Abstract
With growing number of pediatric cases of COVID-19, a unique hyper-inflammatory syndrome, linked to SARS-CoV-2 infection, has emerged in children referred to as multisystem inflammatory syndrome in children (MIS-C). This Kawasaki Disease (KD)-like illness has been described across the world. This syndrome shares features of KD, toxic shock syndrome, and macrophage activation syndrome and is associated with significantly elevated inflammatory markers. Everyday there are new data emerging improving the care of these patients. The Advanced Cardiac Therapies Improving Outcomes Network (ACTION) is a collaborative network designed to improve the outcomes of pediatric patients with end-stage heart failure and involves centers from across North America. The committee gathered information concerning COVID-19 anticoagulation practices at various centers and harmonized the data to formulate a set of recommendations.
Introduction
Coronavirus disease (COVID-19), caused by a novel Coronavirus (SARS-CoV-2), is expanding in the pediatric population with an ever-growing array of clinical presentations. As of March 16, 2021, globally, there were 1119,960,700 confirmed cases of COVID-19, including 2,656,822 deaths, reported to WHO [1]. During the initial wave of the pandemic, children were thought to be relatively spared by this virus. A review of 72,314 cases by the Chinese Center for Disease Control and Prevention showed that only 1% of the cases (416 cases) were in children younger than 10 years of age [2]. In a report by the US-CDC, only 1.7% (2572 cases) occurred in children aged < 18 years and 98% (146,510) occurred in adults aged ≥ 18 years [3].
Similar to adults, there were children who present with mild symptoms or no symptoms at all. However, with growing number of pediatric cases, a unique hyper-inflammatory syndrome, linked to SARS-CoV-2 infection, has emerged in children referred to as multisystem inflammatory syndrome in children (MIS-C). This Kawasaki Disease (KD)-like illness associated with COVID-19 in children was first described in April 2020 in the United Kingdom in 8 pediatric patients [4]. Since then, clusters of children with MIS-C have been described across Europe and the USA [5–8]. This syndrome shares features of KD, toxic shock syndrome, and macrophage activation syndrome and is associated with significantly elevated inflammatory markers [9, 10].
As cases increased, criteria for MIS-C were defined by New York State Department of Health and Centers for Disease Control and Prevention [11, 12]. Systolic myocardial dysfunction in the mild-to-moderate range has been reported most commonly, though severely diminished ventricular function has been reported [13]. Acute COVID-19 patients have been shown to be at a heightened risk of thrombotic complications secondary to pro-inflammatory state, multi-organ vasculitis, and immobilization [14]. While the burden of thrombotic complications is yet to be fully understood in children with ether acute COVID-19 or MIS-C, we hypothesize that the acquired thrombophilia seen in acute COVID-19 infections may also be seen in children with MIS-C.
Children with MIS-C have laboratory findings suggesting that a pro-coagulable state exists in these patients similar to COVID-19 infection. However, the actual reports of thrombotic events in MIS-C patients have been rare. The use of antiplatelet and/or anticoagulation therapies has been reported frequently. Some studies have discussed their practices of anticoagulation, most commonly prophylactic dosing rather than therapeutic dosing with very few providing indications for prophylactic anticoagulation [12]. In a survey study by the International Kawasaki Disease Registry, acetylsalicylic acid (ASA) was felt to be indicated by 91% of respondents and therapeutic anticoagulation dosing would be used for patients with giant coronary artery aneurysms (61%) and those with a severe clinical presentation (33%) [12]. The registry advises consideration of prophylactic dosing of anticoagulation, such as enoxaparin, in patients at higher baseline risk of venous thromboembolism (ex: patients ≥ 12 years old with altered mobility, obesity, known thrombophilia or history of thrombus, critical presentation, etc.), along with pneumatic sequential compression devices [12]. In addition, they recommend consideration of therapeutic dosing of anticoagulation in patients with giant coronary aneurysms, at least moderately diminished ventricular systolic function and other thrombosis concerns [12]. The knowledge regarding this new disease is still scarce. The management of these patients has large variability across centers and depends on local expertise and extrapolation from adult and pediatric data for COVID-19 disease [12, 15]. Guidelines regarding the thrombotic evaluation and anticoagulation management of hospitalized children with MIS-C remains lacking.
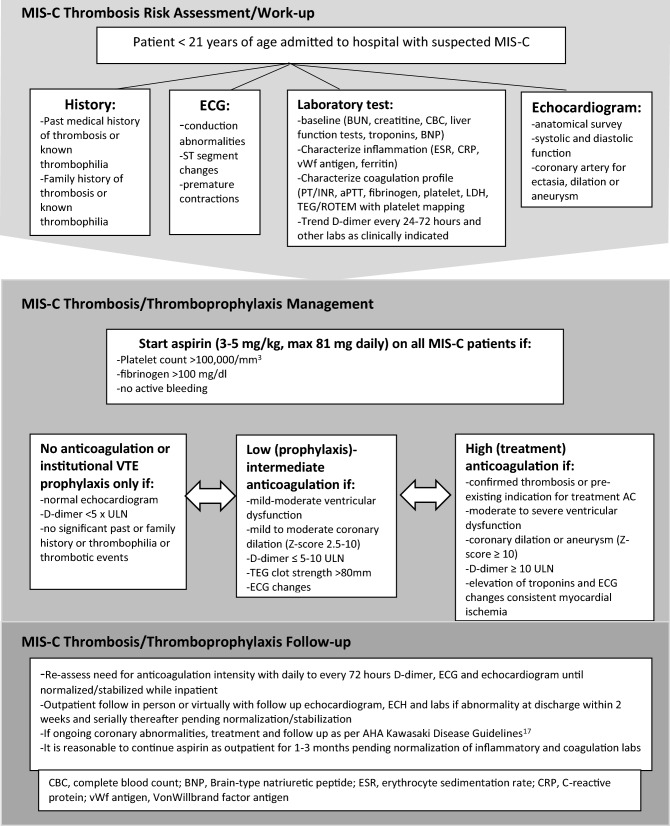
The Advanced Cardiac Therapies Improving Outcomes Network (ACTION) is a collaborative network designed to improve the outcomes of pediatric patients with end-stage heart failure and involves centers from across North America [16]. The ACTION learning network’s anticoagulation committee is a multi-institutional, multidisciplinary collective that includes providers with expertise in antithrombosis management at tertiary care pediatric centers around the world. The committee gathered information concerning COVID-19 anticoagulation practices at various centers and harmonized the data to formulate a set of recommendations. Given the rapid evolution of data, the ACTION network leveraged their expertise in development of collaborative learning pathways to create a consensus document as a shared baseline for clinical care. Consistent with established network practices, this will enable reassessment of guidance over time and across centers, with the ability to recognize and respond to changes in real time as dictated by incoming clinical experience. These are consensus-based recommendations on the use of anticoagulation therapy and thromboprophylaxis in children hospitalized with MIS-C. These guidelines are for patients who are admitted with MIS-C and not for outpatients with suspected MIS-C. Outpatient management should be determined by the primary physician caring for the patient. In addition, these are recommendations and final decision should be individualized by the primary physician according to the patient’s complete clinical picture (Fig. 1).
Fig. 1.
MIS-C thrombosis work-up and management for children admitted to hospital
Approach to MIS-C Thrombosis Risk Evaluation
Patients admitted with confirmed or suspected (when patient doesn’t meet complete CDC criteria or labs are awaited) MIS-C should have the following diagnostics obtained—(i) on admission; (ii) with any change in clinical status or level of care and (iii) at discharge.
- Echocardiogram
-
oEvaluation of anatomy and function with focused examination of coronary arteries for ectasia, dilation, or aneurysm
-
o
- Electrocardiography (ECG)
-
oScreening for any conduction abnormalities, arrhythmias, and/or changes from baseline
-
o
- Baseline anticoagulation laboratory test
-
oComplete blood count (CBC) with differential
-
oRenal function (creatine and BUN)
-
oLiver function (ALP, AST, GGTP, total bilirubin)
-
o
- Characterization of inflammation:
-
oFerritin
-
oVon Willibrand factor antigen
-
oErythrocyte sedimentation rate (ESR)
-
oC-reactive protein (CRP)
-
o
- Characterization of coagulation profile
-
oD-dimer ever 24–72 h during hospitalization
-
oPT/INR
-
oPTT
-
oFibrinogen
-
oPlatelet count
-
oLactate dehydrogenase (LDH)
-
oThromboelastography with platelet mapping (TEG with PM) or rotational thromboelastometry (ROTEM): specifically evaluating for elevated clot strength or maximal amplitude (MA) suggested of hypercoagulability (MA > 80 mm)
-
oHistory of remote or recent thrombotic events
-
oFamily or personal history of thrombophilia
-
o
Approach to MIS-C Thromboprophylaxis/Treatment in Hospitalized Patients
Patients should continue antiplatelet (AP) and/or anticoagulation (AC) for pre-existing conditions unless otherwise specified by provider. May consider holding oral anticoagulation and switching to shorter acting anticoagulant in the setting of critical illness.
All thromboprophylaxis should be reconsidered/discontinued if an alternative diagnosis other than MIS-C is determined to account for the laboratory/clinical findings
- Consider use of aspirin therapy (3-5 mg/kg/day with a maximum of 81 mg daily) in the all hospitalized MIS-C patients unless:
-
oPlatelet count < 100,000/m3
-
oFibrinogen < 100 mg/dL
-
oActive bleeding or concern for high risk of bleeding
-
o
Duration of therapy: Continue for at least one month from diagnosis, regardless of inflammatory marker and coagulation labs or longer until the labs normalize.
-
2.In addition to aspirin, consider full (treatment) anticoagulation (unfractionated heparin, direct thrombin inhibitor, low molecular weight heparin titrated to institutional goal for treatment) for the following:
-
oAcute thrombosis
-
oModerate to severe ventricular dysfunction
-
oCoronary dilation/aneurysm with z-score ≥ 10
-
oD-dimer > 10 × upper limit of normal (ULN)
-
o
Duration of therapy: Continue as indicated for underlying condition or until able to switch to low-dose anticoagulation as echocardiogram and lab data improve.
May consider therapeutic anticoagulation for active malignancy, nephrotic syndrome, flare of underlying inflammatory disease state, heart disease with venous stasis or impaired venous return, personal history of thrombosis, or multiple risk factors – discuss indications with specialist managing underlying condition and/or hematology
-
3.In addition to aspirin, consider low-dose (prophylactic) anticoagulation (unfractionated heparin, direct thrombin inhibitor, low molecular weight heparin, or direct oral anticoagulant to institutional goal for prophylaxis) for the following:
-
oVenous thromboembolism (VTE) prophylaxis based on institutional recommendations
-
oMild to moderate ventricular dysfunction
-
oCoronary dilation/aneurysm with z-score 2.5–10
-
oD-dimer 5–10 × ULN
-
oTEG MA ≥ 80 mm
-
oAny new significant rhythm abnormalities or ECG changes including but not limited to heart block, premature atrial and ventricular contractions, conduction abnormalities, and ST segments changes
-
o
Duration of therapy: Continue as indicated for underlying condition or until echo and lab data improve.
Approach to MIS-C Thromboprophylaxis Management After Discharge
Patients should have an in-person or virtual visit (with in-person testing) with follow-up echocardiogram, ECG, and labs if any abnormality was documented during admission within 2 weeks post discharge and serially thereafter pending normalization/stabilization
It is reasonable to continue anticoagulation and aspirin as outpatient for ~ 1 month post diagnosis. Shorter or longer durations can be considered based on resolution or ongoing abnormalities of inflammatory and coagulation labs, respectively (D-dimer, ferritin, LDH, ESR, CRP, TEG MA).
If patient fulfills criteria for Kawasaki disease, follow American Heart Association recommendations for antiplatelet and anticoagulation as indicated [17].
Conclusion
The COVID-19 pandemic has highlighted the need of sharing clinical experience and data across centers seamlessly and rapidly, so as to tackle a new disease process with the consolidated knowledge from multiple centers and nations. While much is to be learned about MIS-C in children, we have gained and continued to gather invaluable information on how it presents and impacts children in the short and long term. The above guide was created in collaboration with multiple centers in the USA and Canada while the pandemic was unfolding, and experience with COVID-19 infections and MIS-C in children was variable across centers. Having a learning collaborative such as ACTION and Pediatric Heart Transplant Society that was already established greatly facilitated real-time discussion and creation of standardized guides based on the best available evidence and expertise of that time. We acknowledge that a major limitation of this guide is the lack of high levels of evidence derived from controlled trials. Rather we relied upon extrapolation from adult COVID-19 experience, as well as our understanding of similar disease processes such as Kawasaki disease and myocarditis. As such, one must interpret these as reasonable recommendations of care agreed upon by multiple tertiary and quaternary care centers that have expertise in cardiology, thrombosis medicine, critical care, and MIS-C in children. Unfortunately, the progression of the disease had outpaced our acquisition of answers and evidence, but we hope to change that. As more data are gathered on MIS-C, we anticipate that these guidelines will evolve to incorporate higher levels of evidence to support our recommendations and the outcomes of our interventions in treating MIS-C in children.
Declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard (2021) https://covid19.who.int/. Accessed 16 Mar 2021
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a Report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Disease C. in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2019;2020(69):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York city. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebina-Shibuya R, Namkoong H, Shibuya Y, Horita N. Multisystem inflammatory syndrome in children (MIS-C) with COVID-19: insights from simultaneous familial Kawasaki Disease cases. Int J Infect Dis. 2020;97:371–373. doi: 10.1016/j.ijid.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loke YH, Berul CI, Harahsheh AS. Multisystem inflammatory syndrome in children: is there a linkage to Kawasaki disease? Trends Cardiovasc Med. 2020;30:389–396. doi: 10.1016/j.tcm.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaushik A, Gupta S, Sood M, Sharma S, Verma S. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J. 2020;39:e340–e346. doi: 10.1097/INF.0000000000002888. [DOI] [PubMed] [Google Scholar]
- 11.Minocha PK, Phoon CKL, Verma S, Singh RK. Cardiac findings in pediatric patients with multisystem inflammatory syndrome in children associated with COVID-19. Clin Pediatr (Phila) 2021;60:119–126. doi: 10.1177/0009922820961771. [DOI] [PubMed] [Google Scholar]
- 12.Elias MD, McCrindle BW, Larios G, Choueiter NF, Dahdah N, Harahsheh AS, et al. Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the International Kawasaki Disease Registry. CJC Open. 2020;2:632–640. doi: 10.1016/j.cjco.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 14.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldenberg NA, Sochet A, Albisetti M, Biss T, Bonduel M, Jaffray J, et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19-related illness. J Thromb Haemost. 2020;18:3099–3105. doi: 10.1111/jth.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorts A, Smyth L, Gajarski RJ, VanderPluym CJ, Mehegan M, Villa CR, et al. The creation of a pediatric health care learning network: the ACTION quality improvement collaborative. ASAIO J. 2020;66:441–446. doi: 10.1097/MAT.0000000000001133. [DOI] [PubMed] [Google Scholar]
- 17.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]