Abstract
While bacteria typically lack membrane bound organelles, the mechanisms of subcellular organization have been unclear. Bacteria have recently been found to harbor membraneless organelles containing enzymes of many biochemical pathways. These organelles, called biomolecular condensates, have been found to commonly form through the process of liquid-liquid phase separation and are typically enriched in nucleic acid binding proteins. Interestingly, eukaryote and bacterial transcription and RNA decay machinery have been found to form biomolecular condensates. Additionally, DEAD Box ATPases from eukaryotes and bacteria have also been found to modulate biomolecular condensates. The shared ability of RNA metabolic enzymes to assemble into biomolecular condensates across domains suggests that this mode of subcellular organization aids in the control of RNA metabolism.
Keywords: BR-bodies, RNAP condensate, mRNA Decay, mRNA turnover, mRNA transcription, DEAD Box RNA Helicases, biomolecular condensates
Introduction
While eukaryotic cells are known to organize their biochemical pathways via membrane bound organelles, recent investigations of membraneless organelles has revealed their critical and widespread roles. This has been fueled by the finding that these membraneless organelles can often be formed by liquid-liquid phase separation (LLPS) which allows for internal biochemical activity [1–3]. The membraneless organelles formed by this process are often referred to as biomolecular condensates (hereafter referred to as condensates) and include the nucleolus, Cajal bodies, Processing bodies (P-bodies) and stress granules to name a few [2]. Similar condensates have now been identified in bacterial cells [Figure 1a] [4–7], and due to the general lack of membrane bound organelles, have been proposed to play critical roles in organizing the bacterial cytoplasm. Interestingly, transcriptional machinery and RNA decay machinery have been found to form condensates across both eukaryotes and bacteria. This review will focus on recent advances in bacterial RNA metabolism in condensates which will then be compared with functionally related eukaryotic condensates.
Figure 1.
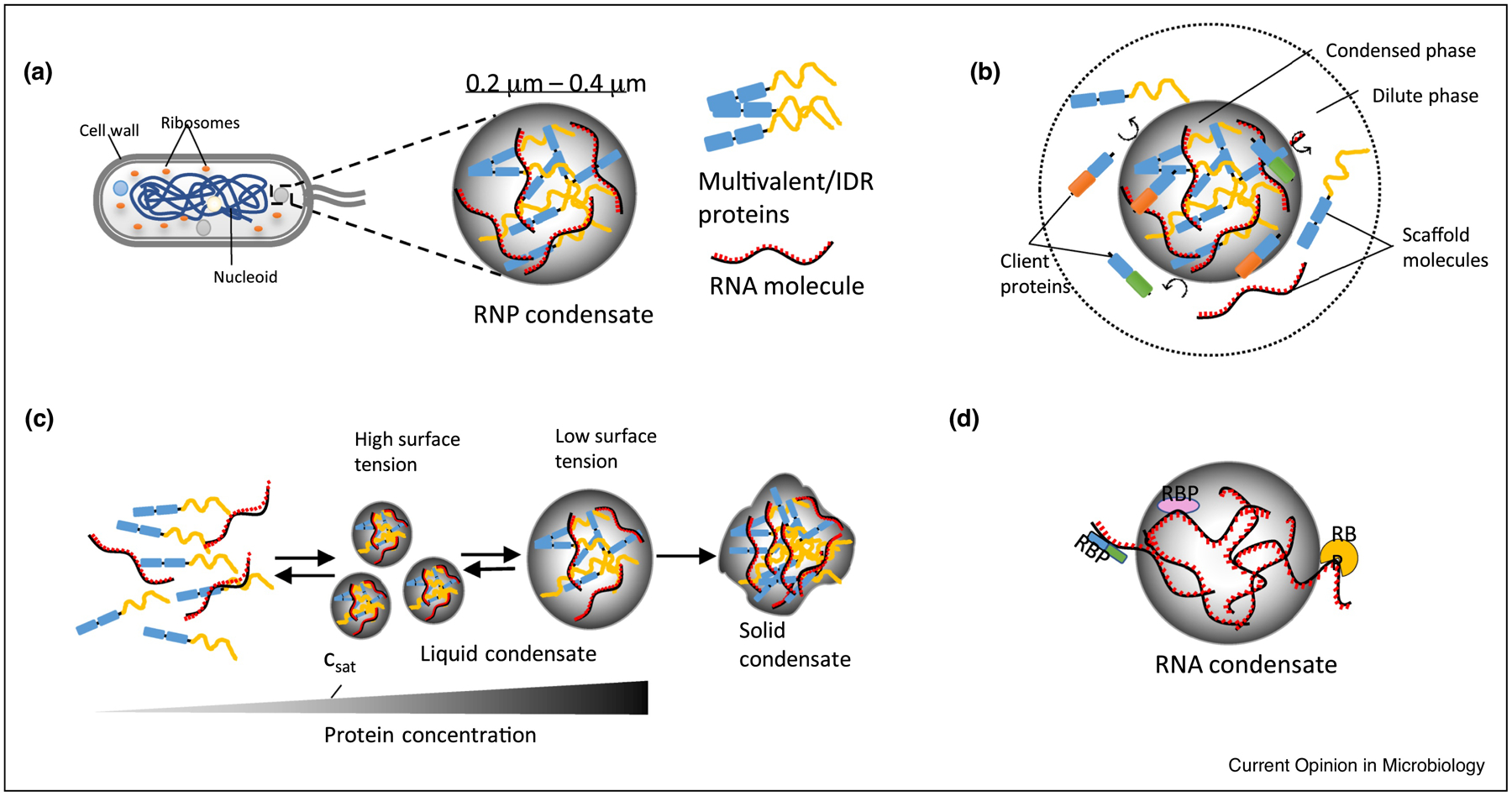
Basic properties of liquid-liquid phase separated condensates. (a) Bacterial cell showing condensates in the cytoplasm with one ribonucleoprotein (RNP) condensate enlarged. RNP condensates are formed by weak multivalent interactions between multivalent/intrinsically disordered proteins (IDPs) and RNA. Multivalency refers to the number of specific intra and as well inter molecular interactions. Multivalent domains can be present on both folded and intrinsically disordered regions (IDRs) of a protein. IDRs lack a defined structure and are enriched in disorder promoting amino acids (Arg, Pro, Gly, Glu, Ser, Ala and Lys). (b) Condensed phase is rich in macromolecules and the dilute phase sparse in macromolecules, nevertheless the system is in thermodynamic equilibrium because of the gain in net free energy (ΔG) in the condensed phase. Condensates are selective in recruiting protein and RNA molecules that may have promoting or inhibitory effect on the condensates. Multivalent/IDR proteins and RNA molecules acts as scaffold molecules in recruiting client protein and RNA molecules into the condensate. (c) The formation of liquid condensates is reversible. At concentrations above the critical saturation concentration (csat), the proteins or RNA molecules condense into droplets, and the smaller droplets can fuse to become bigger droplets as this process lowers the surface tension of smaller droplets. Intracellular or extracellular stimulation may lead to the dissolution of condensates. Liquid condensates can turn into solid condensates by accumulating large number of macromolecules and this process is often irreversible. (d) RNA alone has the intrinsic property to undergo phase separation and RNA molecules can in turn recruit specific RNA binding proteins (RBPs) ultimately forming an RNP granule.
The initial discovery that eukaryotic P-granules behave like liquids [8] has sparked similar findings in many eukaryotic membraneless organelles [9]. A combination of in vivo and in vitro studies have revealed that proteins and RNA undergo LLPS by forming transient and multivalent weak intermolecular interactions driven by an energetically favorable process resulting in a condensed phase (macromolecular rich) and a dilute phase (macromolecular sparse) [Figure 1b]. The largest class of condensates, Ribonucleoprotein (RNP) condensates, are gaining immense interest because of their critical role in organizing RNA transcription, splicing, RNA modification, localization, translation, and decay [10–14]. Condensates aid RNA metabolism through selective permeability of client molecules, enhancement of substrate concentration, and the modulation of chemical reactions [15]. Multivalent RNA binding proteins (RBPs) with intrinsically disordered regions (IDRs) are the key scaffolding proteins involved in condensate formation [2]. IDRs act as “spacers” to provide flexibility, and multivalent weak interactions act as “stickers” to promote dynamic associations between proteins. These interactions can occur between folded domains and IDRs with emerging rules of specificity dictated by the amino acid content [16–18]. These scaffolds can also recruit client proteins [Figure 1b] into the condensate through protein-protein interactions which can bring new biochemical functions [2]. Additionally, RNA has been found to form condensates in the absence of proteins through intermolecular base-pairing, which could recruit specific RBPs [Figure 1d] [19,20]. Overall, the ability to assemble a membraneless organelle through phase-separation may provide a critical role in organizing the biochemical processes within the bacterial cytoplasm.
RNAP condensates as organizers of rRNA transcription
Eukaryotic RNA polymerases (RNAPs) I and II and bacterial RNAP form condensates [6,21,22]. As RNAP I is known to localize in the nucleolus, one of the best characterized eukaryotic condensates that organizes rRNA transcription and ribosome assembly, bacterial RNAP clusters have similar properties [6,23,24] [Figure 2a]. Bacterial RNAP foci are observed in both Escherichia coli and Bacillus subtilis grown in rich media [25–27]. Importantly, E. coli RNAP foci occur in logarithmic growth when cells are doubling most rapidly, and can be reversibly dissociated when hexanediol is added to cells, suggesting that they are in a liquid-like state [6]. Additionally, the RNAP associated anti-termination factor NusA has been found to form similar clusters while the purified protein can phase-separate in vitro [6]. Single-molecule particle tracking experiments found both RNAP and NusA molecules are dynamic, with a significantly higher fraction of slower moving molecules in nutrient rich conditions which likely correspond to those RNAP molecules in foci [6]. Therefore, we will refer to these RNAP foci as RNAP condensates. It has been well established that most RNAP in fast-growing E. coli are known to be engaged in rRNA transcription [28], and 6 of the 7 rRNA loci have been found to colocalize, similar to the colocalization of the rRNA loci in the eukaryotic nucleolus (Figure 2a) [29]. Indeed, super-resolution imaging found that pre-rRNA is colocalized with RNAP clusters, suggesting that these clusters correspond to active RNAP molecules [23]. Interestingly, rRNA synthesis is not an essential requirement for RNAP condensates, as treatment of cells with serine hydroxamate, which induces a starvation response that rapidly halts new rRNA synthesis, does not lead to the complete dissociation of RNAP condensates [23]. In contrast, treatment of E. coli cells with rifampicin, which globally blocks RNAP activity, led to a stronger reduction in RNAP foci. This result suggests that RNAP foci correlate with active mRNA transcription [23]. Removal of 6 of the 7 rRNA genes from the E. coli chromosome did not lead to a complete reduction in RNAP condensates [23], nor did treatment with a DNA gyrase inhibitor or deletion of nucleoid associated protein genes fis or lrp [23], suggesting the nucleoid structure alone is not responsible for RNAP condensate formation. While reduction in rRNA transcription and rRNA gene copy number lead to a reduction in RNAP condensate size and number [23,24,30], further studies will be needed to identify the precise macromolecular rules governing the formation of RNAP condensates. In addition, RNAP I in eukaryotes is responsible for rRNA transcription and resides predominantly in the nucleolus where rRNA genes, processing factors, and modifying enzymes colocalize to allow for cotranscriptional rRNA processing [31]. However, it is currently unknown whether processing factors and modifying enzymes also colocalize with bacterial RNAP condensates to promote cotranscriptional rRNA processing and assembly of ribosomes. In addition, eukaryotic RNAP II has been found to assemble into super enhancer clusters which stimulate mRNA synthesis and affect coordination with splicing condensates[10,32]. It is currently unknown whether bacterial RNAP condensates impact mRNA transcription.
Figure 2.
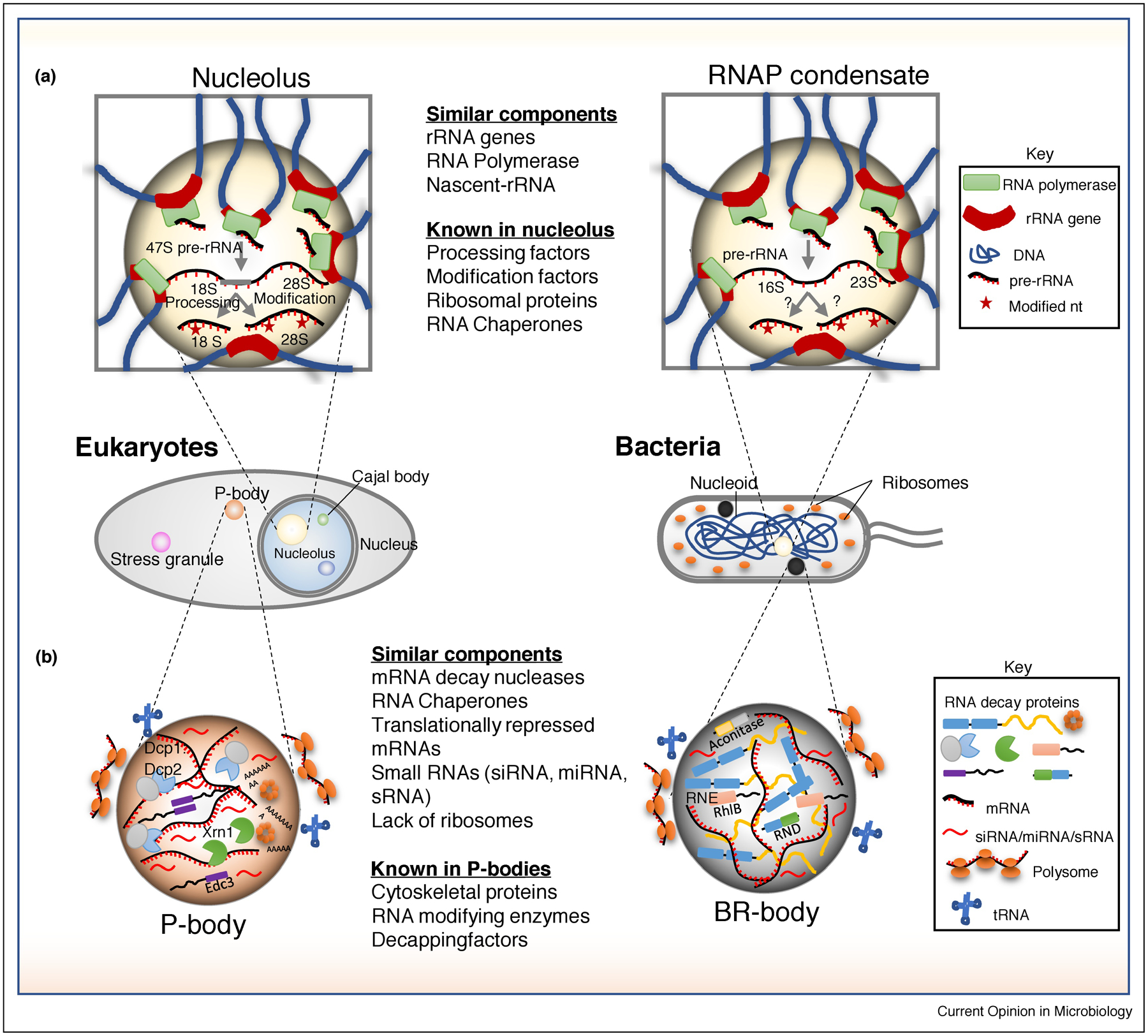
Prokaryotic and eukaryotic RNP granules involved in RNA synthesis and degradation. (a) Left: In eukaryotes, clusters of ribosomal RNA (rRNA) genes are transcribed by RNA polymerase I (RNAP I) and processed in a liquid condensate called nucleolus situated in the nucleus. The processed 18 S and 12 S rRNA are exported into the cytoplasm for the subsequent assembly of ribosomes and translation. Right: In E. coli RNA polymerase (RNAP) foci are shown to colocalize with rRNA operons (In E. coli 6 out of 7 rRNA operons - rrnE, rrnG, rrnD, rrnA, rrnH and rrnB are colocalized). This arrangement of rRNA operons and RNAP in bacteria mimics the nucleolus like compartmentalization seen in eukaryotes, though the transcription and processing of rRNAs in bacterial condensates need to be demonstrated. (b) Processing bodies (P-bodies) in eukaryotes (left) can be compared to the bacterial ribonucleoprotein bodies (BR-bodies) in bacteria (right). There are lot of similarities between these condensates in terms of the presence or absence of functionally equivalent proteins and RNA molecules. While it is agreed that BR-bodies mainly act as RNA decay compartments, there is no consensus whether P-bodies act as RNA decay or storage compartments.
RNA decay machinery condensates stimulate bacterial mRNA decay
Eukaryotic mRNA decay machinery has been identified to localized into cytoplasmic condensates called processing bodies (P-bodies) and stress granules (Figure 2b) [33]. While the major cytoplasmic nuclease Xrn1 is present in both P-bodies and stress granules, bacteria most commonly use different mRNA decay nucleases, the most common of which is RNase E [34,35]. P-bodies and stress granules are conserved from yeast to humans and assemble condensates composed of many mRNA decay-related nucleases, RNA chaperones, and translational repressors [36]. RNA degradosomes, the active multi-protein complexes facilitating mRNA decay in bacteria [37], have been found to form bacterial ribonucleoprotein bodies (BR-bodies) that can be observed as cytoplasmic foci in many bacterial species containing RNase E based RNA degradosomes (C. crescentus, S. meliloti, A. tumefacienes, E. coli, and Cyanobacteria) [4,38], RNase J based degradosomes (H. pylori) [39], and RNase Y based degradosomes (B. subtilis) [40]. Beyond a scaffold nuclease, RNA chaperones and additional RNases are common components of BR-bodies [4]. RNase E in C. crescentus and E. coli and RNase Y in B. subtilis have all been found to undergo foci fusion events, suggesting liquid-like behavior [4,38,40,41]. In addition, the IDR of C. crescentus RNase E was found to be necessary and sufficient for foci formation in cells, and was able to phase-separate into droplets in vitro, suggesting that this protein feature controls condensation of BR-bodies [4]. Deletion of the C-terminal IDR from E. coli or C. crescentus RNase E slows the rate of mRNA decay in the cell [13,41].
Most bacteria initiate bacterial mRNA decay via endonucleolytic cleavage, followed by 3–5’ exonucleolytic degradation of mRNA decay intermediates, such that in wild-type cells virtually no mRNA decay intermediates are detected [42]. Interestingly, RNase E mutants that disrupt association with exonuclease or that lack C-terminal IDRs show increased accumulation of mRNA decay intermediates, suggesting that BR-bodies help ensure mRNA decay fragments are degraded within BR-bodies [Figure 3] [13].
Figure 3.

RNA decay in bacterial ribonucleoprotein bodies (BR-bodies). The existence of various functional enzymes hints their probable coordination in BR-bodies. RNase E, the core component cleaves long RNAs into short RNAs by its endonucleolytic activity. DEAD box ATPase and RNase D (3’−5’ exonuclease) coordinate with RNase E to further degrade RNA. This leads to RNA fragmentation and reduced multivalent interactions between proteins and RNA molecules resulting in the dissolution of BR-body.
One fundamental feature of condensates is selective permeability. BR-bodies enrich long poorly translated mRNAs and small regulatory RNAs (sRNAs) and exclude structured RNAs including rRNAs and tRNAs [13]. Another intriguing observation is the physical exclusion of translating ribosomes from BR-bodies in bacteria and from P-bodies and stress granules in eukaryotes (Figure 2b) [13,36]. This phenomenon has the effect of compartmentalizing mRNA decay processes from translation processes. Enrichment of sRNAs hints at the probable existence of another level of posttranscriptional gene regulation in BR-bodies. Silencing RNAs are also found in eukaryotic RNP granules (P-bodies & stress granules) but their function is poorly understood [36,43]. The bacterial RNA chaperone protein, Hfq, mediates the interaction between sRNAs and mRNAs to regulate the stability and translation of mRNAs [44,45]. sRNA induced mRNA decay is significantly reduced in strains lacking the RNase E C-terminal IDR, and sRNA-mRNA colocalization was significantly increased [46], suggesting that BR-bodies may stimulate the decay of silenced mRNAs. Interestingly, Hfq has been found to form cytoplasmic foci upon nitrogen starvation [47], however, it’s not yet known whether these foci localize with BR-bodies.
While no complete inventory of BR-body associated proteins yet exists, P-bodies and stress granules are known to contain >100 proteins [36, 48, 49]. In P-bodies, a subset of proteins is present at high concentrations forming a “core”, while most P-body proteins were present at lower stoichiometries [50]. While it is hypothesized that the RNA degradosome forms the BR-body core, a systematic investigation of BR-body associated proteins will answer this. One additional question that needs to be investigated is if all the BR-bodies in a cell have a homogenous mixture of client proteins, or if BR-bodies contain a heterogenous client composition based on mRNA-specific requirements of RNA processing/decay factors.
Additionally, while RNA appears to be a critical component for assembly of RNase E based foci in C. crescentus, A. tumefaciens, S. meliloti, and E. coli, it is not required for the RNase Y foci in B. subtilis, suggesting different assembly requirements of BR-bodies across species. The presence of D-foci in mitochondria and RNA stress granules in chloroplasts suggests that similar mechanisms of organization are used in these endosymbiotic organelles [51, 52].
DEAD box ATPases as modulators of bacterial condensates?
DEAD box ATPases belong to helicase super family 2 and are composed of a conserved helicase core made up of two (Rec A-like domains. The name “DEAD-box” derives from the presence of conserved Asp-Glu-Ala-Asp amino acid motif in domain 1 of helicase core [53]. DEAD box ATPases alter RNA structure and remodel RNPs through ATP dependent RNA binding and ATP hydrolysis [54]. Recent investigations in eukaryotic cells have attributed a novel dual role to the DEAD box ATPases in regulating the formation and turnover of condensates depending on the context [Figure 4] [55,56]. DEAD box proteins with IDRs can undergo LLPS and promote the formation of RNP condensates either on their own or with the aid of other protein partners [57]. Additionally, they can promote changes in the condensate RNP composition through ATP binding or hydrolysis, thereby regulating condensate formation [55,56,58]. Most bacteria have DEAD box ATPases that are involved in various steps of RNA metabolism, and several of them have long IDRs or low complexity domains that have the propensity to form condensates [55,59]. For example, E. coli has five DEAD box proteins, RhlB, RhlE, DeaD, SrmB and DbpA, whose functions are involved in various processes including ribosome biogenesis, RNA turnover, and translation initiation [60]. Three of them (DeaD, SrmB and RhlE) have long IDRs and readily form condensates in vitro at their physiological concentrations or when overexpressed in vivo [55]. While RhlB lacks a long IDR and does not phase separate in vitro, it does associate with RNase E and is recruited into BR-bodies, though the role of ATPase activity in the regulation of BR-bodies has not been investigated [4,38]. Yeast Dhh1 DEAD Box ATPase activity has been shown to allow rapid compartment turnover and release of RNA [58] and, it is possible that RhlB ATPase activity may provide a similar function in BR-bodies. DEAD Box ATPase activity may also be able to prevent BR-bodies from solidifying, as has been observed for eukaryotic RNP granules, which may allow them to transition into mRNA storage granules. Another important goal for future research will be to identify whether bacterial DEAD box proteins containing IDRs form independent condensates in vivo. If bacteria can simultaneously generate multiple RNP condensates, this would allow cells to recruit unique client proteins/RNA molecules to each condensate and organize different RNA processes.
Figure 4.
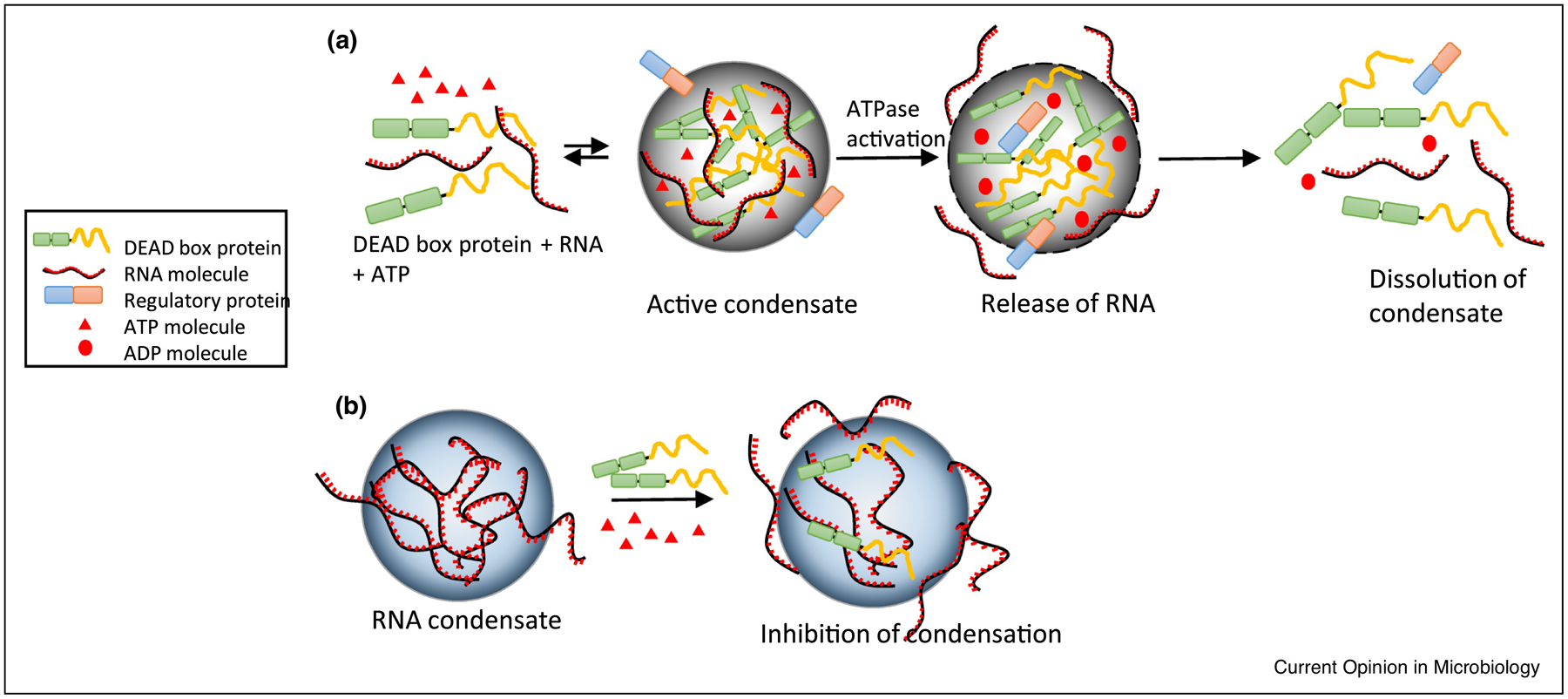
DEAD box proteins regulate the assembly and turnover of condensates. (a) DEAD box proteins undergo oligomerization in the presence of RNA and ATP forming RNP condensates. These RNP condensates may organize various RNA processing reactions depending on the function of a DEAD box ATPase. Upon the stimulation of ATPase activity by a regulatory protein, RNA is released leading to the dissolution of condensate [55]. (b) DEAD box proteins can act as chaperones by reducing the multivalent interactions between RNA molecules in the condensate by means of its ATPase activity. This activity of DEAD box proteins potentially reduces RNA/RNP condensation in the cytoplasm of cells [56].
Conclusions
Although condensate research is only in the early stages in bacteria, there is growing evidence for the idea that condensates play a role in compartmentalizing diverse macromolecules and biochemical processes [61–63]. Organizing biochemical processes in condensates can have multiple advantages, which can be dynamically regulated by their formation and deformation. Condensation can accelerate reactions by concentrating enzymes and substrate. Condensates may also act to suppress reactions by sequestering molecules from their site of action. Additionally, condensation can allow for selective permeability of substrates and enzymes preventing off-pathway reactions and/or premature build-up of pathway intermediates. Many RNA processes are highly coordinated in vivo and it is likely that condensates of different types facilitate the coordination. Now that bacterial RNAP has been found to form a condensate in rapidly growing E. coli cells, it’s possible that these condensates will be coordinated with BR-bodies, as the core BR-body protein RNase E is required for 5S rRNA maturation [64]. Indeed, in C. crescentus, RNase E foci are found to commonly overlap with rRNA genes [65], suggesting RNase E and RNAP condensates coordinate rRNA transcription and processing.. The biochemical functionality of RNAP condensates and coordination with BR-bodies will be an area of immense interest. BR-bodies are comparatively better characterized in terms of their biochemical functions in mRNA decay, being found to accelerate RNA cleavage and prevent the buildup of mRNA decay intermediates, but there are many aspects of their functionality that need to be better understood. How do different RNA-decay enzymes in BR-bodies coordinate decay? Are BR-bodies homogeneous, or heterogeneous within a single cell? How do BR-bodies coordinate with Hfq to mediate sRNA silencing?
As this field grows, one of the key questions to be addressed is how many types of bacterial condensates may exist? It is well known that RBPs often play a central role in condensate formation, yet the true number of bacterial RBPs is unknown, and many well-characterized eukaryotic RNA binding domains are largely absent in bacterial proteins [66]. New unbiased approaches, such as grad-seq and UV-crosslinking phenol-chloroform based mass-spec approaches have identified previously unknown RNA binding proteins with unconventional RNA binding domains (RBDs) [67,68]. Many metabolic enzymes were found to have moonlighting activity as RBPs in E. coli and their RNA binding is strongly conserved with homologues in yeast Saccharomyces cerevisiae [69]. Interestingly, under stress conditions these metabolic enzymes in eukaryotes form condensates together with RNA substrates [70,71]. A comprehensive analysis of bacterial RBPs using global experimental approaches followed by testing in vitro and in vivo condensate forming ability will likely yield new RNP condensates with interesting functions in RNA metabolism.
Highlights.
Biomolecular condensates act as membraneless organelles that assemble via liquid-liquid phase-separation.
Bacterial transcription and mRNA decay machinery were found to form biomolecular condensates.
Bacterial RNP condensates facilitate spatial coordination of multi-step RNA processing pathways.
DEAD box ATPases can act as regulators of bacterial biomolecular condensates.
Acknowledgements
The authors thank members of the Schrader lab for feedback. Funding: This work was supported by the National Institutes of Health [R35GM124733 to J.M.S.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Walter H, Brooks DE: Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation. FEBS Letters 1995, 361:135–139. [DOI] [PubMed] [Google Scholar]
- 2.Banani SF, Lee HO, Hyman AA, Rosen MK: Biomolecular condensates: organizers of cellular biochemistry. Nature Reviews Molecular Cell Biology 2017, 18:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman AA, Weber CA, Jülicher F: Liquid-Liquid Phase Separation in Biology. Annual Review of Cell and Developmental Biology 2014, 30:39–58. [DOI] [PubMed] [Google Scholar]
- 4.Al-Husini N, Tomares DT, Bitar O, Childers WS, Schrader JM: α-Proteobacterial RNA Degradosomes Assemble Liquid-Liquid Phase-Separated RNP Bodies. Molecular Cell 2018, 71:1027–1039.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]; (** This study shows RNase E undergoing LLPS to form BR bodies. This is the first in vivo and in vitro demonstration of LLPS in bacteria)
- 5.Lasker K, von Diezmann L, Zhou X, Ahrens DG, Mann TH, Moerner WE, Shapiro L: Selective sequestration of signalling proteins in a membraneless organelle reinforces the spatial regulation of asymmetry in Caulobacter crescentus. Nature Microbiology 2020, 5:418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladouceur A-M, Parmar BS, Biedzinski S, Wall J, Tope SG, Cohn D, Kim A, Soubry N, Reyes-Lamothe R, Weber SC: Clusters of bacterial RNA polymerase are biomolecular condensates that assemble through liquid–liquid phase separation. PNAS 2020, 117:18540–18549. [DOI] [PMC free article] [PubMed] [Google Scholar]; (** This work provides the first direct evidence for the liquid like behavior of transcriptional condensates in bacteria)
- 7.Saurabh S, Chong TN, Bayas C, Dahlberg PD, Cartwright HN, Moerner WE, Shapiro L: Modulation of kinase activity within a bacterial membraneless organelle. bioRxiv 2020, doi: 10.1101/2020.08.09.232405. [DOI] [Google Scholar]
- 8.Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA: Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324:1729–32. [DOI] [PubMed] [Google Scholar]
- 9.Mitrea DM, Kriwacki RW: Phase separation in biology; functional organization of a higher order. Cell Communication and Signaling 2016, 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, Li CH, Shrinivas K, Manteiga JC, Hannett NM, et al. : Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175:1842–1855.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo YE, Manteiga JC, Henninger JE, Sabari BR, Dall’Agnese A, Hannett NM, Spille J-H, Afeyan LK, Zamudio AV, Shrinivas K, et al. : Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irastortza-Olaziregi M, Amster-Choder O: RNA localization in prokaryotes: Where, when, how, and why. WIREs RNA 2020, n/a:e1615. [DOI] [PubMed] [Google Scholar]
- 13.Al-Husini N, Tomares DT, Pfaffenberger ZJ, Muthunayake NS, Samad MA, Zuo T, Bitar O, Aretakis JR, Bharmal M-HM, Gega A, et al. : BR-Bodies Provide Selectively Permeable Condensates that Stimulate mRNA Decay and Prevent Release of Decay Intermediates. Molecular Cell 2020, 78:670–682.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (* This study shows that BR bodies organize multi step mRNA decay pathway)
- 14.Iserman C, Desroches Altamirano C, Jegers C, Friedrich U, Zarin T, Fritsch AW, Mittasch M, Domingues A, Hersemann L, Jahnel M, et al. : Condensation of Ded1p Promotes a Translational Switch from Housekeeping to Stress Protein Production. Cell 2020, 181:818–831.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyon AS, Peeples WB, Rosen MK: A framework for understanding the functions of biomolecular condensates across scales. Nature Reviews Molecular Cell Biology 2020, doi: 10.1038/s41580-020-00303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Y L, Ds P, Mk R, R P: Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell 2015, 60:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Choi J-M, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, et al. : A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174:688–699.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J-M, Holehouse AS, Pappu RV: Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions. Annu Rev Biophys 2020, 49:107–133. [DOI] [PMC free article] [PubMed] [Google Scholar]; (* This work provides a comprehensive sticker and spacer framework of the molecular determinants of biomolecules that undergo LLPS)
- 19.Van Treeck B, Protter DSW, Matheny T, Khong A, Link CD, Parker R: RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc Natl Acad Sci USA 2018, 115:2734–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]; (* This study shows that eukaryotic RNA can self-assemble to form condensates)
- 20.Boeynaems S, Holehouse AS, Weinhardt V, Kovacs D, Lindt JV, Larabell C, Bosch LVD, Das R, Tompa PS, Pappu RV, et al. : Spontaneous driving forces give rise to protein–RNA condensates with coexisting phases and complex material properties. PNAS 2019, 116:7889–7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehning M, Dugast-Darzacq C, Rankovic M, Hansen AS, Yu T, Marie-Nelly H, McSwiggen DT, Kokic G, Dailey GM, Cramer P, et al. : RNA polymerase II clustering through carboxy-terminal domain phase separation. Nature Structural & Molecular Biology 2018, 25:833–840. [DOI] [PubMed] [Google Scholar]
- 22.Cho W-K, Spille J-H, Hecht M, Lee C, Li C, Grube V, Cisse II: Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361:412–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng X, Bohrer CH, Bettridge K, Lagda AC, Cagliero C, Jin DJ, Xiao J: Spatial organization of RNA polymerase and its relationship with transcription in Escherichia coli. Proc Natl Acad Sci USA 2019, 116:20115–20123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (* This study shows that RNA polymerase clusters in bacteria are active transcription centers engaged in ribosomal RNA transcription)
- 24.Jin DJ, Mata Martin C, Sun Z, Cagliero C, Zhou YN: Nucleolus-like compartmentalization of the transcription machinery in fast-growing bacterial cells. Crit Rev Biochem Mol Biol 2017, 52:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabrera JE, Jin DJ: The distribution of RNA polymerase in Escherichia coli is dynamic and sensitive to environmental cues. Molecular Microbiology 2003, 50:1493–1505. [DOI] [PubMed] [Google Scholar]
- 26.Lewis PJ, Thaker SD, Errington J: Compartmentalization of transcription and translation in Bacillus subtilis. The EMBO Journal 2000, 19:710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.E U, F K, H Sj, C Pr, K An, H M: Multiscale spatial organization of RNA polymerase in Escherichia coli. Biophys J 2013, 105:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremer H, Dennis PP: Modulation of Chemical Composition and Other Parameters of the Cell at Different Exponential Growth Rates. EcoSal Plus 2008, 3. [DOI] [PubMed] [Google Scholar]
- 29.Gaal T, Bratton BP, Sanchez-Vazquez P, Sliwicki A, Sliwicki K, Vegel A, Pannu R, Gourse RL: Colocalization of distant chromosomal loci in space in E. coli : a bacterial nucleolus. Genes Dev 2016, 30:2272–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mata Martin C, Sun Z, Zhou YN, Jin DJ: Extrachromosomal Nucleolus-Like Compartmentalization by a Plasmid-Borne Ribosomal RNA Operon and Its Role in Nucleoid Compaction. Front Microbiol 2018, 9:1115–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, Brangwynne CP: Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 2016, 165:1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho W-K, Spille J-H, Hecht M, Lee C, Li C, Grube V, Cisse II: Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decker CJ, Parker R: P-Bodies and Stress Granules: Possible Roles in the Control of Translation and mRNA Degradation. Cold Spring Harb Perspect Biol 2012, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohanty BK, Kushner SR: Regulation of mRNA Decay in Bacteria. Annu Rev Microbiol 2016, 70:25–44. [DOI] [PubMed] [Google Scholar]
- 35.Hui MP, Foley PL, Belasco JG: Messenger RNA degradation in bacterial cells. Annu Rev Genet 2014, 48:537–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubstenberger A, Courel M, Bénard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot J-B, Munier A, Fradet M, et al. : P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Molecular Cell 2017, 68:144–157.e5. [DOI] [PubMed] [Google Scholar]
- 37.Bandyra KJ, Bouvier M, Carpousis AJ, Luisi BF: The social fabric of the RNA degradosome. Biochim Biophys Acta 2013, 1829:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strahl H, Turlan C, Khalid S, Bond PJ, Kebalo J-M, Peyron P, Poljak L, Bouvier M, Hamoen L, Luisi BF, et al. : Membrane recognition and dynamics of the RNA degradosome. PLoS Genet 2015, 11:e1004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tejada-Arranz A, Galtier E, Mortaji LE, Turlin E, Ershov D, Reuse HD: The RNase J-Based RNA Degradosome Is Compartmentalized in the Gastric Pathogen Helicobacter pylori. mBio 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamouche L, Billaudeau C, Rocca A, Chastanet A, Ngo S, Laalami S, Putzer H: Dynamic Membrane Localization of RNase Y in Bacillus subtilis. mBio 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez PJ, Marchand I, Joyce SA, Dreyfus M: The C-terminal half of RNase E, which organizes the Escherichia coli degradosome, participates in mRNA degradation but not rRNA processing in vivo. Molecular Microbiology 1999, 33:188–199. [DOI] [PubMed] [Google Scholar]
- 42.Hui MP, Foley PL, Belasco JG: Messenger RNA Degradation in Bacterial Cells. Annu Rev Genet 2014, 48:537–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitchiaya S, Mourao MDA, Jalihal AP, Xiao L, Jiang X, Chinnaiyan AM, Schnell S, Walter NG: Dynamic Recruitment of Single RNAs to Processing Bodies Depends on RNA Functionality. Molecular Cell 2019, 74:521–533.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Updegrove TB, Zhang A, Storz G: Hfq: the flexible RNA matchmaker. Curr Opin Microbiol 2016, 30:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assis NG, Ribeiro RA, da Silva LG, Vicente AM, Hug I, Marques MV: Identification of Hfq-binding RNAs in Caulobacter crescentus. RNA Biology 2019, 16:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fei J, Singh D, Zhang Q, Park S, Balasubramanian D, Golding I, Vanderpool CK, Ha T: Determination of in vivo target search kinetics of regulatory noncoding RNA. Science 2015, 347:1371–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McQuail J, Switzer A, Burchell L, Wigneshweraraj S: The RNA-binding protein Hfq assembles into foci-like structures in nitrogen starved Escherichia coli. J Biol Chem 2020, 295:12355–12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R: ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youn J-Y, Dunham WH, Hong SJ, Knight JDR, Bashkurov M, Chen GI, Bagci H, Rathod B, MacLeod G, Eng SWM, et al. : High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies. Mol Cell 2018, 69:517–532.e11. [DOI] [PubMed] [Google Scholar]
- 50.Xing W, Muhlrad D, Parker R, Rosen MK: A quantitative inventory of yeast P body proteins reveals principles of composition and specificity. eLife 2020, 9:e56525.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borowski LS, Dziembowski A, Hejnowicz MS, Stepien PP, Szczesny RJ: Human mitochondrial RNA decay mediated by PNPase–hSuv3 complex takes place in distinct foci. Nucleic Acids Res 2013, 41:1223–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uniacke J, Zerges W: Stress induces the assembly of RNA granules in the chloroplast of Chlamydomonas reinhardtii. J Cell Biol 2008, 182:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linder P, Jankowsky E: From unwinding to clamping — the DEAD box RNA helicase family. Nature Reviews Molecular Cell Biology 2011, 12:505–516. [DOI] [PubMed] [Google Scholar]
- 54.Linder P, Lasko PF, Ashburner M, Leroy P, Nielsen PJ, Nishi K, Schnier J, Slonimski PP: Birth of the D-E-A-D box. Nature 1989, 337:121–122. [DOI] [PubMed] [Google Scholar]
- 55.Hondele M, Sachdev R, Heinrich S, Wang J, Vallotton P, Fontoura BMA, Weis K: DEAD-box ATPases are global regulators of phase-separated organelles. Nature 2019, 573:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]; (** This paper demonstrates the LLPS propensity of DEAD box proteins in prokaryotes and eukaryotes, and ascribes DEAD box proteins as regulators of condensates)
- 56.Tauber D, Tauber G, Khong A, Van Treeck B, Pelletier J, Parker R: Modulation of RNA Condensation by the DEAD-Box Protein eIF4A. Cell 2020, 180:411–426.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sachdev R, Hondele M, Linsenmeier M, Vallotton P, Mugler CF, Arosio P, Weis K: Pat1 promotes processing body assembly by enhancing the phase separation of the DEAD-box ATPase Dhh1 and RNA. eLife 2019, 8:e41415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mugler CF, Hondele M, Heinrich S, Sachdev R, Vallotton P, Koek AY, Chan LY, Weis K: ATPase activity of the DEAD-box protein Dhh1 controls processing body formation. eLife 2016, 5:e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Functions of DEAD-box proteins in bacteria: Current knowledge and pending questions. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2013, 1829:866–877. [DOI] [PubMed] [Google Scholar]
- 60.Redder P, Hausmann S, Khemici V, Yasrebi H, Linder P: Bacterial versatility requires DEAD-box RNA helicases. FEMS Microbiol Rev 2015, 39:392–412. [DOI] [PubMed] [Google Scholar]
- 61.Greening C, Lithgow T: Formation and function of bacterial organelles. Nature Reviews Microbiology 2020, doi: 10.1038/s41579-020-0413-0. [DOI] [PubMed] [Google Scholar]
- 62.Abbondanzieri EA, Meyer AS: More than just a phase: the search for membraneless organelles in the bacterial cytoplasm. Curr Genet 2019, 65:691–694. [DOI] [PubMed] [Google Scholar]
- 63.Azaldegui CA, Vecchiarelli AG, Biteen JS: The emergence of phase separation as an organizing principle in bacteria. Biophysical Journal 2020, doi: 10.1016/j.bpj.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hardwick SW, Chan VSY, Broadhurst RW, Luisi BF: An RNA degradosome assembly in Caulobacter crescentus. Nucleic Acids Res 2011, 39:1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bayas CA, Wang J, Lee MK, Schrader JM, Shapiro L, Moerner WE: Spatial organization and dynamics of RNase E and ribosomes in Caulobacter crescentus. PNAS 2018, 115:E3712–E3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holmqvist E, Vogel J: RNA-binding proteins in bacteria. Nature Reviews Microbiology 2018, 16:601–615. [DOI] [PubMed] [Google Scholar]
- 67.Gerovac M, Mouali YE, Kuper J, Kisker C, Barquist L, Vogel J: Global discovery of bacterial RNA-binding proteins by RNase-sensitive gradient profiles reports a new FinO domain protein. RNA 2020, 26:1448–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hör J, Di Giorgio S, Gerovac M, Venturini E, Förstner KU, Vogel J: Grad-seq shines light on unrecognized RNA and protein complexes in the model bacterium Escherichia coli. Nucleic Acids Res 2020, 48:9301–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shchepachev V, Bresson S, Spanos C, Petfalski E, Fischer L, Rappsilber J, Tollervey D: Defining the RNA interactome by total RNA-associated protein purification. Molecular Systems Biology 2019, 15:e8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin M, Fuller GG, Han T, Yao Y, Alessi AF, Freeberg MA, Roach NP, Moresco JJ, Karnovsky A, Baba M, et al. : Glycolytic Enzymes Coalesce in G Bodies under Hypoxic Stress. Cell Reports 2017, 20:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuller GG, Han T, Freeberg MA, Moresco JJ, Ghanbari Niaki A, Roach NP, Yates JR III, Myong S, Kim JK: RNA promotes phase separation of glycolysis enzymes into yeast G bodies in hypoxia. eLife 2020, 9:e48480. [DOI] [PMC free article] [PubMed] [Google Scholar]


