Abstract
Purpose of Review:
Dry eye disease (DED) is a multifactorial disease affecting approximately 5–50% of individuals in various populations. Contributors to DED include, but are not limited to, lacrimal gland hypofunction, meibomian gland dysfunction, ocular surface inflammation, and corneal nerve dysfunction. Current DED treatments target some facets of the disease, such as ocular surface inflammation, but not all individuals experience adequate symptom relief. As such, this review focuses on alternative and adjunct approaches that are being explored to target underlying contributors to DED.
Recent Findings:
Neuromodulation, stem cell treatments, and oral royal jelly have all been studied in individuals with DED and lacrimal gland hypofunction, with promising results. In individuals with Meibomian gland dysfunction, devices that provide eyelid warming or intense pulsed light therapy may reduce DED symptoms and signs, as may topical Manuka honey. For those with ocular surface inflammation, naturally-derived anti-inflammatory agents may be helpful, with the compound trehalose being farthest along in the process of investigation. Nerve growth factor, blood-derived products, corneal neurotization, and to a lesser degree, fatty acids have been studied in individuals with DED and neurotrophic keratitis (i.e. corneal nerve hyposensitivity). Various adjuvant therapies have been investigated in individuals with DED with neuropathic pain (i.e. corneal nerve hypersensitivity) including nerve blocks, neurostimulation, botulinum toxin, and acupuncture, although study numbers and design are generally weaker than for the other DED sub-types..
Summary:
Several alternative and adjunct DED therapies are being investigated that target various aspects of disease. For many, more robust studies are required to assess their sustainability and applicability.
Keywords: dry eye, alternative therapies, lacrimal, meibomian, corneal nerve
Introduction
Dry eye disease (DED) is “a multifactorial disease characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”[1] It presents with variable symptoms (e.g. dryness, pain, poor visual quality) and signs (e.g. low tear production, tear film instability, ocular surface inflammation), and can occur as an isolated disease or co-morbid to systemic diseases such as Sjögren’s, graft-versus-host disease, migraine, and fibromyalgia. Its symptoms can be debilitating, limiting the ability to work and negatively affecting mental health.[2] Both intrinsic (e.g. age, gender, genetics) and extrinsic (e.g. prolonged computer use, humidity, air pollution) factors can contribute to symptoms and/or signs of DED.[3, 4] With a reported frequency of 5–50% in various populations across studies, DED is a highly prevalent disease and a major source of ocular morbidity in the general population.[4]
DED is often subtyped by the apparent tear film abnormalities noted in a new case. For example, aqueous tear deficiency (ATD) is characterized by decreased tear production/volume, while evaporative tear deficiency (ETD) is characterized by an unstable tear film in the setting of adequate tear production.[5] Both subtypes can be accompanied by ocular surface inflammation and/or abnormal tear composition (high or unstable osmolarity).[1] Abnormalities in the organs involved in producing tear products (meibomian glands, goblet cells) can also accompany both DED sub-types, as can anatomic abnormalities of the eyelids and conjunctiva (e.g. ectropion, conjunctivochalasis) and abnormalities in corneal nerves.[6]
A challenge in DED is that its symptoms and signs are often discordant.[7] Variable nerve function may underlie this noted observation. For example, we have shown that corneal sensitivity varies widely in individuals with DED symptoms. In 403 individuals, the mean mechanical detection threshold was 87 mL/min±46 with a wide range of values (10th percentile 40 mL/min vs. 90th percentile 145 ml/min). Furthermore, 24% of individuals had corneal sensitivity values that fell at or outside this range, 13% were hypersensitive and 11% were hyposensitive.[8*] As such, the symptomatic interpretation of DED signs likely depends on the status of the nerves. In essence, DED may be understood as an umbrella term, characterized by multiple subtypes with different presenting manifestations that occur as a result of distinct underlying tissue injuries. This reality highlights the difficulty in developing a ‘gold standard’ disease definition and suggests that different treatment algorithms will likely be needed to target different underlying disease contributors in an individual patient.
The clinical work-up of DED involves subtyping individuals based on noted abnormalities.[6] Symptoms of DED can be assessed using various questionnaires (e.g. Dry Eye Questionnaire 5 [DEQ5][9] and Ocular Surface Disease Index [OSDI]).[10] Signs of DED are quantified by examination of the ocular surface. Tear stability can be assessed via tear break up time (TBUT), tear production via Schirmer strips, and corneal and conjunctival epithelial abnormalities via fluorescein and lissamine green staining.[11, 12] A slit lamp exam can also detect signs of meibomian gland dysfunction (MGD) or anatomic abnormalities (e.g. conjunctivochalasis). Understanding the symptoms and signs in a patient aids in subtype categorization, which can guide therapeutic decision-making.
Given the complexity of DED, it is not surprising that several therapies are available that target variable aspects of disease. First-line therapies include artificial tears (with and without lipid components) and anti-inflammatories (corticosteroids, cyclosporine, lifitegrast).[13] Other common therapies include punctal plugs for individuals with ATD, and eyelid hygiene or oral antibiotics for individuals with MGD.[14*] Nevertheless, there is constant demand for novel alternative therapies that target different aspects of disease. The goal of this review is to describe emerging alternative and adjunct therapies in DED (Figure 1), focusing on their underlying mechanisms of action and reviewing available human data.
Fig 1. Mapping of alternative therapies for DED based on present underlying pathologies.
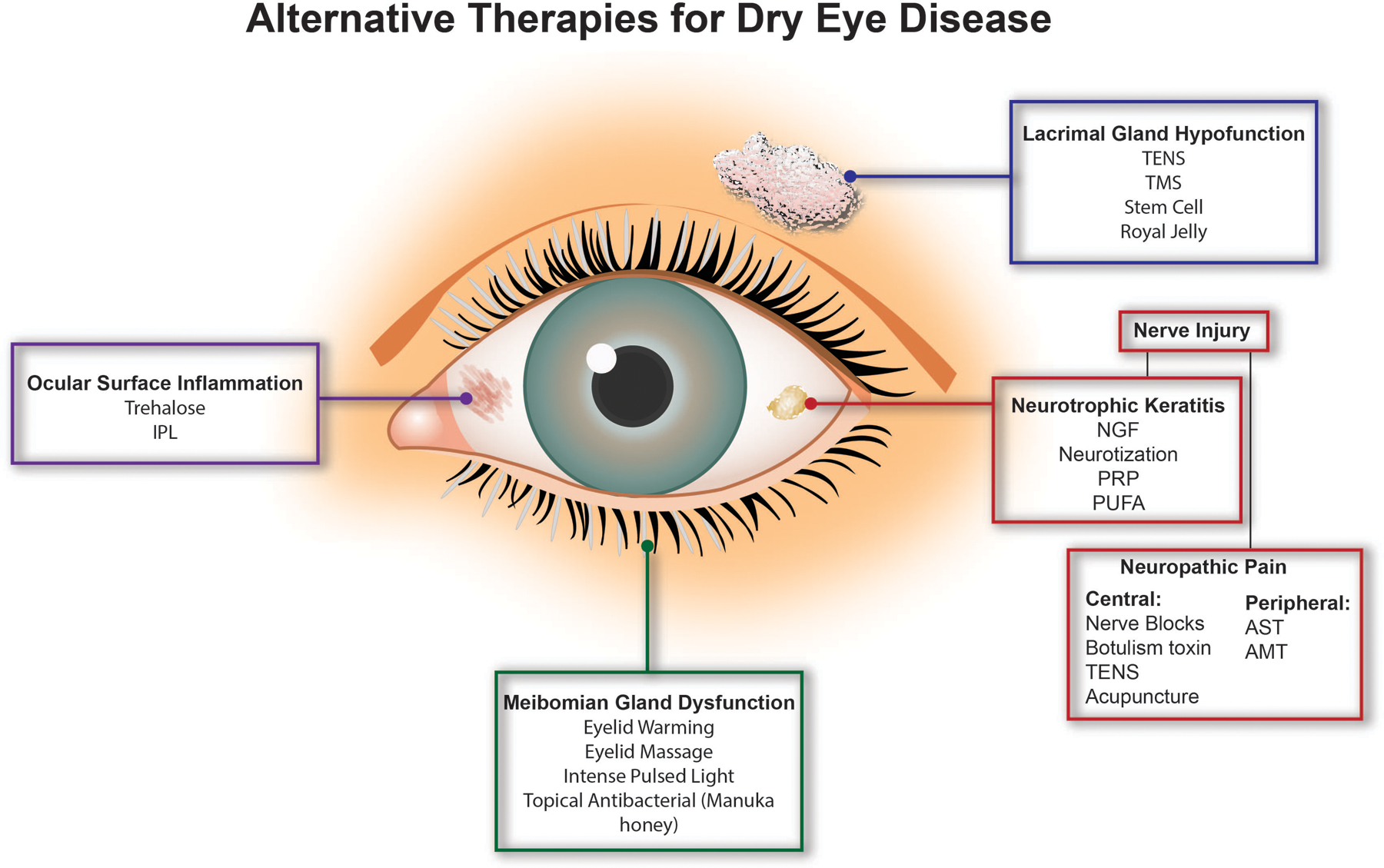
Alternative and adjunct therapies have been developed to treat various underlying causes of DED. Patients with aqueous deficient DED due to lacrimal gland hypofunction may benefit from transcutaneous electrical nerve stimulation (TENS), transcranial magnetic stimulation (TMS), stem cell therapy, or royal jelly therapy. Alternatively, those with evaporative DED due to meibomian gland dysfunction may be treated with office-based eyelid warming and massage devices, intense pulsed light therapy, and topical antibacterial agents, such as Manuka honey. Several therapies are currently available to target DED associated ocular surface inflammation. Trehalose and intense pulsed light therapy are two adjuvant therapies that are also being studied for this purpose. Lastly, nerve dysfunction may also be present in DED. Treatments for neurotrophic keratitis include nerve growth factor (NGF), platelet rich products (PRP), corneal neurotization, and polyunsaturated fatty acids (PUFA). In patients with neuropathic pain, cutaneous nerve blocks, TENS, botulinum toxin, and acupuncture have been used to treat pain of central origin, while autologous serum tears (AST) and amniotic membrane transplant (AMT) have been used to treat pain of peripheral origin.
Therapies for Lacrimal Gland Hypofunction
Lacrimal gland hypofunction, or low production and secretion of tears, is a common cause of ATD, and can occur in isolation or co-morbid with a systemic disease (Sjögren’s, graft-versus-host disease).[15] Lacrimal gland hypofunction can manifest secondary to glandular inflammation, destruction, or denervation, among other causes.[16] Mainstay treatments for ATD include artificial tears and anti-inflammatories,[15] however several alternative therapies are being explored that have been shown to be effective in improving gland output. Some of these therapies include neurostimulation, stem cell therapies, and topical royal jelly.
Peripheral Neurostimulation
Neurostimulation has been previously applied to a host of neurological disorders, including bladder dysfunction, Parkinson’s disease, and neuropathic pain.[17] More recently, it has been applied to lacrimal gland hypofunction.[18*] Neurostimulation can be applied to peripheral (e.g. transcutaneous electrical nerve stimulation [TENS]) or central (e.g. transcranial magnetic stimulation [TMS]) nerves to stimulate innervated tissues.[18*] Commercially available TENS devices induce lacrimation by stimulating anterior ethmoidal nerve afferents (branches of the trigeminal nerve), sending signals to the superior salivatory nucleus as an intermediate, and subsequently activating lacrimal glands, meibomian glands, and goblet cells, aiding in release of their tear film components.[18*] TMS instead applies a coil externally to the scalp and directly stimulates neurons within targeted regions of the brain.[19]
The first peripheral nerve stimulator approved for DED was TrueTear (Allergan, Dublin, Ireland), a previously available handheld device that delivered microcurrents to the anterior ethmoidal nerves via prongs inserted into the nasal cavity.[20**] Several studies demonstrated the use of TrueTear in improving symptoms and signs of ATD. In a randomized study of 48 individuals with ATD (OSDI≥13, Schirmer≤ 10mm, stimulated Schirmer≥7mm higher than basal), all subjects were treated with active and sham treatment one hour apart. The single three-minute active treatment acutely increased tear production (via Schirmer) to a greater degree than sham (active: 5.5±3.0mm to 25.3±1.5mm vs sham: 5.5±3.0mm to 9.2±1.1mm; p<0.0001).[20**] TrueTear’s effect on tear production was also maintained over time. In a randomized study of 58 individuals with ATD (OSDI≥23, Schirmer≤10mm, stimulated Schirmer≥7mm higher than basal), subjects who received active treatment (n=31) for 30s 4–8 times/day over at least 30 days showed greater stimulated change in Schirmer directly after treatment at 0, 7, 14, 30, and 90 days compared to a sham-treated group (n=27) (Δactive: +17.3±2.1mm, +10.4±1.6mm, +7.6±1.5mm, +7.1±1.7mm, +9.0±2.0mm, respectively vs. Δsham: +1.3±1.4mm, −2.0±1.0mm, −5.0±1.3mm, −0.4±0.7mm, +0.4±0.6mm, respectively; p<0.001 for all timepoints). In addition, the active treatment group demonstrated greater improvement in symptoms, TBUT, and corneal staining vs. the sham group, but these improvements did not reach statistical significance.[21*] Interestingly, intranasal stimulation has also been shown to improve aspects of DED beyond tear production. In a prospective, interventional study of 15 individuals with ATD (OSDI≥13, Schirmer≤10mm, stimulated Schirmer≥7mm higher than basal) a single 3-minute treatment improved meibomian gland function in all subjects. Pre-stimulation, meibomian gland area was measured at 2,187.6±635.9μm2 via infrared meibography while post-stimulation, the area was 1,933.2± 538.5μm2, (11.6% decrease; p=0.001), suggesting contraction of the glands due to stimulation.[22*] In all studies, the most frequently reported adverse events included nasal pain or discomfort and epistaxis. Despite these early promising studies, TrueTear was removed from the market in 2020.
iTEAR (Olympic Ophthalmics, Inc., Issaquah, WA) is another TENS device that is currently available on the market. This device mechanically stimulates the external branch of the anterior ethmoidal nerve via an oscillating tip placed against the lateral aspect of the nose.[23**] In an open-label study, 101 individuals with ATD (Schirmer≤10mm, stimulated Schirmer≥7mm higher than basal) used the device at home bilaterally at least 2x/day for 30s for at least 30 days. At 30 days, OSDI was found to have decreased (40.3±22.9 to 25.4±18.6; p=0.05). Schirmer also changed significantly directly after stimulation at 0, 14, and 30 days (+22±7.8mm, +11.1±8.4mm, +9.4±9.3mm, respectively; p<0.05 for all). Noted side effects were minimal but included slight headache, sneezing, and intermittent nasal soreness.[23**]
Peripheral neurostimulation is also possible through use of pharmacologic agents. OC-01 (Oyster Point Pharma, Princeton, NJ), a nasal spray containing the nicotinic acetylcholine receptor agonist varenicline, activates the trigeminal parasympathetic pathway to stimulate tear production.[24**] Demonstrating this, in a study of 123 subjects with ATD (Schirmer≤10mm), subjects were assigned 1:1:1 (n=41 each) to receive a single nasal spray (1.2mg/mL OC-01, 0.6mg/mL OC-01, or placebo) to each nostril 2x/day for 84 days. At day 84, the OC-01 groups showed significantly greater improvements in Schirmer scores in comparison to placebo (0.6mg/mL: 5.5mm to 16.1mm; p<0.05 vs. 1.2mg/mL: 5.4mm to 16.4mm; p<0.05 vs placebo: 5.3mm to 11.5mm; p-value not provided). The most frequently reported adverse reactions in this study included sneezing, blurry vision, and headache.[24**]
TMS has traditionally been used in the treatment of depression and pain[18*], but one company (EpiTech, Kefar Sava, Israel) is currently examining the use of TMS in DED. Demonstrating this potential use, in an open-label study, 29 individuals with DED of mixed etiologies [Sjogren’s (n=13), MGD (n=12), ATD (n=5)] all received a single 11-minute treatment with a TMS device in-office. At week 12 post-treatment, corneal staining was decreased compared to baseline (Δ−1.7; p<0.001), and overall symptoms also decreased (via Patient-Reported Outcomes with LASIK-2 score, range: 0–100: Δ−15.3; p<0.001), with no reported adverse effects.[25**] Given small numbers and the lack of controlled studies, more data are needed to explore the safety and efficacy of TMS in DED.
Overall, these studies demonstrate that devices and compounds that can either electrically or pharmacologically stimulate peripheral or central nerves involved in lacrimal gland function may improve symptoms and signs of DED. Because these modalities target tear production, these studies have mostly focused on individuals with ATD, although future studies may show potential use for these therapies outside the scope of lacrimal hypofunction.
Stem Cell Therapies
Due to their multipotent differentiation capacity, stem cells have been studied as a regenerative strategy in various diseases (e.g. cardiovascular, autoimmune, neurodegenerative, oncologic).[26] Their use in lacrimal gland hyposecretion is more recent. A canine model first documented the therapeutic benefits of mesenchymal stem cell injection into the lacrimal gland as a treatment for ATD.[27] The efficacy of stem cell therapy in ATD was also documented in a Sjögren’s murine model (thrombospondin-1−/− mice)[28], where restoration of lacrimal gland structure, decreased ocular inflammation, and increased tear production were noted after treatment.[29] More recently, human studies have begun to explore this strategy. In an open-label study, 7 subjects with ATD (OSDI>30, TBUT<10s, Schirmer≤5mm) received a transconjunctival injection of adipose-derived stem cells. After 16 weeks, all DE parameters improved from baseline, including OSDI (58.9±20.6 to 34.1±21.6; p<0.002), TBUT (3.7±1.5s to 7.1±1.9s; p<0.002), Schirmer (4.6±0.7mm to 8.1±3.1mm; p<0.03), and staining (Oxford grade; 2.4±0.7 to 1.3±1; p<0.10). Minor adverse reactions were reported, including pain at the site, periorbital edema, ocular discomfort, and blurred vision.[30] Further human studies are needed however, as the majority of studies have thus far been confined to pre-clinical models.
Royal Jelly (RJ)
RJ (made by bees) is another compound shown to promote lacrimal gland activity. Bees release it through hypopharyngeal and mandibular glands into larvae honeycomb chambers, from where beekeepers harvest it.[31] RJ has been noted to have antibacterial, anti-inflammatory, antifungal, and hypotensive properties and has been used in humans to treat a variety of disorders outside the eye including menopause[32], sarcopenia[33], cancer, diabetes, and Alzheimer’s disease.[34] Though its exact mechanisms are not fully understood, RJ has been found to promote mobilization of Ca2+ ions through muscarinic signal transduction pathways within lacrimal glands, allowing for phosphorylation of AMP kinase and preservation of local ATP. As such, preserving intraglandular energy status is a potential mechanism by which RJ improves tear secretion.[35] Demonstrating the use of RJ in ocular disorders, a randomized controlled study divided 43 individuals with ATD (symptoms on DEQ5, TBUT≤5s, Schirmer≤5) into treatment (n=21; 400mg royal jelly tablet 6 times/day orally) and placebo (n=22) groups. At 8 weeks, the treatment vs placebo group had greater improvements in tear stability via TBUT (treatment: 4.5±3.2s to 6.2±2.9s; p=0.04; placebo: 3.8±2.5s to 5.3±2.5s; p=0.3; between groups: p=0.8) and tear production via Schirmer (treatment: 13.6±10.6mm to 19.5±11.7mm; p=<0.001; placebo: 13.8±13.8mm to 14.3±13.4mm; p=1.0; between groups: p=0.8), however the in between group differences did not reach statistical significance. None of the subjects reported any adverse events or side effects.[36] Beneficial effects of RJ were seen in a rat model of ATD (low room temperature and humidity, constant airflow, and placement of rats on a swing to decrease blinking). Rats were treated with 1mL of distilled water (vehicle) or RJ, honey, pollen, larva, or propolis orally once daily for 11 days. After 11 days, rats treated with RJ had the greatest increase in lacrimal protein secretion (ΔRJ:+175% vs Δvehicle: +60%; p<0.001) and tear secretion (RJ: +1.2x vs vehicle: +0.5x; p<0.001) compared to all other compounds.[35] The findings from these studies suggest that RJ may impact lacrimal gland activity, potentially via increasing lacrimal gland energy content.
In summary, neurostimulation, stem cell therapy, and topical RJ are all potential adjuvant/alternative therapies that have been shown to improve lacrimal gland output in individuals with ATD.
Treating Meibomian Gland Dysfunction
Proper incorporation of meibum into the tear film is necessary for preventing evaporation of the inner muco-aqueous layer. Meibum secretion from meibomian glands (MG) can be impaired by MG hypofunction (MG dropout, injury, inflammation), MG duct obstruction (blepharitis, occlusion), and/or altered meibum quality[14*], all of which may manifest as ETD.[37] Current treatments for ETD aim to reduce some of these underlying abnormalities, including inhibiting inflammation, clearing obstructed ducts, and/or softening and physically expressing meibum.[14*] Several emerging therapies have targeted these underlying causes in various ways.
Thermal and Massage Treatments
Inspissated meibum has a high viscosity, reducing its ability to pass through MG ducts. Studies have determined that the optimal temperature required to soften inspissated meibum is 41.5°C[38], but in-home treatments with warm and moistened towels are not efficient at delivering or maintaining this temperature.[39] Solving this issue, multiple devices have been developed for in-office use.
For example, the LipiFlow Thermal Pulsation System (Johnson & Johnson Vision, Jacksonville, Florida) delivers a constant temperature of 42.5°C during a 12-minute treatment session while simultaneously pulsating in order to physically express meibum.[40, 41] In one study, 200 individuals with MGD (OSDI≥13, meibomian gland obstruction, lipid layer≤80mm) were either treated with LipiFlow (n=101, one treatment) or served as controls in a case-crossover design (n=99, treated with warm compresses 2x/day for 3 months then LipiFlow).[42] Three months post-treatment, the treatment group showed greater symptom improvement (ΔOSDI: −24.0±20.9 treatment vs −17.1±18.6 control; p<0.002) and MG expression (ΔMeibomian Gland Evaluator[43] score, examination of 15 glands, range 0–45, lower score indicates greater abnormality; +11.2±9.3 treatment vs +4.5±8.2 control; p<0.0001) than the control group. At 12 months, after all individuals were treated with Lipiflow, the two groups improved from baseline in OSDI (treatment: 44.1±20.4 to 21.6±21.3; p<0.0001 vs crossover: 51.8±23.1 to 24.0±23.2; p<0.001; between groups: p=0.9). Meibomian gland expression scores were similarly improved between the groups (treatment: 6.40±3.70 to 17.3±9.1; p<0.0001 vs crossover: 6.3±3.6 to 18.4±11.1; p<0.001; between groups: p=0.7) scores. While no serious adverse events were reported with LipiFlow treatment, some subjects did experience transient eye/eyelid discomfort (1.5%) and eyelid skin dermatitis (1.5%). Overall, these data highlight the ability of a single LipiFlow treatment to improve symptoms and signs of MGD compared to hot compresses, with sustained effects for at least one year.[42]
Other devices have since entered the market with comparable results at lower price. The TearCare (Sight Sciences, Menlo Park, California) utilizes four adhesives which attach to each tarsal plate to deliver heat (41°C-45°C) to the Meibomian glands for 12 minutes. Following the thermal treatment, manual in-office meibum expression is performed.[44] The major difference from LipiFlow is that there is no massage component and patients are encouraged to blink during treatment to naturally stimulate meibum expression. A randomized clinical trial of 24 subjects with DED (symptoms, TBUT<10s, Schirmer≤10mm) divided subjects into treatment (n=12, one TearCare treatment) or control (n=12, daily warm compress) for 4 weeks. Both groups had evidence of MGD at baseline with Meibomian Gland expression scores (via Meibomian Gland Evaluator) of 6.3±3.6 in the treatment group and 9.0±4.3 in the control group. After 4 weeks, symptoms (via Standardized Patient Evaluation of Eye Dryness (SPEED II, range 0–28): 15.7±5.2 to 7.8±3.5; p-value not given) and signs (TBUT: Δ11.7±2.6s; p<0.0001, corneal staining: 3.5±1.8 to 0.2±0.4; p<0.001) improved to a greater degree in the treatment vs control group, although between group p values were not presented. The treatment group also showed a greater improvement in Meibomian gland expression scores (treatment: 6.3±3.6 to 41.0±2.1; p<0.001 vs. control group: 9.0±4.3 to 8.2±4.0; p-value not given; between groups: p<0.0001). Along with these promising results, no adverse reactions were reported due to treatment.[44]
Another available device for heating the Meibomian gland is iLux (Alcon, Fort Worth, Texas). iLux is a handheld battery-powered device that utilizes inner and outer pads to deliver heat (38°C-42°C) to the palpebral conjunctiva and external eyelids for eight minutes. Advantages over LipiFlow include focus-guided tips that provide customized eyelid warming and compression and a magnifying lens for visualization of Meibomian gland ducts during treatment.[45*] In an open-label study of 142 subjects with ETD (OSDI≥23, TBUT<10s, MG obstruction), subjects were divided 1:1 to receive one bilateral treatment with either iLux or LipiFlow. Compared to baseline, at 4 weeks both groups showed comparable improvements in OSDI (iLux: 50.7±18.6 to 19.5±17.0; p<0.0001 vs. LipiFlow: 50.6±18.7 to 22.6±19.8; p<0.0001; between groups: p=0.3) and TBUT (OD values given; iLux: 3.9±1.9s to 6.7±3.7s; p<0.0001 vs. LipiFlow: 3.9±2.0s to 6.6±3.2s; p<0.0001; between groups: p>0.8). Similar degrees of improvement were also seen in Meibomian gland scores (via Meibomian Gland Evaluator) after 4 weeks (OD values given; iLux: 6.0±3.7 to 23.2±12.1; p<0.0001 vs. LipiFlow: 6.2±4.9 to 24.3±11.2; p<0.0001; between groups: p>0.6). A few subjects in the iLux group experienced transient side effects post-treatment (burning sensations, pain, and petechial hemorrhage. Specific side effects in the LipiFlow group were not presented.[45*]
Overall, these studies demonstrate that devices that heat and/or massage MGs can have a beneficial impact on symptoms and signs of ETD, in some cases to a greater degree than warm compresses alone.
Intense Pulsed Light
Intense pulsed light (IPL) has also been explored for the treatment of ETD. This therapy delivers short pulses of light (wavelengths 500–1200nm) to the skin and is widely used by dermatologists to treat pigmented and vascular lesions.[46] Its application to DED was anecdotally noted in 2002, after individuals with rosacea (a dermatological disease often comorbid with DED[47]) had improvements in OSDI, TBUT, and staining after IPL treatment of facial telangiectasias.[48] IPL has been found to work synergistically with Meibomian gland expression (MGX). In a prospective study, 100 subjects with MGD (Meibomian gland dropout <50%, <6 glands secreting liquid) were divided into 3 treatment groups consisting of MGX (n=32), IPL (n=33), or IPL+MGX (n=35). Three months after last treatment, subjects in the IPL+MGX group had significantly improved OSDI (28.2±16.8 to 15.9±14.6; p=0.002), TBUT (1.7±1.3s to 3.0±0.7s; p=0.003), and MG dropout (21.0±4.0% to 18.0±5.0%; p=0.001) whereas subjects who received IPL alone only showed significant improvement in TBUT (1.7±0.7s to 3.7±1.0s; p<0.001) and subjects who received MGX alone only did not show significant improvement in any parameters. While between groups comparisons were not provided for OSDI or TBUT, Meibomian gland dropout decreased to a greater degree in the IPL+MGX vs MGX group (IPL+MGX: 21±4% to 18±5%; p=0.1 vs MGX: 22±10% to 24±11%; p=0.4; between groups: p<0.01) and in the IPL vs MGX group (IPL: 33±13% to 29±12%; p=0.3 vs MGX: 22±10% to 24±11%; p=0.4; between groups: p<0.01). However, baseline values did not significantly improve in any group, rendering the data less robust.[49] One open-label and one retrospective study similarly reported improvements in signs of MGD (e.g. MG plugging, TBUT) with combined IPL and MGX.[50, 51] Side effects common to participants included mild pain and burning acutely but no long-term adverse effects.
Many hypotheses exist regarding how IPL improves facets of DED, including improved meibum quality due to increased periocular skin temperature, destruction of periocular telangiectasias rich in pro-inflammatory mediators, killing of bacteria, inhibition of inflammatory cytokines (IL-6, tumor necrosis factor (TNF)-α), and reduction of reactive oxidative species.[52] One study nicely demonstrated that IPL reduced inflammatory mediators in tears. In a randomized study, 44 subjects with MGD (SPEED II≥6, Meibomian gland obstruction) received either active IPL (n=44 eyes, 14–16J/cm2) or sham treatment (n=44 eyes, 0J/cm2) 3 times over 12 weeks. At 12 weeks, decreased concentrations of inflammatory mediators were noted in tears of individuals who received active vs sham treatment [Interleukin (IL)-17a (Δactive: −211.7±33.8pg/mL; p<0.001 vs. Δsham: −89.6±22.2pg/mL; p=0.1; between groups: p<0.001), IL-6 (Δactive: −405.6±65.6pg/mL; p<0.01 vs. Δsham: −143.5±26.0pg/mL; p-value not given; between groups: p<0.001), and prostaglandin (PG) E2 (Δactive: −1.6±0.1ng/mL; p-value not given vs. Δsham: −0.7±0.1ng/mL; p-value not given; between groups: p<0.001)]. Interestingly, and similar to what has been noted clinically[53], no significant correlations were noted between inflammatory mediators and DED parameters (SPEED II, TBUT). Negative correlations were noted between inflammatory markers and number of Meibomian glands yielding clear secretions (IL-17a: r=−0.7; p<0.001, IL-6: r=−0.8; p<0.001), indicating a potential relationship between inflammation and altered meibum quality.[54] Overall, IPL improved symptoms and signs of MGD, as well as inflammation, either alone or in combination with MGX. Missing from the literature are comparisons with other therapies, such as LipiFlow. Such studies are needed to understand the patient population best served by IPL.
Manuka Honey
Antibacterial therapies have previously been used in MGD and ETD, most commonly azithromycin and doxycycline.[55, 56] A naturally derived antibacterial agent, Leptospermum spp honey, or Manuka honey, has been incorporated into Optimel eye drops (Melcare Biomedical Pty Ltd, Queensland, Australia). The antibacterial effects of this honey are attributed to its low pH, high osmolarity, and H2O2 content, which inhibits bacterial cell division.[57] A randomized control study of 114 subjects with moderate to severe MGD (MGD grade 1–4[58], TBUT<10s) divided subjects into treatment (98% Manuka gel (n=37) or 16% Manuka drops (n=37), 2x/day) and control (n=40; warm compress) groups. At 8 weeks, improvements were seen within all groups at similar degrees in OSDI (98% Optimel: 45.4±17.3 to 29.1±18.7 vs 16% Optimel: 38.2±15.6 to 24.6±13.6 vs control: 36.2±23.3 to 25.3±16.8; p<0.05 for all; between groups: p=0.5) and TBUT (98% Optimel: 1.8±1.8s to 3.0±1.7s vs 16% Optimel: 1.8±1.8s to 3.9±2.6s vs control: 1.5±1.2s to 3.2±3.8s; p<0.05 for all; between groups: p=0.5). The treatment vs control group did, however, have a more significant improvement in Meibomian gland expressibility (examination of 5 glands, range 0–3; 98% Optimel: 1.7±3.9 to 0.4±0.6; p<0.05 vs 16% Optimel: 1.1±1.0 to 0.7±1.4; p<0.05 vs control: 0.8±0.8 to 0.6±0.8; p=0.1; between groups: p<0.05) and meibum quality (sum of values from 8 glands, range 0–3 each gland, total range 0–24), 98% Optimel: 15.6±5.2 to 8.1±4.4 vs 16% Optimel: 15.2±5.7 to 9.5±6.7 vs control: 14.6±5.2 to 11.2±6.0; p<0.05 for all; between groups: p<0.01). The potential anti-bacterial effects of Manuka honey were noted as colony counts of Staphylococcus epidermidis (present in 42% of participants at baseline) decreased after 8 weeks to a greater degree in the treatment groups vs control group (98% Optimel: 696.0±911.0 to 226.0±442.0; p=0.03 vs 16% Optimel: 421.0±763.0 to 288.0±817.0; p=0.04 vs control: 248.0±391.0 to 155.0±123.0; p=0.1; between groups: p=0.05). However, a confounding factor is that colony counts were higher in the treatment vs control groups at baseline.[59] Unfortunately, another randomized study of 59 subjects with MGD (altered meibum quality and expressibility) found that Manuka honey (n=32; 16% Optimel drops 2x/day) had similar effects to subjects treated with lubricant eye drops and warm compresses (n=27) after 21 days follow-up, including various Meibomian gland parameters.[60*] Noted side effects associated with Optimel drops were redness and stinging.[59, 60*]
In summary, these studies suggest that in-office strategies that warm and massage the eyelids, intense pulsed light therapy, and novel antimicrobial agents, may all have a role as alternative therapies for individuals with MGD and ETD.
Therapies for Ocular Surface Inflammation
Ocular inflammation is a major contributor to DED and can be seen in both ATD and ETD.[61] The source of ocular inflammation has been linked to T-cell activation and subsequent release of pro-inflammatory cytokines, especially IL-1, IL-6, IL-8, and TNF-α, in both humans and DED animal models.[62] Anti-inflammatory medications, including cyclosporine and lifitegrast, are mainstay treatments for DED. More recently, naturally occurring anti-inflammatory substances have been explored as alternative therapies in DED.
One such therapy is trehalose, a naturally occurring disaccharide with high resistance to environmental desiccative and oxidative stress. When suspended in solution and applied to the eyes, it decreases inflammation by activating the transcription factor EB, resulting in autophagosome activation and subsequent destruction of pro-inflammatory cytokines.[63] In an open-label study, 15 subjects with ETD (OSDI>18, TBUT<10, Schirmer>10) received trehalose/hyaluronate tear substitute (one drop/eye, 3x/day x 2 months). Symptoms and signs of DED improved including OSDI (38.7±12.7 to 22.2±2.9; p<0.05), TBUT (6.2±1.9s to 8.6±1.3s; p<0.05), and corneal staining (3.4±0.5 to 1.23±0.6; p<0.05).[64] Another in vitro study demonstrated that trehalose had anti-inflammatory effects. This study examined corneal epithelial cells preincubated for one hour with 0%, 0.1%, 0.5%, 1.0%, 1.5%, 2.0%, 3.0%, or 5.0% trehalose NaCl solution and then exposed to a 450mOsm NaCl solution. Percentage of pro-inflammatory markers after desiccation were significantly lower in the 0.5%, 1.0%, and 1.5% trehalose groups compared to the control group. As compared to control cells, the 1.0% trehalose cells showed the greatest difference in markers (TNFα: 26.1±9.6pg/mL trehalose vs 96.4±12.6pg/mL control; IL-1β: 27.0±8.9pg/mL trehalose vs 87.0±10.8pg/mL control; IL-6: 4.5±0.7ng/mL trehalose vs 7.6±1.4ng/mL control; p<0.05). Interestingly, higher trehalose concentrations (2.0%−5.0%) lost these protective effects and inflammatory markers actually increased.[63] Though preliminary results are promising, randomized trials with a comparison group (placebo) are needed to examine the clinical value of trehalose in DED. This also applies to other anti-inflammatory natural products that have mostly been examined pre-clinically in animal models or in vitro. Other promising anti-inflammatory products for DED include Aster koraiensis extract[65], polydatin[66], Eurya japonica extract[67], L-carnitine[68], pterostilbene[69], alpha-lipoic acid[70], selenoprotein P[71], vitamin B12[68, 72], carotenoids[73], and anthocyanins.[74]
Overall, given the important role of inflammation in DED, there is interest in exploring the effects of naturally-derived anti-inflammatory products in the disease, although these investigations are still in early stages.
Addressing DED with Nerve Abnormalities
Studies have shown that a subset of individuals with DED have underlying nerve abnormalities that may contribute to symptoms. For example, some individuals can have corneal nerve abnormalities causing hypoesthesia along with signs of epitheliopathy, which is categorized as a neurotrophic phenotype, or neurotrophic keratitis (NK). Alternatively, an individual may have a corneal nerve abnormality causing hyperesthesia along with pain out of proportion to ocular surface findings, which is considered a neuropathic phenotype, or neuropathic pain (NP).[75] In some instances, individuals may present with signs of NK and NP simultaneously. Specific treatments have been developed to address nerve abnormalities in both NK and NP.
Neurotrophic Keratitis (NK)
NK is a clinical phenotype characterized by decreased corneal sensitivity, with or without pain, and with signs of corneal epithelial disruption. It can occur in the setting of various disorders, including diabetes, post-viral infection, anesthetic abuse, neurosurgical procedures, and Sjögren’s-associated DED.[76, 77] NK is typically graded based on the severity of epithelial irregularities on a 1–3 scale (1=corneal staining, 2=persistent epithelial defect [PED], 3=ulceration or perforation). Autologous serum tears (AST) and amniotic membrane transplant (AMT)[78] are first-line treatments in NK, along with tarsorrhaphy or therapeutic contact lenses. Recombinant human nerve growth factor (rhNGF) (Oxervate, Cenegermin, Dompe)[79, 80], platelet rich plasma (PRP), and corneal neurotization[81] are newer approaches that have been explored as treatments for NK.
NGF is a naturally-occurring polypeptide that contributes to neuronal growth and differentiation. Released in settings of stress, NGF induces neurite sprouting, eventually resulting in healing and restored function of injured nerves.[82] Numerous tissues express NGF receptors, including those on the ocular surface, and studies have shown that topical application of NGF leads to restoration of the epithelium and prevents corneal melting in NK.[82] The REPARO study randomized 156 subjects with stage 2 or 3 NK to rhNGF 20μg/ml, 10μg/ml, or vehicle (6 drops/day for 8 weeks) in a 1:1:1 ratio. At week 4, a higher frequency of treated subjects achieved a PED size <0.5 mm compared to controls (20 μg/ml: 58.0%, 10 μg/ml: 54.9%, controls: 19.6%; p<0.001). At week 8, outcomes were even better (74.0% vs. 74.5% vs. 43.1%, respectively). Median time to corneal healing (<0.5-mm lesion staining) was significantly faster in both rhNGF groups compared to the vehicle group (rhNGF 20 μg/ml: 28 days vs rhNGF 10 μg/ml: 29 days vs vehicle: 56 days; p=0.002 each). Most individuals treated with rhNGF (>96%) did not have a PED recurrence after the initial treatment for up to 56 weeks.[79] A follow-up multicenter, randomized study found similar results when subjects were treated with rhNGF 20μg/ml (n=24) or vehicle (n=24). At 8 weeks, a higher proportion of the rhNGF group achieved a PED size <0.5 mm than controls (69.6% vs. 29.2%; p<0.01).[80] Overall, these findings indicate a benefit of rhNGF as a therapeutic option for NK.
Interestingly, one study noted positive effects of rhNGF in subjects with non-NK DED. In an open-label study of 40 subjects with ATD (symptoms, TBUT≤10s, Schirmer≤10mm, staining>3) without history of NK, subjects were treated with rhNGF at different concentrations (20 µg/mL [n=20] and 4 µg/mL [n=20], 2x/day for 28 days). Both groups reported similar improvements in symptoms and signs of DED, including OSDI (20 µg/mL: 55.5±21.8 to 32.6±16.2 vs. 4 µg/mL: 52.4±21.8 to 35.7±22.8; p<0.001 for both) and corneal staining via lissamine green (20 µg/mL: 5.2±2.0 to 1.3±2.2 vs. 4 µg/mL: 5.9±2.7 to 3.0±3.3; p<0.001 for both).[83] However, the lack of a control population with which to compare the results limits the strength of the study. Common side effects of rhNGF treatment included hyperemia, pain, and foreign body sensation, but perhaps its most significant limitation is the large cost associated with treatment.[84]
Blood-derived products have long been used in the treatment of ocular surface disorders, owing to their high concentration of growth factors and regenerative capacity.[85–87] More recently, PRP has been explored for the treatment of DED and NK. Benefits of PRP are that platelets can adhere to damaged epithelium and release growth factors (e.g. platelet-derived growth factor (PDGF)), impact fibroblasts, and provide structural support.[88] PRP has in fact been noted to contain higher concentrations of growth factors (e.g. PDGF, NGF) and anti-inflammatory cytokines (e.g. TGF-β) compared to conventional AST.[89*]
PRP have been studied in Sjögren’s[90**], graft-vs-host disease[91], post-LASIK DE[92], and NK. In a retrospective study of 28 individuals with NK due to post-infectious etiologies, 11 of 11 individuals in the PRP group vs 12 of 17 individuals in the AST group achieved re-epithelization. Healing rate was also faster in the PRP vs AST group (PRP: 1.0±0.3mm2/day vs AST: 0.5±0.1mm2/day; p=0.04).[93] PRP (5x/day along with preservative-free artificial tears and vitamin A ointment at night) also healed (n=20) or improved (n=4) NK associated ulcers in 25 subjects with nonhealing PEDs (average size: 3.7±1.1mm x 2.8± 0.9mm).[92] In randomized study, 83 individuals with ATD (OSDI>13, Schirmer<5.5mm) were divided into treatment (n=44, PRP 6 drops/day) and control groups (n=39; artificial tears 6 drops/day bilaterally). At 30 days, greater changes from baseline were seen in the PRP vs control group in OSDI (ΔPRP: −24.9±15.7 vs Δ control: −5.6±5.7; p=0.001), Schirmer (Δ PRP: +1.9±2.1mm vs Δ control: +0.3±1.2mm; p=0.002), and staining (Oxford score; Δ PRP: −2.3±1.1 vs Δ control: −0.3±0.5; p<0.001).[94] Fortunately, no adverse side effects were noted with PRP across studies.
Corneal neurotization is a surgical procedure that involves redirecting sensory innervation from one region of the somatosensory system to another in order to reinnervate a target tissue. This procedure is reserved for the most severe cases of NK and involves grafting a peripheral nerve (e.g. sural nerve) to the trigeminal nerve system in order to restore function to corneal subbasal nerve fibers.[95, 96] Corneal nerves secrete growth factors that help maintain the corneal epithelium and by restoring innervation, neurotization is thought to restore these factors and improve the epitheliopathy.[97] One retrospective study examined 23 subjects with postherpetic NK who underwent neurotization. After treatment (6–20 months post-op), corneal sensation improved on Cochet Bonnet esthesiometry in all patients (1.6 cm to 3.6 cm; p=0.03), with full sensation returning in 2 subjects. In addition, all 4 subjects with a PED had complete resolution without the need for further treatment.[81] In another study, PEDs healed in 3 patients with NK who underwent neurotization. In addition, corneal sensation (0±0mm baseline to 11.7±16.5mm at 3 months to 15.0±21.2mm at 6 months to 30.0±14.4mm at 12 months), and nerve density (0±0n/mm2 baseline to 12.5±5.1n/mm2 at 3 months to 18.7±5.1 at 6 months to 25.1±10.2n/mm2 at 12 months) increased over time.[98] Side effects of neurotization include surgical-related risks and hypoesthesia at the donor site.[95, 96]
Finally, pre-clinical studies have explored the role of omega-3 polyunsaturated fatty acids (PUFA) on nerve regeneration in the setting of DED. Docosahexaenoic (DHA), a PUFA, promotes the release of docosanoids (e.g. neuroprotectin-1), which work in a cascade along with other growth factors to increase synthesis of NGF.[99] One study applied a collagen shield soaked in DHA+PEDF or vehicle (changed every 72 hours over 6 weeks) to rabbit eyes after lamellar keratectomy. Six weeks after initiating treatment, corneal nerve density was higher in the PEDF-DHA vs vehicle group (treatment: 26.7±2.6% vs. vehicle: 11.7±1.7%; p=0.01). Likewise, corneal sensation was greater in the PEDF-DHA vs vehicle group.[100] In humans, one study examined the effect of PUFA in diabetes (a frequent cause of NK). In an open label study, 36 individuals with type 2 diabetes and clinically diagnosed DED were treated with 3 months of oral PUFA (170 mg EPA+115 mg DHA). Marginal improvements in symptoms and signs of DED were noted including OSDI (26.1±8.2 to 22.0±7.5; p<0.001), TBUT (3.4±0.9s to 4.4±1.4s; p<0.001), and Schirmer (5.2±2.7mm to 6.6±2.3mm; p<0.05).[101] However, the lack of a control group limits the strength of this study. Furthermore, a much larger double-blind, randomized controlled study of 535 individuals with ATD (OSDI>25, TBUT<7, Schirmer<7) failed to show a significant difference in symptoms (OSDI) and signs (conjunctival/corneal staining, TBUT, Schirmer) of DED following treatment with either 3000mg oral omega-3 fatty acids (n=349) or placebo (n=186) for 12 months.[102]
Overall, strong evidence is available for the efficacy of rhNGF and PRP as treatments of NK, while weaker data are available regarding the benefits of corneal neurotization and PUFA.
Neuropathic Pain (NP)
NP is defined as “pain caused by a lesion or disease of the somatosensory nervous system.”[103] NP can occur due to an abnormality in corneal sensory nerves (e.g. peripheral neuropathic pain) or central nerves (e.g. central neuropathic pain), or, in some cases, both simultaneously. Certain symptoms are more common in individuals with neuropathic ocular pain (NOP), such as presence of burning, and sensitivity to light and wind.[104] Peripheral NOP can be treated with autologous serum tears (AST) while oral medications (α2γ ligands e.g. gabapentin, selective serotonin-norepinephrine reuptake inhibitors e.g. duloxetine, or tricyclic antidepressants e.g. nortriptyline) are often used when a central component is suspected.[76] For individuals who have persistent pain despite first-line modalities, secondary therapies may be considered.
Cutaneous nerve blocks may be utilized in individuals with NP. This modality entails injection of a nerve-blocking anesthetic that causes reversible blockade of sodium channels that propagate pain, combined with a long-acting steroid to potentiate the pain-blockade.[105] Demonstrating this use, a case series on 11 subjects with clinically diagnosed NOP were treated with periocular (supraorbital, supratrochlear, infratrochlear, and infraorbital) nerve blocks (4mL of 0.5% bupivacaine + 1mL of 80mg/mL methylprednisolone acetate). In total, 7 subjects experienced pain relief lasting from hours to months, while no change was reported in the remaining 4.[106*] Limitations of this study include its retrospective nature, small study population, and lack of long-term follow up. Common side effects of this therapy include discomfort and tenderness at the injection site and adverse reactions to the steroid (swelling, skin discoloration) or anesthetic (temporary headache, nausea).[105]
Transcutaneous electrical stimulation (TENS) has also been studied in subjects with DED and suspected NP. Purported analgesic mechanisms of TENS include segmental “gate control” and supra-spinal descending modulatory mechanisms.[107] A retrospective study found that an in-office 30-minute TENS session improved ocular pain in 14 individuals with ocular surface pain and suspected NP (e.g. burning, photophobia). Mean pain intensity was reduced 5 minutes post-treatment (0–10 numerical rating scale (NRS): OD: 4.5±3.2 baseline to 1.9±2.5 post-treatment; p=0.01; OS: 4.5±3.4 baseline to 2.0±2.4; p=0.01).[108] TENS can further be integrated as an in-home treatment and used with other topical and systemic therapies. For example, in a retrospective study of 10 subjects who used TENS at home for a period of 6.6±3.6 months, pain scores were significantly improved post-treatment, with a mean overall decrease of 27.4% (0–10 NRS: 7.7 baseline vs 5.6 post-treatment; p=0.02), without any adverse events reported during the study period.[109] However, these studies are limited by their retrospective nature, limited number of patients, and lack of control group.
Botulinum toxin type A (BoNT-A) has also been investigated as a treatment for NP.[110] BoNT-A exerts analgesic effects by inhibiting the release of neuroinflammatory substrates (e.g. calcitonin gene-related peptide) and inhibiting unmyelinated C-fiber nociceptors in the meninges, that normally relay signals of photophobia and dryness.[111] A retrospective study of 76 subjects with migraine (≥15 headache days/month for ≥3 months with ≥8 days/month having migranous features) found that ocular surface pain improved after BoNT-A treatment (100U–150U). At 4 to 6 weeks post-treatment, subjects reported decreased migraine severity and interictal photophobia (0–10 NRS: 4.9±3.0 baseline to 3.4±2.5 post-treatment; p<0.001) as well as decreased light sensitivity (Visual Light Symptom Questionnaire, range 8–40: 29.8±5.1 baseline to 27.7±6.5 post-treatment; p=0.002). In those with DED symptoms (DEQ5≥6), symptoms also decreased (DEQ5: 15.4±2.5 baseline to 13.8±4.0 post-treatment; p=0.03).[112] Similar effects were noted in an case series of 4 subjects with ocular pain and photophobia without migraine, where 35U of BoNT-A were injected in 7 areas of the forehead. At one month, all patients noted decreased light sensitivity, with notable improvements in frequency of light sensitivity to outdoor daylight. DED symptoms also improved with notable improvements in frequency and severity of discomfort and dryness.[113] Finally, one study treated 60 subjects with post-LASIK ATD (symptoms, TBUT<10s, Schirmer<5mm) with BoNT-A (n=20) or preservative-free tear substitutes (n=20). After treatment, DED symptoms and signs were less severe in the BoNT-A vs. control group, including in OSDI (BoNT-A: 12.3±8.6 vs. control: 25.3±11.8; p<0.05), TBUT (BoNT-A: 7.1±1.3s vs. control: 4.9±1.5s; p<0.05), and Schirmer (BoNT-A: 8.4±4.4mm vs control: 4.6±2.1mm; p<0.05). These differences lasted up to 3 months post-treatment.[114] Common side effects of BoNT-A injection include ptosis, strabismus, diplopia, pain, and tearing.[110]
Finally, acupuncture, or the use of fine needles placed at acupoints, has been investigated as an adjunct treatment for DED.[115–119] Acupuncture is hypothesized to provide analgesia by dampening sympathetic responses to pain stimuli and/or stimulating the release of analgesic factors like endorphins.[120] In a randomized control trial, 49 subjects with DED symptoms were grouped into acupuncture (n=24; one 45-minute therapy of 12 placed needles at acupoints LI1 and LI2) or sham groups (n=25; 8 needles on the upper shoulders, which do not serve as acupoints). The acupuncture group demonstrated improvements in symptoms at 1 week, 1 month, 3 months, and 6 months post-treatment via OSDI (34.0±17.0 baseline vs. 19.0±17.0, 21.0±17.0, 20.0±21.0, and 16.0±12.0 respectively; p<0.05 for all), however improvements were also noted in the sham group, but to a smaller degree (36.0±20.0 baseline vs. 24.0±22.0, 24.0±21.0, 21.0±20.0, and 25.0±18.0 respectively; p=0.1).[121] Other studies have shown that the BL1[122], TE23, LI4, and ST1[123], acupoints also provide benefit in subjects with ocular pain. Of note, besides conventional acupuncture, laser acupuncture has also shown benefits in improving ocular pain.[124] While these studies did not specifically examine individuals with suspected NP, the studies suggest that acupuncture may improve symptoms by impacting nerve function.
In conclusion, a number of alternative modalities exist for DED with NP, although the number and strength of the studies is lower than for the other DED subtypes. Most robust are the data regarding the use of BoNT-A.
Conclusion
Many therapeutic options exist for the treatment of DED, targeting different contributors of the disease. Potential therapeutics may target lacrimal gland hypofunction, MGD, inflammatory processes, and/or nerve abnormalities. Given the heterogeneity and complexity of DED, adjuvant and alternative therapies should be considered in individuals who fail first-line therapies. While several therapies have been outlined in this review, continued research is needed to identify more options, test current therapies more robustly with control groups comparisons, and identify which subtypes of DED would best benefit from particular treatments.
Key Points.
Dry eye disease (DED) is a common, multifactorial condition and adequate treatment may require addressing multiple disease contributors.
To accurately identify a patient’s underlying cause(s) of DED, a comprehensive ocular examination with measurement of dry eye symptoms and signs is necessary.
Patients who fail conventional treatments may consider alternative or adjunct therapies including, but not limited to, neuromodulation, naturally-derived products, novel technology and devices, and acupuncture.
Continued research in this field will increase the number of treatment options available to those with DED refractory to first line treatments, potentially improving the quality of life of thousands.
Funding sources:
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr. Galor) and Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20–1–0579 (Dr. Galor) and Vision Research Program (VRP) W81XWH-20–1–0820 (Dr. Galor), National Eye Institute R01EY026174 (Dr. Galor) and R61EY032468 (Dr. Galor), NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant (institutional).
Footnotes
Conflicts: None
References
- 1.Craig JP, et al. , TFOS DEWS II Definition and Classification Report. Ocul Surf, 2017. 15(3): p. 276–283. [DOI] [PubMed] [Google Scholar]
- 2.Pouyeh B, et al. , Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am J Ophthalmol, 2012. 153(6): p. 1061–66 e3. [DOI] [PubMed] [Google Scholar]
- 3.Mandell JT, et al. , Impact of Air Pollution and Weather on Dry Eye. J Clin Med, 2020. 9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stapleton F, et al. , TFOS DEWS II Epidemiology Report. Ocul Surf, 2017. 15(3): p. 334–365. [DOI] [PubMed] [Google Scholar]
- 5.Bron AJ, et al. , Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf, 2009. 7(2): p. 78–92. [DOI] [PubMed] [Google Scholar]
- 6.Willcox MDP, et al. , TFOS DEWS II Tear Film Report. Ocul Surf, 2017. 15(3): p. 366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong ES, et al. , Epidemiology of discordance between symptoms and signs of dry eye. Br J Ophthalmol, 2018. 102(5): p. 674–679. [DOI] [PubMed] [Google Scholar]
- *8.Galor A, et al. , Corneal Nerve Pathway Function in Individuals with Dry Eye Symptoms. Ophthalmology, 2020.This study describes corneal nerve abnormalities as causes for discordant symptoms and signs seen in dry eye disease.
- 9.Chalmers RL, Begley CG, and Caffery B, Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye, 2010. 33(2): p. 55–60. [DOI] [PubMed] [Google Scholar]
- 10.Schiffman RM, et al. , Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol, 2000. 118(5): p. 615–21. [DOI] [PubMed] [Google Scholar]
- 11.Wolffsohn JS, et al. , TFOS DEWS II Diagnostic Methodology report. Ocul Surf, 2017. 15(3): p. 539–574. [DOI] [PubMed] [Google Scholar]
- 12.Begley C, et al. , Review and analysis of grading scales for ocular surface staining. Ocul Surf, 2019. 17(2): p. 208–220. [DOI] [PubMed] [Google Scholar]
- 13.Jones L, et al. , TFOS DEWS II Management and Therapy Report. Ocul Surf, 2017. 15(3): p. 575–628. [DOI] [PubMed] [Google Scholar]
- *14.Sabeti S, et al. , Management of meibomian gland dysfunction: a review. Surv Ophthalmol, 2020. 65(2): p. 205–217.This comprehensive review provides an overview of causes and treatments for meibomian gland dysfunction, a major contributor to dry eye disease.
- 15.Messmer EM, The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int, 2015. 112(5): p. 71–81; quiz 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakita T, Regeneration of Lacrimal Gland Function to Maintain the Health of the Ocular Surface. Invest Ophthalmol Vis Sci, 2018. 59(14): p. Des169–des173. [DOI] [PubMed] [Google Scholar]
- 17.Ginn C, Patel B, and Walker R, Existing and emerging applications for the neuromodulation of nerve activity through targeted delivery of electric stimuli. Int J Neurosci, 2019. 129(10): p. 1013–1023. [DOI] [PubMed] [Google Scholar]
- *18.Dieckmann G, Fregni F, and Hamrah P, Neurostimulation in dry eye disease-past, present, and future. Ocul Surf, 2019. 17(1): p. 20–27.Neuromodulation has been used to treat a host of diseases but has recently been shown to treat symptoms and signs of dry eye disease, as evidenced by this novel review.
- 19.Dieckmann G, Goyal S, and Hamrah P, Neuropathic Corneal Pain: Approaches for Management. Ophthalmology, 2017. 124(11s): p. S34–s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Sheppard JD, et al. , Characterization of tear production in subjects with dry eye disease during intranasal tear neurostimulation: Results from two pivotal clinical trials. Ocul Surf, 2019. 17(1): p. 142–150.This study describes two pivotal trials which led to the use of the novel intranasal neurostimulation device, TrueTear, to treat aqueous deficient dry eye disease. This device paved the way for the creation of similar products which have shown great promise in the treatment of this disease.
- *21.Cohn GS, et al. , Randomized, Controlled, Double-Masked, Multicenter, Pilot Study Evaluating Safety and Efficacy of Intranasal Neurostimulation for Dry Eye Disease. Invest Ophthalmol Vis Sci, 2019. 60(1): p. 147–153.This study further proved the efficacy of intranasal neurostimulation devices in the management of dry eye disease caused by lacrimal gland hypofunction.
- *22.Pondelis N, et al. , Infrared meibography allows detection of dimensional changes in meibomian glands following intranasal neurostimulation. Ocul Surf, 2020. 18(3): p. 511–516.This article highlights the novel use of intranasal neurostimulation devices to treat dry eye disease caused by meibomian gland dysfunction and explains how infrared meibography can quantify this effect.
- **23.Ji MH, et al. , Novel Extranasal Tear Stimulation: Pivotal Study Results. Transl Vis Sci Technol, 2020. 9(12): p. 23.This study introduced a new extranasal neurostimulation device, iTear, as a treatment for aqueous deficient dry eye disease. The development of the iTear was especially significant, given the discontinuation of the longstanding TrueTear.
- **24.Pharma OP, Oyster Point MYSTIC Phase 2 Topline Data Call. 2020.The development of Oyster Point’s OC-01 nicotinic acetylcholine receptor agonist eye drops as a menthod of neurostimulation treatment of aqueous deficient dry eye disease is a groundbreaking development as prior such treatments have mainly been provided by physical devices.
- **25.Bar A, et al. , Safety and Effectiveness of Ocular Magnetic Neurostimulation Treatment on Signs and Symptoms in subjects with Moderate to Severe Dry Eye Disease. Investigative Ophthalmology & Visual Science, 2020. 61(7): p. 885–885.As transcranial magnetic stimulation has classically been used in the treatment of depression and pain, the development of EpiTech’s VivEye has introduced the use of this technology in treating dry eye disease as well.
- 26.Miana VV and González EAP, Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience, 2018. 12: p. 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villatoro AJ, et al. , Use of Adipose-Derived Mesenchymal Stem Cells in Keratoconjunctivitis Sicca in a Canine Model. BioMed Research International, 2015. 2015: p. 527926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turpie B, et al. , Sjögren’s syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol, 2009. 175(3): p. 1136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gromova A, et al. , Lacrimal Gland Repair Using Progenitor Cells. Stem Cells Transl Med, 2017. 6(1): p. 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Møller-Hansen M, et al. , Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul Surf, 2021. 19: p. 43–52. [DOI] [PubMed] [Google Scholar]
- 31.Hu H, et al. , In-depth Proteome of the Hypopharyngeal Glands of Honeybee Workers Reveals Highly Activated Protein and Energy Metabolism in Priming the Secretion of Royal Jelly. Mol Cell Proteomics, 2019. 18(4): p. 606–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsushita H, et al. , Effects of royal jelly on bone metabolism in postmenopausal women: a randomized, controlled study. Climacteric, 2020: p. 1–7. [DOI] [PubMed]
- 33.Ali AM and Kunugi H, Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen. Foods, 2020. 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad S, et al. , New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int J Mol Sci, 2020. 21(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imada T, et al. , Oral Administration of Royal Jelly Restores Tear Secretion Capacity in Rat Blink-Suppressed Dry Eye Model by Modulating Lacrimal Gland Function. PLOS ONE, 2014. 9(9): p. e106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue S, et al. , Clinical Evaluation of a Royal Jelly Supplementation for the Restoration of Dry Eye: A Prospective Randomized Double Blind Placebo Controlled Study and an Experimental Mouse Model. PLoS One, 2017. 12(1): p. e0169069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chhadva P, Goldhardt R, and Galor A, Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology, 2017. 124(11S): p. S20–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borchman D, The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf, 2019. 17(2): p. 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang S-P, et al. , Efficacy of Vectored Thermal Pulsation and Warm Compress Treatments in Meibomian Gland Dysfunction: A Meta-Analysis of Randomized Controlled Trials. Cornea, 2019. 38(6): p. 690–697. [DOI] [PubMed] [Google Scholar]
- 40.Li B, et al. , Comparison of the therapeutic effect of Meibomian Thermal Pulsation LipiFlow® on obstructive and hyposecretory meibomian gland dysfunction patients. Int Ophthalmol, 2020. 40(12): p. 3469–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, et al. , Clinical Trial of Thermal Pulsation (LipiFlow) in Meibomian Gland Dysfunction With Preteatment Meibography. Eye Contact Lens, 2016. 42(6): p. 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blackie CA, Coleman CA, and Holland EJ, The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol, 2016. 10: p. 1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meibomian Gland EvaluatorTM. 2021. [cited 2021 February 22]; Available from: https://www.jnjvisionpro.com/products/eye-medical-devices/meibomian-gland-eye-exam-tool.
- 44.Badawi D, A novel system, TearCare(®), for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol, 2018. 12: p. 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Tauber J, et al. , Comparison of the iLUX and the LipiFlow for the Treatment of Meibomian Gland Dysfunction and Symptoms: A Randomized Clinical Trial. Clin Ophthalmol, 2020. 14: p. 405–418.This article describes the iLux, an alternative device for treating dry eye disease caused by meibomian gland dysfunction through eyelid heating. It is proven here to be as effective as the longstanding LipiFlow in softening meibum and improving symptoms and signs of dry eye disease.
- 46.Papageorgiou P, et al. , Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol, 2008. 159(3): p. 628–32. [DOI] [PubMed] [Google Scholar]
- 47.Toyos R, McGill W, and Briscoe D, Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg, 2015. 33(1): p. 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo KY, et al. , Long-term effects of intense pulsed light treatment on the ocular surface in patients with rosacea-associated meibomian gland dysfunction. Cont Lens Anterior Eye, 2018. 41(5): p. 430–435. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, et al. , Comparative evaluation in Intense Pulsed Light therapy combined with or without meibomian gland expression for the treatment of meibomian gland dysfunction. Curr Eye Res, 2020. [DOI] [PubMed]
- 50.Arita R, et al. , Multicenter Study of Intense Pulsed Light for Patients with Refractory Aqueous-Deficient Dry Eye Accompanied by Mild Meibomian Gland Dysfunction. J Clin Med, 2020. 9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albietz JM and Schmid KL, Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin Exp Optom, 2018. 101(1): p. 23–33. [DOI] [PubMed] [Google Scholar]
- 52.Giannaccare G, et al. , Intense Pulsed Light Therapy In The Treatment Of Meibomian Gland Dysfunction: Current Perspectives. Clin Optom (Auckl), 2019. 11: p. 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanza NL, et al. , Dry Eye Profiles in Patients with a Positive Elevated Surface Matrix Metalloproteinase 9 Point-of-Care Test Versus Negative Patients. Ocul Surf, 2016. 14(2): p. 216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu R, et al. , Analysis of Cytokine Levels in Tears and Clinical Correlations After Intense Pulsed Light Treating Meibomian Gland Dysfunction. Am J Ophthalmol, 2017. 183: p. 81–90. [DOI] [PubMed] [Google Scholar]
- 55.Arita R and Fukuoka S, Efficacy of Azithromycin Eyedrops for Individuals With Meibomian Gland Dysfunction-Associated Posterior Blepharitis. Eye Contact Lens, 2021. 47(1): p. 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Benedetti G and Vaiano AS, Oral azithromycin and oral doxycycline for the treatment of Meibomian gland dysfunction: A 9-month comparative case series. Indian J Ophthalmol, 2019. 67(4): p. 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molan PC, Why honey is effective as a medicine. 1. Its use in modern medicine. Bee World, 1999. 80(2): p. 80–92. [Google Scholar]
- 58.Nichols KK, et al. , The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci, 2011. 52(4): p. 1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albietz JM and Schmid KL, Randomised controlled trial of topical antibacterial Manuka (Leptospermum species) honey for evaporative dry eye due to meibomian gland dysfunction. Clin Exp Optom, 2017. 100(6): p. 603–615. [DOI] [PubMed] [Google Scholar]
- *60.Li AL, et al. , Randomised assessor-masked trial evaluating topical manuka honey (Optimel) in treatment of meibomian gland dysfunction. Br J Ophthalmol, 2021.This study introduces the use of novel manuka honey eye drops and eye gel as alternative treatments for meibomian gland dysfunction.
- 61.Lanza NL, et al. , The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul Surf, 2016. 14(2): p. 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hessen M and Akpek EK, Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res, 2014. 9(2): p. 240–50. [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Z, et al. , Trehalose Induces Autophagy Against Inflammation by Activating TFEB Signaling Pathway in Human Corneal Epithelial Cells Exposed to Hyperosmotic Stress. Invest Ophthalmol Vis Sci, 2020. 61(10): p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fariselli C, et al. , Trehalose/hyaluronate eyedrop effects on ocular surface inflammatory markers and mucin expression in dry eye patients. Clinical ophthalmology (Auckland, N.Z.), 2018. 12: p. 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong SC, et al. , In Vivo Anti-Inflammation Potential of Aster koraiensis Extract for Dry Eye Syndrome by the Protection of Ocular Surface. Nutrients, 2020. 12(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park B, et al. , Polydatin Inhibits NLRP3 Inflammasome in Dry Eye Disease by Attenuating Oxidative Stress and Inhibiting the NF-κB Pathway. Nutrients, 2019. 11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L, et al. , Effects of Eurya japonica extracts on human corneal epithelial cells and experimental dry eye. Exp Ther Med, 2020. 20(2): p. 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seen S and Tong L, Dry eye disease and oxidative stress. Acta Ophthalmol, 2018. 96(4): p. e412–e420. [DOI] [PubMed] [Google Scholar]
- 69.Hu L, et al. , Preparation and characterization of a pterostilbene-peptide prodrug nanomedicine for the management of dry eye. Int J Pharm, 2020. 588: p. 119683. [DOI] [PubMed] [Google Scholar]
- 70.Kim H, et al. , Alpha-Lipoic Acid Ameliorates Radiation-Induced Lacrimal Gland Injury through NFAT5-Dependent Signaling. Int J Mol Sci, 2019. 20(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Higuchi A, Development of New Pharmaceutical Candidates With Antioxidant Activity for the Treatment of Corneal Disorders. Cornea, 2019. 38 Suppl 1: p. S45–s49. [DOI] [PubMed] [Google Scholar]
- 72.Yang J, et al. , A new approach of ocular nebulization with vitamin B12 versus oxytocin for the treatment of dry eye disease: an in vivo confocal microscopy study. Drug Des Devel Ther, 2019. 13: p. 2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimokawa T, et al. , Efficacy of high-affinity liposomal astaxanthin on up-regulation of age-related markers induced by oxidative stress in human corneal epithelial cells. J Clin Biochem Nutr, 2019. 64(1): p. 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang SW, et al. , A standardized extract of Rhynchosia volubilis Lour. exerts a protective effect on benzalkonium chloride-induced mouse dry eye model. J Ethnopharmacol, 2018. 215: p. 91–100. [DOI] [PubMed] [Google Scholar]
- 75.Patel S, et al. , Corneal Nerve Abnormalities in Ocular and Systemic Diseases. Exp Eye Res, 2021. 202: p. 108284. [DOI] [PubMed] [Google Scholar]
- 76.Patel S, et al. , Corneal Nerve Abnormalities in Ocular and Systemic Diseases. Experimental Eye Research, 2020: p. 108284. [DOI] [PubMed]
- 77.Sacchetti M and Lambiase A, Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol, 2014. 8: p. 571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaheen BS, Bakir M, and Jain S, Corneal nerves in health and disease. Survey of Ophthalmology, 2014. 59(3): p. 263–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonini S, et al. , Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology, 2018. 125(9): p. 1332–1343. [DOI] [PubMed] [Google Scholar]
- 80.Pflugfelder SC, et al. , Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology, 2020. 127(1): p. 14–26.This study introduces a novel agent, Cenegermin, to treat neurotrophic keratopathy by delivering recombinant human nerve growth factor topically to the ocular surface.
- 81.Kim JS, Rafailov L, and Leyngold IM, Corneal Neurotization for Postherpetic Neurotrophic Keratopathy: Initial Experience and Clinical Outcomes. Ophthalmic Plast Reconstr Surg, 2021. 37(1): p. 42–50. [DOI] [PubMed] [Google Scholar]
- 82.Bonini S, et al. , Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology, 2000. 107(7): p. 1347–1351. [DOI] [PubMed] [Google Scholar]
- 83.Sacchetti M, et al. , Effect of recombinant human nerve growth factor eye drops in patients with dry eye: a phase IIa, open label, multiple-dose study. British Journal of Ophthalmology, 2020. 104(1): p. 127.This trial described the clinical use of recombinant human nerve growth factor eye drops in treating moderate to severe dry eye disease.
- 84.Sheha H, et al. , Update On Cenegermin Eye Drops In The Treatment Of Neurotrophic Keratitis. Clinical ophthalmology (Auckland, N.Z.), 2019. 13: p. 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valencia Castillo SL, et al. , Autologous serum eye drops improve tear production, both lachrymal flow and stability tests and conjunctival impression cytology with transfer in dry eye disease. Blood Transfus, 2021. 19(1): p. 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erikitola OO, et al. , Fingerprick Autologous Blood in the Treatment of Severe Dry Eyes and Ocular Surface Disease. Cornea, 2020. Publish Ahead of Print. [DOI] [PubMed]
- 87.Balal S, et al. , Finger-Prick Autologous Blood in the Treatment of Persistent Corneal Epithelial Defects. Cornea, 2020. 39(5): p. 594–597. [DOI] [PubMed] [Google Scholar]
- 88.Hartwig D, et al. , Epitheliotrophic capacity of a growth factor preparation produced from platelet concentrates on corneal epithelial cells: a potential agent for the treatment of ocular surface defects? Transfusion, 2004. 44(12): p. 1724–1731. [DOI] [PubMed] [Google Scholar]
- *89.You J, et al. , Human Platelets and Derived Products in Treating Ocular Surface Diseases - A Systematic Review. Clin Ophthalmol, 2020. 14: p. 3195–3210.This study highlights the importance of blood-derived products in treating ocular surface diseases, including dry eye disease, by delivering a mixture of growth factors.
- **90.Avila MY, Igua AM, and Mora AM, Randomised, prospective clinical trial of platelet-rich plasma injection in the management of severe dry eye. British Journal of Ophthalmology, 2019. 103(5): p. 648–653.This trial introduces the use of platelet-rich plasma, a compound used to treat other conditions, in dry eye disease, opening an avenue for further research into this alternative treatment method.
- 91.Zallio F, et al. , A Single-Center Pilot Prospective Study of Topical Application of Platelet-Derived Eye Drops for Patients with Ocular Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant, 2016. 22(9): p. 1664–1670. [DOI] [PubMed] [Google Scholar]
- 92.Javaloy J, et al. , Effect of platelet-rich plasma in nerve regeneration after LASIK. 2013. 29(3): p. 213. [DOI] [PubMed] [Google Scholar]
- 93.Kim KM, Shin Y-T, and Kim HK, Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Japanese Journal of Ophthalmology, 2012. 56(6): p. 544–550. [DOI] [PubMed] [Google Scholar]
- 94.García-Conca V, et al. , Efficacy and safety of treatment of hyposecretory dry eye with platelet-rich plasma. Acta Ophthalmologica, 2019. 97(2): p. e170–e178. [DOI] [PubMed] [Google Scholar]
- 95.Kim JS, Rafailov L, and Leyngold IM, Corneal Neurotization for Postherpetic Neurotrophic Keratopathy: Initial Experience and Clinical Outcomes. Ophthalmic Plastic & Reconstructive Surgery, 9000. Publish Ahead of Print. [DOI] [PubMed]
- 96.Terzis JK, Dryer MM, and Bodner BI, Corneal Neurotization: A Novel Solution to Neurotrophic Keratopathy. Plastic and Reconstructive Surgery, 2009. 123(1). [DOI] [PubMed] [Google Scholar]
- 97.Cortina M, et al. , Recovery of Corneal Sensitivity, Calcitonin Gene-Related Peptide-Positive Nerves, and Increased Wound Healing Induced by Pigment Epithelial-Derived Factor Plus Docosahexaenoic Acid After Experimental Surgery. Archives of ophthalmology, 2011. 130: p. 76–83. [DOI] [PubMed] [Google Scholar]
- 98.Giannaccare G, et al. , In Vivo and Ex Vivo Comprehensive Evaluation of Corneal Reinnervation in Eyes Neurotized With Contralateral Supratrochlear and Supraorbital Nerves. Cornea, 2020. 39(2). [DOI] [PubMed] [Google Scholar]
- 99.Pham TL and Bazan HEP, Docosanoid signaling modulates corneal nerve regeneration: effect on tear secretion, wound healing, and neuropathic pain. J Lipid Res, 2020. [DOI] [PMC free article] [PubMed]
- 100.He J, et al. , The PEDF Neuroprotective Domain Plus DHA Induces Corneal Nerve Regeneration After Experimental Surgery. Investigative Ophthalmology & Visual Science, 2015. 56(6): p. 3505–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Georgakopoulos CD, et al. , Effect of Omega-3 Fatty Acids Dietary Supplementation on Ocular Surface and Tear Film in Diabetic Patients with Dry Eye. Journal of the American College of Nutrition, 2017. 36(1): p. 38–43. [DOI] [PubMed] [Google Scholar]
- 102.Dry Eye A, et al. , n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N Engl J Med, 2018. 378(18): p. 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.IASP Terminology. 2020; Available from: https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698.
- 104.Kalangara JP, et al. , Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens, 2017. 43(3): p. 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scholz A, et al. , Complex Blockade of TTX-Resistant Na+ Currents by Lidocaine and Bupivacaine Reduce Firing Frequency in DRG Neurons. Journal of Neurophysiology, 1998. 79(4): p. 1746–1754. [DOI] [PubMed] [Google Scholar]
- *106.Small LR, et al. , Oral Gabapentinoids and Nerve Blocks for the Treatment of Chronic Ocular Pain. Eye & Contact Lens, 2020. 46(3).This study explains the use of gabapentin as a treatment of ocular pain, providing another method of pain control for patients with ocular pain.
- 107.Vance CG, et al. , Using TENS for pain control: the state of the evidence. Pain Manag, 2014. 4(3): p. 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sivanesan E, et al. , Noninvasive Electrical Stimulation for the Treatment of Chronic Ocular Pain and Photophobia. Neuromodulation: Technology at the Neural Interface, 2018. 21(8): p. 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zayan K, et al. , Transcutaneous Electrical Nerve Stimulation for the Long-Term Treatment of Ocular Pain. Neuromodulation, 2020. 23(6): p. 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Venkateswaran N, et al. , Onabotulinum toxin A improves photophobia and sensations of dryness independent of ocular surface parameters. Investigative Ophthalmology & Visual Science, 2019. 60(9): p. 6757–6757. [Google Scholar]
- 111.Diel RJ, et al. , Botulinum Toxin A for the Treatment of Photophobia and Dry Eye. Ophthalmology, 2018. 125(1): p. 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Diel RJ, et al. , Photophobia and sensations of dryness in patients with migraine occur independent of baseline tear volume and improve following botulinum toxin A injections. British Journal of Ophthalmology, 2019. 103(8): p. 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Venkateswaran N, et al. , Periorbital botulinum toxin A improves photophobia and sensations of dryness in patients without migraine: Case series of four patients. American Journal of Ophthalmology Case Reports, 2020. 19: p. 100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fouda SM and Mattout HK, Comparison Between Botulinum Toxin A Injection and Lacrimal Punctal Plugs for the Control of Post-LASIK Dry Eye Manifestations: A Prospective Study. Ophthalmology and Therapy, 2017. 6(1): p. 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Na J-H, et al. , Therapeutic effects of acupuncture in typical dry eye: a systematic review and meta-analysis. Acta Ophthalmologica. n/a(n/a). [DOI] [PubMed] [Google Scholar]
- 116.Kim BH, et al. , Optimizing acupuncture treatment for dry eye syndrome: a systematic review. BMC Complement Altern Med, 2018. 18(1): p. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu D, Gao C, and Zhong YM, [Clinical observation of dry eye syndrome treated with acupuncture]. Zhongguo Zhen Jiu, 2019. 39(8): p. 837–40. [DOI] [PubMed] [Google Scholar]
- 118.Cheng J, et al. , [Clinical observation of eye acupuncture combined with conventional acupuncture on dry eye syndrome with yin deficiency of liver and kidney]. Zhongguo Zhen Jiu, 2019. 39(9): p. 945–9. [DOI] [PubMed] [Google Scholar]
- 119.Xie W, et al. , [Guiding-qi acupuncture for dry eye syndrome]. Zhongguo Zhen Jiu, 2018. 38(2): p. 153–8. [DOI] [PubMed] [Google Scholar]
- 120.Bäcker M, et al. , Acupuncture in migraine: investigation of autonomic effects. Clin J Pain, 2008. 24(2): p. 106–15. [DOI] [PubMed] [Google Scholar]
- 121.Dhaliwal DK, et al. , Acupuncture and dry eye: current perspectives. A double-blinded randomized controlled trial and review of the literature. Clin Ophthalmol, 2019. 13: p. 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang X, et al. , Efficacy and safety of acupuncture at a single BL1 acupoint in the treatment of moderate to severe dry eye disease: Protocol for a randomized, controlled trial. Medicine, 2018. 97(22): p. e10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin YH, et al. , An Association Rule Analysis of Combined Acupoints for the Treatment of Patients with Dry Eye Disease. Complement Med Res, 2020: p. 1–8. [DOI] [PubMed]
- 124.Hu WL, et al. , Effect of laser acupuncture on dry eye: A study protocol for a 2-center randomized controlled trial. Medicine (Baltimore), 2018. 97(22): p. e10875. [DOI] [PMC free article] [PubMed] [Google Scholar]


