Abstract
Autogenous odontogenic materials are a new, highly biocompatible option for jaw restoration. The inorganic component of autogenous teeth acts as a scaffold to maintain the volume and enable donor cell attachment and proliferation; the organic component contains various growth factors that promote bone reconstruction and repair. The composition of dentin is similar to that of bone, which can be a rationale for promoting bone reconstruction. Recent advances have been made in the field of autogenous odontogenic materials, and studies have confirmed their safety and feasibility after successful clinical application. Autogenous odontogenic materials have unique characteristics compared with other bone-repair materials, such as the conventional autogenous, allogeneic, xenogeneic, and alloplastic bone substitutes. To encourage further research into odontogenic bone grafts, we compared the composition, osteogenesis, and development of autogenous odontogenic materials with those of other bone grafts. In conclusion, odontogenic bone grafts should be classified as a novel bone substitute.
Keywords: AutoBT, Autograft, Bone reconstruction, Implant, Tooth material
Introduction
An ideal bone graft has four characteristics: osteoconductivity (provides a scaffold for bone regeneration and energy transfer), osteoinductivity (contains growth and regulation factors that induce bone formation), osteogenesis (has cells that promote bone formation), and bone binding (tightly integrated with bone tissue) [1]. Since autogenous bone grafts possess all these desirable properties and good biocompatibility, they have always been regarded the gold standard for bone grafting [2]. To overcome the several disadvantages of autogenous bone, such as inevitable additional surgery, resorption, and limited amount, there are allogeneic, xenogeneic, and inorganic bone substitutes, such as bone cement, hydroxyapatite (HA), and tricalcium phosphate (TCP) [3]. However, these bone substitutes have insufficient bone formation ability due to their manufacturing process, poor porosity, and an unfavorable host response [4].
The main mineral components of autogenous dental bone grafts include four types of calcium phosphate [HA, TCP, octacalcium phosphate (OCP), and amorphous calcium phosphate (ACP)] [5]. The histological composition of teeth is divided into enamel, dentin, and cementum. Previous studies have found several similarities between dentin and bone. Dentin contains 70–75% inorganic content, 20% organic content, and 10% water; the inorganic, organic, and water contents in alveolar bone are 65%, 25%, and 10%, respectively [6]. Type I collagen accounts for 90% of the organic contents in dentin and plays a supporting and connecting role during bone formation [7]. The remaining 10% of the organic portion of dentin is composed of non-collagenous proteins (NCPs) that can induce bone formation, such as bone morphogenetic protein (BMP), insulin-like growth factor-II (IGF-II) and transforming growth factor-beta (TGF-β) [7]. In the relationship between non-collagen organic material and jaw bone repair, BMP is a key factor in bone repair [8].
Since growth factors can be easily released after the process of demineralization [6], a demineralized dentin matrix (DDM) has a greater osteogenic effect than a non-demineralized dentin matrix. Kim et al. conducted animal experiments to study the effects on human teeth, and found that the bone inductivity of autogenous teeth comes from the dentin. They used an autogenous tooth material and a series of steps such as dehydration, degreasing, and ethylene oxide disinfection to manufacture a new type of autogenous tooth granular bone substitute called autogenous tooth bone graft material (AutoBT®, Korea Tooth Bank, Korea). Subsequently, Korea established a dental bank in 2009 [6, 9]. Other studies reported that dentin contains many polypeptides and signaling molecules, isolated in the mineralized matrix. Therefore, the proteins in the dentin matrix can promote bone healing. After demineralization, the dentinal tubules are enlarged, releasing the collagen fibers and basic proteins [10, 11]. These proteins can promote angiogenesis and bone formation; hence, DDM has osteoinductive effects [12, 13]. In recent years, many researchers have studied autogenous dental materials to demonstrate their osteogenic properties [13] and the amount of stability provided to the height of the jaw [14] and dental implants [6, 15]. Till date, several studies have shown that autogenous odontogenic materials can be used clinically [16]. The aim of this study was to compare the composition, osteogenesis, and development of autogenous odontogenic materials with those of other bone grafts.
Comparison with various bone grafts (Table 1)
Table 1.
The main characteristics of various bone grafts [34]
| Graft | Definition | Advantages | Disadvantages | Examples |
|---|---|---|---|---|
| Autogenous bone | The patient’s own bone | Excellent biocompatibility, bone conductivity, and bone induction and contains living osteoblasts [17] | Needs additional surgery, which can cause complications such as nerve damage or arterial injury [22] | Cortical or cancellous bone |
| Allogeneic bone | A graft taken from a genetically dissimilar member of the same species as the recipient | Has various tissue cells, growth factors, extracellular matrix, and other factors [26] | Antigenic and low risk of spreading disease | Cadaver cortical or cancellous bone, FDBA, DFDBA |
| Xenogeneic bone | Grafts derived from a genetically different species than the recipient | High volume, and some grafts have excellent bone conductivity | Highly antigenic and high risk of spreading disease | Bio-Oss, coralline HA, red algae |
| Alloplastic (synthetic materials) bone | Fabricated graft materials | Can be manufactured and stored in large quantities | Causes inflammation and poor bone induction [116] | Calcium sulfate, bioactive glass, HA, NiTi |
FDBA freeze-dried bone allograft, DFDBA demineralized freeze-dried bone allograft; HA hydroxyapatite
Autogenous bone grafts
Being the gold standard in bone grafts, autogenous bone has excellent biocompatibility, osteoconductivity, and osteoinductivity and contains living osteoblasts [17]. Klijn et al. [18] systematically evaluated the clinical applications and confirmed that autogenous bone is indeed the ideal bone graft in sinus augmentation surgeries. Although the most common donor site is the iliac crest [19], the mandible can be harvested to treat atrophy of the jaws. Autogenous bone can be combined with the surrounding bone, and its osteoinductivity can accelerate the speed of bone regeneration [20]. Bell et al. found that autogenous bone grafts could restore and maintain an adequate volume of mandible required to provide support and stability to dental implants [21]. In contrast, another experimental study found that autogenous bone grafts showed large volume loss [22].
The cementum layer of AutoBT has a highly crystalline structure, which can resist resorption during the healing period. For example, while increasing the horizontal width of the alveolar ridge and in staged implant placement, the absorption of AutoBT is significantly lower than that of autogenous bone [23]. Many researchers studied and compared the efficacy of autogenous dentin from root and autogenous bone mass, and found similarities in bone expansion area, bone-implant contact, and degree of wound healing [23, 24]. Another trial evaluating the bone healing capacity of dentin treated with liquid nitrogen and autogenous bone grafts, revealed a similar degree of integration with the surrounding regenerated tissue, and a comparable amount of new bone formation between the autogenous dentin and autogenous bone graft [25]. These experimental data show that autogenous tooth grafts promote bone regeneration comparable to autogenous bone. The autogenous tooth grafts and cortical bone have similar physical and chemical properties, including good biodegradability [3]. Furthermore, autogenous tooth grafts have the advantage of not requiring an additional surgery to obtain the graft, and being able to maintain the grafted volume for a long period of time.
Allogeneic bone
The most widely used allograft is the freeze-dried bone allograft (FDBA), which is lyophilized during its manufacture to reduce its antigenicity. However, the osteoblasts are damaged during this process, thereby limiting its osteoinductive ability [26]. Due to an inevitable immune response associated with FDBA, the integration period with the surrounding tissues is longer, compared with that of autogenous bone material [27]. Piattelli et al. [28] found that demineralized FDBA (DFDBA) was reabsorbed and surrounded by connective tissue, leaving some bone cell cavity in a hollow state.
The possibility of spreading infection through allogeneic bone grafts has always been a crucial point to consider during its production. The U.S. Food and Drug Administration (FDA) has established some standard protocols that must be strictly followed [29]. With these precautions, the possibility that an allogeneic bone graft could cause a viral infection remains one in 8 million to one in 2 million [30]. Nonetheless, Barry et al. concluded that the success rate of massive osseous and osteochondral grafts using allogeneic bone was about 60–90%, proving its feasibility as a bone graft [31]. Compared with allogeneic bone materials, autogenous tooth materials are safer because they are obtained from the same individual. Furthermore, the non-immunogenicity of dentin is attributed to its acellular matrix [32].
Xenogeneic bone
Xenogeneic bone has been extensively researched owing to its excellent availability in large quantities. However, xenogeneic bones are highly antigenic in nature because they carry organic matter from a different species. Murugan et al. used high temperatures to obtain only inorganic bone and thereby avoid immune reactions. They found that after a sequential treatment at temperatures of 500 °C, 700 °C, and 900 °C, the collagen content in bovine tibia samples was 0, and X-ray diffraction (XRD) analysis showed that the bone gradually became more crystalline as the temperature increased during treatment. The bone treated at 500 °C appeared similar to the low crystalline structure of HA, whereas the bones treated at 700 °C and 900 °C had similarly high crystallinity (Fig. 1). A lower crystalline state is easily absorbed by the bone [33].
Fig. 1.

Compared with the samples at 300 °C and 500 °C, the X-ray diffraction spectrum of bone heated at 700 °C and 900 °C has a higher crystalline phase. Compared with raw bone and bone heated at 300 °C and 500 °C, heat treatment at 700 °C and 900 °C caused a significant change in the Bragg diffraction peaks: A raw bone, B bone heated at 300 °C, c bone heated at 500 °C, d bone heated at 700 °C, and e bone heated at 900 °C [33]. Reproduced with permission of BULLETIN OF MATERIALS SCIENCE
In the existing research, the most well-known xenogeneic bone material is bovine in origin (Bio-Oss®, Geistlich Pharma, Switzerland) [34]. Bio-Oss contains naturally occurring, deproteinized bovine cancellous bone minerals in bulk or small particles, and is biocompatible and osteoconductive as well [35]. Wu et al. [36] conducted a human study using AutoBT and Bio-Oss to compare the level of horizontal bone loss around dental implants and reported a similar osteogenic effect. In another study, clinical and imaging examinations comparing AutoBT and Bio-Oss in sinus bone transplantation, showed that the new bone formation in AutoBT was higher than that in Bio-Oss [37]. Therefore, it is believed that the unsatisfactory results of xenogeneic bone grafts are due to antigenicity from a different species, and a damaged osteoinductive effect caused by the process of removing antigenicity. However, it is worth noting that there are also a few reports demonstrating better bone formation with xenogeneic bone grafts [32, 33, 38].
Alloplastic bone
There are many kinds of synthetic bone grafts; however, both the organic and inorganic materials have some common disadvantages that reduce their clinical value. Among the polymer bone grafts, aliphatic polyesters have been most studied, including polylactic acid, polycaprolactone, polyglycolic acid, and their copolymers and derivatives. Some of them, such as polypropylene fumarate, can adversely affect natural bone tissue by causing the release of acid compounds, while undergoing the process of degradation [39]. Inorganic synthetic bone materials include TCP, biological glass (BG), glass ionomer, alumina, calcium sulfate, calcium phosphate, and synthetic HA [34]. Among them, BG provides a greater stimulation for bone regeneration, and it has been used clinically in at least 1.5 million patients [40].
Compared with autogenous teeth and bone materials, Hassan et al. found that the autogenous bone grafts performed better than synthetic bone grafts (through guided bone regeneration), in immediate implants [41]. Kim et al. [42] reported that autogenous teeth were more stable bone grafts than the synthetic grafts, for resorption around dental implants following maxillary sinus-lift procedures. Unlike autogenous tooth materials, calcium sulfate is easily softened and broken in a humid environment. They do not provide enough mechanical support to the body, and synthetic HA is fragile. In an experiment, β-TCP was found to generate a new bone that was smaller than the absorbed volume of the material, despite mixing it with other non-absorbable materials [43]. Although autogenous dental materials have demonstrated good biocompatibility, they are yet to overcome shortcomings such as bone resorption.
Composition of healthy human teeth
Inorganic components
The main elements of teeth are calcium and phosphorus, along with chlorine, iron, zinc, and other elements, whereas the bones also contain nickel [44]. In dried fat-free teeth, 95% of the enamel is mineralized with calcium, phosphorus, and carbonate, accounting for 36%, 17%, and 2.4%, respectively. Minerals account for 70% of the dentin with calcium, phosphorus, and carbonate constituting 26%, 13%, and 3.2%, respectively [45]. Although tooth is composed of multiple crystals, the crystallinity of tooth enamel is better and the crystal size is larger than that of dentin [46]. Crystallinity is related to the dissolution rate and solubility; apatite with high crystallinity is more difficult to dissolve than apatite with low crystallinity [47].
Organic components
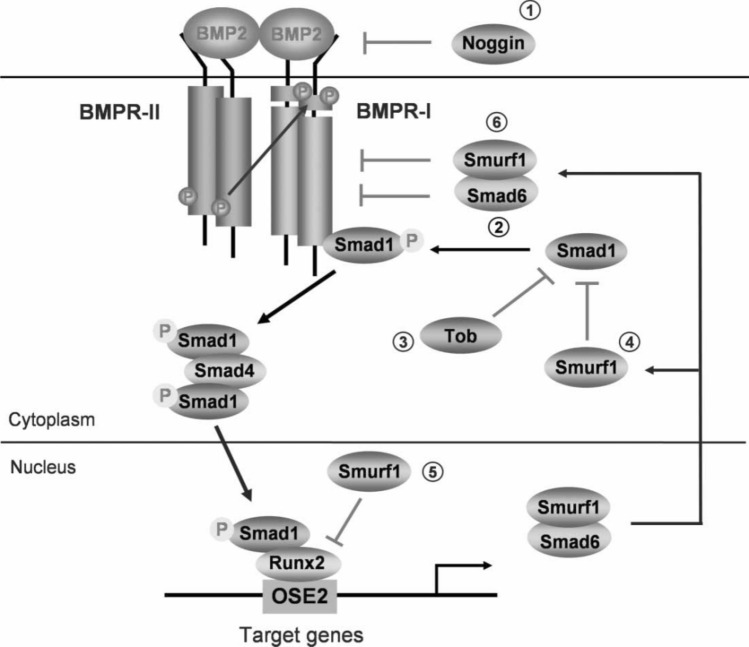
Similar to bone, dentin is composed of 90% collagen, mainly type I, and lacks the soft connective tissue proteins, fibronectin and type III collagen [48]. Half or more than half of the non-collagenous components are hyperphosphorylated proteins, and the remaining are acidic glycoproteins, Gla-proteins, serum proteins, proteoglycans, etc. Several NCPs also promote bone regeneration, such as dentin sialoproteins (DSP) and dentin phosphoproteins (DPP) [49, 50]. BMPs existing in teeth play a significant role in their development (Fig. 2) [51]. Under their (such as osteogenic) regulation, new bone tissue is produced, and differentiation of bone marrow mesenchymal stem cells into a bone-producing tissue is increased, which also plays a positive role in promoting mineralization [52]. Recently, TGF-β found in teeth has been shown to promote tooth formation [53]. TGF-β also promotes the aggregation and differentiation of mesenchymal stem cells (MSCs) and bone healing [54]. In the following sections, we have discussed the fabrication, composition, and limitations of several types of odontogenic bone grafts.
Fig. 2.
Bone morphogenetic protein (BMP) signaling and its regulation. BMP signals are mediated by type I and II BMP receptors and their downstream molecules Smad 1, 5, and 8. Phosphorylated Smad 1, 5, and 8 proteins form a complex with Smad 4 and are then translocated into the nucleus where they interact with other transcription factors such as Runx2 in osteoblasts. BMP signaling is regulated at different molecular levels [51]. Reproduced with permission of GROWTH FACTORS
Types of autogenous tooth materials (Table 2)
Table 2.
The main characteristics of various autogenous tooth materials
| Graft | Component | Manufacture step | Advantages | Disadvantages | Healing effect | Implant |
|---|---|---|---|---|---|---|
| AutoBT | HA, TCP ACP, OCP, Collagen, Non-collagenous proteins | Dehydration, degreasing, demineralization, freeze-drying and sterilization |
Osteoconduction Osteoinduction Physical and chemical properties are similar to those of human cortical bone The immune response and infection are negligible |
Relatively limited sources | New bone formation occurred in most of the areas of interest [111] | Stability is good, implant stability increases over time [117] |
| DDM | HA, TCP ACP, OCP, Collagen, Non-collagenous proteins | Dehydration, degreasing, demineralization, freeze-drying and sterilization |
Osteoconduction Osteoinduction As the carrier of rh-BMP |
The organic material was damaged Osteoinduction is worse than that of AutoBT |
As a scaffold for inducing bone formation [117] | Low bone resorption rate after implant loading [114] |
| Tooth ash | Mainly HA and β-TCP | Cleaning, heating grinding to remove any impurities |
Osteoconduction Less cross-infection and immune responses Absorbable Easy to prepare, store and use |
Without osteoinduction Large mobility |
Only has osteoconduction Capacity [65] |
Bone fusion at meager levels [65] |
DDM (Fig. 3)
Fig. 3.
Common scanning electron microscopy images of demineralized dentin matrix (DDM) [115]. Reproduced with permission of SCIENTIFIC REPORTS
Production and application
To prepare DDM powder, human teeth are extracted and stored in an alcohol solution until further use. Each tooth is sectioned into the crown and the root. The root is crushed into powder and soaked in distilled water and hydrogen peroxide solution. Then, the soft tissues and foreign debris are removed, and the powder is dehydrated, degreased, demineralized with hydrochloric acid (HCl). Subsequently, it is freeze-dried, and sterilized with ethylene oxide [55]. Teeth can be manually ground under liquid nitrogen cooling. Ethanol and ether are generally used for dehydration and degreasing, respectively, phosphate buffered saline or distilled water is used for cleaning, and HCl is generally used for demineralization. It is to be noted that HCl can inactivate organic substances with osteoinductive effects [55, 56]. Pang et al. reported a clinical study that DDM has been shown to be comparable as Bio-Oss in inducing bone healing [57]. In few cases, the use of recombinant human BMP-2 (rhBMP-2) along with DDM, during clinical application, further improved the bone-healing effects. The average loss of height and width reduced from the conventional 1.53 mm to 0.27 mm, and from 3.87 mm to 0.47 mm, respectively. The amount of bone formation was slightly greater than with DDM alone. However, the ideal rhBMP-2 concentration still remains to be studied [55].
Composition
The mineral content of powdered and bulk DDM is 5–10% and 10–30%, respectively. It mainly consists of HA, TCP, OCP, and ACP, of which HA is the most important component. We speculate that the presence of these components can provide a basis for the bone conductivity of the material. DDM contains a variety of NCPs, such as phosphoprotein, sialoprotein, glycoprotein, proteoglycan, osteopontin (OPN), human osteocalcin, and dentin matrix protein (DMP) [58].
AutoBT (Korea Tooth Bank, Seoul, Korea)
Effects of using the material
In a study, AutoBT, a novel autogenous dental material was produced using teeth kept in the freezer to remove the soft tissue, and then ground, washed, dehydrated, degreased, and freeze-dried (Fig. 4) [11]. Kim et al. [59] reported a case wherein AutoBT grafted in a 40-year-old patient was gradually absorbed and replaced by new bone, as observed in a histological section. Owing to the presence of a large amount of lamellar bone, the bone reconstruction process in the mouth was relatively rapid, and a stable bone structure could be generated within five months. The level of alveolar bone near the implants was stable for an average of 31 months; two of the cases almost healed without resorption of the graft, and most of the wounds had good bone healing with new bone.
Fig. 4.
A–C Extracted teeth ready to be fabricated into autogenous tooth bone graft (AutoBT) by Korea tooth bank (A). Powdered form of AutoBT (B). Block form of AutoBT (C) [7]. Reproduced with permission of JOURNAL of the KOREAN ASSOCIATION of ORAL MAXILLOFACIAL SURGEONS
Inorganic components
Kim et al. stored extracted teeth in alcohol, prepared them as AutoBT, and stored them in a dry environment at 100 °C. Using scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy, and XRD analyses, it was found that AutoBT contains HA, TCP, ACP, and OCP. However, the composition ratio varies in different parts of the teeth. The crown is mainly composed of enamel, and the ratio of calcium and phosphorus in the crown is close to 1.75. The ratio of dentin in the root is larger, where the ratio of calcium to phosphorus is close to 1.32 of ACP; dentin and cementum contain more carbon than enamel [5]. In a comparative analysis of AutoBT and autogenous bone, the XRD images were similar, indicating that both have low crystallinity and that the root of the tooth is easily absorbed by the bone [3].
Organic components
Kim et al. found that type I collagen in dentin accounts for 90% of the organic components in AutoBT, and the remaining 10% contains lactate, citrate, lipids, biopolymers, and a variety of NCPs [7]. Although no rhBMP-2 was found in the electrophoresis conducted for this experiment, new bone formation was found in mice. The degree of healing in the bone defect demonstrated the osteoinductive effect of the material [49].
Tooth ash (Dental Plaster; Mungyo Industrial Co, Gimhae, Korea)
Production method
Tooth ash requires sintering at a high temperature after the following steps. After collecting the tooth, it is soaked in hydrogen peroxide and then cleaned with ultrasonic waves to remove the soft tissue. The tooth is dried, disinfected with ethanol, bleached, and then subjected to a high temperature. The tooth powder is held at 1200 °C for at least 1 h to remove the impurities, and then disinfected with ethylene oxide. This high temperature treatment removes harmful substances and prevents inflammation. Since it is difficult to hold tooth ash in bone defects, it is usually mixed with plaster in a 2:1 weight ratio [60].
Tooth ash application
Before use, tooth ash is generally mixed with other materials. When dental ash and gypsum were applied to mandibular defects in 10 patients, no adverse reactions occurred, such as inflammation and infection, and the healing was good [59]. In an osteoporosis model with 60 rats, a mixture of dental ash and plaster accelerated new bone formation and was more stable than the control group [61]. Gu et al. [62] used human dental ash to treat created bone defects in dog alveolar bone and found the presence of osteoclasts in surrounding tissue indicating an active bone remodeling process. Therefore, this material is simple and easy to obtain, convenient to use, and promotes bone healing. A mixture of tooth ash and platelet-rich plasma could demonstrate better final bone healing effects [6, 63]. Appropriate demineralization of materials can release organic components, and the presence of various proteins can promote bone regeneration [64]. According to a study by Murata et al., DDM does not inhibit the release of BMP, which means that BMP can be used to promote bone healing [9]. Furthermore, Kim et al. [65] compared dental powder and dental ash produced at 60 °C and 1000 °C, respectively, and found that, although both exhibited better healing performance than the non-filled materials tested, the materials produced at 1000 °C had a higher rate of bone formation.
Characteristics of tooth ash
First of all, one of the advantages of tooth ash is that high temperature treatment can better remove the sticky epithelium of the tooth and other organic matter that affects bone healing after implantation. Besides, the composition of tooth ash is close to that of the bone and can be better absorbed in the human body compared to other bone graft materials. At the same time, it also has the advantages of low cost, low pollution, no significant foreign body reaction, and easy handling [61, 66]. The shortcomings, the first is that the high temperature inactivates the cytokines that induce bone healing and promote bone formation in the tooth components [67]. Secondly, because of its small particles and strong fluidity in a humid environment, the tooth ash is usually mixed with materials such as plaster that act as a "binder" during use [61].
Osteogenesis of autogenous dental materials
As explained earlier, an ideal bone graft has four properties [14]. Autogenous tooth material has physical and chemical properties similar to autogenous cortical bones, and has been shown to have excellent osteogenic ability [3]. Several studies have provided evidence that the histological composition of teeth, especially dentin, is similar to that of bones. Dentin and alveolar bone are composed of similar inorganic and organic substances (65:35) [3, 7]. SEM results showed that the structural pattern of AutoBT was similar to that of autogenous cortical bone. In XRD analysis, the AutoBT crown was found to be mainly composed of highly crystalline calcium phosphate. Materials with high crystal content are not easily broken down by osteoclasts, resulting in poor osteoconduction. In contrast, AutoBT root has a low crystalline structure, enabling osteoinduction and osteoconduction (Fig. 5). In the calcium phosphate ion dissolution test, the amount of ion dissolution in AutoBT showed a pattern similar to that of autogenous cortical bone [3, 68, 69]. Moreover, the density, roughness, and homology of AutoBTs are similar to those of autogenous cortical bone [3]. The embryological origins of teeth, cartilage, and maxillofacial bones are the same; all are derived from the migration and differentiation of the neural crest cells [7].
Fig. 5.
The similarity in crystalline structures between AutoBT (Korea tooth bank, Korea) and autogenous bones [3]. Reproduced with permission of ORAL SURGERY ORAL MEDICINE ORAL PATHOLOGY ORAL RADIOLOGY
Many scholars have conducted research on autogenous dental materials to demonstrate their osteogenic potential in lateral alveolar ridge augmentation and explore the stability of implants following placement of the graft. As early as 1967, Yeomans et al. transplanted decalcified dentin from rabbit teeth into muscle tissue and found osteoinduction after four weeks, following which a stable bone system was formed [70]. Formation of the surrounding bone could be observed, when using a mixture of human tooth ash and gypsum [71]. Schwarz et al. found that autogenous tooth root had sufficient osteogenesis to support the alveolar crest and the implantation of second-stage implants [23]. Additionally, Xu et al. found that autogenous tooth-derived materials had osteogenic effects, and the tooth graft-bone interface was gradually replaced by new bone. All these experiments indicate that autogenous teeth have a good osteogenic potential [72].
Osteoinduction of autogenous dental materials
Tooth minerals are composed of five biological calcium phosphates: HA, TCP, OCP, ACP, and dehydrated dicalcium phosphate. These five types of calcium phosphate interact with each other for good bone remodeling. Dentin NCPs (dNCPs) are a group of proteins secreted by dentin, such as dentin-specific proteins and various growth factors [7]. Dentin-specific proteins are synthesized and secreted only by dentinal cells and include DPP and DSP [73]. Growth factors in dentin include TGF-β (especially BMP), LIM mineralized protein-1 (LMP-1), IGF, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and matrix metalloproteinase (MMP) [7, 74–76]. NCPs act as signaling molecules in biological processes such as bone formation, absorption, and conversion [77]. Several recent studies have found that NCPs also play a role in the mechanical behavior of the composition and structural integrity of the bone matrix [77, 78]. The presence of these components (mainly dNCPs) gives the teeth its osteoinductive properties that make autogenous teeth increasingly valued as bone grafts.
Growth factors of autogenous dental materials
Autogenous tooth materials contain several growth factors that can regulate cell growth, proliferation, and differentiation, including TGF-β (especially BMP), LMP-1, IGF, PDGF, FGF, VEGF, EGF, and MMPs. TGF found in teeth, play a role in promoting tooth formation [7, 53], and it has been shown to attract the aggregation of MSCs and regulate subsequent bone formation [79]. Bessho et al. extracted BMP from bone, dentin, and wound tissue after tooth extraction in rabbits and implanted them into the body after a series of purification procedures. In other biological experiments on xenografts, different sources of BMP induced new bone growth, demonstrating that the antigenicity of BMP remains unclear [80]. The presence of BMP plays a certain role in osteogenesis, and the degree of demineralization can affect the activity of BMP. Koga et al. found that partially demineralized dentin matrix (PDDM, 70% demineralized) is superior to non-demineralized dentin [81]. In another experiment, DDM induced bone formation 4 weeks after implantation, whereas non-demineralized dentin did not induce any bone formation until 8–12 weeks after implantation [70]. This might be because demineralization exposed the organic matter inside the teeth increasing the surface area and porosity, which would enhance the osteogenesis ability of the DDM. However, complete demineralization of DDM damages the internal BMP, which in turn affects its osteogenesis ability [82].
LMP-1 expression is found in predentin, odontoblasts, and vascular endothelial cells in healthy teeth, and in non-mineralized reparative dentin, odontoblastoid cells, and dental pulp fibroblasts in caries and pulpitis [74]. LMP-1 is an indispensable positive regulator in the initiation of osteoblast differentiation, and affects the intermediate link in the BMP-6 signaling pathway [83]. Thus, LMP-1 plays an important role in the osteoinduction of autogenous teeth.
Human collagenases exist in three distinct molecules, MMP-1, MMP-8, and MMP-13 [84]. MMPs are Zn2+ and Ca2+, which require enzymes capable of degrading almost all of the extracellular matrix and basement membrane components in normal tissue remodeling [76]. In an in vitro culture [85], dentin has been found to contain MMPs, and mature odontoblasts contain MMP-2 and MMP-9. The expression and synthesis of MMP-8 collagenase in mature human odontoblasts and pulp has been demonstrated [84]. MMPs have been shown to be involved in bone resorption. Gelatinase B is an MMP-9 produced by osteoclasts that has been found to regulate early bone formation. The gelatinase B transcript is concentrated in the area of rapid new bone growth and has an inhibitory effect on bone resorption [86]. The activity of bone cells depends on the direct degradation of bone matrix components by MMPs, in addition to the activation of mature osteoclasts by osteoblasts, and the attachment, migration, and fusion of osteoclasts through the action of MMPs.
The dentin matrix contains several important angiogenic factors, such as high concentrations of PDGF, low concentrations of VEGF and FGF, and extremely low concentrations of EGF [75]. In dentin, rhPDGF factor-BB has been found to promote wound healing and osteoinduction by promoting the proliferation and recruitment of new bone cells [87]. VEGF plays a key role in the regulation of angiogenesis. Osteoblasts can produce VEGF, indicating that osteoclastic bone activity and bone marrow formation have active neovascularization [88].
Meanwhile, the presence of FGF-1 has been found to stimulate bone formation and increase bone density through an osteoinductive effect [6, 89]. EGF stimulates the production of prostaglandin E2 (PGE2), and PGE2 can influence bone resorption and bone formation to stimulate the synthesis of IGF [90]. IGF-I and IGF-II have been isolated from human dentin, where IGF-I is known to stimulate skeletal growth, increase bone matrix production by a direct effect on the differentiated function of the osteoblast (i.e., enhanced bone collagen production) and an increase in osteoprogenitor cell replication, resulting in a larger number of functional osteoblasts [91].
Other factors of autogenous dental materials
Studies have shown that the bone induction effect of dentin stems not only from NCPs, but also from other components such as DMP-1, dentin sialophospoprotein (DSPP), osteocalcin (OC), OPN, alkaline phosphatase (ALP), and some special transcription factors.
DMP-1 was originally isolated from rat dentin, and was believed to be a dentin-specific protein. However, many studies have shown that DMP-1 is a bone- and tooth-specific protein found in brain gray matter, brainstem, salivary gland, pancreas, kidney, and some epithelial-derived tumors, and the expression level in bone tissue is significantly higher than that in dentin [92, 93]. DMP-1 is a highly phosphorylated acidic NCP that is predominantly expressed in osteoblasts during embryonic development and is required for the mineralization of bone and dentin. In mineralized tissues, it takes the form of proteolytic fragments. The proteolysis process of DMP-1 is crucial for the formation and mineralization of dentin, cementum, and jaw bone and plays a key role in the development of bone and cartilage [94].
DSPP is the main NCP in dentin blasts, and it can be proteolytically converted into DSP and DPP [95]. Earlier studies found that DSPP is highly expressed in odontoblasts, and DSPP expression is highly regulated during amelogenesis by ameloblasts. This could be related to the potential nucleation promoting effect of DSPP on HA crystal formation [96]. DSPP, a non-specific tooth protein, is also found in bones.
Dentin sialophosphate is expressed by sialophosphate genes of osteoblasts in both bone and dentin, and some analyses showed significant differences in the regulatory and action mechanisms of DSPP expression [97, 98]. OC is a rich, non-collagen, vitamin-K-dependent protein secreted by osteoblasts. OC can regulate bone mineralization, affect the activity of osteoblasts and osteoclasts, and maintain the balance of bone formation [99, 100]. Bone connexin is a tissue-specific protein that initiates active mineralization by connecting the mineral and collagen phases in bone during the initial formation of minerals, and helps the continuous attachment of crystals to collagen fibers [101]. OPN is a multifunctional protein that plays a role in bone remodeling by promoting osteoblast and osteoclast activity [102]. ALP is necessary for biomineralization, which is a unique developmental process in teeth, bones, and other hard tissues. Genes related to ALP are expressed during the entire process of bone healing, especially in the later stage, which is consistent with the formation of increased mineralized bone [103]. PG (RunX-2) is a transcription factor that plays a key role in inducing immature bone cells to differentiate into mature osteoblasts and mediate bone formation through a stable pathway that regulates the expression of an alternative lymphoid enhancer binding factor (Lef1) transcript during osteoblast maturation. Lef1 is a transcriptional effector that can regulate osteoblast differentiation, bone mineral density, and bone strength [104]. Other studies have found that human RunX-2 can maintain the balance between bone formation and absorption [99].
Supportive and bone-binding effects of autogenous tooth materials
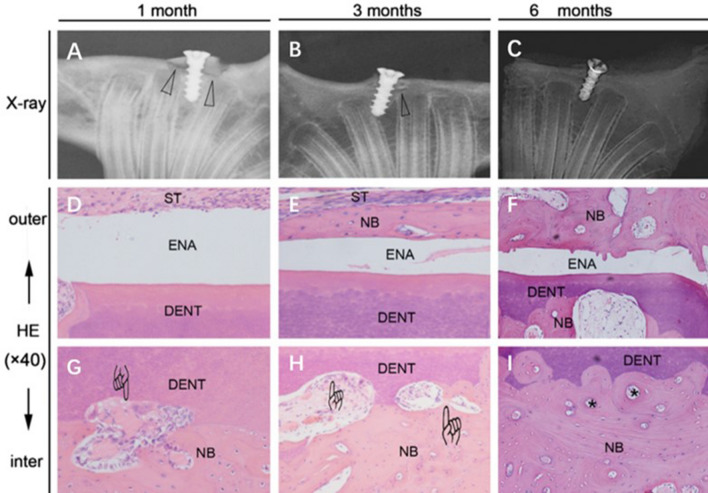
After AutoBT implantation, histological analyses showed that over time, the bone graft was gradually absorbed and connected with the surrounding bone, with the edges in close contact and forming a stable structure [6]. DDM particles were soaked in a simulated body fluid under specific conditions for surface modification, and then implanted into autonomous tissues on the backs of rats. The surface structure of the particles became dense, and the particles were covered by a thin fibrous film, all of which indicated a high degree of bone binding [105]. In another study [72], radiographical and histological observations showed a gradual increase in the rate of contact between autogenous tooth grafts and the surrounding bone over a six-month period (Fig. 6).
Fig. 6.
A–C A graft heals with the original bone, and the low-density region represented by the triangles which gradually disappears. Histologically, D the enamel layer is covered with soft tissue, E with a thin layer of new bone, and F with newly remodeled bone on the surface of enamel. G–I Around dentin, vascularization and osteoclast-like cells (finger mark) and new bone formation are observed with bony lacunae (asterisk) which indicates bone remodeling process [72]. Reproduced with permission of DENTAL TRAUMATOLOGY *DENT dentin of tooth, ENA enamel of tooth, NB new bone, ST soft tissue
DDM combines the properties of grafts (osteoinductivity, osteoconductivity, osteogenesis, and bone binding) and acts as a carrier of rhBMP-2 [106]. Kim et al. fixed rhBMP-2 on inorganic bovine bone, TCP, DDM powder, and DDM chips, and then analyzed the amount of rhBMP-2 released, including the amount and location of new bone formation. They concluded that DDM was a better bone graft with a greater potential as an effective scaffold material for rhBMP-2 [56]. Jung et al. used rhBMP-2/DDM and compared it with DDM and deproteinized bovine bone with collagen (DBBC) alone. The results showed that rhBMP-2/DDM produced higher formation of new bone than the others, confirming the great potential of DDM as a BMP carrier [107]. In a human study of autogenous PDDM (APDDM) and particulate cancellous bone and marrow (PCBM), the new bone volume remained stable, indicating the increased value of APDDM as a bone marrow carrier and scaffold [108].
Comparison of the healing properties and implant stability of three kinds of odontogenic materials (Table 2)
AutoBT, DDM, and tooth ash are the most studied autogenous tooth bone grafts at this stage. Most of the research objects of the predecessors are mammalian experiments, or experiments on permanent teeth. After searching, we found other branches of autogenous tooth bone graft, such as demineralized deciduous tooth powder (DDTP). Mirae Park et al. studied demineralized deciduous teeth and found that although the chemical and mechanical properties of permanent teeth and deciduous teeth are different, deciduous teeth are more widely used than permanent teeth. The deciduous teeth have more organic matter, which enhances the adhesion of osteoblasts and improves the resorption rate of the material after transplantation. In his experiments, it was found that deciduous teeth showed similar physical and chemical characteristics as permanent teeth except for faster demineralization and larger surface area [57].
Because AutoBT, DDM, tooth ash these three materials have been shown to have clinically used research papers published, and other tooth bone grafts are basically similar. For example, homogenous demineralized dentin matrix (HDDM) can induce bone formation without host immune rejection, after a series of operations such as demineralization and deantigenization, and demineralization does not change its bone-promoting properties [7]. Jingtao Wu et al. also studied the mechanism of tooth ash called calcined tooth powder (CTP) that was processed at 300 °C to promote bone healing. They found that CTP affects the proliferation of hDPSCs in vitro and promotes the bone/tooth differentiation of hDPSCs through the MAPK signaling pathway [66]. Therefore, in the following we mainly explain the comparison of the three main tooth bone grafts. After searching the literature, we have not found that anyone has conducted experiments on these three materials to compare the final bone healing. Therefore, by describing the experimental results and indirect comparisons, we objectively let the readers have a more intuitive understanding of the autogenous tooth bone grafts from the data and description (Table 2).
First of all, because the three kinds of odontogenic materials are made in different ways, the parts of the teeth used are different, and their components are also different. As mentioned above, teeth are divided into enamel, dentin and cementum. In previous studies, the tooth composition of AutoBT and tooth ash is different from that of DDM. The content of this section has been described in detail in 4.1, 4.2, and 4.3. Porosity in enamel and cytokines that promote bone healing account for less than dentin, and dentin and bone have similar organic and inorganic compositions and ratios [58]. Therefore, theoretically, the osteogenic effects and material absorption rates of these three autogenous tooth bone grafts are different. Jeong et al. used AutoBT on the maxillary sinus and found that during the healing process of 3–6 months, 46–87% of the area had new bone [109]. However, Kim et al. applied tooth ash to adult dogs. After 12 months of implantation, 75 ± 23% of new bone was observed in the area of interest. In this article, the stability of the implant is used to indirectly reflect the state of bone healing. However, there are many factors that affect the stability of implants. For example, Reddi et al. found that the geometry of the transplanted tooth block determines the oxygen tension of the surrounding tissues [110]. Therefore, the implants in different articles may be different, so it cannot be used as an absolute standard. In a retrospective review, among the 182 analyzed implants with AutoBT filling and restoration, the survival rate was 97.7% and the failure rate was 2.3%. And the average ISQ of the first stage implants is 67.3, and the average ISQ of the second stage implants is 75.5. Therefore, when using AutoBT, the stability of the implants will increase over time [111].
As a bone graft material, the absorption of DDM is slower than that of autogenous bone, because DDM is denser, which seems to be a common problem of autogenous tooth bone grafts [112]. Although both DDM and AutoBT have osteoinductivity. But Togari et al. [113] proved that DDM can be used as a scaffold for bone regeneration to induce a high degree of new bone formation. Not only the edge of the implant material can be used as a scaffold to induce bone formation but the entire dental implant block. Compared with the other two types of autogenous tooth bone grafts, DDM has the advantage that it has enough cavities for the attachment, differentiation, growth of osteoblasts and blood vessels in the surrounding tissues. After performing a radiological evaluation of the remodeling of the DDM block, Lee found that after 14 or 15 months of implant loading, the graft block was completely replaced by newly formed bone from the host [114]. After experimentation, it was found that dental ash only has osteoconductivity, and the bone integration with the implant in the body is lower than normal [65]. Therefore, relatively, other autogenous tooth bone grafts that have both osteoconductivity and osteoinduction have more advantages.
Conclusions
Teeth are a unique organ in the body with similar characteristics of the bone, and researchers continue to conduct experiments to improve the adhesion of autogenous dental materials in various ways. Clearly, autogenous tooth materials have many advantages. In addition to their high biocompatibility and convenient acquisition, they do not require high temperature or other processing procedures to change their internal structure; therefore, they preserve a large amount of organic matter to enhance bone regeneration. They have osteoinductive effects, and the dental tissue can act as a scaffold for bone repair. Therefore, autogenous dental materials should be classified as a novel type of bone substitute, which can be used as bone grafts, and is expected to have broad development prospects.
Acknowledgement
The authors thank the CONVERSATIONALIST club of Shandong First Medical University.
Compliance with ethical standards
Conflict of interest
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuxin Zhang, Xuehan Li and Yanxin Qi have contributed equally to this work.
Contributor Information
Shuxin Zhang, Email: ph.dzhangsx@outlook.com.
Xuehan Li, Email: xuehanlii@outlook.com.
Yanxin Qi, Email: wanyang1792528724@outlook.com.
Xiaoqian Ma, Email: maxiaoqianzi@outlook.com.
Shuzhan Qiao, Email: qsz15506541976@outlook.com.
HongXin Cai, Email: caihongxin11@outlook.com.
Bing Cheng Zhao, Email: ad9665@126.com.
Heng Bo Jiang, Email: hengbojiang@hotmail.com.
Eui-Seok Lee, Email: ees225@hanmail.net.
References
- 1.Janicki P, Schmidmaier G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury Int J Care Inj. 2011;42:77–81. doi: 10.1016/j.injury.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury Int J Care Inj. 2011;42:3–15. doi: 10.1016/j.injury.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Kim YK, Kim SG, Yun PY, Yeo IS, Jin SC, Oh JS, et al. Autogenous teeth used for bone grafting: a comparison with traditional grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:39–45. doi: 10.1016/j.oooo.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Liu H, Liu X, Lian X, Guo Z, J H-J, et al. Improved workability of injectable calcium sulfate bone cement by regulation of self-setting properties. Mater Sci Eng C Mater Biol Appl. 2013;33:1048–53. doi: 10.1016/j.msec.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, et al. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol. 2011;11:7442–5. doi: 10.1166/jnn.2011.4857. [DOI] [PubMed] [Google Scholar]
- 6.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Kim YK, Lee J, Um IW, Kim KW, Murata M, Akazawa T, et al. Tooth-derived bone graft material. J Korean Assoc Oral Maxillofac Surg. 2013;39:103–11. doi: 10.5125/jkaoms.2013.39.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herford AS, Boyne PJ. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2) J Oral Maxillofac Surg. 2008;66:616–624. doi: 10.1016/j.joms.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Murata M, Akazawa T, Mitsugi M, Um IW, Kim YK. Human dentin as novel biomaterial for bone regeneration. Ch 6 (InTech, Europe/China 2011)
- 10.Um IW, Ku JK, Lee BK, Yun PY, Nam JH. Postulated release profile of recombinant human bone morphogenetic protein-2 (rhBMP-2) from demineralized dentin matrix. J Korean Assoc Oral Maxillofac Surg. 2019;45:123–128. doi: 10.5125/jkaoms.2019.45.3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Um IW, Ku JK, Kim YK, Lee BK, Leem DH. Histological review of demineralized dentin matrix as a carrier of rhBMP-2. Tissue Eng Part B Rev. 2020;26:284–293. doi: 10.1089/ten.teb.2019.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atiya BK, Shanmuhasuntharam P, Huat S, Abdulrazzak S, Oon H. Liquid nitrogen-treated autogenous dentin as bone substitute: an experimental study in a rabbit model. Int J Oral Maxillofac Implants. 2014;29:165–170. doi: 10.11607/jomi.te54. [DOI] [PubMed] [Google Scholar]
- 13.Reis-Filho CR, Silva ER, Martins AB, Pessoa FF, Gomes PVN, Araújo MSC, et al. Demineralised human dentine matrix stimulates the expression of VEGF and accelerates the bone repair in tooth sockets of rats. Arch Oral Biol. 2012;57:469–76. doi: 10.1016/j.archoralbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Nampo T, Watahiki J, Enomoto A, Taguchi T, Ono M, Nakano H, et al. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010;81:1264–72. doi: 10.1902/jop.2010.100016. [DOI] [PubMed] [Google Scholar]
- 15.Jung MH, Lee JH, Wadhwa P, Jiang HB, Jang HS, Lee ES. Bone regeneration in peri-implant defect using autogenous tooth biomaterial enriched with platelet-rich fibrin in animal model. Appl Sci. 2020;10:1939. doi: 10.3390/app10061939. [DOI] [Google Scholar]
- 16.Kim YK, Lee JH, Um IW, Cho WJ. Guided bone regeneration using demineralized dentin matrix: long-term follow-up. J Oral Maxillofac Surg. 2015;1:e1–e9. [DOI] [PubMed]
- 17.Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J. 2016;98:6–9. doi: 10.1302/0301-620X.98B.36350. [DOI] [PubMed] [Google Scholar]
- 18.Klijn RJ, Meijer GJ, Bronkhorst EM, Jansen JA. A meta-analysis of histomorphometric results and graft healing time of various biomaterials compared to autologous bone used as sinus floor augmentation material in humans. Tissue Eng Part B Rev. 2010;16:493–507. doi: 10.1089/ten.teb.2010.0035. [DOI] [PubMed] [Google Scholar]
- 19.Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93:2227–2236. doi: 10.2106/JBJS.J.01513. [DOI] [PubMed] [Google Scholar]
- 20.Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg. 2012;23:323–327. doi: 10.1097/SCS.0b013e318241dcba. [DOI] [PubMed] [Google Scholar]
- 21.Bell RB, Blakey GH, White RP, Hillebrand DG, Molina A. Staged reconstruction of the severely atrophic mandible with autogenous bone graft and endosteal implants. J Oral Maxillofac Surg. 2002;60:1135–1141. doi: 10.1053/joms.2002.34986. [DOI] [PubMed] [Google Scholar]
- 22.Zins JE, Whitaker LA. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 1983;72:778–785. doi: 10.1097/00006534-198312000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz F, Hazar D, Becker K, Sader R, Becker J. Efficacy of autogenous tooth roots for lateral alveolar ridge augmentation and staged implant placement. A prospective controlled clinical study. J Clin Periodontol. 2018;45:996–1004. doi: 10.1111/jcpe.12977. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz F, Golubovic V, Becker K, Mihatovic I. Extracted tooth roots used for lateral alveolar ridge augmentation: a proof-of-concept study. J Clin Periodontol. 2016;43:345–353. doi: 10.1111/jcpe.12481. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Prasad M, Gao T, Wang X, Zhu Q, D'Souza R, et al. Failure to process dentin matrix protein 1 (DMP1) into fragments leads to its loss of function in osteogenesis. J Biol Chem. 2010;285:31713–22. doi: 10.1074/jbc.M110.137059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borie E, Fuentes R, Sol MD, Oporto G, Engelke W. The influence of FDBA and autogenous bone particles on regeneration of calvaria defects in the rabbit: a pilot study. Ann Anat. 2011;193:412–417. doi: 10.1016/j.aanat.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Gomes KU, Carlini JOL, Biron C, Rapoport AO, Dedivitis RA. Use of allogeneic bone graft in maxillary reconstruction for installation of dental implants. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2008;66:2335–2338. doi: 10.1016/j.joms.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Piattelli A, Scarano A, Corigliano M, Piattelli M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: a histological and histochemical study in man. Biomaterials. 1996;17:1127–1131. doi: 10.1016/0142-9612(96)85915-1. [DOI] [PubMed] [Google Scholar]
- 29.Vangsness CT, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31:474. doi: 10.1177/03635465030310032701. [DOI] [PubMed] [Google Scholar]
- 30.Buck BE, Resnick L, Shah SM, Malinin TI. Human immunodeficiency virus cultured from bone. Implications for transplantation. Clin Orthop Relat Res. 1990;251:249. [PubMed] [Google Scholar]
- 31.Eppley BL, Pietrzak WS, Blanton MW. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16:981–989. doi: 10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- 32.Kim SG, Kim YK, Park JS. Scientific evidence for autogenous tooth bone graft material (AutoBT) J Korean Dent Sci. 2009;2:42–45. [Google Scholar]
- 33.Murugan R, Rao KP, Kumar TSS. Heat-deproteinated xenogeneic bone from slaughterhouse waste: physico-chemical properties. Bull Mater Sci. 2003;26:523–528. doi: 10.1007/BF02707351. [DOI] [Google Scholar]
- 34.Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am. 2007;19:513–521. doi: 10.1016/j.coms.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Sartori S, Silvestri M, Forni F, Cornaglia A, Tesei P, Cattaneo V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin Oral Implants Res. 2003;14:369–72. doi: 10.1034/j.1600-0501.2003.140316.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu D, Zhou L, Lin J, Chen J, Huang W, Chen Y. Immediate implant placement in anterior teeth with grafting material of autogenous tooth bone vs xenogenic bone. BMC Oral Health. 2019;19:266. doi: 10.1186/s12903-019-0970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jun SH, Ahn JS, Lee JI, Ahn KJ, Yun PY, Kim YK. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J Adv Prosthodont. 2014;6:528–38. doi: 10.4047/jap.2014.6.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su-Gwan K, Hak-Kyun K, Sung-Chul L. Combined implantation of particulate dentine, plaster of Paris, and a bone xenograft (Bio-Oss) for bone regeneration in rats. J Craniomaxillofac Surg. 2001;29:282–288. doi: 10.1054/jcms.2001.0236. [DOI] [PubMed] [Google Scholar]
- 39.Haugen HJ, Lyngstadaas SP, Rossi F, Perale G. Bone grafts: which is the ideal biomaterial? J Clin Periodontol. 2019;46:92–102. doi: 10.1111/jcpe.13058. [DOI] [PubMed] [Google Scholar]
- 40.Jones JR, Brauer DS, Hupa L, Greenspan DC. Bioglass and bioactive glasses and their impact on healthcare. Int J Appl Glass Sci. 2016;7:423–434. doi: 10.1111/ijag.12252. [DOI] [Google Scholar]
- 41.Hassan KS, Kassim A, Ogaly AURA. A comparative evaluation of immediate dental implant with autogenous versus synthetic guided bone regeneration. Oral SurgOral Med Oral Pathol Oral Radiol Endod. 2008;106:8–15. doi: 10.1016/j.tripleo.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Kim YK, Lee J, Yun JY, Yun PY, Um IW. Comparison of autogenous tooth bone graft and synthetic bone graft materials used for bone resorption around implants after crestal approach sinus lifting: a retrospective study. J Periodontal Implant Sci. 2014;44:216–221. doi: 10.5051/jpis.2014.44.5.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costantino PD, Friedman CD. Synthetic bone graft substitutes. Otolaryngol Clin North Am. 1994;27:1037–1074. doi: 10.1016/S0030-6665(20)30622-8. [DOI] [PubMed] [Google Scholar]
- 44.Calonius PE, Visapaeae A. The inorganic constituents of human teeth and bone examined by X-ray emission spectrography. Arch Oral Biol. 1965;10:9–13. doi: 10.1016/0003-9969(65)90051-8. [DOI] [PubMed] [Google Scholar]
- 45.Zipkin I. The Inorganic Composition of Bones and Teeth Ch 3. US: Springer; 1970. [Google Scholar]
- 46.Xue J, Zhang L, Zou L, Liao Y, Li W. High-resolution X-ray microdiffraction analysis of natural teeth. J Synchrotron Radiat. 2008;15:235–238. doi: 10.1107/S0909049508003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fulmer MT, Ison IC, Hankermayer CR, Constantz BR, Ross J. Measurements of the solubilities and dissolution rates of several hydroxyapatites. Biomaterials. 2002;23:751–755. doi: 10.1016/S0142-9612(01)00180-6. [DOI] [PubMed] [Google Scholar]
- 48.Linde A. Dentin matrix proteins: composition and possible functions in calcification. Anat Rec Adv Integr Anat Evolut Biol. 1989;224:154–166. doi: 10.1002/ar.1092240206. [DOI] [PubMed] [Google Scholar]
- 49.Kim YK, Lee J, Kim KW, Um IW, Ito K. Analysis of organic components and osteoinductivity in autogenous tooth bone graft material. Phytochemistry. 2013;35:2337–2345. [Google Scholar]
- 50.Ritchie HH, Ritchie DG, Wang LH. Six decades of dentinogenesis research. Historical and prospective views on phosphophoryn and dentin sialoprotein. Eur J Oral Sci. 1998;106:211–220. doi: 10.1111/j.1600-0722.1998.tb02178.x. [DOI] [PubMed] [Google Scholar]
- 51.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2003;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 52.Hollinger J, Mark DE, Bach DE, Reddi AH, Seyfer AE. Calvarial bone regeneration using osteogenin. J Oral Maxillofac Surg. 1989;47:1182–1186. doi: 10.1016/0278-2391(89)90009-8. [DOI] [PubMed] [Google Scholar]
- 53.Smith AJ, Matthews JB, Hall RC. Transforming growth factor-beta1 (TGF-beta1) in dentine matrix. Ligand activation and receptor expression. Eur J Oral Sci. 1998;106:179–184. doi: 10.1111/j.1600-0722.1998.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, Huang HC, et al. Recruitment of mesenchymal stem cells and expression of TGF-beta 1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526–34. doi: 10.1016/j.orthres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Um IW, Kim YK, Park JC, Lee JH. Clinical application of autogenous demineralized dentin matrix loaded with recombinant human bone morphogenetic-2 for socket preservation: A case series. Clin Implant Dent Relat Res. 2019;21:4–10. doi: 10.1111/cid.12710. [DOI] [PubMed] [Google Scholar]
- 56.Kim YK, Um IW, An HJ, Kim KW, Hong KS, Murata M. Effects of demineralized dentin matrix used as an rhBMP-2 carrier for bone regeneration. J Hard Tissue Biol. 2014;23:415–22. doi: 10.2485/jhtb.23.415. [DOI] [Google Scholar]
- 57.Pang KM, Um IW, Kim YK, Woo JM, Kim SM, Lee JH. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: a prospective randomized clinical trial in comparison with anorganic bovine bone. Clin Oral Implants Res. 2017;28:809–15. doi: 10.1111/clr.12885. [DOI] [PubMed] [Google Scholar]
- 58.Um IW, Kim YK, Mitsugi M. Demineralized dentin matrix scaffolds for alveolar bone engineering. J Indian Prosthodont Soc. 2017;17:120. doi: 10.4103/jips.jips_62_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim YK, Kim SG, Bae JH, Um IW, Jeong KI. Guided bone regeneration using autogenous tooth bone graft in implant therapy: case series. Implant Dent. 2014;23:138–143. doi: 10.1097/ID.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 60.Min B, Song JS, Kim SO, Kim KM, Park WS, Lee JH. Osteoconduction capacity of human deciduous and permanent teeth ash in a rat calvarial bone defect model. Cell Tissue Bank. 2015;16:361–9. doi: 10.1007/s10561-014-9480-7. [DOI] [PubMed] [Google Scholar]
- 61.Kim SY, Kim SG, Lim SC, Bae CS. Effects on bone formation in ovariectomized rats after implantation of tooth ash and plaster of Paris mixture. J Oral Maxillofac Surg. 2004;62:852–857. doi: 10.1016/j.joms.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 62.Gu HR, Jang HS, Kim SW, Park JC, Kim BO. Periodontal regeneration using the mixture of human tooth-ash and plaster of paris in dogs. J Korean Acad Periodontol. 2006;36:15–26. doi: 10.5051/jkape.2006.36.1.15. [DOI] [Google Scholar]
- 63.Jeong KI, Kim SG, Oh JS, Lee SY, Cho YS, Yang SS, et al. Effect of platelet-rich plasma and platelet-rich fibrin on peri-implant bone defects in dogs. J Biomed Nanotechnol. 2013;9:535–7. doi: 10.1166/jbn.2013.1503. [DOI] [PubMed] [Google Scholar]
- 64.Sarala C, Chauhan M, Sandhya PS, Dharmendra CH, Mitra N. Autogenous tooth bone graft: Ingenious bone regeneration material. Indian J Dent Sci. 2018;10:56–59. doi: 10.4103/IJDS.IJDS_129_17. [DOI] [Google Scholar]
- 65.Kim JH, Kim SG, Lim SC, Oh JS, You JS, Jeong MA. Histomorphometric analysis of bone formation in bony defects around implants in adult dogs: a comparison of grafts of low and high heat-treated autogenous tooth ash. Implant Dent. 2013;22:639–44. doi: 10.1097/01.id.0000433933.20759.25. [DOI] [PubMed] [Google Scholar]
- 66.Wu J, Li N, Fan Y, Wang Y, Gu Y, Li Z, Pan Y, et al. The conditioned medium of calcined tooth powder promotes the osteogenic and odontogenic differentiation of human dental pulp stem cells via MAPK signaling pathways. Stem Cells Int. 2019;19:1–13. doi: 10.1155/2019/4793518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.BongKyun K, SuGwan K, SeoYoon K, SungChul L, YoungKyun K. A comparison of bone generation capability in rabbits using tooth ash and plaster of Paris with platelet-rich plasma or fibrin sealant. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e8–e14. doi: 10.1016/j.tripleo.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 68.Young-Kyun K, Hyo-Jung L, Kyung-Wook K, Su-Gwan K, In-Woong U. Guide bone regeneration using autogenous teeth: case reports. J Korean Assoc Oral Maxillofac Surg. 2011;37:142–147. doi: 10.5125/jkaoms.2011.37.2.142. [DOI] [Google Scholar]
- 69.Kim GW, Yeo IS, Kim SG, Um IW, Kim YK. Analysis of crystalline structure of autogenous tooth bone graft material: X-ray diffraction analysis. J Korean Assoc Oral Maxillofac Surg. 2011;37:225–228. doi: 10.5125/jkaoms.2011.37.3.225. [DOI] [Google Scholar]
- 70.Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12:999–1006. doi: 10.1016/0003-9969(67)90095-7. [DOI] [PubMed] [Google Scholar]
- 71.Ku HR, Jang HS, Kim SG, Jeong MJ, Park JC, Kim HJ, et al. Guided tissue regeneration of the mixture of human tooth-ash and plaster of paris in dogs. KEM. 2007;330–332:1327–30. doi: 10.4028/www.scientific.net/KEM.330-332.1327. [DOI] [Google Scholar]
- 72.Qin X, Raj RM, Liao XF, Shi W, Ma B, Gong SQ, et al. Using rigidly fixed autogenous tooth graft to repair bone defect: an animal model. Dent Traumatol. 2014;30:380–4. doi: 10.1111/edt.12101. [DOI] [PubMed] [Google Scholar]
- 73.Butler WT. Dentin matrix proteins. Eur J Oral Sci. 1998;106:204–210. doi: 10.1111/j.1600-0722.1998.tb02177.x. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Zhang Q, Chen Z, Zhang L. Immunohistochemical localization of LIM mineralization protein 1 in pulp-dentin complex of human teeth with normal and pathologic conditions. J Endod. 2008;34:143–147. doi: 10.1016/j.joen.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013–1016. doi: 10.1016/S0003-9969(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 76.Qian Y, Huang HZ. The role of RANKL and MMP9 in the bone resorption caused by ameloblastoma. J Oral Pathol Med. 2010;39:592–598. doi: 10.1111/j.1600-0714.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 77.Morgan S, Poundarik AA, Vashishth D. Do non-collagenous proteins affect skeletal mechanical properties? Calcif Tissue Int. 2015;97:281–291. doi: 10.1007/s00223-015-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, et al. Dilatational band formation in bone. Proc Natl Acad Sci USA. 2012;109:19178–83. doi: 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, Huang HC, et al. Recruitment of mesenchymal stem cells and expression of TGF-$beta;1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526–34. doi: 10.1016/j.orthres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Bessho K, Tagawa T, Murata M. Purification of rabbit bone morphogenetic protein derived from bone, dentin, and wound tissue after tooth extraction. J Oral Maxillofac Surg. 1990;48:162–169. doi: 10.1016/S0278-2391(10)80204-6. [DOI] [PubMed] [Google Scholar]
- 81.Takamitsu K, Tokutaro M, Yosuke K, Kei-ichiro M, Takashi I, Yuya N, et al. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLoS ONE. 2016;11:e0147235. doi: 10.1371/journal.pone.0147235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park M, Mah YJ, Kim DH, Kim ES, Park EJ. Demineralized deciduous tooth as a source of bone graft material: its biological and physicochemical characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:307–314. doi: 10.1016/j.oooo.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 83.Boden SD, Liu Y, Hair GA, Helms JA, Hu D, Racine M, et al. LMP-1, a LIM-domain protein, mediates BMP-6 effects on bone formation. Endocrinology. 1998;139:5125–34. doi: 10.1210/endo.139.12.6392. [DOI] [PubMed] [Google Scholar]
- 84.Palosaari H, Wahlgren J, Larmas M, Rönkä H, Sorsa T, Salo T. The expression of MMP-8 in human odontoblasts and dental pulp cells is down-regulated by TGF-beta1. J Dent Res. 2000;79:77–84. doi: 10.1177/00220345000790011401. [DOI] [PubMed] [Google Scholar]
- 85.Tjäderhane L, Salo T, Larjava H, Larmas M, Overall CM. A novel organ culture method to study the function of human odontoblasts in vitro: gelatinase expression by odontoblasts is differentially regulated by TGF-β1. J Dent Res. 1998;77:1486–1496. doi: 10.1177/00220345980770070301. [DOI] [PubMed] [Google Scholar]
- 86.Rice DPC, Kim HJ, Thesleff I. Detection of gelatinase B expression reveals osteoclastic bone resorption as a feature of early calvarial bone development. Bone. 1997;21:479–486. doi: 10.1016/S8756-3282(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 87.Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restor Dent. 2003;23:213–225. [PubMed] [Google Scholar]
- 88.Shen WD, Masakazu M, Hiroshi D, Kanji S. Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology. 1997;138:2953–2962. doi: 10.1210/endo.138.11.5546. [DOI] [PubMed] [Google Scholar]
- 89.Dunstan CR, Boyce R, Boyce BF, Garrett IR, Izbicka E, Burgess WH, et al. Systemic administration of acidic fibroblast growth factor (FGF-1) prevents bone loss and increases new bone formation in ovariectomized rats. J Bone Miner Res. 1999;14:953–9. doi: 10.1359/jbmr.1999.14.6.953. [DOI] [PubMed] [Google Scholar]
- 90.Mccarthy TL, Centrella M, Raisz LG, Canalis E. Prostaglandin E2 stimulates insulin-like growth factor I synthesis in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1991;128:2895–2900. doi: 10.1210/endo-128-6-2895. [DOI] [PubMed] [Google Scholar]
- 91.Finkelman RD, Mohan S, Jennings JC, Taylor AK, Jepsen S, Baylink DJ. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J Bone Miner Res. 1990;5:717–23. doi: 10.1002/jbmr.5650050708. [DOI] [PubMed] [Google Scholar]
- 92.Huang B, Maciejewska I, Sun Y, Peng T, Qin D, Lu Y, et al. Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tissue Int. 2008;82:401–10. doi: 10.1007/s00223-008-9140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ogbureke KUE, Nikitakis NG, Warburton G, Ord RA, Sauk JJ, Waller JL, et al. Up-regulation of SIBLING proteins and correlation with cognate MMP expression in oral cancer. Oral Oncol. 2007;43:920–32. doi: 10.1016/j.oraloncology.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 94.Sun Y, Lu Y, Chen L, Gao T, Souza RD, Feng JQ, et al. DMP1 processing is essential to dentin and jaw formation. J Dent Res. 2011;90:624. doi: 10.1177/0022034510397839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, et al. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009;28:221–9. doi: 10.1016/j.matbio.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macdougall M, Nydegger J, Gu TT, Simmons D, Luan X, Ca VA, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2010;39:25–37. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, et al. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81:392–4. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- 98.Qin C, Brunn JC, Cadena E, Ridall A, Butler WT. Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect Tissue Res. 2003;44:179–183. doi: 10.1080/03008200390152296. [DOI] [PubMed] [Google Scholar]
- 99.Handschin AE. Cbfa-1 (Runx-2) and osteocalcin expression by human osteoblasts in heparin osteoporosis in vitro. Clin Appl Thromb Hemost. 2006;12:465–472. doi: 10.1177/1076029606293433. [DOI] [PubMed] [Google Scholar]
- 100.Wolf G. Function of the bone protein osteocalcin: definitive evidence. Nutr Rev. 1996;54:332–333. doi: 10.1111/j.1753-4887.1996.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 101.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 102.Amarjot S, Gurveen G, Harsimrat K, Mohamed A, Harpal J. Role of osteopontin in bone remodeling and orthodontic tooth movement: a review. Prog Orthod. 2018;19:18. doi: 10.1186/s40510-018-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodrigues WC, Silva Fabris AL, Hassumi JS, Gonçalves A, Sonoda CK, Okamoto R. Kinetics of gene expression of alkaline phosphatase during healing of alveolar bone in rats. Br J Oral Maxillofac Surg. 2016;54:531–5. doi: 10.1016/j.bjoms.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 104.Kim JM, Yang YS, Park KH, Ge X, Xu R, Li N, et al. A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation. Nat Commun. 2020;11:2289. doi: 10.1038/s41467-020-16038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akazawa T, Murata M, Hino J, Nagano F, Shigyo T, Nomura T, et al. Surface structure and biocompatibility of demineralized dentin matrix granules soaked in a simulated body fluid. Appl Surf Sci. 2012;262:51–5. doi: 10.1016/j.apsusc.2012.01.053. [DOI] [Google Scholar]
- 106.Um IW. Demineralized dentin matrix (DDM) as a carrier for recombinant human bone morphogenetic proteins (rhBMP-2) Adv Exp Med Biol. 2018;1077:487–499. doi: 10.1007/978-981-13-0947-2_26. [DOI] [PubMed] [Google Scholar]
- 107.Jung GU, Jeon TH, Kang MH, Um IW, Song IS, Ryu JJ, et al. Volumetric, radiographic, and histologic analyses of demineralized dentin matrix combined with recombinant human bone morphogenetic protein-2 for ridge preservation: a prospective randomized controlled trial in comparison with xenograft. Appl Sci. 2018;8:1288. doi: 10.3390/app8081288. [DOI] [Google Scholar]
- 108.Umebayashi M, Ohba S, Kurogi T, Noda S, Asahina I. Full regeneration of maxillary alveolar bone using autogenous partially demineralized dentin matrix and PCBM for implant-supported full arch rehabilitation. J Oral Implantol. 2020;46:122–127. doi: 10.1563/aaid-joi-D-19-00315. [DOI] [PubMed] [Google Scholar]
- 109.Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA, Park JJ. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011;20:471–5. doi: 10.1097/ID.0b013e3182386d74. [DOI] [PubMed] [Google Scholar]
- 110.Reddi AH, Huggins CB. Influence of geometry of transplanted tooth and bone on transformation of fibroblasts. Proc Soc Exp Biol Med. 2016;143:634–637. doi: 10.3181/00379727-143-37381. [DOI] [PubMed] [Google Scholar]
- 111.Gual-Vaqués P, Polis-Yanes C, Estrugo-Devesa A, Ayuso-Montero R, López-López J. Autogenous teeth used for bone grafting: a systematic review. Medicina Oral Patologia oral y cirugia bucal. 2017;23:e112. doi: 10.4317/medoral.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Pergamon. 1967;12:999. doi: 10.1016/0003-9969(67)90095-7. [DOI] [PubMed] [Google Scholar]
- 113.Kim Y-K, Kim S-G, Um I-W, Kim K-W. Bone grafts using autogenous tooth blocks: a case series. Implant Dent. 2013;22:584–589. doi: 10.1097/ID.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 114.Lee EG, Lee JY, Kim YK, Um IW, Choi JH. Delayed implant placement after extraction socket reconstruction and ridge augmentation using autogenous tooth bone graft material: case reports. Dentistry. 2013;03:2161–1122. [Google Scholar]
- 115.Tanoue R, Ohta K, Miyazono Y, Iwanaga J, Koba A, Natori T, et al. Three-dimensional ultrastructural analysis of the interface between an implanted demineralised dentin matrix and the surrounding newly formed bone. Sci Rep. 2018;8:2858. doi: 10.1038/s41598-018-21291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meikle MC, Papaioannou S, Ratledge TJ, Speight PM, Watt-Smith SR, Hill PA, Reynolds JJ. Effect of poly DL-lactide-co-glycolide implants and xenogeneic bone matrix-derived growth factors on calvarial bone repair in the rabbit. Biomaterials. 1994;15:513–21. doi: 10.1016/0142-9612(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 117.Togari K, Miyazawa K, Yagihashi K, Tabuchi M, Maeda H, Kawai T, et al. Bone regeneration by demineralized dentin matrix in skull defects of rats. J Hard Tissue Biol. 2012;21:25–34. doi: 10.2485/jhtb.21.25. [DOI] [Google Scholar]