Abstract
Chronic stress is a major risk for cardiovascular disease. Perivascular adipose tissue (PVAT) has been shown to regulate vascular function, however, the impact of chronic stress and the comorbidity of metabolic syndrome (MetS) on thoracic (t)PVAT are unknown. Additionally, aerobic exercise training (AET) is known to combat the pathology of MetS and chronic stress, but the role of tPVAT in these actions are also unknown. Therefore, the purpose of this study is to examine the effects of unpredictable chronic mild stress (UCMS) on the tPVAT regulation of aortic function and the preventative effect of AET.
Methods & Results:
Lean (LZR) and obese (OZR) Zucker rats (16–17-week-old) were exposed to 8 weeks of UCMS with and without treadmill exercise (AET). In LZR, UCMS impaired aortic endothelial-dependent dilation (EDD) (assessed ex-vivo by wire-myography), and aortic stiffness (assessed by elastic modulus) with no change in OZR UCMS. However, both LZR and OZR UCMS tPVAT impaired EDD compared to respective controls. LZR and OZR UCMS had higher oxidative stress production, diminished adiponectin, and impaired aortic nitric oxide levels. Divergently, UCMS induced greater inflammatory cytokine production in LZR UCMS tPVAT, but not in OZR UCMS tPVAT. AET prevented the tPVAT impairment of aortic relaxation with UCMS in LZR and OZR. Additionally, AET reduced aortic stiffness in both LZR and OZR. These beneficial effects on tPVAT regulation of the aorta are likely due to AET preservation of adiponectin, reduced oxidative stress and inflammation, and enhanced nitric oxide.
Conclusion:
UCMS impaired tPVAT-regulated aortic function in LZR, and augmented MetS-induced EDD in OZR. Conversely, AET in combination with UCMS largely preserved aortic function and the tPVAT environment, in both groups.
Keywords: Perivascular adipose tissue, chronic stress, metabolic syndrome, inflammation, aldosterone, exercise, aortic stiffness
Introduction:
Depressive psychological disorders are a common worldwide affliction affecting approximately 264 million people, making it a leading global cause of disability (Disease et al., 2018). The burden of depressive states weighs heaviest in the USA with a prevalence rate of roughly 17% (National Survey on Drug Use and Health (U.S.) & United States. Substance Abuse and Mental Health Services Administration. Office of Applied Studies., 2015). The complex psychophysiological interactions of depressive states elicit numerous health issues resulting in depressive states being an independent risk factor of cardiovascular disease (CVD) (Van der Kooy et al., 2007). The links between depressive states and arterial function has slowly come to light in recent history. Clinical and animal model evaluations suggest arterial stiffness and impaired endothelial function may underlie this association, nonetheless the exact mechanisms remain enigmatic (Rajagopalan et al., 2001; Mattace-Raso et al., 2006; d’Audiffret et al., 2010; Seldenrijk et al., 2011; Golbidi et al., 2015). In addition, there is a high co-prevalence of depressive states with obesity/metabolic syndrome (MetS) (Carey et al., 2014; Pratt & Brody, 2014), a disease state which already presents with increased oxidative stress, and inflammation, with subsequent arterial dysfunction (Brooks et al., 2015; DeVallance et al., 2015; Branyan et al., 2018; Brooks et al., 2018a; DeVallance et al., 2018a; DeVallance et al., 2019b). Our previous work in the obese Zucker Rat (OZR, model of MetS) suggests that the dysfunctional thoracic perivascular adipose tissue (tPVAT) impairs endothelial function of the underlying aorta (DeVallance et al., 2018a). Specifically, tPVAT-derived tumor necrosis factor-alpha (TNFα) mediates activation of aortic reactive oxygen species (ROS) driving the impairment of aortic function (DeVallance et al., 2018a). Despite the growing evidence of depressive states inducing vasculopathies, the effect on tPVAT regulation of aortic function is unknown especially combined with MetS.
The unpredictable chronic mild stress (UCMS) protocol is a well-defined model to induce depressive-like behavior in rodents (Willner, 1997; Mineur et al., 2006, 2007; Jayatissa et al., 2010) that impairs cerebral and skeletal muscle micro-vessel function (Branyan et al., 2018; Brooks et al., 2018a). Further, aerobic exercise training (AET) is an effective therapy to alleviate the contributions of both UCMS and MetS on arterial health (Gleeson et al., 2011; Golbidi et al., 2012; Donley et al., 2014; Branyan et al., 2018; Brooks et al., 2018a; DeVallance et al., 2019a). AET promotes arterial protection through its anti-inflammatory and anti-oxidative effects (Gleeson et al., 2011; Golbidi et al., 2012). However, the effect of AET on tPVAT and tPVAT regulation of aortic function under UCMS conditions or with comorbidity of UCMS and MetS is unknown. These gaps in knowledge are of global-clinical importance based on the high prevalence of depressive states and their high co-prevalence with MetS. Our previous work shows that tPVAT mediates aortic impairment in MetS (DeVallance et al., 2018a) and that 8wks of AET reduces tPVAT inflammation, which prevents tPVAT-mediated aortic impairments (DeVallance et al., 2019a). Thus, the purpose of this study is to examine the effects of UCMS with or without MetS on the tPVAT regulation of aortic function. Secondly, we wanted to determine the role of AET on alleviating the potential negative actions of UCMS and MetS on tPVAT. We hypothesized that UCMS-induced changes in tPVAT would impair aortic function in LZR and exacerbate tPVAT-mediated impairments in OZR EDD. Further, we hypothesized that AET would prevent or limit the tPVAT-mediated impairment of aortic function with UCMS.
Methods & Materials
Ethical Approval:
Zucker rats from Envigo were used to conduct the experiments reported in this manuscript. Protocol #1603000971 was approved by the West Virginia University Health Science Center (WVUHSC) Animal Care and Use Committee. This protocol meets the NIH guidelines for care and use of laboratory animals and complies with the animal use ethics checklist set forth by the Journal of Experimental Physiology.
Animals:
Male lean (LZR, n=24) and OZR (n=24) were purchased from Envigo Laboratories at 7–9 weeks of age. Animals received standard chow and tap water ad libitum and housed on a reversed light/dark cycle. LZR and OZR were randomly assigned into control (LZR-Con, n=8 & OZR-Con, n=8), UCMS (LZR-UCMS, n=8 & OZR-UCMS, n=8), or combination of UCMS and AET groups (LZR UCMS+AET, n=8 & OZR UCMS+AET, n=8). This study was part of a large multi-group study and as such the UCMS and exercise groups have been previously reported (Branyan et al., 2018). Importantly, the focus of this paper, which examined the effects of UCMS and UCMS with AET on tPVAT has not been previously published and thus novel.
UCMS Protocol:
The UCMS protocol is a well-defined model to induce a depressive-like behavior in rodents (Willner, 1997; Mineur et al., 2006). Rodents undergoing UCMS manifest with clinically relevant depressive symptoms such as anhedonia and learned helplessness (Willner, 1997; Mineur et al., 2006) with alterations in brain structure and function parallel to clinical depression (Mineur et al., 2007; Jayatissa et al., 2010). Rats were singly housed in UCMS groups, and exposed to the following mild environmental stressors in randomly chosen sequences for 8 hours each day (starting at 9am), 5 days/week, over the course of 8 weeks:
Damp bedding – 10 oz. of water was added to each standard cage
Bath – all bedding was removed and ~0.5 inches of water was added to empty cage. Water temperature was room temperature, ~24°C
Cage Tilt - cage was tilted to 45 degrees without bedding
Social stress – each rat was switched into a cage of a neighboring rat
No bedding – all bedding was removed from the cage
Alteration of light/dark cycles –turning lights off/on in random increments for scheduled period.
Coat score was monitored throughout the study as previously indicated (Branyan et al., 2018). The score was calculated by giving a score of 0 (clean) or 1 (dirty) to eight regions of the body (head, neck, back, forelimbs, stomach, hindlimbs, tail, genitals) (Yalcin et al., 2005).
UCMS and Exercise Combination Protocol:
LZR UCMS+AET and OZR UCMS+AET underwent 8 weeks of treadmill running, following our previously published protocol (Branyan et al., 2018; DeVallance et al., 2019a). Animals ran 5 days/week in individual lanes on a motor driven treadmill at a 5% grade. During the first week, animals were acclimatized to the treadmill by progressively increasing running time (10 minutes/ day) from 20 minutes until a duration of 60 minutes was achieved. A maximum speed test was then performed by increasing the treadmill speed 2 m/min every 2 minutes until the rat was unable to stay on the belt. Target running speed was set for 60–70% of that maximum. Workouts for the following 7 weeks were 60 minutes (8am – 9am) in duration and consisted of 15 minutes of gradual increases in speed until reaching target speed, which was maintained for remaining 45 minutes. Mild electrical stimulation was used to encourage running. Treadmill running was performed first thing in the morning immediately followed by subjection to the UCMS protocol as described above.
Stress Markers:
Fasting blood was drawn intravenously from anesthetized rats into lithium-heparin coated blood tubes and transported immediately to the laboratory for analysis. Levels of triglycerides (TG) were measured by the clinical laboratory service at Ruby Memorial Hospital (Morgantown, WV). Blood glucose was measured using a commercially available glucometer (FreeStyle, Abbott). Using a commercially available ELISA Kit (Cayman Chemical, Item #501320) serum corticosterone, collected at time of terminal surgery, was measured in duplicate accordingly to the manufacturer’s instructions. During terminal procedures adrenal glands were removed and cleaned under a dissecting microscope. Subsequently, adrenal glands were patted dry and weighed individually and then averaged together for each animal and then normalized to body weight.
Terminal Procedures:
Terminal procedures were performed a minimum of 48 hours following the last bout of AET or UCMS to eliminate the acute effects of AET or UCMS on experiments. At time of terminal procedures, animals were weighed then deeply anesthetized with pentobarbital sodium (50 mg/kg i.p). All rats then received carotid artery and jugular vein cannulation to measure mean arterial pressure and to administer heparin, respectfully. The aorta with the surrounding tPVAT was removed, processed and assessed as previously described in detail (DeVallance et al., 2018a; DeVallance et al., 2019a). Hereafter experimental methods are briefly described.
Measurement of ROS:
Measurement of hydrogen peroxide (H2O2) abundance, tPVAT and treated aortic lysates were analyzed by coumarin boronic acid (CBA, Cayman #14051) assay as previously described (DeVallance et al., 2019a). CBA probe preparation and protocol were modified from Zielonka et al. [22]. In brief, 10μg of donor aortic rings exposed to tPVAT and tPVAT sample lysates were loaded, in triplicate, into wells of a 384 well black sided clear bottom plate. Subsequently, assay buffer composed of HBSS supplemented with 25 mM HEPES, 1% BSA, 10uM DTPA, 100uM l-NAME, 1 mM Taurine was added and then CBA was added to each well at a final concentration of 0.5 mM. One well from each biological sample received an additional 1 KU/ml bovine liver catalase to act as a negative control. Upon addition of the CBA, plates were placed in a Biotek plate reader preheated to 37°C and read kinetically at excitation 350 nm and emission 450 nm. The average rate of fluorescence was determined over the linear portion of the response and then normalized by subtracting out the rate of fluorescence from the negative control.
NO Bioavailability:
Aortic rings were placed in individual wells of a 96 well plate containing HEPES buffer and 4-Amino-5-Methylamino-2′,7′- Difluorofluorescein Diacetate (DAF-FM-DA, Invitrogen) supplemented with L-Arginine (100μM, MP biomedical Inc. 100736), with the following treatments: control (no added drug), PVAT, or nitro-L- arginine methyl ester (L-NAME, a potent inhibitor of NO synthase, Sigma-Aldrich N5751) and then stimulated with Acetyl-β-methylcholine chloride (methacholine (MCh), 1×10−6, Sigma-Aldrich A2251). The conditioned solution was read in a plate reader excitation/emission at 495/515nm wavelength. Fluorescence was normalized to aorta length and L-NAME value.
tPVAT Cytokine Profile:
tPVAT at a ratio of 200mg/1mL was incubated in HEPES buffer for 1 hours at 37°C. The tPVAT was then removed and the media was snap frozen and stored at −80°C. The conditioned media was then run on MSD multiplex rat inflammatory panel 2 (Mesoscale discovery, V-plex K15059D-2), MMP-9 activity ELISA (GE), and High molecular weight adiponectin ELISA (Mybiosource MBS020496). Additionally, tPVAT homogenates were prepared and run on MSD inflammation panel 1 rat (Mesoscale discovery, K15179C-9). All assays were run per manufacturer’s instructions. tPVAT homogenates were used to run SOD activity assay (Sigma-Aldrich 19160-1KT-F) to manufacturer’s specifications.
Aortic Reactivity:
Endothelial dependent dilation (EDD) and the effect of tPVAT on EDD were assessed in aortic rings cleaned and mounted in a myobath chamber between a fixed point and a force transducer (World Precision Instruments). The equilibrated aortic rings were constricted with phenylephrine and exposed to increasing doses of MCh (1×10−9 M to 1×10−5 M). Additional responses were carried out in the presence of tPVAT. As we previously described (DeVallance et al., 2018a; DeVallance et al., 2019a) 3 mm thoracic aortic rings (cleaned of surrounding tissue) were rinsed in physiological salt solution and mounted in a myobath chamber between a fixed point and a force transducer (World Precision Instruments) pre-stretched, and allowed to equilibrate for 1-hr in Krebs Henseleit Buffer aerated with 95%O2 and 5%CO2 at 37°C. After equilibration, aortic baseline tension was adjusted to 1 g and vessel viability was checked with 50 mM of KCl and rings not generating a rapid response were excluded from the study. To test endothelial dependent dilation (EDD), aortic rings were pre-constricted with phenylephrine PE, 1×10−7 M Sigma-Aldrich P6126) and a stable tension was reached and recorded followed by increasing doses of MCh (1×10−9 M-1×10−5 M). Following the MCh curve, the system was washed again and allowed to return to baseline. To test the effect of tPVAT, exudate was added to the bath and rings incubated for 30 min and the MCh curves were repeated. tPVAT exudate was obtained as follows: tPVAT at a ratio of 200mg/1mL was incubated in HEPES buffer for 2 hours at 37°C. The tPVAT was removed and the media (exudate) was used for the tPVAT incubation studies. Relaxation was calculated as %relaxation for each dose of MCh from the following equation:
where z=tension after PE 1×10−7M, x=tension following a given does of MCh, and y=baseline tension.
Aortic Stiffness:
Aortic rings were incubated in Ca2+ free Van Breemen solution to elicit a passive state. The rings were then mounted on an automated motorized force transducer (Aurora Scientific Inc. model 6350*358) and force output was recorded in LabChart software by Powerlab (AD Instruments). After preconditioning rings’ internal diameter and wall thickness were measured and stretched to 10mN of force for 3 minutes. Subsequently, the automated force transducer increased the aortic ring diameter by 25% of initial internal diameter every 3 minutes until mechanical failure (determined by a drop in force following a stretch). Elastic modulus was determined as the slope of the stress-strain relationship (DeVallance et al., 2018a). Stress and strain were calculated as follows:
Δd=change in diameter, d(i)=initial diameter) L=one-dimensional load applied, H=wall thickness, and D=length of vessel.
PVAT Culture Studies:
To determine the direct impact of PVAT on mechanical stiffness, LZR aortic rings (n=3–4/treatment) were cultured for 72-hours in RMPI + GlutaMAX™ + 25 mM HEPES media (Gibco® by Life Techonologies™) with streptomycin and kept in a CO2 cell incubator at 37°C under 5% CO2, under the following conditions; control (culturing healthy donor aortas in the culture media without any tPVAT), LZR tPVAT, OZR tPVAT, LZR UCMS tPVAT, OZR UCMS tPVAT, LZR UCMS+AET tPVAT or OZR UCMS+AET tPVAT. Media was discarded and replenished daily. Following the 72-hours of culture aortic rings were subjected to the protocol described above to generate an elastic modulus.
Statistical Analysis:
We have previously shown that in LZR that tPVAT improved aortic dilation by 5%, and that OZR tPVAT reduced EDD by 10% (DeVallance et al., 2018a). As such, we performed a power analysis using PASS software with a repeated measures analysis. The analysis was powered off the interaction term between tPVAT and UCMS group to establish a 10 ± 5% (SD) impairment in aortic dilation with tPVAT in the LZR UCMS group, for an effect of 1.66. Assuming 0.5 correlation between time points and alpha set to 0.05, total of 4 rats per group was needed to detect the interaction effect with 0.97 power. However, we doubled that sample size to account for potential experimental issues within the combined UCMS+AET groups. Data are represented as mean ± SD or individual data points with mean indicated by black bar. All experiments were run in duplicate and the average of the reads used as the mean for each animal. Data analysis and graphing were conducted using GraphPad Prism 8 software (GraphPad Software, Inc.) and p ≤ 0.05 was set for statistical significance. Comparisons between groups were conducted using a one-way ANOVA, and a repeated measure ANOVA for aortic reactivity with Holm-Sidak post-hoc test used to determine differences between groups.
Results:
Animal Characteristics
Body mass was greater in OZR vs. LZR, both UCMS and UCMS+AET slightly reduced mass in both OZR and LZR groups (Table 1). Importantly, MAP was not affected by UCMS or UCMS+AET within LZR or OZR groups (Table 1). Of note, stress markers (corticosterone, coat scores, adrenal weights) were elevated after UCMS in LZR and OZR groups and they remained higher in the UCMS+AET groups vs. control groups (Table 1).
Table 1.
Animal Characteristics
| LZR | OZR | |||||
|---|---|---|---|---|---|---|
| Control | UCMS | UCMS+AET | Control | UCMS | UCMS+AET | |
| Body Mass (g) | 400±36 | 361±25* | 343±21* | 641±54 | 585±39* | 567±46* |
| MAP (mmHg) | 112±8 | 122±13 | 120±11 | 139±14 | 142±16 | 132±13 |
| Glucose (mg/dl) | 98±15 | 124±17 | 115±15 | 184±29 | 230±34 | 219±36 |
| TG (mg/dl) | 25±7 | 54±40 | 36±8 | 124±19 | 114±35 | 103±40 |
| Corticosterone (ng/ml) | 7.0±0.4 | 8.8±1.7* | 10.7±4 | 13.8±1.4 | 17.3±5.2* | 15.8±4.0* |
| Coat Score (AU) | 0.6±0.4 | 1.5±0.4* | 1.8±0.8* | 2.4±0.4 | 4.8±0.8* | 4.5±0.4* |
| Adrenal weight (mg) | 16.8±2.3 | 25.2±4.2* | 21.4±3.1* | 27.2±3.9 | 32.7±6.5 | 32.0±6.7 |
| Normalized adrenal weight (mg/g) | 0.040±0.003 | 0.068±0.008* | 0.063±0.006* | 0.045±0.006 | 0.056±0.008* | 0.061±0.008* |
| Aorta Wall Thickness (μm) | 120±16 | 116±14 | 119±17 | 138±11 | 141±16 | 118±8* |
Data represented as Mean ± SD,
denotes statistical difference compared to with specie control (i.e., LZR or OZR) measured by ANOVA with Holm-Sidak Post-Hoc p<0.05 (n=6–8).
MAP, mean arterial pressure; TG, triglyceride. p=0.06 for glucose and MAP in LZR con vs UCMS; p=0.06 glucose OZR con vs UCMS.
Effect of UCMS and AET on Aortic Reactivity
In LZR control, max aortic EDD reached ~85%, and incubating with its own tPVAT slightly improved aortic EDD (p<0.05). Following 8-weeks of UCMS, aortic EDD (without tPVAT) was impaired by ~10% in LZR UCMS vs. LZR controls. Adding the LZR UCMS-tPVAT further impaired aortic EDD in the LZR UCMS group (Fig.1A). Aortic EDD (without tPVAT) in LZR UCMS+AET was not statistically different from LZR controls, and was slightly greater, albeit not significantly, compared to the LZR UCMS group (Fig.1B). Incubating the LZR UCMS+AET with its own tPVAT did not impact, negatively or positively, aortic EDD. However, aortic EDD was greater (p<0.01) in the LZR UCMS+AET group with tPVAT compared to the LZR UCMS group with tPVAT (Fig.1B).
Figure 1. Unpredictable chronic mild stress (UCMS) results in thoracic aortic perivascular adipose tissue impairment of aortic endothelial dependent dilation (EDD), which is prevented by aerobic exercise (AET) in both LZR and OZR rats.
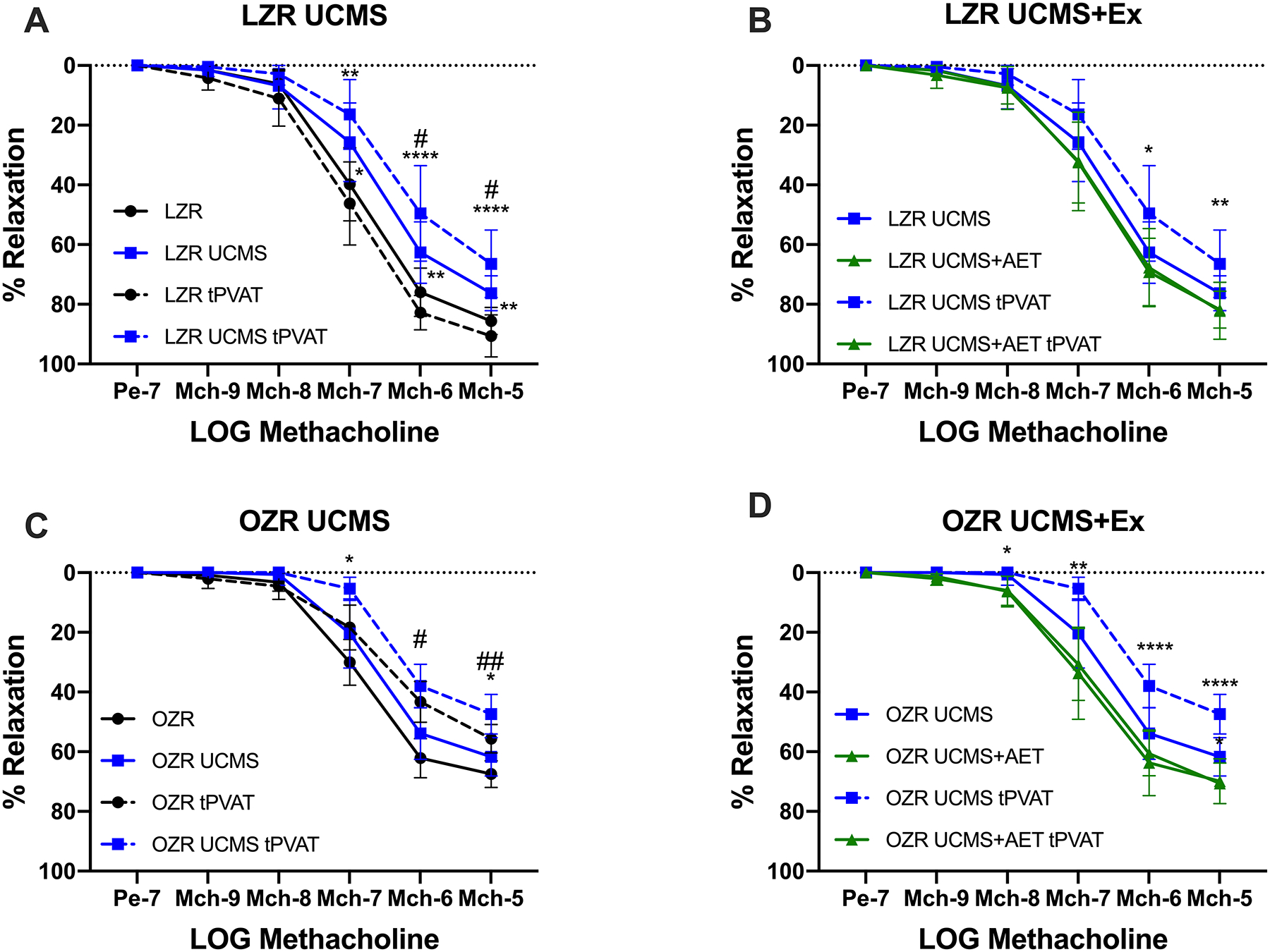
The impact of UCMS and UCMS+AET on EDD with and without tPVAT was tested by methacholine-induced relaxation of thoracic aortic rings using wire myograph in LZR (A&B) and OZR (C&D) groups. n=6–8 in each group or treatment. *denotes significant differences within treatments i.e., aortic ring with tPVAT relaxation compared to aortic ring with tPVAT relaxation or aortic ring only compared to aortic ring only. #denote significant effect of tPVAT, i.e., comparing aortic ring only to aortic ring with tPVAT. *p<0.05, **p<0.01, *** p<0.001, **** p<0.0001, #p<0.05, ##p<0.01 as measured by two-way repeated measures ANOVA with holm-sidak post-hoc analysis.
In OZR controls, max aortic EDD reached ~70%, and incubating with its own tPVAT significantly reduced aortic EDD (p<0.05). Following 8-weeks of UCMS, aortic EDD (without tPVAT) was similar between OZR controls and UCMS groups (Fig 1C). Adding the OZR UCMS-tPVAT further impaired aortic EDD in the OZR UCMS group (Fig.1C). Of note, the impaired EDD by OZR UCMS tPVAT was significantly greater than aortic EDD impairment induced by the OZR tPVAT (Fig. 1C). AET improved aortic EDD in the OZR UCMS+AET compared to the OZR UCMS group. Further, unlike OZR controls, or OZR UCMS groups, adding the tPVAT from the OZR UCMS+AET did not negatively affect aortic EDD, that is aortic EDD was similar in the OZR UCMS+AET group with and without its own tPVAT (Fig. 1D).
Effect of UCMS and AET on Aortic Stiffness
Aortic stiffness, assessed by elastic modulus, was increased in LZR UCMS compared to LZR control group (302±59 vs. 372±39 kPA, p<0.05, Fig. 2A). This increased aortic stiffness was prevented by AET in LZR UCMS+AET group (311±45 vs. 372±39 kPA, p<0.05, Fig. 2A). OZR controls and OZR UCMS had similar levels of aortic stiffness (588±122 vs. 536±117, Fig 2B). AET lowered aortic stiffness in OZR UCMS+AET compared to both OZR controls and OZR UCMS groups (368±91 kPA, p<0.05, Fig.2B).
Figure 2. Unpredictable chronic mild stress (UCMS)-induced thoracic aortic perivascular adipose tissue-mediated aortic stiffness in LZR but not OZR.
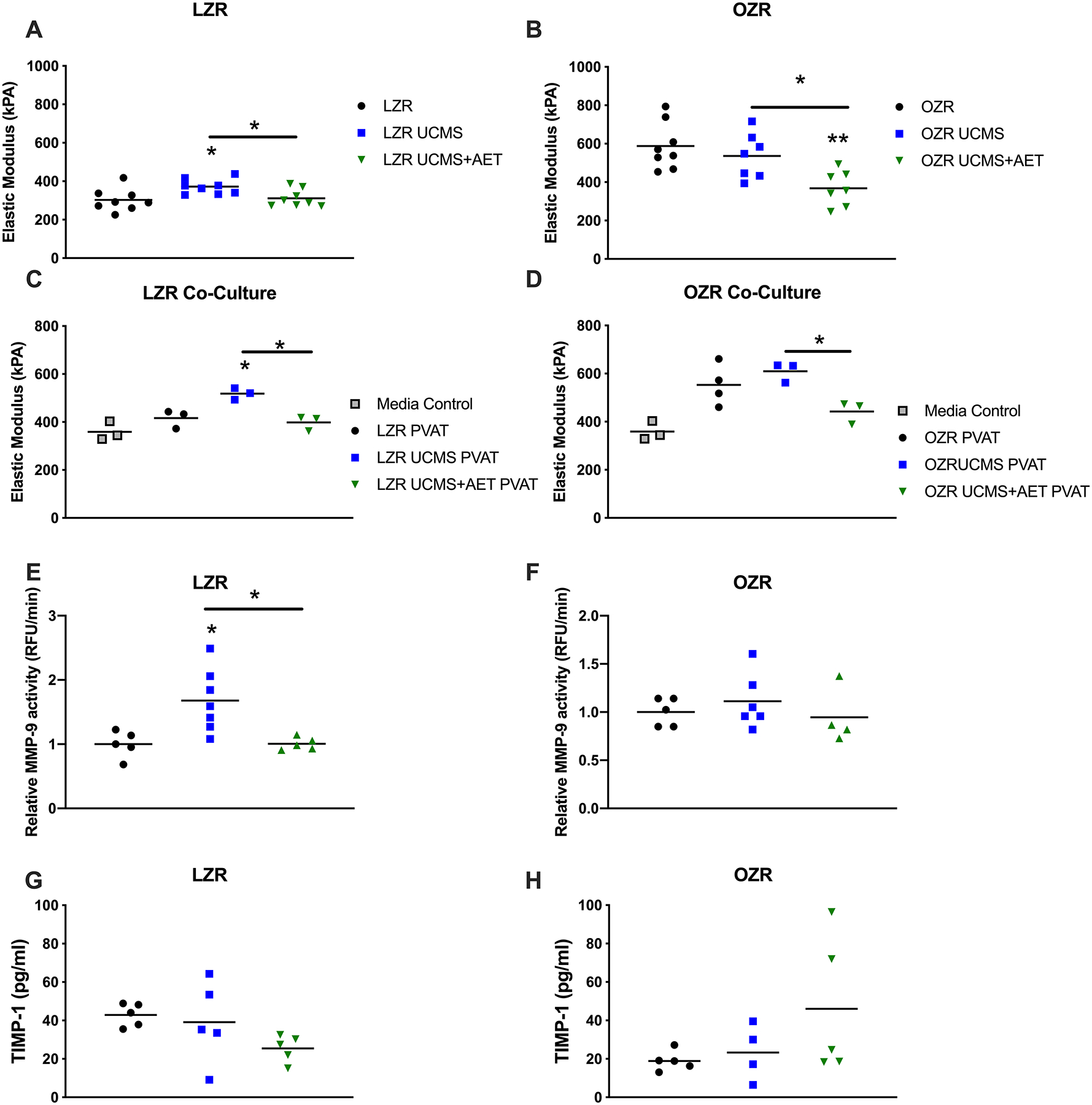
Stiffness was measured in aortic rings passively stretched on an automatic force transducer to calculate the Elastic modulus (kPA). Aortic rings collected from LZR (A, n=8) and OZR (B, n=7–8) were made passive by incubation in a calcium free solution and passively stretched. To more directly assess tPVAT-mediated aortic stiffness health donor aortic rings were collected and co-cultured with tPVAT for 72 hours and then mounted on the automated force transducer to access stiffness (C&D, n=3–4). The vascular remodeling factors MMP-9 (E&F, n=4–7) assessed by ELISA based activity assay and TIMP-1 (G&H, n=5) assessed as part of a multiplex assay in tPVAT. * p<0.05, ** p<0.01
To address the direct role of tPVAT on aortic stiffness, we co-cultured healthy LZR vessels with tPVAT from the various experimental groups. Similar to elastic modulus in the uncultured aortas, treatment of the healthy LZR aorta with LZR UCMS tPVAT increased aortic stiffness compared to LZR tPVAT (416±38 vs. 518±24 kPA, p<0.05, Fig.2C). AET prevented the tPVAT-mediated increase in aortic stiffness in LZR UCMS+AET group (398±30 kPA, p<0.05, Fig.2C). In OZRs, UCMS did not increase aortic stiffness compared to OZR (553±85 vs. 609±41 kPA). However, AET decreased tPVAT-mediated stiffness in OZR UMCS+AET (Fig.2D) compared to OZR UCMS (609±41 vs. 442±46 kPA) p<0.05).
Given the role of MMP-9 on aortic stiffening we next examine the activity of MMP-9 in the tPVAT. MMP-9 activity was increased by ~65% in the tPVAT from the LZR UCMS group (p<0.05, Fig.2E), while it remained unchanged in all OZR groups (Fig.2F). Finally, the MMP-9 inhibitor, TIMP-1, was decreased in LZR UCMS+AET group compared to control (43±6 vs. 26±7 pg/ml, p<0.05) and unaffected in OZR groups (Fig. 2G and H).
Nitric Oxide, Superoxide Dismutase, and Reactive Oxygen Species
NO measured by DAF-FM fluorescence in aortic segments was significantly reduced in LZR UCMS vs. LZR control, however, AET prevented this decrease (Fig 3A). Aortic NO production was enhanced ~25% by tPVAT treatment in LZR controls (Fig 3A, p<0.01). In contrast, LZR UCMS tPVAT treatment further reduced aortic NO bioavailability (p<0.05 vs. LZR UCMS AO, p<0.001 vs. LZR AO+tPVAT). Aortic NO bioavailability in the presence of tPVAT from the LZR UCMS+AET group was similar to levels reported in the LZR control tPVAT group, but greater compared to LZR UCMS tPVAT group (p<0.01, Fig. 3A). In OZRs, no difference in basal aortic NO bioavailability were noted between OZR controls, UCMS, and UCMS+AET. OZR aorta treated with OZR tPVAT significantly reduced NO (~20%), an effect that was exacerbated in OZR UCMS tPVAT treatment (p<0.001 vs. both OZR AO+tPVAT and OZR UCMS AO). AET in OZR UCMS group, increased aortic NO bioavailability in the presence of tPVAT compared to OZR UCMS, and OZR controls tPVAT (p<0.01 vs. OZR AO+tPVAT and p<0.001 vs. OZR UCMS AO+tPVAT, Fig. 3B).
Figure 3. Unpredictable chronic mild stress (UCMS) impairment of nitric oxide levels and increase in ROS production are prevented by exercise.
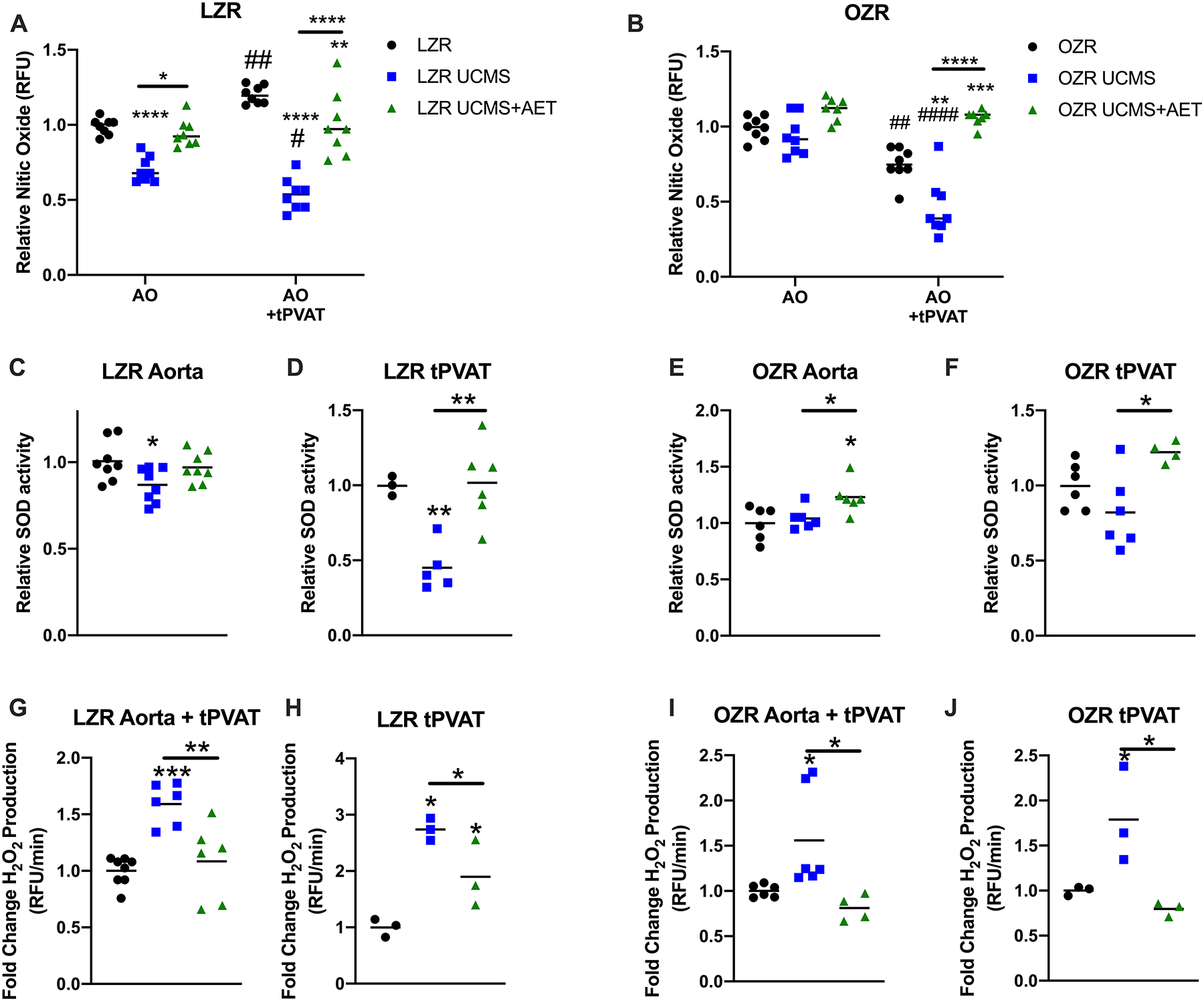
Aortic nitric oxide (NO) production was tested using a fluorescent DAF probe and normalized by aortic rings pre-treated with L-NAME to determine the background fluorescence. Relative NO production was tested with and without tPVAT treatment in LZR (A) and OZR (B) (n=7–8). Superoxide dismutase (SOD) activity was tested in LZR (C&D) and OZR (E&F) aortic rings (n=6–8) and tPVAT (n=3–6). Finally, relative ROS production measured by coumarin boronic acid was tested in donor aortic lysates following the treatment with tPVAT (n=4–8) and in tPVAT lysates (n=3) of LZR (G&H) and OZR (I&J), *denotes significant differences within treatments i.e., aortic ring with tPVAT compared to aortic ring with tPVAT or aortic ring only compared to aortic ring only. #denote significant effect of tPVAT, i.e., comparing aortic ring only to aortic ring with tPVAT. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, #p<0.05, ##p<0.01, ####p<0.0001 as measured by one-way ANOVA with holm-sidak post-hoc analysis.
UCMS in LZR reduced SOD activity in both aorta (~15%, p<0.05, Fig. 3C) and tPVAT (~50%, p<0.01, Fig. 3D). However, AET prevented the decreased SOD activity in tPVAT LZR UCMS+AET group (p<0.01, Fig. 3D). In OZRs, SOD activity was similar in the aorta and tPVAT between OZR and OZR UCMS. However, SOD activity in both the aorta and tPVAT was increased in the OZR UCMS+AET group compared to OZR UCMS group (~23%, p<0.05 and ~40%, p<0.05 respectively, Fig. 3 E&F).
ROS production measured in healthy donor aorta treated with tPVAT was ~50% elevated in LZR UCMS compared to LZR control groups, which was largely prevented in the LZR UCMS+AET group (p<0.01) (Fig. 3G). ROS measured directly in tPVAT lysate was 2.5-fold elevated in LZR UCMS compared to controls (p<0.05) and incompletely prevented in LZR UCMS+AET (Fig. 3 G&H). In OZRs, UCMS significantly enhanced ROS production in tPVAT (1.7-fold, p<0.05), and also the tPVAT treated aorta (1.5-fold, p<0.05). This response was completely prevented in the OZR UCMS+AET (p<0.05, Fig. 3 I&J).
Effect of UCMS and Exercise on tPVAT-derived Cytokines and Hormones
The tPVAT release of pro-inflammatory cytokines TNFα (16 ±3 v. 30 ±4 pg/ml p<0.001), IL-1β (113 ±53 v. 317 ±28 pg/ml p<0.01), IFN-γ (15 ±4 v. 31 ±8 pg/ml p<0.05), IL-6 (324 ±43 v. 1314 ±207 pg/ml p<0.001), and TSP-1 (179 ±8 v. 376 ±73 ng/ml p<0.001) were significantly increased in LZR UCMS vs. LZR control. However, AET prevented the increase in TNFα (30 ±4 v. 23 ±3 pg/ml p<0.05) and IL-1β (317 ±13 v. 239 ±10 pg/ml p<0.05) LZR UCMS+AET (Fig 4). The tPVAT release of pro-inflammatory cytokines was similar in OZR and OZR UCMS groups. However, AET lowered (p<0.05) TNFα (OZR 99 ±50 v. 54 ±3 pg/ml p<0.05), IL-1β (OZR UCMS 479 ±72 v. 341 ±80 pg/ml p<0.05), and IL-6 (684 ±194 v. 1621 ±355 pg/ml OZR 684 ±194 v. 1677 ±151 pg/ml OZR UCMS, both p<0.001) (Fig 5). Anti-inflammatory cytokines IL-4 (35 ±3 v. 12 ±8 pg/ml p<0.001), IL-5 (336 ±23 v. 99 ±6 pg/ml p<0.001), IL-13 (40 ±1 v. 13 ±4 pg/ml p<0.001), IL-10 (81 ±3 v. 22 ±3 pg/ml p<0.001), HMW Adiponectin (3.9 ±0.2 v. 1.2 ±0.4 μg/ml p<0.001) were significantly reduced in LZR UCMS compared to LZR control. In LZR UCMS+AET, AET partly restored IL-5 (99±6 vs. 145 ±3 pg/ml p<0.01) and IL-10 (22±3 vs. 39 ±4 pg/ml p<0.001 compared to LZR UCMS. However, AET in LZR UCMS completely restored HMW adiponectin levels to that noted in LZR controls (3.9±0.2 vs. 4.3±0.5 μg/ml). In OZR UCMS, anti-inflammatory cytokines were largely unchanged compared to OZR controls with the exception of a 3-fold reduction in HMW adiponectin (1.5±0.4 vs. 0.5±0.2 μg/ml p<0.01), which was prevented by AET in OZR UCMS group (0.5±0.2 vs. 2.2±0.5 μg/ml p<0.001). Additionally, IL-10 was increased in OZR UCMS+AET group compared to OZR controls (79±60 vs. 23±5 pg/ml p<0.01) and OZR UCMS (79±60 vs. 26±1 pg/ml p<0.01). Elevated aldosterone levels were observed in the OZR UCMS group (4.1±0.3 vs. 6.1±1.6 pg/mg p<0.05) compared to OZR controls, which was prevented by AET in OZR UCMS (6.1±1.6 vs. 3.2±1.1 pg/mg p<0.01, Fig. 5).
Figure 4. Unpredictable chronic mild stress (UCMS) negatively impacts inflammatory cytokine and hormone production of tPVAT, which is partially prevented by exercise in LZR rats.
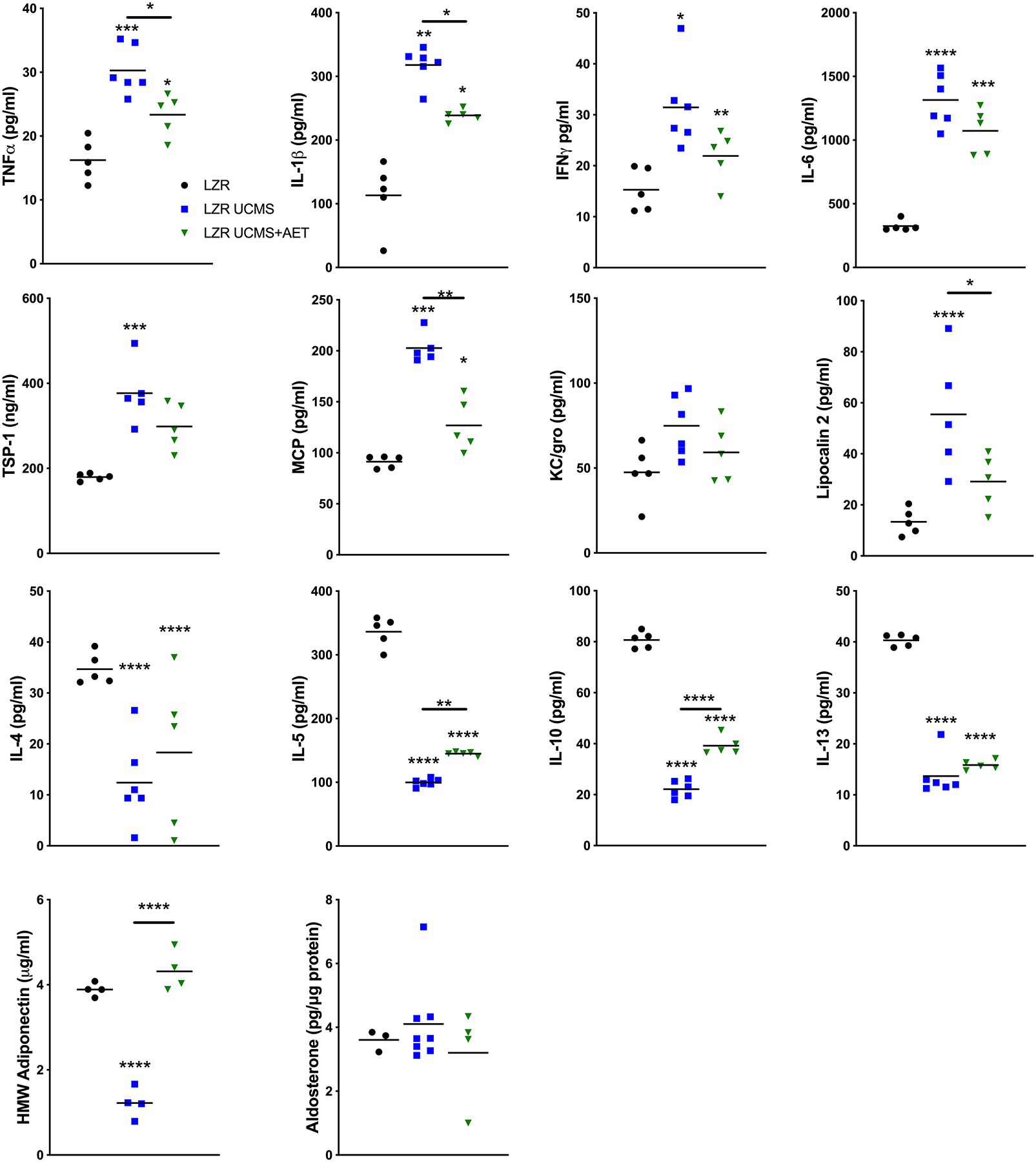
Pro- & anti- inflammatory cytokines were tested in tPVAT by MSD multiplex and ELISA. n=3–6 per group, * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001, as measured by one-way ANOVA with holm-sidak post-hoc analysis.
Figure 5. Unpredictable chronic mild stress (UCMS) negatively impacts inflammatory cytokine and hormone production of tPVAT, which is partially prevented by exercise in OZR rats.
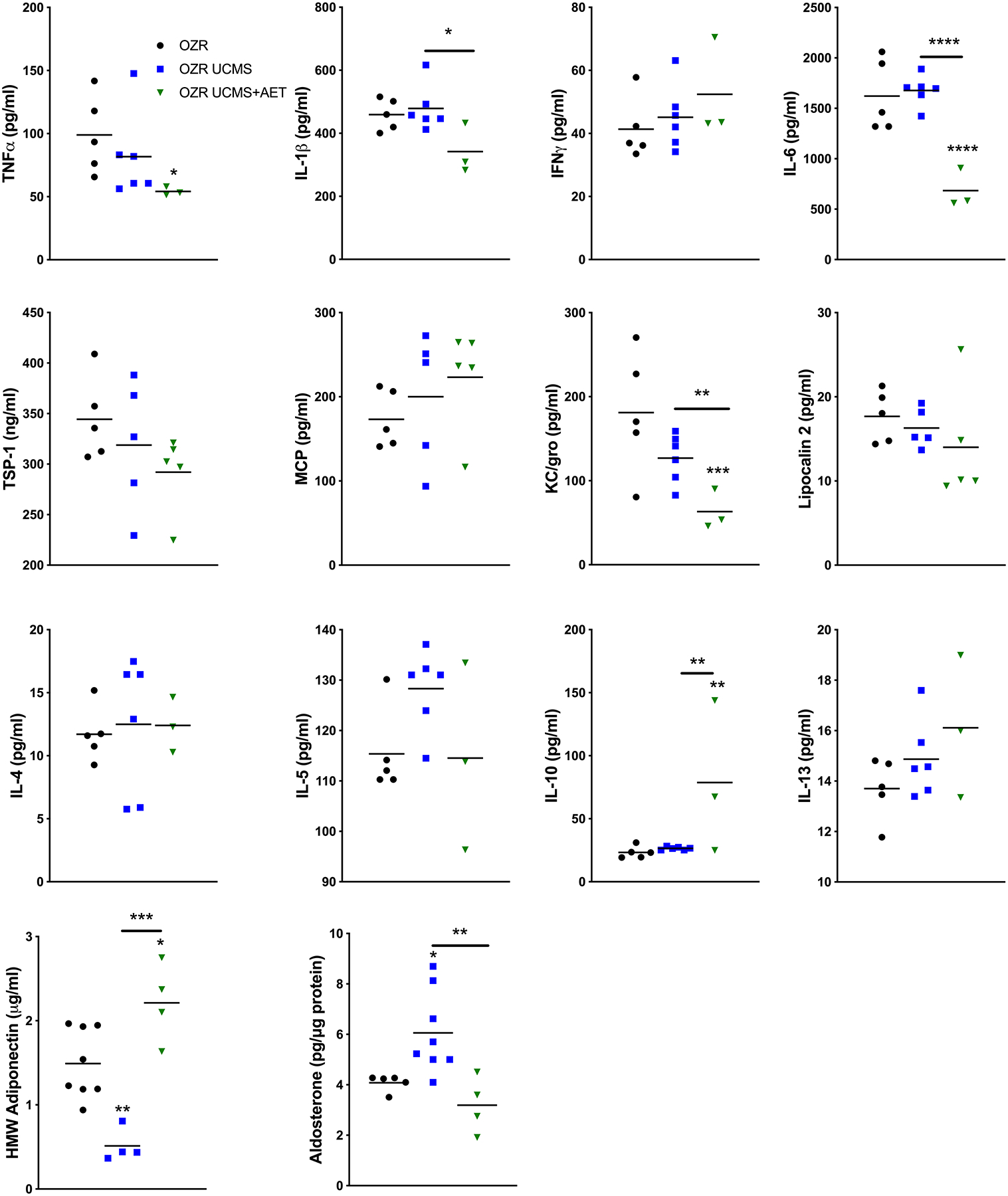
Thoracic aortic PVAT lysates were analyzed by aldosterone ELISA. N = 3–6* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 as measured by one-way ANOVA with holm-sidak post-hoc analysis.
Discussion
For first time our study showed 3 important findings regarding the effect of UCMS alone and with MetS on tPVAT and tPVAT regulation of aortic function: 1) UCMS in LZR increased production of TNFα and ROS in tPVAT with subsequent activation of aortic ROS, reduced NO bioavailability, diminished EDD, and increased aortic stiffness; 2) OZR-UCMS did not alter aortic NO or EDD compared to controls without the presence of tPVAT. However, with tPVAT, aortic ROS was activated with a reduction in NO and aortic EDD in OZR-UCMS compared to OZR controls, likely due to increased production of aldosterone in tPVAT; and 3) AET increased HMW adiponectin in tPVAT, which removed the UCMS associated detrimental effects of tPVAT on EDD.
Role of UCMS on tPVAT dysfunction
tPVAT secretes a number of paracrine signaling molecules (Gollasch, 2012; Nosalski & Guzik, 2017; DeVallance et al., 2018b) that reach the medial and endothelial layers via direct diffusion, through the vasa vasorum, and/or via a dense reticular network of collagenous conduits connecting the medial layer with the underlying adventitia (Chatterjee et al., 2009; Rajsheker et al., 2010; Gil-Ortega et al., 2015). Our previous works showed in MetS aortic dysfunction was mediated in part by an upregulation of oxidative products and pro-inflammatory cytokines in tPVAT (DeVallance et al., 2018a). Our current data suggests that UCSM induced a similar “obese-like” phenotype in lean rats, reflected by an increase in oxidative products and cytokine levels. Indeed, the loss of the ‘healthy’ tPVAT phenotype was highlighted by a reduction in the homeostatic cytokines IL-4, IL-5, IL-10, and IL-13 (Guzik et al., 2017). These cytokines can suppress inflammation and promote insulin sensitivity (Molofsky et al., 2013; Lizcano et al., 2017). Thus, the loss of these cytokines with UCMS suggests a deviation from the normal healthy phenotype of tPVAT, potentially driven by an overactivation of the sympathetic nervous system (Saxton et al., 2019). We and others have previously shown that the actions of MetS on tPVAT are multifactorial (Galvez et al., 2006; Park et al., 2014; Mikolajczyk et al., 2016; DeVallance et al., 2018a; DeVallance et al., 2019a), reflected by an increased release of adipokines (resistin, leptin and visfatin), cytokines (IL‐6, IL-1, TNF‐α etc), chemokines (RANTES, CCL5, MCP-1, CCL2) from the tPVAT. Within the stroma vascular fraction of tPVAT, it is speculated that increased M1 macrophages and T-cells contribute to these changes (Guzik et al., 2007; Lu et al., 2011). The mechanism driving these tPVAT changes in UCMS are unknown. However, it has been shown that in both UCMS and MetS, increased activation of the sympathetic nervous system mediates an upregulation of inflammation (Carlson et al., 2000; Huggett et al., 2004; Esler et al., 2006). Similarly, the activation of the sympathetic nervous system and the release of cortisol/corticosterone may have disrupted the redox balance and upregulated pro-inflammatory cytokines (Carlson et al., 2000; Huggett et al., 2004; Esler et al., 2006; Wu et al., 2019). In the context that UCMS did not elevate tPVAT production of pro-inflammatory cytokines in OZR, we speculate that the already heighted activation of the sympathetic nervous system in OZR (Morgan et al., 1995; Lambert et al., 2015) and recognized glucocorticoid resistance in MetS (Mattsson et al., 2003) may account for this finding. Additionally, it has been shown that factors associated with MetS can drive neurological changes in UCMS (Liu et al., 2015). Ultimately, it appears UCMS is not an additive insult in the context of MetS-tPVAT inflammation. Despite the lack of change in tPVAT inflammation in the OZR UCMS group we did find increased levels of aldosterone and diminished adiponectin compared to OZR controls, which may influence vascular function (Virdis et al., 2002; Pu et al., 2003).
Role of UCMS on Aortic Dysfunction Mediated by tPVAT
The pathophysiological adaptations that manifest in the presence of chronic stress, especially with the co-occurrence of MetS have important implications on CVD. We have shown that chronic stress reduced aortic EDD in LZR, which is supported by previous work in mice (d’Audiffret et al., 2010) and in resistance vessels of rats (Branyan et al., 2018). However, in the OZR UCMS did not further impair aortic EDD compared to OZR controls. Increased aortic stiffness is a classic CVD risk factor, with significant clinical relevance, and is an independent predictor of CV morbidity and mortality (Laurent et al., 2001; Laurent et al., 2003). Our data showed increased aortic stiffness in the LZR-UCMS, however, no effect was noted in the OZR-UCMS vs. OZR controls.
When we examined the effects of LZR UCMS tPVAT, aortic EDD was impaired, which is in stark contrast to the effect of LZR tPVAT. In the context of aortic stiffness, our data showed that factors released from LZR UCMS tPVAT can directly mediate stiffening of a “healthy donor” aorta. Together these findings suggest that UCMS caused the release of factors that hinder normal vascular function in LZR. While there were no basal changes in EDD in the OZR UCMS group, EDD in the presence of OZR UCMS tPVAT was significantly blunted. An effect that was exaggerated in comparison to the documented impairment imparted by OZR tPVAT on EDD.
The increased tPVAT levels of TNFα and IL-1β in LZR UCMS are known activators of oxidative enzymes (DeVallance et al., 2019b). These factors likely contributed to the increased activation of aortic ROS in the LZR UCMS group especially TNFα, as we previously documented this in OZRs (DeVallance et al., 2018a). Additionally, increased tPVAT levels of TSP-1 in the LZR UCMS group could directly inhibit the activation of eNOS (Isenberg et al., 2009) and activate ROS production (Novelli et al., 2019), thus also contributing to the reduction in NO bioavailability and aortic dysfunction (Mandler et al., 2017). While IFN-γ is known to impair NO bioavailability at high levels (Javanmard & Dana, 2012), these levels are far above the concentrations recorded in our study and thus unlikely to have contributed to the aortic dysfunction. In addition, to these effects on the aorta these cytokines may act through autocrine signaling pathways to further promote pro-inflammatory gene expression (Stylianou et al., 1992; Abu-Amer et al., 1998; Turner et al., 2007), and impair dilatory-mediating cytokine expression (IL-10 and adiponectin) (Wang & Scherer, 2008). Indeed, our data showed greatly reduced levels of IL-10 and adiponectin released from tPVAT. HMW adiponectin promotes NO production through rapid non-genomic and long term genomic pathways (Wang & Scherer, 2008). This would suggest that the reduced levels observed in the current study may have contributed to the diminished NO bioavailability and EDD after UCMS in both the LZR and OZR groups. We have previously shown that increased expression of TNFα can directly impair NO bioavailability and downregulate IL-10 and adiponectin through an autocrine signaling pathway (DeVallance et al., 2018a). Thus, with chronic stress TNFα may play a key central role in the tPVAT and aortic dysfunction. While it is not as well established, in addition to actions of the sympathetic nervous system on pro-inflammatory cytokines, its overactivation has been speculated to directly reduce PVAT adiponectin release (Saxton et al., 2019).
A potential pathway by which tPVAT may further impaired aortic EDD with UCMS in the OZR (given that inflammatory cytokines were not changed) is the observed increase in aldosterone, which has been implicated in obesity related arterial dysfunction (Briones et al., 2012). Aldosterone activation of ROS may further link aldosterone to arterial dysfunction (Virdis et al., 2002; Pu et al., 2003). Additionally, aldosterone is proposed to increase endothelin sensitivity and cause endothelial swelling and rigidity (Oberleithner, 2005), which may exacerbate aortic dysfunction as rigid endothelial cells produce less NO (Oberleithner et al., 2007). Conversely, aldosterone has been shown to improve endothelial function through increased eNOS activation (Liu et al., 2003). However, in MetS or UCMS, eNOS may be uncoupled (Lee et al., 2013; Galougahi et al., 2014) suggesting that aldosterone activation of eNOS might perpetuate ROS production via monomeric “uncoupled” eNOS. Our data suggests elevated aldosterone might be the factor, which promotes the exacerbated tPVAT-mediated impairment of aortic EDD and elevated ROS activation in OZR. However, the signaling pathways which mediate the upregulation of aldosterone in the comorbid state are unknown and warrant further investigation. Contrary to our findings, it has been reported that human PVAT does not produce aldosterone (Assersen et al., 2018). However, these experiments were conducted in small resistance arteries isolated from humans and PVAT is very heterogeneous along the vascular tree. Therefore, further assessment of human tPVAT is warranted to assess aldosterone production to determine whether this is a clinically relevant pathway.
In terms of aortic stiffness, the increased ROS production and the potential for an increased sympathetic nervous activity induced by UCMS in LZR likely contributed to the aortic stiffening. However, our aorta-tPVAT culture studies provided some insight to the role of tPVAT on aortic stiffness. LZR UCMS tPVAT increased aortic stiffness of a LZR control aorta, which was also supported by our finding of increased MMP9 activity in LZR-UCMS tPVAT exudate. This increased MMP9 activity may, in part, be due to the increased TNFα levels, which mediates the activation of MMP9 (Wu et al., 2013). We previously showed a similar effect in OZR tPVAT, which was dependent on TNFα (DeVallance et al., 2018a). Increased elastic modulus of a healthy donor aorta co-cultured with LZR UCMS tPVAT supports the idea that tPVAT can elicit structural changes independent of preexisting disease pathology in the aorta. However, circulating factors, such as corticosterone, are likely playing a supportive role in increasing aortic stiffness (Arnold et al., 2017) in vivo in LZR UCMS. Under stress conditions, cortisol levels can be more than double in humans (Burrage et al., 2018). Inference of the chronic impact of cortisol on arterial stiffness can be gained from patients with Cushing’s Syndrome who exhibit elevated vascular stiffness (Whitworth et al., 2005; Paolo Bassareo et al., 2014) and impaired endothelial function (Baykan et al., 2007). We acknowledge the limitation that our co-culture experiments lack the physiological input of intraluminal flow, which is important for shear stress mediated release of NO and regulation of stiffness. However, the use of a media control helps to account for the increase in stiffness due to the lack of flow.
Effect of Exercise in Preventing UCMS induced Aortic and tPVAT Dysfunction
Regularly performed exercise induces structural and functional adaptations to large and small vessels. Indeed, we have shown that AET in OZR leads to the classical arterial adaptive response whereby aortic ROS was less and NO abundance was greater with improved aortic EDD (DeVallance et al., 2018b). Interestingly, our previously published work suggested AET-mediated aortic improvements were dependent on AET-induced changes in tPVAT. Additionally, our work showed that 8 weeks of treadmill running in OZR limited the development of an inflammatory tPVAT phenotype (DeVallance et al., 2019a). In the current study, we show that concurrent AET with UCMS did not improve aortic EDD back to none UCMS levels in either LZR or OZR groups in the absence of tPVAT. The main effect of AET was on limiting the UCMS-induced tPVAT pro-inflammatory/pro-oxidative environment, and thus the detrimental actions of tPVAT on aortic function. Indeed, AET normalized the aortic production of NO following treatment with tPVAT and greatly reduced the tPVAT ROS and activation of aortic ROS. This is potentially due to the restoration of the NO promoting (Wang & Scherer, 2008) adiponectin levels in tPVAT of both AET LZR and OZR groups.
Furthermore, AET in the LZR UCMS group decreased aortic stiffness supported by the lack of tPVAT mediated stiffness in the co-culture experiments. AET in OZR UCMS reduced aortic stiffness compared to both OZR control and OZR UCMS, and the co-culture experiments demonstrated a reduced role of tPVAT on aortic stiffness. AET decreasing tPVAT-activated aortic ROS and restoration of NO levels are the likely mechanisms of the preserved elasticity of the aorta. More specifically, we have previously shown TNFα as an essential factor mediating tPVAT-induced aortic stiffening in OZR (DeVallance et al., 2018a). Suggesting the reduced TNFα observed in AET groups might account for improved aortic stiffness. These data show for the first time that AET promoted tPVAT-induced aortic compliance in UCMS and the UCMS+MetS comorbid state. However, future studies are needed to uncover the exact mechanisms through which AET reduces tPVAT regulation of aortic stiffness in these disease states.
AET is known to be anti-inflammatory and reduce oxidative stress in the vasculature as well as other tissues (Golbidi et al., 2012). However, AET effects on UCMS induced tPVAT dysfunction were previously unknown. There are many purposed mechanisms by which AET improve inflammation including reduced immune infiltration into adipose tissue, repolarization of tissue resident immune cells, and endocrine signaling from AET induced release of anti-inflammatory cytokines. These mechanisms of AET signaling are beyond the scope of the study but are discussed in detailed elsewhere (Gleeson et al., 2011). In the current study, AET with UCMS in LZR showed mixed success in reducing pro-inflammatory cytokines with a significant reduction noted in TNFα, and IFN-γ. As previously stated, these cytokines impair endothelial function and their reduction following AET was likely a key mechanism in preserving NO and EDD. In the OZR UCMS group, AET had little impact on the cytokine levels except for a reduction in the pro-inflammatory cytokine TNFα compared to the OZR controls. Similarly, we previously published that AET in MetS alone reduced tPVAT TNFα (DeVallance et al., 2019a). The partial success of AET in limiting the inflammatory changes due to UCMS, may be due to corticosterone levels remaining elevated. That is the corticosterone-induced pro-inflammatory pathways may be opposing the well-documented anti-inflammatory actions of AET. The one uniform effect of AET was the preservation/restoration of adiponectin levels. This AET-mediated effect on adiponectin levels may shift the balance towards the promotion of NO and away from oxidant production (Masaki et al., 2004; Wang & Scherer, 2008; Zemse et al., 2010), which we observed in the current study.
Another potential mechanism by which AET prevented the tPVAT-mediated aortic dysfunction in OZR UCMS was our novel finding that AET reduced tPVAT production of aldosterone. Previous reports have implicated aldosterone-induced ROS in PVAT-mediated arterial dysfunction (Withers et al., 2011). The role of AET on aldosterone is supported by findings in the two-kidney, one-clip (2K-1C) hypertensive rats, which showed decreased circulating aldosterone in both control and 2k-1C groups following AET. Reduced levels of tPVAT aldosterone in the OZR UCMS+AET coincide with a reduced tPVAT-mediated activation of aortic ROS. The mechanism through which AET reduces tPVAT aldosterone has not been explored however it may be related to AET-induced reductions of sympathetic outflow (Mueller, 2007).
Limitations:
A limitation of our co-culture experiment was the lack of intraluminal flow in the aortic rings, which is an important for shear stress mediated release of NO and regulation of stiffness. However, the use of a media control helps to account for the increase in stiffness due to the lack of flow. Aortic function experiments were conducted ex-vivo and may not reflect the magnitude of impairment imposed by tPVAT in-vivo. However, many logistical hurdles remain in assessing tPVAT regulation of the aorta in-vivo. We believe our data clearly shows UCMS negatively impacts tPVAT which can activate aortic ROS and impair EDD and stiffness. Previous work established that females are partially protected from the vascular consequences of UCMS (Brooks et al., 2018b). Our current study was only conducted in male rats based on the basal differences in UCMS response. Future studies will examine the role of sex hormones in the modulation of tPVAT-mediated responses to UCMS.
Clinical relevance:
Chronic stress-induced tPVAT-mediated vascular impairments reported in this manuscript (LZR UCMS group) have a number of clinical implications. Vascular ROS and diminished NO are associated with atherosclerotic initiation and progression along with the development of vascular stiffness (Forstermann et al., 2017; Yang et al., 2017; DeVallance et al., 2019b). Increased vascular stiffness is an independent predictor of cardiovascular and all-cause mortality (Ecobici & Voiculescu, 2017) (Laurent et al., 2001). As for potential impact of chronic stress with MetS, no impact was seen with stiffness, however, the greater vascular ROS and EDD dysfunction may heighten CVD risk.
Conclusion:
In conclusion, this study presents novel data that highlights the pathophysiological role of tPVAT to chronic stress. UCMS in LZR resulted in a “bad” tPVAT phenotype characterized by an altered cytokine profile which resulted in tPVAT activation of aortic ROS, reduced NO, reduced EDD, and increased aortic stiffness. While in the comorbid state, UCMS had limited impact on inflammatory cytokines but did elevate aldosterone levels. Resulting in a similar upregulation of aortic ROS, reduced NO, and reduced EDD. In large, AET was able to prevent the tPVAT-mediated impairment of aortic function. While AET prevents some of the strain (LZR vs. OZR) specific UCMS-induced dysfunction it also uniformly restored levels of the vasoactive cytokine, adiponectin, in both AET groups. Suggesting, a key benefit of AET across disease states is enhanced adiponectin levels in tPVAT. Collectively, our study suggests a previously unidentified role of tPVAT in mediating the vasculopathies associated with chronic stress and that beneficial effects of AET in reversing the vascular dysfunction is in part mediated through modifications in tPVAT.
New Findings:
What is the central question of this study?
tPVAT is known to, in part, regulate aortic function. However, the role of tPVAT in aortic impairment associated with chronic exposure to psychosocial stressors (Unpredictable Chronic Mild Stress; UCMS) is unknown. Thus, the purpose of this study is to examine the effects of UCMS on the tPVAT regulation of aortic function. Secondly, we wanted to determine the role of exercise training on alleviating the potential negative actions of UCMS on tPVAT.
What is the main finding and its importance?
The main finding of this study is that UCMS causes tPVAT to disrupt endothelial dependent dilation. UCMS increased inflammatory cytokine production and diminished tPVAT-adiponectin. Exercise training proved efficacious in preventing tPVAT-mediated disruption of aortic function. Together, our data support a tPVAT-mechanism through which chronic stress negatively impacts vascular health and adds to our knowledge of how psychological disorders might increase the risk of cardiovascular disease.
Funding:
This study was supported by the American Heart Association grants IRG14330015, pre- doctoral fellowship AHA (14PRE20380386), post-doctoral fellowship AHA 19TPA34850089; National Institutes of Health R01 DK124510-01 (EEK), and National Institute of General Medical Sciences of the National Institutes of Health (U54GM104942, and 5P20GM109098).
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Reference:
- Abu-Amer Y, Ross FP, McHugh KP, Livolsi A, Peyron JF & Teitelbaum SL (1998). Tumor necrosis factor-alpha activation of nuclear transcription factor-kappaB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of Ikappa Balpha. J Biol Chem 273, 29417–29423. [DOI] [PubMed] [Google Scholar]
- Arnold N, Gori T, Schnabel RB, Schulz A, Prochaska JH, Zeller T, Binder H, Pfeiffer N, Beutel M, Espinola-Klein C, Lackner KJ, Blankenberg S, Munzel T & Wild PS (2017). Relation between Arterial Stiffness and Markers of Inflammation and Hemostasis - Data from the Population-based Gutenberg Health Study. Sci Rep 7, 6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assersen KB, Jensen PS, Briones AM, Rasmussen LM, Marcussen N, Toft A, Vanhoutte PM, Jensen BL & Hansen PBL (2018). Periarterial fat from two human vascular beds is not a source of aldosterone to promote vasoconstriction. Am J Physiol Renal Physiol 315, F1670–F1682. [DOI] [PubMed] [Google Scholar]
- Baykan M, Erem C, Gedikli O, Hacihasanoglu A, Erdogan T, Kocak M, Durmus I, Korkmaz L & Celik S (2007). Impairment of flow-mediated vasodilatation of brachial artery in patients with Cushing’s Syndrome. Endocrine 31, 300–304. [DOI] [PubMed] [Google Scholar]
- Branyan KW, Devallance ER, Lemaster KA, Skinner RC, Bryner RW, Olfert IM, Kelley EE, Frisbee JC & Chantler PD (2018). Role of Chronic Stress and Exercise on Microvascular Function in Metabolic Syndrome. Med Sci Sports Exerc 50, 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD & Touyz RM (2012). Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 59, 1069–1078. [DOI] [PubMed] [Google Scholar]
- Brooks S, Branyan KW, DeVallance E, Skinner R, Lemaster K, Sheets JW, Pitzer CR, Asano S, Bryner RW, Olfert IM, Frisbee JC & Chantler PD (2018a). Psychological stress-induced cerebrovascular dysfunction: the role of metabolic syndrome and exercise. Exp Physiol 103, 761–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SD, DeVallance E, d’Audiffret AC, Frisbee SJ, Tabone LE, Shrader CD, Frisbee JC & Chantler PD (2015). Metabolic Syndrome Impairs Reactivity and Wall Mechanics of Cerebral Resistance Arteries in Obese Zucker Rats. Am J Physiol Heart Circ Physiol 309, H1846–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, Shoemaker JK, Jackson DN & Frisbee JC (2018b). Protection from vascular dysfunction in female rats with chronic stress and depressive symptoms. Am J Physiol Heart Circ Physiol 314, H1070–H1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage E, Marshall KL, Santanam N & Chantler PD (2018). Cerebrovascular dysfunction with stress and depression. Brain Circ 4, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M, Small H, Yoong SL, Boyes A, Bisquera A & Sanson-Fisher R (2014). Prevalence of comorbid depression and obesity in general practice: a cross-sectional survey. Br J Gen Pract 64, e122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SH, Shelton J, White CR & Wyss JM (2000). Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension 35, 403–408. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S & Weintraub NL (2009). Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 104, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Audiffret AC, Frisbee SJ, Stapleton PA, Goodwill AG, Isingrini E & Frisbee JC (2010). Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol (1985) 108, 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVallance E, Branyan KW, Lemaster K, Olfert IM, Smith DM, Pistilli EE, Frisbee JC & Chantler PD (2018a). Aortic dysfunction in metabolic syndrome mediated by perivascular adipose tissue TNFalpha and NOX2 dependent pathway. Exp Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVallance E, Branyan KW, Lemaster K, Olfert IM, Smith DM, Pistilli EE, Frisbee JC & Chantler PD (2018b). Aortic dysfunction in metabolic syndrome mediated by perivascular adipose tissue TNFalpha- and NOX2-dependent pathway. Exp Physiol 103, 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVallance E, Branyan KW, Lemaster KC, Anderson R, Marshall KL, Olfert IM, Smith DM, Kelley EE, Bryner RW, Frisbee JC & Chantler PD (2019a). Exercise training prevents the perivascular adipose tissue-induced aortic dysfunction with metabolic syndrome. Redox Biol 26, 101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVallance E, Fournier SB, Donley DA, Bonner DE, Lee K, Frisbee JC & Chantler PD (2015). Is obesity predictive of cardiovascular dysfunction independent of cardiovascular risk factors? Int J Obes (Lond) 39, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVallance E, Li Y, Jurczak MJ, Cifuentes-Pagano E & Pagano PJ (2019b). The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid Redox Signal 31, 687–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disease GBD, Injury I & Prevalence C (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donley DA, Fournier SB, Reger BL, DeVallance E, Bonner DE, Olfert IM, Frisbee JC & Chantler PD (2014). Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985) 116, 1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecobici M & Voiculescu M (2017). Importance of arterial stiffness in predicting cardiovascular events. Rom J Intern Med 55, 8–13. [DOI] [PubMed] [Google Scholar]
- Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G & Lambert E (2006). Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48, 787–796. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Xia N & Li H (2017). Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ Res 120, 713–735. [DOI] [PubMed] [Google Scholar]
- Galougahi KK, Liu CC, Gentile C, Kok C, Nunez A, Garcia A, Fry NA, Davies MJ, Hawkins CL, Rasmussen HH & Figtree GA (2014). Glutathionylation mediates angiotensin II-induced eNOS uncoupling, amplifying NADPH oxidase-dependent endothelial dysfunction. J Am Heart Assoc 3, e000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M & Fernandez Alfonso MS (2006). Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 26, 1297–1302. [DOI] [PubMed] [Google Scholar]
- Gil-Ortega M, Somoza B, Huang Y, Gollasch M & Fernandez-Alfonso MS (2015). Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metab 26, 367–375. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS & Nimmo MA (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11, 607–615. [DOI] [PubMed] [Google Scholar]
- Golbidi S, Badran M & Laher I (2012). Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res 2012, 941868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbidi S, Frisbee JC & Laher I (2015). Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am J Physiol Heart Circ Physiol 308, H1476–1498. [DOI] [PubMed] [Google Scholar]
- Gollasch M (2012). Vasodilator signals from perivascular adipose tissue. Br J Pharmacol 165, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C & Harrison DG (2007). Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204, 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Skiba DS, Touyz RM & Harrison DG (2017). The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res 113, 1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett RJ, Burns J, Mackintosh AF & Mary DA (2004). Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension 44, 847–852. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Martin-Manso G, Maxhimer JB & Roberts DD (2009). Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer 9, 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanmard SH & Dana N (2012). The effect of interferon gamma on endothelial cell nitric oxide production and apoptosis. Adv Biomed Res 1, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatissa MN, Henningsen K, Nikolajsen G, West MJ & Wiborg O (2010). A reduced number of hippocampal granule cells does not associate with an anhedonia-like phenotype in a rat chronic mild stress model of depression. Stress 13, 95–105. [DOI] [PubMed] [Google Scholar]
- Lambert EA, Straznicky NE, Dixon JB & Lambert GW (2015). Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity? Am J Physiol Heart Circ Physiol 309, H244–258. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P & Benetos A (2001). Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37, 1236–1241. [DOI] [PubMed] [Google Scholar]
- Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B & Boutouyrie P (2003). Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 34, 1203–1206. [DOI] [PubMed] [Google Scholar]
- Lee DY, Wauquier F, Eid AA, Roman LJ, Ghosh-Choudhury G, Khazim K, Block K & Gorin Y (2013). Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: role of mitochondrial reactive oxygen species. J Biol Chem 288, 28668–28686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Schmuck S, Chorazcyzewski JZ, Gros R & Feldman RD (2003). Aldosterone regulates vascular reactivity: short-term effects mediated by phosphatidylinositol 3-kinase-dependent nitric oxide synthase activation. Circulation 108, 2400–2406. [DOI] [PubMed] [Google Scholar]
- Liu YN, Peng YL, Liu L, Wu TY, Zhang Y, Lian YJ, Yang YY, Kelley KW, Jiang CL & Wang YX (2015). TNFalpha mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytokine Netw 26, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano F, Vargas D, Gomez A & Torrado A (2017). Human ADMC-Derived Adipocyte Thermogenic Capacity Is Regulated by IL-4 Receptor. Stem Cells Int 2017, 2767916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Su LY, Lee RM & Gao YJ (2011). Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol 656, 68–73. [DOI] [PubMed] [Google Scholar]
- Mandler WK, Nurkiewicz TR, Porter DW & Olfert IM (2017). Thrombospondin-1 mediates multi-walled carbon nanotube induced impairment of arteriolar dilation. Nanotoxicology 11, 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M & Yoshimatsu H (2004). Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology 40, 177–184. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM & Witteman JC (2006). Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113, 657–663. [DOI] [PubMed] [Google Scholar]
- Mattsson C, Lai M, Noble J, McKinney E, Yau JL, Seckl JR & Walker BR (2003). Obese Zucker rats have reduced mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type 1 expression in hippocampus-implications for dysregulation of the hypothalamic-pituitary-adrenal axis in obesity. Endocrinology 144, 2997–3003. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D, Sagan A, Wu J, Vinh A, Marvar PJ, Guzik B, Podolec J, Drummond G, Lob HE, Harrison DG & Guzik TJ (2016). Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J 30, 1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Belzung C & Crusio WE (2006). Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res 175, 43–50. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Belzung C & Crusio WE (2007). Functional implications of decreases in neurogenesis following chronic mild stress in mice. Neuroscience 150, 251–259. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A & Locksley RM (2013). Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 210, 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DA, Anderson EA & Mark AL (1995). Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension 25, 834–838. [DOI] [PubMed] [Google Scholar]
- Mueller PJ (2007). Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol 34, 377–384. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health (U.S.) & United States. Substance Abuse and Mental Health Services Administration. Office of Applied Studies. (2015). Results from the … National Survey on Drug Use and Health. National findings. In National Survey on Drug Use and Health series, pp. 7 volumes. Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies, Rockville, MD. [Google Scholar]
- Nosalski R & Guzik TJ (2017). Perivascular adipose tissue inflammation in vascular disease. Br J Pharmacol 174, 3496–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli EM, Little-Ihrig L, Knupp HE, Rogers NM, Yao M, Baust JJ, Meijles D, St Croix CM, Ross MA, Pagano PJ, DeVallance ER, Miles G, Potoka KP, Isenberg JS & Gladwin MT (2019). Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 316, L1150–L1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H (2005). Aldosterone makes human endothelium stiff and vulnerable. Kidney Int 67, 1680–1682. [DOI] [PubMed] [Google Scholar]
- Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE & Hausberg M (2007). Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A 104, 16281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolo Bassareo P, Maria Zedda A & Mercuro G (2014). Impairment of Arterial Compliance in Cushing’s Syndrome. Eur Endocrinol 10, 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kim KH, Seo KW, Bae JU, Kim YH, Lee SJ, Lee WS & Kim CD (2014). Resistin derived from diabetic perivascular adipose tissue up-regulates vascular expression of osteopontin via the AP-1 signalling pathway. J Pathol 232, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA & Brody DJ (2014). Depression and obesity in the U.S. adult household population, 2005–2010. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Pu Q, Neves MF, Virdis A, Touyz RM & Schiffrin EL (2003). Endothelin antagonism on aldosterone-induced oxidative stress and vascular remodeling. Hypertension 42, 49–55. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E & Pitt B (2001). Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol 88, 196–198, A197. [DOI] [PubMed] [Google Scholar]
- Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL & Weintraub NL (2010). Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol 10, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton SN, Withers SB & Heagerty AM (2019). Emerging Roles of Sympathetic Nerves and Inflammation in Perivascular Adipose Tissue. Cardiovasc Drugs Ther 33, 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldenrijk A, van Hout HP, van Marwijk HW, de Groot E, Gort J, Rustemeijer C, Diamant M & Penninx BW (2011). Depression, anxiety, and arterial stiffness. Biol Psychiatry 69, 795–803. [DOI] [PubMed] [Google Scholar]
- Stylianou E, O’Neill LA, Rawlinson L, Edbrooke MR, Woo P & Saklatvala J (1992). Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J Biol Chem 267, 15836–15841. [PubMed] [Google Scholar]
- Turner NA, Mughal RS, Warburton P, O’Regan DJ, Ball SG & Porter KE (2007). Mechanism of TNFalpha-induced IL-1alpha, IL-1beta and IL-6 expression in human cardiac fibroblasts: effects of statins and thiazolidinediones. Cardiovasc Res 76, 81–90. [DOI] [PubMed] [Google Scholar]
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C & Beekman A (2007). Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 22, 613–626. [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves MF, Amiri F, Viel E, Touyz RM & Schiffrin EL (2002). Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 40, 504–510. [DOI] [PubMed] [Google Scholar]
- Wang ZV & Scherer PE (2008). Adiponectin, cardiovascular function, and hypertension. Hypertension 51, 8–14. [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Williamson PM, Mangos G & Kelly JJ (2005). Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag 1, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134, 319–329. [DOI] [PubMed] [Google Scholar]
- Withers SB, Agabiti-Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA & Heagerty AM (2011). Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arterioscler Thromb Vasc Biol 31, 908–913. [DOI] [PubMed] [Google Scholar]
- Wu C, Zhang H, Zhang J, Zhang H, Zeng Y, Fang S, Li P, Zhang Y, Lin X, Wang L, Xue Y & Guan M (2019). Increased oxidative stress, inflammation and fibrosis in perirenal adipose tissue of patients with cortisol-producing adenoma. Adipocyte 8, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HT, Sie SS, Kuan TC & Lin CS (2013). Identifying the regulative role of NF-kappaB binding sites within promoter region of human matrix metalloproteinase 9 (mmp-9) by TNF-alpha induction. Appl Biochem Biotechnol 169, 438–449. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Aksu F & Belzung C (2005). Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol 514, 165–174. [DOI] [PubMed] [Google Scholar]
- Yang X, Li Y, Li Y, Ren X, Zhang X, Hu D, Gao Y, Xing Y & Shang H (2017). Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front Physiol 8, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemse SM, Chiao CW, Hilgers RH & Webb RC (2010). Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-alpha on the endothelium of murine aorta. Am J Physiol Heart Circ Physiol 299, H1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]


