Abstract
Christensenellaceae is a family of subdominant commensal bacteria found in humans. It is thought to play an important role in gut health by maintaining microbial symbiosis. Indeed, these bacteria occur at significantly lower levels or are absent in individuals suffering from inflammatory bowel diseases (IBDs). Here, we explored if type species Christensenella minuta (strain: DSM 22607) could have the potential to help treat IBDs. We assessed key properties displayed by the bacterium using a combination of in vitro and in vivo assays. We found that while C. minuta is a strict anaerobe, it is also oxygen tolerant. Additionally, we observed that the species produces high levels of acetate and moderate levels of butyrate. We performed deep phenotyping using Biolog microarrays. Using human intestinal cell lines, we discovered that C. minuta demonstrated strong anti-inflammatory activity, resulting in reduced levels of proinflammatory IL-8 cytokines via the inhibition of the NF-κB signaling pathway. Furthermore, C. minuta protected intestinal epithelial integrity in vitro. Finally, in two distinct animal models of acute colitis, C. minuta prevented intestinal damage, reduced colonic inflammation, and promoted mucosal healing. Together, these results indicate that C. minuta has potent immunomodulatory properties, underscoring its potential use in innovative microbiome-based IBD biotherapies.
Subject terms: Bacteriology, Bacterial host response
Introduction
Inflammatory bowel diseases (IBDs) are disorders characterized by the chronic abnormal inflammation of the gastrointestinal tract, which triggers an uncontrolled and deleterious inflammatory response1,2 during which levels of interleukine-8 (IL-8) cytokines increase3,4 and reactive oxygen species (ROS) are overproduced5. The two main types of IBDs are Crohn's disease (CD) and ulcerative colitis (UC)6, which each display distinct physiological symptoms. Although the aetiology of IBDs remains poorly understood, we do know that a complex set of interconnected environmental7, genetic8, and immune9 factors are involved. IBD triggers are also little characterized, but the best-supported hypothesis is that immune system disruptions provoke imbalances in crosstalk between gut commensal bacteria and human hosts2. Indeed, in multiple studies, individuals suffering from CD or UC have been found to display microbial dysbiosis, characterized by a decrease in commensal bacteria in the phyla Firmicutes and Bacteroidetes, allowing an increase in bacteria in the class Gammaproteobacteria10–13. This dysbiosis is accompanied by changes in short-chain fatty acid (SCFA) production14,15, which can affect inflammation pathways and immune system modulation14.
Because proper balance within the intestinal microbiota helps ensure health, strategies have been developed to address IBD-related dysbiosis. To date, fecal microbiota transplantation (FMT)16 and treatments using isolated bacteria17 have yielded promising results. FMT has been shown to be efficient in treating Clostridium difficile infections18; it may also help restore the microbiota of those suffering from IBDs, but more research is needed to support its routine use in IBD cases19. Indeed, this procedure can be difficult to set up and control. Donor choice is an especially important consideration20 because donor incompatibility can lead to alterations in nutrient absorption, promote the onset of chronic disease, or transfer undesired microorganisms21. Recently, in murine models, treatments employing single bacterial strains have been successfully used to modulate gut microbial populations22 and restore gastrointestinal health by reducing tissue damage and levels of pro-inflammatory cytokines12,23–26. Moreover, in humans, these approaches can correct dysbiosis and decrease quantities of inflammatory mediators, leading to remission27.
In 2017, research showed that the recently described family Christensenellaceae plays a major role in gut health28: these bacteria serve as keystone species in the development of the symbiotic gut microbiome. Christensenella is part of the phylum Firmicutes and the order Clostridiales, and its members are strict anaerobic, Gram-negative, non-sporulating, and non-motile bacteria29. This family has been described as a highly heritable taxon and serves as a hub in a co-occurrence network that includes other heritable taxa30. Studies have found that individuals suffering from CD or UC have significantly lower levels of or completely lack Christensenellaceae in their intestinal microbiota31,32. Furthermore, in individuals with CD, such decreases in abundance are highly predictive of flare-ups33. Recently, it was discovered that IBD-related alterations in gut microbiota contribute to inflammation dynamics as well as the loss of commensal bacteria that are key to restoring balance and general homeostasis34. Taken together, these findings suggest a strong link exists between the abundance of Christensenellaceae and the occurrence of IBDs, indicating that these commensal bacteria could play a central role in gut physiology.
Here, we hypothesized that Christensenella species have anti-inflammatory properties that protect the intestinal mucosa. To test this hypothesis, we first characterized various properties displayed by the type species Christensenella minuta DSM 22607, namely its oxygen sensitivity, metabolic profile, and ability to produce SCFAs. Then, in vitro, we assessed how well the bacterium modulated inflammation in human colonic cells and protected intestinal barrier integrity during inflammation. Finally, we explored whether C. minuta DSM 22607 could reduce inflammation in vivo in two distinct preclinical colitis models in rodents. We found support for our hypothesis—this bacterial species may hold promise for microbiome-based IBD biotherapies.
Results
An oxygen tolerant anaerobe that produces short-chain fatty acids
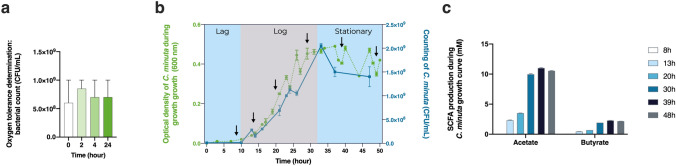
To better characterize C. minuta DSM 22607, we first assessed the strain’s oxygen sensitivity. We found that the bacterium was not extremely oxygen sensitive (EOS) because it could tolerate the presence of oxygen for at least 24 h (Fig. 1a). We then quantified its production of SCFAs during different growth phases (latent, exponential, and stationary) (Fig. 1b,c). We observed that C. minuta was able to produce high levels of acetate and moderate levels of butyrate, as also seen elsewhere29; in contrast, no propionate was generated. Our results show that, during all three growth phases, SCFA production ratios remained the same: approximately 1 mol of butyrate for each 5 mol of acetate.
Figure 1.
Oxygen sensitivity and metabolite production in Christensenella minuta. (a) Bacterial counts (CFUs/mL) after 0, 2, 4, and 24 h of oxygen exposure. (b) Growth over time expressed in terms of optical density and colony forming units (CFUs). The arrows show the different sampling points. (c) Butyrate and acetate production at different sampling points.
The bacterium’s detailed metabolic phenotype
We obtained an extensive phenotypic profile for C. minuta using Biolog microassays. We performed three independent replicates. We found that C. minuta can metabolize N-acetyl-d-glucosamine, d-arabitol, arbutin, d-cellobiose, dextrin, d-fructose, l-fucose, d-galactose, α-d-glucose, maltotriose, d-mannitol, d-mannose, 3-methyl-d-glucose, palatinose, salicin, turanose, fumaric acid, pyruvic acid, l-phenylalanine, 2′-deoxyadenosine, inosine, and uridine (Table 1).
Table 1.
Metabolic profile of Christensenella minuta strain DSM 22607.
| Target compound | C. minuta | Target compound | C. minuta | Target compound | C. minuta |
|---|---|---|---|---|---|
| Water | − | Turanose | + | 3-Methyl-d-glucose | + |
| N-Acetyl-d-galactosamine | − | Acetic acid | − | α-Methyl-d-galactoside | − |
| N-Acetyl-d-glucosamine | + | Formic acid | − | β-Methyl-d-galactoside | − |
| N-Acetyl-β-d-mannosamine | − | Fumaric acid | + | α-Methyl-d-glucoside | − |
| Adonitol | − | Glyoxylic acid | − | β-Methyl-d-glucoside | – |
| Amygdalin | − | α-Hydroxybutyric acid | − | Palatinose | + |
| d-Arabitol | + | β-Hydroxybutyric acid | − | d-Raffinose | − |
| Arbutin | + | Itaconic acid | − | l-Rhamnose | − |
| d-Cellobiose | + | α-Ketobutyric acid | – | Salicin | + |
| α-Cyclodextrin | − | α-Ketovaleric acid | − | d-Sorbitol | − |
| β-Cyclodextrin | − | D,l-Lactic Acid | − | Stachyose | − |
| Dextrin | + | l-Lactic acid | − | Sucrose | − |
| Dulcitol | − | d-Lactic acid methyl ester | − | d-Trehalose | − |
| i-Erythritol | − | d-Malic acid | − | Glycyl-l-aspartic acid | − |
| d-Fructose | + + | l-Malic acid | − | Glycyl-l-glutamine | − |
| l-Fucose | + + | Propionic acid | − | Glycyl-l-methionine | − |
| d-Galactose | + | Pyruvic acid | + | Glycyl-l-proline | − |
| d-Galacturonic acid | – | Pyruvic acid methyl ester | − | l-Methionine | − |
| Gentiobiose | − | d-Saccharic acid | − | l-Phenylalanine | + |
| d-Gluconic acid | − | Succinamic acid | − | l-Serine | − |
| d-Glucosaminic acid | – | Succinic acid | − | l-Threonine | − |
| α-d-Glucose | + + | Succinic acid monomethyl ester | − | l-Valine | – |
| Glucose-1-phosphate | − | m-Tartaric acid | − | l-Valine plus l-aspartic acid | – |
| Glucose-6-phosphate | − | Urocanic acid | − | 2′-Deoxyadenosine | + |
| Glycerol | − | l-Alaninamide | − | Inosine | + |
| D, l-α-Glycerol phosphate | − | l-Alanine | − | Thymidine | − |
| m-Inositol | − | l-Alanyl-l-glutamine | − | Uridine | + |
| α-d-Lactose | − | l-Alanyl-l-histidine | − | Thymidine-5′-monophosphate | − |
| Lactulose | – | l-Alanyl-l-threonine | − | Uridine-5′-monophosphate | − |
| Maltose | − | l-Asparagine | − | d-Mannose | + + |
| Maltotriose | + | l-Glutamic acid | − | d-Melezitose | − |
| d-Mannitol | + + | l-Glutamine | − | d-Melibiose | − |
An ability to limit IL-8 production and inhibit NF-κB activation in HT-29 cells
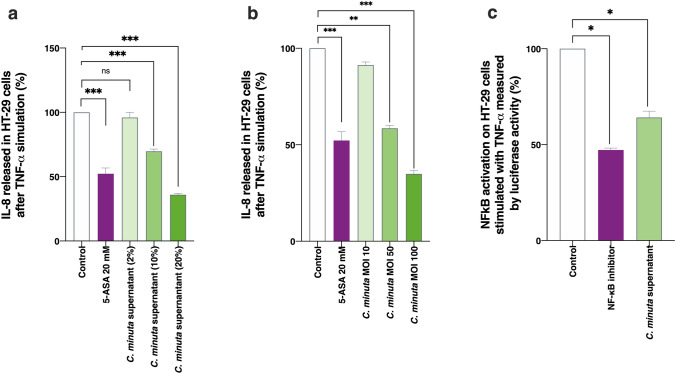
Using human HT-29 cells, we analyzed how well C. minuta and its supernatant could modulate TNF-α induced secretion of IL-8 and thus limit inflammation. IL-8 is a major proinflammatory cytokine, and, therefore, bacteria capable of inhibiting its secretion have anti-inflammatory effects35. Both the supernatant (Fig. 2a) and the bacterium itself (Fig. 2b) demonstrated anti-inflammatory properties: IL-8 production decreased by around 50% in both cases. This level of inhibition resembles that associated with 5-ASA, a compound commonly used to treat IBDs. When co-incubation occurred at different multiplicities of infection (MOIs), we observed a dose–response effect, where percent inhibition ranged from 0% (MOI 10) to 50% (MOI 50) (Fig. 2b). Similar results were obtained with 2% (v/v), 10% (v/v), and 20% (v/v) of the supernatant. We also explored C. minuta’s effects on the NF-κB pathway, which plays a key role in inflammation by regulating immune responses36, including IL-8 production37. The bacterial supernatant decreased NF-κB activation by 40%, an effect similar to that of the control NF-κB inhibitor BAY 11-7082 (10 µm) (Fig. 2c). In contrast, no effects were observed when the bacterium alone was used (data not shown). We thus concluded that C. minuta is likely secreting a potent anti-inflammatory effector into its culture medium.
Figure 2.
Anti-inflammatory properties of Christensenella minuta in vitro. IL-8 production by HT-29 cells exposed to TNF-α in presence of (a) C. minuta supernatant or (b) C. minuta bacteria. (c) Levels of NF-κB activation in HT-29 cells transfected with a reporter system and exposed to TNF-α. Results of Mann Whitney U tests comparing the control groups to the other groups: *p < 0.05, **p < 0.01, and ***p < 0.001.
An ability to maintain barrier integrity in Caco-2 cells
We assessed whether C. minuta could maintain intestinal barrier integrity in an in vitro cell model by measuring transepithelial electrical resistance (TEER) in Caco-2 cells exposed to TNF-α, which disrupts tight junctions, increases epithelial barrier permeability, and causes inflammation. Measurements were made immediately prior to and 24 h after TNF-α exposure. We observed that the TEER ratio remained stable when Caco-2 cells were treated with C. minuta in Dulbecco's modified Eagle’s medium (DMEM) for 3 h beforehand (Fig. 3). This result indicates that barrier integrity had been maintained, seemingly via the anti-inflammatory action of different effectors that protected the intestinal barrier.
Figure 3.
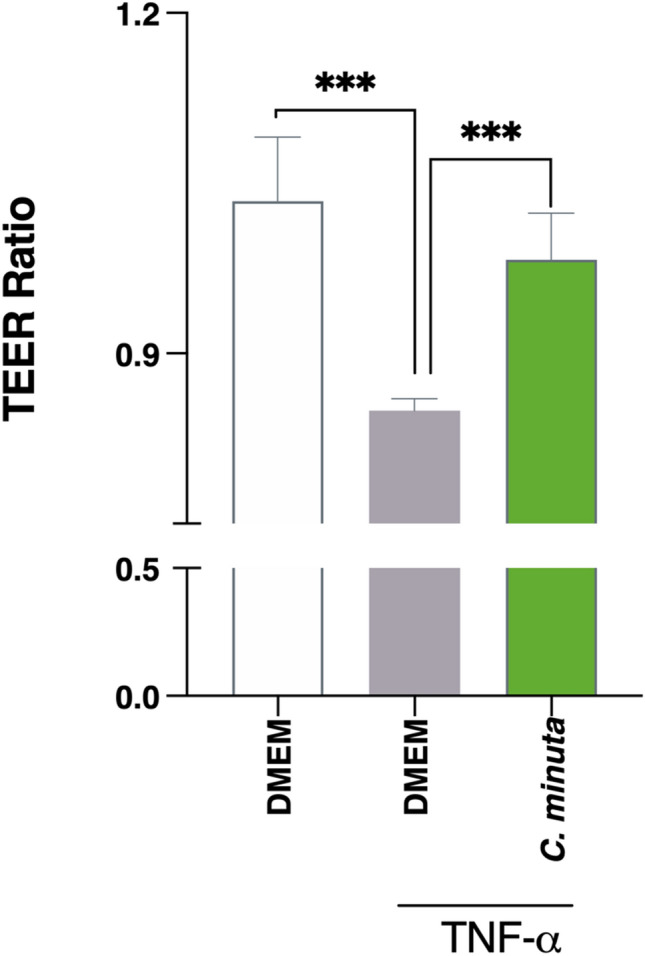
Effects of Christensenella minuta on intestinal barrier permeability. Polarized monolayers of Caco-2 cells were exposed to TNF-α to disrupt the intestinal barrier. TEER was measured immediately prior to and 24 h after TNF-α exposure. Results of Mann Whitney U tests comparing the DMEM + TNF-α group to the other groups: *p < 0.05, **p < 0.01, and ***p < 0.001.
A faculty to prevent and protect against DNBS-induced colitis
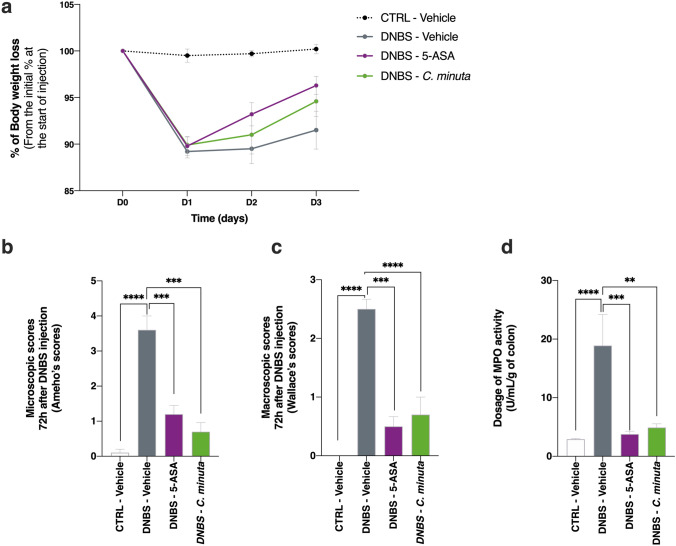
We performed an experiment to determine whether the anti-inflammatory properties of C. minuta seen in vitro were also observed in vivo. Treatment mice were given daily doses of C. minuta for 14 days. Colitis was then induced by an intrarectal injection of DNBS, and the mice were euthanized 3 days later. We found that the treatment group tended to gain body mass faster than the DNBS-vehicle group (Fig. 4a), although this difference was not significant. We also observed a decrease in the microscopic scores in the treatment group, reflecting restored colonic epithelial structure and reduced immune cell infiltration (Fig. 4b). A similar pattern was seen in the macroscopic scores (Fig. 4d). To evaluate the bacterium’s anti-inflammatory effects on colonic tissue, we characterized the activity of myeloperoxidase (MPO), an enzyme found in the intracellular granules of neutrophils38. We observed that DNBS-induced inflammation resulted in increased neutrophil infiltration and MPO activity; these effects were significantly less pronounced in the treatment group gavaged with C. minuta (Fig. 4e).
Figure 4.
Effects of Christensenella minuta on DNBS-induced inflammation in mice. (a) Change in body mass after colitis was induced; (b) colon microscopic scores; (c) colon macroscopic scores; and (d) levels of MPO activity. Results of Mann Whitney U tests comparing the DNBS-Vehicle group to the three other groups: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
An ability to prevent and protect against TNBS-induced colitis
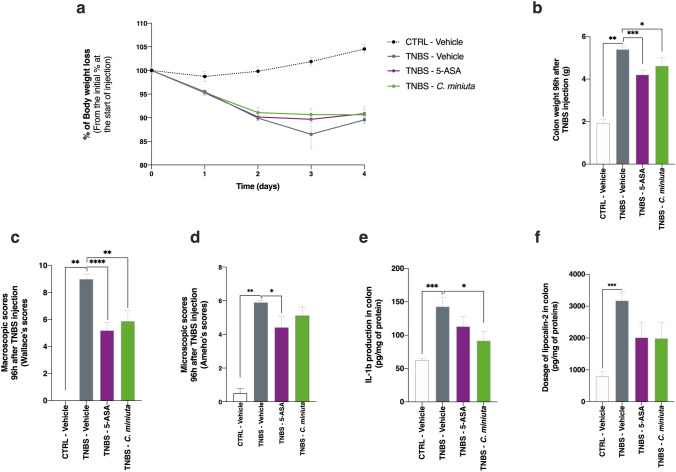
To obtain additional confirmation of the bacterium’s anti-inflammatory effects in vivo, we repeated the experiment in a second model—TNBS-induced colitis in rats, known to be more susceptible to inflammation39. Treatment rats were given daily doses of C. minuta for 14 days. Colitis was then induced by an intrarectal injection of TNBS, and the rats were euthanized 4 days later. Unlike the the mouse model, the treatment group had no effect on body mass gain, compared to TNBS-vehicle group at the end of the experiment (Fig. 5a). However, colon mass was lower in the treatment group (Fig. 5b), which indicates that intestinal transit was improved by C. minuta. Remarkably, the macroscopic scores (i.e., Wallace scores40; Fig. 5c) provided support for the idea that C. minuta could be as efficient as 5-ASA, a compound used to treat UC41, in protecting colonic tissue. Moreover, the microscopic scores for the treatment group showed that inflammatory profiles seemed to be improved at the histological level (Fig. 5d), compared to what was seen in the TNBS-vehicle group. Furthermore, the C. minuta treatment appeared to induce an immunomodulatory response by decreasing IL-1β secretion (Fig. 5e). This result specifically indicates that the TNBS-induced Th1 response was dampened. IL-6 and IL-10 production (of Th2 and Th1 cytokines, respectively) was not affected by the TNBS injection (data not shown). Finally, we used lipocalin-2 (LCN-2) as a non-invasive biological marker of intestinal inflammation. The C. minuta treatment tended to decrease the concentration of LCN-2 in the colon (Fig. 5f). These results validated our in vitro findings, demonstrating the bacterium’s anti-inflammatory properties in two in vivo colitis models.
Figure 5.
Effects of Christensenella minuta on TNBS-induced inflammation in rats. (a) Body mass over the course of the experiment; (b) colon mass at the end of the experiment; (c) colon microscopic scores; (d) colon macroscopic scores; colon IL-1β levels (e); and (f) levels of LCN-2, a proxy for colon neutrophil infiltration. Results of Mann Whitney U tests comparing the TNBS-Vehicle group to other three groups: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Discussion
IBDs are debilitating chronic diseases for which no curative treatments are currently available. Our research is grounded in the idea that microbiome-based therapies offer an innovative approach to healing intestinal mucosa. Indeed, there is increasing evidence that some commensal bacteria possess anti-inflammatory properties that can improve IBD symptoms10–12.
In 2012, Morotomi et al.42 described a new family of bacteria, the Christensenellaceae, whose presence was later found to be correlated with gut microbiota health28. Although this taxon is a subdominant member of the microbiome, co-occurrence analyses have revealed that it plays a central role within a broader network of heritable bacteria in the gut ecosystem30,43. Indeed, individuals with IBDs display drastically lower levels of these bacteria in their intestines, suggesting that intestinal inflammation is greater when the abundance of Christensenella species is lower31,44,45. Consequently, we explored the anti-inflammatory properties of Christensenella minuta (strain DSM 22607) to determine whether it could be used in IBD treatments.
First, we characterized the bacterium’s metabolic phenotype to gain insight into its overall metabolic capacities. We found that, while C. minuta is an anaerobe, it was highly tolerant of oxygen, unlike other anaerobic commensal bacteria, such as Faecalibactium prausnitzii, which are EOS. Indeed, recent research has discovered that Christensenella occurs in different parts of the human intestine that vary in oxygen concentrations (i.e., the terminal ileum13,44, cecum46, and distal colon47). These findings support the idea that C. minuta could have beneficial effects within the upper gastrointestinal tract and, particularly, in the distal ileum, a major site of inflammation in Crohn’s disease; in contrast, other candidate EOS bacteria only occur in the colon. Since IBDs are associated with oxidative stress and high levels of ROS5, the ability of C. minuta to tolerate oxygen might confer resistance to inflammation-induced oxidative stress in the gut. The bacterium might thus be well suited to creating environmental conditions that allow the establishment of more sensitive anaerobes species. Indeed, the increasing presence of facultative anaerobes observed in the colon during IBD48 could give a major advantage to C. minuta as a biotherapy against EOS candidates. The bacterium’s oxygen tolerance could also facilitate its use in industrial manufacturing processes, a practical consideration if C. minuta’s benefits are to be translated into microbiome-based clinical treatments.
We also confirmed that C. minuta produces high levels of acetate and moderate levels of butyrate29,49 and demonstrated that the acetate:butyrate production ratio was 5:1 over all three growth phases. Widely produced by gut bacteria, SCFAs result from carbohydrate fermentation and, to a lesser extent, from protein fermentation50. Interestingly, a number of bacteria have been identified as either acetate or butyrate producers but rarely both51. SCFAs are crucial compounds since they modulate host pathways through interactions with G-protein-coupled receptors (GPRs), which are found in colonic, hepatic, muscular, and adipose tissues52. These interactions influence multiple important functions related to cell differentiation and energy metabolism. The butyrate receptor GPR109a also occurs in intestinal epithelial cells, adipocytes, and immune cells, where it helps control inflammation and cell proliferation53. Decreases in SCFA production could have deleterious effects, mainly by influencing host-microbe interactions34. Consequently, balanced SCFA production is essential to gut homeostasis.
Because SCFAs can affect microbiota-host crosstalk via their immunomodulatory properties54, we ascertained whether C. minuta had an influence on inflammation. We discovered that both C. minuta bacteria and their its supernatant displayed potent anti-inflammatory properties, decreasing the secretion of IL-8 cytokines by HT-29 cells following TNF-α induced inflammation. Such anti-inflammatory properties have also been seen in other bacteria, including Faecalibacterium prausnitzii55, several strains of Lactobacillus56, and Akkermensia muciniphila24.
To further explore the bacterium’s anti-inflammatory effects, we tested the impact of both the bacteria and its supernatant against HT-29 cells transfected with a NF-κB reporter system known to regulate IL-8 production37. Only the supernatant decreased NF-κB activation. This result, combined with the findings of the previous experiment, suggest that at least two different bacterial effectors were responsible for the effects observed. Past work using a variety of commensal and pathogenic microorganisms has shown that bacteria utilize a variety of mechanisms to modulate the canonical NF-κB pathway57. It is possible that butyrate concentrations in the supernatant (which were about 10 times lower than physiological concentrations58) helped inhibit the NF-κB pathway59. It may also be that other compounds, such as polysaccharides60, peptidoglycans61, and proteins62, were secreted into the supernatant or exposed on the surface of the bacterial membrane63. Further research is needed to decipher the underlying mechanisms at work.
We then evaluated how well C. minuta could protect epithelial cells from TNF-α-induced permeability using a Caco-2 cell line. We found that the bacterium successfully maintained the integrity of the epithelial cell monolayer following induced inflammation. Individuals with IBDs have very low levels of the adhesion molecules that regulate intestinal permeability64; C. minuta could help restore proper permeability and limit any damage that has occurred. Recent work has highlighted that Escherischia coli Nissle 1917 could attenuate declines in TEER induced by TNF-α and IFNγ, notably by inhibiting the NF-κB-mediated activation of the MLCK-P-MLC signaling pathway65. F. prausnitzii and Roseburia intestinalis have also been found to help reverse impaired epithelial barrier function by modulating the expression of tight junction proteins and decreasing paracellular permeability66. It would be worthwhile to decipher the precise mechanism in use by C. minuta.
To ascertain whether C. minuta displayed the same anti-inflammatory properties in vivo, we performed experiments using two different animal models of colitis: a mouse model of moderate, DNBS-induced colitis and a rat model of severe, TNBS-induced colitis39. Based on the macroscopic scores, treatment with C. minuta significantly limited colon damage in both models. To characterize the bacterium’s immunomodulatory effects, we assessed neutrophil infiltration in colonic tissues by monitoring MPO activity (in the mice) and LCN-2 levels (in the rats). In both models, the metrics were lower in C. minuta-treated animals. Similar studies found comparable effects in a mouse model of TNBS-induced colitis using a treatment based on Parabacteroides distasonis67 and in a mouse model of DNBS-induced colitis using a treatment based on F. prausnitizii68 and different Lactobaccillus strains56. Taken together, these findings demonstrate that using single-strain colitis treatments could be effective6. It has been shown that IL-8 secretion induces neutrophil activation in inflammed regions69,70. Given that C. minuta decreased IL-8 secretion and NF-κB activation in vitro, this signaling pathway could have been involved in the reduction of neutrophil activation. Furthermore, a decrease in IL-1β was seen in the C. minuta-treated rats (Fig. 5e). IL-1β signaling is mediated by multiple transcription factors, including NF-κB71. It is possible that cytokine release in the TNBS-induced colitis model was partially modulated by C. minuta’s secretion of NF-κB inhibitors. A similar mode of action has been seen in F. prausnitzii in different colitis models62, notably via the release of the microbial anti-inflammatory molecule (MAM). Although the NF-κB signaling pathway serves as a major line of defense against pathogens, it can have deleterious effects when overactivated due to the increased production of proinflammatory cytokines72. Consequently, it is important to determine which molecules help control pathway activation so that the development of inflammation can be halted. For example, SCFAs such as butyrate can limit inflammation via their inhibitory action73.
In conclusion, our study is the first to show that C. minuta displays strong immunomodulatory properties in vitro and in vivo. Our findings open the door to intriguing new research questions. Although additional research is obviously needed to better understand the bacterium’s effects and their underlying mechanisms, our work underscores that C. minuta holds promise for treating IBDs and merits further study with a view to developing single-strain biotherapies.
Methods
Culturing the bacteria
Christensenella minuta (DSM 22607) was cultured in Gifu anaerobic medium (GAM broth, HyServe) in an anaerobic chamber (5%/5%/90% CO2, H2, N2) kept at 37 °C. Granulated agar (15 g/L, Difco) was added when necessary. Bacterial cultures were centrifuged at 2500×g and then resuspended in 1X Dulbecco’s phosphate-buffered saline (DPBS, Gibco). We then employed these cultures in the in vitro and in vivo experiments described below. To establish the growth curves, cultures were followed for their entire growth cycle (up to 54 h). We used a spectrophotometer (Ultrospec 10) to measure optical density (OD600) and thus estimate bacterial counts. Samples of cultures were collected at different timepoints and centrifuged at 4000×g at 4 °C for 15 min. We recovered the supernatants and stored them at − 20 °C until we could measure short-chain fatty acid (SCFA) concentrations.
Characterizing short-chain fatty acid concentrations
Bacterial supernatants were deproteinized overnight at 4 °C via the addition of phosphotungstic acid (10% [v/v]); Sigma). We then centrifuged the resulting samples for 15 min at 12,000×g. Concentrations of SCFAs were determined using a gas chromatograph (GC; Agilent 6890 N Network) equipped with a split-splitless injector (GC Agilent 7890B), a flame-ionization detector, and a capillary column (15 m × 0.53 mm × 0.5 µm) packed with SP 1000 (Nukol; Supelco 25,236). The flow rate of hydrogen, the carrier gas, was 10 mL/min; the temperatures of the injector, column, and detector were 200 °C, 100 °C, and 240 °C, respectively. We used 2-ethylbutyrate as the internal standard in our samples and employed a panel of SCFA standards. Two replicates were performed for each sample. We collected the SCFA data and integrated the peaks using the GC’s default software (Agilent). To determine the final concentrations of SCFAs, the supernatants were weighed before and after protein precipitation to obtain the appropriate multiplication factor (i.e., the supernatant to sample mass ratio).
Assessing oxygen sensitivity
To evaluate C. minuta’s sensitivity to oxygen, we used bacteria from the cultures described above55. Briefly, we grew C. minuta for 48 h in a liquid medium. Then, we took 10-µL samples of different concentrations of the bacteria (range of final concentrations: 104–109 CFU/mL) and deposited them on Petri dishes. The dishes were placed outside of the anaerobic chamber and exposed to oxygen for 2, 4, and 24 h.
Establishing a metabolic profile
We established a metabolic profile for C. minuta using AN MicroPlate™ technology (Biolog) in accordance with the manufacturer’s instructions. Briefly, cultures were streaked twice on Biolog Universal Anaerobe Agar (BUA; Biolog) supplemented with 5% (w/v) defibrinated sheep blood (Alliance Bio Expertise). We allowed growth to occur for 4 days at 37 °C under anaerobic conditions. Bacteria were swabbed and transferred into prereduced anaerobic inoculating fluid until 65% transmittance was reached. Then, 100 mL of this bacterial suspension was used to inoculate each well of AN MicroPlates™ under anaerobic conditions. We incubated the plates for 24 h at 37 °C under anaerobic hydrogen-free conditions using a GENbox and anaerobic jar system (bioMérieux). Color shifts in each well were evaluated visually and via optical density measurements made at 590 nm (FLUOstar Omega, BMG Labtech).
Culturing eukaryotic cells
We obtained the human colon adenocarcinoma cell line HT-29 from the European Collection of Authenticated Cell Cultures (ECACC; Sigma). Cells were grown in McCoy’s 5A medium (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco) and 1% (v/v) penicillin/streptomycin (P/S; Sigma). The cultures were maintained at 37 °C under conditions of 5% CO2 until 80% confluence was reached. We obtained the Caco-2 cell line from the American Type Tissue Collection (ATCC®) and maintained it in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with glutaMAX™ (Gibco), 20% heat-inactivated FBS, and 1% non-essential amino acids (Gibco). Cells were kept at 37 °C under conditions of 10% CO2 until 80% confluence was reached.
Characterizing immunomodulation in HT-29 cells
First, we seeded HT-29 cells into 24-well plates (3 × 105 cells per well). After 24–48 h, confluence was reached, and the complete medium was replaced by a McCoy’s 5A medium supplemented with 2% (v/v) FBS. After 24 h, we replaced this medium with McCoy’s 5A medium supplemented with 2% (v/v) FBS and TNF-α (5 ng/mL, InvivoGen) to which we added either (1) supernatant obtained from the culture medium during C. minuta’s stationary phase (at concentrations of 2%, 10%, or 20%) or (2) bacteria (at MOIs of 10, 50, or 100—ratio of bacteria to eukaryotic cells). PBS glycerol was used as a control. After 6 h of coincubation, supernatants were obtained from the cell cultures and stored at -80 °C until interleukine-8 (IL-8) concentrations could be quantified. The latter process was performed using a Human IL-8 ELISA MAX Standard Set (BioLegend); absorbance was measured at 450 nm using a FLUOstar® Omega microplate reader (BMG Labtech).
Characterizing immunomodulation in HT-29 cells transfected with a NF-κB luciferase reporter vector
HT-29 cells at a density of 3 × 105 cells per well were reverse-transfected with 200 ng pRelA-luc and 10 ng pRL-TK using X-tremeGENE HP DNA Transfection Reagent (Roche) in 24-well plates. Briefly, we prepared the transfection reagent:DNA complex as follows: we added the appropriate quantity of diluted plasmids to serum and antibiotics-free medium (final volume: 50 µL). The mixture was gently combined, and the transfection reagent was added at a ratio of 3:1. We gently mixed the transfection complex, which was then incubated for 15 min at room temperature. We subsequently seeded the complex with fresh cells (i.e., still in suspension). The plates were incubated for 24 h, and the medium was then remplaced by McCoy’s 5A medium supplemented with 2% (v/v) FBS. After 24 h of serum starvation, the medium was removed and replaced by McCoy’s 5A medium supplemented with 2% (v/v) FBS and 5 ng/mL of TNF-α (InvivoGen) and either (1) supernatant obtained from the culture medium during C. minuta’s stationary phase (concentration: 10%) or (2) bacteria and culture medium (10%) as a control. After 6 h of coincubation, we washed the cells twice with cold 1X PBS and lyzed them via exposure to 50 µL of Passive Lysis Buffer (Promega) for 15 min at room temperature under conditions of gentle shaking. Next, the lysates were transferred to microtubes. The Dual-Luciferase® Reporter Assay System (Promega) was used largely in accordance with the manufacturer’s instructions; a few minor modifications were made. Briefly, we transferred 2 × 20 µL samples of the lysates to white 96-well plates; then, we added 50 µL of LAR II solution to each well. First, we quantified levels of firefly luciferase activity. Then, we added 50 µL of Stop & Glo® Reagent, and we quantified levels of Renilla luciferase activity using a FLUOstar® Omega microplate reader (BMG Labtech). NF-κB activity was quantified via the ratio of firefly activity to Renilla activity.
Assessing effects on intestinal permeability by measuring transepithelial electrical resistance
We used the Caco-2 cell line to determine whether C. minuta could affect the epithelial barrier, as previously described74. Briefly, Caco-2 cells were grown on Transwell® inserts. When optimal transepithelial electrical resistance (TEER) values were reached (REMS AutoSampler, World Precision Instruments), fresh DMEM was added. Then, the C. minuta treatment (bacteria at MOI 40) or the control (PBS 1X) was applied to the apical compartment of the cells. Three hours later, 100 ng/mL of TNF-α (Peprotech) was added to the basal compartment of the cells. TEER was measured just before and 24 h after the treatments. The results were normalized (i.e., relative to basal TEER).
Assessing effects on DNBS-induced colitis in mice
We assessed the effects of a C. minuta treatment on DNBS-induced colitis in mice. We obtained 40 7-week-old male C57BL/6J mice from the Janvier Lab and maintained them under specific pathogen-free (SPF) conditions in the animal facilities of the French National Research Institute for Agriculture, Food, and Environment (IERP Experimental Unit, INRAE). They were housed in cages of five. Our experiments were performed in accordance with European Union legislation on animal welfare and were approved by COMETHEA, our local committee on animal experimentation (n°16744-201807061805486) and in compliance with the ARRIVE relevant guidelines. After a 7-day acclimation period, the 40 mice were divided into 4 groups (n = 10 mice/group): the vehicle control group (no inflammation; CTRL-Vehicle), the inflamed control group (inflammation induced; DNBS-Vehicle), the treatment group (inflammation induced, treatment with C. minuta; DNBS-C. minuta), and the anti-inflammatory control group (inflammation induced, treatment with 5-ASA; DNBS-5-ASA). For two weeks, we gave the vehicle and inflamed control mice an oral dose of PBS (150 µL) containing 16% (v/v) glycerol and the treatment mice were given an oral dose of C. minuta (109 CFU/mL). The anti-inflammatory control mice were given an oral dose of 5-ASA (100 mg/kg; Sigma) from the day of the DNBS injection. Gavages were performed daily until the end of the experiment. Then, we anesthetized the mice using an intraperitoneal injection of 0.1% ketamine and 0.06% xylazine; we subsequently gave them an intrarectal injection of DNBS (175 mg/kg) dissolved in 30% ethanol (w/v). The vehicle control group received an intrarectal injection of 30% ethanol. Three days after the injections, the mice were euthanized. During the experiment, we measured body mass daily. Colon microscopic scores (Ameho), macroscopic scores (Wallace), and myeloperoxidase (MPO) activity levels were characterized as previously described75.
Assessing effects on TNBS-induced colitis in rats
We assessed the effects of a C. minuta treatment on TNBS-induced colitis in rats. We used Sprague Dawley rats and performed this research at an accredited contract research organization (Intestinal Biotech Development, Lille) in accordance with governmental regulations and in compliance with the ARRIVE relevant guidelines. The rats were divided into 4 different groups. For the first 14 days of the experiment, the vehicle (CTRL-Vehicle) and inflamed control rats (TNBS-vehicle) were gavaged with an oral dose of PBS (150 µL) containing 1% (v/v) glycerol. The rats were given an oral dose of C. minuta (109 CFU/mL) (TNBS-C. minuta); 5-ASA granules, the anti-inflammatory control group, were mixed into the rats’ food. Then, we anesthetized the rats for 2 h and administered an intrarectal injection of TNBS (80 mg/kg) dissolved in 40% ethanol (w/v) to induce colitis. The rats were euthanized four days after the injection, and the effects of the treatment and controls were assessed, as was colon mass. During the experiment, we measured body mass daily. Colon microscopic and macroscopic scores (Ameho and Wallace, respectively) were characterized as previously described40,76. We quantified inflammation by assessing the levels of the proinflammatory cytokines IL-1β and IL-6 and the anti-inflammatory cytokine IL-10 (eBioscience); the level of lipocalin-2 (LCN-2) (Cliniscience) in the colon was determined using ELISA. Briefly, a 1-cm stretch of the distal colon was recovered and homogenized (50 mg/mL) in Tris–HCl buffer containing protease inhibitors (Sigma) and ceramic beads (diameter: 1.4 and 2.8 mm) using a Precellys tissue homogenizer. Samples were centrifuged for 20 min, and the supernatant was frozen at − 80 °C.
Statistical analysis
All results were expressed as means ± standard error of the mean (SEM). We performed non-parametric statistical analyses—two-sided Mann Whitney U tests—using GraphPad Prism (v. 8.2.1; GraphPad Software). We employed an alpha level of 0.05.
Acknowledgements
This work has benefited from the facilities and expertise of @BRIDGe (Université Paris-Saclay, INRAE, AgroParisTech, GABI, 78350 Jouy-en-Josas, France). We wish to thank the staff of the INRAE Infectiology of Fishes and Rodents Facility (IERP-UE907, Jouy-en-Josas Research Center, France) in which animal experiments have been performed. IERP Facility belongs to the National Distributed Research Infrastructure for the Control of Animal and Zoonotic Emerging Infectious Diseases through In Vivo Investigation. R.M and P.L. would like to especially thank Patrizia Brigidi and Silvia Maravolta for introducing them in Christensenella´s world.
Author contributions
C.K., K.L.C., K.R., K.T., C.M. and F.C. performed the experiements. G.R., P.L., S.C. and R.M. conceived and supervised the project. C.K. wrote the first draft of the manuscript. G.R., P.L., S.C. and R.M. corrected the manuscript. All the authors have read and accepted the final version of the manuscript.
Competing interests
CK, KLC, KR, CM, GR and SC are employees of Ysopia Bioscience, during the conduct of the study; The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McDowell, C., Farooq, U. & Haseeb, M. Inflammatory bowel disease. In StatPearls (StatPearls Publishing, 2021). [PubMed]
- 2.Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020;145:16–27. doi: 10.1016/j.jaci.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J. Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, et al. CXCL8 chemokine in ulcerative colitis. Biomed. Pharmacother. 2021;138:111427. doi: 10.1016/j.biopha.2021.111427. [DOI] [PubMed] [Google Scholar]
- 5.Bourgonje AR, et al. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 2020;26:1034–1046. doi: 10.1016/j.molmed.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Flynn S, Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg. Clin. North Am. 2019;99:1051–1062. doi: 10.1016/j.suc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 7.van der Sloot KWJ, Weersma RK, Alizadeh BZ, Dijkstra G. Identification of environmental risk factors associated with the development of inflammatory bowel disease. J. Crohns Colitis. 2020;14:1662–1671. doi: 10.1093/ecco-jcc/jjaa114. [DOI] [PubMed] [Google Scholar]
- 8.Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature. 2020;578:527–539. doi: 10.1038/s41586-020-2025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019;16:531–543. doi: 10.1038/s41575-019-0172-4. [DOI] [PubMed] [Google Scholar]
- 10.Frank DN, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3:417–427. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokol H, et al. Prominence of ileal mucosa-associated microbiota to predict postoperative endoscopic recurrence in Crohn’s disease. Gut. 2020;69:462–472. doi: 10.1136/gutjnl-2019-318719. [DOI] [PubMed] [Google Scholar]
- 14.Bhaskaran N, et al. Role of Short Chain Fatty Acids in Controlling Tregs and Immunopathology During Mucosal Infection. Front. Microbiol. 2018;9:1995. doi: 10.3389/fmicb.2018.01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang X, et al. Systematic review and meta-analysis: Short-chain fatty acid characterization in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2019;25:1751–1763. doi: 10.1093/ibd/izz188. [DOI] [PubMed] [Google Scholar]
- 16.Kilinçarslan S, Evrensel A. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with inflammatory bowel disease: An experimental study. Actas Esp. Psiquiatr. 2020;48:1–7. [PubMed] [Google Scholar]
- 17.Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. BioMed Res. Int. 2015;2015:505878. doi: 10.1155/2015/505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voth E, Khanna S. Fecal microbiota transplantation for treatment of patients with recurrent Clostridioides difficile infection. Expert Rev. Anti Infect. Ther. 2020 doi: 10.1080/14787210.2020.1752192. [DOI] [PubMed] [Google Scholar]
- 19.Levy AN, Allegretti JR. Insights into the role of fecal microbiota transplantation for the treatment of inflammatory bowel disease. Ther. Adv. Gastroenterol. 2019 doi: 10.1177/1756284819836893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan P, Li X, Shen J, Feng Q. Fecal microbiota transplantation for the treatment of inflammatory bowel disease: An update. Front. Pharmacol. 2020;11:1409. doi: 10.3389/fphar.2020.574533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagliardi A, et al. Rebuilding the gut microbiota ecosystem. Int. J. Environ. Res. Public. Health. 2018;15:1679. doi: 10.3390/ijerph15081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill C, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 23.Martín R, et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015;15:67. doi: 10.1186/s12866-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai R, et al. Strain-specific anti-inflammatory properties of two akkermansia muciniphila strains on chronic colitis in mice. Front. Cell. Infect. Microbiol. 2019;9:239. doi: 10.3389/fcimb.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach Knudsen KE, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;10:1499. doi: 10.3390/nu10101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X-F, et al. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021 doi: 10.1007/s00394-021-02503-5. [DOI] [PubMed] [Google Scholar]
- 27.Basso PJ, Câmara NOS, Sales-Campos H. Microbial-based therapies in the treatment of inflammatory bowel disease: An overview of human studies. Front. Pharmacol. 2019;9:1571. doi: 10.3389/fphar.2018.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancabelli L, et al. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol. Ecol. 2017;93:fix153. doi: 10.1093/femsec/fix153. [DOI] [PubMed] [Google Scholar]
- 29.Morotomi N, et al. Evaluation of intestinal microbiotas of healthy Japanese adults and effect of antibiotics using the 16S ribosomal RNA gene based clone library method. Biol. Pharm. Bull. 2011;34:1011–1020. doi: 10.1248/bpb.34.1011. [DOI] [PubMed] [Google Scholar]
- 30.Goodrich JK, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascal V, et al. A microbial signature for Crohn’s disease. Gut. 2017;66:813–822. doi: 10.1136/gutjnl-2016-313235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pittayanon R, et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology. 2020;158:930–946.e1. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- 33.Braun T, et al. Individualized dynamics in the gut microbiota precede Crohnʼs disease flares. Am. J. Gastroenterol. 2019;114:1142–1151. doi: 10.14309/ajg.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 34.Lee M, Chang EB. Inflammatory bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. 2021;160:524–537. doi: 10.1053/j.gastro.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kechaou N, et al. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl. Environ. Microbiol. 2013;79:1491–1499. doi: 10.1128/AEM.03075-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nennig SE, Schank JR. The role of NFkB in drug addiction: Beyond inflammation. Alcohol Alcohol. Oxf. Oxfs. 2017;52:172–179. doi: 10.1093/alcalc/agw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee BC, Kim SH, Choi SH, Kim TS. Induction of interleukin-8 production via nuclear factor-κB activation in human intestinal epithelial cells infected with Vibrio vulnificus. Immunology. 2005;115:506–515. doi: 10.1111/j.1365-2567.2005.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odobasic D, Kitching AR, Holdsworth SR. Neutrophil-mediated regulation of innate and adaptive immunity: The role of myeloperoxidase. J. Immunol. Res. 2016 doi: 10.1155/2016/2349817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaCroix-Fralish ML, Austin J-S, Zheng FY, Levitin DJ, Mogil JS. Patterns of pain: Meta-analysis of microarray studies of pain. Pain. 2011;152:1888–1898. doi: 10.1016/j.pain.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- 41.Bokemeyer B, Hommes D, Gill I, Broberg P, Dignass A. Mesalazine in left-sided ulcerative colitis: Efficacy analyses from the PODIUM trial on maintenance of remission and mucosal healing. J. Crohns Colitis. 2012;6:476–482. doi: 10.1016/j.crohns.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Morotomi M, Nagai F, Watanabe Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam nov. Int. J. Syst. Evol. Microbiol. 2012;62:144–149. doi: 10.1099/ijs.0.026989-0. [DOI] [PubMed] [Google Scholar]
- 43.Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018;16:e2006842. doi: 10.1371/journal.pbio.2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zakrzewski M, et al. IL23R -protective coding variant promotes beneficial bacteria and diversity in the ileal microbiome in healthy individuals without inflammatory bowel disease. J. Crohns Colitis. 2019;13:451–461. doi: 10.1093/ecco-jcc/jjy188. [DOI] [PubMed] [Google Scholar]
- 45.Kummen M, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66:611–619. doi: 10.1136/gutjnl-2015-310500. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Indias I, et al. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am. J. Transl. Res. 2016;8:5672. [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan C, Graham M, Staley C, Subramanian S. Mucosal microbiota and metabolome along the intestinal tracts reveals location specific relationship. Mystems. 2018 doi: 10.1101/454496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013;7:1256–1261. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruaud A, et al. Syntrophy via interspecies H2 transfer between Christensenella and Methanobrevibacter underlies their global cooccurrence in the human gut. MBio. 2020;11:e03235-19. doi: 10.1128/mBio.03235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.den Besten G, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015;74:13–22. doi: 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- 52.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 53.Parada Venegas D, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorburn AN, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015;6:7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 55.Martín R, et al. Functional Characterization Of Novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: A step forward in the use of F. prausnitzii as a next-generation probiotic. Front. Microbiol. 2017;8:1226. doi: 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres-Maravilla E, et al. Identification of novel anti-inflammatory probiotic strains isolated from pulque. Appl. Microbiol. Biotechnol. 2016;100:385–396. doi: 10.1007/s00253-015-7049-4. [DOI] [PubMed] [Google Scholar]
- 57.Johannessen M, Askarian F, Sangvik M, Sollid JE. Bacterial interference with canonical NFκB signalling. Microbiology. 2013;159:2001–2013. doi: 10.1099/mic.0.069369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosignoli P, et al. Protective activity of butyrate on hydrogen peroxide-induced DNA damage in isolated human colonocytes and HT29 tumour cells. Carcinogenesis. 2001;22:1675–1680. doi: 10.1093/carcin/22.10.1675. [DOI] [PubMed] [Google Scholar]
- 59.Lenoir M, et al. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes. 2020;12:1–16. doi: 10.1080/19490976.2020.1826748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danne C, et al. A large polysaccharide produced by Helicobacter hepaticus induces an anti-inflammatory gene signature in macrophages. Cell Host Microbe. 2017;22:733–745.e5. doi: 10.1016/j.chom.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macho Fernandez E, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60:1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- 62.Breyner NM, et al. Microbial anti-inflammatory molecule (MAM) from Faecalibacterium prausnitzii shows a protective effect on DNBS and DSS-induced colitis model in mice through inhibition of NF-κB pathway. Front. Microbiol. 2017;8:114. doi: 10.3389/fmicb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piqué N, Berlanga M, Miñana-Galbis D. Health benefits of heat-killed (tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michielan A, D’Incà R. Intestinal permeability in inflammatory bowel disease: Pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157. doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo S, et al. Escherichia coli Nissle 1917 protects intestinal barrier function by inhibiting NF-κB-mediated activation of the MLCK-P-MLC signaling pathway. Mediators Inflamm. 2019;2019:5796491. doi: 10.1155/2019/5796491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohebali N, Ekat K, Kreikemeyer B, Breitrück A. Barrier protection and recovery effects of gut commensal bacteria on differentiated intestinal epithelial cells in vitro. Nutrients. 2020;12:2251. doi: 10.3390/nu12082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cuffaro B, et al. In vitro characterization of gut microbiota-derived commensal strains: Selection of parabacteroides distasonis strains alleviating TNBS-induced colitis in mice. Cells. 2020;9:2104. doi: 10.3390/cells9092104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martín R, et al. The Commensal Bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm. Bowel Dis. 2014;20:417–430. doi: 10.1097/01.MIB.0000440815.76627.64. [DOI] [PubMed] [Google Scholar]
- 69.Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993;64:456–460. [PubMed] [Google Scholar]
- 70.de Oliveira S, et al. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J. Immunol. Baltim. Md. 2013;1950(190):4349–4359. doi: 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaudhry SI, et al. Autocrine IL-1β-TRAF6 signalling promotes squamous cell carcinoma invasion through paracrine TNFα signalling to carcinoma-associated fibroblasts. Oncogene. 2013;32:747–758. doi: 10.1038/onc.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng C, Ouyang Y, Lu N, Li N. The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: Recent advances. Front. Immunol. 2020;11:1387. doi: 10.3389/fimmu.2020.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee C, et al. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017;51:47–56. doi: 10.1016/j.intimp.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 74.Chamignon C, et al. Evaluation of the probiotic properties and the capacity to form biofilms of various lactobacillus strains. Microorganisms. 2020;8:1053. doi: 10.3390/microorganisms8071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barone M, et al. A versatile new model of chemically induced chronic colitis using an outbred murine strain. Front. Microbiol. 2018;9:565. doi: 10.3389/fmicb.2018.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ameho CK, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor α production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–493. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]