Abstract
Purpose of review:
Hematopoiesis is co-regulated by innate immunity, which is an ancient evolutionary defense mechanism also involved in the development and regeneration of damaged tissues. This review seeks to shed more light on the workings of the Nlrp3 inflammasome, which is an intracellular innate immunity pattern recognition receptor (PRR) and sensor of changes in the hematopoietic microenvironment, and focus on its role in hematopoeisis.
Recent findings:
Hematopoietic stem progenitor cells (HSPCs) are exposed to several external mediators of innate immunity. Moreover, since hemato/lymphopoietic cells develop from a common stem cell, their behavior and fate are coregulated by intracellular innate immunity pathways. Therefore, the Nlrp3 inflammasome is functional both in immune cells and in HSPCs and affects hematopoiesis in either a positive or negative way, depending on its activity level. Specifically, while a physiological level of activation regulates the trafficking of HSPCs and most likely maintains their pool in the bone marrow, hyperactivation may lead to irreversible cell damage by pyroptosis and HSPC senescence and contribute to the origination of myelodysplasia and hematopoietic malignancies.
Summary:
Modulation of the level of Nrp3 inflammasome activation will enable improvements in HSPC mobilization, homing, and engraftment strategies. It may also control pathological activation of this protein complex during HSPC senescence, GvHD, the induction of cytokine storms, and the development of hematopoietic malignancies.
Keywords: Hematopoietic stem cells, innate immunity, Nlrp3 inflammasome, hematopoietic stem cell trafficking
Introduction
The Nlrp3 inflammasome is, so far, the best-studied member of the inflammasome family, which consists of several members [1–6]. This intracellular protein complex is formed by multiprotein oligomers, each consisting of Nlrp3 protein, apoptosis-associated speck-like protein containing a CARD (ASC, also known as PYCARD), and pro-caspase 1 [7–11]. Upon activation, these multiprotein oligomers aggregate to form the inflammasome (also known as a speck complex). Nlrp3-mediated activation of caspase 1 promotes proteolytic cleavage and maturation of two pro-inflammatory cytokines, interleukin-1β (IL-1β) and interleukin 18 (IL-18), and cleavage of gasdermin D, which forms cell membrane pores involved in secretion of mature IL-1β and IL-18 [12–14]. Moreover, these pores secrete several danger-associated molecular pattern (DAMP) molecules [15,16]. Formation of gasdermin D pores may lead to irreversible leakage of cytoplasm content and a pro-inflammatory form of programmed cell death known as pyroptosis [17,18]. Recent results indicate that physiological activation of the Nlrp3 inflammasome is involved in i) mobilization of HSPCs from bone marrow (BM) into peripheral blood (PB), ii) HSPC homing to BM after transplantation, and iii) HSPC expansion and aging [19*, 20–25, 26*]. On the other hand, hyperactivation of the Nlrp3 inflammasome is involved in certain hematological pathologies, including i) myelodysplastic syndrome, ii) myeloproliferative neoplasms, iii) leukemia, and iv) post-transplantation graft-versus-host disease (GvHD) [27–37]. Thus, the biological impact of the Nlrp3 inflammasome on hematopoiesis depends on the overall level and timing of its activation [31*].
HSPCs require the Nlrp3 inflammasome for normal migration.
One of the essential features of HSPCs is their inborn ability to migrate, both during prenatal development and later on in the postnatal period of life. Based on reports that the Nlrp3 inflammasome is required for migration of T lymphocytes [38–40] and macrophages [41–44], we hypothesized that it could also be involved in the chemotactic responsiveness of HSPCs. In fact, by employing the Transwell migration system, we found that blockade of the Nlrp3 inflammasome by the small-molecule inhibitor MCC950 impaired migration of HSPCs in response to the major BM chemoattractant stromal-derived factor 1 (SDF-1) as well as two other supportive homing factors, namely, extracellular adenosine triphosphate (eATP) and sphingosine-1-phosphate (S1P) [19]. This finding has been subsequently reproduced with BMMNCs isolated from Nlrp3-KO mice, and we obtained similar results with human HSPCs exposed to MCC950 [19].
Overall, the migration of HSPCs in response to chemoattractants is regulated by membrane lipid rafts (MLRs), which are microdomains of cell membrane enriched in glycosphingolipids and cell-surface protein receptors [45,46]. As reported, HSPCs respond much more strongly to an SDF-1 chemotactic gradient if its specific receptor (CXCR4) is incorporated into the MLRs [47,48]. This association facilitates a stronger transduction between activated CXCR4 and the downstream signaling pathways involved in cell migration [49]. Our recent report revealed that activation of the Nlrp3 inflammasome in HSPCs enhances incorporation of CXCR4 into MLRs, resulting in better responsiveness of these cells to BM-expressed chemotactic factors [19].
These in vitro results inspired us to investigate the role of the Nlrp3 inflammasome in the trafficking of HSPCs in i) a murine model of HSPC mobilization into PB and ii) homing to BM after hematopoietic transplantation [19,20,27].
Nlrp3 inflammasomes expressed in the BM microenvironment and in HSPCs are required for proper mobilization of HSPCs.
HSPCs are retained in a quiescent state in stem cell niches spread throughout the BM microenvironment. Several mechanisms have been proposed that regulate their detachment and egress from these niches in response to a gradient of HSPC-specific chemotactic factors [50–56]. A crucial role in this process is the induction of a proteolytic and lipolytic microenvironment [57–62], which results from a state of sterile inflammation in the BM microenvironment in response to cues that promote egress of HSPCs from BM into PB [51,63,64]. From a clinical point of view, the forced release of HSPCs is observed after systemic administration of the cytokine granulocyte colony-forming factor (G-CSF) or the SDF-1 receptor (CXCR4) antagonist AMD3100. This process is employed in the clinic to harvest HSPCs from PB for transplantation and is called pharmacological mobilization [65*, 66*, 67–72].
Our recent results indicate that a crucial role in the initiation of the sterile inflammation state in BM, which promotes the mobilization process, is played by the activation of purinergic signaling and, in particular, the release of eATP [26,27]. In response to pro-mobilizing agents, a source of this extracellular signaling nucleotide is innate immunity cells, including granulocytes, monocytes, and dendritic cells [73–76]. It is well known that mice deficient in these cells are poor mobilizers [55,77,78].
eATP released in response to G-CSF or AMD3100 administration as one of the DAMPs that is secreted by pannexin 1 channels. It has been demonstrated that blockade of pannexin 1 channels by specific inhibitory peptides significantly decreases the pharmacological mobilization efficiency of HSPCs in mice [79]. Interestingly, our previously published results indicate that the pannexin 1 channel must be functional for optimal mobilization, not only in HSPCs but also in cells present in the hematopoietic microenvironment [80]. Supporting this finding, mice that were exposed to a pannexin-1-blocking peptide before mobilization mobilized poorly, which indicates a role for eATP in activating in this microenvironment pathways that are involved in HSPC egress into PB [80]. Based on this observation, we became interested in a potential link between eATP and intracellular activation of Nlrp3 inflammasomes.
The Nlrp3 inflammasome is activated in HSPCs and the BM microenvironment in an eATP–P2X4- and eATP–P2X7-dependent manner.
eATP is an activating ligand for several purinergic receptors from both the ionotropic P2X and metabotropic P2Y families, which consist of 7 and 8 receptors, respectively [81–83]. Our results indicate that the most important receptors in the mobilization of HSPCs are the ionotropic P2X4 and P2X7 receptors [79,84,85]. These receptors are highly expressed on the surfaces of both human and murine HSPCs. The importance of these receptors was confirmed by the finding that their blockade by small-molecule inhibitors, PSB12054 and A438079, respectively, impaired the chemotactic responsiveness of murine and human HSPCs to SDF-1, eATP, and S1P gradients [84]. Moreover, like the blockade of pannexin 1 channels, the lack of functional P2X4 and P2X7 receptors on the surfaces of cells in the BM microenvironment also results in poor G-CSF- and AMD3100-induced mobilization in mice [84,85].
Explaining the role of eATP–P2X4 and eATP–P2X7 signaling in HSPC mobilization, both of these ligand–receptor axes are strong activators of the Nlrp3 inflammasome [86–88], which, as a component of innate immunity, functions as an intracellular pattern recognition receptor (PRR) for DAMPs [7,8,10]. eATP is the most important DAMP of the molecules in this functional category, such as high mobility group box 1 (HMGB1) and S100A9 immunoregulatory proteins. All these DAMPs are released in an Nlrp3 inflammasome-dependent manner, both from innate immunity cells and HSPCs, while eATP, by employing positive autocrine/paracrine feedback loop, maintains activation of this PRR [14,89,90]. At the same time other DAMPs, such as HMGB1 and S1009A, activate the complement cascade (ComC) [91], which has been reported to be crucial for optimal egress of HSPCs into PB [92]. Supporting this finding, mice that do not activate the ComC are normally poor mobilizers [78,93,94]. In addition to DAMPs, the Nlrp3 inflammasome may also be activated by reactive oxygen species (ROS)[95–97], and it is worth mentioning that ROS have been reported as important factors promoting the migration and mobilization of HSPCs [98–100]. This connection between the Nlrp3 inflammasome and ROS may explain why the biological effects of this PRR, like ROS, depend on the level of its expression (concentration) [12]. It is known that a balanced expression of ROS is crucial for maintaining the stem cell pool and host immunity, both under homeostatic steady-state conditions and during stress situations [98]. The same seems to be true for the Nlrp3 inflammasome.
Finally, very recently published results have demonstrated that when Nlrp3 inflammasome-associated caspase 1 is knocked out in mice, the resulting caspase-1-KO mice are poor mobilizers [101]. This finding has been explained by the role caspase 1 plays in the release of DAMPs, which promote mobilization (via eATP) and activate the ComC (via HMGB1 and S1009A). All these DAMPs together are required for normal egress of HSPCs from BM into PB.
Interestingly, the normal basic level of Nlrp3 inflammasome expression in HSPCs and innate immunity cells is regulated by intestinal Gram-negative bacteria-derived liposaccharide (LPS) [8,102–104]. By interacting with Toll-like receptor 4 (TLR4), LPS maintains expression of Nlrp3 inflammasome components in innate immunity cells and HSPCs. That LPS circulating in PB is derived from intestinal bacteria explains why mice depleted of this Gram-negative flora are poor mobilizers [105]. We propose that these results are most likely to be explained by the deficient expression of Nlrp3 inflammasome components in innate immunity cells and HSPCs.
Figure 1 illustrates all the major components of innate immunity that are part of the Nlrp3 inflammasome activation pathway and for which a deficiency results in poor mobilization.
Figure 1. The elements of innate immunity involved in Nlrp3 inflammasome activation whose attenuation decreases HSPC mobilization.
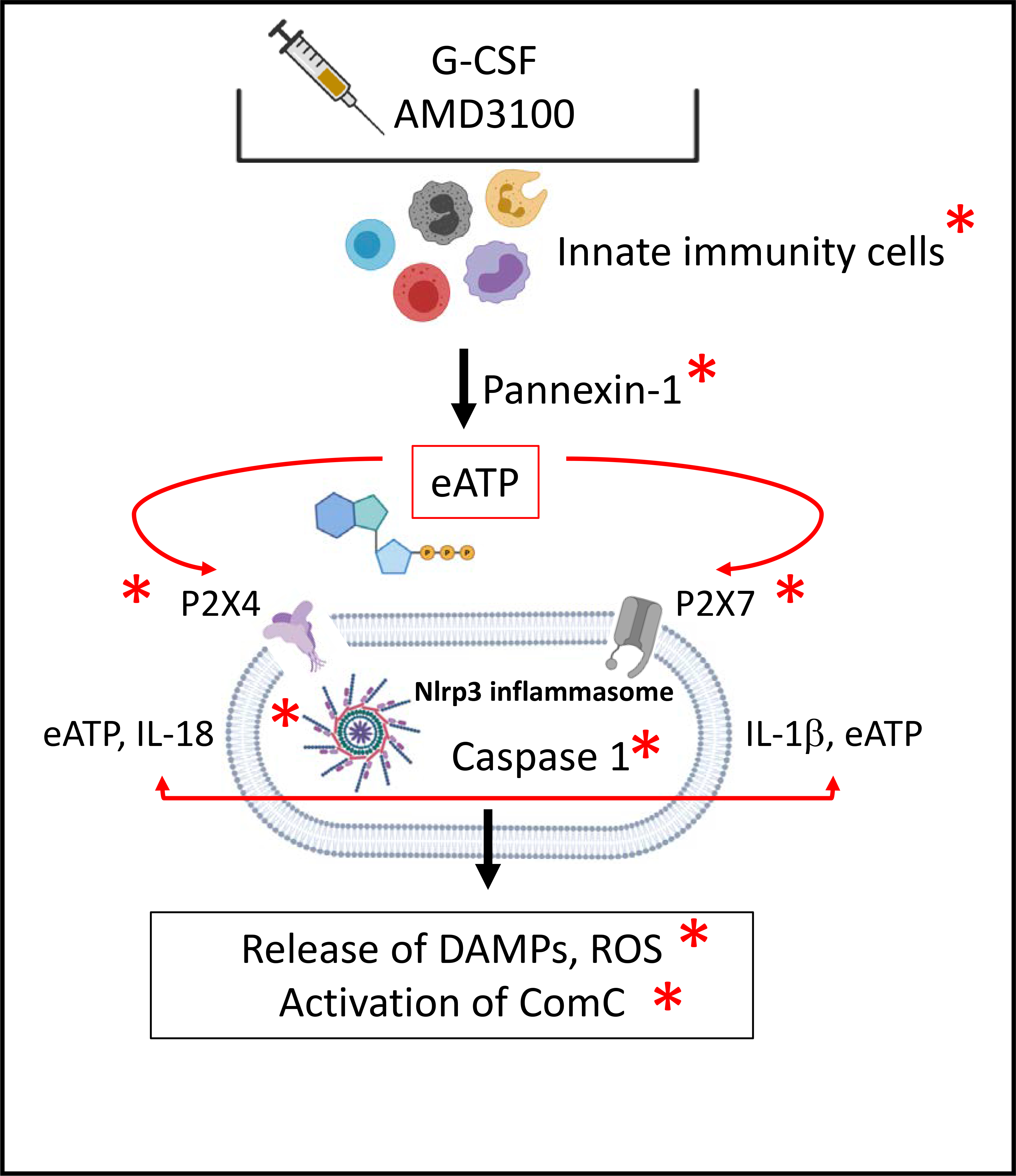
Pro-mobilizing agents (G-CSF or AMD3100) stimulate the release of eATP from innate immunity cells (granulocytes, monocytes, and dendritic cells) in a pannexin-1-channel-dependent manner. Once released from these cells, eATP activates Nlrp3 inflammasomes in HSPCs via the P2X4 and P2X7 receptors, which subsequently activates caspase 1 to release active IL-1β and IL-18. Autocrine/paracrine stimulation of HSPCs, innate immunity cells, and cells in the BM microenvironment leads to a release of several types of DAMPs and activation of the complement cascade (ComC). Red asterisks indicate elements whose attenuation results in poor mobilization in our recently published or preliminary results.
Evidence that the Nlrp3 inflammasome directs homing and engraftment of transplanted HSPCs.
As presented above, the Nlrp3 inflammasome controls the normal chemotactic responsiveness of HSPCs to major BM chemoattractants. Therefore, besides its role in mobilization, we also became interested in the role of this PRR in the homing and engraftment of HSPCs. Again, we focused on Nlrp3 inflammasome expression, both directly in HSPCs, which must migrate to BM niches, and in cells in the BM microenvironment in transplantation recipients. As expected, we found that HSPCs from Nlrp3-KO mice show a decrease in homing (i.e., seeding efficiency) and engraftment in regular myeloablated hosts compared with normal control mouse HSPCs [19]. Since myeloablative conditioning for transplantation by lethal irradiation also induces a state of sterile inflammation in BM, in another set of experiments we studied the role of BM microenvironment-expressed Nlrp3 inflammasomes in facilitating the homing of transplanted BMMNCs. We found that Nrlp3-KO transplantation recipient mice engrafted poorly with normal BMMNCs compared with normal control animal recipients [19]. This finding indicates that the response of the Nlrp3 inflammasome to conditioning for transplantation here plays an important role in preparing the BM microenvironment to be seeded by HSPCs. Similarly, we recently demonstrated that the Nlrp3 inflammasome is highly activated in the BM of mice conditioned for transplantation by lethal irradiation and is involved in the regulation of SDF-1 expression and activation of the complement cascade, which has been demonstrated to promote optimal engraftment of HSPCs [78,92–94].
Moreover, in addition to Nlrp3 inflammasome deficiency, impaired homing and engraftment was also observed in animals deficient for other elements of purinergic signaling pathways related to Nlrp3 inflammasome activation (Figure 2). This again was observed both for transplanted cells and for animals that served as transplantation recipients. Supporting this finding, in addition to the abovementioned blockade of the pannexin 1 channel, poor homing and engraftment was observed following transplantations performed with P2X4 and P2X7 receptor-deficient BMMNCs [85,106]. We also observed poor homing and engraftment when transplanted recipient mice were deficient for P2X4 and P2X7 receptors [106] as well as caspase 1 [101**]. These findings support the role of Nlrp3 inflammasomes expressed in cells in the BM microenvironment in facilitating the seeding efficiency of transplanted HSPCs.
Figure 2.
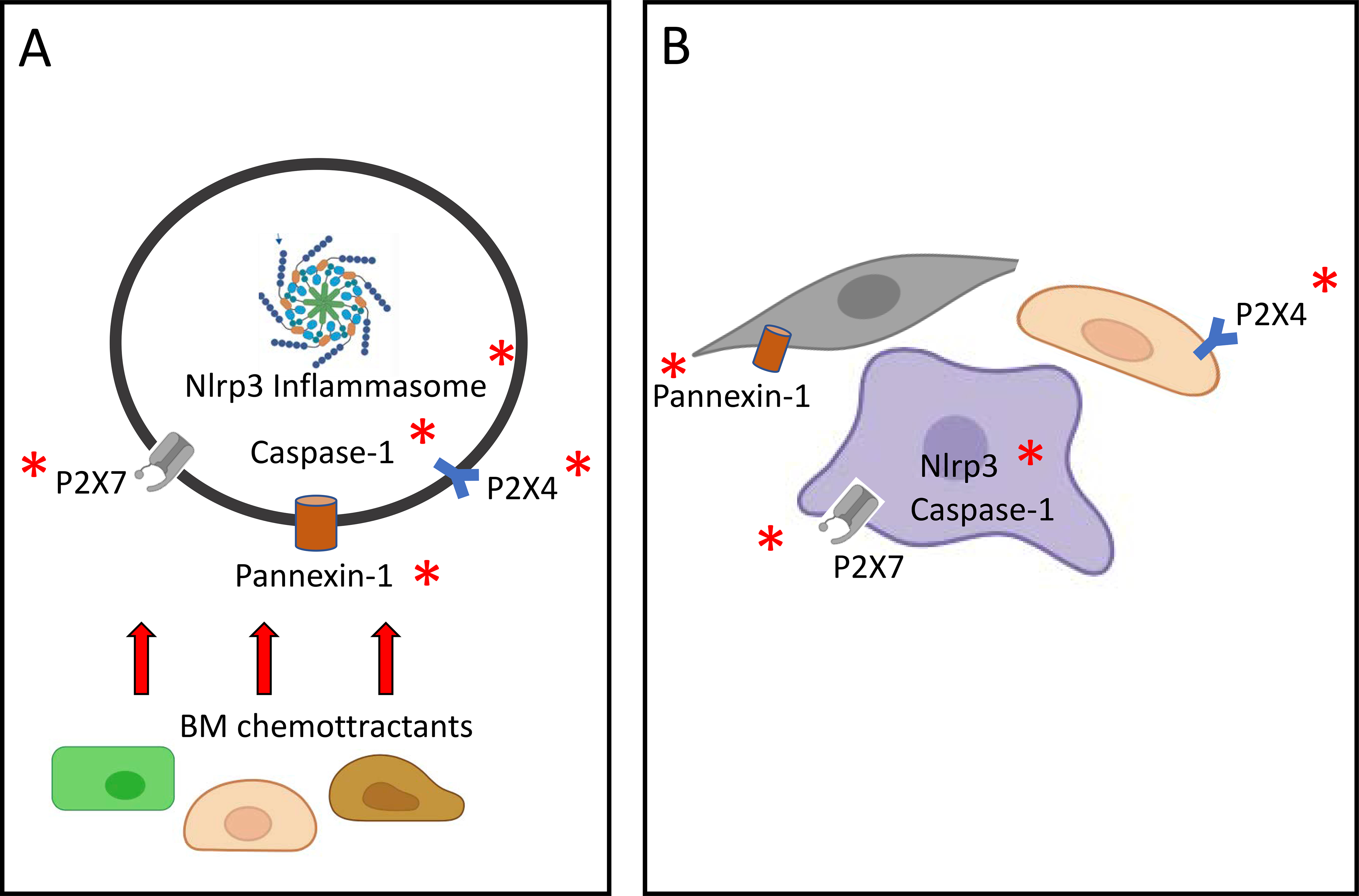
A, The elements of innate immunity that are involved in Nlrp3 inflammasome activation and whose attenuation decreases HSPC migration after transplantation into recipient BM. Migration of transplanted HSPCs up a chemotactic gradient of BM chemoattractants is decreased by impaired Nlrp3 function as well as pannexin 1, P2X4, P2X7, and caspase 1 deficiency. B, The same effect is shown for Nlrp3 inflammasome activation components in the BM microenvironment of transplantation recipients. To home and engraft transplanted HSPCs, BM microenvironment cells require normal expression of the Nlrp3 inflammasome, pannexin 1, P2X4, P2X7, and caspase 1. Red asterisks indicate elements whose attenuation results in poor homing and engraftment in our recently published or preliminary results.
eATP and its extracellular metabolite adenosine (eAdo) regulate, in opposite ways, activation of Nlrp3 inflammasomes and cell migration.
In addition to eATP, another central mediator of purinergic signaling is its metabolite, extracellular adenosine (eAdo). As depicted in Figure 3, eATP released from stressed or activated cells is converted into eAdo in the extracellular space by two cell-surface ectonucleotidases, CD39 and CD73 [81,83]. This eATP-derived metabolite interacts with the P1 family of purinergic receptors, which consists of four G protein-coupled members. Of these four receptors, HSPCs positively express A2A and A2B, which after binding eAdo negatively affect migration of HSPCs [84,107,108].
Figure 3. Cell migration-promoting mechanisms at the leading surface/edge and negative feedback mechanisms at the retracting surface/edge of migrating HSPCs.
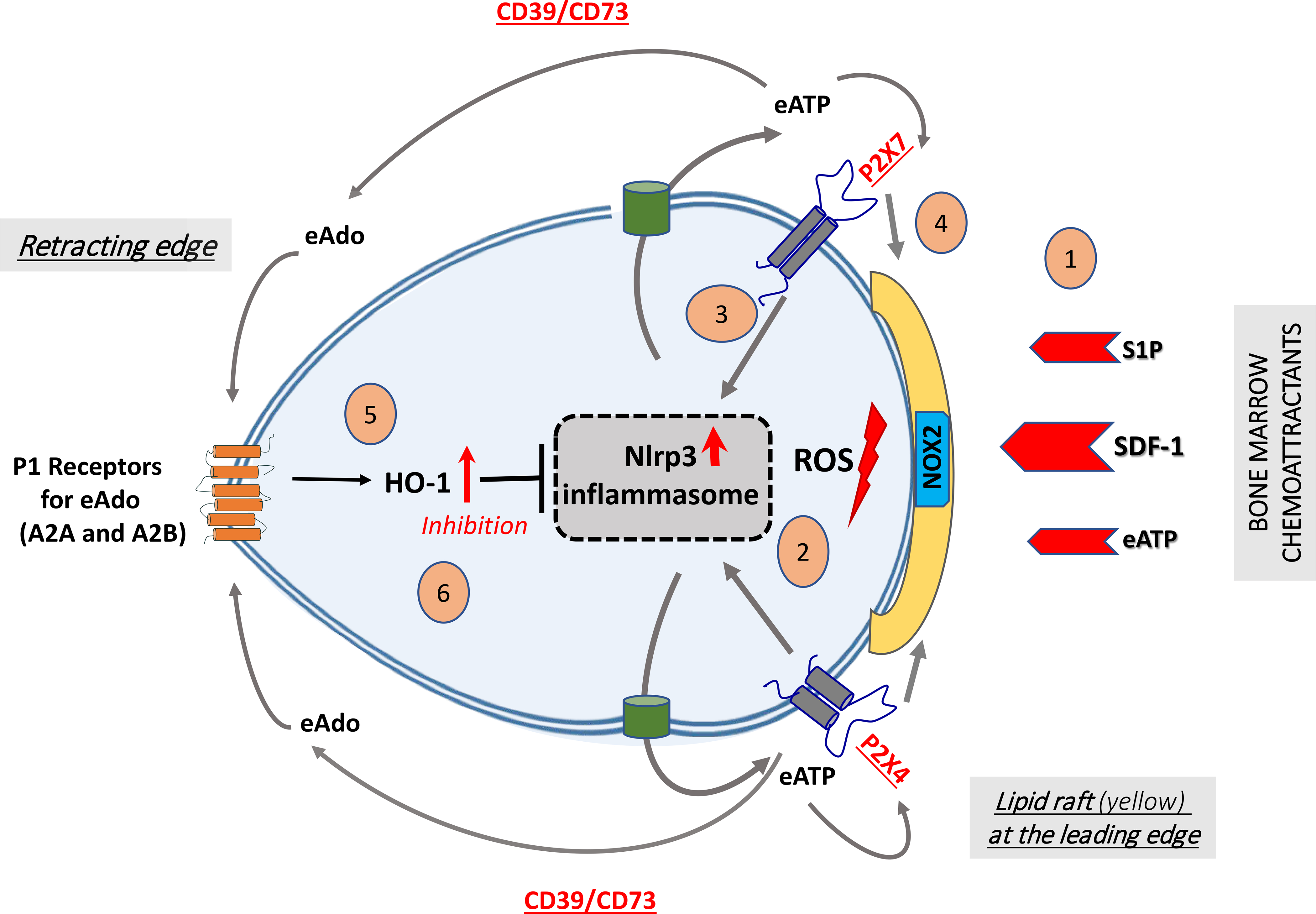
We propose that, in response to BM chemoattractants, HSPCs activate Nox2 (shown as a green box in a membrane lipid raft [MLR], depicted in yellow), which is an MLR-associated enzyme and a source of ROS [1]. ROS activates the Nlrp3 inflammasome [2], which releases ATP into the extracellular space surrounding HSPCs [3]. In a positive-feedback mechanism, extracellular ATP (eATP) activates the Nlrp3 inflammasome and MLR formation so that cells respond more robustly to BM chemoattractants [4]. In a negative-feedback mechanism, eATP is converted by the cell surface-expressed ectonucleotidases CD39 and CD73 into extracellular adenosine (eAdo), which, via the P1 receptors (A2a, A2b), activates heme oxygenase 1 (HO-1) [5], a negative regulator of the Nlrp3 inflammasome [6]. (Modified from ref. 128).
Figure 3 also shows HSPCs migrating to BM after transplantation in response to SDF-1, S1P, and eATP gradients released from BM stroma conditioned for transplantation by myeloablative radio- or chemotherapy. HSPCs migrating in response to all these chemoattractants activate the Nlrp3 inflammasome, which initiates release from cells of several DAMPs, including eATP, HMGB1, and S1009A. While eATP, by employing P2X4 and P2X7 receptor autocrine loops, activates Nlrp3 inflammasomes to maintain the activity required for cell migration [85,106*,109], two other DAMPs activate the complement cascade, whose cleavage fragments, including anaphylatoxin C3a and C5a and non-lytic C5b-C9 (membrane attack complex), additionally stimulate Nlrp3 inflammasomes, both in migrating HSPCs and in the BM microenvironment [91,93].
As depicted in Figure 3, autocrine-secreted eATP promotes and maintains formation of MLRs. As reported these cell membrane microdomains incorporate CXCR4 and other homing receptors to connect them better with downstream signaling pathways. This facilitates optimal navigation of HSPCs to BM niches [47, 65*]. At the same time, eATP is processed by CD39 and CD73 to eAdo, which, by interacting with P1 receptors, activates intracellular heme oxygenase 1 (HO-1), which in turn has an inhibitory effect on Nlrp3 inflammasomes and MLR formation [19,110–112]. These are all coordinated processes that require both local cell migration-stimulating and cell migration-inhibiting mechanisms. Specifically, chemotactic factors, including CXCR4, S1P1R, P2X4, and P2X7, are expressed at the leading surface of migrating cells, while the migration-inhibitory P1 receptors, A2A and/or A2B, are expressed at the receding surface [75,113]. Thus, migration of HSPCs is balanced by eATP-, SDF-1-, and S1P-promoted chemotaxis and controlled negatively by eAdo.
The potential role of Nlrp3 inflammasomes in maintaining the pool of HSPCs and expanding these cells after transplantation into the recipient BM.
One of the challenging questions still to be answered is the potential effect of the Nlrp3 inflammasome on HSPC proliferation. Our recent results indicate that Nlrp3-KO mice have ~20% fewer Sca-1+Kit+Lin– (SKL) HSPCs in BM than do WT animals [19]. This raises the legitimate question of whether the Nlrp3 inflammasome affects the proliferation and expansion of these cells. In support of this possibility, it has been reported that in the developing murine embryo, the Nlrp3 inflammasome is involved in expansion of CD41+ HSPCs [22]. Moreover, loss of Nlrp3 inflammasome components prevented the proliferation of embryonic HSPCs, and positive multilineage expansion results were obtained with human induced pluripotent stem cell-derived hemogenic cells in the presence of Nlrp3 inflammasome activators [114].
Another question is whether the proliferation of HSPCs is also affected by the eATP metabolite eAdo, which has an effect opposite to eATP on HSPC migration, as discussed above. Interestingly, in a zebrafish embryo model, somewhat surprisingly, eAdo has been proposed as a positive regulator of hematopoiesis [115]. However, in our hands, eAdo did not affect the proliferation of either murine or human HSPCs. This apparent discrepancy between zebrafish and human hematopoiesis can be explained by species differences. Nevertheless, the impact of the Nlrp3 inflammasome on proliferation of HSPCs needs further study. This question could be addressed in a model of stress-induced hematopoiesis, for example, by evaluating hematopoietic recovery in sublethally irradiated Nlrp3-KO mice.
Hyperactivation of the Nlrp3 inflammasome as the culprit behind pyroptosis, senescence of HSPCs, induction of cytokine storms, GvHD, and the origin of hematopoietic malignancies.
While, as demonstrated above, the physiological level of Nlrp3 inflammasome activation regulates trafficking of HSPCs [19,20] and most likely maintains the pool of these cells in BM [116], hyperactivation in HSPCs may lead to several adverse effects, including irreversible cell damage through the mechanisms of pyroptosis and senescence and even contribute to the origin of hematopoietic malignancies [29–31,33,35,36,117–119]. On the other hand, uncontrolled hyperactivation of Nlrp3 inflammasomes in innate immunity cells may induce a cytokine storm [34] and initiate GvHD after hematopoietic transplantation (Figure 4). Moreover, while excessive hyperactivation of Nlrp3 inflammasomes in HSPCs may lead to cell death by pyroptosis, in a recent elegant paper it has been shown that Nlrp3 inflammasomes activated due to mitochondrial stress in aged HSPCs contribute to their aging [120**]. This process could be mediated by mitochondria-derived ROS, which, as mentioned above, are potent activators of Nlrp3 inflammasomes [96]. Another problem to consider is the involvement of Nlrp3 inflammasomes in myelodysplastic syndromes as part of aberrant activation of innate immunity and a “smoldering” pro-inflammatory state in the hematopoietic microenvironment [29,121]. This process potentiates BM inflammation and leads to hematopoietic cell damage, chromosomal abnormalities, expansion of BM myeloid-derived suppressor cells, and initiates myeloproliferative disorders and even leukemia. These effects are a consequence of inflammaging, which is driven, at least partially, by prolonged excessive activation of the Nlrp3 inflammasome [23–25,32].
Figure 4. Positive and negative effects of the Nlrp3 inflammasome on hematopoiesis.
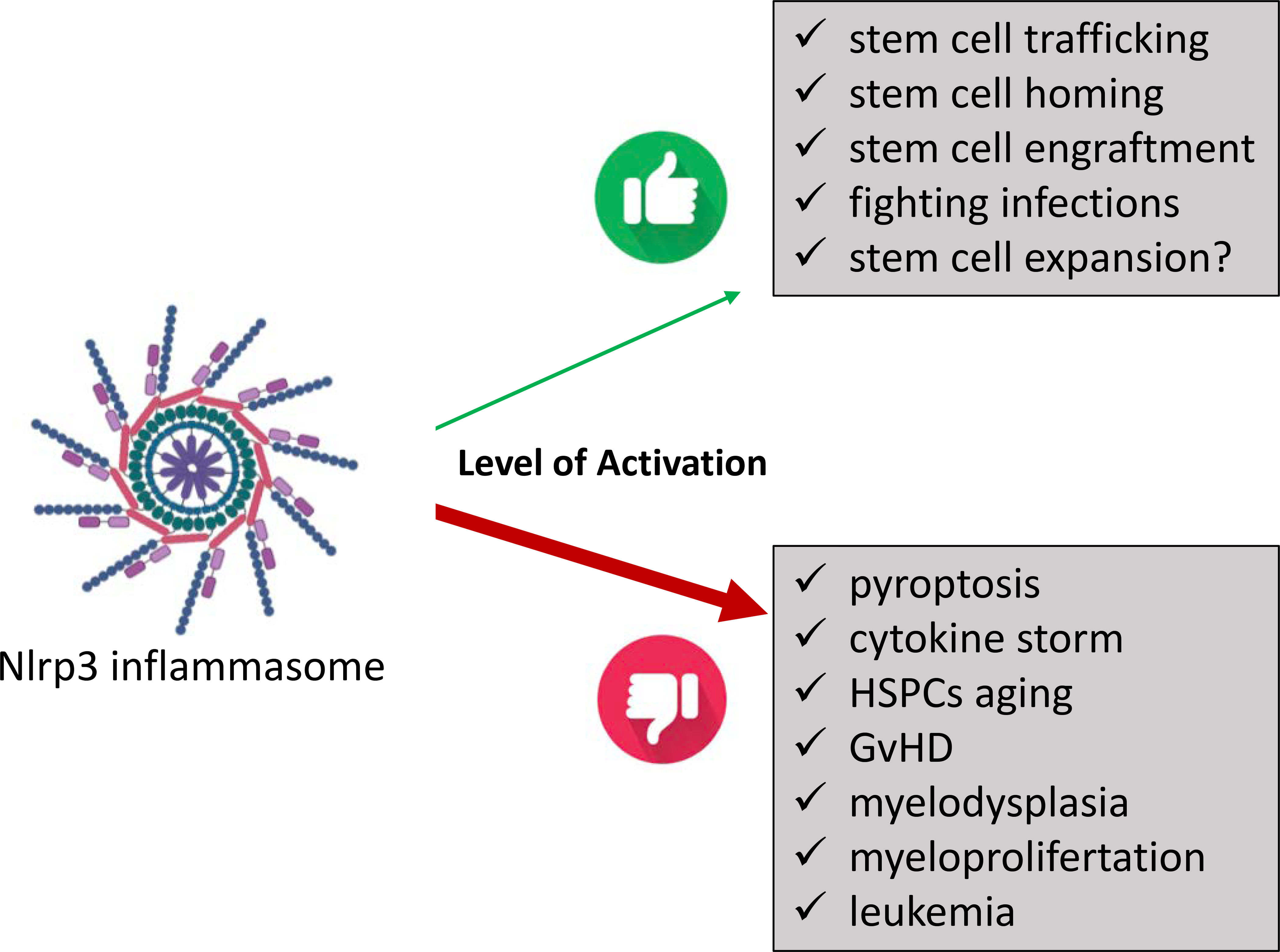
A physiologically low level of activation regulates stem cell trafficking, stem cell homing, stem cell engraftment, fighting infections, and, very likely, stem cell expansion. Hyperactivation is involved in hematopoietic cell pyroptosis, induction of cytokine storms, HSPC aging, induction of post-transplantation GvHD, myelodysplasia, and myeloproliferative disorders and may contribute to leukemia.
Another complication of Nlrp3 hyperactivation in innate immunity cells is initiation of cytokine storms, as seen, for example, in COVID19-infected patients [122*–124*]. or patients undergoing CAR-T cell therapy [125,126]. Finally, the Nlrp3 inflammasome may be one of the contributing factors in initiating GvHD after hematopoietic transplantation [34,127]. Evidence indicates that these effects are regulated by modulating Nlrp3 inflammasome activity by positive and negative eATP- and eAdo- mediated signaling, respectively [128*].
Conclusions
The Nlrp3 inflammasome has recently become a “rising star” in studies on normal and pathological hematopoiesis. This popularity is reflected by the increasing number of publications related to the role of this exciting protein complex in maintaining BM homeostasis or its involvement in the pathogenesis of several disorders. It is expected that modulation of the level of Nrp3 inflammasome activation will allow for the development of better stem cell mobilization, homing, and engraftment strategies. On the other hand, controlling pathological activation of this protein complex may ameliorate HSPC senescence, GvHD, cytokine storms, and the development of hematopoietic malignancies. However, more investigation is still needed to elucidate the mechanistic aspects of Nlrp3 inflammasome action. Finally, in addition to the existing small-molecule inhibitor of the Nlrp3 inflammasome, MCC950, other potent new drugs are under investigation, and the first clinical trials to control activation of the Nlrp3 inflammasome in patients have been initiated. This progress is expected to lead to the application of these compounds in benign and malignant hematology.
Key points.
Hematopoiesis is co-regulated by several innate immunity mediators and intracellular innate immunity pathways.
Nlrp3 inflammasome is an innate immunity intracellular pattern recognition receptor that in addition to innate immunity cells is also functional in HSC and affects hematopoiesis in either a positive or negative way, depending on its activity level.
Novel evidence demonstrates that physiological level of Nlrp3 inflammasome activation regulates the trafficking of HSC and most likely maintains their pool in the bone marrow.
In contrast hyperactivation of Nlrp3 inflammasome may lead to irreversible cell damage by pyroptosis, HSC senescence, myelodysplasia and hematopoietic malignancies.
Acknowledgments:
This work was supported by the NIH grants 2R01 DK074720, the Stella and Henry Hoenig Endowment, and the OPUS grant UMO-2018/29/B/NZ4/01470 to MZR.
Footnotes
Conflict-of-interest statement
The authors have no financial interests to disclose.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Chen GY, Nunez G: Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Nardo D, Latz E: NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol 2011; 32:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G: The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franchi L, Munoz-Planillo R, Nunez G: Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012; 13:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder K, Tschopp J: The inflammasomes. Cell 2010; 140:821–832. [DOI] [PubMed] [Google Scholar]
- 6.Schroder K, Zhou R, Tschopp J: The NLRP3 inflammasome: a sensor for metabolic danger? Science 2010; 327:296–300. [DOI] [PubMed] [Google Scholar]
- 7.Groslambert M, Py BF: Spotlight on the NLRP3 inflammasome pathway. J Inflamm Res 2018; 11:359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y, Hara H, Nunez G: Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci 2016; 41:1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo EK, Kim JK, Shin DM, Sasakawa C: Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 2016; 13:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Place DE, Kanneganti TD: Recent advances in inflammasome biology. Curr Opin Immunol 2018; 50:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stutz A, Horvath GL, Monks BG, Latz E: ASC speck formation as a readout for inflammasome activation. Methods Mol Biol 2013; 1040:91–101. [DOI] [PubMed] [Google Scholar]
- 12.Tschopp J, Schroder K: NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 2010; 10:210–215. [DOI] [PubMed] [Google Scholar]
- 13.Yabal M, Calleja DJ, Simpson DS, Lawlor KE: Stressing out the mitochondria: Mechanistic insights into NLRP3 inflammasome activation. J Leukoc Biol 2019; 105:377–399. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Wang H, Kouadir M, Song H, Shi F: Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis 2019; 10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latz E, Xiao TS, Stutz A: Activation and regulation of the inflammasomes. Nat Rev Immunol 2013; 13:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Zeng X, Li X, Cui C, Hou R, Guo Z, Mehta JL, Wang X: Advances in the molecular mechanisms of NLRP3 inflammasome activators and inactivators. Biochem Pharmacol 2020; 175:113863. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Gao W, Shao F: Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 2017; 42:245–254. [DOI] [PubMed] [Google Scholar]
- 18.Zhou R, Yazdi AS, Menu P, Tschopp J: A role for mitochondria in NLRP3 inflammasome activation. Nature 2011; 469:221–225. [DOI] [PubMed] [Google Scholar]
- 19 *.Adamiak M, Abdel-Latif A, Bujko K, Thapa A, Anusz K, Tracz M, Brzezniakiewicz-Janus K, Ratajczak J, Kucia M, Ratajczak MZ: Nlrp3 Inflammasome Signaling Regulates the Homing and Engraftment of Hematopoietic Stem Cells (HSPCs) by Enhancing Incorporation of CXCR4 Receptor into Membrane Lipid Rafts. Stem Cell Rev Rep 2020; 16:954–967.This paper provides an evidence that Nlrp3 inflammasome enhances incorporation of homing receptors expressed by HSPCs into membrane lipid rafts.
- 20.Adamiak M, Ciechanowicz A, Skoda M, Cymer M, Tracz M, Xu B, Ratajczak MZ: Novel Evidence that Purinergic Signaling - Nlrp3 Inflammasome Axis Regulates Circadian Rhythm of Hematopoietic Stem/Progenitor Cells Circulation in Peripheral Blood. Stem Cell Rev Rep 2020; 16:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbore G, Kemper C: A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur J Immunol 2016; 46:1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frame J, Long T, Schuster-Kubaczka C, Esain V, Lim S, Daley G, North T: Inflammasome-Mediated Regulation of Hematopoiesis in the Vertebrate Embryo. Blood 2018; 132:330. [Google Scholar]
- 23.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A: Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018; 14:576–590. [DOI] [PubMed] [Google Scholar]
- 24.Gritsenko A, Green JP, Brough D, Lopez-Castejon G: Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotamisligil GS: Inflammation, metaflammation and immunometabolic disorders. Nature 2017; 542:177–185. [DOI] [PubMed] [Google Scholar]
- 26 *.Ratajczak MZ, Adamiak M, Thapa A, Bujko K, Brzezniakiewicz-Janus K, Lenkiewicz AM: NLRP3 inflammasome couples purinergic signaling with activation of the complement cascade for the optimal release of cells from bone marrow. Leukemia 2019; 33:815–825.This paper describes an invovlement of Nlrp3 inflammasome in connecting purinergic signaling with activation of complement cascade in release of HSPCs from BM into PB.
- 27.Lenkiewicz AM, Adamiak M, Thapa A, Bujko K, Pedziwiatr D, Abdel-Latif AK, Kucia M, Ratajczak J, Ratajczak MZ: The Nlrp3 Inflammasome Orchestrates Mobilization of Bone Marrow-Residing Stem Cells into Peripheral Blood. Stem Cell Rev Rep 2019; 15:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apostolova P, Zeiser R: The Role of Purine Metabolites as DAMPs in Acute Graft-versus-Host Disease. Front Immunol 2016; 7:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basiorka AA, McGraw KL, Eksioglu EA, et al. : The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 2016; 128:2960–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM: Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest 2013; 123:4695–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. *.Christgen S, Kanneganti TD: Inflammasomes and the fine line between defense and disease. Curr Opin Immunol 2020; 62:39–44.It is an excellent review on a role of inflammasome family in regulating immune response against infections.
- 32.Feldman N, Rotter-Maskowitz A, Okun E: DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev 2015; 24:29–39. [DOI] [PubMed] [Google Scholar]
- 33.Hamarsheh S, Zeiser R: NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front Immunol 2020; 11:1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jankovic D, Ganesan J, Bscheider M, et al. : The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med 2013; 210:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lussana F, Rambaldi A: Inflammation and myeloproliferative neoplasms. J Autoimmun 2017; 85:58–63. [DOI] [PubMed] [Google Scholar]
- 36.Moossavi M, Parsamanesh N, Bahrami A, Atkin SL, Sahebkar A: Role of the NLRP3 inflammasome in cancer. Mol Cancer 2018; 17:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeiser R, Penack O, Holler E, Idzko M: Danger signals activating innate immunity in graft-versus-host disease. J Mol Med (Berl) 2011; 89:833–845. [DOI] [PubMed] [Google Scholar]
- 38.Martin BN, Wang C, Zhang CJ, et al. : T cell-intrinsic ASC critically promotes T(H)17-mediated experimental autoimmune encephalomyelitis. Nat Immunol 2016; 17:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue M, Williams KL, Gunn MD, Shinohara ML: NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 2012; 109:10480–10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goossens GH, Blaak EE, Theunissen R, Duijvestijn AM, Clement K, Tervaert JW, Thewissen MM: Expression of NLRP3 inflammasome and T cell population markers in adipose tissue are associated with insulin resistance and impaired glucose metabolism in humans. Mol Immunol 2012; 50:142–149. [DOI] [PubMed] [Google Scholar]
- 41.Elliott EI, Sutterwala FS: Monocytes Take Their Own Path to IL-1beta. Immunity 2016; 44:713–715. [DOI] [PubMed] [Google Scholar]
- 42.Erlich Z, Shlomovitz I, Edry-Botzer L, et al. : Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat Immunol 2019; 20:397–406. [DOI] [PubMed] [Google Scholar]
- 43.Netea MG, Nold-Petry CA, Nold MF, et al. : Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 2009; 113:2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finucane OM, Sugrue J, Rubio-Araiz A, Guillot-Sestier MV, Lynch MA: The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1beta-dependent manner in macrophages. Sci Rep 2019; 9:4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sowa G, Pypaert M, Sessa WC: Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci U S A 2001; 98:14072–14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan JL: Cell biology. Integrins, rafts, Rac, and Rho. Science 2004; 303:773–774. [DOI] [PubMed] [Google Scholar]
- 47.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak MZ: Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood 2005; 105:40–48. [DOI] [PubMed] [Google Scholar]
- 48.Wu W, Kim CH, Liu R, Kucia M, Marlicz W, Greco N, Ratajczak J, Laughlin MJ, Ratajczak MZ: The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia 2012; 26:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ratajczak MZ, Adamiak M: Membrane lipid rafts, master regulators of hematopoietic stem cell retention in bone marrow and their trafficking. Leukemia 2015; 29:1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christopherson KW 2nd, Hangoc G, Mantel CR, Broxmeyer HE: Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004; 305:1000–1003. [DOI] [PubMed] [Google Scholar]
- 51.Golan K, Vagima Y, Ludin A, et al. : S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood 2012; 119:2478–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoggatt J, Singh P, Tate TA, et al. : Rapid Mobilization Reveals a Highly Engraftable Hematopoietic Stem Cell. Cell 2018; 172:191–204 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chavez JS, Pietras EM: Hematopoietic Stem Cells Rock Around The Clock: Circadian Fate Control via TNF/ROS Signals. Cell Stem Cell 2018; 23:459–460. [DOI] [PubMed] [Google Scholar]
- 54.Karpova D, Rettig MP, Ritchey J, et al. : Targeting VLA4 integrin and CXCR2 mobilizes serially repopulating hematopoietic stem cells. J Clin Invest 2019; 129:2745–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Supakorndej T, Krambs JR, Rao M, Abou-Ezzi G, Ye RY, Li S, Trinkaus K, Link DC: Bone marrow dendritic cells regulate hematopoietic stem/progenitor cell trafficking. J Clin Invest 2019; 129:2920–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinho S, Frenette PS: Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 2019; 20:303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J, Ratajczak MZ: Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia 2012; 26:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC: Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood 2004; 104:65–72. [DOI] [PubMed] [Google Scholar]
- 59.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J: Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia 2010; 24:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juarez JG, Harun N, Thien M, et al. : Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood 2012; 119:707–716. [DOI] [PubMed] [Google Scholar]
- 61.Adamiak M, Poniewierska-Baran A, Borkowska S, Schneider G, Abdelbaset-Ismail A, Suszynska M, Abdel-Latif A, Kucia M, Ratajczak J, Ratajczak MZ: Evidence that a lipolytic enzyme--hematopoietic-specific phospholipase C-beta2--promotes mobilization of hematopoietic stem cells by decreasing their lipid raft-mediated bone marrow retention and increasing the promobilizing effects of granulocytes. Leukemia 2016; 30:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seitz G, Boehmler AM, Kanz L, Mohle R: The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann N Y Acad Sci 2005; 1044:84–89. [DOI] [PubMed] [Google Scholar]
- 63.Winkler IG, Pettit AR, Raggatt LJ, et al. : Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia 2012; 26:1594–1601. [DOI] [PubMed] [Google Scholar]
- 64.Mendelson A, Frenette PS: Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med 2014; 20:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65 *.Huang X, Guo B, Capitano M, Broxmeyer HE: Past, present, and future efforts to enhance the efficacy of cord blood hematopoietic cell transplantation. F1000Res 2019; 8.It is an up-to-date excellent review on mechanisms involved in mobilization of HSPCs from BM into PB and a role of membrane lipid rafts in this phenomenon.
- 66 *.Karpova D, Rettig MP, DiPersio JF: Mobilized peripheral blood: an updated perspective . F1000Res 2019; 8.It is an up-to-date excellent review on mechanisms involved in mobilization of HSPCs from BM into PB.
- 67.Pelus LM, Broxmeyer HE: Peripheral blood stem cell mobilization; a look ahead. Curr Stem Cell Rep 2018; 4:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broxmeyer HE, Orschell CM, Clapp DW, et al. : Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 2005; 201:1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dar A, Schajnovitz A, Lapid K, et al. : Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia 2011; 25:1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonig H, Papayannopoulou T: Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia 2013; 27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenbaum AM, Link DC: Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia 2011; 25:211–217. [DOI] [PubMed] [Google Scholar]
- 72.Schuettpelz LG, Borgerding JN, Christopher MJ, Gopalan PK, Romine MP, Herman AC, Woloszynek JR, Greenbaum AM, Link DC: G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling. Leukemia 2014; 28:1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM: ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 2007; 282:2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM: The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood 2012; 120:2365–2375. [DOI] [PubMed] [Google Scholar]
- 75.Junger WG: Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 2011; 11:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y, Yao Y, Sumi Y, et al. : Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal 2010; 3:ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wysoczynski M, Adamiak M, Suszynska M, Abdel-Latif A, Ratajczak J, Ratajczak MZ: Poor Mobilization in T-Cell-Deficient Nude Mice Is Explained by Defective Activation of Granulocytes and Monocytes. Cell Transplant 2017; 26:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, Ratajczak J, Ratajczak MZ: Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia 2010; 24:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adamiak M, Abdel-Latif A, Ratajczak MZ: Purinergic signaling regulates mobilization of hematopoietic stem cells. Oncotarget 2018; 9:36052–36054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cymer M, Brzezniakiewicz-Janus K, Bujko K, Thapa A, Ratajczak J, Anusz K, Tracz M, Jackowska-Tracz A, Ratajczak MZ, Adamiak M: Pannexin-1 channel “fuels” by releasing ATP from bone marrow cells a state of sterile inflammation required for optimal mobilization and homing of hematopoietic stem cells. Purinergic Signal 2020; 16:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burnstock G: Blood cells: an historical account of the roles of purinergic signalling. Purinergic Signal 2015; 11:411–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Filippin KJ, de Souza KFS, de Araujo Junior RT, Torquato HFV, Dias DA, Parisotto EB, Ferreira AT, Paredes-Gamero EJ: Involvement of P2 receptors in hematopoiesis and hematopoietic disorders, and as pharmacological targets. Purinergic Signal 2020; 16:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burnstock G: The therapeutic potential of purinergic signalling. Biochem Pharmacol 2018; 151:157–165. [DOI] [PubMed] [Google Scholar]
- 84.Adamiak M, Bujko K, Brzezniakiewicz-Janus K, Kucia M, Ratajczak J, Ratajczak MZ: The Inhibition of CD39 and CD73 Cell Surface Ectonucleotidases by Small Molecular Inhibitors Enhances the Mobilization of Bone Marrow Residing Stem Cells by Decreasing the Extracellular Level of Adenosine. Stem Cell Rev Rep 2019; 15:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bujko K, Adamiak M, Thapa A, Kucia M, Ratajczak J, Ratajczak MZ: Novel evidence that extracellular adenosine triphosphate (ATP), as a purinergic signaling mediator, activates mobilization by engaging a P2X4 ligand-gated channel receptor expressed on the surface of hematopoietic and innate immunity cells. Blood 2019; 134:4472. [Google Scholar]
- 86.Amores-Iniesta J, Barbera-Cremades M, Martinez CM, Pons JA, Revilla-Nuin B, Martinez-Alarcon L, Di Virgilio F, Parrilla P, Baroja-Mazo A, Pelegrin P: Extracellular ATP Activates the NLRP3 Inflammasome and Is an Early Danger Signal of Skin Allograft Rejection. Cell Rep 2017; 21:3414–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zha QB, Wei HX, Li CG, Liang YD, Xu LH, Bai WJ, Pan H, He XH, Ouyang DY: ATP-Induced Inflammasome Activation and Pyroptosis Is Regulated by AMP-Activated Protein Kinase in Macrophages. Front Immunol 2016; 7:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bauernfeind FG, Horvath G, Stutz A, et al. : Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009; 183:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kelley N, Jeltema D, Duan Y, He Y: The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci 2019; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Savage CD, Lopez-Castejon G, Denes A, Brough D: NLRP3-Inflammasome Activating DAMPs Stimulate an Inflammatory Response in Glia in the Absence of Priming Which Contributes to Brain Inflammation after Injury. Front Immunol 2012; 3:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ratajczak MZ, Adamiak M, Bujko K, Thapa A, Pensato V, Kucia M, Ratajczak J, Ulrich H: Innate immunity orchestrates the mobilization and homing of hematopoietic stem/progenitor cells by engaging purinergic signaling-an update. Purinergic Signal 2020; 16:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adamiak M, Abdelbaset-Ismail A, Suszynska M, Abdel-Latif A, Ratajczak J, Ratajczak MZ: Novel evidence that the mannan-binding lectin pathway of complement activation plays a pivotal role in triggering mobilization of hematopoietic stem/progenitor cells by activation of both the complement and coagulation cascades. Leukemia 2017; 31:262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adamiak M, Lenkiewicz AM, Cymer M, Kucia M, Ratajczak J, Ratajczak MZ: Novel evidence that an alternative complement cascade pathway is involved in optimal mobilization of hematopoietic stem/progenitor cells in Nlrp3 inflammasome-dependent manner. Leukemia 2019; 33:2967–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ: Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia 2009; 23:2052–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abais JM, Xia M, Zhang Y, Boini KM, Li PL: Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 2015; 22:1111–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martinon F: Signaling by ROS drives inflammasome activation. Eur J Immunol 2010; 40:616–619. [DOI] [PubMed] [Google Scholar]
- 97.Harijith A, Ebenezer DL, Natarajan V: Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol 2014; 5:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ludin A, Gur-Cohen S, Golan K, Kaufmann KB, Itkin T, Medaglia C, Lu XJ, Ledergor G, Kollet O, Lapidot T: Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal 2014; 21:1605–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tesio M, Golan K, Corso S, et al. : Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 2011; 117:419–428. [DOI] [PubMed] [Google Scholar]
- 100.Zhu H, Kwak HJ, Liu P, et al. : Reactive Oxygen Species-Producing Myeloid Cells Act as a Bone Marrow Niche for Sterile Inflammation-Induced Reactive Granulopoiesis. J Immunol 2017; 198:2854–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101 **.Thapa A, Adamiak M, Bujko K, Ratajczak J, Abdel-Latif AK, Kucia M, Ratajczak MZ. Danger associated molecular pattern molecules take unexpectedly a central stage in Nlrp3 inflammasome-caspase-1 mediated trafficking of hematopoietic stem/progenitor cells. Leukemia 2021. (accepted in press).This paper for a first time described a role of danger associated molecular pattern molecules (DAMPs) released from hematopoietic cells in promnoting activation of complement cascade and mobilization of HSPCs.
- 102.Zhao Z, Wang Y, Zhou R, et al. : A novel role of NLRP3-generated IL-1beta in the acute-chronic transition of peripheral lipopolysaccharide-elicited neuroinflammation: implications for sepsis-associated neurodegeneration. J Neuroinflammation 2020; 17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang J, Wise L, Fukuchi KI: TLR4 Cross-Talk With NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front Immunol 2020; 11:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grishman EK, White PC, Savani RC: Toll-like receptors, the NLRP3 inflammasome, and interleukin-1beta in the development and progression of type 1 diabetes. Pediatr Res 2012; 71:626–632. [DOI] [PubMed] [Google Scholar]
- 105.Velders GA, van Os R, Hagoort H, Verzaal P, Guiot HF, Lindley IJ, Willemze R, Opdenakker G, Fibbe WE: Reduced stem cell mobilization in mice receiving antibiotic modulation of the intestinal flora: involvement of endotoxins as cofactors in mobilization. Blood 2004; 103:340–346. [DOI] [PubMed] [Google Scholar]
- 106.Adamiak M, Thapa A, Bujko K, Pensato V, Kucia M, Ratajczak J, Ratajczak MZ: A Novel Underappreciated Role for the Extracellular Adenosine Triphosphate (ATP)–P2X4 Purinergic Receptor Axis in the Homing and Engraftment of HSPCs. Blood 2020:2332. [Google Scholar]
- 107.Lemoli RM, Ferrari D, Fogli M, et al. : Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood 2004; 104:1662–1670. [DOI] [PubMed] [Google Scholar]
- 108.Feng W, Wang L, Zheng G: Expression and function of P2 receptors in hematopoietic stem and progenitor cells. Stem Cell Investig 2015; 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ratajczak MZ, Bujko K, Cymer M, Thapa A, Adamiak M, Ratajczak J, Abdel-Latif AK, Kucia M: The Nlrp3 inflammasome as a “rising star” in studies of normal and malignant hematopoiesis. Leukemia 2020; 34:1512–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wysoczynski M, Ratajczak J, Pedziwiatr D, Rokosh G, Bolli R, Ratajczak MZ: Identification of heme oxygenase 1 (HO-1) as a novel negative regulator of mobilization of hematopoietic stem/progenitor cells. Stem Cell Rev Rep 2015; 11:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Adamiak M, Abdelbaset-Ismail A, Kucia M, Ratajczak J, Ratajczak MZ: Toll-like receptor signaling-deficient mice are easy mobilizers: evidence that TLR signaling prevents mobilization of hematopoietic stem/progenitor cells in HO-1-dependent manner. Leukemia 2016; 30:2416–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Adamiak M, Moore JBt, Zhao J, Abdelbaset-Ismail A, Grubczak K, Rzeszotek S, Wysoczynski M, Ratajczak MZ: Downregulation of Heme Oxygenase 1 (HO-1) Activity in Hematopoietic Cells Enhances Their Engraftment After Transplantation. Cell Transplant 2016; 25:1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ledderose C, Liu K, Kondo Y, et al. : Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest 2018; 128:3583–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frame J, Long T, Schuster-Kubaczka C, Esain V, Eun Lim S, Daley GQ, North T: Inflammasome-mediated expansion of the hematopoietic system in the vertebrate embryo. Experimental Hematology 2019; Volume 76:S66. [Google Scholar]
- 115.Jing L, Tamplin OJ, Chen MJ, et al. : Adenosine signaling promotes hematopoietic stem and progenitor cell emergence. J Exp Med 2015; 212:649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116 *.Yang L, Hu M, Lu Y, Han S, Wang J: Inflammasomes and the Maintenance of Hematopoietic Homeostasis: New Perspectives and Opportunities. Molecules 2021; 26.An excellent up-to-date review summarizng a role of inflammasomes in regulating hematopoietic homeostasis.
- 117.Croker BA, Silke J, Gerlic M: Fight or flight: regulation of emergency hematopoiesis by pyroptosis and necroptosis. Curr Opin Hematol 2015; 22:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Torre-Minguela C, Mesa Del Castillo P, Pelegrin P: The NLRP3 and Pyrin Inflammasomes: Implications in the Pathophysiology of Autoinflammatory Diseases. Front Immunol 2017; 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Geyer HL, Dueck AC, Scherber RM, Mesa RA: Impact of Inflammation on Myeloproliferative Neoplasm Symptom Development. Mediators Inflamm 2015; 2015:284706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120 **.Luo H, Mu WC, Karki R, Chiang HH, Mohrin M, Shin JJ, Ohkubo R, Ito K, Kanneganti TD, Chen D: Mitochondrial Stress-Initiated Aberrant Activation of the NLRP3 Inflammasome Regulates the Functional Deterioration of Hematopoietic Stem Cell Aging. Cell Rep 2019; 26:945–954 e944.First publication that demonstrated expression of Nlrp3 inflammasome in normal HSPCs.
- 121.Sallman DA, Cluzeau T, Basiorka AA, List A: Unraveling the Pathogenesis of MDS: The NLRP3 Inflammasome and Pyroptosis Drive the MDS Phenotype. Front Oncol 2016; 6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. *.Freeman TL, Swartz TH: Targeting the NLRP3 Inflammasome in Severe COVID-19. Front Immunol 2020; 11:1518.This work supports a role of Nlrp3 inflammasome in COVID19 infection and proposes new therapeutic strategy aimed at inhibition of this intracellular protein immune complex.
- 123 *.Ratajczak MZ, Kucia M: SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia 2020; 34:1726–1729.This paper presents a concept that activation of Nlrp3 inflammasome leads to cytokine storm in COVID19 infected poatients.
- 124 **.Rodrigues TS, de Sa KSG, Ishimoto AY, et al. : Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med 2021; 218.A recent report supporting a role of Nlrp3 in flammasome activation in induction of cytokine storm in COVID19 patients.
- 125.Karan D: Inflammasomes: Emerging Central Players in Cancer Immunology and Immunotherapy. Front Immunol 2018; 9:3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J: T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature 2009; 460:269–273. [DOI] [PubMed] [Google Scholar]
- 127.Koehn BH, Apostolova P, Haverkamp JM, et al. : GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood 2015; 126:1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128 *.Ratajczak MZ, Kucia M. Extracellular adenosine triphosphate (eATP) and its metabolite, extracellular adenosine (eAdo), as opposing “Yin–Yang” regulators of Nlrp3 inflammasome in the trafficking of hematopoietic stem/progenitor cells. Frontiers in Immunology. 11:603942. doi: 10.3389/fimmu.2020.603942.A comprehensive review on opposite role of eATP and eAdo in trafficking of HSPCs.


