Abstract
The coronavirus disease 2019 (COVID-19) has affected more than 29 million people and led to more than 542,000 deaths in the United States.1 Older age, comorbidities, and racial and ethnic minority status are associated with severe COVID-19.2 Among patients with inflammatory bowel disease (IBD), racial and ethnic minorities have worse outcomes, mediated in part by inequitable health care access.3 Racial and ethnic minority patients with IBD and COVID-19 may be an especially vulnerable population. The purpose of this study was to evaluate racial and ethnic disparities in COVID-19 outcomes among IBD patients and the impact of non-IBD comorbidities on observed disparities.
Abbreviations used in this paper: aOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; IBD, inflammatory bowel disease; SECURE-IBD, Surveillance of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease
The coronavirus disease 2019 (COVID-19) has affected more than 29 million people and led to more than 542,000 deaths in the United States.1 Older age, comorbidities, and racial and ethnic minority status are associated with severe COVID-19.2 Among patients with inflammatory bowel disease (IBD), racial and ethnic minorities have worse outcomes, mediated in part by inequitable health care access.3 Racial and ethnic minority patients with IBD and COVID-19 may be an especially vulnerable population. The purpose of this study was to evaluate racial and ethnic disparities in COVID-19 outcomes among IBD patients and the impact of non-IBD comorbidities on observed disparities.
Methods
The Surveillance of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) database was established in March 2020 to determine the impact of COVID-19 on patients with IBD and evaluate factors affecting COVID-19 outcomes. Details of the data collection and quality control have been described in a previous publication.4 Details of statistical analyses are provided in the Supplementary Methods section.
Results
Population Characteristics
We evaluated 2019 US cases reported to SECURE-IBD, of which 161 (8.0%) were Hispanic, 211 (10.5%) were non-Hispanic black, 1502 (74.4%) were non-Hispanic white, and 145 (7.2%) were reported as unknown or another race/ethnicity. In the entire cohort, the mean age was 35.7 years, 52.1% were female, and 23.4% were obese. Other baseline characteristics are described in Supplementary Table 1.
Across race/ethnicity categories, minority groups had a higher prevalence of obesity, active IBD, and comorbid conditions including diabetes, hypertension, and lung disease. There was no difference across race/ethnicity categories in the proportion of patients on each IBD medication type.
Associations Between Race, Ethnicity, and Coronavirus Disease 2019 Outcomes Among Inflammatory Bowel Disease Patients in the United States
Hospitalization occurred in 12.4% of Hispanic, 7.1% of non-Hispanic white, and 20.4% of non-Hispanic black individuals (P < .001) (Supplementary Table 2). No reported deaths occurred outside of the hospital. Severe COVID-19 occurred in 3.1% of Hispanic, 1.6% of non-Hispanic white, and 7.6% of non-Hispanic black individuals (P < .001).
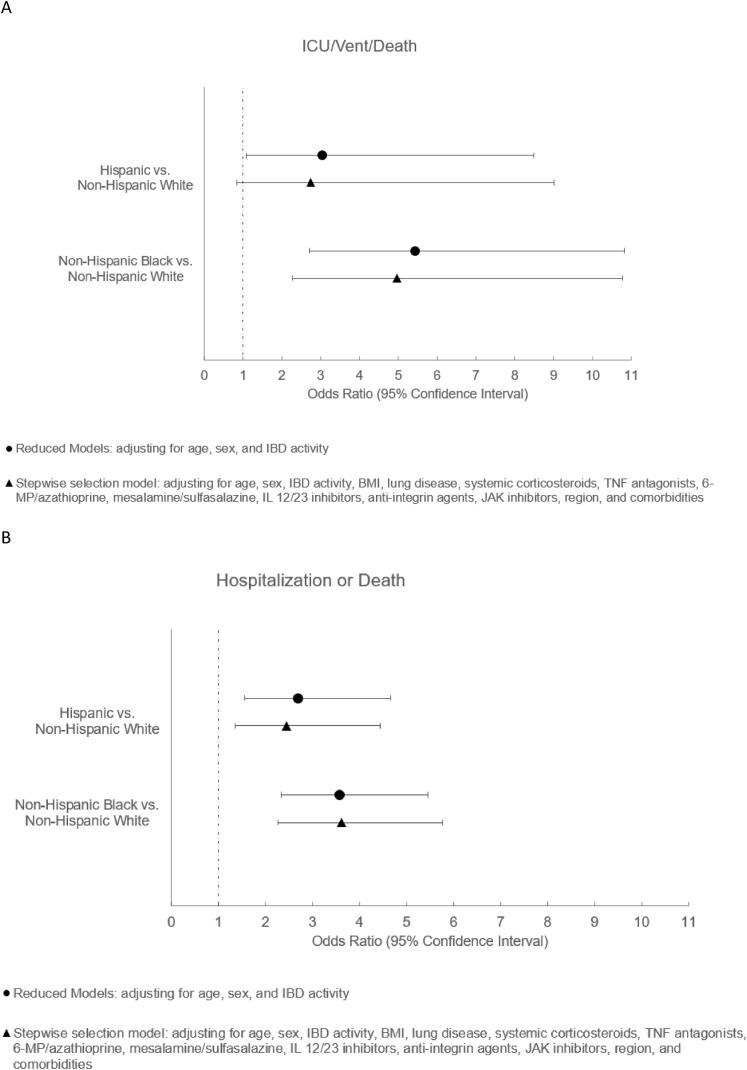
In the first set of models, compared with non-Hispanic white individuals, Hispanic individuals had an increased risk of hospitalization (adjusted odds ratio [aOR], 2.70; 95% CI, 1.56–4.66; P = .0004) and severe COVID-19 (aOR, 3.04; 95% CI, 1.09–8.49; P = .03) (Figure 1 ). After additional adjustment for comorbidities, region, and IBD medications, the effect estimates were attenuated slightly compared with the initial analyses (hospitalization: aOR, 2.45; 95% CI, 1.35–4.44; P = .003; severe COVID-19: aOR, 2.74; 95% CI, 0.84–9.01; P = .096) (Figure 1).
Figure 1.
Estimates of coronavirus disease 2019 (COVID-19) outcomes and 95% CIs by race and ethnicity from Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) cases in the United States. (A) Odds ratios of hospitalization resulting from COVID-19 among Hispanic vs non-Hispanic white individuals and among non-Hispanic black vs non-Hispanic white individuals. (B) Odds ratios of severe COVID-19 outcomes (intensive care unit [ICU] stay, mechanical ventilation, or death) among Hispanic vs non-Hispanic white individuals and among non-Hispanic black vs non-Hispanic white individuals. BMI, body mass index; IL, interleukin; JAK, Janus kinase; 6-MP, 6-mercaptopurine; TNF, tumor necrosis factor.
Similarly, in the first set of models, compared with non-Hispanic white individuals, non-Hispanic black individuals had higher odds of hospitalization (aOR, 3.58; 95% CI, 2.34–5.47; P < .0001) and severe COVID-19 (aOR, 5.43; 95% CI, 2.72–10.83; P < .0001). After further adjustment for comorbidities, region, and IBD medications, the aOR for hospitalization was 3.62 (95% CI, 2.27–5.77; P < .0001), and for severe COVID-19 the aOR was 4.96 (95% CI, 2.28–10.88; P < .0001). Effect estimates were similar without adjustment for IBD medications in otherwise identical models.
Discussion
We report that Hispanic and black individuals had higher odds of hospitalization and severe COVID-19 compared with non-Hispanic white individuals. Upon adjusting for comorbid conditions, region, and IBD medications, the effect estimates, although slightly attenuated, remained significant for hospitalization in both groups, and for severe COVID-19 among black individuals.
Underlying comorbid conditions in both groups partially explain these findings, and are consistent with previous data. In a study of 3626 patients in Louisiana, the odds of hospitalization and death were higher among black individuals compared with white individuals, the latter explained by comorbid conditions and social determinants of health.5 Limited access to health care, economic and educational disadvantages, and disparities in social determinants of health contributed directly to worse COVID-19 outcomes, and indirectly through a higher prevalence of comorbid conditions.6 The role of a biological basis for differences in COVID-19 outcomes by race/ethnicity is less clear.7 , 8
Our study had several strengths. In this large collaborative registry, we have data on COVID-19 in IBD patients from most US states for more than 1 year. Limitations included the risk of unmeasured confounding, reporting bias with potential under-representation of vulnerable groups, missing data (although <4% for all variables except ethnicity), and lack of data on social determinants of health.
In summary, we highlight disparities in COVID-19 outcomes in IBD patients based on race and ethnicity, mediated in part by comorbid conditions. These findings underscore the clinical importance of considering social determinants of health and higher risk in minority groups. Ultimately, health policies are needed to bridge these gaps and improve health care access and outcomes for all.
Acknowledgments
The authors thank other members of the Surveillance of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) Advisory Committee including Richard B. Gearry, MBChB, PhD, FRACP, FRCPC, and Jean-Francois Rahier, MD, PhD. The authors thank Fox E. Underwood, MSc, for the design and implementation of the SECURE-IBD interactive map. The authors acknowledge the physicians and other health care providers worldwide who have reported cases to the SECURE-IBD database and the organizations who supported or promoted the SECURE-IBD database (reporter names are available at www.covidibd.org/reporter-acknowledgment, and organization names are available at www.covidibd.org/our-partners).
Footnotes
Conflicts of interest These authors disclose the following: Manasi Agrawal has received research support from the Dickler Family Fund, New York Community Trust, and the Helmsley Charitable Trust Fund for Surveillance of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease; Gilaad G. Kaplan has received honoraria for speaking from AbbVie, Janssen, Pfizer, and Takeda, has received research support from Ferring, Janssen, AbbVie, GlaxoSmith Kline, Merck, and Shire, has consulted for Gilead, shares ownership of a patent (Treatment of Inflammatory Disorders, Autoimmune Disease, and PBC), and holds a UTI Limited Partnership, assignee patent WO2019046959A1, PCT/CA2018/051098; Siew C. Ng has received research grants from Ferring, Olympus, and AbbVie, and speaker’s fees from Pfizer, AbbVie, Takeda, Janssen, Ferring, Menarini, and Tillotts Pharmaceuticals; Walter Reinisch has served as a speaker for Abbott Laboratories, AbbVie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, and Yakult, has consulted for Abbott Laboratories, AbbVie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Arena Pharmaceuticals, Astellas, Astra Zeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernest & Young, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pharmacosmos, Philip Morris Institute, Pfizer, Procter & Gamble, Prometheus, Protagonist, Provention, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Therakos, Theravance, Tigenix, UCB, Vifor, Zealand, Zyngenia, and 4SC, has been an advisory board member for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, DSM, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia, and 4SC, and has received research funding from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnsotik, and MSD; Flavio Steinwurz is a speaker and consultant for AbbVie, Eurofarma, Ferring, Janssen, Pfizer, Sanofi, Takeda, and UCB; James D. Lewis has received personal fees from Johnson & Johnson Consumer, Inc, Eli Lilly and Company, Samsung Bioepis, UCB, Bristol-Myers Squibb, Bridge Biotherapeutics, Celgene, Merck, Gilead, Arena Pharmaceuticals, Protagonist Therapeutics, and Entasis Therapeutics, grants, personal fees, and other from Takeda Pharmaceuticals, personal fees and nonfinancial support from AbbVie, grants and personal fees from Janssen Pharmaceuticals, Nestle Health Science, and personal fees and other from Pfizer; Michele Kissous-Hunt has served as a speaker/consultant for AbbVie, Janssen, and Takeda; Irene Modesto is an employee and shareholder of Pfizer, Inc; Ryan C. Ungaro has served as a consultant and/or advisory board member for Bristol Myers Squibb, Eli Lilly, Janssen, Pfizer, and Takeda, and has received research support from AbbVie, Boehringer Ingelheim, and Pfizer; Jean-Frederic Colombel has received research grants from AbbVie, Janssen Pharmaceuticals, and Takeda, has received payment for lectures from AbbVie, Amgen, Allergan, Inc, Ferring Pharmaceuticals, Shire, and Takeda, has received consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix, and Viela bio, and holds stock options in Intestinal Biotech Development and Genfit; and Michael D. Kappelman has consulted for AbbVie, Janssen, Pfizer, and Takeda, is a shareholder in Johnson & Johnson, and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Celtrion, and Arenapharm. Funding for this work was provided in part by Pfizer, Takeda, Janssen, AbbVie, Eli Lilly, Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Celtrion, and Arenapharm. The work was also supported by research grants from Janssen Pharmaceuticals (J.W.Y.M.).
Funding This work was funded by the Helmsley Charitable Trust (2003-04445), National Center for Advancing Translational Sciences (UL1TR002489), National Institutes of Health training grant T32DK007634 (E.J.B.), and National Institutes of Health training grant K23KD111995-01A1 (R.C.U.).
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2021.05.060.
Supplementary Methods
Statistical Analysis
We included US cases reported to the registry between March 2020 and March 2021. We used descriptive statistics to summarize demographic and disease characteristics. The outcomes were as follows: adverse COVID-19 outcomes, a composite of hospitalization or death from COVID-19; and severe COVID-19, a composite of intensive care unit stay, mechanical ventilation, and/or death.
We categorized race/ethnicity into Hispanic, non-Hispanic black, non-Hispanic white, and other. We excluded individuals with missing race/ethnicity data and those in the other category (n = 4 and 149, respectively). We used multivariable logistic regression to analyze the impact of race/ethnicity on outcomes. In the first set of models, we included race/ethnicity, age, sex, and IBD activity by physician global assessment. Because IBD medications were not associated with race/ethnicity on bivariate analysis except for a heterogeneous other IBD medications category, we did not include this variable in the models.
For each outcome variable, we conducted a second analysis. In these models, we included race/ethnicity, age, sex, IBD activity, obesity (body mass index, ≥30 kg/m2), systemic corticosteroids (vs not), tumor necrosis factor antagonists (vs not), 6-mercaptopurine/azathioprine (vs not), and mesalamine/sulfasalazine (vs not) a priori. We then used backward selection of all variables associated with each outcome (P ≤ .10 on bivariate analysis), including comorbid conditions, US Census regions (Northeast, Midwest, South and West), interleukin 12/23 inhibitor (vs not), anti-integrin agent (vs not), and Janus kinase inhibitor (vs not). Medications included in the model a priori were the most commonly used IBD medications, while less common medications were subject to backward selection. We additionally performed otherwise identical models without adjustment for IBD medications. SAS version 9.4 (SAS Institute, Cary, NC) was used for data preparation and analyses.
Supplementary Table 1.
Baseline Demographic and Clinical Characteristics for US Cases Reported to SECURE-IBD: Overall and Stratified by Race/Ethnicitya
| Characteristic | All patients |
Hispanic |
Non-Hispanic black |
Non-Hispanic white (reference) |
P valueb | Other/unknown |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Total patients, n | 2019 | 100.0 | 161 | 8.0 | 211 | 10.5 | 1502 | 74.4 | 145 | 7.2 | |
| Age (mean, SD) | 35.7 | 17.87 | 30.0 | 15.06 | 36.6 | 19.07 | 36.3 | 17.95 | .121 | 34.9 | 17.20 |
| Female sex | 1051 | 52.1 | 75 | 46.6 | 126 | 59.7 | 784 | 52.2 | 66 | 45.5 | |
| US Census region | <.001 | ||||||||||
| Midwest | 638 | 31.6 | 19 | 11.8 | 64 | 30.3 | 511 | 34.0 | 44 | 30.3 | |
| Northeast | 521 | 25.8 | 49 | 30.4 | 38 | 18.0 | 386 | 25.7 | 48 | 33.1 | |
| South | 568 | 28.1 | 42 | 26.1 | 96 | 45.5 | 402 | 26.8 | 28 | 19.3 | |
| West | 230 | 11.4 | 46 | 28.6 | 7 | 3.3 | 153 | 10.2 | 24 | 16.6 | |
| Other or missing | 62 | 3.1 | 5 | 3.1 | 6 | 2.8 | 50 | 3.3 | 1 | 0.7 | |
| BMI | <.001 | ||||||||||
| <30 | 1359 | 67.3 | 107 | 66.5 | 116 | 55.0 | 1040 | 69.2 | 96 | 66.2 | |
| ≥30 | 473 | 23.4 | 37 | 23.0 | 79 | 37.4 | 329 | 21.9 | 28 | 19.3 | |
| Missing | 187 | 9.3 | 17 | 10.6 | 16 | 7.6 | 133 | 8.9 | 21 | 14.5 | |
| Disease type | .038 | ||||||||||
| Crohn’s disease | 1297 | 64.2 | 88 | 54.7 | 140 | 66.4 | 982 | 65.4 | 87 | 60.0 | |
| Ulcerative colitis | 658 | 32.6 | 66 | 41.0 | 68 | 32.2 | 477 | 31.8 | 47 | 32.4 | |
| IBD-unspecified | 52 | 2.6 | 7 | 4.3 | 3 | 1.4 | 33 | 2.2 | 9 | 6.2 | |
| IBD disease activityc | .002 | ||||||||||
| Remission | 1145 | 56.7 | 80 | 49.7 | 114 | 54.0 | 884 | 58.9 | 67 | 46.2 | |
| Mild | 396 | 19.6 | 34 | 21.1 | 48 | 22.7 | 273 | 18.2 | 41 | 28.3 | |
| Moderate | 263 | 13.0 | 27 | 16.8 | 30 | 14.2 | 186 | 12.4 | 20 | 13.8 | |
| Severe | 62 | 3.1 | 9 | 5.6 | 12 | 5.7 | 32 | 2.1 | 9 | 6.2 | |
| Unknown | 119 | 5.9 | 10 | 6.2 | 6 | 2.8 | 97 | 6.5 | 6 | 4.1 | |
| IBD medicationd | |||||||||||
| Any medication | 1905 | 94.4 | 151 | 93.8 | 196 | 92.9 | 1429 | 95.1 | .328 | 129 | 89.0 |
| Sulfasalazine/mesalamine | 302 | 15.0 | 32 | 19.9 | 26 | 12.3 | 217 | 14.4 | .107 | 27 | 18.6 |
| Budesonide | 67 | 3.3 | 5 | 3.1 | 9 | 4.3 | 47 | 3.1 | .680 | 6 | 4.1 |
| Oral/parenteral steroids | 94 | 4.7 | 11 | 6.8 | 9 | 4.3 | 69 | 4.6 | .420 | 5 | 3.4 |
| 6MP/AZAe | 80 | 4.0 | 9 | 5.6 | 7 | 3.3 | 55 | 3.7 | .443 | 9 | 6.2 |
| Methotrexatee | 12 | 0.6 | 0 | 0.0 | 2 | 0.9 | 9 | 0.6 | .490 | 1 | 0.7 |
| Anti-TNFf | 807 | 40.0 | 68 | 42.2 | 87 | 41.2 | 606 | 40.3 | .881 | 46 | 31.7 |
| Anti-TNF + IMM | 178 | 8.8 | 16 | 9.9 | 17 | 8.1 | 133 | 8.9 | .819 | 12 | 8.3 |
| Anti-integrin | 272 | 13.5 | 17 | 10.6 | 28 | 13.3 | 213 | 14.2 | .437 | 14 | 9.7 |
| IL12/23 inhibitor | 287 | 14.2 | 21 | 13.0 | 26 | 12.3 | 217 | 14.4 | .654 | 23 | 15.9 |
| JAK inhibitor | 41 | 2.0 | 5 | 3.1 | 3 | 1.4 | 30 | 2.0 | .512 | 3 | 2.1 |
| Other IBD medication | 62 | 3.1 | 8 | 5.0 | 8 | 3.8 | 40 | 2.7 | .202 | 6 | 4.1 |
| Comorbid conditions | |||||||||||
| Any condition | 594 | 29.4 | 53 | 32.9 | 90 | 42.7 | 410 | 27.3 | <.001 | 41 | 28.3 |
| Cardiovascular disease | 93 | 4.6 | 6 | 3.7 | 13 | 6.2 | 68 | 4.5 | .485 | 6 | 4.1 |
| Diabetes | 78 | 3.9 | 10 | 6.2 | 15 | 7.1 | 46 | 3.1 | .004 | 7 | 4.8 |
| Lung disease | 183 | 9.1 | 14 | 8.7 | 33 | 15.6 | 129 | 8.6 | .004 | 7 | 4.8 |
| Hypertension | 192 | 9.5 | 15 | 9.3 | 37 | 17.5 | 129 | 8.6 | <.001 | 11 | 7.6 |
| Cancer | 25 | 1.2 | 0 | 0.0 | 3 | 1.4 | 20 | 1.3 | .333 | 2 | 1.4 |
| History of stroke | 12 | 0.6 | 1 | 0.6 | 4 | 1.9 | 7 | 0.5 | .051 | 0 | 0.0 |
| Chronic renal disease | 40 | 2.0 | 3 | 1.9 | 6 | 2.8 | 30 | 2.0 | .708 | 1 | 0.7 |
| Chronic liver disease | 55 | 2.7 | 7 | 4.3 | 8 | 3.8 | 36 | 2.4 | .210 | 4 | 2.8 |
| Other | 197 | 9.8 | 19 | 11.8 | 27 | 12.8 | 130 | 8.7 | .085 | 21 | 14.5 |
| Current smoker | 51 | 2.5 | 3 | 1.9 | 11 | 5.2 | 34 | 2.3 | .033 | 3 | 2.1 |
AZA, azathioprine; BMI, body mass index; COVID-19, coronavirus disease 2019; IL, interleukin; IMM, immunomodulator (includes 6-mercaptopurine, azathioprine, methotrexate); JAK, Janus kinase; SECURE-IBD, Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease; 6MP, 6-mercaptopurine; TNF, tumor necrosis factor.
Unless otherwise specified, percentages do not include missing values or unknown values. For all characteristics, less than 4% of data was missing and unknown, respectively, for each category. Percentages and numbers from each subcategory may not add up to the exact number of total reported cases owing to missing values and/or non–mutually exclusive variables.
P values include Hispanic, non-Hispanic white, and non-Hispanic black. They do not include other/unknown.
By physician global assessment at time of COVID-19 infection.
At time of COVID-19 infection. Medication categories are not mutually exclusive unless otherwise noted.
Monotherapy indicates no concomitant TNF antagonist, anti-integrin, anti-interleukin 12/23, or JAK inhibitor.
Monotherapy.
Supplementary Table 2.
COVID-19 Outcomes for US Cases Reported to SECURE-IBD, Overall and Stratified by Race/Ethnicity
| All patients |
Hispanic |
Non-Hispanic black |
Non-Hispanic white (reference) |
P valuea | Other/unknown |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Hospitalization | 187 | 9.3 | 20 | 12.4 | 43 | 20.4 | 106 | 7.1 | <.001 | 18 | 12.4 |
| ICU/ventilator/death | 47 | 2.3 | 5 | 3.1 | 16 | 7.6 | 24 | 1.6 | <.001 | 2 | 1.4 |
COVID-19, Coronavirus Disease 2019; ICU, intensive care unit; SECURE-IBD, Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease.
P values include Hispanic, non-Hispanic white, and non-Hispanic black. They do not include other/unknown.
References
- 1.Johns Hopkins University and Medicine CRC . Johns Hopkins University & Medicine; Baltimore, MD: 2020. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [Google Scholar]
- 2.Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir Med. 2020;8:547–548. doi: 10.1016/S2213-2600(20)30228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes E.L., Loftus E.V., Jr., Kappelman M.D. Effects of race and ethnicity on diagnosis and management of inflammatory bowel diseases. Gastroenterology. 2021;160:677–689. doi: 10.1053/j.gastro.2020.08.064. [DOI] [PubMed] [Google Scholar]
- 4.Brenner E.J., Ungaro R.C., Gearry R.B., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price-Haywood E.G., Burton J., Fort D., et al. Hospitalization and mortality among black patients and white patients with covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeberg H., Paabo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020;587:610–612. doi: 10.1038/s41586-020-2818-3. [DOI] [PubMed] [Google Scholar]
- 8.Bunyavanich S., Grant C., Vicencio A. Racial/ethnic variation in nasal gene expression of transmembrane serine protease 2 (TMPRSS2) JAMA. 2020;324:1567–1568. doi: 10.1001/jama.2020.17386. [DOI] [PMC free article] [PubMed] [Google Scholar]