ABSTRACT
Background
Formulas often contain high amounts of added sugars, though little research has studied their connection to obesity.
Objectives
This study assessed the contribution of added sugars from formulas during complementary feeding on total added sugar intakes, and the association between these sugars and upward weight-for-age percentile (WFA%) crossing (i.e., participants crossing a higher threshold percentile were considered to have an upward crossing).
Methods
Data from three 24-hour dietary recalls for infants (n = 97; 9–12 months) and toddlers (n = 44; 13–15 months) were obtained in this cross-sectional analysis. Foods and beverages with added sugars were divided into 17 categories. Pearson's correlations were used to test relations between added sugar intake and upward WFA% crossing, followed by multivariable regressions when significant. ANOVA compared intakes of all, milk-based, and table foods between primarily formula-fed compared with breastfed participants. Multivariable regressions were used to test effects of added sugars and protein from all foods compared with added sugars and protein from milk-based sources on upward WFA% crossing.
Results
Added sugars from formulas comprised 66% and 7% of added sugars consumed daily by infants and toddlers, respectively. A significant association was observed between upward WFA% crossing and added sugars from milk-based sources after controlling for gestational age, sex, age, introduction to solid foods, mean energy intakes, and maternal pre-pregnancy BMI and education (β = 0.003; 95% CI, 0.000–0.007; P = 0.046). Primarily formula-fed participants consumed nearly twice the energy from added sugars (P = 0.003) and gained weight faster (upward WFA% crossing = 1.1 ± 1.2 compared with 0.3 ± 0.6, respectively; P < 0.001) than their breastfed counterparts.
Conclusions
Added sugars in formulas predict rapid weight gain in infants and toddlers. Educating mothers on lower-sugar options may enhance childhood obesity prevention.
Keywords: infant formula, added sugars, complementary feeding, infant rapid weight gain, childhood obesity
See corresponding commentary on page 1375.
Introduction
Over the past few decades, the suitability of formulas as an alternative to breast milk has attracted attention due to their association with excessive weight gain (1). Given that breast milk possesses a far more varied and complex nutritional profile than commercialized formulas (2), elucidating the reasons underlying this phenomenon has been challenging. The high protein content in some types of formulas has been hypothesized to drive early rapid weight gain (3), as trials comparing intakes of low-protein compared with high-protein formulas (4) or of breast milk compared with low- and high-protein formulas (3, 5–7) have found a positive relationship between protein and excessive weight gain. And yet, a sizeable number of studies have also failed to identify any connection to body weight (8–12) or adiposity (13–15), suggesting that other or additional factors are at play.
Against this background, research comparing dietary intakes and growth rates in infants consuming cow's milk formula (CMF; 2.1 g protein/100 kcal) or protein hydrolysate formula (PHF; 2.8 g protein/100 kcal) may offer clues into formula's obesogenic properties (16). Noteworthily, Mennella and colleagues (10, 11) discovered that infants who consumed the lower protein option of CMF gained more weight than PHF-fed infants, which implies that the protein content may not be the sole explanation for this phenomenon. To this end, a recent nutritional assessment of 257 formula products from 11 countries demonstrated that formulas contain a mean of 5.9 g of added sugars per 100 mL (range, 1.1–9.8 g) (17).
To our knowledge, little has been reported on the relationship between added sugars [i.e., sugars or sweeteners added to foods during processing, such as sucrose, dextrose, syrup, honey, and concentrates from fruits and vegetables (18)] in formulas and obesity risk. High intake of added sugars is positively correlated with overweight and obesity in children and adults (19). Our previous work also demonstrated that rapid weight gain among infants/toddlers in this cohort was positively associated with total added sugar intake from all sources during the complementary feeding period (20). However, the relation between added sugars specifically from infant formulas and rapid weight gain is not clear. Therefore, we aimed to determine the contribution of added sugars in formulas compared with those in beverages and foods (namely, table foods) consumed during the complementary feeding period on total infant added sugar intakes (Aim 1), and the association between added sugars in formulas compared with those in table foods on rapid weight gain (Aim 2). Given that formula-fed individuals are introduced to added sugars much earlier than those who are breastfed, we also examined the amount of added sugars consumed during the complementary feeding period from table foods between primarily formula-fed compared with breastfed infants/toddlers (Aim 3). As an ancillary analysis, we compared the effect size of added sugar versus protein intakes on rapid weight gain (Aim 4). To accomplish these aims, we divided our cohort into infants (9–12 months; n = 97) and toddlers (13–15 months; n = 44) because we expected the sources of added sugars to differ between them. Currently, the CDC recommends feeding breast milk or formula for a year before fully transitioning to table foods (21), so we classified consumption of breast milk and/or formula as a milk-based diet and intake of all other energy sources as a diet of table foods.
Methods
Participants
Participants comprised a convenience sample of individuals (n = 141; ages 9–15 months at baseline) recruited between 2017 and 2019 for involvement in an ongoing longitudinal intervention. To ensure that the sample was generally healthy, we excluded individuals if they were born preterm (<37 weeks gestation); born with a low birth weight (<2500 g); born with known medical problems; on any special diets; experiencing developmental delays or disabilities; born to a mother who was <18 years at the time of birth; born to a mother who smoked, used controlled substances, or consumed excessive alcohol during pregnancy; born in a high-risk pregnancy (i.e., to a mother with gestational diabetes mellitus, pre-eclampsia, etc.); or not a singleton. In the analyses, we also excluded data from 3 participants who consumed calories that were ±2 SDs from their estimated energy requirements, per the equation published by the Institute of Medicine's Food and Nutrition Board (22).
Procedures
Parents who expressed interest were screened via a telephone interview, and eligible dyads were scheduled for a 1-hour laboratory appointment. The University at Buffalo'sInstitutional Review Board approved the study (IRB #: STUDY0000472) and all parents provided written, informed consent for their infant's/toddler's participation. During the laboratory visit, parents received a packet of information explaining the collection of dietary data and then had their infant's/toddler's anthropometrics measured.
Pregnancy history and feeding practices questionnaire
Information on breastfeeding duration and timing of solid food introduction were obtained from parents through a shortened version of the feeding questionnaire used in the Infant Feeding Practices Study II (23). Since data on exclusive breastfeeding were not collected, we relied upon information regarding the duration of breastfeeding to create a primarily breastfed group and a formula-fed group. Those breastfed ≥3 months were classified as primarily breastfed (n = 110) and those breastfed ≤2 months were classified as primarily formula-fed (n = 31).
Infant/toddler dietary collection
Upon receiving the packet for collecting data on dietary intakes, parents were informed that they would be contacted via telephone on 3 separate occasions (i.e., on 2 weekdays and 1 weekend day) to report three 24-hour recalls. All telephone calls took place within 10 days of obtaining anthropometric measurements at a time deemed suitable for the participating parent. At the beginning of the call, research staff (i.e., dietetic students and interns who had been extensively trained by a registered dietitian) always inquired whether the infant/toddler had a normal eating day 24 hours prior; if that was not the case, a new day to complete the recall would be scheduled. The procedure used was a modified version of that utilized in the Feeding Infants and Toddlers Study (24), wherein information on foods and beverages given by the parent or other caregivers (i.e., daycare providers) was collected. (Note: if the infant/toddler was still breastfeeding, the parent reported their duration at the breast.) In order to maintain protocol integrity and consistency, the staff utilized a script for each recall, which was captured using the USDA's Automated Multiple-Pass Method (25).
At the beginning of the telephone calls, parents verbalized a quick list of everything that their infant/toddler had consumed 24 hours prior. Next, a research member would look over the list they had written down for any major feeding gaps and inquire about possible foods or beverages consumed during those times. Following this, the research member verbalized what they had written down and asked the parent whether they wanted them to modify anything. After completing the first run-through, the research member would ask detailed questions about the infant's/toddler's intakes by going through each item on the list, and would also seek clarity when something seemed unusual or unclear. If the parent struggled in estimating the amount of a food/beverage consumed, the research member would refer them to the serving size guide in their packet. Information pertaining to supplements, medications, and the eating typicality of the day in question was gathered at the end of the call, though this was not included in final analyses since it was not the main focus of the current study.
Nutrition analysis
Research staff entered the dietary recalls into Nutrition Data System for Research (NDSR) software (version 2019; Nutrition Coordinating Center, University of Minnesota) for analysis (16). This software allows for the entry of 24-hour dietary recalls using a multiple pass method, and it analyzes the nutritional profiles of foods, beverages, and personal recipes. Its database is supremely comprehensive and contains over 18,000 foods (∼7500 are brand-specific; >1000 are baby foods) but if on a rare occasion a specific food was lacking, a research member would select a generic food with a comparable nutrition profile. Failing to locate a decent substitute, the Nutrition Coordinating Center would be contacted to update their database. This approach was utilized to ensure that energy and macronutrient intakes were recorded as accurately as possible. In total, 15 foods were added.
The method described by the Feeding Infants and Toddlers Study (8) was used for participants who were still breastfeeding. For exclusively breastfed infants aged 7–12 months, research members would input 600 mL of breast milk per day into their calculations; for infants who consumed both breast milk and formula, the volume of formula listed in their dietary recalls was subtracted from 600 mL and the remainder was entered as breast milk. The new formula volume was always entered in separately. For toddlers >12 months of age, 1 fluid ounce (29.6 mL) per every 5 minutes of feeding was entered. NDSR offers “milk, human” as their only breast milk representation, so this was selected whenever breast milk was noted in a dietary recall (16). Supplementary Table 1 lists the formula products consumed by the formula-fed participants, which vary in their protein and added sugar contents.
Once all dietary recalls were entered into the NDSR, mean intakes of calories, carbohydrates, protein, fat, and total and added sugars were extracted (16). Considering added sugars, which the Nutrition Coordinating Center had made available since 2005, were the main focus of this study, that information was extracted for further analysis. Foods and beverages with added sugars were then grouped into 16 categories (i.e., yogurt, baby snacks and sweets, quick breads, savory snacks, sweet bakery products, flavored milks and dairy drinks, fruits, fruit drinks, sugar and candy, ready-to-eat cereals, breads/rolls/tortillas, ice cream/pudding/gelatin, mixed dishes, protein foods, baby and cooked cereals, and sweetened beverages), as described by Herrick et al. (26), and an additional category was created for infant formulas. All condiments were put into the “mixed food” category because they were likely to be eaten with another food. Items such as peanut butter and wheat bread were separated into their respective groups (i.e., peanut butter under protein foods and wheat bread under breads/rolls/tortillas).
Infant/toddler and parent anthropometrics
Research staff measured each infant's/toddler's weight using a Seca 374 Digital Baby Scale, and length (in the supine position) using a Seca 416 Infantometer, per the WHO's Multicentre Growth Reference Study protocols (27). All length measurements were taken and recorded independently by 2 staff members, who then compared values. If the difference between their values was greater than 7.0 mm, the infant/toddler would be remeasured and the mean of the last 2 values would be recorded as the length (27). The infant's/toddler's weight-for-length, weight-for-age, and length-for-age z-scores were calculated using the WHO infant growth chart (0 to 5 years) (28). The infant's/toddler's birth weight was reported by the participating parent. Rapid weight gain was classified using upward weight-for-age percentile (WFA%) crossing (29), an established method that we have employed previously (20). In brief, it calculates the number of major percentile (5th, 10th, 25th, 50th, 75th, 90th, and 95th) thresholds that have been crossed from birth to the time of the baseline assessment. Participants crossing a higher threshold percentile were considered to have an upward crossing; participants crossing a lower threshold or staying in the same band between major percentiles were considered to have no upward crossing (29). Participating parents (all of whom were mothers) reported their pre-pregnancy BMI. Individuals were classified as normal weight if their BMI was between 18.5–24.9 kg/m2 and overweight/obese if their BMI was ≥25 kg/m2 (30).
Data analysis
Descriptive statistics are presented as means (± SDs), ranges, or percentages. Added sugars consumed by individuals in our cohort were extracted from 3-day dietary recalls for further analysis to address Aim 1. To determine the percentage contribution of added sugars from each category to an infant's/toddler's total added sugar intakes, the amount of added sugars (g) from each category was divided by the total amount consumed and then multiplied by 100. To address Aim 2, Pearson's correlation coefficients were used to test bivariate relationships between intakes from different energy sources and rapid weight gain (i.e., upward WFA% crossing). If significant correlations were observed, a multivariable regression analysis was performed, controlling for covariates (i.e., gestational age, sex, age, first introduction to solid foods, and mean energy intakes, as well as maternal pre-pregnancy BMI and education). We addressed Aim 3 by conducting an ANOVA to compare dietary intakes from all, milk-based, and table foods between those who were primarily formula-fed compared with breastfed. Pertaining to Aim 4, we created 2 multivariable regression models (which employed the aforementioned covariates) for predicting rapid weight gain: 1 analyzed intakes of added sugar and protein from all food sources, and the other analyzed intakes of added sugar and protein from milk-based sources. We calculated appropriate effect sizes for these 2 regression models using Cohen's f 2 (31). Of note, an f 2 value of 0.35 is considered to be a large effect size, an f 2 value of 0.15 is considered to be a medium effect size, and an f 2 value of 0.02 is considered to be a small effect size (32). SYSTAT 11 (Systat Software, 2004) was used to conduct all analyses.
Results
Participant characteristics
Characteristics of the participants and their mothers are in Table 1. On the whole, the sample consisted of highly educated families of Caucasian ethnicity, with mothers having a mean age of 32 years (±4.4 years) and a mean pre-pregnancy BMI of 28.3 kg/m2 (±7.2 kg/m2).
TABLE 1.
Participant characteristics1
| Child | |
| Male sex, n (%) | 63 (44.7) |
| Age, mo | 11.9 ± 1.9 (9.1–15.8) |
| Caucasian, n (%) | 110 (78.0) |
| Refuse to answer, n (%) | 4 (2.8) |
| Gestational age, wk | 39.4 ± 1.3 (37–43) |
| Birthweight, kg | 3.5 ± 0.5 (2.5–5.2) |
| Weight-for-length z-score | 0.6 ± 0.9 (-1.3 to 3.1) |
| Weight-for-age z-score | 0.2 ± 0.9 (-2.4 to 2.9) |
| Length-for-age z-score | −0.4 ± 1.1 (-3.1 to 2.9) |
| Exclusively formula-fed, n (%) | 4 (2.8) |
| Breastfeeding duration, mo | 7.7 ± 4.6 (0–15.8) |
| ≤1 mo, n (%) | 18 (12.8) |
| ≤2 mo, n (%) | 9 (6.4) |
| ≥6 mo, n (%) | 96 (68.1) |
| First introduction to solid foods | 5.3 ± 1 (2–9) |
| <4 mo, n (%) | 5 (3.5) |
| 4–5 mo, n (%) | 62 (44.0) |
| ≥6 mo, n (%) | 74 (52.5) |
| Mother | |
| Age, y | 33 ± 4.4 (24–46) |
| Education level | |
| Some college or less, n (%) | 36 (25.5) |
| College graduate or more, n (%) | 103 (87.1) |
| Refuse to answer, n (%) | 2 (1.4) |
| Parity | |
| Nulliparous, n (%) | 79 (56) |
| Parous ≥ 1, n (%) | 62 (44) |
| Pre-pregnancy BMI, kg/m² | 28.3 ± 7.2 (19–52.9) |
| Normal weight, 18.5–24.9 kg/m2, n (%) | 54 (38.6) |
| Overweight/obese, ≥25 kg/m2, n (%) | 86 (61.4) |
| Household total income | |
| <$30,000, n (%) | 13 (9.2) |
| $30,000–$69,999, n (%) | 35 (24.8) |
| $70,000–$109,999, n (%) | 53 (37.6) |
| ≥$110,000, n (%) | 33 (23.4) |
| Refuse to answer, n (%) | 7 (5) |
Values are means ± SD (range), unless otherwise indicated. n = 141. Participant weight-for-length z-score, weight-for-age z-score, and length-for-age z-score were calculated using the WHO's growth charts (28).
Sources of added sugars between infants and toddlers
Pertaining to Aim 1, infants consumed a mean of 863 ± 200 kcal daily. Mean intakes from a milk-based diet were 368 ± 175 kcal, which translated to 42.6% of an infant's energy intakes. Almost 90% consumed a milk-based diet during the 9–12-month period (n = 37 consumed only breast milk; n = 37 consumed only formulas; n = 13 consumed both). Additionally, the majority of infants (97.3%) consumed added sugars on any given day, which contributed to 7% of their energy intakes (15.0 ± 13.8 g; 60.2 ± 55.1 kcal). In total, 65.5% of the added sugars they consumed came from formulas. Table 2 lists their top 8 sources of added sugars.
TABLE 2.
Percent contribution of added sugars in the diets of infants (9–12 months) and toddlers (13–15 months) from the top 8 sources they consumed1
| Source of added sugars | Contribution, % |
|---|---|
| Infants, n = 97 | |
| Formula | 65.5 |
| Baby snacks and sweets | 5.69 |
| Sweet bakery products | 5.44 |
| Yogurt | 4.08 |
| Mixed dishes | 3.16 |
| Ready-to-eat cereals | 2.83 |
| Sugar and candy | 2.20 |
| Quick breads and bread products | 1.89 |
| Toddlers, n = 44 | |
| Sweet bakery products | 20.6 |
| Yogurt | 16.8 |
| Mixed dishes | 11 |
| Cooked and baby cereals | 7.47 |
| Formula | 7.37 |
| Sugar and candy | 6.06 |
| Ready-to-eat cereals | 5.72 |
| Bread, rolls, tortillas | 5.48 |
Percent contribution of added sugars was calculated by dividing the amount of added sugars consumed from a specific category by the total amount consumed, according to participants’ dietary recalls. Sources of added sugars consumed were categorized into 16 groups [as described by Herrick et al. (26)], plus an additional category for formula.
Toddlers consumed a mean of 1030 ± 232 kcal daily. Mean intakes from a milk-based diet were 88.9 ± 134.5 kcal (8.7% of their daily energy intakes), and the majority of their energy intakes came from table foods (937 ± 263 kcal). Just 19 toddlers consumed a milk-based diet (n = 16 consumed only breast milk; n = 3 consumed only formulas). All toddlers consumed added sugar daily (10.7 ± 7.0 g; 42.8 ± 27.9 kcal, or 4.2% of total energy intake). Table 2 lists their top 8 sources of added sugars. Importantly here, formulas were not a major source of added sugars.
Added sugars in formulas and rapid weight gain
In our previous publication, we showed that added sugar intakes from all sources correlated positively with upward WFA% crossing. To address Aim 2, we broke down the sources of added sugars into either milk–based or table foods. We observed a significant correlation between upward WFA% crossing and added sugars from the milk-based diet (r = 0.218; P < 0.001), and a positive but nonsignificant correlation between upward WFA% crossing and added sugars from table foods (Table 3). The association between upward WFA% crossing and added sugars from the milk-based diet remained significant after controlling for gestational age, sex, age, first introduction to solid foods, and mean energy intakes, as well as maternal pre-pregnancy BMI and education (β = 0.003; 95% CI, 0.000–0.007; P = 0.046).
TABLE 3.
Pearson's correlation coefficients for the relationship between intakes of different energy sources and rapid weight gain (upward weight-for-age percentile crossing) among infants (9–12 months) and toddlers (13–15 months)1
| r | |||
|---|---|---|---|
| All, n = 141 | Infants, n = 97 | Toddlers, n = 44 | |
| All food sources | |||
| Energy, kcal/d | 0.073 | −0.026 | 0.3182 |
| Carbohydrates, kcal/d | 0.073 | −0.059 | 0.4033 |
| Protein, kcal/d | 0.025 | −0.012 | 0.161 |
| Fat, kcal/d | 0.036 | 0.026 | 0.065 |
| Added sugars, kcal/d | 0.2804 | 0.2693 | 0.3853 |
| Milk-based sources | |||
| Energy, kcal/d | 0.059 | 0.118 | −0.099 |
| Carbohydrates, kcal/d | 0.076 | 0.141 | −0.090 |
| Protein, kcal/d | 0.111 | 0.186 | −0.102 |
| Fat, kcal/d | 0.032 | 0.076 | −0.104 |
| Added sugars, kcal/d | 0.2184 | 0.2633 | 0.080 |
| Table food sources | |||
| Energy, kcal/d | 0.012 | −0.092 | 0.3312 |
| Carbohydrates, kcal/d | 0.017 | −0.121 | 0.3983 |
| Protein, kcal/d | −0.003 | −0.056 | 0.169 |
| Fat, kcal/d | 0.004 | −0.033 | 0.129 |
| Added sugars, kcal/d | 0.143 | 0.061 | 0.3702 |
Pearson's correlation coefficients assessed bivariate relationships between intakes from different energy sources and upward weight-for-age percentile crossing. Then, if significant correlations were observed, a multivariable regression analysis was performed, controlling for covariates (i.e., gestational age, sex, age, first introduction to solid foods, and mean energy intakes, as well as maternal pre-pregnancy BMI and education). Weight-for-age percentile was calculated using the WHO's growth charts (29).
P < 0.05.
P < 0.01.
P < 0.001.
As shown in the results for Aim 1, the sources of energy based on food sources were vastly different between the infants and toddlers, so we further broke them down (Table 3). Among infants, we observed a significant correlation between upward WFA% crossing and added sugars from milk-based sources (r = 0.263; P = 0.009) but not table foods. Among toddlers, contrarily, we observed a significant correlation between upward WFA% crossing and added sugars from table foods (r = 0.370; P = 0.013) but not milk-based sources.
Amounts of added sugars from table foods between primarily breastfed compared with formula-fed participants
Pertaining to Aim 3, when we examined overall diets, there were no differences in terms of total calorie, carbohydrate, protein, and fat intakes, but a significant difference was observed for added sugar energy intake (48.3 ± 45.9 kcal/d for the breastfed group compared with 77.5 ± 53.0 kcal/d for the formula-fed group; P = 0.003; Table 4; Figure 1). Primarily formula-fed participants consumed greater amounts of added sugars from milk-based sources and table foods than primarily breastfed participants (40.7 ± 55.0 kcal/d vs. 24.4 ± 43.7 kcal/d from milk-based sources, P = 0.088; 36.8 ± 29.6 kcal/d vs. 23.9 ± 22.1 kcal/d from table foods, P < 0.001) . The formula-fed individuals also experienced significantly faster weight gain than the breastfed group (upward WFA% crossing = 1.1 ± 1.2 compared with 0.3 ± 0.6, respectively; P < 0.001).
TABLE 4.
Different energy sources consumed by participants who were primarily formula-fed (breastfed ≤ 2 months) or breastfed (breastfed ≥ 3 months) and the upward weight-for-age percentile crossed1
| Formula-fed, n = 31 | Breastfed, n = 110 | P | |
|---|---|---|---|
| All food sources | |||
| Energy, kcal/d | 934 (224) | 906 (223) | 0.466 |
| Carbohydrates, kcal/d | 461 (119) | 436 (131) | 0.335 |
| Protein, kcal/d | 149 (65.2) | 127 (61.4) | 0.087 |
| Fat, kcal/d | 343 (98.4) | 368 (118) | 0.295 |
| Added sugars, kcal/d | 77.5 (53) | 48.3 (45.9) | 0.003 |
| Milk-based sources | |||
| Energy, kcal/d | 190 (232) | 306 (195) | 0.006 |
| Carbohydrates, kcal/d | 82.8 (101) | 125 (81.1) | 0.017 |
| Protein, kcal/d | 17.0 (20.5) | 21.1 (15.0) | 0.216 |
| Fat, kcal/d | 90.9 (111) | 163 (103) | 0.001 |
| Added sugars, kcal/d | 40.7 (55.0) | 24.4 (43.7) | 0.088 |
| Table food sources | |||
| Energy, kcal/d | 749 (348) | 600 (336) | 0.033 |
| Carbohydrates, kcal/d | 378 (161) | 310 (164) | 0.044 |
| Protein, kcal/d | 132 (76.7) | 106 (71.1) | 0.080 |
| Fat, kcal/d | 252 (150) | 205 (155) | 0.130 |
| Added sugars, kcal/d | 36.8 (29.6) | 23.9 (22.1) | 0.009 |
| Upward WFA% crossed | 1.1 (1.2) | 0.3 (0.6) | <0.001 |
Values are means (SDs). Differences in intakes from all, milk-based, and table food sources were calculated using an ANOVA. Weight-for-age percentile was calculated using the WHO's growth charts (29).
FIGURE 1.
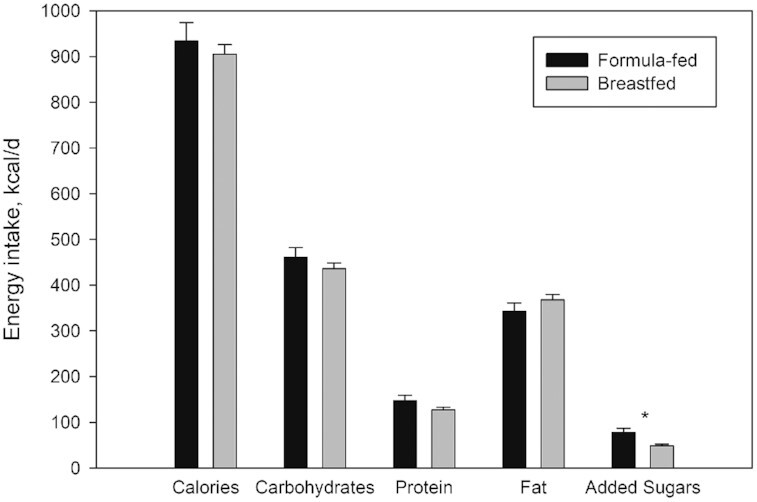
Daily energy intakes in total and from specific macronutrients by infants and toddlers who were primarily formula-fed (breastfed ≤ 2 months; n = 31) compared with those who were primarily breastfed (breastfed ≥ 3 months; n = 110). Values are means ± SDs. *Different from breastfed at P = 0.003.
Effect size of added sugar compared with protein intakes on rapid weight gain
To address Aim 4, the full multivariable regression model for overall intakes of protein and added sugars demonstrated that added sugars were a significant predictor of rapid weight gain (P = 0.022), while protein was not (Table 5). We observed a larger effect size (f 2 = 0.042) for added sugars than for protein (f 2 < 0.001) in predicting rapid weight gain. For the model specifically focusing on milk-based protein and added sugars (Table 6), milk-based added sugars did not reach statistical significance but the effect size (f 2 = 0.024) was still bigger than that of milk-based protein (f 2 < 0.001).
TABLE 5.
Multivariable regression model for predicting rapid weight gain (upward weight-for-age percentile crossing) by infants and toddlers from protein and added sugar from all food sources1
| Effect | β | t | P | f 2 |
|---|---|---|---|---|
| Child | ||||
| Sex | 0.042 | 0.296 | 0.767 | 0.001 |
| Age, mo | 0.008 | 0.158 | 0.874 | 0.001 |
| Gestational age, wk | −0.124 | −2.207 | 0.029 | 0.039 |
| Maternal | ||||
| Pre-pregnancy BMI, kg/m² | 0.017 | 1.653 | 0.101 | 0.024 |
| Education level, y | −0.015 | −0.409 | 0.684 | 0.002 |
| Dietary intakes | ||||
| Introduction of solid foods, mo | −0.046 | −0.627 | 0.532 | 0.004 |
| Total energy, kcal/d | 0.000 | 0.081 | 0.936 | 0.001 |
| All protein, kcal/d | 0.000 | 0.145 | 0.885 | 0.001 |
| All added sugars, kcal/d | 0.004 | 2.316 | 0.022 | 0.042 |
Weight-for-age percentile crossing was calculated using the WHO's growth charts (29).
TABLE 6.
Multivariable regression model for predicting rapid weight gain (upward weight-for-age percentile crossing) by infants and toddlers from protein and added sugar in milk-based sources1
| Effect | β | t | P | f 2 |
|---|---|---|---|---|
| Child | ||||
| Sex | 0.051 | 0.356 | 0.722 | 0.001 |
| Age, mo | 0.012 | 0.203 | 0.840 | 0.000 |
| Gestational age, wk | −0.130 | −2.28 | 0.024 | 0.044 |
| Maternal | ||||
| Pre-pregnancy BMI, kg/m² | 0.018 | 1.758 | 0.081 | 0.024 |
| Education level, y | −0.023 | −0.646 | 0.519 | 0.005 |
| Dietary intakes | ||||
| Introduction of solid foods, mo | −0.056 | −0.749 | 0.455 | 0.005 |
| Total energy, kcal/d | 0.000 | 0.792 | 0.430 | 0.005 |
| Milk-based protein, kcal/d | −0.002 | −0.327 | 0.744 | 0.001 |
| Milk-based added sugars, kcal/d | 0.004 | 1.77 | 0.080 | 0.024 |
Weight-for-age percentile crossing was calculated according to the WHO's growth charts (29).
Discussion
Presently, limited research has examined the link between added sugars in formulas and the obesity risk, though this is not too surprising when considering that few government mandates exist to list the inclusion of added sugars on product labels or to make them distinguishable from other carbohydrates (33, 34). Presumably, this in turn causes many to believe the primary or only carbohydrate type in formulas is lactose. This oversight or misconception is apparent in the contemporary National Health and Nutrition Examination Survey, wherein Herrick and colleagues (26) neglected to account for the added sugars in formulas when assessing sources of this ingredient in the diets of infants and toddlers.
As a cross-sectional study, we found that infants consumed more added sugars daily than toddlers. Our analyses also revealed that added sugars from all sources were significantly related to rapid weight gain, and these sources differed by age group. Specifically, the majority of added sugars consumed by infants were from formulas, while the majority of added sugars consumed by toddlers were from table foods. Although there was no difference in mean energy intakes between primarily formula-fed and breastfed participants, the former ate more added sugars from all sources during the complementary feeding period. It is well documented, for better or worse, that early dietary exposures shape food preferences that endure through adulthood (35, 36) and modulate risks of later obesity (37). Thus, it was likely that the primarily formula-fed participants in our study, having been introduced to added sugars earlier than their breastfed counterparts, would also eat table foods with higher contents of added sugars and display accelerated weight gain. This is concerning because 30–50% of commercial foods for infants and toddlers (i.e., snacks, desserts, and juices/drinks) contain at least 1 type of added sugar (38).
To date, reasons for rapid weight gain during infancy are many and are still being explored, yet factors like responsive parenting (39), infant sleep (40), and maternal obesity (41, 42) have been identified as some of the prominent contributors. Maternal obesity has been associated with a variety of offspring health problems (41). In particular, the Danish National Birth Cohort (n = 3768) discovered that a high BMI prior to pregnancy related positively to child weight gain up through 12 months (β = 18.6; SE = 3.9; P < 0.0001) (42). In our study, although we did not capture data on all of the abovementioned variables, we were able to determine that the correlation between a mother's pre-pregnancy BMI and an infant's rapid weight gain was r = 0.276. This effect size is similar to those we observed between added sugar intakes and rapid weight gain (ranging from 0.280 to 0.385). Thus, we speculate that the effect size of added sugar intakes is just as strong as that of maternal pre-pregnancy BMI in estimating weight gain among infants/toddlers.
Furthermore, we demonstrated that the contribution from added sugars in predicting rapid weight gain was stronger than that of protein. Approximately 80% of formulas consumed in the United States are made from cow's milk (43), which is lower in protein than PHF but markedly higher in added sugars. Among the formula products consumed by our participants, the protein content per serving (100 kcal) differed slightly (1.97–2.78 g) while the variance for added sugar content per serving (100 kcal) was much wider (2.94–7.73 g; Supplementary Table 1). This range of added sugar contents per serving is marginally smaller than that found in the global study conducted by Bridge et al. (17) and is probably due, in part, to underreporting and the lack of a universal definition for added sugars (18, 33, 44). A multiplicity of studies has reported that infants ingest more when feeding on CMF over PHF (10, 11, 45–48), so it is plausible that higher infant intakes of CMF are predominantly tied to its sweetness, given infants’ innate preferences for this basic taste (49). As such, the higher amount of added sugars in CMF, in conjunction to the higher intakes of CMF in infants, might shed light on their higher weight gain.
The early protein hypothesis proposes that high protein intakes promote rapid infant weight gain by triggering release of insulin and insulin-like growth factor 1 (3). However, data on the role of added sugars (i.e., glucose and fructose) are sparse. Increased levels of blood glucose trigger the release of insulin to promote its uptake into peripheral tissues, where it is then stored as glycogen or fat (triglycerides) (50). Fructose, in comparison, is mostly metabolized and stored in the liver as glycogen and triglycerides (51), and thus it does not stimulate secretion of insulin or leptin (52). Since these hormones are responsible for energy storage and satiety (52), low levels of fructose could be perceived by the brain as an energy deficit, thereby leading to overeating and subsequent weight gain. Emerging human data indicate a positive correlation between the fructose content in breast milk and infant growth and body composition (53), although studies evaluating the effect of fructose in infant formulas are necessary to confirm this relationship.
This study had many strengths, including assessments made during a critical window of development and the use of three 24-hour dietary recalls, which is an optimal measure for estimating nutrient intakes (54). Furthermore, our study focused uniquely on consumption of added sugars, in contrast with related investigations that assessed types or groups of foods (e.g., sweetened beverages). Measuring this allowed us to employ it as a continuous variable in statistical analyses and draw meaningful associations to infant/toddler growth. Lastly, we performed our nutrition analysis using the research-graded program NDSR, which provided detailed information on the added sugar contents for all foods consumed by the participants (16).
There are limitations to this study. Dietary intakes of infants and toddlers were based on maternal reports. Research evaluating the accuracy of 24-hour recalls performed by mothers of infants and toddlers has noted that they tend to overestimate energy intakes (55). Furthermore, although participants’ birth weights were reported by their mothers, evidence shows that a maternal report of a child's birth weight is quite accurate, especially if the mother hails from a higher socioeconomic status background or if the infant was of a normal weight, as predominantly observed here (56). In addition, our sample consisted primarily of families with a high socioeconomic background, so our findings may not generalize to more diverse cohorts. This may be a reason why the majority (∼68%) of parents in our study breastfed their infants/toddlers beyond 6 months of age. Even though data on breastfeeding duration were collected, we did not obtain information on exclusive breastfeeding, and instead needed to rely upon the dichotomous variables of “primarily breastfed” and “primarily formula-fed” for our analyses. According to the 2018 breastfeeding report card from the CDC, only about a third of babies are breastfed exclusively at 3 months (57). Moreover, daily amounts of added sugars consumed by our participants were calculated based on values from NDSR, which were gathered from the USDA National Nutrient Database for Standard Reference, other nutritional databases, and scientific publications rather than laboratory analyses (58). Lastly, due to the cross-sectional nature of our study, we do not have information on the types or brands of formulas consumed by participants prior to the point of contact. Research demonstrates that most infants consume the same type of formula during the first year of life if they don't have allergic reactions (59), and the prevalence of allergic reactions necessitating adjustments in formula selections is modest, ranging from 0.25%–5% (60, 61).
In conclusion, our work has deepened insight pertaining to the etiology of infant rapid weight gain. Added sugars are pervasive throughout the entire US food system. The present findings reveal that their inclusion in formulas predicts rapid weight gain as early as 9 months, and daily amounts of added sugars consumed by primarily formula-fed individuals are almost double those of primarily breast-fed individuals. Efforts by policymakers and pediatricians to educate mothers on lower-sugar options when breastfeeding is not feasible may enhance preventive measures of childhood obesity.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows – KLK: initiated and developed the research question and study design, led the analysis, and drafted the manuscript; BB: initiated and developed the research question, drafted the manuscript, and made major contributions to revising the manuscript; KSM: contributed to the development of the research question, data collection, nutrition analysis, and revision of the manuscript; TR: contributed to the development of the research question, nutrition analysis, and revision of the manuscript; HRH, DBK: contributed to the interpretation of results and revision of the manuscript; RAP: contributed to the data analyses, interpretation of the results, and revision of the manuscript; and all authors: agree to be accountable for all aspects of the work and read and approved the final manuscript.
Notes
This work was supported by funding from the National Institute of Child Health and Human Development of the NIH (grant number: R01 HD087082-01).
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CMF, cow's milk formula; NDSR, Nutrition Data System for Research; PHF, protein hydrolysate formula; WFA%, weight-for-age percentile.
Contributor Information
Kai Ling Kong, Baby Health Behavior Lab, Division of Health Services and Outcomes Research, Children’s Mercy Research Institute, Children’s Mercy Hospital, Kansas City, MO, USA; Department of Pediatrics, University of Missouri- Kansas City, Kansas City, MO, USA; Department of Pediatrics, University of Kansas Medical Center, Kansas City, KS, USA.
Brenda Burgess, Division of Behavioral Medicine, Department of Pediatrics, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY, USA.
Katherine S Morris, Division of Behavioral Medicine, Department of Pediatrics, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY, USA.
Tyler Re, Baby Health Behavior Lab, Division of Health Services and Outcomes Research, Children’s Mercy Research Institute, Children’s Mercy Hospital, Kansas City, MO, USA.
Holly R Hull, Department of Dietetics and Nutrition, Kansas University Medical Center, University of Kansas, Kansas City, KS, USA.
Debra K Sullivan, Department of Dietetics and Nutrition, Kansas University Medical Center, University of Kansas, Kansas City, KS, USA.
Rocco A Paluch, Division of Behavioral Medicine, Department of Pediatrics, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY, USA.
Data Availability
Deidentified participant data (including data dictionaries), study protocols, statistical analysis plan, and informed consent forms will be made available to researchers who provide methodologically sound proposals. All proposals should be sent to: kkong@cmh.edu.
References
- 1. Uwaezuoke SN, Eneh CI, Ndu IK. Relationship between exclusive breastfeeding and lower risk of childhood obesity: A narrative review of published evidence. Clin Med Insights Pediatr. 2017;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin CR, Ling P-R, Blackburn GL. Review of infant feeding: Key features of breast milk and infant formula. Nutrients. 2016;8(5):279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Anton B, Gruszfeld Det al. Can infant feeding choices modulate later obesity risk. ? Am J Clin Nutr. 2009;89(5):1502s–8s. [DOI] [PubMed] [Google Scholar]
- 4. Singhal A, Kennedy K, Lanigan J, Fewtrell M, Cole TJ, Stephenson T, Elias-Jones A, Weaver LT, Ibhanesebhor S, MacDonald PDet al. Nutrition in infancy and long-term risk of obesity: Evidence from 2 randomized controlled trials. Am J Clin Nutr. 2010;92(5):1133–44. [DOI] [PubMed] [Google Scholar]
- 5. Roche AF, Guo S, Siervogel RM, Khamis HJ, Chandra RK. Growth comparison of breast-fed and formula-fed infants. Can J Public Health. 1993;84(2):132–5. [PubMed] [Google Scholar]
- 6. Axelsson IEM, Ivarsson SA, Raiha NCR. Protein intake in early infancy: Effects on plasma amino acid concentrations, insulin metabolism, and growth. Pediatric Research. 1989;26(6):614–5. [DOI] [PubMed] [Google Scholar]
- 7. Inostroza J, Haschke F, Steenhout P, Grathwohl D, Nelson SE, Ziegler EE. Low-protein formula slows weight gain in infants of overweight mothers. J Pediatr Gastroenterol Nutr. 2014;59(1):70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Räihä N, Minoli I, Moro G. Milk protein intake in the term infant. I. Metabolic responses and effects on growth. Acta Paediatr Scand. 1986;75(6):881–6. [DOI] [PubMed] [Google Scholar]
- 9. Turck D, Grillon C, Lachambre E, Robiliard P, Beck L, Maurin JL, Kempf C, Bernet J-P, Marx J, Lebrun Fet al. Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J Pediatr Gastroenterol Nutr. 2006;43(3):364–71. [DOI] [PubMed] [Google Scholar]
- 10. Mennella JA, Ventura AK, Beauchamp GK. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics. 2011;127(1):110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mennella JA, Inamdar L, Pressman N, Schall JI, Papas MA, Schoeller D, Stallings VA, Trabulsi JC. Type of infant formula increases early weight gain and impacts energy balance: A randomized controlled trial. Am J Clin Nutr. 2018;108(5):1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. German Infant Nutritional Intervention Study Group . Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at ≤6 y of age: results from the German Infant Nutritional Intervention Study. Am J Clin Nutr. 2009;89(6):1846–56. [DOI] [PubMed] [Google Scholar]
- 13. Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am J Clin Nutr. 1993;58(2):152–61. [DOI] [PubMed] [Google Scholar]
- 14. Kramer MS, Guo T, Platt RW, Vanilovich I, Sevkovskaya Z, Dzikovich I, Michaelsen KF, Dewey K; Promotion of Breastfeeding Intervention Trials Study Group . Feeding effects on growth during infancy. J Pediatr. 2004;145(5):600–5. [DOI] [PubMed] [Google Scholar]
- 15. Bell KA, Wagner CL, Feldman HA, Shypailo RJ, Belfort MB. Associations of infant feeding with trajectories of body composition and growth. Am J Clin Nutr. 2017;106(2):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schakel SF. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products–A research perspective. J Food Comp and Anal. 2001;14:315–22. [Google Scholar]
- 17. Bridge G, Lomazzi M, Bedi R. A cross-country exploratory study to investigate the labelling, energy, carbohydrate and sugar content of formula milk products marketed for infants. Br Dent J. 2020;228(3):198–212. [DOI] [PubMed] [Google Scholar]
- 18. United States Food and Drug Administration . Added sugars on the new nutrition facts label. U.S. Department of Health and Human Services; 2020; [Internet]. Available from: https://www.fda.gov/food/new-nutrition-facts-label/added-sugars-new-nutrition-facts-label. [Google Scholar]
- 19. Siervo M, Montagnese C, Mathers JC, Soroka KR, Stephan BC, Wells JC. Sugar consumption and global prevalence of obesity and hypertension: An ecological analysis. Public Health Nutr. 2014;17(3):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kong K, Burgess B, Morris KS, Faith MS, Paluch RA. High intake of added sugars is linked to rapid weight gain in infancy, breastfeeding ≥ 12 months may protect against this: A preliminary investigation. Pediatric Obesity. 2021, 16:(3);e12728.doi:10.1111/ijpo.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention . Guidelines & recommendations. U.S. Department of Health and Human Services; 2020; [Internet]. Available from: https://www.cdc.gov/breastfeeding/recommendations/index.htm. [Google Scholar]
- 22. Food and Nutrition Board . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academy Press; 2005. [Google Scholar]
- 23. Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant feeding practices study II: Study methods. Pediatrics. 2008;122(Suppl 2):S28–35. [DOI] [PubMed] [Google Scholar]
- 24. Anater AS, Catellier DJ, Levine BA, Krotki KP, Jacquier EF, Eldridge AL, Bronstein KE, Harnack LJ, Lorenzana Peasley JM, Lutes AC. The Feeding Infants and Toddlers Study (FITS) 2016: Study design and methods. J Nutr. 2018;148(Suppl 3):1516S–24S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raper N, Perloff B, Ingwersen L, Steinfeldt L, Anand J. An overview of USDA's dietary intake data system. J Food Compos Anal. 2004;17(3-4):545–55. [Google Scholar]
- 26. Herrick KA, Fryar CD, Hamner HC, Park S, Ogden CL. Added sugars intake among US infants and toddlers. J Acad Nutr Diet. 2020;120(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25(Suppl 1):S27–36. [DOI] [PubMed] [Google Scholar]
- 28. WHO Multicentre Growth Reference Study Group . WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 29. Ekelund U, Ong K, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rössner S. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: The Stockholm Weight Development Study (SWEDES). Am J Clin Nutr. 2006;83(2):324–30. [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention . Defining adult overweight and obesity. U.S. Department of Health & Human Services; 2020; [Internet]. Available from: https://www.cdc.gov/obesity/adult/defining.html. [Google Scholar]
- 31. Cohen JE. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 32. Cohen JE. A power primer. Psych Bull. 1992;112(1):155–9. [DOI] [PubMed] [Google Scholar]
- 33. UNICEF . The international code of marketing of breastmilk substitutes. Baby friendly initiative. 2018, World Health Organization, Switzerland. [Google Scholar]
- 34. Office of the Register . Part 107–Infant Formula. Office of the Federal Register; 2020; [Internet]. Available from: https://www.ecfr.gov/cgi-bin/text-idx?SID=a54dc6470436e8496112b86909d4b683&mc=true&node=pt21.2.107&rgn=div5#sp21.2.107.b. [Google Scholar]
- 35. Mennella JA. Ontogeny of taste preferences: Basic biology and implications for health. Am J Clin Nutr. 2014;99(3):704S–11S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nicklaus S, Boggio V, Chabanet C, Issanchou S. A prospective study of food preferences in childhood. Food Qual Prefer. 2004;15(7):805–18. [Google Scholar]
- 37. Ambrosini GL. Childhood dietary patterns and later obesity: A review of the evidence. Proc Nutr Soc. 2014;73(1):137–46. [DOI] [PubMed] [Google Scholar]
- 38. Cogswell ME, Gunn JP, Yuan K, Park S, Merritt R. Sodium and sugar in complementary infant and toddler foods sold in the United States. Pediatrics. 2015;135(3):416–23. [DOI] [PubMed] [Google Scholar]
- 39. Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: A randomized clinical trial. JAMA Pediatr. 2016;170(8):742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, Rich-Edwards JW, Gillman MW. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–8. [DOI] [PubMed] [Google Scholar]
- 41. Santangeli L, Sattar N, Huda SS. Impact of maternal obesity on perinatal and childhood outcomes. Best Pract Res Clin Obstet Gynaecol. 2015;29(3):438–48. [DOI] [PubMed] [Google Scholar]
- 42. Baker JL, Michaelsen KF, Rasmussen KM, Sørensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80(6):1579–88. [DOI] [PubMed] [Google Scholar]
- 43. Oliveira V FE, Smallwood D. Rising infant formula costs to the WIC Program: Recent trends in rebates and wholesale prices. Report No.: Economic Research Report 93. Washington, DC: US Department of Agriculture; 2010. [Google Scholar]
- 44. EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010;8(3):1462–539. [Google Scholar]
- 45. Mennella JA, Beauchamp GK. Developmental changes in the acceptance of protein hydrolysate formula. J Dev Behav Pediatr. 1996;17(6):386–91. [DOI] [PubMed] [Google Scholar]
- 46. Hyams JS, Treem WR, Etienne NL, Weinerman H, MacGilpin D, Hine P, Choy K, Burke G. Effect of infant formula on stool characteristics of young infants. Pediatrics. 1995;95(1):50–4. [PubMed] [Google Scholar]
- 47. Hauser B, Keymolen K, Blecker U, Suys B, Bougatef A, Loeb H, Vandenplas Y. A comparative evaluation of whey hydrolysate and whey-predominant formulas. How well do infants accept and tolerate them?. Clin Pediatr (Phila). 1993;32(7):433–7. [DOI] [PubMed] [Google Scholar]
- 48. Vandenplas Y, Hauser B, Blecker U, Suys B, Peeters S, Keymolen K, Loeb H. The nutritional value of a whey hydrolysate formula compared with a whey-predominant formula in healthy infants. J Pediatr Gastroenterol Nutr. 1993;17(1):92–6. [DOI] [PubMed] [Google Scholar]
- 49. Rosenstein D, Oster H. Differential facial responses to four basic tastes in newborns. Child Dev. 1988;59(6):1555–68. [PubMed] [Google Scholar]
- 50. Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148(5):852–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun SZ, Empie MW. Fructose metabolism in humans–What isotopic tracer studies tell us. Nutr Metab (Lond). 2012;9(1):89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Havel PJ. Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63(5):133–57. [DOI] [PubMed] [Google Scholar]
- 53. Goran MI, Martin AA, Alderete TL, Fujiwara H, Fields DA. Fructose in breast milk is positively associated with infant body composition at 6 months of age. Nutrients. 2017;9(2):146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma Y, Olendzki BC, Pagoto SL, Hurley TG, Magner RP, Ockene IS, Schneider KL, Merriam PA, Hébert JR. Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol. 2009;19(8):553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fisher JO, Butte NF, Mendoza PM, Wilson TA, Hodges EA, Reidy KC, Deming D. Overestimation of infant and toddler energy intake by 24-h recall compared with weighed food records. Am J Clin Nutr. 2008;88(2):407–15. [DOI] [PubMed] [Google Scholar]
- 56. Walton KA, Murray LJ, Gallagher AM, Cran GW, Savage MJ, Boreham C. Parental recall of birthweight: A good proxy for recorded birthweight?. Eur J Epidemiol. 2000;16(9):793–6. [DOI] [PubMed] [Google Scholar]
- 57. Kuehn B. Breastfeeding report card. JAMA. 2018;320(14):426. [DOI] [PubMed] [Google Scholar]
- 58. Nutrition Coordinating Center . Appendix 22: Sources of food and nutrient data. NDSR Manual, Minneapolis, MN, University of Minnesota. 2020. [Google Scholar]
- 59. Brill H. Approach to milk protein allergy in infants. Can Fam Physician. 2008;54(9):1258–64. [PMC free article] [PubMed] [Google Scholar]
- 60. Høst A. Frequency of cow's milk allergy in childhood. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):33–7. [DOI] [PubMed] [Google Scholar]
- 61. Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: A whole population birth cohort study. Pediatrics.2001;108(2):E33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data (including data dictionaries), study protocols, statistical analysis plan, and informed consent forms will be made available to researchers who provide methodologically sound proposals. All proposals should be sent to: kkong@cmh.edu.


