Abstract
Introduction:
This study aimed to compare demographics, disease characteristics, and outcomes of HIV-infected patients with non-small-cell lung cancer (NSCLC) with the general NSCLC population.
Patients/Methods:
A retrospective cohort study was used to compare HIV-infected and uninfected groups. Medical records of all HIV+ patients diagnosed with NSCLC between 2000–2016 at Yale New Haven Hospital (New Haven, CT) were reviewed and compared with the general Yale NSCLC population regarding demographics, NSCLC characteristics, treatment, and survival. Log-rank tests and Kaplan-Meier curves were used to analyze survival differences. Unadjusted and adjusted Cox Proportional Hazard models were used to assess predictors of survival.
Results:
35 HIV-NSCLC patients and 5,187 general NSCLC patients were identified. Median age at cancer diagnosis was 54 (IQR 49–59) for HIV-NSCLC versus 68 (IQR 61–76) for NSCLC (p<0.001). Both groups had high rates of tobacco use. At the time of NSCLC diagnosis, 80% of HIV-NSCLC were on antiretroviral therapy, 60% had an HIV-1 RNA <400 copies/ml, and median CD4 was 407 cells/µL (IQR 218–592). Histology, cancer stage, and first-line cancer treatment regimens were not significantly different between groups. Overall median survival was 12.4 months (95% CI: 7.2–20.4) for HIV-NSCLC versus 22.8 months (95% CI: 21.2–24.1) for general NSCLC. HIV-NSCLC had decreased survival at 2 years (p=0.028) and 3 years (p=0.014) compared with general NSCLC. HIV status was an independent risk factor for poorer outcomes when controlling for other factors (HR 1.8; 95% CI: 1.24–2.62).
Conclusion:
Despite similar histology, stage, and treatment between groups, HIV-infected patients had worse outcomes for NSCLC.
Keywords: non small cell lung cancer (NSCLC), lung cancer, HIV, AIDS
MicroAbstract
This retrospective cohort study of HIV-infected and HIV-uninfected patients with non-small cell lung cancer (NSCLC) from a single institution during the years 2000–2016 found that HIV-infected patients had decreased survival despite comparable histology, stage at diagnosis, and treatment regimens. Additionally, HIV status was an independent risk factor for worse outcomes. HIV infection may adversely impact NSCLC outcomes.
Introduction
Since the advent of combination antiretroviral therapy (ART) and despite a significant decline in AIDS-related morbidity and mortality, we have witnessed a significant rise in the number of non-AIDS defining cancers (NADC).1–5 This increase is likely related to multiple factors, including increased life expectancy, high rates of tobacco and alcohol use, increased prevalence of co-infection with oncoviruses, and the effects of chronic HIV-mediated immunosuppression and inflammation.6–12
Lung cancer is now the most common NADC among persons living with HIV (PLWH) and has become a leading cause of mortality, accounting for up to 40% of all cancer deaths and 10% of non-HIV related deaths.7,10,13–16 While a high prevalence of tobacco use may contribute to increased rates of lung cancer among PLWH, large cohort studies have found HIV to be an independent risk factor for lung cancer when adjusted for smoking and age, with a two to four fold risk of lung cancer for PLWH compared with the general population.2,17–19 The underlying mechanism is likely multifactorial, and some have hypothesized chronic HIV-related immune activation may play a role.20 Additionally, at time of lung cancer diagnosis PLWH may present at a mean age 20 years younger than the general population.15,21 Most PLWH are symptomatic at the time of diagnosis with locally advanced or metastatic disease in 70–96% of cases.13,22,23 Though several studies suggest that patients with HIV-associated NSCLC have elevated mortality compared to the general NSCLC population,18,19,24–27 some studies have reported similar outcomes between both groups.28,29
While large cohort studies have provided insight into general trends of lung cancer in PLWH decades after ART introduction, they have been limited in the inability to provide individual data on ART use and HIV disease characteristics including CD4+ T cell count trajectories, HIV viral load, and presence of AIDS-defining illnesses (ADI), as well as detailed information about cancer treatment. This study sought to provide an in-depth characterization of patients from a single academic center in the antiretroviral era through comprehensive medical record review and analysis. By limiting review to a single institution, variation across geographic and institutional approaches to cancer are minimized. Specifically, we sought to characterize differences in cancer treatment and cancer outcomes between HIV-infected and uninfected individuals with the same medical oncology providers treating both groups of patients.
Patients and Methods
After an institutional review board approval, electronic medical record databases at Yale New Haven Hospital (YNHH; New Haven, CT) were queried using International Classification of Diseases, 9th or 10th Revision, Clinical Modification (ICD-9-CM and ICD-10-CM) codes for HIV (V08, V042, B20, Z21) and lung cancer (162.0–162.9, 197.0, C34) between 2000 and 2016. This initial screen produced 97 individual subjects. Charts were then reviewed so only those with documented HIV infection and primary NSCLC with confirmed pathology data were included. Subjects were required to have at least one-year follow-up from time of cancer diagnosis to be included. This resulted in a total of 35 patients with primary NSCLC and HIV infection. The remainder of patients had coding errors and did not meet criteria for study analysis.
Data was then extracted from electronic medical records using a standardized data collection form. This included demographic data, HIV risk factors, age at cancer diagnosis, history of ADI as defined by the Center for Disease Control,30 most recent CD4+ T cell count, CD4:CD8 ratio, and HIV-1 RNA (each within 6 months or less prior to cancer diagnosis); CD4+ T cell count nadir, duration of HIV infection, ART regimen, hepatitis C co-infection status, histological cancer type, cancer stage based on American Joint Committee on Cancer (AJCC) TNM system,31 smoking history, substance use history, cancer treatment, and survival. The YNHH Tumor Registry was also queried for all patients diagnosed with NSCLC at YNHH from 2000 to 2016 with at least one-year follow up time, or had died within one year. Data on tumor registry patients included sex, age, smoking status, TNM stage, histology, treatment, and vital status. Systemic therapy for lung cancer included chemotherapy, immunotherapy and targeted therapy. Data for 35 PLWH-NSCLC patients were confirmed with the tumor registry, and any differences from medical chart review was re-reviewed and reconciled. Additionally, longitudinal data available from 2009 to 2016 recorded from the two YNHH HIV outpatient clinic sites was used to compare demographic and clinical characteristics of the HIV-NSCLC population with the general HIV population.
Patient baseline characteristics were summarized using median and interquartile range for continuous variables and frequency and percentage for categorical variables. Wilcoxon rank sum tests and Chi-square or Fisher’s Exact test were used to determine the association between patients’ HIV status and baseline characteristics. Log-rank tests were conducted and Kaplan-Meier curves were created to show survival differences between HIV-positive and negative groups. Association between patient baseline characteristics and survival was tested using unadjusted and adjusted Proportional Hazard Cox models. All p-values were 2-tailed, and p-values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.4.3 (SAS Institute, Cary, NC).
Results
Demographics and HIV characteristics
A total of 35 patients were identified on chart review to have HIV infection and primary NSCLC and were included in the analysis (HIV-NSCLC cohort). One patient had two primary lung cancers at different sites diagnosed a year apart; only the first cancer diagnosis was recorded and used for analysis. A total of 5,187 HIV-uninfected NSCLC patients from the YNHH tumor registry were identified (Table 1a). 66% of the HIV-NSCLC cohort were male, compared with 51% male in the general NSCLC population (p=0.09). At time of cancer diagnosis, median age was 54 (IQR: 49–59), compared with a median age of 68 (IQR: 61–76) among the general NSCLC population (p<0.001). The majority of patients were non-Hispanic black (63%) in the HIV-NSCLC group, compared with the majority being non-Hispanic white (84%) in the general NSCLC population (p<0.001). In the HIV-NSCLC group, 97% had a history of tobacco use (17% former smokers and 80% current smokers) with a median 30 pack-year smoking history (IQR 19.5–40). In comparison, 90% of the general NSCLC population were past or present smokers (p=0.25), but detailed smoking history was unavailable.
Table 1a:
Demographics and Clinical Characteristics of HIV+ and all NSCLC Patients
| Patient Characteristics | HIV+ (N=35) | All NSCLC (N=5,187) | P-value |
|---|---|---|---|
| Median Age at time of cancer diagnosis (IQR) | 54 (49, 59) | 68 (61, 76) | <0.001 |
| Male | 23 (66%) | 2669 (51%) | 0.09 |
| Female | 12 (34%) | 2518 (49%) | |
| Race/Ethnicity | <0.001 | ||
| Black, non-Hispanic | 22 (63%) | 441 (8.5%) | |
| White, non-Hispanic | 9 (26%) | 4353 (84%) | |
| Hispanic | 4 (11%) | 195 (3.8%) | |
| Other/Unknown | 0 | 198 (3.8%) | |
| Tobacco Use | 0.25 | ||
| Former/Present | 34 (97%) | 4392 (90%) | |
| Never | 1 (3%) | 471 (10%) | |
| Unknown | 324 | ||
| Histology | 0.49 | ||
| Adenocarcinoma | 23 (66%) | 3855 (74%) | |
| Squamous Cell | 10 (28%) | 1069 (21%) | |
| Large Cell/NOS | 2 (6%) | 263 (5%) | |
| Stage | 0.93 | ||
| Stage I | 11 (31%) | 1612 (31%) | |
| Stage II | 3 (9%) | 481 (9%) | |
| Stage III | 9 (26%) | 1096 (21%) | |
| Stage IV | 12 (34%) | 1998 (39%) | |
| Year of cancer diagnosis | 0.06 | ||
| 2000–2005 | 7 (20%) | 745 (14%) | |
| 2006–2010 | 12 (34%) | 1087 (21%) | |
| 2011–2016 | 16 (46%) | 3355 (65%) | |
Among the HIV-NSCLC cohort, the majority of patients (n=28; 80%) were on ART and 21 (60%) had HIV-1 RNA < 400 copies/ml (Table 1b). At time of lung cancer diagnosis, the median CD4+ T-cell count was 407 cells/µL (IQR: 218–592) and 18 patients (51%) had a documented ADI in their medical history. Median documented CD4+ T cell nadir was 162 cells/µL and median CD4:CD8 ratio was 0.48, though this data was unknown for 12 and 11 patients, respectively.
Table 1b:
Clinical Characteristics of HIV+ Patients at time of NSCLC Diagnosis
| Clinical characteristics | HIV-NSCLC, N= 35 (%) |
|---|---|
| History of Tobacco Use | 34 (97%) |
| Former | 6 (17%) |
| Current | 28 (80%) |
| Median smoking pack years (IQR) | 30 (19.5, 40) |
| History of Alcohol Use | 21 (60%) |
| Former | 8 (23%) |
| Current | 13 (37%) |
| Unknown | 4 (11%) |
| History of Illicit Drug Use | 22 (63%) |
| Former | 17 (49%) |
| Current | 5 (14%) |
| Unknown | 3 (9%) |
| On ART | 28 (80%) |
| Not on ART | 7 (20%) |
| Type of ART | |
| NRTI in regimen | 28 (100%) |
| NNRTI in regimen | 5 (18%) |
| Protease Inhibitor in regimen | 14 (50%) |
| Integrase Inhibitor in regimen | 8 (29%) |
| Unknown | 5 (18%) |
| HIV VL | |
| <400 | 21 (60%) |
| >400 | 12 (34%) |
| Unknown | 2 (6%) |
| Median CD4 (IQR)a | 407 (218, 575) |
| Median CD4/CD8 ratio (IQR)b | 0.48 (0.34, 0.69) |
| Median past CD4 nadir (IQR)c | 162 (88, 231) |
| History of AIDS-defining illness (ADI) | 18 (51%) |
| No history of ADI | 11 (31%) |
| Unknown | 6 (17%) |
| Median duration of HIV infection in years | 13.5 |
| Mode of HIV Transmission d | |
| IVDU | 14 (54%) |
| Heterosexual sex | 9 (35%) |
| MSM | 2 (8%) |
| Blood Transfusion | 1 (3%) |
| HCV co-infection | 22 (63%) |
NRTI= Nucleoside Reverse Transcriptase Inhibitor. NNRTI= Non-nucleoside Reverse Transcriptase Inhibitor.
Missing data for 2 patients;
Missing data for 11 patients;
Missing data for 12 patients
Unknown route of HIV transmission for 9 patients (26%)
The median interval of time from HIV diagnosis to lung cancer diagnosis was 13.5 years. The majority of patients had past or present alcohol use (60%) and illicit drug use (63%). Among the HIV-NSCLC population, there were higher rates of Hepatitis C virus (HCV) co-infection (63%), compared with a rate of 30% in the general HIV population at YNHH. Of the patients with HCV, 50% had a history of IVDU and 100% were past or present smokers. Mode of HIV transmission in HIV-NSCLC cohort versus the general HIV clinic population was significantly different (p<0.01): HIV-NSCLC transmission risk was 54% intravenous drug use (IVDU), 35% heterosexual sex, 8% men who have sex with men (MSM), and 4% from blood transfusion. Comparatively, the general YNHH HIV population transmission risk was 18% IVDU, 44% heterosexual sex, 24% MSM, and 1% from blood transfusion.
Lung Cancer Presentation, Treatment and Survival
Between the HIV-NSCLC and general NSCLC cohorts respectively, histology was notable for 66% vs 74% adenocarcinoma, 28% vs 21% squamous cell carcinoma, and 6% vs 5% large cell (p=0.49). In the HIV-NSCLC cohort, 60% of patients were stage III or IV at diagnosis, as were 60% of the general NSCLC cohort. Use of systemic therapy, radiation, or surgery for first-line treatment of NSCLC was comparable for both groups when evaluated by stage (Table 2).
Table 2:
First course of treatment for all NSCLC by stage at diagnosis
| Early Stage (Stage I-II) | Locally Advanced (Stage III) | Metastatic (Stage IV) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV+ N=13 | HIV−N=2093 | P-value | HIV+N=9 | HIV−N=1096 | P-value | HIV+N=13 | HIV−N=1998 | P-value | |
| Surgery | 9 (69%) | 1406 (67%) | 0.99 | 1 (11%) | 311 (28%) | 0.46 | 0 | 156 (8%) | 0.62 |
| Radiation | 4 (31%) | 647 (31%) | 0.99 | 6 (67%) | 737 (67%) | 0.99 | 4 (31%) | 1101 (55%) | 0.08 |
| Systemic therapy | 3 (23%) | 402 (19%) | 0.72 | 6 (67%) | 835 (78%) | 0.45 | 7 (54%) | 1221 (61%) | 0.59 |
Overall median survival was 12.4 months (95% CI: 7.2–20.4) in the HIV-NSCLC cohort versus 22.8 months (95% CI: 21.2–24.1) for the general NSCLC cohort. Kaplan-Meier methods were done to compare survival between both cohorts (Figure 1). One-year survival in HIV-infected versus HIV-uninfected patients was 57.14% (95% CI: 39.28– 71.52) versus 64.41% (95% CI: 63.08–65.71, p=0.39). HIV-NSCLC patients had significantly worse survival at 2 years: 31.43% (95% CI: 17.09– 46.84) versus 48.71% (95% CI: 47.31–50.09) (p=0.028); and at 3 years: 22.86% (95% CI: 10.76–37.64) versus 40.40% (95% CI: 38.99–41.80) (p=0.014). After 3 years, the differences were not significant and limited by small patient numbers in the HIV-NSCLC group.
Figure 1: Survival among HIV-NSCLC and the general NSCLC population.
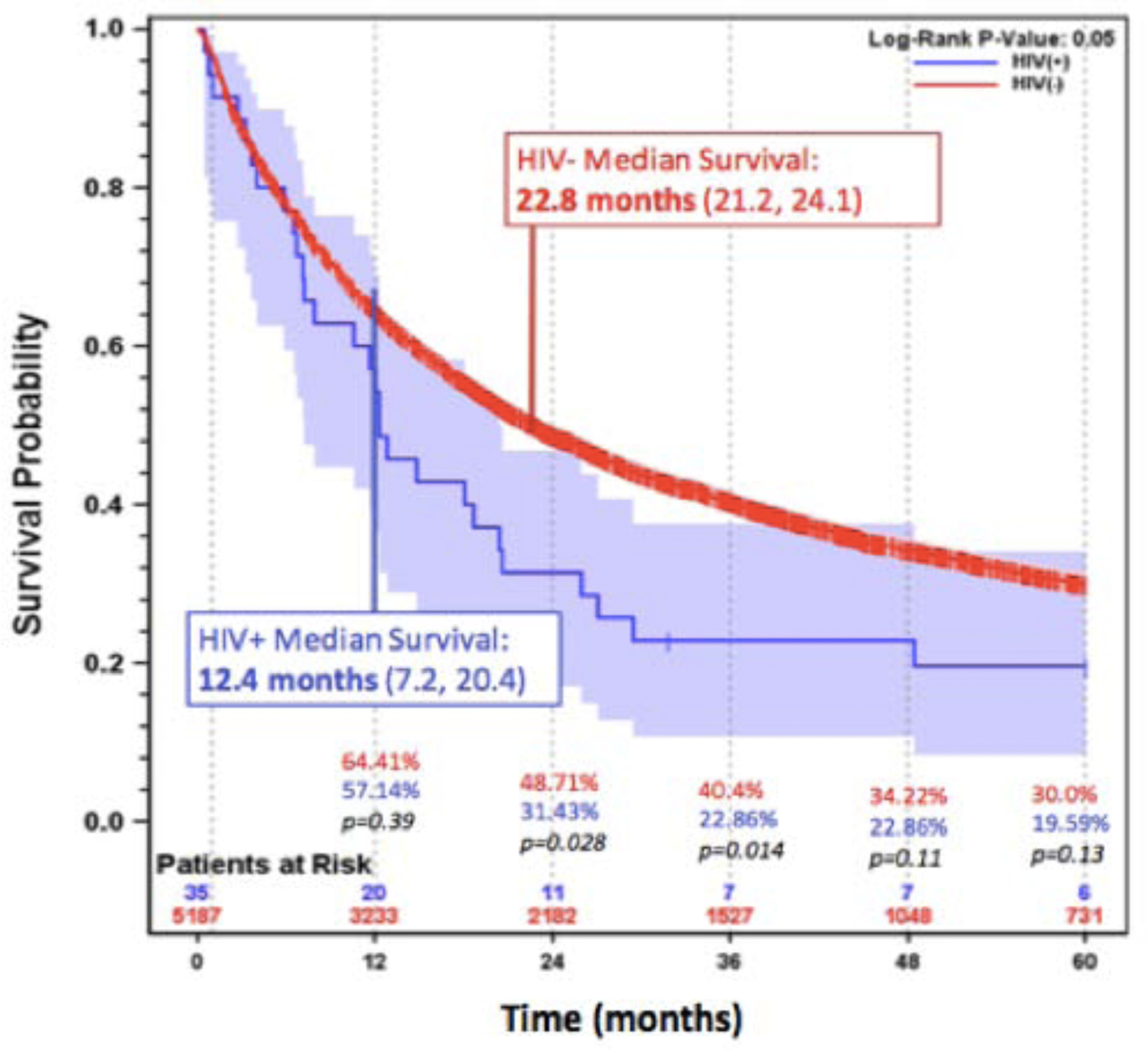
Kaplan Meier Survival for HIV-NSCLC and general NSCLC patients with median survival for each cohort displayed. HIV-NSCLC patients had decreased survival at 2 years (p=0.028) and 3 years (p=0.014) after cancer diagnosis, compared with general NSCLC patients. After 3 years, the differences are not significant.
Using multivariate Cox regression analysis, HIV, age, gender, race, and NSCLC stage were all independently associated with survival (Table 3). HIV status was an independent risk factor for poorer outcomes when controlling for age, sex, race, and stage of cancer with a HR of 1.8 (1.24–2.62). Among PLWH, earlier stage of cancer diagnosis and HIV-1 RNA < 400 copies/ml were associated with better survival (Table 4). There was no association between survival and age, sex, ethnicity, ART status, CD4+ T cell count, history of ADI, or HCV co-infection.
Table 3:
Factors associated with worse survival among all patients with NSCLC
| Multivariate Model | Univariate Model | |||
|---|---|---|---|---|
| Variables | Hazard Ratio | P-value | Hazard Ratio | P-value |
| HIV+ status | 1.80 (1.24, 2.63) | 0.002 | 1.30 (0.90, 1.88) | 0.16 |
| Increased Age | 1.02 (1.02, 1.02) | <0.001 | 1.01 (1.01, 1.02) | <0.001 |
| Male Sex | 1.32 (1.23, 1.41) | <0.001 | 1.44 (1.35, 1.54) | <0.001 |
| Black Race | 1.12 (1.00, 1.25) | 0.05 | 1.19 (1.06, 1.33) | 0.002 |
| Early Stage (I/II) Cancer | 0.26 (0.24, 0.28) | <0.001 | 0.27 (0.25, 0.29) | <0.001 |
Table 4:
Predictors of Survival among HIV-NSCLC Patients
| Mean Survival Time in years (95% CI) | Log-rank P-value | |
|---|---|---|
| Age | ||
| <62 years | 0.99 (0.55, 1.54) | 0.12 |
| >62 years | NA (1.48, NA) | |
| Male | 0.99 (0.55, 1.54) | 0.69 |
| Female | 1.46 (0.27, 3.98) | |
| Black, non-Hispanic | 1.10 (0.54, 2.42) | 0.42 |
| White, non-Hispanic | 1.01 (0.07, 1.54) | |
| Hispanic | 2.18 (0.96, 15.25) | |
| History of ADI | 1.00 (0.48, 1.69) | 0.53 |
| No history of ADI | 1.48 (0.30, NA) | |
| HIV Viral Load | ||
| <400 copies/ml | 1.48 (0.87, 2.42) | 0.023 |
| >400 copies/ml | 0.43 (0.08, 1.68) | |
| On ART | 1.22 (0.59, 2.22) | 0.26 |
| Off ART | 0.66 (0.22, 2.13) | |
| HCV co-infection | 0.97 (0.55, 1.68) | 0.75 |
| None | 1.22 (0.48, 2.42) | |
| Stage I | 15.25 (0.87, 15.25) | <0.001 |
| Stage II | 2.22 (1.48, NA) | |
| Stage III | 0.96 (0.08, 1.68) | |
| Stage IV | 0.54 (0.22, 0.99) | |
| Hazard Ratio per 100 unit increase | P value | |
| CD4 cell count | 0.97 (0.83, 1.14) | 0.752 |
Discussion
Lung cancer is a leading cause of death among people living with HIV (PLWH).4,32 This current study investigated presentation and outcomes of patients with HIV-associated NSCLC compared to the general NSCLC population from a single institution in the combination ART era. We confirm prior reports of younger age at presentation and high prevalence of tobacco use. Patients in this HIV-NSCLC cohort were largely engaged in HIV care with a relatively high percentage of patients on ART and over half with viral suppression. Yale New Haven Hospital has the oldest tumor registry in the country and by utilizing a large group of over 5,000 HIV-uninfected individuals presenting to the same institution with NSCLC, our study was uniquely able to characterize outcomes of patients cared for by the same oncology practice over the same time period. Though presenting with similar stages and histologic types of NSCLC, and receiving generally comparable treatments, patients with HIV had significantly impaired outcomes. In addition, in a multivariate analysis, HIV was found to be significant independent predictor of worse outcome, and only detectable peripheral viral load (> 400 copies/mL) and advanced stage of cancer were predictors of worse outcome among PLWH with NSCLC.
Both HIV-infected and general NSCLC groups had a similar distribution of histological subtypes and stage at lung cancer diagnosis. While some studies have raised concerns for possible oncologic treatment disparities among PLWH,33,34 at our institution, patients with HIV received comparable first-line treatment modalities to the general population, across all stages. HIV-infected individuals also received appropriate chemotherapeutic regimens based on NSCLC treatment guidelines. One limitation is that specific dosing or specific treatment type was not available for the general NSCLC group, precluding direct comparison of completion of treatment regimen. Thus, there remains a possibility that the HIV-infected and general NSCLC groups had diffences in treatment intensity due to tolerability, toxicity, and adherance. Additionally, we note that immunotherapy was not commonly administered to patients in either cohort during the time-period examined While recent studies indicate first-line treatment for NSCLC is effective and tolerable for PLWH35, further studies on use of immunotherapy are needed given the now frequent use of checkpoint inhibitor-based treatment for lung cancer, and the possibility of differential impact on outcomes in PLWH.36–39 Another limitation is that we only had sequencing for oncogenic mutations on a limited number of patients and thus did not include in this analysis. However, prior studies have shown no significant difference in proportions of driver mutations among HIV-associated NSCLC.40
In this study the HIV-NSCLC group had significantly lower overall survival than the general NSCLC cohort (12 vs 23 months), suggesting that disparities in outcomes of NSCLC in patients with HIV requires further investigation. Median two-year and three-year survival was also significantly lower among PLWH. Findings after three years were not significant, but this was likely due to wide confidence intervals from a limited sample size. Additionally, we found that HIV infection itself was an independent risk factor for worse outcomes when adjusted for age, race, sex, and cancer stage. Of note, in a univariate analysis, HIV was not an independent risk factor, emphasizing the importance of controlling for critical variables such as age, sex, race and stage on cancer-related mortality. Though some studies have found that HIV is a predictor for poor outcomes,41 others have not.28,29 The discordance with the latter studies may be due to inability to perform multivariate analyses or small sample size. Deeper investigation into possible NSCLC treatment differences for PWLH, as well as possible biologic differences in HIV-associated cancers should be further explored.
Among the HIV-NSCLC cohort, better survival was associated with HIV-1 RNA < 400 copies/ml and earlier stage at diagnosis. The role of viral replication on treatment outcome may be representative of patients who were potentially less engaged in longitudinal care. However, this finding may also reflect the role of HIV replication on higher levels of underlying inflammation with resulting immune deficits contributing to poor anti-tumor immune responses. Of note, neither CD4+ T cell count or history of ADI were found to be predictors of worse outcomes. Multiple studies have now shown that CD4+ T cell count is not associated with poor outcomes in lung cancer,17,18,42 suggesting immunodeficiency as defined by T cell depletion alone is not a good predictor either for cancer incidence nor for HIV-associated cancer prognosis, supporting the need for more detailed characterization of immune deficits in HIV to understand differences within tumor microenvironment. The lack of correlation of CD4+ T cell count with outcome should be an important consideration when decisions are made about types of cancer-related treatment regimens in these patients.
We also demonstrate with detailed, individualized patient data that there is a high percentage of patients with history of IVDU and Hepatitis C co-infection among patients with HIV-associated NSCLC, higher than the overall HIV population in the New Haven area, raising additional questions about the potential for increased risk of NSCLC among these patient populations. HCV infection has been noted as a risk factor for non-liver related cancers such as lung cancer, head and neck cancer, and lymphoma in the general population, suggesting a role of systemic inflammation in the setting of chronic viral infections.43–46 In addition, the impact of IVDU on lung cancer incidence should be further explored. A single report from Italy found that HIV patients with history of IVDU had increased risk for lung cancer which was not seen in MSM.47 It has also been shown in multiple studies that a history of recurrent bacterial pneumonias are risk factors for lung cancer among PLWH.20,48 Additionally, those with use of intravenous and other illicit drug use are at increased of bacterial pulmonary infections.49 Further study on biology and epidemiology of illicit drug use on cancer risk should be explored.
It is also important to note that demographics different significantly between our HIV-infected group and HIV-uninfected groups and underlying socioeconomic disparities were unable to be measured due to limited medical record data on details such as income, education level, primary language, and housing status. These important social determinants of health could also have contributed to disparate outcomes in this cohort and warrant consideration in clinical practice, in additional to further investigation in future studies.
Recently updated guidance by the U.S. Preventative Services Task Force has proposed screening for lung cancer with annual low-dose computed tomography in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years50, an expansion from prior guidance.51 Even with this expansion, in our cohort 10 patients (28.6%) would not meet criteria by age, and four patients who were within age criteria would not have met it by smoking pack year criteria (11.4%). Therefore, 14 patients (40%) would not have met criteria for lung cancer screening. This suggests that even with expanded guidelines, a substantial proportion of PLWH who are at risk for lung cancer could be missed, in large part due to the younger age at presentation. Further research is ongoing to determine if HIV infected smokers may benefit from lung cancer screening starting at a younger age.
Conclusion
This study provides detailed characterization of PLWH diagnosed with lung cancer from a single urban institution. At the time of cancer diagnosis, the majority of patients were on anti-retroviral therapy and virally suppressed with a relatively high CD4 cell count. Presentation occurred at a younger age with high rates of smoking. High rates of HCV infection and history of IVDU were also noted in this population. PLWH received similar treatment regimens at each stage of diagnosis compared to those without HIV. Overall median survival in PLWH was significantly shorter than the HIV-uninfected population and HIV infection was found to be an independent risk factor for worse outcomes. Among the PLWH, earlier stage of cancer diagnosis and HIV-1 RNA < 400 copies/ml were each independently associated with better survival.
Clinical Practice Points.
This retrospective cohort study importantly highlights a single institution experience with HIV-associated non-small cell lung cancer (NSCLC) over the past two decades. Though there have been registry studies reviewing outcomes in HIV-associated lung cancer, utilizing experience at a single institution has allowed us to obtain detailed patient characteristics and treatment decisions using robust medical record review. Moreover, with the same oncology providers treating both HIV-NSCLC patients and the larger NSCLC population with use of equally aggressive cancer treatments, we see a divergence in outcomes, particularly with early mortality, which has not previously been shown. In addition, we show that in a multivariate analysis, HIV is an independent risk factor for poor outcomes, and that CD4 T-cell count is not.
The medical community of oncology providers should be aware of these findings as lung cancer is now the most common non-AIDS defining malignancy in persons living with HIV and likely to remain a growing problem given the high rates of smoking in this aging population. Further efforts should be made for deeper investigation into possible NSCLC treatment differences for patients with HIV, as well as possible biologic differences in HIV-associated cancers.
Acknowledgments:
We appreciate the support of the Yale Specialized Programs of Research Excellence (SPORE) in Lung Cancer, the Yale Cancer Center Tumor Board Registry, Yale AIDS Care Program.
Funding: Funding received from the National Cancer Institute (NCI), National Institutes of Health (NIH), (R01CA206483, T32AI007433) and NCI, NIH Lung SPORE Developmental Research Program (P50 CA196530).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Sarah Goldberg reports grants and personal fees from AstraZeneca, personal fees from Bristol-Myers Squibb, personal fees from Genentech, personal fees from Eli Lilly, personal fees from Amgen, personal fees from Spectrum, personal fees from Boehringer Ingelheim, all outside the submitted work. All other authors have no financial conflicts of interest to disclose.
References
- 1.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. Journal of the National Cancer Institute. 2015;107(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. International journal of cancer. 2008;123(1):187–194. [DOI] [PubMed] [Google Scholar]
- 3.Vandenhende MA, Roussillon C, Henard S, et al. Cancer-Related Causes of Death among HIV-Infected Patients in France in 2010: Evolution since 2000. PloS one. 2015;10(6):e0129550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. Journal of the National Cancer Institute. 2011;103(9):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morlat P, Roussillon C, Henard S, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS (London, England). 2014;28(8):1181–1191. [DOI] [PubMed] [Google Scholar]
- 6.Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Current opinion in oncology. 2012;24(5):506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet (London, England). 2007;370(9581):59–67. [DOI] [PubMed] [Google Scholar]
- 8.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS (London, England). 2009;23(8):875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette smoking in the HIV-infected population. Proceedings of the American Thoracic Society. 2011;8(3):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess Mortality among HIV-Infected Individuals with Cancer in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(7):1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigel K, Makinson A, Thaler J. Lung cancer in persons with HIV. Curr Opin HIV AIDS. 2017;12(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadranel J, Garfield D, Lavole A, Wislez M, Milleron B, Mayaud C. Lung cancer in HIV infected patients: facts, questions and challenges. Thorax. 2006;61(11):1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet F, Burty C, Lewden C, et al. Changes in cancer mortality among HIV-infected patients: the Mortalite 2005 Survey. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(5):633–639. [DOI] [PubMed] [Google Scholar]
- 15.Winstone TA, Man SFP, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest. 2013;143(2):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiels MS, Cole SR, Wegner S, et al. Effect of HAART on incident cancer and noncancer AIDS events among male HIV seroconverters. Journal of acquired immune deficiency syndromes (1999). 2008;48(4):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS (London, England). 2012;26(8):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirk GD, Merlo C, P OD, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(1):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiels MS, Cole SR, Mehta SH, Kirk GD. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. Journal of acquired immune deficiency syndromes (1999). 2010;55(4):510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shebl FM, Engels EA, Goedert JJ, Chaturvedi AK. Pulmonary infections and risk of lung cancer among persons with AIDS. Journal of acquired immune deficiency syndromes (1999). 2010;55(3):375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Annals of internal medicine. 2010;153(7):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. The Lancet Respiratory medicine. 2014;2(7):583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non–Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clinic proceedings Mayo Clinic. 2008;83(5):584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigel K, Crothers K, Dubrow R, et al. Prognosis in HIV-infected patients with non-small cell lung cancer. British journal of cancer. 2013;109(7):1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biggar RJ, Engels EA, Ly S, et al. Survival after cancer diagnosis in persons with AIDS. Journal of acquired immune deficiency syndromes (1999). 2005;39(3):293–299. [DOI] [PubMed] [Google Scholar]
- 26.Marcus JL, Chao C, Leyden WA, et al. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(8):1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirelli U, Spina M, Sandri S, et al. Lung carcinoma in 36 patients with human immunodeficiency virus infection. The Italian Cooperative Group on AIDS and Tumors. Cancer. 2000;88(3):563–569. [DOI] [PubMed] [Google Scholar]
- 28.Hleyhel M, Belot A, Bouvier AM, et al. Trends in survival after cancer diagnosis among HIV-infected individuals between 1992 and 2009. Results from the FHDH-ANRS CO4 cohort. International journal of cancer. 2015;137(10):2443–2453. [DOI] [PubMed] [Google Scholar]
- 29.Powles T, Thirwell C, Newsom-Davis T, et al. Does HIV adversely influence the outcome in advanced non-small-cell lung cancer in the era of HAART? British journal of cancer. 2003;89(3):457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider E, Whitmore S, Glynn KM, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years--United States, 2008. MMWR Recomm Rep. 2008;57(RR-10):1–12. [PubMed] [Google Scholar]
- 31.Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer Staging Manual, 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 32.Winstone TA, Man SF, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest. 2013;143(2):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suneja G, Shiels MS, Melville SK, Williams MA, Rengan R, Engels EA. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS (London, England). 2013;27(3):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suneja G, Shiels MS, Angulo R, et al. Cancer treatment disparities in HIV-infected individuals in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(22):2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavole A, Greillier L, Mazières J, et al. First-line carboplatin plus pemetrexed with pemetrexed maintenance in HIV-positive patients with advanced non-squamous non-small cell lung cancer: the phase II IFCT-1001 CHIVA trial. The European respiratory journal. 2020;56(2). [DOI] [PubMed] [Google Scholar]
- 36.Cook MR, Kim C. Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients With HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. 2019. [DOI] [PubMed]
- 37.Jain P, Jain C, Velcheti V. Role of immune-checkpoint inhibitors in lung cancer. Ther Adv Respir Dis. 2018;12:1753465817750075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uldrick TS, Gonçalves PH, Abdul-Hay M, et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol. 2019;5(9):1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spano JP, Veyri M, Gobert A, et al. Immunotherapy for cancer in people living with HIV: safety with an efficacy signal from the series in real life experience. AIDS (London, England). 2019;33(11):F13–f19. [DOI] [PubMed] [Google Scholar]
- 40.Thaler J, Sigel C, Beasley MB, et al. Clinically significant mutations in HIV-infected patients with lung adenocarcinoma. British journal of cancer. 2017;117(9):1392–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YH, Shen XD. Human immunodeficiency virus infection and mortality risk among lung cancer patients: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(15):e0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hessol NA, Martinez-Maza O, Levine AM, et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS (London, England). 2015;29(10):1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omland LH, Farkas DK, Jepsen P, Obel N, Pedersen L. Hepatitis C virus infection and risk of cancer: a population-based cohort study. Clin Epidemiol. 2010;2:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, De Luca A, Smith C, et al. Chronic Hepatitis B and C Virus Infection and Risk for Non-Hodgkin Lymphoma in HIV-Infected Patients: A Cohort Study. Annals of internal medicine. 2017;166(1):9–17. [DOI] [PubMed] [Google Scholar]
- 45.Mahale P, Sturgis EM, Tweardy DJ, Ariza-Heredia EJ, Torres HA. Association Between Hepatitis C Virus and Head and Neck Cancers. Journal of the National Cancer Institute. 2016;108(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allison RD, Tong X, Moorman AC, et al. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006–2010. J Hepatol. 2015;63(4):822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serraino D, Boschini A, Carrieri P, et al. Cancer risk among men with, or at risk of, HIV infection in southern Europe. AIDS (London, England). 2000;14(5):553–559. [DOI] [PubMed] [Google Scholar]
- 48.Hooker CM, Meguid RA, Hulbert A, et al. Human immunodeficiency virus infection as a prognostic factor in surgical patients with non-small cell lung cancer. The Annals of thoracic surgery. 2012;93(2):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. The New England journal of medicine. 1995;333(13):845–851. [DOI] [PubMed] [Google Scholar]
- 50.USPSTF Proposes Expanded Lung Cancer Screening. Cancer discovery. 2020;10(9):Of1. [DOI] [PubMed] [Google Scholar]
- 51.Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]


