Abstract
BACKGROUND:
In recent years, three-dimensional (3D)-printing of tissue-engineered cartilaginous scaffolds is intended to close the surgical gap and provide bio-printed tissue designed to fit the specific geometric and functional requirements of each cartilage defect, avoiding donor site morbidity and offering a personalizing therapy.
METHODS:
To investigate the role of 3D—bioprinting scaffolding for nasal cartilage defects repair a systematic review of the electronic databases for 3D-Bioprinting articles pertaining to nasal cartilage bio-modelling was performed. The primary focus was to investigate cellular source, type of scaffold utilization, biochemical evaluation, histological analysis, in-vitro study, in-vivo study, animal model used, length of research, and placement of experimental construct and translational investigation.
RESULTS:
From 1011 publications, 16 studies were kept for analysis. About cellular sources described, most studies used primary chondrocyte cultures. The cartilage used for cell isolation was mostly nasal septum. The most common biomaterial used for scaffold creation was polycaprolactone alone or in combination. About mechanical evaluation, we found a high heterogeneity, making it difficult to extract any solid conclusion. Regarding biological and histological characteristics of each scaffold, we found that the expression of collagen type I, collagen Type II and other ECM components were the most common patterns evaluated through immunohistochemistry on in-vitro and in-vivo studies. Only two studies made an orthotopic placement of the scaffolds. However, in none of the studies analyzed, the scaffold was placed in a subperichondrial pocket to rigorously simulate the cartilage environment. In contrast, scaffolds were implanted in a subcutaneous plane in almost all of the studies included.
CONCLUSION:
The role of 3D—bioprinting scaffolding for nasal cartilage defects repair is growing field. Despite the amount of information collected in the last years and the first surgical applications described recently in humans. Further investigations are needed due to the heterogeneity on mechanical evaluation parameters, the high level of heterotopic scaffold implantation and the need for quantitative histological data.
Keywords: Cartilage, Nasal, Bioprinting, Chondrocytes, Polycaprolactone
Introduction
Three-dimensional (3D) printing technology was first introduced by Hull [1] who originally named it as stereolithography or additive manufacturing. Over the last two decades, despite a relevant gap in its widespread adoption, this technology has acquired a significant reputation and is being prevalently used at the research and manufacturing levels in several fields [2–4]. More recently, diverse medical sub-specialties have started considering novel 3D bioprinting approaches, based on the concept of combining living cells and biomaterials, controlling cell proliferation, attachment, and migration within 3D printed scaffolds. Although still far from clinical use, these techniques could represent an initial step to create complex organs.
The nasal cartilage represents a specialized connective tissue devoid of the nerves, blood vessels, and lymphatic vessels. This tissue presents unique challenges for tissue engineering as it has relatively low cellularity (composed of approximately 1% chondrocytes and 99% extracellular matrix [ECM]), specific mechanical characteristics, and a low intrinsic self-repair capacity [5, 6].
Nasal defects that affect the craniofacial composition may arise from several etiologies, such as congenital malformation or cartilage absence, iatrogenic secondary to functional, oncological resection, or trauma. Importantly, nasal defects are related to both cosmetic and functional deficits [7]. Currently, treatment is based on reconstruction with local flaps or autologous cartilage grafts. However, these techniques are associated with increased donor-site morbidity, and the amount of tissue obtained is limited in size and shape depending on the availability of donor tissue. Thus, alternative techniques that take into consideration the complexity related to the anatomical 3D geometry are essential for the treatment of these pathologies.
Historically, the lack of autologous cartilage analogues for nasal reconstruction has led surgeons to try and develop a variety of allogeneic and synthetic grafts, which have thus far failed to replace the current gold standard. The efficacy of each material tested and their associated complications, such as extrusion, risk of infection, and foreign body reaction, are well described in the literature [8, 9]. Moreover, allogeneic grafts can be affected by immune rejection and have an associated risk of disease transmission [8, 9].
In recent years, 3D printing of tissue-engineered cartilaginous scaffolds is intended to close this gap and provide bioprinted tissue designed to fit the specific geometric and functional requirements of each defect, avoiding donor-site morbidity and personalizing therapy that best responds to patient needs. To achieve this goal, it is necessary to consider some criteria, such as the size, shape, and mechanical properties of the nasal cartilage [10]. The main goal of any tissue engineering strategy is to replicate such conditions and provide an engineered construct as similar as possible to the native tissue.
This review aims to analyze the current literature on the use of 3D bioprinting for nasal cartilage scaffolding, including up-to-date evidence.
Methods
This review involved a systematic search of MEDLINE/PubMed, Embase, Google Scholar, and Scopus databases for 3D bioprinting articles pertaining to nasal cartilage biomodeling between January 2014 and May 2020. The review was performed by two independent authors (C.CH. & X.A.). The following search terms were included: “tissue engineering,” “regenerative medicine,” “cartilage,” “nose,” “nasal,” “bioprinting,” “3D-printing,” and “nasal reconstruction.” Each abstract was individually reviewed to determine the primary focus: nasal anatomic region, cellular source, scaffold utilization, biochemical evaluation, histological analysis, in-vitro study, in-vivo study, animal model, length of research, orthotopic or heterotopic placement of experimental construct, and translational investigation. Articles that did not relate to any of these subjects were excluded. Review and opinion articles were also excluded.
This systematic review followed the guidelines proposed by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [11].
The methodological quality of evidence of the identified human and animal studies was appraised by the Oxford Center for Evidence-Based Medicine (OCEBM) Levels of Evidence [12] and Grading of Recommendations Assessment, Development, and Evaluation (GRADE), [13] respectively.
Results
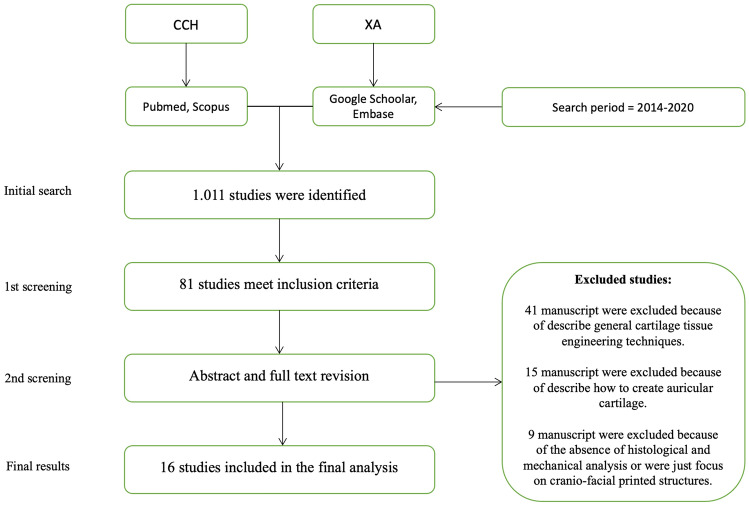
A total of 1,011 manuscripts were revised, and 81 studies met the inclusion criteria. Of these, 65 were excluded for the following reasons: (i) description of general cartilage tissue engineering techniques (n = 41), (ii) focus on the auricle (not nasal) cartilage (n = 15), and (iii) absence of histological and mechanical analyses or focus on craniofacial printed structures (n = 9) (Fig. 1). In total, 16 studies were included in the final analysis. All 16 articles were studied and analyzed; the information is summarized in Tables 1 and 2 [14–30].
Fig. 1.
Prisma flowchart
Table 1.
Characteristics of studies included
| Anatomic region | Translational study/Model | Cellular source & 3D printing method | Mechanical evaluation | Biochemical and histological evaluation | In-Vitro/In-Vivo Study | Orthotopic or Heterotopic placement | References |
|---|---|---|---|---|---|---|---|
| Nasal septum | No |
Human nasal septum derived progenitors undergoing septoplasty or septorhinoplasty FFFM |
No | Collagen Type II | Yes/Yes | N/A | [14] |
| Nasal and auricular cartilage | Yes/Porcine |
Chondrocytes from porcine auricular cartilage SLS |
No | Type I collagen–, hyaluronic acid–, and TGF-b2. | Yes/Yes | Heterotopic | [15] |
| Human nasal alar cartilage | Yes/Mice |
Chondrocytes from cadaveric human nasal alar cartilage CADP |
Yes | Expression of type II collagen | Yes/Yes | Heterotopic | [16] |
| Nasal dorsum cartilage | Yes/Rabbit |
Rabbit knee chondrocytes FFFM |
No | SEM confirmed that the chondrocytes seeded into the spaces between the PCL fibers. | Yes/Yes | Heterotopic | [17] |
| Nasal cartilage | Yes/Rabbit |
No FFFM |
No | Collagen type I. | No/Yes | Ortotopic | [18] |
| Nasal cartilage | Yes/Mice |
Human nasal chondrocytes & human bone marrow–derived mesenchymal stem cells Inkjet 3D printing |
Yes | GAGs and type II collagen | No/Yes | Heterotopic | [19] |
| Nasal cartilage | Yes/Mice |
Human nasal chondrocytes & human bone marrow–derived mesenchymal stem cells Inkjet 3D printing |
No | GAG | No/Yes | Heterotopic | [20] |
| Nasal cartilages and subchondral bone reconstruction | No |
Murine fibroblast gelatin isolated from pigskin was used for ossein-hydroxyapatite complex (OHC) also containing osteocalcin and type I collagen was used as bioactive powder.electrospinning process Ossein-hydroxyapatite complex (OHC) also containing osteocalcin and type I collagen was used as bioactive powder. FDM/Electrospinning |
Yes | Adhesion morphology. | Yes/No | N/A | [21] |
| Craniofacial cartilage | Yes/Rat |
Porcine adipose-derived stem cells and chondrocytes SLS |
No | Type II collagen | Yes/Yes | Heterotopic | [22] |
| Nasal septal deformities | Yes/Human & porcine |
No FFFM |
Yes | Porosity. | Yes/Yes | Orthotopic | [23] |
| Nasal & ear cartilage | Yes/Mice & goat |
Goat auricular cartilage derived chondrocytes Inkjet 3D printing |
Yes | Chondrocytes and ECM. | Yes/Yes | Heterotopic | [24] |
| Nasal cartilage | Yes/Porcine |
DECM of porcine nasal cartilage & human primary nasoseptal chondrocytes FFFM /DECM |
No | GAG, collagen type I, collagen type II. | Yes/No | N/A | [25] |
| Nasal cartilage | Yes/Mice |
Human adipose-derived stem-cells hyalin cartilage-decellularized ECM pregel pMSTL |
No | SOX9, ACAN, and COL21A. | No/Yes | Heterotopic | [26] |
| Nasal cartilage | Yes/Human |
Human nasal septal cartilage chondrocytes FFFM |
No | CD9, CD44, CD73, CD90, CD105, CD166, CD14, CD19, CD34, and HLA-DR. | Yes/No | N/A | [27] |
| Nasal cartilage | No |
Cartilage tissue was from sheep condyle FFFM |
Yes | Proliferation of the cells was confirmed by measuring DNA content which showed a gradual increase. | Yes/No | N/A | [28] |
| Nasal cartilage | No |
Primary human chondrocytes, isolated from normal human articular cartilage from the knee and hip joints FDM |
Yes | Chondrocyte proliferation and Collagen type II expression. | Yes/No | N/A | [29] |
| Nasal cartilage with functional electronic olfaction | No |
Nasal cartilage chondrocytes GELMA/Hibrid |
Yes | Collagen II and GAGs deposition | Yes/No | N/A | [30] |
N/A: not apply; PLLA = poly (L-lactide); PCL = polycaprolactone; PGA = poly (glycolic acid); FTIR = fourier transform infrared; WAXS = wide angle x-ray scattering; ECM = extracellular matrix; GAG = glycosaminoglycans; DECM = decellularized extracellular matrix; GelMA = gelatin-methacrylamide; PEGDMA = polyethylene glycol dimethacrylate; hNCs = human nasal chondrocytes; hBMSCs = human bone marrow–derived mesenchymal stem cells. Type of 3D printed method used in each study. FFFM = fused filament fabrication method; FDM = fused deposition method; SLS = selective laser sintering; CADP = computer assisted deposition printing; PLS = plastic laser-sintering system; DECM = decellularized extracellular cartilage matrix; pMSTL = projection-based microstereolithography
Table 2.
Summary of study conclusions
| Study conclusion | References |
|---|---|
| Authors demonstrate that electrospun constructs maintain NSP proliferation and differentiation, and that the aligned nanofibrous scaffolds can significantly enhance chondrogenic differentiation of nasal septum derived progenitors. | [14] |
| Authors describe image-based CAD/CAM 3D printing process in the production of bioresorbable PCL scaffolds with defined porous architecture for cartilaginous frameworks. | [15] |
| In this study, the authors demonstrate that three-dimensional printing–aided tissue engineering can achieve precise three-dimensional shapes of human nasal alar cartilage. | [16] |
| In this manuscript a new PCL scaffold designed by 3D printing method seeded with fibrin/chondrocytes to be used as a biocompatible augmentation material in rhinoplasty in the future was described. | [17] |
| In this manuscript author introduce a PCL scaffold between the pericondrium and the septal cartilage. According to their result, they suggest that PCL can be used for nasal reconstruction such as nasal augmentation. | [18] |
| In vivo chondrogenesis in a 3D bioprinted human cell-laden hydrogel construct has been demonstrated. | [19] |
| In this study, authors described the creation of viable cartilage in vivo using a 3D-bioprinted construct. The lineage with human nasal chondrocytes showed good proliferative ability in terms of cell number and cartilage-cluster formation. Furthermore, they observed that the addition of MSCs enhanced chondrocyte proliferation. | [20] |
| Here authors demonstrated the properties of nanofibrous gelatin membranes were strongly influenced by the concentration of gelatin. Also, the obtained layered PLLA/gelatin/Osteo scaffold could be potentially suitable for nasal cartilages and subchondral bone reconstruction. | [21] |
| The viability of a Tissue-engineered cartilage successfully produced on a 3D-printed bioresorbable scaffolds using an adipose-derived stem cell and chondrocyte co-culture technique was demostrated. | [22] |
| Authors demonstrated the clinical feasibility of 3-D printed, homogeneous, composite, microporous polycaprolactone nasal human implant, demonstrated proper mechanical support and thinness with excellent biocompatibility and surgical manipulability. | [23] |
| In this study, authors established a novel scaffold-fabricated strategy for native polymers (Gelatin & Hyaluronic) and provided a novel natural 3D printed scaffold with satisfactory outer shape, pore structure, mechanical strength, degradation rate, and weak immunogenicity for cartilage regeneration. | [24] |
| The authors demonstrate that the addition of DECM particles to a PCL scaffold leads to a significantly higher cartilage regeneration compared to pure PCL scaffolds in vitro. | [25] |
| Properties of hNC–PCL complex may be a valuable therapeutic agent for implantation into injured cartilage tissue, and can be used clinically to repair cartilaginous skeletal defects. | [26] |
| In this study, authors show the feasibility of manufacturing neocartilage using chondrocytes/GelMA/PCL 3D bioprinted porous constructs which could be applied as a method for fabricating implants for nose reconstruction. | [27] |
| In this work, authors evaluate the possibility of preparing 3D scaffolds from composite polycaprolactone/Graphene. | [28] |
| A new hybrid device consisting of a dual bioink printed nose construct with an integrated biosensing system was described in this study. | [29] |
Abbreviations: N/A: not apply; PLLA = poly (L-lactide); PCL = polycaprolactone; PGA = poly (glycolic acid); FTIR = fourier transform infrared; WAXS = wide angle x-ray scattering; ECM = extracellular matrix; GAG = glycosaminoglycans; DECM = decellularized extracellular matrix; GelMA = gelatin-methacrylamide; PEGDMA = polyethylene glycol dimethacrylate; hNCs = human nasal chondrocytes; hBMSCs = human bone marrow–derived mesenchymal stem cells
From the first publication in 2014, an increasing yearly publication rate was observed. Of the 16 studies included, 11 were related to translational research, 10 in animal models [15–20, 22–26] and one in human beings [27]. The most common animal model used was laboratory mice (n = 5). [16, 19, 20, 24, 26]
The cellular sources described in the studies were more heterogeneous. Two studies included acellular constructs [18, 23]. Most studies used primary chondrocyte cultures (n = 12) [15–17, 19, 20, 22, 24, 25, 27–30]. The following cartilages were used for cell isolation: nasal septum (n = 7) [14, 16, 19, 20, 25, 27, 30], auricle [15, 24, 29], knee or hip joints [17, 29], and condyle [28]. Four studies used mesenchymal stem cells, derived either from the bone marrow[19, 20] or adipose tissue[22, 26], and they were used alone[26] or in combination with primary chondrocytes [19, 20, 22]. One study used chondrocytes derived from stem/progenitor cells [14] and one utilized primary fibroblasts [21]. With regard to the species from which cells were derived, most studies used human cells (n = 9); [14, 16, 19, 20, 25–27, 29, 30] however, porcine, [15, 22] rabbit [17], mouse, [21] goat [24], and sheep[28] cells were also used (Table 1).
The most common biomaterial used for scaffold creation was polycaprolactone (PCL), alone or in combination (n = 9) [14, 15, 17, 18, 22, 23, 25–27, 29]. Other materials used for scaffold creation were gelatin-methacrylamide [24, 28, 30] and poly L-lactic acid [14, 16, 21]. The mechanical evaluation of the scaffolds used was not performed in nine studies. Among the studies that included the mechanical evaluation, we found a high heterogeneity, making it difficult to derive any definitive conclusion (Table 1).
Regarding the biological and histological characteristics of each scaffold, we found that the expression of type I and II collagen and other ECM components were the most common patterns evaluated through immunohistochemistry in various in-vitro and in-vivo studies. Other histological factors considered were proteoglycans expression, transforming growth factor-beta 2, or glycosaminoglycans (GAGs). The authors usually evaluated protein expression at 4, 8, and 12 weeks. Scanning electron microscopy was typically performed to confirm chondrocyte seeding and adhesion to the scaffold fibers. The level of cytotoxicity was assessed only by Rajzer et al. [21] Regarding biomaterial porosity, Kim et al. [23] highlighted the importance of scaffold porosity of 50%, which increased the ingrowth of surrounding tissues. According to Wiggenhauser et al. [25] GAG formation was significantly higher on PCL scaffolds than on any other type of scaffold. Yi et al. [26] highlighted that the cartilage-derived hydrogel scaffold produced more type II collagen than cells seeded in alginate scaffolds, and Kim et al. [27] demonstrated the expression of CD9, CD44, CD73, CD90, CD105, and CD166 chondrogenic markers. (Table 1).
Only two studies performed an orthotopic placement of the scaffolds [18, 23]. However, the scaffold was not placed in a subperichondrial pocket to rigorously simulate the cartilage environment in any of the study included in this review. In contrast, scaffolds were implanted in a subcutaneous plane in almost all of the included studies.
The most common type of 3D bioprinting method used was the fused filament fabrication method (FFFM) in seven studies [14, 17, 18, 23, 25, 27, 28]. The type of 3D bioprinting methods used in the remaining studies are described in Table 1. Regarding the type of cartilage or extent of the nose to be replicated, 14 studies tried to fabricate a 3D printed construct to replicate the nasal septum [14, 15, 18–29]. In one study, the authors tried to replicate the alar nasal cartilage [16], and in another study, the authors tried to replicate the nasal dorsum cartilage [17] (Table 1).
According to the OCEBM grading system, the only study involving humans received a level 3 qualification [27]. According to the GRADE method, six studies were graded as 1b, [16, 19, 20, 26] six as 2b, [15, 17, 18, 21, 22, 28] and three as 2c [14, 25, 29] (Table 3).
Table 3.
Quality of evidence appraisal
| OCEBM | GRADE | References |
|---|---|---|
| 2c | [14] | |
| 2b | [15] | |
| 1b | [16] | |
| 2b | [17] | |
| 2b | [18] | |
| 1b | [19] | |
| 1b | [20] | |
| 2b | [21] | |
| 2b | [22] | |
| 1b | [23] | |
| 1b | [24] | |
| 2c | [25] | |
| 1b | [26] | |
| Level 3 | [27] | |
| 2b | [28] | |
| 2c | [29] |
Abbreviations: OCEBM = oxford center for evidence-based medicine, GRADE = grading of recommendations assessment, development and evaluation
Discussion
Nowadays, tissue engineering investigations are widely conducted in the fields of regeneration, restoration, or replacement of defective or injured functional living organs and tissues [31–33]. This represents the main reason for understanding the basic concept of 3D bioprinting as a tool to produce a 3D structure combining living cells and biomaterials, controlling cell proliferation, attachment, differentiation, and migration within 3D structures. Therefore, a significant challenge in reconstructive plastic surgery is the need for repair or replacement of damaged or absent cartilaginous structures, such as the nose [34].
Current surgical procedures have several drawbacks and complications, such as infections, tissue necrosis, and pain. Moreover, a limited number of surgeons have mastered the art of using autogenous cartilage, such as the rib or conchal cartilage [35], despite this being the current gold standard for nasal cartilage framework reconstruction. This situation is probably related to the highly complicated and time-consuming multi-staged procedure, with different anesthetic exposures and risks depending on patients’ comorbidities and the type of defect. Furthermore, the outcome of surgery is often less than perfect [35–39].
An alternative option for autologous rib reconstruction is the use of prefabricated, synthetic implant, MedPor (Stryker Corporation, Kalamazoo, Michigan), or porous, high-density polyethylene implants [40]. The benefits of this technique include avoiding donor-site morbidity from autologous cartilage harvesting and lower variability with the framework appearance, bypassing the technically demanding carving of the framework. However, personalized frameworks are not available for patient-specific anatomical defects. Although MedPor is approved by the Food and Drug Administration, it has a greater incidence of framework extrusion and soft tissue necrosis when compared to autologous cartilage reconstruction [41].
Biomedical scaffolds made of natural or synthetic polymers have emerged in recent years from the biomedical and tissue engineering fields as a potential tool for nasal cartilage repair, [42, 43] to replace or functionally and structurally regenerate native tissues. A scaffold has the following functions: it should provide internal pathways for cell attachment, differentiation, and migration; it must permit trafficking of various growth factors (GFs) and waste products; it should maintain its shape when the cells are growing, while being permissive to partial remodeling; and it should have adequate mechanical properties [44]. Ideally, the scaffold manufacturing process should be individually calibrated to achieve these functions, modifying porosity (pore-size distribution, pore volume, and pore interconnectivity) of scaffolds to increase cell affinity, proliferation, migration, attachment, and differentiation, and even enabling nutrient and oxygen transport in a purpose-built manner [45, 46].
All these advantages are increasing the interests of scientists in the application of scaffolds with well-designed and specific architectures that can efficiently provide a native niche for cells in ex-vivo/in-vitro environments and in animal and human models [47, 48]. Generally, ECM is composed of a 3D ultrafine fibrous architecture with certain physical and mechanical properties that are specific to each tissue in the body. Concurrently, ECM modulates cell morphology and functions, such as adhesion, proliferation, and differentiation [49]. Therefore, scaffolding strategies should consider these properties as essential during the design and fabrication process.
As mentioned above, scaffolds are intended to support cell growth and environment equilibrium. Hence, the secretion of ECM represents a critical stage during chondrocyte maturation. This phenomenon was detected via the observation of ECM-like structures on stem cell-seeded scaffolds after chondrogenic induction [14]. Among the various proteins present in the ECM, type II collagen is the major component of hyaline-like cartilage [50, 51]. Nasal cartilage is composed of hyaline cartilage, which consists of densely packed collagen and proteoglycan-based ECM embedded with chondrocytes [52]. Moreover, it is a relatively simple structure with mechanically robust and elastic properties than the other tissues in the body. These characteristics justify a concerted effort to replicate this type of tissue [53].
One of the main problems in translating this technology from the laboratory to surgical or clinical facilities is the need to source autologous chondrocytes. However, some solutions are on the horizon. Ear, nose, and throat surgeons usually perform septal surgeries. They represent an opportunity for harvesting the nasal cartilage from an easy and a less-invasive surgery, with reduced morbidity [54].
Human nasal chondrocytes proliferate approximately four times faster than human articular chondrocytes in monolayer culture and have markedly higher chondrogenic capacity in in-vitro tissue-engineered constructs [55]. In addition, nasal chondrocytes exhibit a higher proliferation and chondrogenic capacity than articular chondrocytes [56].
Chondrocytes induce chondrogenic differentiation of mesenchymal stem cells (MSCs) via the production of exogenous GFs, such as cytokine-like protein 1, bone morphogenetic protein-2, parathyroid hormone-related peptide, and transforming growth factor-beta, and paracrine, juxtacrine, and gap-junction signaling pathways [57–60]. In this way, chondrocytes provide the chondrogenic niche required for the commitment of MSCs to the chondrogenic phenotype, circumventing the need for exogenous GF delivery. Additionally, chondrocytes provide a matrix for MSC migration and prevent ossification of MSC-derived chondrocytes [61].
Limitation of utilizing chondrocytes solely for cartilage tissue engineering is the large number of cells needed to seed human-sized craniofacial frameworks [62–66]. The number of chondrocytes available from autologous cartilage is limited, and passaging chondrocytes induce dedifferentiation with loss of type II collagen and s-GAG production [67, 68]. MSCs, of which ample cell quantities are available, have been posited as a solution to seeding requirements. A variety of MSC types, including adipose-derived stromal cells (ASCs) and bone marrow stromal cells, are capable of chondrogenic differentiation [69, 70]. However, the chondrogenic commitment of MSCs requires exogenous delivery of GFs for weeks, and cells can demonstrate a propensity for ossification [71, 72]. Additionally, neovascularization surrounding 3D tissue-engineered cartilaginous constructs has proven to be a challenge for the long-term stability of these constructs, particularly with the fragility of ASCs in hypoxic tissue environments [73].
Another relevant factor regarding the histological characteristics of the scaffold is the evaluation of cellular proliferation. All the studies included in this review, except the one reported by Apelgren et al., described a qualitative description of chondrocyte proliferation. In this study, the results revealed quantitative evidence of the proliferation of bioprinted chondrocytes, and data confirming the presence of the boosting effect derived from co-culture with MSCs [20].
According to our results, PCL was the most commonly used polymer. PCL represents a biocompatible and biodegradable synthetic polymer with adequate mechanical strength and durability needed for cartilage regeneration. The superior rheological and viscoelastic properties of this polymer render PCL easy to manufacture and manipulate into different implants and devices [18]. Its excellent mechanical properties (it resists deformity from scar contraction during the healing process) and slow biodegradation support the use of this polymer in nasal reconstruction [17]. However, it is important to note that the mechanical properties of bioengineered cartilage are modulated by the deposition of GAGs and collagens and by the cell type used [74].
In a previous publication, Ferril et al. reported the characteristics that define an ideal alloplastic material. The authors highlighted that implants need to be noncarcinogenic, nonallergenic, readily available, resistant to mechanical strain, and entirely absorbable, while still providing the desired outcome [75]. Polycaprolactone implants are produced with a compressive stiffness range between 2.74 and 55.95 MPa, according to the processing parameters, and this range is inclusive of native cartilage parameters (12.8–22.5 MPa) [76, 77]. Thus, the mechanical properties of PCL implants can match those of cartilage without difficulty.
However, in line with the purpose of this review, we need to mention unfavorable properties of synthetic materials for cartilage regeneration, such as their low biocompatibility, low bioactivity, and the possibility of aseptic inflammation caused by their degradation products when implanted into immunocompetent large animal models and humans. Some authors have argued against the use of synthetic polymers for cartilage regeneration. Conversely, those authors claim that the use of natural polymers improves biocompatibility, biological activity, reduced immunogenicity, and cytotoxicity from their degradation products becoming favorable as biomimetic scaffolds for cartilage regeneration [78, 79]. Hence, natural ECM-derived scaffolds have become popular in recent years owing to their natural composition and natively structured microenvironment [80]. Wiggenhauser et al. [25] recommend the use of porcine septal cartilage to develop a matrix derivative that is highly suitable for cartilage scaffolding with human chondrocytes as a decellularized extracellular cartilage matrix. Furthermore, these authors had previously demonstrated the efficacy and safety of these types of scaffolds [81–84].
As highlighted by Moller et al. [19], hydrogel-based scaffolds also constitute a valid alternative. Their high water content gives them a structural similarity to the cartilage ECM, conceptually improving the regeneration environment [85, 86]. In the case of hydrogels, one of the shortcomings is their viscoelastic properties, which hamper good printing fidelity. Another problem is their mechanical properties, such as strength and stiffness, which make them difficult to handle during transplantation. Moller et al. demonstrated the viability of co-culture of human nasal chondrocytes (hNCs) and human bone marrow-derived MSCs, rather than a single cell type alone, using a specific combination of 20/80 ratio, which is an optimal ratio for the induction of cartilage regeneration [87–92].
Regarding the 3D bioprinting method used in the studies included in this review, the FFFM was the most common method. This technique is one of the most reliable methods for 3D printed scaffold fabrication because of its ease of use, variety of biomaterials available, and good mechanical properties. However, some disadvantages are the material restrictions related to thermoplastic polymers and lack of guarantee that it can be printed with cells effectively owing to the high manufacturing temperatura [93]. Other methods are Inkjet 3D bioprinting technology, a method that allows creating different structures using a rapid prototyping and layered manufacturing technology, and the use of polymeric bio-ink printing for applications in biological and tissue engineering fields [93]. The selective laser sintering technology utilizes selective laser printing from 3D modeling software on the surface of a powder bed and prints using several different materials, such as ceramics, metals, and polymers [15]. Projection-based microstereolithography utilizes a software that is able to convert a two-dimensional facial picture into a 3D facial model. Further, an algorithm of the nasal graft model is generated using the two nasal surface data extracted from the original and modified nasal models. This facial model is transformed into a mesh surface model and exported to a stereolithography file format to print the scaffold [26].
The limitations identified in this review were as follows: the lack of a control group in almost all of the studies, heterogeneity in mechanical evaluation parameters, high level of heterotopic scaffold implantation, use of a subcutaneous plane and not the subperichondrial plane to replicate the cartilage environment, and need for more quantitative histological data. Studies examining the combination of hNCs and PCL are rare in the context of craniofacial defects or degenerative diseases. The effectiveness and quality of septal chondrocytes combined with PCL must be evaluated for the clinical application of this type of construct.
Acknowledgements
Conceptualization, C.M.C.E. and A.A.; methodology, C.M.C.E.; validation, A.I., X.A. and I.G.; formal analysis, C.M.C.E. and R.H; investigation, C.R. and A.D.; data curation, J.P. and J.A; writing—original draft preparation, C.M.C.E.; writing—review and editing, C.M.C.E.; supervision. All authors have read and agreed to the published version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declares that they don’t have any conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hull CW. Inventor; Uvp Inc., original assignee. Apparatus for production of three-dimensional objects by stereolithography. US Patent US4575330A (1986). https://www.google.com/patents/US4575330. Accessed 11 Oct 2020.
- 2.Kruth JP, Leu MC, Nakagawa T. Progress in additive manufacturing and rapid prototyping. CIRP Ann Manuf Technol. 1998;47:525–540. doi: 10.1016/S0007-8506(07)63240-5. [DOI] [Google Scholar]
- 3.Melchels FP, Domingos MA, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012;37:1079–1104. doi: 10.1016/j.progpolymsci.2011.11.007. [DOI] [Google Scholar]
- 4.Bak D. Rapid prototyping or rapid production? 3D printing processes move industry towards the latter. Assem Autom. 2003;23:340–345. doi: 10.1108/01445150310501190. [DOI] [Google Scholar]
- 5.Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999;7:2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- 6.Jackson DW, Scheer MJ, Simon TM. Cartilage substitutes: overview of basic science and treatment options. J Am Acad Orthop Surg. 2001;9:37–52. doi: 10.5435/00124635-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5:335–341. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 8.Kushnaryov A, Yamaguchi T, Briggs K, Reuther MS, Watson D, Masuda K, et al. Evaluation of autogenous engineered septal cartilage grafts in rabbits: a minimally invasive preclinical model. Otolaryngol Head Neck Surg. 2013;149:37–38. doi: 10.1177/0194599813495815a17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulco I, Miot S, Haug MD, Barbero A, Wixmerten A, Feliciano S, et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet. 2014;384:337–346. doi: 10.1016/S0140-6736(14)60544-4. [DOI] [PubMed] [Google Scholar]
- 10.Niermeyer WL, Rodman C, Li MM, Chiang T. Tissue engineering applications in otolaryngology-the state of translation. Laryngoscope Investig Otolaryngol. 2020;5:630–648. doi: 10.1002/lio2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA group. preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OCEBM Levels of Evidence Working Group. “The Oxford 2011 levels of evidence.” Oxford centre for evidence-based medicine. 2011. http://www.cebm.net/index.aspx?o=5653.
- 13.Wei D, Tang K, Wang Q, Estill J, Yao L, Wang X, et al. The use of GRADE approach in systematic reviews of animal studies. J Evid Based Med. 2016;9:98–104. doi: 10.1111/jebm.12198. [DOI] [PubMed] [Google Scholar]
- 14.Shafiee A, Seyedjafari E, Sadat Taherzadeh E, Dinarvand P, Soleimani M, Ai J. Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Mater Sci Eng C Mater Biol Appl. 2014;40:445–454. doi: 10.1016/j.msec.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Zopf DA, Mitsak AG, Flanagan CL, Wheeler M, Green GE, Hollister SJ. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngol Head Neck Surg. 2015;152:57–62. doi: 10.1177/0194599814552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Fan F, Kang N, Wang S, You J, Wang H, et al. Tissue engineering of human nasal alar cartilage precisely by using three-dimensional printing. Plast Reconstr Surg. 2015;135:451–458. doi: 10.1097/PRS.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Shin YS, Park DY, Choi JW, Park JK, Kim DH, et al. The application of three-dimensional printing in animal model of augmentation rhinoplasty. Ann Biomed Eng. 2015;43:2153–2162. doi: 10.1007/s10439-015-1261-3. [DOI] [PubMed] [Google Scholar]
- 18.Park SH, Yun BG, Won JY, Yun WS, Shim JH, Lim MH, et al. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope. 2017;127:1036–1043. doi: 10.1002/lary.26400. [DOI] [PubMed] [Google Scholar]
- 19.Möller T, Amoroso M, Hägg D, Brantsing C, Rotter N, Apelgren P, et al. In vivo chondrogenesis in 3D bioprinted human cell-laden hydrogel constructs. Plast Reconstr Surg Glob Open. 2017;5:e1227. doi: 10.1097/GOX.0000000000001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apelgren P, Amoroso M, Lindahl A, Brantsing C, Rotter N, Gatenholm P, et al. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS One. 2017;12:e0189428. doi: 10.1371/journal.pone.0189428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajzer I, Kurowska A, Jabłoński A, Jatteau S, Śliwka M, Ziąbka M, et al. Layered gelatin/PLLA scaffolds fabricated by electrospinning and 3D printing- for nasal cartilages and subchondral bone reconstruction. Mater Des. 2018;155:297–306. doi: 10.1016/j.matdes.2018.06.012. [DOI] [Google Scholar]
- 22.Morrison RJ, Nasser HB, Kashlan KN, Zopf DA, Milner DJ, Flanangan CL, et al. Co-culture of adipose-derived stem cells and chondrocytes on three-dimensionally printed bioscaffolds for craniofacial cartilage engineering. Laryngoscope. 2018;128:E251–E257. doi: 10.1002/lary.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Lim MH, Jeun JH, Park SH, Lee W, Park SH, et al. Evaluation of polycaprolactone-associated human nasal chondrocytes as a therapeutic agent for cartilage repair. Tissue Eng Regen Med. 2019;16:605–614. doi: 10.1007/s13770-019-00210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia H, Zhao D, Zhu H, Hua Y, Xiao K, Xu Y, et al. Lyophilized scaffolds fabricated from 3D-printed photocurable natural hydrogel for cartilage regeneration. ACS Appl Mater Interfaces. 2018;10:31704–31715. doi: 10.1021/acsami.8b10926. [DOI] [PubMed] [Google Scholar]
- 25.Wiggenhauser PS, Schwarz S, Koerber L, Hoffmann TK, Rotter N. Addition of decellularized extracellular matrix of porcine nasal cartilage improves cartilage regenerative capacities of PCL-based scaffolds in vitro. J Mater Sci Mater Med. 2019;30:121. doi: 10.1007/s10856-019-6323-x. [DOI] [PubMed] [Google Scholar]
- 26.Yi HG, Choi YJ, Jung JW, Jang J, Song TH, Chae S, et al. Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty. J Tissue Eng. 2019;10:2041731418824797. doi: 10.1177/2041731418824797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DH, Yun WS, Shim JH, Park KH, Choi D, Park MI, et al. Clinical application of 3-dimensional printing technology for patients with nasal septal deformities: a multicenter study. JAMA Otolaryngol Head Neck Surg. 2018;144:1145–1152. doi: 10.1001/jamaoto.2018.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Cantu L, Gleadall A, Faris C, Segal J, Shakesheff K, Yang J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 2020;109:110578. doi: 10.1016/j.msec.2019.110578. [DOI] [PubMed] [Google Scholar]
- 29.Rajzer I, Kurowska A, Jabłoński A, Kwiatkowski R, Piekarczyk W, Hajduga MB, et al. Scaffolds modified with graphene as future implants for nasal cartilage. J Mater Sci. 2020;55:4030–42. doi: 10.1007/s10853-019-04298-7. [DOI] [Google Scholar]
- 30.Jodat YA, Kiaee K, Vela Jarquin D, De la Garza Hernández RL, Wang T, Joshi S, et al. A 3D-printed hybrid nasal cartilage with functional electronic olfaction. Adv Sci (Weinh) 2020;7:1901878. doi: 10.1002/advs.201901878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;2011:290602. doi: 10.1155/2011/290602. [DOI] [Google Scholar]
- 32.Atala A. Tissue engineering of reproductive tissues and organs. Fertil Steril. 2012;98:21–29. doi: 10.1016/j.fertnstert.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 33.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 34.Lindahl A. From gristle to chondrocyte transplantation: treatment of cartilage injuries. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140369. doi: 10.1098/rstb.2014.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brent B. The correction of mi-rotia with autogenous cartilage grafts: I. The classic deformity. Plast Reconstr Surg. 1980;66:1–12. doi: 10.1097/00006534-198007000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Firmin F. State-of-the-art autogenous ear reconstruction in cases of microtia. In: Staudenmaier R, editor. Aesthetics and functionality in ear reconstruction. Basel: Karger Publishers; 2010. pp. 25–52. [DOI] [PubMed] [Google Scholar]
- 37.Zopf DA, Iams W, Kim JC, Baker SR, Moyer JS. Full-thickness skin graft overlying a separately harvested auricular cartilage graft for nasal alar reconstruction. JAMA Facial Plast Surg. 2013;15:131–134. doi: 10.1001/jamafacial.2013.25. [DOI] [PubMed] [Google Scholar]
- 38.Firmin F, Marchac A. A novel algorithm for autologous ear reconstruction. Semin Plast Surg. 2011;25:257–264. doi: 10.1055/s-0031-1288917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osorno G. Autogenous rib cartilage reconstruction of congenital ear defects: report of 110 cases with Brent's technique. Plast Reconstr Surg. 1999;104:1951–1962. doi: 10.1097/00006534-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Romo T, 3rd, Presti PM, Yalamanchili HR. Medpor alternative for microtia repair. Facial Plast Surg Clin North Am. 2006;14:129–136. doi: 10.1016/j.fsc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Zhao YY, Zhuang HX, Jiang HY, Jiang WJ, Hu XG, Hu SD, et al. Clinical application of three methods for total ear reconstruction. Zhonghua Zheng Xing Wai Ke Za Zhi. 2008;24:287–290. [PubMed] [Google Scholar]
- 42.Tian H, Tang Z, Zhuang X, Chen X, Jing X. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog Polym Sci. 2012;37:237–280. doi: 10.1016/j.progpolymsci.2011.06.004. [DOI] [Google Scholar]
- 43.O’Brien FJ. Biomaterials and scaffolds for tissue engineering. Mater Today (Kidlington) 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- 44.Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today (Kidlington) 2013;16:496–504. doi: 10.1016/j.mattod.2013.11.017. [DOI] [Google Scholar]
- 45.Gauvin R, Chen YC, Lee JW, Soman P, Zorlutuna P, Nichol JW, et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33:3824–3834. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin JR, Gupta MK, Page JM, Yu F, Davidson JM, Guelcher SA, et al. A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials. 2014;35:3766–3776. doi: 10.1016/j.biomaterials.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teh TK, Toh SL, Goh JC. Aligned hybrid silk scaffold for enhanced differentiation of mesenchymal stem cells into ligament fibroblasts. Tissue Eng Part C Methods. 2011;17:687–703. doi: 10.1089/ten.tec.2010.0513. [DOI] [PubMed] [Google Scholar]
- 48.Unadkat HV, Hulsman M, Cornelissen K, Papenburg BJ, Truckenmüller RK, Carpenter AE, et al. An algorithm-based topographical biomaterials library to instruct cell fate. Proc Natl Acad Sci U S A. 2011;108:16565–16570. doi: 10.1073/pnas.1109861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas V, Jose MV, Chowdhury S, Sullivan JF, Dean DR, Vohra YK. Mechano-morphological studies of aligned nanofibrous scaffolds of polycaprolactone fabricated by electrospinning. J Biomater Sci Polym Ed. 2006;17:969–984. doi: 10.1163/156856206778366022. [DOI] [PubMed] [Google Scholar]
- 50.Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 51.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 52.Homicz MR, McGowan KB, Lottman LM, Beh G, Sah RL, Watson D. A compositional analysis of human nasal septal cartilage. Arch Facial Plast Surg. 2003;5:53–58. doi: 10.1001/archfaci.5.1.53. [DOI] [PubMed] [Google Scholar]
- 53.Bas O, De-Juan-Pardo EM, Meinert C, D'Angella D, Baldwin JG, Bray LJ, et al. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication. 2017;9:025014. doi: 10.1088/1758-5090/aa6b15. [DOI] [PubMed] [Google Scholar]
- 54.Kafienah W, Jakob M, Démarteau O, Frazer A, Barker MD, Martin I, et al. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8:817–826. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 55.Rotter N, Bonassar LJ, Tobias G, Lebl M, Roy AK, Vacanti CA. Age dependence of biochemical and biomechanical properties of tissue-engineered human septal cartilage. Biomaterials. 2002;23:3087–3094. doi: 10.1016/S0142-9612(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 56.Tay AG, Farhadi J, Suetterlin R, Pierer G, Heberer M, Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue Eng. 2004;10:762–770. doi: 10.1089/1076327041348572. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, et al. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31:9406–9414. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 58.Keller B, Yang T, Chen Y, Munivez E, Bertin T, Zabel B, et al. Interaction of TGFbeta and BMP signaling pathways during chondrogenesis. PLoS One. 2011;6:e316421. doi: 10.1371/journal.pone.0016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JS, Ryoo ZY, Chun JS. Cytokine-like 1 (Cytl1) regulates the chondrogenesis of mesenchymal cells. J Biol Chem. 2007;282:29359–29367. doi: 10.1074/jbc.M700965200. [DOI] [PubMed] [Google Scholar]
- 60.Choi YS, Lim SM, Shin HC, Lee CW, Kim SL, Kim DI. Chondrogenesis of human periostiumderived progenitor cells in atelocollagen. Biotechnol Lett. 2007;29:323–329. doi: 10.1007/s10529-006-9240-2. [DOI] [PubMed] [Google Scholar]
- 61.Mo XT, Guo SC, Xie HQ, Deng L, Zhi W, Xiang Z, et al. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45:42–51. doi: 10.1016/j.bone.2008.07.240. [DOI] [PubMed] [Google Scholar]
- 62.Shieh SJ, Terada S, Vacanti JP. Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials. 2004;25:1545–1557. doi: 10.1016/S0142-9612(03)00501-5. [DOI] [PubMed] [Google Scholar]
- 63.Zhou L, Pomerantseva I, Bassett EK, Bowley CM, Zhao X, Bichara DA, et al. Engineering ear constructs with a composite scaffold to maintain dimensions. Tissue Eng Part A. 2011;17:1573–1581. doi: 10.1089/ten.tea.2010.0627. [DOI] [PubMed] [Google Scholar]
- 64.Xue J, Feng B, Zheng R, Lu Y, Zhou G, Liu W, et al. Engineering earshaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials. 2013;34:2624–2631. doi: 10.1016/j.biomaterials.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Ruszymah BH, Chua KH, Mazlyzam AL, Aminuddin BS. Formation of tissue engineered composite construct of cartilage and skin using high density polyethylene as inner scaffold in the shape of human helix. Int J Pediatr Otorhinolaryngol. 2011;75:805–810. doi: 10.1016/j.ijporl.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Yanaga H, Imai K, Fujimoto T, Yanaga K. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plast Reconstr Surg. 2009;124:817–825. doi: 10.1097/PRS.0b013e3181b17c0e. [DOI] [PubMed] [Google Scholar]
- 67.von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 68.Kusuhara H, Isogai N, Enjo M, Otani H, Ikada Y, Jacquet R, et al. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009;17:136–146. doi: 10.1111/j.1524-475X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 69.Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 70.Martin I, Shastri VP, Padera RF, Yang J, Mackay AJ, Langer R, et al. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. J Biomed Mater Res. 2001;55:229–235. doi: 10.1002/1097-4636(200105)55:2<229::AID-JBM1009>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 71.Hwang NS, Elisseeff J. Application of stem cells for articular cartilage regeneration. J Knee Surg. 2009;22:60–71. doi: 10.1055/s-0030-1247728. [DOI] [PubMed] [Google Scholar]
- 72.Hickok NJ, Haas AR, Tuan RS. Regulation of chondrocyte differentiation and maturation. Microsc Res Tech. 1998;43:174–190. doi: 10.1002/(SICI)1097-0029(19981015)43:2<174::AID-JEMT9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 73.Merceron C, Vinatier C, Portron S, Masson M, Amiaud J, Guigand L, et al. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010;298:C355–C364. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37:1–57. doi: 10.1615/CritRevBiomedEng.v37.i1-2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferril GR, Wudel JM, Winkler AA. Management of complications from alloplastic implants in rhinoplasty. Curr Opin Otolaryngol Head Neck Surg. 2013;21:372–378. doi: 10.1097/MOO.0b013e3283628e40. [DOI] [PubMed] [Google Scholar]
- 76.Glasgold MJ, Kato YP, Christiansen D, Hauge JA, Glasgold AI, Silver FH. Mechanical properties of septal cartilage homografts. Otolaryngol Head Neck Surg. 1988;99:374–379. doi: 10.1177/019459988809900404. [DOI] [PubMed] [Google Scholar]
- 77.Li X, Cui R, Sun L, Aifantis KE, Fan Y, Feng Q, et al. 3D-printed biopolymers for tissue engineering application. Int J Polym Sci. 2014;2014:829145. doi: 10.1155/2014/829145. [DOI] [Google Scholar]
- 78.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliv Rev. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 79.Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiggenhauser PS, Schantz JT, Rotter N. Cartilage engineering in reconstructive surgery: auricular, nasal and tracheal engineering from a surgical perspective. Regen Med. 2017;12:303–314. doi: 10.2217/rme-2016-0160. [DOI] [PubMed] [Google Scholar]
- 81.Elsaesser AF, Bermueller C, Schwarz S, Koerber L, Breiter R, Rotter N. In vitro cytotoxicity and in vivo effects of a decellularized xenogeneic collagen scaffold in nasal cartilage repair. Tissue Eng Part A. 2014;20:1668–1678. doi: 10.1089/ten.tea.2013.0365. [DOI] [PubMed] [Google Scholar]
- 82.Goldberg-Bockhorn E, Schwarz S, Elsässer A, Seitz A, Körber L, Dürselen L, et al. Physical characterization of decellularized cartilage matrix for reconstructive rhinosurgery. Laryngorhinootologie. 2014;93:756–763. doi: 10.1055/s-0034-1384531. [DOI] [PubMed] [Google Scholar]
- 83.Schwarz S, Elsaesser AF, Koerber L, Goldberg-Bockhorn E, Seitz AM, Bermueller C, et al. Processed xenogenic cartilage as innovative biomatrix for cartilage tissue engineering: effects on chondrocyte differentiation and function. J Tissue Eng Regen Med. 2015;9:E239–E251. doi: 10.1002/term.1650. [DOI] [PubMed] [Google Scholar]
- 84.Schwarz S, Koerber L, Elsaesser AF, Goldberg-Bockhorn E, Seitz AM, Durselen L, et al. Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Eng Part A. 2012;18:2195–2209. doi: 10.1089/ten.tea.2011.0705. [DOI] [PubMed] [Google Scholar]
- 85.Guillotin B, Guillemot F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 2011;29:183–190. doi: 10.1016/j.tibtech.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 86.Frampton JP, Hynd MR, Shuler ML, Shain W. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed Mater. 2011;6:015002. doi: 10.1088/1748-6041/6/1/015002. [DOI] [PubMed] [Google Scholar]
- 87.Martínez Ávila H, Feldmann EM, Pleumeekers MM, Nimeskern L, Kuo W, de Jong WC, et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials. 2015;44:122–133. doi: 10.1016/j.biomaterials.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 88.Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17:1425–1436. doi: 10.1089/ten.tea.2010.0517. [DOI] [PubMed] [Google Scholar]
- 89.Zuo Q, Cui W, Liu F, Wang Q, Chen Z, Fan W. Co-cultivated mesenchymal stem cells support chondrocytic differentiation of articular chondrocytes. Int Orthop. 2013;37:747–752. doi: 10.1007/s00264-013-1782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012;18:1542–1551. doi: 10.1089/ten.tea.2011.0715. [DOI] [PubMed] [Google Scholar]
- 91.de Windt TS, Hendriks JA, Zhao X, Vonk LA, Creemers LB, Dhert WJ, et al. Concise review: unraveling stem cell cocultures in regenerative medicine: which cell interactions steer cartilage regeneration and how? Stem Cells Transl Med. 2014;3:723–733. doi: 10.5966/sctm.2013-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leijten JC, Georgi N, Wu L, van Blitterswijk CA, Karperien M. Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue Eng Part B Rev. 2013;19:31–40. doi: 10.1089/ten.teb.2012.0273. [DOI] [PubMed] [Google Scholar]
- 93.Chiesa-Estomba C, González-Fernández I, Iglesias-Otero M. 3D printing for biomedical applications: where arewe now? Eur Med J. 2017;2:16–22. [Google Scholar]