Abstract
Purpose
Two types of intraocular lenses (IOLs), namely ultraviolet-filtering IOL (UVF-IOL) and blue-light-filtering IOL (BF-IOL), are used to replace the aging lens in cataract patients. This provides a clinical scenario to investigate the BF and UVF effects on circadian rhythm. We revisited this topic and conducted an updated meta-analysis investigating the effects of UVF-IOL and BF-IOL on sleep quality.
Methods
A literature search was conducted using the PubMed, Embase, and Cochrane Library databases, and finally, four randomized controlled trials, one nonrandomized controlled study, and two cohort studies were included in this meta-analysis.
Results
The fixed-effect model revealed a significantly larger sleep quality improvement in the UVF-IOL group than in the BF-IOL group (standard mean difference [SMD] = 0.10, 95% confidence interval [CI]: 0.00–0.21) at 3–8 weeks but not 7–12 months after IOL implantation (SMD = 0.03, 95% CI: −0.08 to 0.13). The random effects model revealed no difference between groups at 3–8 weeks (SMD = 0.16, 95% CI: −0.07 to 0.39) and 7–12 months (SMD = 0.03, 95% CI: −0.08 to 0.13) after IOL implantation.
Conclusions
Our study found some weak evidence supporting that UVF-IOL implantation demonstrated a greater improvement in subjective sleep quality than the BF-IOL implantation only in a shorter period but not in a longer period. More trials should be conducted before further recommendations. Nevertheless, our study provides some insights into the effects of short wavelength electromagnetic radiation on the circadian rhythm. PROSPERO registration number: CRD42019128832.
Subject terms: Quality of life, Perception
Introduction
Light, an entity of a combination of electromagnetic waves, affects the activities of possibly all organisms on earth [1]. The circadian rhythm, entrained by light input, is mediated by intrinsically photosensitive retinal ganglion cells (ipRGC) [2]. These retinal ganglion cells contain melanopsin—a photopigment that is sensitive to short wavelengths, especially the spectrum around 480 nm [3–7]. The axons of the ipRGC mainly project to the suprachiasmatic nucleus in the hypothalamus and regulate the secretion of melatonin by the pineal gland [8, 9], with peak production at 464 nm [10].
The normal human crystalline lens absorbs ultraviolet (UV; wavelength: 10–400 nm) and harmful short wavelengths in visible electromagnetic radiation, namely blue light (wavelength: 400–495 nm) [11]. Age-related cataracts present with a yellow-brownish discoloration, which indicates an accumulation of chromophores that preferentially absorb short wavelengths in the visible spectrum [12]; the discoloration predominantly reduces the input of short wavelength light and reduces the light input by more than 70% [13]. Currently, replacing the aging lens with an artificial intraocular lens (IOL) is the only treatment for improving light transmission in cataract patients [14]. A wide range of IOLs is available, and two types of IOL, namely the conventional UV-filtering IOL (UVF-IOL) and blue-light-filtering IOL (BF-IOL), are commonly used for implantation in patients with cataract [15]. These two types of IOLs are used to reduce damage caused by retinal pigment epithelial cells exposed to short wavelengths, mainly UV light (10–400 nm) and blue light (400–495 nm), and therefore reduce the risk of age-related macular degeneration [15–17]. A meta-analysis showed that postoperative visual performance did not differ between patients with BF-IOLs and those with UVF-IOL, but the colour vision was significantly compromised in blue light under mesopic conditions in patients with BF-IOL [18]. A systematic review of various types of studies based on the Oxford Centre for Evidence-Based Medicine criteria [19] and a systematic review of randomized controlled trials (RCTs) [20] did not provide a consensus regarding which of the two types of IOL offers greater photoprotection. Nevertheless, the choice between the two types of IOL in cataract surgery provides a clinical scenario for the investigation of the effects of BF and UVF on the circadian rhythm. Meta-analyses of nonrandomized controlled studies (NRSs) [21] and RCTs [22] have not provided evidence that either BF or UVF might be better than the other in terms of a normal circadian rhythm. Because new controlled trials and studies comparing the effects of UVF-IOL and BF-IOL implantation have been published recently, we revisited this issue and conducted an updated meta-analysis to investigate the effects of UVF and BF on sleep quality.
Methods
Search strategy and study eligibility
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines in this study. A systematic literature search was performed using the PubMed, Embase, and Cochrane Library databases until May 2019. A combination of keywords, namely sleep, cataract, and blue light in the form of title words or medical subject headings were used in the search. Two reviewers (T-ML and DW) performed the literature search independently. Discrepancies were resolved through discussion and consultation with the other two reviewers (K-WT and E-WL). The reference lists of all retrieved articles were manually examined to determine whether listed articles met the inclusion criteria. The proposal of our systematic review and meta-analysis was reviewed and accepted by the PROSPERO (registration number: CRD42019128832).
Inclusion and exclusion criteria
We investigated controlled trials or studies that compared UVF-IOL and BF-IOL replacement in cataractous eyes. Studies were included if they enroled patients diagnosed with cataract with nuclear opacification grades of ≥2 according to the Lens Opacities Classification System II; they were randomized or nonrandomized trials or controlled studies focusing on the effect of cataract surgery on sleep quality; and if patients had undergone either UVF-IOL or BF-IOL implantation following phacoemulsification. Studies were excluded if they were conference abstracts, single-arm trials or studies, or duplicate publications.
Bias assessment
Two methods were used for assessing the risk of bias in this study. The risk of bias of NRSs and cohort studies was assessed according to the Cochrane Methodology of Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) [23]. RCTs were assessed according to the revised Cochrane risk-of-bias tool for randomized trials (Rob 2.0, released 15 March 2019) [24]. The ROBINS-I included the following seven aspects: confounding bias, selection of participant bias, classification of intervention bias, deviations from intended intervention bias, missing data bias, outcome measurement bias, and selection of reporting bias. The Rob 2.0 included the following five aspects: bias arising from the randomization process, deviation from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Two reviewers (T-ML and DW) completed the risk-of-bias assessment independently. Disagreements were resolved through discussion and consultation with the other two reviewers (K-WT and E-WL).
Data extraction and outcomes of interest
Two reviewers (T-ML and DW) extracted data and compared results. Discrepancies were resolved through discussion and consultation with the other two reviewers (K-WT and E-WL). The outcome was primarily subjective sleep quality as measured by using the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Score (ESS), pictorial sleepiness scale (PSS), or other similar scales. A lower score in PSQI, ESS, and PSS implicates better sleep quality.
Data synthesis and statistical analysis
Changes in scores before and after surgery were used for meta-analyses. For trials that reported the baseline and endpoints, changes in scores were calculated using the method recommended in the Cochrane Handbook (a correlation between baseline and endpoint measurement of 0.5 was used) [25].
Continuous outcome data were analysed using the standard mean difference (SMD). The precision of each effect size was reported as a 95% confidence interval (CI). Cochran’s Q test was conducted, and the I2 was calculated to evaluate statistical heterogeneity and inconsistency between treatment effects across trials. To facilitate reporting, we tentatively assigned levels of heterogeneity, namely low, moderate, and high, to I2 values of 25–50%, 51–75%, and 76–100%, respectively, [26].
We reported both random effects [27] and fixed-effect [28] models in the meta-analyses. All analyses were performed using the Review Manager (version 5.3; The Cochrane Collaboration, 2014, the Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Literature search
The sampling procedures are delineated in Fig. 1. A total of 68 articles were identified in the initial search and a related trial was found in the reference list. After duplicates were removed, 43 articles were potentially relevant. Then, 20 irrelevant articles were excluded. After further examination of the contents of remaining articles, one reply letter, one conference paper, seven reviews and/or meta-analyses, two protocols, and five single-arm trials were excluded. Finally, seven articles, namely four RCTs [13, 29–31], one NRS [15], and two cohort studies [14, 32], were selected. The characteristics of the included trials and studies are summarized in Table 1. Their sample size ranged from 63 to 961 patients. Most of these trials and studies reported age information of the participants except one trial. The inclusion criteria of these trials and studies varied, so as the sides of implanted eyes (unilateral or bilateral), the interval to fellow eye surgery, and the assessment scale and time.
Fig. 1. Risk of bias.
Rob 2.0 revised Cochrane risk-of-bias tool was used to assess the randomized trials and ROBINS-I risk of bias was used to assess the nonrandomized studies of interventions. Sampling flowchart.
Table 1.
Characteristics of included trials and studies.
| Study | Size (M/F), Age | Inclusion criteria | SIFE | Scale, Assessment time |
|---|---|---|---|---|
| Brøndsted [30] | 76 (35/41), 73.7 | Departmental guidelines. Bilateral senile cataract eligible for cataract surgery. The eye with the lowest visual acuity was included. | 3 weeks | PSQI, 3 weeks |
| Brøndsted [29] | 76 (NI/NI), NI | Departmental guidelines. Bilateral senile cataract eligible for cataract surgery. The eye with the lowest visual acuity was included. | 3 weeks | PSQI, 12 months |
| Schmoll [13] | 59 (NI/NI), 71 | Unilateral or bilateral cataract, graded by degree of nuclear brunescence in either eye according to the Oxford Clinical Cataract Classification and Grading System. | NI | EES, 1 month |
| Zambrowski [31] | 204 (86/118), 76.2 ± 7.4 | Bilateral and best-corrected visual acuity worse than 20/32 in each eye (Monoyer chart at 5 m and converted to Snellen acuity). | 1–3 months | PSQI, 3–5 months |
| Feng [15] | 119 (52/48), 74.5 ± 5.7 | Bilateral, lens opacity ≥ N2, Lens Opacity Classification System II | NI | PSQI, 1 and 12 months |
| Alexander [14] | 961 (412/549), 76.9 ± 5.5 | Eye side not mentioned. National Health Service Guidelines, usually with vision worse than 6/12 (Snellen chart) | NI | PSQI, 1 and 12 months |
| Ayaki [32] | 206 (83/123), 74.1 ± 8.8 | Unilateral or bilateral. Posterior sub-capsular cataracts, nuclear sclerosis (Grade III or IV on the Emery Little classification), or a central cortical opacity disturbing the optical axis | 2–14 days | PSQI, 2 and 7 months |
Bias assessment
The assessment results are summarized in Table 2. Zambrowski et al. [31] demonstrated a low risk of bias in all five risk domains. Both Brøndsted et al. [30] and Brøndsted et al. [29] demonstrated a low risk of bias arising from the randomization process, missing outcome data, measurement of the outcome, and selection of the reported result and some concerns in the bias arising from deviation from intended interventions. Schmoll et al. [13] demonstrated some concerns in the bias arising from the randomization process, deviation from intended interventions, measurement of the outcome, and selection of the reported result and high risk of bias arising from missing outcome data. Deviation from intended intervention appeared to be common in the included RCTs mainly because of the deficiency of related information in the articles. In the NRS [15] and cohort studies [14, 32], none of the domains accessed exhibited a serious risk, whereas a moderate risk was predominant over confounding, deviations from intended intervention, measurement of outcomes, and selection of reported results. All three studies had a low risk in the classification of intervention. For the selection of participants into study and missing data, both the cohort studies demonstrated a low risk, whereas the NRS demonstrated a moderate risk.
Table 2.
Risk of bias.
| RoB 2.0 | Schmoll [13] | Brøndsted [30] | Brøndsted [29] | Zambrowski [31] |
|---|---|---|---|---|
| RCT | RCT | RCT | RCT | |
| Randomization process | Some concerns | Low risk | Low risk | Low risk |
| Deviation from intended interventionsa | Some concerns | Some concerns | Some concerns | Low risk |
| Missing outcome data | High risk | Low risk | Low risk | Low risk |
| Measurement of the outcome | Some concerns | Low risk | Low risk | Low risk |
| Selection of the reported result | Some concerns | Low risk | Low risk | Low risk |
| Overall | High risk | Some concerns | Some concerns | Low risk |
| ROBIN-I | Alexander [14] | Ayaki [32] | Feng [15] | |
|---|---|---|---|---|
| Cohort | Cohort | NRS | ||
| Confounding | Moderate | Moderate | Moderate | – |
| Selection of participants into study | Low | Low | Moderate | – |
| Classification of intervention | Low | Low | Low | – |
| Deviations from intended intervention | Moderate | Moderate | Moderate | – |
| Missing data | Low | Low | Moderate | – |
| Measurement of outcomes | Moderate | Moderate | Moderate | – |
| Selection of reported results | Moderate | Moderate | Moderate | – |
aEffect adhering to intervention.
Subjective sleep quality
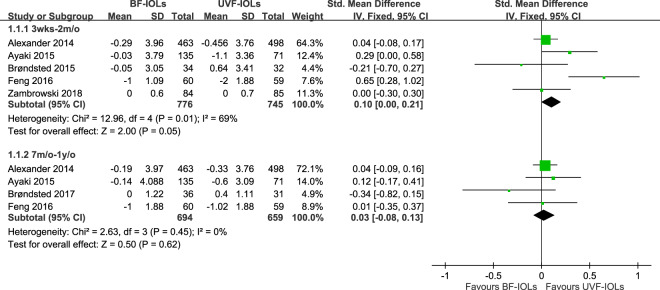
All included studies compared the effect of the two types of IOL on subjective sleep quality. Among them, four included trials and studies compared the effect of the two types of IOL on PSQI 3–8 weeks [14, 15, 30, 32] and four on 7–12 months [14, 15, 29, 32] after implantation; one included study reported the finding using a PSS with the change of score calculated from the 1-week average 2 months after surgery and the 1-week average before surgery [31], and one included study reported a non-significant finding on ESS without providing any statistical detail [13]. Finally, six studies with statistical results were used in the meta-analysis. Figure 2 demonstrates the pooled effects of the two types of IOL on changes in subjective sleep quality in a fixed-effect model, and Fig. 3 demonstrates the effects in a random effects model. The fixed-effect model revealed a significantly larger amplitude of subjective sleep quality improvement in the UVF-IOL group than in the BF-IOL group (SMD = 0.10, 95% CI: 0.00–0.21) 3–8 weeks after implantation with moderate heterogeneity across trials (p = 0.01, I2 = 69%). The between-group difference 7–12 months after implantation (SMD = 0.03, 95% CI: −0.08 to 0.13) and the heterogeneity across trials (p = 0.45, I2 = 0%) were not significant. No difference was observed between the two analyses (p = 0.31, I2 = 2.0%).
Fig. 2. Forest plot comparing ultraviolet-filtering intraocular lenses (UVF-IOLs) and blue-light-filtering IOL (BF-IOLs) in the fixed-effect model.
Test for subgroup difference: χ2 = 1.02, df = 1 (p = 0.31, I2 = 2.0%).
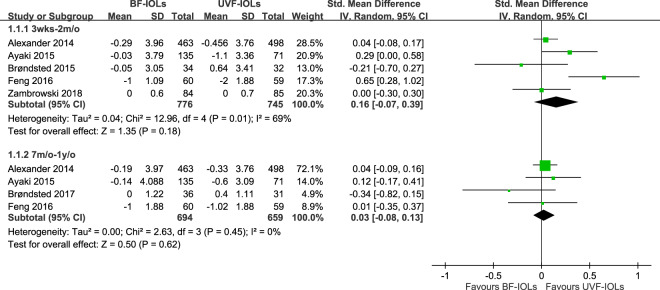
Fig. 3. Forest plot comparing ultraviolet-filtering intraocular lenses (UVF-IOLs) and blue-light filtering.
IOL (BF-IOLs) in the random effects model. Test for subgroup difference: χ2 = 1.02, df = 1 (p = 0.31, I2 = 1.8%).
The random effects model, though demonstrates a trend in the same direction, did not reveal a statistical between-group difference at 3–8 weeks (SMD = 0.16, 95% CI: −0.07 to 0.39) after implantation. Similar to that observed in the fixed model, no difference was found in 7–12 months (SMD = 0.03, 95% CI: −0.08 to 0.13). Moderate heterogeneity across the trials was observed 3–8 weeks after implantation (p = 0.01, I2 = 69%) but not 7–12 months after implantation (p = 0.45, I2 = 0%). Similarly, there was no difference between two analyses (p = 0.31, I2 = 1.8%).
Discussion
Our study demonstrated that UVF-IOL implantation significantly reduced the PSQI score compared with BF-IOL implantation in cataract patients 3–8 weeks after surgery according to the fixed-effect model. No significant difference was observed between the two types of implantation 7–12 months after surgery, either by random- or fixed-effect models.
On most occasions, the random effects model is used in a meta-analysis. Similarly, two previous meta-analyses examining the blue-filtering effect on cataract patients used the random effects model in their meta-analyses. The fixed-effect model may be used in a meta-analysis in the following conditions: (1) all studies included in the analysis are functionally identical and (2) the study goal is to compute the common effect size for an identical population and not generalize the findings to other populations [28, 32, 33]. These conditions were met in our study. The results of the fixed-effect model appeared more reasonable because physiological changes caused by IOL implantation are attributable only to a change of wavelength in a small, closed, and fixed environment in cataract patients. In other words, the meta-analysis performed using the fixed-effect model is appropriate in our study because all included trials are functionally identical, and the inference is limited to the specific population included in the analysis. However, it should be reminded that the significant findings of our study are rather weak.
In addition to trials and studies that reported changes in subjective sleep quality prospectively, investigations using other experimental designs have been published. Lander et al. conducted a retrospective nonrandomized study comparing the effects of UVF-IOL and BF-IOL on sleep quality [34]. The study reported no difference between the groups. The results of single-arm interventions for PSQI scores [2, 3], ESS scores [3], number of poor sleepers [2], melatonin secretion profiles [35], and other sleep quality indicators [2, 35] described the changes before and after the implantation of the types of IOL examined. Similarly, the meaning of meta-analysis [22] of the single-arm interventions simply means that the types of IOL investigated is effective in improving subjective sleep quality in cataract patients.
The short-term difference between UVF-IOL and BF-IOL implantation in cataract patients may be partially explained by the circadian system reacting to acute changes in light exposure, which eventually habituates and restores to the pre-exposure status [14, 15, 36]. Cataract surgery would increase blue-light transmission by ~250% in cataract patients with BF-IOL implantation and 300% in cataract patients with UVF-IOL implantation [30]. The additional blue-light transmission, which benefits sleep quality, appears to be adapted by the circadian system and returns to the norm after a period. However, both types of IOLs probably effectively improve sleep quality, and the difference can be detectable in the early period after IOL implantation but not distinguishable after 7–10 months. The adaption phenomenon was also observed in the RCT conducted by Schmoll et al. that the ESS reduction was significant for cataract patients who had one-eye surgery but not for those who had two-eye surgery as compared with control [13]. Additional studies are required to test this hypothesis. Furthermore, the possibility of a ceiling effect cannot be excluded; the effect could have resulted from treatments having reached their highest capacity or effects being not measurable. Although BF-IOLs are promoted to preserve the macular health and prevent age-related macular degeneration [31], decreased light transmission in BF-IOLs may affect colour vision, contrast sensitivity, and scotopic sensitivity, in addition to sleep quality and circadian rhythms [37]. Nevertheless, recent systematic reviews have demonstrated that BF-IOL and UVF-IOL do not differ in terms of best-corrected visual acuity, short-term contrast sensitivity, macular pigment optical density, contrast sensitivity, colour discrimination, daytime alertness, reaction time, or patient satisfaction [20, 37, 38].
Both methodological and clinical factors might have contributed to the heterogeneity observed in this study. First, our included studies adopted different experimental designs, namely RCT, NRS, and cohort studies, meaning different levels of measuring errors. Second, different diagnostic criteria were used for the included studies, implying that patient populations might consist of cataract patients of different severity, and thus, might need different recovery speed after surgery and time length for optical and circadian adjustments. Third, although three controlled trials conducted implantations for both eyes of their patients, one cohort study included those who underwent unilateral or bilateral implantation, and the other cohort study did not report information on this issue. This poses a question of light dose effects.
Our study has a few limitations. First, the scenario occurred in cataract patients. The question of whether blue-filtering effects may be generalized to the general population, for example, people using blue-filtering spectacles and screens, remains unanswered. Second, most of the cataract patients in the included trials were elderly patients and had severe cataract. Thus, the findings of this study may not be applied to younger patients with cataract or patients with mild cataract.
Conclusions
Our study found some weak evidence demonstrating that UVF-IOL implantation causes a greater improvement in subjective sleep quality than BF-IOL implantation within a short period but not after a longer period as revealed in the fixed model. The strength of evidence has not yet indicated a need of concerning UVF-IOL in priority. The choices should be based on ophthalmological indications and considerations. Nevertheless, our findings provide some insights into the effects of short wavelength electromagnetic radiation on the circadian rhythm. Additional trials and studies with larger sample sizes are warranted to clarify this issue.
Summary
What was known before
UVF-IOL and BF-IOL are commonly used for implantation in cataract patients. Both are designed to reduce the potential damage of short wavelengths on the retina and retinal pigment epithelium, while BF-IOL was introduced later to prevent chromatic aberration and cyanopsia; further synthesis showed that BF-IOL compromises the colour vision in blue light under mesopic conditions.
The choice of IOL provides a clinical scenario for the investigation of the effects of BF and UVF on the circadian rhythm.
Previous syntheses did not find any evidence that UVF-IOL and BF-IOL affect the circadian rhythm differently.
What this study adds
Our updated synthesis found weak evidence that UVF-IOL implantation causes a greater improvement in subjective sleep quality than BF-IOL implantation within a short period.
The findings have little impact on clinical practice but provide further insight into the effects of light on the circadian rhythm.
Acknowledgements
This study was funded by the Taipei Medical University for newly employed teaching staff (grant number: TMU107-AE1-B05).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tsung-Min Lee, El-Wui Loh
References
- 1.Turek FW. Circadian rhythms. Recent Prog Horm Res. 1994;49:43–90. doi: 10.1016/b978-0-12-571149-4.50007-6. [DOI] [PubMed] [Google Scholar]
- 2.Wei X, She C, Chen D, Yan F, Zeng J, Zeng L, et al. Blue-light-blocking intraocular lens implantation improves the sleep quality of cataract patients. J Clin Sleep Med. 2013;9:741–45. doi: 10.5664/jcsm.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shenshen Y, Minshu W, Qing Y, Yang L, Suodi Z, Wei W. The effect of cataract surgery on salivary melatonin and sleep quality in aging people. Chronobiol Int. 2016;338:1064–72. doi: 10.1080/07420528.2016.1197234. [DOI] [PubMed] [Google Scholar]
- 4.Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 2013;280:20122987. doi: 10.1098/rspb.2012.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–4. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 6.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–54. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 7.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–73. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 8.Turner PL, Mainster MA. Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92:1439–44. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benarroch EE. Suprachiasmatic nucleus and melatonin: reciprocal interactions and clinical correlations. Neurology. 2008;71:594–8. doi: 10.1212/01.wnl.0000324283.57261.37. [DOI] [PubMed] [Google Scholar]
- 10.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simunovic MP. On seeing yellow: the case for, and against, short-wavelength light-absorbing intraocular lenses. Arch Ophthalmol. 2012;130:919–26. doi: 10.1001/archophthalmol.2011.1642. [DOI] [PubMed] [Google Scholar]
- 12.Kessel L, Siganos G, Jørgensen T, Larsen M. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep. 2011;34:1215–9. doi: 10.5665/SLEEP.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmoll C, Khan A, Aspinall P, Goudie C, Koay P, Tendo C, et al. New light for old eyes: comparing melanopsinmediated non-visual benefits of blue-light and UV-blocking intraocular lenses. Br J Ophthalmol. 2014;98:124–8. doi: 10.1136/bjophthalmol-2013-304024. [DOI] [PubMed] [Google Scholar]
- 14.Alexander I, Cuthbertson FM, Ratnarajan G, Safa R, Mellington FE, Foster RG, et al. Impact of cataract surgery on sleep in patients receiving either ultraviolet-blocking or blue-filtering intraocular lens implants. Investig Ophthalmol Vis Sci. 2014;55:4999–5004. doi: 10.1167/iovs.14-14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng X, Xu K, Hao Y, Qi H. Impact of blue-light filtering intraocular lens implantation on the quality of sleep in patients after cataract surgery. Medicine. 2016;95:e5648. doi: 10.1097/MD.0000000000005648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downes SM. Ultraviolet or blue-filtering intraocular lenses: what is the evidence? Eye. 2016;30:215–21. doi: 10.1038/eye.2015.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augustin AJ. Reliable UV-light protection in intraocular lenses–scientific rationale and quality requirements. Klin Monbl Augenheilkd. 2014;231:901–8. doi: 10.1055/s-0034-1368566. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XF, Zou HD, Yu YF, Sun Q, Zhao NQ. Comparison of blue light-filtering IOLs and UV light-filtering IOLs for cataract surgery: a meta-analysis. PloS ONE. 2012;7:e33013. doi: 10.1371/journal.pone.0033013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Kelly D, Nolan JM, Dennison JL, Beatty S. The evidence informing the surgeon’s selection of intraocular lens on the basis of light transmittance properties. Eye. 2017;31:258–72. doi: 10.1038/eye.2016.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downie LE, Busija L, Keller PR. Blue-light filtering intraocular lenses (IOLs) for protecting macular health. Cochrane Database Syst Rev. 2018;5:Cd011977. doi: 10.1002/14651858.CD011977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erichsen JH, Brøndsted AE, Kessel L. Effect of cataract surgery on regulation of circadian rhythms. J Cataract Refractive Surg. 2015;41:1997–2009. doi: 10.1016/j.jcrs.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Zheng L, Wu XH, Lin HT. The effect of cataract surgery on sleep quality: a systematic review and Meta-analysis. Int J Ophthalmol. 2017;10:1734–41. doi: 10.18240/ijo.2017.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10:29–31. [Google Scholar]
- 25.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; 2011. www.cochrane-handbook.org.
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Nikolakopoulou A, Mavridis D, Salanti G. Demystifying fixed and random effects meta-analysis. Evid Based Ment Health. 2014;17:53–7. doi: 10.1136/eb-2014-101795. [DOI] [PubMed] [Google Scholar]
- 29.Brøndsted AE, Haargaard B, Sander B, Lund-Andersen H, Jennum P, Kessel L. The effect of blue-blocking and neutral intraocular lenses on circadian photoentrainment and sleep one year after cataract surgery. Acta Ophthalmol. 2017;95:344–51. doi: 10.1111/aos.13323. [DOI] [PubMed] [Google Scholar]
- 30.Brøndsted AE, Sander B, Haargaard B, Lund-Andersen H, Jennum P, Gammeltoft S, et al. The effect of cataract surgery on circadian photoentrainment: a randomized trial of blue-blocking versus neutral intraocular lenses. Ophthalmology. 2015;122:2115–24. doi: 10.1016/j.ophtha.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 31.Zambrowski O, Tavernier E, Souied EH, Desmidt T, Le Gouge A, Bellicaud D, et al. Sleep and mood changes in advanced age after blue-blocking (yellow) intra ocular lens (IOLs) implantation during cataract surgical treatment: a randomized controlled trial. Aging Ment Health. 2018;22:1351–6. doi: 10.1080/13607863.2017.1348482. [DOI] [PubMed] [Google Scholar]
- 32.Ayaki M, Negishi K, Suzukamo Y, Tsubota K. Color of intra-ocular Lens and cataract type sre prognostic determinants of health indices after visual and photoreceptive restoration by surgery. Rejuvenation Res. 2015;18:145–52. doi: 10.1089/rej.2014.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. 1st ed. Chichester, West Sussex, UK: John Wiley & Sons; 2009. pp. 77–85.
- 34.Landers JA, Tamblyn D, Perriam D. Effect of a blue-light-blocking intraocular lens on the quality of sleep. J Cataract Refract Surg. 2009;35:83–8. doi: 10.1016/j.jcrs.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Hosoe K, Hamada T, Morita T. Change in sleep state of the elderly before and after cataract surgery. J Physiol Anthropol. 2010;29:219–24. doi: 10.2114/jpa2.29.219. [DOI] [PubMed] [Google Scholar]
- 36.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–55. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 37.Cuthbertson FM, Peirson SN, Wulff K, Foster RG, Downes SM. Blue light-filtering intraocular lenses: review of potential benefits and side effects. J Cataract Refract Surg. 2009;35:1281–97. doi: 10.1016/j.jcrs.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 38.Augustin AJ. Blue light-filtering IOLs—currently available data. Klin Monbl Augenheilkd. 2010;227:617–23. doi: 10.1055/s-0029-1245524. [DOI] [PubMed] [Google Scholar]