Abstract
On recreational sandy beaches, there are guidelines for the management of bacterial pollution in coastal waters regarding untreated sewage, urban wastewater, and industrial wastewater. However, terrestrial plant debris on coastal beaches can be abundant especially after floods and whilst it has rarely been considered a concern, the bacterial population associated with this type of pollution from the viewpoint of public health has not been adequately assessed. In this study, microbes associated with plant debris drifting onto Kizaki Beach in Japan were monitored for 8 months throughout the rainy season, summer, typhoon season, and winter. Here we show that faecal-indicator bacteria in the plant debris and sand under the debris were significantly higher than the number of faecal bacteria in the sand after a 2015 typhoon. When we focused on specific pathogenic bacteria, Brevundimonas vesicularis and Pseudomonas alcaligenes were commonly detected only in the plant debris and sand under the debris during the survey period. The prompt removal of plant debris would therefore help create safer beaches.
Subject terms: Microbiology, Environmental sciences
Introduction
Beaches are valuable recreational areas. They are also a tourism resource and the areas surrounding the locations of beaches are economically important. In order to promote the sustainable development of beaches and surrounding areas, an international system, “The Blue Flag Programme (BF)”, has been established by the Foundation for Environmental Education1, and the certification of beaches is rapidly spreading to various countries around the world. The BF consists of four categories (water quality, environmental management, environmental education, safety), of which water quality is an important category and requires the monitoring of coastal water quality. In particular, the number of fecal indicator bacteria in coastal waters due to the inflow of industrial wastewater and sewage is very important, and should be closely monitored when flooding occurs during torrential rain events. In contrast, standards have not yet been set for the quality of the beach sand that users come into direct contact with since information on the effects of bacterial contamination in beach sand is also important.
An enormous amount of terrestrial plant debris accumulates during heavy rainfall events such as mature cyclones (i.e., typhoons) and washes ashore on a coastal sand beach in the Pacific Ocean Hyuganada, which is directly affected by flooding from river, used for various recreational activities such as sea bathing, surfing, and sightseeing (Fig. 1a). Herein, we focused on the terrestrial plant debris that flowed outward from the land and accumulated on a coastal beach. Since plants are natural in origin the general public does not perceive any threat. Consequently, information on coastal plant debris is extremely scarce compared to marine plastic debris. Since plant debris has high water retention and a large specific surface area, it is very important from the viewpoint of a microbial habitat and may be a great concern with regard to public health, especially at recreational beaches. In addition to the inflow of treated sewage and wastewater onto the beach due to flooding, the drifting and accumulation of plant debris also causes bacterial contamination in beach sand and increases the health risk for beach users. Intervention by managers and care by users are required for sound management and usage based on hazards and risks as related to sandy beaches2. Beach management should also consider the threat to users due to sand contamination by bacteria.
Figure 1.
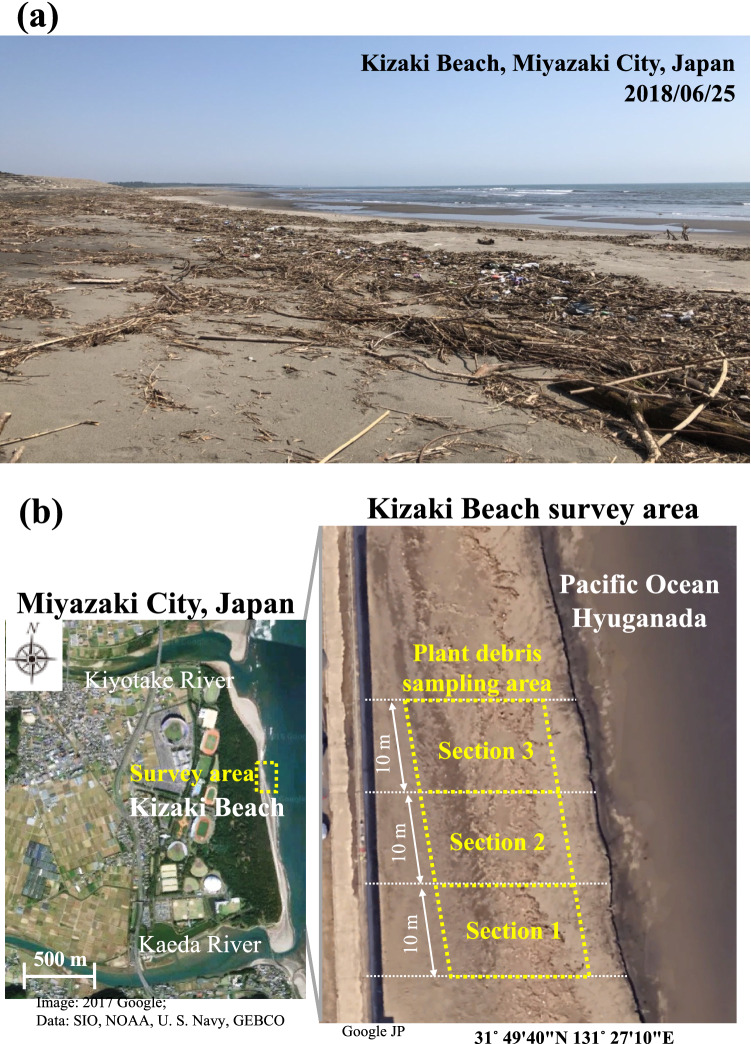
(a) Plant debris on Kizaki Beach on June 25, 2018. (b) Locations of sampling points on Kizaki beach, Miyazaki City, Japan. Most of the plant debris collected in this survey which accumulated near the high-water line of Kizaki Beach were branches and withered grass. All the plant debris was collected from three consecutive sections (Sections 1–3) along the high-water line at 10 m intervals (b) obtained from Google Earth (https://earth.google.com/web/@31.82602436,131.4436947,5.75042276a,5202.77770972d,35y,0h,0t,0r: https://earth.google.com/web/@31.82805704,131.45286349,1.42880734a,100.50282223d,35y,0h,0t,0r) and explanation and illustration were drawn in Microsoft Power Point (Version 16.16.27).
Bacterial contamination of sandy beaches, although not well known, is extremely important from a public health perspective. Sand pollution is recognised as a significant risk to public health3–10. Faecal bacteria can accumulate and survive for long periods of time in sand and their concentrations can be 10 to 100 times greater than those in beach water11–15. In addition, after a typhoon event, heterotrophic bacteria, coliform bacteria, Escherichia coli, and enterococci can be 10–103 times higher than what is normally detected in beach sand outside the water line15. The risk of beach users contracting an infectious disease can be strongly associated with the presence of pathogenic bacteria in the sand11,16–21. In addition to the inflow of sewage or drainage, plant debris is another likely source of bacterial contamination on beaches. For instance, the same genotype of Enterococci identified in river water has been detected in beach seawater after flooding22. Therefore, it is possible that terrestrial plant debris are exposed to treated sewage and/or drainage that flows outward at the time of flooding, and that fecal bacteria remain attached to drifting plant debris and accumulate on the beach. However, no study to date has investigated bacterial contamination associated with terrestrial plant debris drifting onto beaches based on continuous monitoring and bacterial florae analysis.
As a model area for this study, we evaluated Kizaki Beach, which faces the Hyuganada Sea in the Pacific Ocean and borders the Kyushu District in Japan, and is an important recreational base where the 2019 International Surfing Association World Surfing Games were held. Kizaki Beach is primarily intended for recreation by beach users and is not a major area for functioning conservation of natural beach ecosystems. The amount of plant debris drifting onto the beach and the number of faecal-indicator bacteria in the plant debris and the sand below the plant debris were monitored. Detection of pathogenic bacteria was attempted for each sample using bacterial florae analysis targeting the 16S rRNA gene. Additionally, laboratory experiments examined the survival of bacteria due to drought stress of plant debris and regrowth after the addition of water to simulate the conditions of dry weather and rainfall.
Results
Amount of plant debris on Kizaki Beach
The locations and weight of plant debris in three 10 m sections of beach front in relation to the high-water line measured monthly are shown in Figs. 1b and 2. The amount of plant debris varied depending on the survey date. On June 25, 2018, the debris weight was 10 times larger (average 132.4 kg/10 m) than that on May 24. Flooding triggered by consecutive heavy rainfalls for three days (92.5 mm/day) presumably transported a large amount of plant debris from the watersheds of the Kiyotake and Kaeda Rivers before the survey in June. The debris weight on October 10 was 40 times larger (204.6 kg/10 m) than that on September 26 (5.3 kg/10 m). During this period, a September 28 typhoon caused severe flooding with localised heavy rain beginning October 4 (precipitation, 92.5 mm/day, rainfall intensity 34.0 mm/h). The amount of plant debris that drifted onto Kizaki Beach varied from 5 to 200 kg over 10 m up to the high-water line due to flooding caused by the rainfall. Regardless of the survey month, the major plants composing the debris were Phragmites spp., Phyllostachys spp., and Ambrosia spp., which are common to the floodplains of Japan. Kizaki beach is a straight, shallow, sandy beach, and debris of coastal plant products such as seaweed or seagrass are rarely washed ashore. Seaweed and seagrass were not present in the samples of plant debris in this survey.
Figure 2.
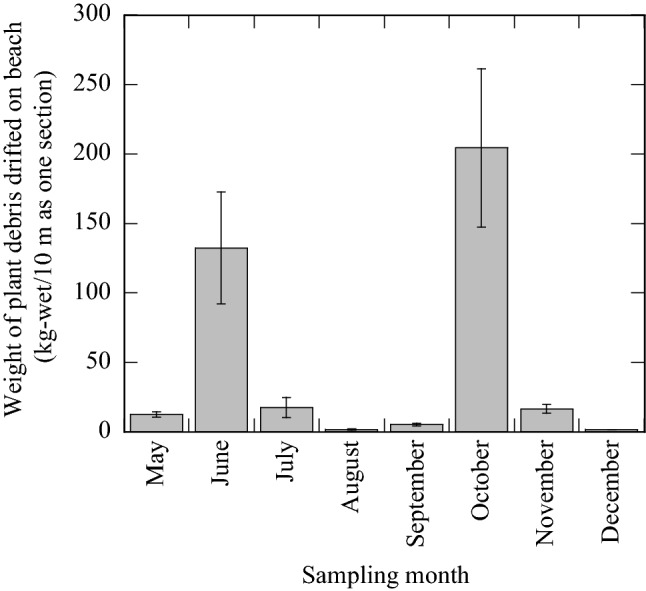
The monthly weight of plant debris accumulated on sections of Kizaki beach. Data shows the mean of three consecutive sections and error bars represent standard deviations (SD).
The changes in water content and temperature of the plant debris and sand under the debris during the survey period are shown in Supplementary Figure S1. The water content of the debris varied greatly (range 8.2–31.3%). It was lowest in midsummer (August) when there was no rainfall. The water content of the sand just under the debris was stable and was within the range of 3.2–6.3% (average 4.6 ± 1.3%). The temperature of the sand was also stable at 36 °C in the summer season (July to September). It is assumed that the water content and temperature of the sand was stable because it was covered with debris.
Bacterial pollution of the beach sand
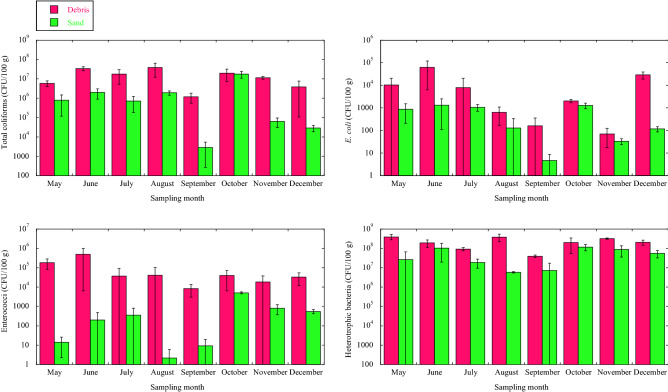
The changes in the numbers of total coliforms, E. coli, enterococci, and heterotrophic bacteria in the plant debris and the sand under the debris of the beach during the entire survey period are shown in Fig. 3. During the survey period, no association was found between the weight of plant debris which had drifted onto the beach and the numbers of bacteria. In all the bacterial parameters, the bacterial counts associated with the plant debris were the highest, followed by the sand under the debris. The high number of total coliforms were detected continuously in the plant debris, ranging from 1.2 × 105 to 3.8 × 107 CFU/100 g (average: 1.7 × 107 CFU/100 g). In the sand under the plant debris, the count of total coliforms was large (range 2.8 × 103–1.7 × 107 CFU/100 g (average: 2.9 × 106 CFU/100 g)). E. coli, as a noticeable parameter, was detected in high concentrations in the plant debris (1.0 × 104 CFU/100 g on May 24, 6.3 × 104 CFU/100 g on June 25). Although the total number of coliforms and E. coli in the sand under the plant debris was lower than that in the debris, the change in counts tended to follow the amount of plant debris. In contrast, the change of the number of enterococci in the debris was different from the sand. The enterococci count in the plant debris was markedly high on May 24, June 25, August 30, and September 26 (ranging from 8.3 × 103 to 5.0 × 105 CFU/100 g); more than 103 times greater than in the sand under the debris. On the other hand, the number of heterotrophic bacteria in the debris and sand was high and fluctuated to a lesser extent ranging from 5.2 × 107 in the sand to 2.3 × 108 CFU/100 g in the debris on average.
Figure 3.
The changes in the numbers of total coliforms, E. coli, enterococci, and heterotrophic bacteria in the plant debris and the sand under the debris, during the entire survey period. Data are expressed as means ± standard deviations. ND, none detected.
Comparison of the bacterial Genera between plant debris and sand
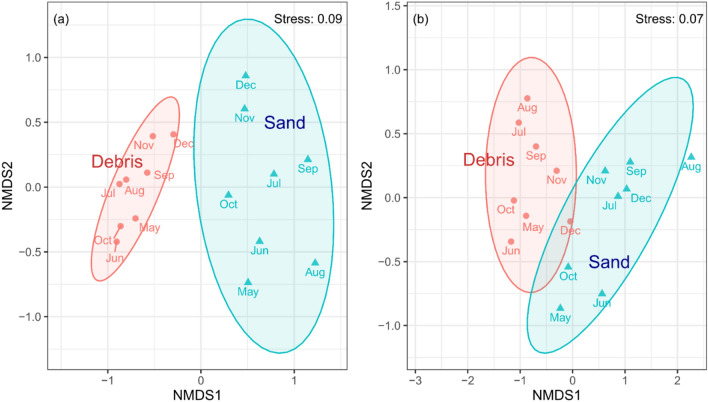
The samples of plant debris and sand under the debris averaged 23,834 ± 4166, (24 samples: 3 sections and 8 months) and 22,120 ± 4229 (24 samples: 3 sections and 8 months) operational taxonomic units (OTUs), respectively, according to next-generation sequencing results. We identified 1249, and 1989 bacterial Genera in the samples of plant debris and sand under the debris, respectively. Based on the OTU results of non-metric multi-dimensional scaling (NMDS), the bacterial communities were clearly segregated by plant debris and sand under the debris (Fig. 4). Figure 4a depicts the relative abundances of all bacterial Genera, and Fig. 4b includes only pathogenic bacterial Genera. In each sample, the total bacterial flora showed independent construction (Fig. 4a).
Figure 4.
Non-metric multidimensional scaling with Bray–Curtis distance based on relative abundances of all bacterial Genera: (a) and 84 pathogenic bacterial Genera; (b) in debris (circle) and sand under the debris (triangle) from May to December. Ellipses surrounding each environmental group indicate 90% confidence levels.
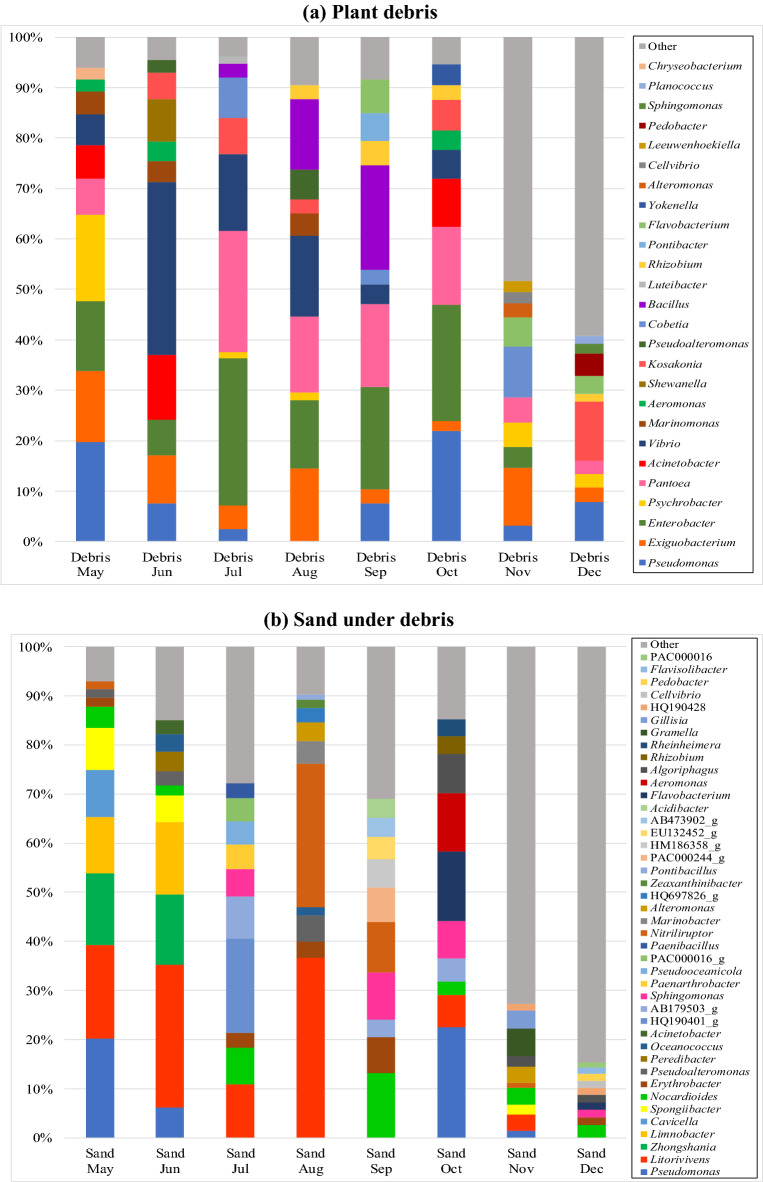
The distributions of the 10 most abundant Genera in these samples are shown in Fig. 5a. The Genera displayed variety and changed each month due to changes in environmental conditions such as water content and temperature. The major Genera detected in the plant debris throughout the survey period were: Enterobacter (4.1–29.2%), Vibrio (3.7–34.2%), and Pantoea (2.6–24.0%). The changes in the distributions of the 10 most abundant orders in the beach sand under the debris are shown in Fig. 5b with the major Genera throughout the survey being Litorivivens (3.4–36.6%), Pseudomonas (1.4–22.5%), and Nocardioides (2.6–13.2%).
Figure 5.
The distributions of the 10 most abundant Genera in these sample sets.
Variation of potential pathogen bacteria
The pathogens that posed potential risks to humans were extracted from the OTU datasets based on the list of pathogenic bacterial Genera presented by Fang et al.23. Eighty-four of the 145 Genera in the pathogenic bacterial list were detected. Based on the NMDS analysis of potential pathogenic bacteria, the 90% confidence level debris cluster partially overlapped with the sand under the debris cluster suggesting a partial relationship between these two bacterial groups (Fig. 4b). From a comprehensive view of the NMDS analysis for potential pathogenic bacterial Genera, it can be inferred that the plant debris and sand under the debris form distinctive community structures, but partly depend on each other based on physical characteristics among the environments.
A total of 35 major pathogenic bacteria with an abundance ratio (OTU number of each bacterium/OTU number of all bacteria) of 0.1% or more were detected. Bacillus, Mycobacterium, Pseudomonas, and Sphingomonas were detected in the samples during the survey period (Supplementary Figure S2). Permutational multivariate analysis of variance (PERMANOVA) revealed significant differences among the plant debris and sand under the debris (p < 0.01). The prevalence of potential pathogenic bacteria in the plant debris is significantly high than the sand under the debris. The debris samples showed a greater difference when compared to the sand under the debris based on adjusted p values (< 0.001). Enterobacter, Vibrio, Pantoea, and Pseudomonas were detected at relatively high concentrations in the plant debris, and the average abundances were 10.1%, 8.23%, 8.16%, and 7.02%, respectively. Pseudomonas, Sphingomonas, Aeromonas, and Bacillus were detected at relatively high concentrations in the beach sand just under the plant debris with the average abundances being 3.77%, 1.46%, 0.91%, and 0.48%, respectively. It was characteristic that Enterobacter, an attenuated bacterium of the Enterobacteriaceae family, was detected in more than 10% of the debris samples, and that the potentially pathogenic Genus Pseudomonas, was mainly detected in both debris and sand under the debris. Vibrio is a common bacterial Genus in coastal substrates15. These results suggest that plant debris has the highest potential to hold pathogenic bacteria on sandy beaches.
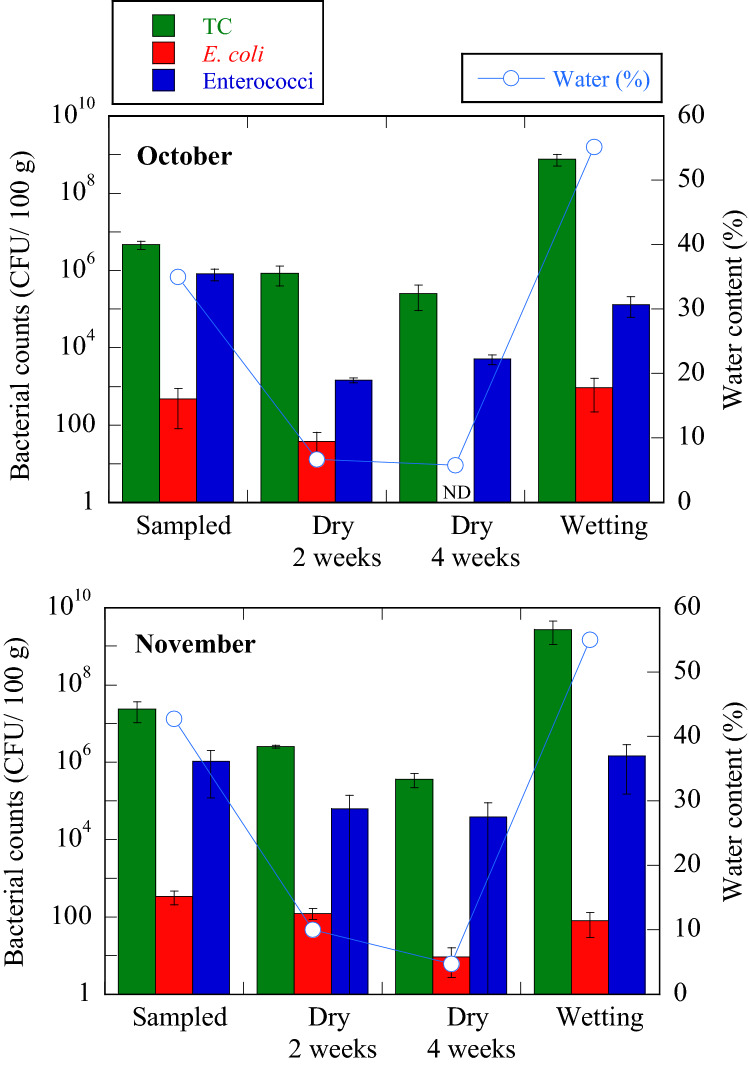
Change in the bacterial count and flora under drying and wetting conditions
The changes in the bacterial counts of plant debris under drying and wetting conditions are shown in Fig. 6. Immediately after harvesting debris from the beach in October 2019, the number of total coliforms was found to be 4.6 × 106 CFU/100 g with a 35% water content. After drying for 2 weeks, the water content of the plant debris dropped significantly to 6.7%, and the number of total coliforms decreased to 8.3 × 105 CFU/100 g. The dried debris was in a ‘dry state’ with no visible moisture. Four weeks later, the water content had decreased to 5.8% and the number of total coliforms remained relatively unchanged (105 CFU/100 g). When the dried debris were soaked in sterile distilled water and incubated for 24 h (water content, 55.2%), the number of total coliforms drastically increased to 7.6 × 108 CFU/100 g. E. coli, which had been undetectable after 4 weeks of drying, was detected at 9.3 × 102 CFU/100 g after wetting. The number of enterococci which had decreased from 8.2 × 105 to 1.5 × 103 CFU/100 g after 2 weeks of debris drying and which was detected at 5.1 × 103 CFU/100 g after drying for 4 weeks, increased to 1.3 × 105 CFU/100 g after wetting. In drying and wetting repeat experiments using different plant debris collected in November 2019, total coliforms and enterococci were active in the debris dried for 4 weeks at 3.6 × 105 CFU/100 g and 3.9 × 104 CFU/100 g, respectively with 4.7% wet content. In contrast, E. coli was affected by drying with counts decreasing from 3.4 × 102 to 9.3 CFU/100 g after 4 weeks of drying, which then increased to 79 CFU/100 g after wetting.
Figure 6.
The change in the bacterial count of plant debris under drying and wetting conditions. The plant debris used in the drying-wetting experiment was collected from Kizaki Beach. The raw plant debris were dried for 4 weeks in the dark at 37 °C and then immersed in sterile distilled water for 1 h. The wet plant debris were then incubated at 30 °C for 24 h. TC, total coliforms, ND, not detected, CFU, colony forming units.
As a result of the florae analysis of the plant debris at each stage of the two drying and wetting experiments, a total of 1249 bacterial Genera were identified from all the samples. Among the bacterial Genera detected in each sample, the top 10 Genera with the highest relative abundances are shown in Supplementary Figure S3. The bacterial florae of plant debris collected in October and November were quite different. However, Bacillus, Erythrobacter, Rhizobium, Nocardiodes, Sphingomonas, and Novoshingobium were commonly identified as the 10 major Genera under dry conditions. Bacillus, Enterobacter, and Rhizobium, which were detected in the just-collected debris, are extremely resistant to drought conditions, and their relative abundance in dried debris after 4 weeks was 5.2%, 4.4%, and 4.4%, respectively.
Discussion
Plant debris as bacterial pollution sources on sandy beaches
From the viewpoint of public health for beach users, BF requires that beach managers monitor the quality of coastal waters and post and publish the results24. As a source of water quality pollution, sewage and drainage of urban wastewater due to torrential rain and are the most important management items. Particular attention has been paid to faecal bacteria, and the limits for E. coli and enterococci in coastal water are strictly set at 250 CFU/100 ml and 100 CFU/100 ml, respectively. In order to protect the health of beach users, preventive measures must be taken to address the increased bacterial load after flooding events. On the other hand, although water quality standards have been set, standards or monitoring items related to sand quality have not. However, the accumulation of plant debris on beaches due to flooding has a large effect on the sand quality.
E. coli was detected in high concentrations in the plant debris with the average being 1.4 × 104 CFU/100 g throughout the monitoring period. Furthermore, the average number of enterococci (1.1 × 105 CFU/100 g) was 10 times greater than that of E. coli in the debris. The bacterial impact is significantly greater in the plant debris than what was detected after the typhoon. It can be assumed that 106 CFU/100 g of faecal-indicator bacteria can be routinely detected from rotting plants and sand under the plants on most beaches. However, it has been reported that there is a trend of increasing risk for Enterococcus > 13 CFU/g (= 1.3 × 103 CFU/100 g) in beach sand with enteric illnesses among beachgoers20. After evaluating the number of enterococci in each sample based on the 13 CFU/g level, all debris and the sand under the debris collected in October exceeded the level of increasing risk for Enterococcus. Therefore, leaving plant debris on recreational beaches may increase the risk of enteric illnesses for beach users.
When we focused on potentially pathogenic species, 44 among the list of 538 pathogenic bacterial species27, were extracted from the OTU data. In addition, the abundance ratio was 0.012 to 15.3% (Supplementary Figure S4). The highest ratio for the plant debris and sand under the debris were 6.25% in July and 0.79% and in October respectively. Brevundimonas vesicularis and Pseudomonas alcaligenes were commonly detected in the plant debris and sand under the debris during the survey period. B. vesicularis is widely known as a phytopathogenic bacteria. Pseudomonas spp. is a major component of plant root bacteria. Therefore, the route of transmitted B. vesicularis and P. alcaligenes to the sand would be via plant debris. The NMDS ordination (Fig. 4b), which shows the link between debris and sand, supports this hypothesis.
Based on the results of drying-wetting experiments of plant debris, we confirmed that total coliforms and enterococci survived at the range of 105 to 106 CFU/100 g and 103 to 104 CFU/100 g, respectively, even in very dry conditions (5% moisture content). In general, total coliforms include potentially pathogenic bacteria. It has been recognized that enterococci, which cause opportunistic infections, are detected at high concentrations in dry beach sand and have a high tolerance for dry conditions25,26. Therefore, plant debris will most likely be the main source of enterococci pollution on beaches as seen on Kizaki Beach. Although E. coli had a weaker resistance to drying than total coliforms and enterococci, E. coli regrew under wet conditions. Both Bacillus and Erythrobacter are included in the list of 145 bacterial Genera suspected of being pathogenic. Furthermore, the pathogenic bacteria Bacillus pumilus and Pseudomonas alcaligenes were detected in the dried debris. In both of the two experiments, even when the dried plant debris were returned to humid conditions, the debris showed completely different florae from when it was collected. However, Pantoea was a common Genus that proliferated in humid conditions. It was inferred that the florae associated with plant debris on sandy beaches changed due to repeated drying and wetting conditions.
There is a definite need to assess pollution on beaches in terms of human health and safety risks2. Our research can contribute to the provision of information related to establishing new risk assessments.
Sustainable management for creating safe beaches
In 2009, Japan enacted policies which ensured the conservation of good coastal landscapes and environments through treatment of marine debris that drifted ashore and effectively reduced their generation. Coastal managers must take measures necessary for the treatment of marine debris to keep coastal land under their control clean in terms of landforms, landscapes, ecosystems, and other natural conditions, whilst considering the utilisation, economic activities, and other social opportunities of the coast. Many other countries have laws and/or regulations with similar purposes for marine debris. In the European Union (EU), the most important initiative to address marine litter is the Marine Strategy Framework Directive (MSFD) established in 2008. Leading the United States’ Federal Government's measures against marine debris is the Marine Debris Research, Prevention, and Reduction Act of 2006. Currently, the BF, as an international environmental certification, shows the criteria for solving problems related to beach environmental conservation and management including marine debris and beach waste24, and it is being promoted in various countries around the world, including Japan1.
Among the marine debris and beach waste, plant debris has a natural origin. Therefore, although the plant debris evaluated in this study are quantitatively more abundant than plastic debris on Kizaki Beach, the general public does not perceive any threat. However, as demonstrated in the present study, faecal-indicator bacteria as well as pathogenic bacteria were retained at high densities on the plant debris that drifted ashore with heavy rain and typhoon events, indicating that plant debris is a suitable habitat for such bacteria. The bacteria in plant debris are extremely resistant to drought, and can survive for 2–4 weeks with a water content of only 5%. Furthermore, it was also shown that even if the numbers of bacteria were reduced by drying, the bacteria re-grew after wetting. For recreational beach users, the drifting and accumulation of plant debris increases the risk of illness due to contact and infection by pathogenic bacteria at a high density.
Since beach conservation has a variety of targets and purposes regarding public health, ecosystem conservation, and landscape conservation, cleaning the plant debris on the beach is required for each purpose28.To reduce the health risks for users of recreational sandy beaches, plant debris should be given a high priority in maintenance management and should be considered a potential health hazard for infectious diseases, and not just harmless naturally-occurring products. Based on this study, we propose that to address the public health issues of beaches and coasts, it is necessary to collect and treat plant debris to the same extent as plastic marine debris since the prompt removal of plant debris can create safer recreational beaches. The coastal managers should monitor beaches and develop a plan for the collection of plant debris in the event of flood events which can occur just after heavy rain and typhoon events. On the other hand, beach management requires a budget, so it is important to discuss these issues with local users, tourists, and administrative managers. For the management of Kizaki Beach, a council of local governments, local contractors, and users has been established. We propose to consider the collection and adequate disposal of plant debris for the purpose of protecting the user’s health.
Materials and methods
Sampling sites
Kizaki Beach, one of the main recreational beaches in Japan, is situated between the outlets of the Kiyotake and Kaeda Rivers (Fig. 2). Kizaki Beach is a prefectural general sports park (total area 154 hectares) that has advanced facilities designed to respect the natural environment, so there are no houses, factories, or farms near the beach. In addition, there are no drain pipes connected to Kizaki Beach; therefore, there is no inflow from domestic or agricultural sources. The Kiyotake and Kaeda Rivers encompass 166.4 and 53.8 km2, respectively, and their corresponding lengths are 28.8 and 17.5 km, respectively. Although, there are small-scale sewage treatment plants along the Kiyotake River, they use a discrete and segregated system so sewage does not flow directly into the river even during heavy rains. Moreover, > 90% of the catchment areas of the Kaeda River are forests. Therefore, the coastal water around Kizaki Beach is classified as the highest quality by the Ministry of the Environment, Japan. Normally, the numbers of E. coli and enterococci in the coastal waters are 1 CFU/100 mL and 0–1 CFU/100 mL, respectively15. Topographically, since Kizaki Beach is linearly shallow for 2 km facing the Pacific Ocean, the beach is typically an open system. However, plant debris, which includes driftwood, branches, and withered grass, accumulates along the high-water line on Kizaki Beach. During large-scale rainfall events such as typhoons, a large amount of plant debris accumulates and seriously hinders the recreational use of this beach. Since it is frequently impacted by drifting plant debris, it was selected as the model beach for this study.
Sampling of plant debris and beach sand
We selected the narrow area of plant debris washed ashore on the beach as the survey area (Fig. 1b), and focussed on the plant debris and the sand under the debris. To monitor the effects of plant debris on bacterial contamination of the beach, this survey was conducted monthly for eight months (May 24—December 18, 2018) at a fixed location and included the rainy season and the typhoon season from summer to autumn. The plant debris collected were ≤ 30 mm in diameter and ≤ 100 cm in length. Most of the plant debris accumulated near the high-water line on Kizaki Beach collected during this survey were branches and withered grass. Plant debris was collected in plastic bags from three consecutive sections (Sections 1, 2, and 3) along the high-water line at 10 m intervals. We assumed that the amount of collected debris was a rough standing stock estimate for each survey month. From the three sections where plant debris were collected, sand (surface layer − 10 cm) just under the plant debris was collected using an acrylic column. For each section, three points were randomly selected, and sand was composited to obtain each section sample.
The collected plant debris was taken back to the laboratory within 30 min after collection, and each 10 m sample section was measured for wet weight (kg wet/10 m as one section). This wet weight of plant debris was defined as the existing amount of plant debris per 10 m of section on each survey day. On June 25 and October 10, 2018, when it was difficult to collect the entire amount of plant debris which had drifted onto the beach, plant debris was collected within a range of 1–2 m along the high-water line and the weight was measured and then converted to a weight which represented 10 m. After measuring the weight, the plant debris was pulverized to a length of about 5 cm using a pulveriser (MGS-1510Si, Minato Electric Industries, Oita, Japan). The pulverized plant debris and sand were dried at 110 °C for 24 h, and the dry weight was measured. The water content (%) was determined from the wet and dry weight of each sample.
Enumeration of faecal-indicator bacteria
Plant debris (300 g) was thoroughly mixed with 3 L of sterilised physiological saline solution in a sterilised 5 L polyethylene bottle for 2 min. Sand (5 g) was mixed for 2 min with 40 mL of sterilised physiological saline solution. After settling for 1 min, the supernate was aliquoted, and the extract was serially diluted. CHROMagar ECC agar plates (CHROMagar, Paris, France) were used to differentiate and count Escherichia coli and other coliform bacteria. The diluted supernate was filtered through a 0.45-μm pore membrane filter (47-mm diameter, sterile, mixed cellulose ester; Advantec, Tokyo, Japan) and incubated on ECC agar plates for 24 h at 37 °C. Blue colonies were identified as E. coli isolates. Mauve colonies were designated isolates of other coliform species. Enterococci were enumerated on membrane-Enterococcus indoxyl-β-d-glucoside (mEI) agar plates using the membrane filter method29. The diluted supernates were passed through a membrane filter and the filtrate was used to inoculate membrane-Enterococcus indoxyl-β-d-glucoside (mEI) agar plates for 24 h at 41 °C. Blue colonies were identified as enterococci isolates. Heterotrophic bacteria were enumerated on agar plates (agar 1.5%, Difco Marine Broth 2216; Becton, Dickinson and Company, MD, USA). The diluted supernate was spread onto the plates and incubated for 7 days at 22.5 °C. After incubation, colonies were counted as heterotrophic bacteria.
The number of coliform bacteria, E. coli, enterococci, and heterotrophic bacteria of all samples was determined from the mean number of colony-forming units (CFUs) of 3 replicates. The bacterial count was expressed as CFU/100 g of debris or sand. The detection limits of each bacterial count in the plant debris and sand samples were 3 × 101 CFU/100 g, and 7 CFU/100 g, respectively.
The supernates extracted from the plant debris and sand were then filtered through a membrane filter. DNA was extracted from the bacteria collected on the membrane filters using a DNeasy PowerWater kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. The concentrations of DNA in the extracts were determined using a Quantus fluorometer (Promega, WI, USA) and an appropriate kit.
16S rRNA-gene sequencing and data analysis
The V3–4 region of the bacterial 16S rRNA genes of each sample were amplified using specific primers that included an overhang adapter sequence linked to the 5′ ends of the primers (forward primer, 5′-ACACTCTTTCCCTACACGACGCTCTTCCGATCT-NNNNN-CCTACGGGNGGCWGCAG-3′; and reverse primer, 5′-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-NNNNN-GACTACHVGGGTATCTAATCC-3′).
The polymerase chain reaction (PCR) reaction solution (total 10 µL) was mixed with 1.0 µL of 10 × Ex Taq Buffer (TaKaRa, Tokyo, Japan), 0.8 µL of dNTP Mixture (TaKaRa), 0.5 µL of each primer, 2.0 µL of Template DNA, and 0.1 µL of Ex Taq Hot Start Version (TaKaRa), and 5.1 µL of deionized distilled water (DDW). The PCR reactions were performed as follows: initial denaturation at 94 °C for 2 min, 30 cycles of denaturation at 94 °C, annealing at 55 °C for 30 s, extension at 72 °C for 30 s, and final extension at 72 °C for 5 min. The PCR products were purified using Agencourt AMPure (Beckman Coulter, CA, USA).
The purified PCR products were subjected to a second-round of PCR amplification with (2nd forward primer, 5′-AATGATACGGCGACCACCGAGATCTACAC-Index-ACACTCTTTCCCTACACGACGC-3′; and 2nd reverse primer, 5′-CAAGCAGAAGACGGCATACGAGAT-Index-GTGACTGGAGTTCAGACGTGTG-3′). The polymerase chain reaction (PCR) reaction solution (total 10 µL) was mixed with 1.0 µL of 10 × Ex Taq Buffer, 0.8 µL of dNTP Mixture, 0.5 µL of each primer, 2.0 µL of Template DNA, and 0.1 µL of Ex Taq Hot Start Version, and 5.1 µL of DDW. The PCR reactions were performed as follows: initial denaturation at 94 °C for 2 min, 10 cycles of denaturation at 94 °C, annealing at 60 °C for 30 s, extension at 72 °C for 30 s, and final extension at 72 °C for 5 min. The PCR products were purified using Agencourt AMPure, and sequenced 2 × 300-bp paired-end by the MiSeq platform (Illumina, Inc., CA, USA) using the MiSeq v3 Reagent Kit (Illumina). The sequencing data were analysed using Qiime ver. 1.9.0 (Pycogent, GitHub Inc., CA, USA). Raw paired-end reads were trimmed to remove low-quality sequences and then merged. All the sequences that were not determined to be chimeras were extracted and was performed to create Operational Taxonomic Units (OTUs) sequence. The merged reads were clustered into OTUs at 97% sequence identity based on the EzBioCloud 16S database. A representative sequence of each OTU was identified to the phylogenetic division or genus or species.
Drying-wetting experiments of plant debris in an incubator
Plant debris that drifted to the shore presumably experienced repetitive drying and wetting depending on the weather. The number of bacteria attached to the debris must also be significantly affected by water content. Therefore, the drying condition of plant debris was artificially controlled in a laboratory incubator, and the inactivation and reactivation of bacteria by drying and wetting was investigated. The plant debris used in the drying-wetting experiment was collected from targeted areas on Kizaki Beach. The raw plant debris was dried for 4 weeks in the dark at 37 °C. Aliquots of the dried debris were sampled every two weeks. Furthermore, the plant debris dried for 4 weeks were immersed in sterile distilled water for 1 h. The wet plant debris were wrapped in aluminium foil to prevent evaporation and incubated at 30 °C for 24 h. For all the debris samples (3 aliquots for each sample): raw debris, 2- and 4-week dried debris, and re-wetted debris; the bacterial counts and water content were determined as described previously. In addition, DNA was also extracted from the debris samples for bacterial florae analyses. Drying-wetting experiments were repeated twice using different plant debris which were collected in October and November, 2019.
Statistical analysis
Based on the relative abundances (OTU number) of the bacterial Genera determined by sequencing analysis, we derived a Bray–Curtis distance between the plant debris and sand samples among the sampling periods. The distances measured were subsequently used for performing non-metric multidimensional scaling (NMDS) using the metaMDS function from the vegan package (R v.3.6.3). Florae differences among the debris and sand samples were also tested using permutational multivariate analysis of variance (PERMANOVA) with the pairwise.adonis function in the pairwiseAdonis package in R ver. 3.6.3. The test used the Bray–Curtis distance and was based on 10,000 permutations. Subsequently, post-hoc pairwise comparisons of the similarity between the samples were performed with an adjusted p value based on the Bonferroni correction using the pairwise.adonis function in the pairwiseAdonis package (R v.3.6.3).
Supplementary information
Acknowledgements
We would like to thank Professor Aya Nishiwaki (Faculty of Agriculture, University of Miyazaki) for his expertise in classifying the plant debris. In addition, we would also like to thank Professor Satoshi Ishii (Department of Soil, Water, and Climate, University of Minnesota) for his helpful comments in revising the manuscript. Furthermore, we would like to express our sincere gratitude to Dr. Lucia Fanini for providing valuable comments and information in the review of this manuscript. The authors would also like to thank Enago (www.enago.com) for manuscript review and editing support.
Author contributions
Y.S. designed the research. Y.S., H.S., T.K. performed all the sampling. T.K. analysed the samples. Y.T. performed the drying-wetting experiments. Y.S., H.S., and K.N. processed and analysed the data. Y.S. wrote the manuscript with assistance from K.N.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91066-w.
References
- 1.Foundation for Environmental Education (FEE). Blue Flag & the Sustainable Development Goals. FEE (2019).
- 2.Fanini L, Dereo O, Elliott M. Advances in sandy beach research—Local and global perspectives. Estuar. Coast. Shelf Sci. 2020;234:106646. doi: 10.1016/j.ecss.2020.106646. [DOI] [Google Scholar]
- 3.Sato MIZ, et al. Sanitary quality of sands from marine recreational beaches of São Paulo, Brazil. Braz. J. Microbiol. 2005;36:321–326. doi: 10.1590/S1517-83822005000400003. [DOI] [Google Scholar]
- 4.Beversdorf LJ, Bornstein-Forst SM, McLellan SL. The potential for beach sand to serve as a reservoir for Escherichia coli and the physical influences on cell die-off. J. Appl. Microbiol. 2007;102:1372–1381. doi: 10.1111/j.1365-2672.2006.03177.x. [DOI] [PubMed] [Google Scholar]
- 5.Halliday E, Gast RJ. Bacteria in beach sands: An emerging challenge in protecting coastal water quality and bather health. Environ. Sci. Technol. 2011;45:370–379. doi: 10.1021/es102747s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabino R, et al. Pathogenic fungi: an unacknowledged risk at coastal resorts? New insights on microbiological sand quality in Portugal. Mar. Pollut. Bull. 2011;62:1506–1511. doi: 10.1016/j.marpolbul.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Enns AA, et al. Spatial and temporal variation in indicator microbe sampling is influential in beach management decisions. Water Res. 2012;46:2237–2246. doi: 10.1016/j.watres.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira E, et al. Microbiological and mycological beach sand quality in a volcanic environment: Madeira archipelago, Portugal. Sci. Total. Environ. 2013;461–462:469–479. doi: 10.1016/j.scitotenv.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Halliday E, McLellan SL, Amaral-Zettler LA, Sogin ML, Gast RJ. Comparison of bacterial communities in sands and water at beaches with bacterial water quality violations. PLoS ONE. 2014;9:e90815. doi: 10.1371/journal.pone.0090815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abreu R, et al. Sediment characteristics and microbiological contamination of beach sand—A case-study in the archipelago of Madeira. Sci. Total Environ. 2016;573:627–638. doi: 10.1016/j.scitotenv.2016.08.160. [DOI] [PubMed] [Google Scholar]
- 11.Bonilla TD, et al. Prevalence and distribution of fecal indicator organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Mar. Pollut. Bull. 2007;54:1472–1482. doi: 10.1016/j.marpolbul.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Imamura GJ, Thompson RS, Boehm AB, Jay JA. Wrack promotes the persistence of fecal indicator bacteria in marine sands and seawater. FEMS Microbiol Ecol. 2011;77:40–49. doi: 10.1111/j.1574-6941.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 13.Phillips MC, Solo-Gabriele HM, Piggot AM, Klaus JS, Zhang Y. Relationships between sand and water quality at recreational beaches. Water Res. 2011;45:6763–6769. doi: 10.1016/j.watres.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, He X, Yan T. Differential decay of wastewater bacteria and change of microbial communities in beach sand and seawater microcosms. Environ. Sci. Technol. 2015;49:8531–8540. doi: 10.1021/acs.est.5b01879. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Teranishi K, Matsuwaki T, Nukazawa K, Ogura Y. Effects of bacterial pollution caused by a strong typhoon event and the restoration of a recreational beach: Transitions of fecal bacterial counts and bacterial flora in beach sand. Sci. Total Environ. 2018;640–641:52–61. doi: 10.1016/j.scitotenv.2018.05.265. [DOI] [PubMed] [Google Scholar]
- 16.Whitman RL, Przybyla-Kelly K, Shively DA, Nevers MB, Byappanahalli MN. Hand-mouth transfer and potential for exposure to E. coli and F+ coliphage in beach sand, Chicago. Illinois. J. Water Health. 2009;7:623–629. doi: 10.2166/wh.2009.115. [DOI] [PubMed] [Google Scholar]
- 17.Fleisher JM, et al. The BEACHES Study: Health effects and exposures from non-point source microbial contaminants in subtropical recreational marine waters. Int. J. Epidemiol. 2010;39:1291–1298. doi: 10.1093/ije/dyq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelzaher AM, et al. Daily measures of microbes and human health at a non-point source marine beach. J. Water Health. 2011;9:443–457. doi: 10.2166/wh.2011.146. [DOI] [PubMed] [Google Scholar]
- 19.Heaney CD, et al. Contact with beach sand among beachgoers and risk of illness. Am. J. Epidemiol. 2009;170:164–172. doi: 10.1093/aje/kwp152. [DOI] [PubMed] [Google Scholar]
- 20.Heaney CD, et al. Fecal indicators in sand, sand contact, and risk of enteric illness among beachgoers. Epidemiology. 2012;23:95–106. doi: 10.1097/EDE.0b013e31823b504c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solo-Gabriele HM, et al. Beach sand and the potential for infectious disease transmission: Observations and recommendations. J. Mar. Biol. Assoc. UK. 2015;96:101–120. doi: 10.1017/S0025315415000843. [DOI] [Google Scholar]
- 22.Furukawa T, Yoshida T, Suzuki Y. Application of PFGE to source tracking of fecal pollution in coastal recreation area: A case study in Aoshima Beach, Japan. J. Appl. Microbiol. 2011;110:688–696. doi: 10.1111/j.1365-2672.2010.04918.x. [DOI] [PubMed] [Google Scholar]
- 23.Fang T, et al. Distribution comparison and risk assessment of free-floating and particle-attached bacterial pathogens in urban recreational water: Implications for water quality management. Sci. Total Environ. 2018;613–614:428–438. doi: 10.1016/j.scitotenv.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Foundation for Environmental Education (FEE). Blue Frag Beach Criteria and Explanatory Notes 2017. FEE (2017).
- 25.Feng Z, Reniers A, Haus BK, Solo-Gabriele HM. Modeling sediment-related enterococci loading, transport, and inactivation at an embayed nonpoint source beach. Water Resour. Res. 2013;49:693–712. doi: 10.1029/2012WR012432. [DOI] [Google Scholar]
- 26.Halliday E, Ralston DK, Gast RJ. Contribution of sand-associated enterococci to dry weather water quality. Environ. Sci. Technol. 2015;49:451–458. doi: 10.1021/es504908h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li WC, Tse HF, Fok L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016;566–567:333–349. doi: 10.1016/j.scitotenv.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 28.Zielinski S, Botero CM, Yanes A. To clean or not to clean? A critical review of beach cleaning methods and impacts. Mar. Pollut. Bull. 2019;139:390–401. doi: 10.1016/j.marpolbul.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 29.United States Environmental Protection Agency. Method 1600: Enterococci in water by membrane filtration using membrane-enterococcus indoxyl-β-D-glucoside agar (mEI) (ed. U.S Environmental Protection Agency) EPA-821-R-02–022 (U.S Environmental Protection Agency Office of Water, 2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.