ctrl n=11, Glu n=11; 50 Hz Mann-Whitney U test p=0.023; 100 Hz Independent Samples t-Test p=0.013), but muscle spindle afferents that entrained to vibrations (4 of 11) continued to entrain upon glutamate addition. This suggests that overall muscle spindle afferent excitability is increased in the presence of glutamate, but it does not override the ability to entrain to vibration. Following XA, muscle spindle afferent firing in response to vibration was trending down and completely eliminated in 2 afferents, but the differences were not significant (XA n=17; Mann-Whitney U test 50 Hz p=0.547; 100 Hz p=0.458; Fig 2B-E, G-H, J). Interestingly, 3 of the 5 muscle spindle afferents that showed complete elimination of firing following XA during ramp-and-hold stretch did fire during vibrations. In one case, the afferent could still entrain to 50 and 100 Hz vibrations (Fig 1C & 2C). This suggests that dynamic muscle stretch could still depolarize the afferents to threshold, presumably through current via PIEZO2 and/or other mechanically sensitive ion channels. Overall, our pharmacological studies suggest that glutamate released from synaptic-like vesicles regulates muscle spindle afferent excitability and is most important for maintaining firing during the static phase of a stretch.
Figure 2. Glutamate is not important for mediating dynamic sensitivity.

A. Representative raw neural response to 1 mM glutamate (Glu) in an afferent that could not entrain to 50 Hz vibration. Instantaneous frequency of muscle spindle afferent firing before (black) and after 1 mM glutamate (Glu; blue) shown overlaid below. All 4 afferents that could entrain to vibration before Glu continued to entrain post-Glu. Similar representative traces following 3 mM xanthurenic acid (XA; red) that caused a reduction in firing during stretch but no change in the ability to entrain to 100 Hz (B; same unit as Fig. 1B) and a unit that ceased firing during the static phase of stretch following XA but could still entrain to 50 Hz vibration (C; same unit as Fig. 1C). Example of 1 of 2 units following XA that eliminated firing during both ramp-and-hold stretch and vibration (D). Time course of the change in average firing rate during 50 Hz (E) or 100 Hz vibrations (H) shown for no drug controls (Ctrl; black solid line; n=11), 1 mM Glu (blue dashed line; n=11) and 3 mM XA (red dotted line; n=17). Time when drug was added shown in gray bar. All values expressed as the % of the average response during the 20 min baseline (%BL). Error bars denote ± standard deviation. Maximum increases in firing during the 40 min drug exposure shown as individual points for Ctrl and Glu during 50 Hz (F) and 100 Hz vibrations (I). Individual points for maximum decreases in firing for Ctrl and XA during 50 Hz (G) and 100 Hz vibrations (J). Gray lines denote the median. * denotes 50 Hz Average Firing Frequency p=0.023, Mann-Whitney U test and 100 Hz Average Firing Frequency p=0.013, Independent samples t-test.
Decreased VGLUT1 levels reduces muscle spindle afferent static stretch sensitivity
Due to the variability of our pharmacological studies, we attempted to confirm our findings using mice with one copy of the VGLUT1 gene (VGLUT1+/−) or wild type (WT) littermates. Complete elimination of VGLUT1 is lethal after 3 weeks without supportive care and there is no floxed allele currently available to target the knockout to muscle proprioceptors (Fremeau et al., 2004). As in the XA drug addition studies, there was heterogeneity in the responses of individual afferents, presumably due to variable VGLUT1 protein levels in individual afferents and/or compensatory changes in other regulators of neuronal excitability. In muscle spindle afferents of WT mice, the response to stretch varied in terms of absolute change in frequency to stretch, with some spindle afferents more responsive than others (Fig. 3A-B). However, muscle spindle afferents in all WT mice fired continuously during the entire period of stretch. In contrast, this consistent firing pattern was present in most (Fig 3C), but not all muscle spindle afferents from VGLUT1+/− mice. Three out of 14 muscle spindle afferents could not fire throughout the entire stretch (Fig. 3D, E), which was similar to the inability to fire through the hold phase of stretch that was also observed in 5 of 17 XA-treated muscle spindle afferents (Fig. 1C). Average firing rates at the beginning (IST) and end (FST) of stretch were significantly lower in the VGLUT1+/− group (Fig. 3F-G; two-way ANOVA, main effect of genotype IST p = 0.011; FST p = 0.010; n=14 for both groups). In previous studies, FST firing rates increased linearly over the range of stretch lengths given here (Wilkinson et al., 2012). The FST firing rate at 7.5% Lo stretch was the same or less than the FST firing rate at 2.5% Lo only in 4 of 14 VGLUT1+/− afferents (Fig. 3H). However, the difference in FST firing rate at the highest and lowest stretch lengths (FST at 7.5% minus FST at 2.5% Lo stretch) was not significantly different between genotypes (Mann-Whitney U test, p = 0.194). The significantly decreased firing rates during the plateau phase of stretch that we observed are strikingly similar to those seen with pharmacological blockade of VGLUT1 and support the hypothesis that vesicle-released glutamate is needed to maintain the firing of muscle spindle afferents during sustained stretch.
Figure 3. Muscle spindle afferents of VGLUT1+/− mice have lower firing frequencies during static phase of stretch.
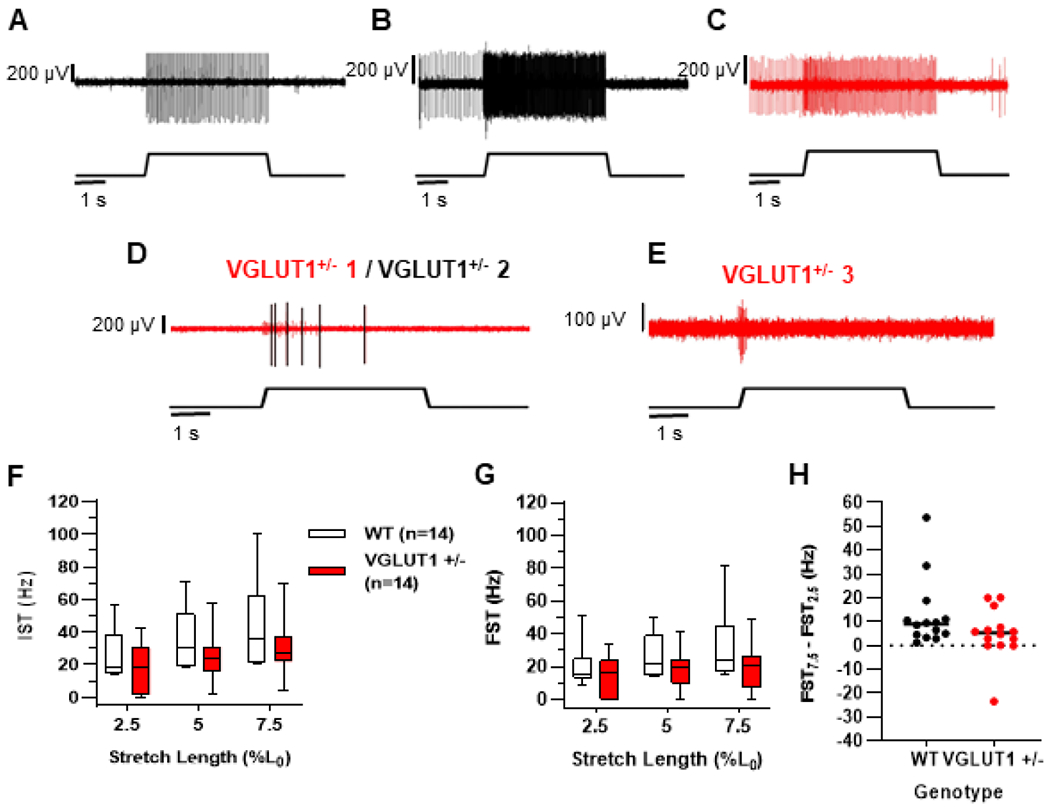
Characteristic responses of muscle spindle afferents isolated from WT mice to static stretch (A & B). Normal stretch response in firing pattern of VGLUT1+/− mice during the ramp-and-hold stretch seen in 11 of 14 afferents (C). Three out of 14 afferents of VGLUT1+/− mice failed to consistently fire throughout stretch (D; afferent VGLUT1+/−1 shown in red and VGLUT1+/−2 shown in black; E afferent VGLUT1+/−3; same afferents shown in Fig. 4B). Boxplots of firing rates of muscle spindle afferents during IST (F) and FST (G) at all 3 stretch lengths. Line represents group median and bars represent maximum and minimum group values. Differences in average firing rates between the WT (black; n = 14) and VGLUT1+/− afferents (red; n = 14) during IST and FST significantly different as determined by a two-way ANOVA (main effect of genotype, IST p = 0.011 and FST p = 0.10). (H) Difference in firing at FST at the 7.5% Lo stretch and that of the 2.5% Lo stretch were compared between genotypes. The black line represents the mean for a given group. No significant group differences observed (p = 0.194; Mann-Whitney UTest), but 4 VGLUT1+/− afferents had values of 0 or less, something never observed in WT afferents.
Decreased VGLUT1 levels do not change dynamic sensitivity
There was a less pronounced trend for lower firing rates in the VGLUT1+/− mice during the dynamic phase of ramp-and-hold stretch that was not statistically significant (Two-way ANOVA, main effect on genotype, p = 0.190 for DP and p = 0.736 for DI). To further measure dynamic sensitivity of muscle spindle afferents, we also measured the response to sinusoidal vibrations. Based on raw neural data, responses to vibrations appeared phenotypically normal for most muscle spindle afferents of either WT or VGLUT1+/− mice, with a majority of animals belonging to both groups able to respond at least to the largest vibration amplitudes for the duration of the vibration (Fig 4A). Notably, only 1 of 3 muscle spindle afferents that failed to fire for the entire static stretch could not respond to any vibrations, while the other 2 had normal or greater than normal responses to vibration (Fig 4B; filled red circles on 4C-D). In fact, one of the afferents that could not maintain firing during static stretch was the only afferent of any genotype that could entrain to all vibrations of 25 μm amplitude or greater, including the 100 Hz vibrations (Fig 4C). This was similar to what was previously seen following XA administration, where 3 of 5 XA-treated muscle spindle afferents that exhibited an absence of firing during ramp-and-hold stretches had normal or slightly reduced firing during vibration (Fig. 2 B-C, G & J). There was no significant difference between the average firing rate during vibration in VGLUT1+/− afferents (Fig. 4C-D; three-way mixed ANOVA, main effect on genotype, p = 0.904). In contrast to the decreased afferent responsiveness to static stretch, VGLUT1+/− afferents were as sensitive to dynamic muscle movement during vibrations as WT controls. These results are similar to what was seen following XA administration and further support the hypothesis that vesicle-released glutamate does not contribute appreciably to afferent dynamic sensitivity.
Figure 4. There is no difference in response to vibration in VGLUT1+/− afferents.

Most afferents of both genotypes had fairly normal responses to vibration (A). Top trace is raw neural firing of a typical WT (black) and bottom trace a VGLUT1+/− (red) muscle spindle afferent to a 50 μm/100 Hz vibration (length change on bottom). Firing patterns of the 3 muscle spindle afferents of VGLUT1+/− mice that failed to fire consistently throughout ramp-and-hold stretches (B; afferent labels the same as those used in raw traces Fig 3D-E) to a 25 μm/50 Hz vibration. 2 of 3 afferents responded at levels at or above the average change in firing rate for WT afferents. The third unit, VGLUT1+/−2, did not fire at all during vibration. Individual average firing rates in response to 25 μm (C) or 50 μm (D) vibrations of 4 amplitudes (x axis). WT afferents (n=14) are shown by filled black circles. VGLUT1+/− afferents shown in open red circles. Filled red circles denote the response of the 3 afferents that did not maintain firing during static stretch. Gray lines denote group median. Data from 5 μm and 100 μm vibrations not shown, but followed a similar pattern. There was no effect of genotype on average firing frequency during vibration (3 factor ANOVA, main effect of genotype p= 0.904).
Discussion
In this study we investigated the role of vesicle-released glutamate in muscle spindle afferent function. Specifically, we tested the hypothesis that vesicle-released glutamate maintains afferent excitability during sustained stretch, but is unnecessary for dynamic sensitivity. By following identified single muscle spindle afferents, we found that glutamate increased afferent firing during the static phase of stretch and that its effects were usually apparent within minutes (Fig 1), which confirms what others have reported when looking at whole nerve firing in rats (Bewick et al., 2005). In contrast, glutamate did not alter dynamic sensitivity, including peak firing during ramp-and-hold stretch (DP). Similarly, glutamate could increase overall excitability and firing rates in afferents that were not entraining to 50 or 100 Hz vibrations, but did not alter the firing rate of those afferents that were already entraining pre-drug (Fig 2). There was a lot of variability in individual afferent responses, with many afferents unaffected by glutamate. Given the totality of our results, we think that this is most likely due to individual differences in effective glutamate dose at a given afferent, as glutamate may be degraded or transported into cells by excitatory amino acid transporters before reaching our recorded afferent and the spindle capsule is selectively permeable and a barrier to diffusion (Dow et al., 1980). However, we have not formally eliminated the possibility that certain afferents are more reliant on glutamate signaling.
To further confirm that glutamate released from vesicles increases static but not dynamic sensitivity, we decreased glutamate packaging into vesicles by VGLUT1 either by pharmacological blockade with XA or by eliminating one copy of the VGLUT1 gene. We used both approaches because although XA is a potent glutamate transport inhibitor, it can also act as a Group II metabotropic glutamate receptor agonist (Copeland et al., 2013). It is unclear whether spindle afferent endings have Group II metabotropic glutamate receptors (mGluR2 and mGluR3), although developing muscle proprioceptors do appear to have mGluR3 expression (Wu et al., 2019). Both approaches yielded qualitatively similar results that also supported the results seen following glutamate administration and by others in rats (Bewick et al., 2005). Firing rates during the beginning and end of the plateau phase of stretch were decreased or eliminated in the majority of afferents treated with XA (Fig 1), although we saw similar individual variability in response to pharmacological block with XA as we did with glutamate. In afferents where firing was completely eliminated during stretch, firing during the later phase of stretch (FST) was the first to be eliminated or decreased and in some cases firing only occurred during the ramp phase of stretch (Fig 1). We also observed this pattern in 3 of 14 VGLUT1+/− afferents which could not maintain firing during stretch, something never observed in WT animals. Additionally, average firing rates during static stretch were significantly lower in the VGLUT1+/− afferents (Fig 3). Our extracellular recording technique relies on finding units that respond to stretch. As such, those afferents with the highest stretch sensitivity are more likely to be sampled and we would completely miss any that had low enough glutamate release to eliminate firing during stretch as we saw in the XA experiments. Given the heterogeneous responses of VGLUT1+/− afferents, there was potentially individual variability in VGLUT1 protein levels between afferents. Mice with both copies of the VGLUT1 gene eliminated do not survive without intensive support after 3 weeks (Fremeau et al., 2004), but if we were able to completely eliminate VGLUT1 in muscle spindle afferents, we think it is likely that we would have seen more dramatic changes in static stretch sensitivity. Overall, though, there is consensus between both the XA and transgenic studies that confirms an important role for vesicle-released glutamate in maintaining afferent excitability, especially during the static phase of stretch.
Firing rates during vibration were less affected by limiting glutamate release than during ramp-and-hold stretch, similar to what was observed following glutamate addition. In fact, in 3 of 5 afferents where firing could not be maintained during static stretch after XA, the response to vibration was either normal or less markedly reduced than the response during stretch (Fig 2). Similarly, in 2 of the 3 VGLUT1+/− afferents which could not maintain firing throughout ramp-and-hold stretch, the response to vibration was similar or slightly greater than the WT average (Fig 4). In fact, the only afferent that could entrain to all vibrations except the 5 μm amplitude vibrations, was a VGLUT1+/− afferent which could not maintain firing throughout ramp-and-hold stretch. There was no difference in the response to vibration when all VGLUT1+/− afferents were compared to WT (Fig 4), which contrasts sharply with the significantly decreased response during static stretch. This suggests that depolarizing current through PIEZO2 and/or other mechanically sensitive channels is enough during dynamic stretch to depolarize the afferent to threshold even with reduced glutamate release. Compensatory changes in the expression of other membrane potential modulating proteins could contribute to the relatively unchanged dynamic sensitivity in VGLUT1+/− afferents and this interesting possibility should be explored in future studies. Overall, the totality of our results support an important role for vesicle-released glutamate in maintaining afferent excitability during static stretch, which is not important for the dynamic response to stretch.
Potential model for spindle afferent mechanotransduction
The ability to produce both static and dynamic sensitivity to muscle movement likely involves many molecular components and the unique mechanical forces transmitted by the specific intrafusal fiber(s) contacted by a given muscle spindle afferent. Recent work has shown that the rapidly-adapting mechanically activated non-selective cation channel PIEZO2 is necessary for mechanosensitivity in muscle proprioceptors (Woo et al., 2015a) as well as many other sensory neurons (Kefauver et al., 2020). Muscle spindle afferents have synaptic-like vesicles containing glutamate that are released in a stretch and calcium dependent manner (Bewick et al., 2005). In both rats (Bewick et al., 2005) and the mice studied here, glutamate is essential for afferent excitability during static stretch. It seems likely that muscle spindle afferent synaptic-like-vesicles are the relevant source of vesicle-released glutamate mediating the responses we see when VGLUT1 activity is reduced since that is the only glutamate transporter expressed on muscle spindle afferent endings (Wu et al., 2004). Even though the VGLUT1+/− mice we used had a whole body reduction in VGLUT1 levels, in our reduced muscle-nerve preparation we know of no other cell type that both has VGLUT1 and is likely to alter the firing properties of muscle spindle afferents. Glutamate is co-released at the neuromuscular junction (Colombo & Francolini, 2019), but we did not observe any changes in muscle tension to suggest this was causing an appreciable effect on muscle fibers. VGLUT3 and not VGLUT1 seems to be the main vesicular glutamate transporter at the neuromuscular junction in leg muscles (Boulland et al., 2004; Kraus et al., 2004), which further eliminates the possibility that our results were due to alterations in muscle or muscle spindle length.
As calcium can enter through PIEZO2 (Coste et al., 2010), this provides a pathway for initial afferent depolarization to be coupled to glutamate containing synaptic-like vesicle release. We note that there is also evidence for acetylcholine containing vesicles in the afferent endings and that acetylcholine acts as a negative regulator of muscle afferent excitability (Zhang et al., 2014; Gerwin et al., 2019). We would predict that calcium entry through PIEZO2 likely regulates acetylcholine containing vesicle release as well. The target for synaptic-like vesicle released glutamate has not been definitively identified, but a metabotropic glutamate autoreceptor seems likely. Blocking ionotropic receptors with kynurenic acid does not block the effect of glutamate on whole nerve firing in a rat model (Bewick et al., 2005), suggesting ionotropic receptors are not involved in the response. A specific antagonist to the phospholipase-D coupled metabotropic glutamate receptor (PLD mGluR) can eliminate stretch-evoked firing in a rat model, but it took over 2 hrs to see any effect (Bewick et al., 2005; Zanato et al., 2014). This is much longer than it took for us to observe changes following glutamate addition or pharmacological blockade of glutamate packaging. The existence of a PLD coupled mGluR has been suggested pharmacologically (Pellegrini-Giampietro et al., 1996), but the structure and sequence is as of yet undefined which hinders study of this receptor. Others have shown that 1 μM kainic acid can increase whole nerve firing during stretch in rats and that this effect can be blocked with a PLD mGluR antagonist (Zanato et al., 2014). In contrast, we found no evidence that kainic acid at much higher doses (30-100 μM) could alter muscle spindle afferent firing in mice in the hour timeframe where we saw both glutamate and XA effects. We found recruitment of non-muscle spindle afferents with high doses of glutamate and think it is possible that this discrepancy is due to the recruitment of non-muscle spindle afferents by kainic acid in the previous study, since muscle spindle afferent identity was not confirmed. It is also possible there is a longer latency effect on spindle afferent excitability mediated through kainic acid sensitive receptors that is separate from the processes that we observed. However, we cannot rule out the possibility that diffusion limitations or species differences contributed to our inability to see a response to kainic acid, especially since we found quite a bit of variability in individual afferent responses to all drugs tested in this study. Spindle afferents in the masseter muscle show high immunohistochemical labeling of mGluR5, which seems like a strong alternative candidate for mediating the glutamate effect (Lund et al., 2010). Recent advances in single cell profiling of muscle spindle afferents holds great promise for identifying candidate glutamate receptors that can then be tested using a combination of pharmacological and transgenic approaches (Wu et al., 2019; Kim et al., 2020; Oliver et al., 2020).
Recordings of the muscle spindle afferent receptor potential show that the majority of the ionic current is from sodium (Hunt et al., 1978), suggesting other sodium channels in addition to PIEZO2 are contributing to afferent depolarization. In fact, the drugs riluzole and phenytoin, which share only the ability to block persistent inward sodium currents (INaP), cause specific decreases in static phase firing which can be rescued with superimposed vibration (Vincent et al., 2016). This ability for dynamic stretch to overcome firing inhibition exactly matches our observation when we reduced glutamate release by decreasing VGLUT1 activity. Candidates for mediating this INaP include the voltage gated sodium channel subtypes NaV1.6, NaV1.1, or NaV1.7, which are all detected either on the sensory endings, pre-terminal axons, and/or heminodes (Carrasco et al., 2017). Interestingly, metabotropic glutamate signaling via mGluR5 in CA1 dendrites can increase NaV1.6 mediated INaP and repetitive firing, an effect also blocked by riluzole (Yu et al., 2018a; Yu et al., 2018b). This is a very attractive potential pathway connecting glutamate release to INaP via mGluR5, which is known to be present on muscle spindle afferents (Lund et al., 2010). However, future studies are necessary determine whether glutamate increases afferent excitability by directly altering voltage gated sodium channel activity or through a redundant parallel pathway.
In summary, we propose that PIEZO2 is the first channel to open during muscle movement and that depolarizing current through that channel is necessary to elicit the initial action potentials (Woo et al., 2015a). The calcium entering through PIEZO2 causes the release of synaptic-like vesicles containing glutamate which then act on an as yet unidentified glutamate autoreceptor, potentially a PLD coupled mGluR (Bewick et al., 2005) or mGluR5 (Lund et al., 2010). Glutamate signaling then leads to additional sodium current that acts to maintain muscle spindle afferent excitability during prolonged stretch. A promising candidate to mediate this activity is the NaV1.6 channel (Carrasco et al., 2017), although additional experiments are necessary to confirm this model. We note that this model (Fig. 5) is qualitatively similar for one proposed to mediate mechanotransduction in the Merkel cell-neurite complex where PIEZO2 mediated current in the innervating neuron is necessary for the initial response to stretch and neurotransmitters released from the Merkel cell in a PIEZO2 dependent manner are necessary to maintain firing (Woo et al., 2015b). However, our model is incomplete, as many additional molecular contributors have been identified that modulate stretch responses in muscle spindle afferents, including the mechanically gated DEG/ENaC (Simon et al., 2010; Lin et al., 2016) and Tentonin channels (Hong et al., 2016), voltage dependent potassium channels including Kv3.3 (Housley et al., 2020), calcium activated potassium channels (Shenton et al., 2014), the PDZ-scaffold protein Whirlin (de Nooij et al., 2015), vesicle released acetylcholine (Gerwin et al., 2019), and likely many others. This model also does not account for the dynamic inhibition of firing in response to the release of stretch.
Figure 5. Potential model for muscle spindle afferent mechanotransduction.

Schematic of the muscle spindle (top) and potential signaling pathway for synaptic-like vesicle released glutamate (bottom; modified from (Bewick et al., 2005)). Muscle stretch opens PIEZO2 which allows both Na+ and Ca++ entry into the cell and is likely one of the primary drivers of membrane depolarization during the dynamic phase of stretch. Ca++ can cause the fusion of glutamate containing synaptic-like vesicles and the released glutamate likely binds to a receptor on the afferent membrane. Potential candidates are a phospholipase-D coupled metabotropic glutamate receptor (PLD mGluR; (Bewick et al., 2005)) or metabotropic glutamate receptor 5 (mGluR5; (Lund et al., 2010)). Glutamate binding leads to additional depolarizing current potentially by increasing the activity of voltage-gated sodium channels, possibly NaV1.6, NaV1.7, and/or NaV1.1, which are all located in the afferent endings (Carrasco et al., 2017). Alternatively, glutamate increases depolarization via another unidentified mechanism. Figure created using BioRender.com.
Potential therapeutic relevance of muscle spindle afferent released glutamate
Our results have confirmed that vesicle-released glutamate is essential for maintaining muscle spindle afferent excitability, especially during prolonged stretch. Interestingly, treatment with the chemotherapy drug oxiplatin leads to a similar reduction in muscle spindle afferent firing during static muscle stretch (Vincent et al., 2016), that is even more pronounced when combined with a cancer model in rats (Housley et al., 2020). While other proteins like the voltage-gated potassium subunit Kv3.3 currently seem more promising for mediating these deficits, cancer and oxiplatin do alter expression of genes involved in glutamatergic synaptic transmission (Housley et al., 2020). Even if alterations in afferent ending glutamate release is not causal to the dysfunction observed, increasing muscle spindle afferent glutamate release or signaling may help normalize muscle spindle afferent activity depending on the causal change that occurs during cancer and chemotherapy.
In addition to its role in maintaining muscle spindle afferent excitability, there is some evidence that synaptic-like vesicle released glutamate may also be involved in mediating nociception during some models of muscle pain. Masseter muscle spindle afferents have increased excitability during a model of delayed onset muscle soreness produced by two injections of acidic saline (Lund et al., 2010). The development of both ipsilateral and contralateral allodynia can be blocked by injecting either ionotropic or metabotropic glutamate receptor antagonists into the ipsilateral muscle. There are small caliber fibres with both mGluR5 and nociceptive markers, like CGRP, Substance P and TRPV1, in close proximity to muscle spindle afferent annulospiral endings that have the potential to be activated by spindle afferent released glutamate (Lund et al., 2010). Increased activity in muscle spindle afferents is also seen following one hypertonic saline injection in jaw muscles (Ro & Capra, 2001) and in a rat model of chronic fatigue syndrome (Yasui et al., 2019). Joint immobilization in the chronic fatigue syndrome model led to decreased pain behavior (Yasui et al., 2019), suggesting increased muscle spindle afferent activity could have been driven by sensitization in the periphery. This allows for the possibility that the efficacy of muscle-injected glutamate antagonists in blocking pain behavior (Lund et al., 2010) may have been at least partially due to decreased peripheral muscle spindle afferent excitability. Future studies should investigate the importance of muscle spindle afferent released glutamate to the development of muscle pain conditions.
Summary
In summary, vesicle-released glutamate is essential for maintaining muscle spindle afferent excitability, but is unnecessary for dynamic sensitivity. A better understanding of the signaling pathway that underlies this increased excitability has important implications for understanding not only mechanosensation in spindle afferents but may also define targets for therapeutic intervention during diseases with lower or higher afferent excitability than normal.
Supplementary Material
Key Points.
Muscle spindle afferents are slowly adapting low threshold mechanoreceptors that report muscle length and movement information critical for motor control and proprioception.
The rapidly adapting cation channel PIEZO2 has been identified as necessary for muscle spindle afferent stretch sensitivity, but the properties of this channel suggest additional molecular elements are necessary for mediating the complex slowly adapting response of muscle spindle afferents.
We report that glutamate increases muscle spindle afferent static sensitivity in an ex vivo mouse muscle nerve preparation, while blocking glutamate packaging into vesicles by the sole vesicular glutamate transporter, VGLUT1, either pharmacologically or by transgenic knock out of one allele of VGLUT1 decreases muscle spindle afferent static but not dynamic sensitivity.
Our results confirm that vesicle-released glutamate is an important contributor to maintained muscle spindle afferent excitability and may suggest a therapeutic target for normalizing muscle spindle afferent function.
Acknowledgements
The authors would like to thank Sulekha Anand for advice on the statistical analyses used and Stephan Kröger for his thoughtful suggestions which improved the article.
Funding Sources
This work was supported by a National Institutes of Health (NIH) research grant (SC3 GM127195; KAW), NIH Research Initiative for Scientific Enhancement (RISE) Fellowship (#5R25GM71381; SO, AS, SRV, NKV), and NIH Maximizing Access to Research Careers (MARC) fellowship (#2T34GM008253; NKV).
Footnotes
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.
Competing Interests
The authors declare no competing interests.
References
- Assaraf E, Blecher R, Heinemann-Yerushalmi L, Krief S, Carmel Vinestock R, Biton IE, Brumfeld V, Rotkopf R, Avisar E, Agar G & Zelzer E. (2020). Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nature communications 11, 3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GS, Reid B, Richardson C & Banks RW. (2005). Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic-like vesicles: evidence from the rat muscle spindle primary sensory ending. J Physiol 562, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT Jr, Edwards RH, Storm-Mathisen J & Chaudhry FA. (2004). Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. Journal of Comparative Neurology 480, 264–280. [DOI] [PubMed] [Google Scholar]
- Carrasco DI, Vincent JA & Cope TC. (2017). Distribution of TTX-sensitive voltage-gated sodium channels in primary sensory endings of mammalian muscle spindles. J Neurophysiol 117, 1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, Hayes LH, Alter K, Zampieri C & Stanley C. (2016). The role of PIEZO2 in human mechanosensation. New England Journal of Medicine 375, 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo MN & Francolini M. (2019). Glutamate at the Vertebrate Neuromuscular Junction: From Modulation to Neurotransmission. Cells 8, 996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland CS, Neale SA & Salt TE. (2013). Actions of Xanthurenic Acid, a putative endogenous Group II metabotropic glutamate receptor agonist, on sensory transmission in the thalamus. Neuropharmacology 66, 133–142. [DOI] [PubMed] [Google Scholar]
- Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G & Steen U. (2013). Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proceedings of the National Academy of Sciences 110, 4667–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE & Patapoutian A. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooij JC, Simon CM, Simon A, Doobar S, Steel KP, Banks RW, Mentis GZ, Bewick GS & Jessell TM. (2015). The PDZ-Domain Protein Whirlin Facilitates Mechanosensory Signaling in Mammalian Proprioceptors. The Journal of Neuroscience 35, 3073–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow PR, Shinn SL & Ovalle WK Jr. (1980). Ultrastructural study of a blood-muscle spindle barrier after systemic administration of horseradish peroxidase. American Journal of Anatomy 157, 375–388. [DOI] [PubMed] [Google Scholar]
- Franco JA, Kloefkorn HE, Hochman S & Wilkinson KA. (2014). An in vitro adult mouse muscle-nerve preparation for studying the firing properties of muscle afferents. Journal of visualized experiments : JoVE, 51948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA & Edwards RH. (2004). Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science 304, 1815–1819. [DOI] [PubMed] [Google Scholar]
- Gerwin L, Haupt C, Wilkinson KA & Kröger S. (2019). Acetylcholine receptors in the equatorial region of intrafusal muscle fibres modulate mouse muscle spindle sensitivity. J Physiol 597, 1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G-S, Lee B, Wee J, Chun H, Kim H, Jung J, Cha JY, Riew T-R, Kim GH & Kim I-B. (2016). Tentonin 3/TMEM150c confers distinct mechanosensitive currents in dorsal-root ganglion neurons with proprioceptive function. Neuron 91, 107–118. [DOI] [PubMed] [Google Scholar]
- Housley SN, Nardelli P, Carrasco DI, Rotterman TM, Pfahl E, Matyunina LV, McDonald JF & Cope TC. (2020). Cancer Exacerbates Chemotherapy-Induced Sensory Neuropathy. Cancer Research 80, 2940–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C, Wilkinson R & Fukami Y. (1978). Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J Gen Physiol 71, 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D & Gu JG. (2014). Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 157, 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefauver JM, Ward AB & Patapoutian A. (2020). Discoveries in structure and physiology of mechanically activated ion channels. Nature 587, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Franke V, Brandt B, Spuler S, Akalin A & Birchmeier C. (2020). Single-nucleus transcriptomics reveals functional compartmentalization in syncytial skeletal muscle cells. bioRxiv, 2020.2004.2014.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus T, Neuhuber WL & Raab M. (2004). Vesicular glutamate transporter 1 immunoreactivity in motor endplates of striated esophageal but not skeletal muscles in the mouse. Neurosci Lett 360, 53–56. [DOI] [PubMed] [Google Scholar]
- Lin SH, Cheng YR, Banks RW, Min MY, Bewick GS & Chen CC. (2016). Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nature communications 7, 11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JP, Sadeghi S, Athanassiadis T, Caram Salas N, Auclair F, Thivierge B, Arsenault I, Rompre P, Westberg KG & Kolta A. (2010). Assessment of the potential role of muscle spindle mechanoreceptor afferents in chronic muscle pain in the rat masseter muscle. PLoS One 5, e11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A & Lumpkin EA. (2014). Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall KL, Saade D, Ghitani N, Coombs AM, Szczot M, Keller J, Ogata T, Daou I, Stowers LT & Bönnemann CG. (2020). PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature 588, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kühnemund J, Francisco AG, Keenan WT, Dubin AE, Lewin GR & Patapoutian A. (2018). The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Science Translational Medicine 10, eaat9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura K, Woo S-H, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD & Patapoutian A. (2017). Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Florez-Paz DM, Badea TC, Mentis GZ, Menon V & de Nooij JC. (2020). Molecular development of muscle spindle and Golgi tendon organ sensory afferents revealed by single proprioceptor transcriptome analysis. bioRxiv, 2020.2004.2003.023986. [Google Scholar]
- Pellegrini-Giampietro DE, Torregrossa SA & Moroni F. (1996). Pharmacological characterization of metabotropic glutamate receptors coupled to phospholipase D in the rat hippocampus. Br J Pharmacol 118, 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U & Gandevia SC. (2012). The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol Rev 92, 1651–1697. [DOI] [PubMed] [Google Scholar]
- Ro JY & Capra NF. (2001). Modulation of jaw muscle spindle afferent activity following intramuscular injections with hypertonic saline. Pain 92, 117–127. [DOI] [PubMed] [Google Scholar]
- Shenton F, Bewick GS & Banks RW. (2014). A study of the expression of small conductance calcium-activated potassium channels (SK1-3) in sensory endings of muscle spindles and lanceolate endings of hair follicles in the rat. PLoS One 9, e107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Shenton F, Hunter I, Banks RW & Bewick GS. (2010). Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol 588, 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczot M, Liljencrantz J, Ghitani N, Barik A, Lam R, Thompson JH, Bharucha-Goebel D, Saade D, Necaise A & Donkervoort S. (2018). PIEZO2 mediates injury-induced tactile pain in mice and humans. Science translational medicine 10, eaat9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JA, Wieczerzak KB, Gabriel HM, Nardelli P, Rich MM & Cope TC. (2016). A novel path to chronic proprioceptive disability with oxaliplatin: distortion of sensory encoding. Neurobiology of disease 95, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KA, Kloefkorn HE & Hochman S. (2012). Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation. PLoS One 7, e39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-H, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA & Patapoutian A. (2015a). Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 18, 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-H, Lumpkin EA & Patapoutian A. (2015b). Merkel cells and neurons keep in touch. Trends Cell Biol 25, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-H,Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL & Patapoutian A. (2014). Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Schieren I, Qian Y, Zhang C, Jessell TM & de Nooij JC. (2019). A role for sensory end organ-derived signals in regulating muscle spindle proprioceptor phenotype. Journal of Neuroscience 39, 4252–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-X, Koshimizu Y, Feng Y-P, Okamoto K, Fujiyama F, Hioki H, Li Y-Q, Kaneko T & Mizuno N. (2004). Vesicular glutamate transporter immunoreactivity in the central and peripheral endings of muscle-spindle afferents. Brain Res 1011, 247–251. [DOI] [PubMed] [Google Scholar]
- Yasui M, Menjyo Y, Tokizane K, Shiozawa A, Tsuda M, Inoue K & Kiyama H. (2019). Hyperactivation of proprioceptors induces microglia-mediated long-lasting pain in a rat model of chronic fatigue syndrome. Journal of neuroinflammation 16, 67–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Kwon J, Sohn J-W, Lee SH, Kim S & Ho W-K. (2018a). mGluR5-dependent modulation of dendritic excitability in CA1 pyramidal neurons mediated by enhancement of persistent Na+ currents. J Physiol 596, 4141–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Sohn J-W, Kwon J, Lee S-H, Kim S & Ho W-K. (2018b). Enhancement of dendritic persistent Na+ currents by mGluR5 leads to an advancement of spike timing with an increase in temporal precision. Molecular Brain 11, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanato C, Watson S, Bewick GS, Harrison WT & Zanda M. (2014). Synthesis and biological evaluation of (−)-kainic acid analogues as phospholipase D-coupled metabotropic glutamate receptor ligands. Organic & biomolecular chemistry 12, 9638–9643. [DOI] [PubMed] [Google Scholar]
- Zeng W-Z, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD & Patapoutian A. (2018). PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362, 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wesolowski M, Karakatsani A, Witzemann V & Kröger S. (2014). Formation of cholinergic synapse-like specializations at developing murine muscle spindles. Dev Biol 393, 227–235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


