Abstract
Aim
To assess the predictors and influence of resection margins and the role of neoadjuvant and adjuvant therapy on survival for a national cohort of patients with resected pancreatic cancer.
Methods
Using the National Cancer Data Base between 2004 and 2016, 56,532 patients were identified who underwent surgical resection for pancreatic adenocarcinoma. Univariate and multivariate models were employed to identify factors predicting R0/R1 resection and assess the impact on survival.
Results
In total, 48,367 (85.6%) patients were found to have negative margins (R0) compared to 8165 (14.4%) who had microscopic residual tumor (R1). Factors predicting positive margin on univariate analysis included male gender, Medicare, advanced stage, moderately or poorly differentiated tumor, lymphovascular invasion, and tumors > 2 cm. Factors predicting R0 resection included receipt of neoadjuvant therapy and treatment at an Academic/Research Center. Following adjustment for other factors, margin status remained an independent predictor for overall survival (HR: 1.24; 95% CI 1.22–1.27, p < 0.001) (1-, 3-, and 5-year overall survival rates (R0: 77%, 37%, and 25% vs R1: 62%, 19%, and 10%).
Conclusions
A positive margin predicts a poorer survival than R0 resections regardless of stage and receipt of adjuvant therapy. Several modifiable factors significantly predict the likelihood of R0 resection including neoadjuvant treatment and treatment at Academic/Research Programs. Knowledge about these factors can help guide patient management by offering neoadjuvant treatment modalities at Academic as well as Community hospitals.
Keywords: Margin status, survival, predictors of R1 resection, pancreatic cancer
Introduction
In 2020, it is estimated that 57,600 Americans will be diagnosed with pancreatic cancer and more than 47,050 will die from the disease.1 Pancreatic cancer is now the 3rd leading cause of cancer-related deaths in the USA surpassing breast cancer.2 Curative resection is crucial for survival of patients with pancreatic adenocarcinoma (PDAC); however, only 20 to 30% of patients with pancreatic cancer have resectable disease at time of diagnosis.3 Long-term outcomes remain poor, even after resection, with an approximate survival rate of 37% for localized and 12% for regional disease.4 The long-term prognosis of patients undergoing surgery is determined by both pathologic and molecular characteristics of the tumor. Pathologic prognostic factors include stage, grade, size, and the resection margin status. 5-8 Knowledge of these factors can help with the selection of patients who should receive neoadjuvant or adjuvant treatment.9
Despite optimization and standardization of surgical procedures by highly trained experts, surgical resection is not always successful at removing the tumor in its entirety. The presence of positive surgical margins after resection of PDAC is a major factor associated with poor patient prognosis, and rates of margin-positive resection are often considered as quality metrics in research studies.10 A positive margin is often correlated with the quality of surgery and pathological examination of the specimen, as low R1 rates are often seen as an indicator of a high-quality care in high-volume centers. In addition, R1 rates could also reflect a more aggressive tumor biology. The current incidence of R0 resection varies widely within the literature from 15–92% with median overall 5-year survival rates of 24.9% with R0 vs 18.7% with R1 resection.3
The wide variation of reported predictors and rates of positive margins in the literature coming from single or multi-institutional studies preclude meaningful comparison of data.
The aim of the current study is to determine predictors of a positive margin and its true prognostic value. Furthermore, we will analyze the role of neoadjuvant therapy on survival outcomes and the benefit of adjuvant therapy based on margin status.
Methods
Design and Data Sources
A retrospective cohort study was conducted using data from the National Cancer Database (NCDB). The NCDB was established by the American College of Surgeons and Commission on Cancer in 1989 and includes data from all Commission on Cancer-accredited hospitals in the USA and Puerto Rico. It is estimated to include approximately 70% of new cancer diagnoses and is comprised of more than 30 million records from 1500 hospitals. The database also includes census tract-level data from the US Census Bureau’s American Community Survey, which provides estimates of patient income, educational attainment, and urban/rural status.
Participants and Variables
We included all patients aged 18 or older who were diagnosed with pancreatic adenocarcinoma and underwent surgery between the years of 2004 and 2016. Patients were included if they had R0 (negative margin) or R1 (microscopically positive margin) resection performed. Exclusion criteria included macroscopic-positive margins, unknown margin status, the presence of metastatic disease at time of diagnosis, no surgery performed, missing information about chemotherapy, and pathologic staging. Demographic data including age, sex, race/ethnicity, and insurance type were collected at patient level, while proxy measures of socioeconomic status were derived from the 2012 American Community Survey for each patient’s home ZIP code. These included ZIP code-level measures of median household income and educational attainment measured as the proportion of patients in the ZIP code with less than a high school diploma. Survival data on the cohort was available from the years 2004–2015. Patient urban/rural location was determined at the ZIP code level from the 2012 American Community Survey, and travel distance was measured as the haversine distance in miles between the center of the patient’s ZIP code and the address of the hospital where they underwent surgery.
Statistical Analysis
To identify factors associated with margin status, the Wilcoxon rank-sum test and chi-square or Fisher’s exact test were used to compare baseline characteristics for each outcome of interest. We used univariable logistic regression to calculate unadjusted odds ratios and 95% confidence intervals and included variables reaching significance level of P < 0.20 in a multivariable logistic regression model. Overall survival rates were calculated as the time from date of diagnosis to death or last follow-up. Overall survival was estimated by the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazards modeling was used to evaluate the impact of margin status on survival while adjusting for potential confounders. All analyses were conducted using Stata 13 (StataCorp, College Station, TX).
Results
Characteristics of R0 and R1
A total of 56,532 patients with clinically diagnosed pancreatic adenocarcinoma who underwent surgery between 2004 and 2016 were identified. Microscopically negative margins (R0) were found in 48,367 (85.6%), whereas microscopic residual tumor (R1) was present in 8165 (14.4%) patients. Patients with a positive margin were more likely to be above 70 years, male, on Medicare, had a higher Charlson-Deyo Score, and were treated at non-academic centers. In addition, R1 patients were found to have more often poorly differentiated tumors and stage II/III disease, and fewer patients received neoadjuvant treatment.
Patients with R0 resection had significantly shorter hospital stays with a median of 8 days (ICR 6–12 days) vs 9 days (ICR 7–14 days) in R1 patients (p < 0.001). Unplanned 30-day readmission rate was higher in patients with R1 resection (8.2% vs 6.9%, p < 0.001). Thirty-day and 90-day mortality were 4.4% and 9.0% in patients with R1 resection vs 2.7% and 5.4%, respectively (p < 0.001). Patient, clinicopathologic, and treatment characteristics of R0 vs R1 are listed in Table 1.
Table 1.
Characteristics of patients with R0 vs R1 resection margin
| R0 48,367 |
R1 8165 |
P value | |
|---|---|---|---|
| Characteristics | |||
| Age at diagnosis, median (IQR) | 67 (59, 74) | 67 (59, 74) | < 0.001 |
| Age < 70 | 29,162 (60.3%) | 4750 (58.2%) | < 0.001 |
| Age ≥ 70 | 19,205 (39.7%) | 3415 (41.8%) | |
| Sex, N (%) | < 0.001 | ||
| Male | 24,261 (50.2%) | 4322 (52.9%) | |
| Female | 24,106 (49.8%) | 3843 (47.1%) | |
| Race, N (%) | 0.005 | ||
| White | 39,397 (81.5%) | 6710 (82.2%) | |
| African-American | 4688 (9.7%) | 703 (8.6%) | |
| Asian/PI | 1239 (2.6%) | 242 (3.0%) | |
| Other/unknown | 2228 (4.6%) | 387 (4.7%) | |
| Insurance type, N (%) | 0.001 | ||
| Private | 18,352 (37.9%) | 2973 (36.4%) | |
| Medicaid | 2234 (4.6%) | 367 (4.5%) | |
| Medicare | 25,431 (52.6%) | 4479 (54.9%) | |
| Not insured | 687 (1.4%) | 94 (1.2%) | |
| Other/unknown | 1663 (3.4%) | 252 (3.1%) | |
| Income ($USD), N (%) | 0.074 | ||
| < $38,000 | 7637 (15.8%) | 1199 (14.7%) | |
| $38,000–$47,999 | 10,786 (22.4%) | 1843 (22.7%) | |
| $48,000–$62,999 | 12,925 (26.8%) | 2183 (26.8%) | |
| > $63 k | 16,835 (34.9%) | 2906 (35.7%) | |
| Education, N (%) | 0.230 | ||
| 21%+ | 7134 (14.8%) | 1161 (14.3%) | |
| 13–20.9% | 12,164 (25.2%) | 2057 (25.3%) | |
| 7–12.9% | 16,017 (33.2%) | 2785 (34.2%) | |
| < 7% | 12,888 (26.7%) | 2129 (26.2%) | |
| Patient urban/rural location, N (%) | 0.252 | ||
| Metro areas | 39,358 (81.4%) | 6701 (82.1%) | |
| Urban Metro-Adjacent | 4890 (10.1%) | 775 (9.5%) | |
| Urban Not Metro-Adjacent | 1944 (4.0%) | 311 (3.8%) | |
| Rural | 2175 (4.5%) | 378 (4.6%) | |
| Charlson-Deyo Score, N (%) | < 0.001 | ||
| 0 | 31,827 (65.8%) | 5096 (62.4%) | |
| 1 | 12,472 (25.8%) | 2272 (27.8%) | |
| 2 | 2912 (6.0%) | 560 (6.9%) | |
| >3 | 1156 (2.4%) | 237 (2.9%) | |
| Differentiation | < 0.001 | ||
| Well differentiated, differentiated, NOS | 4891 (10.1%) | 671 (8.2%) | |
| Moderately differentiated, moderately well differentiated, intermediate differentiation | 22,266 (46.0%) | 3814 (46.7%) | |
| Poorly differentiated | 14,434 (29.8%) | 2915 (35.7%) | |
| Undifferentiated, anaplastic, unknown | 6776 (14.1%) | 765 (9.4%) | |
| Stage | |||
| Stage I | 8828 (18.3%) | 469 (5.7%) | < 0.001 |
| Stage II | 38,328 (79.2%) | 7173 (87.9%) | |
| Stage III | 1211 (2.5%) | 523 (6.4%) | |
| Neoadjuvant treatment | < 0.001 | ||
| No neoadjuvant | 35,672 (83.2%) | 6159 (87.1%) | |
| Neoadjuvant chemoradiation (CRT) | 3530 (8.2%) | 358 (5.1%) | |
| Neoadjuvant chemotherapy | 3564 (8.3%) | 539 (7.6%) | |
| Neoadjuvant radiation | 121 (0.3%) | 15 (0.2%) | |
| Adjuvant treatment | 17,658 (41.2%) | 2453 (34.7%) | |
| No adjuvant | 17,658 (41.2%) | 2453 (34.7%) | |
| Adjuvant chemoradiation | 9716 (22.7%) | 2531 (35.8%) | |
| Adjuvant chemotherapy | 14,960 (34.9%) | 1926 (27.2%) | |
| Adjuvant radiation | 553 (1.3%) | 161 (2.3%) | |
| Surgical approach | < 0.001 | ||
| Open or approach unspecified | 20,721 (77.8%) | 3463 (80.6%) | |
| Robotic assisted | 892 (3.3%) | 134 (3.1%) | |
| Robotic converted to open | 136 (0.5%) | 32 (0.7%) | |
| Laparoscopic | 3690 (13.8%) | 439 (10.2%) | |
| Laparoscopic converted to open | 1207 (4.5%) | 231 (5.4%) | |
| Type of surgery | < 0.01 | ||
| Partial pancreatectomy | 7661 (15.8%) | 1057 (12.9%) | |
| Local or partial pancreatectomy and duodenectomy | 3729 (7.7%) | 544 (6.7%) | |
| Without distal/partial gastrectomy | 4590 (9.5%) | 851 (10.4%) | |
| With partial gastrectomy (Whipple) | 22,464 (46.4%) | 4216 (51.6%) | |
| Total pancreatectomy | 1825 (3.8%) | 233 (2.9%) | |
| Total pancreatectomy and subtotal gastrectomy/duodenectomy | 4332 (9.0%) | 761 (9.3%) | |
| Extended pancreatoduodenectomy | 2730 (5.6%) | 425 (5.2%) | |
| Other | 1036 (2.1%) | 414 (1.0%) | |
| Hospital type, N (%) | 1682 (3.5%) | 320 (4.0%) | < 0.001 |
| Community Cancer Program | |||
| Comprehensive Community Cancer Program | 12,456 (26.2%) | 2284 (28.2%) | |
| Academic/Research Program | 27,373 (57.6%) | 4192 (51.8%) | |
| Integrated Network Cancer Program | 6050 (12.7%) | 1297 (16.0%) | |
| Surgical inpatient stay, days from surgery, median (IQR) | 8 (6, 12) | 9 (7, 14) | < 0.001 |
| Readmission within 30 days of surgical discharge | < 0.001 | ||
| Unplanned readmission within 30 days of discharge | 3354 (6.9%) | 672 (8.2%) | |
| 30-day mortality | < 0.001 | ||
| Patient died 30 or fewer days after surgery performed | 1149 (2.7%) | 324 (4.4%) | |
| 90-day mortality | < 0.001 | ||
| Patient died 90 or fewer days after surgery performed | 2314 (5.4%) | 671 (9.0%) |
Factors Predicting Resection Margin
Male gender, age > 70, lower education, stage II or greater, tumor size > 2 cm, moderately or poorly differentiated tumors, and presence of lymphovascular invasion were associated with a positive margin using univariable logistic regression. In contrast, patients who had neoadjuvant chemoradiation, neoadjuvant chemotherapy, laparoscopic surgery, or treatment at Academic/Research Programs or Integrated Network Cancer Programs were more likely to have R0 resection. Among patients who received neoadjuvant therapy, the addition of radiation to chemotherapy improved the negative margin rate. On multivariable analysis, factors including tumor size, stage II or greater, poor differentiation, presence of lymphovascular invasion, and Medicare remained significant factors for a positive margin. Neoadjuvant chemoradiation and treatment at Academic/Research Programs remained independent predictors of R0 resection (Table 2).
Table 2.
Factors associated with R1 margin following pancreas resection (univariable and multivariable analysis)
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Characteristics | OR (95% conf. interval | P value | HR (95% conf. interval | P value |
| Age (cutoff) (70 vs greater 70) | 1.09 (1.04–1.15) | 0.001 | 0.99 (0.90–1.08) | 0.863 |
| Sex | < 0.001 | |||
| Male | 1.00 Reference | 1.00 Reference | ||
| Female | 0.90 (0.85–0.93) | < 0.001 | 0.91 (0.84–0.96) | 0.009 |
| Race | 0.005 | 0.062 | ||
| White | 1.00 Reference | 1.00 Reference | ||
| Afr Am | 0.88 (0.91–0.96) | 0.003 | 0.92 (0.81–1.05) | 0.229 |
| Asian/PI | 1.15 (0.99–1.32) | 0.055 | 1.17 (0.94–1.46) | 0.159 |
| Hispanic | 1.02 (0.91–1.14) | 0.729 | 0.99 (0.84–1.19) | 0.988 |
| Other/unknown | 0.89 (0.73–1.07) | 0.216 | 0.65 (0.46–0.94) | 0.021 |
| Income | 0.072 | 0.712 | ||
| < 38,000 | 1.00 Reference | 1.00 Reference | ||
| 38,000–47,999 | 1.09 (1.01–1.18) | 0.034 | 1.06 (0.94–1.20) | 0.990 |
| 48,000–62,999 | 1.08 (1.00–1.16) | 0.059 | 1.03 (0.91–1.16) | 0.471 |
| > 63 k | 1.10 (1.02–1.18) | 0.010 | 1.01 (0.90–1.13) | 0.181 |
| Insurance | < 0.001 | < 0.001 | ||
| No insurance | 1.00 Reference | 1.00 Reference | ||
| Medicaid | 1.01 (0.90–1.14) | 0.815 | 0.93 (0.77–1.12) | 0.431 |
| Medicare | 1.09 (1.03–1.14) | 0.001 | 1.12 (1.02–1.22) | 0.018 |
| Not insured | 0.94 (0.81–1.07) | 0.343 | 0.90 (0.73–1.11) | 0.322 |
| Other/unknown | 0.84 (0.68–1.05) | 0.131 | 1.10 (0.88–1.57) | 0.605 |
| Education | 0.233 | |||
| 21%+ | 1.00 Reference | |||
| 13–20.9% | 1.10 (1.00–1.17) | 0.034 | ||
| 7–12.9% | 1.08 (0.99–1.16) | 0.059 | ||
| < 7% | 1.10 (1.02–1.18) | 0.010 | ||
| Size | < 0.001 | < 0.001 | ||
| < 2 cm | 1.00 Reference | 1.00 Reference | ||
| 2–5 cm | 2.17 (1.97–2.39) | < 0.001 | 1.76 (1.52–2.04) | < 0.001 |
| > 5 cm | 2.70 (2.42–3.02) | < 0.001 | 1.97 (1.67–2.33) | < 0.001 |
| Tumor grade | < 0.001 | < 0.001 | ||
| Well differentiated | 1.00 Reference | 1.00 Reference | ||
| Moderately differentiated, moderately well differentiated, intermediate differentiation | 1.24 (1.14–1.36) | 0.001 | 1.09 (0.94–1.25) | 0.245 |
| Poorly differentiated | 1.47 (1.35–1.61) | 0.001 | 1.24 (1.08–1.44) | 0.003 |
| Undifferentiated, anaplastic, unknown | 1.18 (0.94–1.48 | 0.154 | 0.84 (0.58–1.22) | 0.365 |
| Stage | < 0.001 | < 0.001 | ||
| Stage 0/1 | 1.00 Reference | 1.00 Reference | ||
| Stage II | 3.52 (3.12–3.88) | < 0.001 | 2.70 (2.29–3.19) | < 0.001 |
| Stage III | 8.13 (7.08–9.34) | < 0.001 | 6.71 (5.31–8.48) | < 0.001 |
| Neoadjuvant treatment | < 0.001 | < 0.001 | ||
| No neoadjuvant | 1.00 Reference | 1.00 Reference | ||
| Neoadjuvant chemoradiation (CRT) | 0.59 (0.53–0.66) | 0.001 | 0.68 (0.57–0.79) | < 0.001 |
| Neoadjuvant chemotherapy | 0.88 (0.80–0.96) | 0.006 | 0.96 (0.83–1.11) | 0.570 |
| Neoadjuvant radiation | 0.72 (0.42–1.23) | 0.227 | 0.86 (0.39–1.92) | 0.717 |
| Surgical approach | < 0.001 | < 0.001 | ||
| Open | 1.00 Reference | 1.00 Reference | ||
| Robotic assisted | 0.90 0.75–1.08) | 0.259 | 0.91 (0.72–1.14) | 0.412 |
| Robotic converted to open | 1.41 (0.96–2.10) | 0.083 | 1.08 (0.63–1.84) | 0.774 |
| Laparoscopic | 0.71 (0.64–0.79) | < 0.001 | 0.72 (0.63–0.81) | < 0.001 |
| Laparoscopic converted to open | 1.15 (0.99–1.32) | 0.067 | 1.04 (0.64–0.81) | 0.684 |
| LN - examined | ||||
| < 12 | 1.00 Reference | 1.00 Reference | ||
| > = 12 | 1.1 (1.0–1.10) | 0.045 | 1.04 (0.96–1.13) | 0.340 |
| Lymphovascular invasion | < 0.001 | < 0.001 | ||
| Not present | 1.00 Reference | 1.00 Reference | ||
| Present | 1.93 (1.81–2.06) | < 0.001 | 1.57 (1.45–1.70) | < 0.001 |
| Facility type | < 0.001 | < 0.001 | ||
| Community Cancer Program | 1.00 Reference | 1.00 Reference | ||
| Comprehensive Community Cancer Program | 0.96 (0.85–1.09) | 0.571 | 0.78 (0.60–1.01) | 0.060 |
| Academic/Research Program | 0.8 (0.71–0.91) | 0.001 | 0.58 (0.45–0.75) | < 0.001 |
| Integrated Network Cancer Program | 1.13 (0.99–1.29) | 0.080 | 0.74 (0.56–0.96) | 0.023 |
Neoadjuvant and Adjuvant Treatment Strategies Based on Margin Status
Information on treatment strategies was available in 49,958 patients. Patients undergoing neoadjuvant chemoradiation (OR 0.59, 95% CI 0.53–0.66, p < 0.001) or neoadjuvant chemotherapy (OR 0.88, 95% CI 0.80–0.96, p = 0.006) were more likely to achieve R0 status compared to those without neoadjuvant treatment (Table 1). Adjuvant treatment strategies were significantly different between both groups. Patients with R1 resection were more likely to receive some form of adjuvant therapy (R1 65.3% vs R0 58.8%, p < 0.001).
Survival and Margin Status
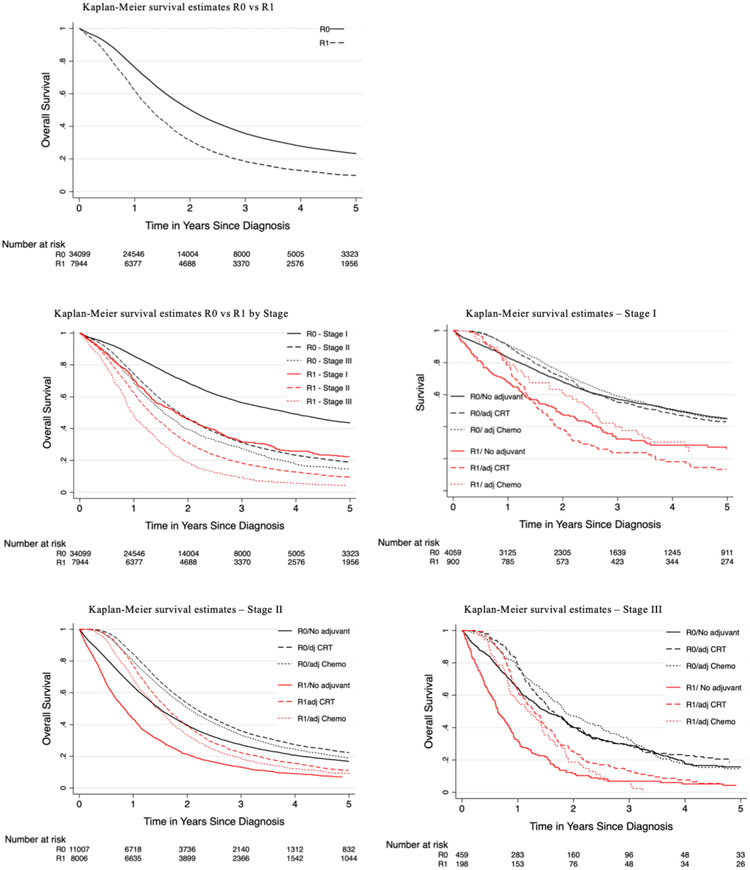
Significant factors predicting survival on univariable analysis included female gender (HR 0.97, 95% CI 0.94–0.99, p < 0.049), income > 48 k (HR 0.90, 95% CI (0.87–0.93), p < 0.001), neoadjuvant chemoradiation (HR 0.86, 95% CI (0.83–0.90), p < 0.001), neoadjuvant chemotherapy (HR 0.80, 95% CI 0.77–0.84, p < 0.001), adjuvant chemoradiation (HR 0.85, 95% CI 0.83–0.87, p < 0.001), and adjuvant chemotherapy (HR 0.80, 95% CI (0.84–0.88), p < 0.001). Following adjustment, the following factors remained independent predictors: female gender (HR 0.96, 95% CI (0.94–0.99), p < 0.049), neoadjuvant chemoradiation (HR 0.89, 95% CI (0.84–0.95), p < 0.001) or chemotherapy (HR 0.84, 95% CI (0.79–0.89), p < 0.001), adjuvant chemoradiation (HR 0.64, 95% CI (0.60–0.66), p < 0.001), adjuvant chemotherapy (HR 0.72, 95% CI (0.69–0.74), p < 0.001), and treatment at an Academic/Research Program (HR 0.76, 95% CI (0.67–0.85), p < 0.001). Factors with negative prognosis included tumor size > 2 cm (HR 1.38, 95% CI (1.31–1.46), p < 0.001), stage II (HR 1.68, 95% CI (1.60–1.78), p < 0.001) or stage III (HR 2.13, 95% CI (1.92–2.36), p < 0.001), non-private insurance (Medicaid HR 1.18, 95% CI (1.10–1.28), p < 0.001), poor differentiation (HR 1.87, 95% CI (1.76–2.00), p < 0.001), and lymphovascular invasion (HR 1.26, 95% CI (1.22–1.31), p < 0.001) (Table 3). 1-, 2-, 3-, and 5-year overall survival rates were significantly better for patients who had R0 vs R1 resection (R0 77%, 52%, 37%, 25% versus 62%, 32%, 19%, 10%) combining all stages. Patients with R0 resection demonstrated consistently better survival as compared to R1 regardless of the use of adjuvant chemoradiation or chemotherapy for patients with R1 resection margin (Fig. 1). Patients also had improved survival with R0 resection at every stage compared with those patients who underwent R1 resection (Fig. 2).
Table 3.
Factors predicting survival following pancreatic surgery
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Characteristics | HR (95% conf. interval | P value | HR (95% conf. interval | P value |
| Age (cutoff) (70 vs greater 70) | 1.24 (1.21–1.27) | 0.001 | 1.16 (1.12–1.21) | < 0.001 |
| Sex | ||||
| Male | 1.00 Reference | 1.00 Reference | ||
| Female | 0.93 (0.91–0.95) | < 0.001 | 0.96 (0.94–0.99) | 0.049 |
| Race | < 0.001 | < 0.001 | ||
| White | 1.00 Reference | 1.00 Reference | ||
| Afr Am | 0.96 (0.93–0.99) | 0.029 | 0.98 (0.93–1.03) | 0.440 |
| Asian/PI | 0.80 (0.75–0.87) | < 0.001 | 0.82 (0.74–0.91) | < 0.001 |
| Hispanic | 0.85 (0.80–0.89) | < 0.001 | 0.86 (0.80–0.94) | < 0.001 |
| Other/unknown | 0.90 (0.83–0.85) | 0.013 | 0.83 (0.73–0.95) | 0.008 |
| Income | < 0.001 | < 0.001 | ||
| < 38,000 | 1.00 Reference | 1.00 Reference | ||
| 38,000–47,999 | 0.96 (0.93–1.00) | 0.035 | 0.93 (0.88–0.98) | 0.008 |
| 48,000–62,999 | 0.90 (0.87–0.93) | < 0.001 | 0.90 (0.85–0.95) | < 0.001 |
| > 63 k | 0.83 (0.81–0.86) | < 0.001 | 0.82 (0.77–0.87) | < 0.001 |
| Insurance | < 0.002 | < 0.001 | ||
| Private insurance | 1.00 Reference | 1.00 Reference | ||
| Medicaid | 1.16 (1.11–1.23) | < 0.001 | 1.18 (1.10–1.28) | < 0.001 |
| Medicare | 1.32 (1.30–1.35) | < 0.001 | 1.15 (1.11–1.20) | < 0.001 |
| Not insured | 1.07 (1.00–1.14) | 0.023 | 1.11 (1.02–1.21) | 0.016 |
| Other/unknown | 1.18 (1.08–1.29) | < 0.001 | 1.06 (0.91–1.24) | 0.437 |
| Education | < 0.001 | 0.213 | ||
| > 21% | 1.00 Reference | 1.00 Reference | ||
| 13–20.9% | 0.99 (0.96–1.02) | 0.568 | 1.05 (0.99–1.10) | 0.095 |
| 7–12.9% | 0.92 (0.89–0.95) | < 0.001 | 1.04 (0.99–1.10) | 0.98 |
| <7% | 0.87 (0.84–0.90) | < 0.001 | 1.01 (0.95–1.08) | 0.95 |
| Size | < 0.001 | < 0.001 | ||
| < 2 cm | 1.00 Reference | 1.00 Reference | ||
| 2–5 cm | 1.60 (1.56–1.66) | < 0.001 | 1.38 (1.31–1.46) | < 0.001 |
| > 5 cm | 1.67 (1.60–1.74) | < 0.001 | 1.54 (1.45–1.65) | < 0.001 |
| Grade | < 0.001 | < 0.001 | ||
| Well differentiated | 1.00 Reference | 1.00 Reference | ||
| Moderately differentiated, moderately well differentiated, intermediate differentiation | 1.56 (1.52–1.64) | < 0.001 | 1.46 (1.37–1.55) | < 0.001 |
| Poorly differentiated | 2.06 (1.98–2.15) | < 0.001 | 1.87 (1.76–2.00) | < 0.001 |
| Undifferentiated, anaplastic, unknown | 1.88 (1.71–2.07) | < 0.001 | 1.72 (1.49–1.99) | < 0.001 |
| Stage | < 0.001 | < 0.001 | ||
| Stage 0/1 | 1.00 Reference | 1.00 Reference | ||
| Stage II | 2.00 (1.93–2.06) | < 0.001 | 1.68 (1.60–1.78) | < 0.001 |
| Stage III | 2.56 (2.41–2.72)) | < 0.001 | 2.13 (1.92–2.36) | < 0.001 |
| Neoadjuvant treatment | < 0.001 | < 0.001 | ||
| No neoadjuvant | 1.00 Reference | 1.00 Reference | ||
| Neoadjuvant chemoradiation (CRT) | 0.86 (0.83–0.90) | < 0.001 | 0.89 (0.84–0.95) | < 0.001 |
| Neoadjuvant chemotherapy | 0.80 (0.77–0.84) | < 0.001 | 0.84 (0.79–0.89) | < 0.001 |
| Neoadjuvant radiation | 0.92 (0.74–1.15) | 0.090 | 0.76 (0.53–1.1) | 0.14 |
| Adjuvant treatment | < 0.001 | < 0.001 | ||
| No adjuvant | 1.00 Reference | 1.00 Reference | ||
| Adjuvant CRT | 0.85 (0.83–0.87) | 0.001 | 0.64 (0.60–0.66) | 0.001 |
| Adjuvant chemo | 0.86 (0.84–0.88) | 0.001 | 0.72 (0.69–0.74) | 0.001 |
| Adjuvant XRT | 0.92 (0.84–1.01) | 0.090 | 0.84 (073–0.97) | 0.021 |
| Surgical approach | < 0.001 | < 0.001 | ||
| Robotic assisted | 1.00 Reference | 1.00 Reference | ||
| Robotic converted to open | 1.25 (0.97–1.61) | 0.085 | 1.08 (0.83–1.40) | 0.591 |
| Laparoscopic | 1.10 (0.98–1.21) | 0.113 | 1.06 (0.95–1.18) | 0.310 |
| Laparoscopic converted to open | 1.32 (1.17–1.49) | < 0.001 | 1.15 (1.01–1.31) | 0.029 |
| Open or approach unspecified | 1.28 (1.16–1.42) | < 0.001 | 1.19 (1.07–1.31) | 0.001 |
| LN-examined | ||||
| < 12 | 1.00 Reference | 1.00 Reference | ||
| > = 12 | 1.1 (1.0–1.10) | 0.045 | 1.04 (0.96–1.13) | 0.340 |
| Margin status | ||||
| R0 | 1.00 Reference | 1.00 Reference | ||
| R1 | 1.34 1(1.26–1.42) | < 0.001 | 1.24 (1.22–1.27) | < 0.001 |
| Lymphovascular invasion | ||||
| Not present | 1.00 Reference | 1.00 Reference | ||
| Present | 1.54 (1.49–1.58) | < 0.001 | 1.26 (1.22–1.31) | < 0.001 |
| Type of facility | < 0.001 | < 0.001 | ||
| Community Cancer Program | 1.00 Reference | 1.00 Reference | ||
| Comprehensive Community Cancer Program | 1.06 (1.0–1.22) | 0.047 | 0.94 (0.83–1.06) | 0.342 |
| Academic/Research Program | 0.92 (0.87–0.97) | 0.003 | 0.76 (0.67–0.85) | < 0.001 |
| Integrated Network Cancer Program | 1.08 (1.01–1.14) | 0.017 | 0.89 (0.78–1.00) | 0.058 |
| Year of diagnosis | < 0.001 | < 0.001 | ||
Fig. 1.
Kaplan–Meier survival curves demonstrating 5-year survival for pancreatic adenocarcinoma comparing patients with R0 vs R1 resection margin with/without adjuvant treatment
Fig. 2.
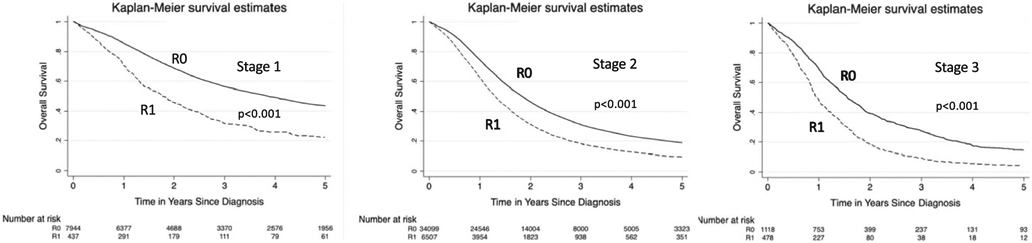
Kaplan–Meier survival graphs by stage. Kaplan–Meier curves demonstrating 5-year survival for pancreatic adenocarcinoma comparing patients with R0 vs R1 resection margin at every stage
Discussion
Curative resection is crucial for survival in patients with pancreatic cancer; despite medical and surgical advances, this is not always achieved.11 The presence of positive surgical margins remains one of the crucial factors which has been associated with poor prognosis.10,12,13 However, the impact of microscopically positive resection margins (R1) on patient outcomes and survival differs broadly in the literature.14 In the current study, we found several variables that were independent predictors for R0 resection including neoadjuvant treatment and treatment at Academic Centers. Furthermore, we found that a positive margin independently predicted a worse survival at every stage despite the use of adjuvant therapy.
Several clinical trials have studied variables that affect outcomes in patients with pancreatic cancer, and many have shown a survival benefit of adjuvant therapy following curative resection.15-17 However, the downside of adjuvant therapy is that close to 50% of patients drop out and fail to complete adjuvant therapy.17 With this in mind, emphasis has been put on the use neoadjuvant chemotherapy in resectable and borderline resectable disease.15 Neoadjuvant therapy can help with downstaging and treatment of occult metastasis which are present in 17% of patients with resectable disease, better patient compliance, and increase chance for margin negative resection.18-20 Our analysis identified several factors that were predictors for positive margin following resection; in addition, we found that neoadjuvant treatment was an independent predictor that increased the likelihood for achieving R0 resection status. It is therefore important to consider neoadjuvant therapy for patients with resectable disease if they have known risk factors for a positive margin.
With more patients suffering from pancreatic cancer, studies have also compared the perioperative, recurrence, and overall survival outcomes between different types of hospitals which have started a debate on centralization for certain surgical procedures.21-23 Several studies have shown improved outcomes based on a variety of performance metrics and increased disease-free and overall survival in patients being cared for at Academic Centers. Differences in the outcomes following R0 resection and long-term survival between high and low volume centers are not necessarily just related to surgical expertise. It is important to consider the role of general competence and the availability of a multidisciplinary team including oncology and interventional radiology, in addition to enhanced ICU care which is more frequently established at high volume Academic Centers.22,24
Furthermore, resection margin status is believed to be an important key prognostic factor. The rates of margin involvement, local tumor recurrence, and overall survival of pancreatic cancer patients are often conflicting.14 Recent studies have raised the concern that the discrepancies between margin status and clinical outcome are caused by frequent underreporting of microscopic margin involvement.13,14 In addition, there remains a lack of standardization of pathological examination, different nomenclature, as well as involvement and underreporting of microscopic/macroscopic-positive resections margins by pathologists or surgeons.13 Controversy regarding the microscopic margin is also present as definitions have changed over the last decades (R0: > 1 mm vs no tumor on ink).25 All these factors have resulted in the broad variety of reported R1 rates that preclude meaningful comparison of clinicopathological correlation and outcomes.14 In the current study, we compared patients with negative (R0) and those with microscopically positive resection margins (R1). We found that patients with R0 margin status had better overall survival regardless of stage and adjuvant treatment strategies compared to the R1 group. These findings are supported by Gnerlich et. al. who showed in a prospective trial that patients with positive posterior margin had significantly poorer local recurrence-free survival compared with patients with a negative margin regardless of lymph node involvement.13
As with any retrospective cohort study, the current analysis has some limitations. Although NCDB is a powerful resource for studying national trends and hospital-level variation, it does not include the level of granularity necessary to reach substantial conclusions that could otherwise be provided by randomized trials. One limitation is the lack consensus of R0 vs R1 resection margin; it is unclear if the defined R0 resection margin in the database is R0 equal to 1 mm or no tumor on ink as definitions have changed over recent years. It is therefore unclear what the reliable rates of R0 and R1 resection margin in the study population are. Another limitation is the lack of granularity of neoadjuvant and adjuvant chemoradiation or chemotherapy, it is unclear what regimen patients received, the duration, and how many patients finished their treatment or dropped out. However, the data presented in this study is novel, has been derived from a large comprehensive cancer database, and highlights the incidence, factors, and prognosis associated with surgical margin and use of neoadjuvant therapy.
Conclusion
The current study analyzed a large cohort of patients with pancreatic cancer and found several factors that predicted the likelihood of R1 resection. Those included male gender, age > 70, lower education (univariate analysis) as well as tumor size, advanced stage, poor differentiation, presence of lymphovascular invasion, and Medicare (multivariate analysis). There was a higher chance of patients achieving R0 resection following neoadjuvant chemoradiation and treatment at Academic/Research Programs. It is therefore important to consider using neoadjuvant treatment strategies for patients with resectable disease to improve long-term survival.
Footnotes
Conflict of interest statement All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund: C.K. S.K.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD & Jemal A Cancer statistics, 2020. CA Cancer J Clin 70, 7–30 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Morrison AH, Byrne KT & Vonderheide RH Immunotherapy and prevention of pancreatic cancer. Trends Cancer 4, 418–428(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tummers WS et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br. J. Surg. 106, 1055–1065 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Survival Rates for Pancreatic Cancer. at <https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html>
- 5.Baldwin S et al. Pancreatic cancer metastatic to a limited number of lymph nodes has no impact on outcome. HPB (Oxford) 18, 523–528 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elshaer M, Gravante G, Kosmin M, Riaz A & Al-Bahrani A A systematic review of the prognostic value of lymph node ratio, number of positive nodes and total nodes examined in pancreatic ductal adenocarcinoma. Ann R Coll Surg Engl 99, 101–106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari D et al. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br. J. Surg. 104, 600–607 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Bilici A Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J. Gastroenterol. 20, 10802–10812 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motoi F & Unno M Adjuvant and neoadjuvant treatment for pancreatic adenocarcinoma. Jpn. J. Clin. Oncol (2020). doi: 10.1093/jjco/hyaa018 [DOI] [PubMed] [Google Scholar]
- 10.Ghaneh P et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann. Surg 269, 520–529 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Acher AW, Bleicher J, Cannon A & Scaife C Advances in surgery for pancreatic cancer. J. Gastrointest. Oncol 9, 1037–1043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ocaña J et al. Relevance of positive resection margins in ductal pancreatic adenocarcinoma and prognostic factors. Cir Esp 98, 85–91 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Gnerlich JL et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch. Surg 147, 753–760 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Verbeke CS & Menon KV Redefining resection margin status in pancreatic cancer. HPB (Oxford) 11, 282–289 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seufferlein T & Ettrich TJ Treatment of pancreatic cancer-neoadjuvant treatment in resectable pancreatic cancer (PDAC). Transl. Gastroenterol. Hepatol 4, 21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenocchio E et al. Is there a standard adjuvant therapy for resected pancreatic cancer?. Cancers (Basel) 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert A et al. An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol 11, 1758835919875568 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo S, Ammori J, Eads J & Dorth J The role of neoadjuvant therapy in pancreatic cancer: a review. Future Oncol 12, 669–685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motoi F et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol 49, 190–194 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Datta SK et al. Survival outcomes between surgery with adjuvant therapy compared to neoadjuvant therapy with surgery in stage I pancreatic adenocarcinoma: Results from a large national cancer database. J. Clin. Oncol 37, 335–335 (2019). [Google Scholar]
- 21.White MG et al. A tale of two cancers: traveling to treat pancreatic and thyroid cancer. J. Am. Coll. Surg 225, 125–136.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Søreide JA, Sandvik OM & Søreide K Improving pancreas surgery over time: Performance factors related to transition of care and patient volume. Int J Surg 32, 116–122 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Raoof M et al. Centralization of pancreatic cancer surgery: travel distances and disparities. J. Am. Coll. Surg 223, e166 (2016). [Google Scholar]
- 24.Fong Y, Gonen M, Rubin D, Radzyner M & Brennan MF Long-term survival is superior after resection for cancer in high-volume centers. Ann. Surg 242, 540–4; discussion 544 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strobel O et al. Pancreatic Cancer Surgery: The New R-status Counts. Ann. Surg. 265, 565–573 (2017). [DOI] [PubMed] [Google Scholar]